The discovery by Garcia et al. 1 that 20-HETE activates GPR75 and signals via Gαq/11/PLC/PKC and c-Src/EGFR pathways to elicit vascular effects represents a transformative milestone in the field of cytochrome P450 eicosanoids. It is the first demonstration that a member of this class of eicosanoids acts via a GPR. The GPR75 was previously deorphanized and the chemokine, RANTES/CCL5 was identified as its endogenous ligand. Activation of this receptor was reported to protect hippocampus from amyloid β toxicity and to stimulate insulin secretion in pancreatic islet cells.2,3
Studies over the past 35 years have revealed that 20-HETE is a major metabolite of AA produced by enzymes of CYP 4A and 4F families in the blood vessels, kidney, heart, lung and other tissues. It plays a critical role in the regulation of vascular reactivity, sodium transport, endothelial dysfunction, oxidative stress, cell proliferation, vascular hypertrophy, inflammation, angiogenesis and the control of blood pressure. 4,5 Increased levels of 20-HETE are associated with hypertension, stroke, myocardial infarction, vasospasm, and vascular restenosis. 4,5 The existence of 20-HETE receptors was first foreseen by the finding that inactive analogues of 20-HETE are competive antagonists of its vasoconstrictor actions. 6 Subsequent studies indicating that the vasoconstrictor, and natriuretic actions of 20-HETE are PLC/PKC-dependent, while its effects on cell migration and proliferation, endothelial dysfunction, inflammation are associated with activation of the c-Src and MAPK pathways, futher suggest that 20-HETE act via GPRs. 4,5,7,8 However, the identification of this elusive receptor using binding studies has been fraught with difficulties because 20-HETE is rapidly esterified into membrane phospholipids, avidly binds to proteins, and distributes intracellularly. 4 Garcia et al. 1 overcome these limitations utilizing a novel multi-step strategy by cross-linking a relatively polar and photoactive 20-HETE antagonist to the cell surface, then using click chemistry to attach a fluorescent tag, followed by the isolation of the labeled proteins, proteomics, and bioinformatics to identify binding partners and ultimately the receptor. Their successful approach indicates that this strategy is a viable template for identification of receptors for other CYP eicosanoids and lipid meditators.
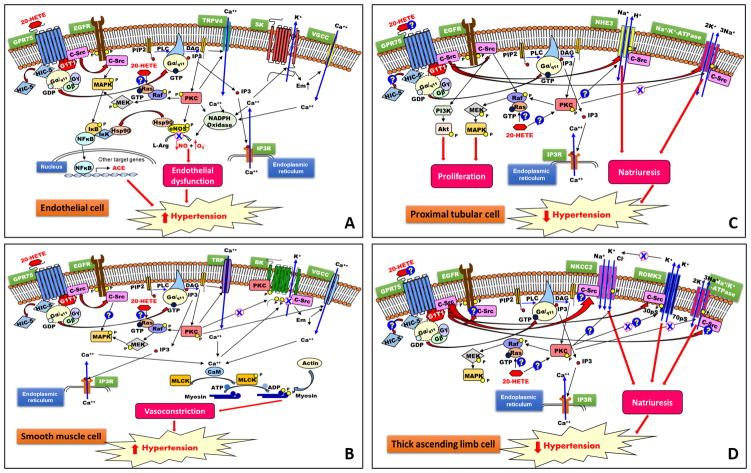
Garcia et al. 1 went on to developed an antibody to GPR75 for immunoprecipitation studies to determine the mechanisms of the G protein signaling. They demonstrated that activation of GPR75 by 20-HETE in human endothelial cells promotes dissociation of the Gαq/11 subunit and release of c-Src from G1T1 which is bound to the receptor (Figure 1A). Gaq/11 activates PLC that in turn hydrolyzes PIP2 to IP3 and DAG, which promotes phosphorylation, activation and translocation of PKC. 9 c-Src released from GPR75 binds to and phosphorylates the EGFR, which activates the MAPK/IKK-β/NF-κB pathway. This leads to uncoupling of eNOS, endothelial dysfunction, and increased expression of ACE. 8 Similarly, the authors found that 20-HETE activation of GPR75 in rat aortic VSMCs promoted disassociation of Gαq/11 which is known to activate the PLC/DAG/IP3/PKC pathway to increase intracellular calcium and the vasoconstrictor response to Gq receptor agonists. They also demonstrated increased association of PKCα and c-Src and enhanced tyrosine phosphorylation of the BK β channel subunit in VSMCs. This is consistent with previous reports that the vasoconstrictor response to 20-HETE is associated with increased TRPC6 and decreased BK channel activities that depolarize the membrane and promote calcium entry through voltage-sensitive calcium channels. 5,10 One limitation of the present study, however, is that the authors did not directly show that knockdown of GPR75 blocks the vasoconstrictor effect of 20-HETE or its inhibitory effect on BK channel activity.
Figure 1.
Hypothetical incorporation of the GPR75 signaling pathways in attempt to explain the established vascular and renal tubular effects of 20-HETE. The effects of 20-HETE to uncouple eNOS, promote endothelial dysfunction and increase the expression of ACE in the endothelium are presented in Panel A. Panel B presents the vasoconstrictor action of 20-HETE in VSMCs. The natriuretic effects of 20-HETE in the proximal tubule and thick ascending loop of Henle are summarized in Panels C and D. Arrows indicate increased activity, while crosses indicate inhibitory actions. Question marks indicate actions that are not fully defined.
The most exciting aspect of the study of Garcia et al 1 is they established a role for GPR75 in a 20-HETE dependent mouse model of hypertension. Mutations in CYP4A11 and CYP4F2 are associated with the development of hypertension in man. 11,12 Studies in CYP4A14 KO, inducible CYP4A12 transgenic and DHT-treated mouse models indicate that increased vascular 20-HETE production contributes to the development of hypertension. These models are associated with uncoupling of eNOS, endothelial dysfunction, increased vascular reactivity and hypertrophy, and activation of the local RAS. 12 Inhibition of 20-HETE also lowers pressure in a variety of angiotensin II-dependent models of hypertension. 11 Garcia et al. 1 has now demonstrated that knockdown of the expression of GPR75 mimics the effects of 20-HETE inhibitors to prevent the development of hypertension and vascular hypertrophy in a CYP4A12 transgenic mouse model. These findings imply that GPR75 may be a viable target for the treatment of hypertension.
The identification of GPR75 as the first 20-HETE receptor raises many questions and opportunities for followup investigation. First, is GPR75 the only receptor for 20-HETE? Additional bands were labeled by the 20-HETE antagonist. Does this suggest the existence of other receptors or binding partners? For example, there is some evidence that 20-HETE, like DAG, may serve as an intracellular second messenger and directly activate PKC and/or small G-proteins. 4 It is vitally important to determine whether GPR75 is expressed and 20-HETE is produced in all the tissues where it acts. GPR75 is highly expressed in the brain. CYP4A enzymes have been identified in pial vessels, astrocytes and neonatal hippocampal neurons, 4,5 but it is difficult to detect nonesterfied 20-HETE in brain tissue. 13 20-HETE inhibitors reduce infarct size after stroke and protect neonatal hippocampal neurons from hypoxic injury. 4,5 However, it is not known whether the neurodegenerative effects of 20-HETE are mediated by activation GPR75. In contrast, activation of GPR75 with its endogenous ligand, RANTES/CCL5, is neuroprotective.2 Moreover, administration of CCL5 did not activate GPR75 signaling in endothelial cells, and the size of GPR75 protein isolated was smaller than expected.1 This suggests that multiple isoforms or splice variants of GPR75 might be expressed different tissues.
The study of Garcia et al. 1 has established that the signaling pathways activated by 20-HETE via GPR75 are consistent with its known actions in the endothelium and VSMCs (Figures 1A, B). 20-HETE also inhibits sodium reabsorption in the PT and TALH in part by activating PKC which phosphorylates serine 23 to inhibit Na+/K+-ATPase. 14 Moreover, 20-HETE inhibits the NHE3 exchanger in the PT via a signaling pathway that has not been fully determined, and blocks a 70 pS ROMK potassium channel possibly via c-Src 15 which recycles potassium essential for reabsorption of sodium by the NKCC transporter in the TALH (Figures 1C, D). 4,5 Data presented in the study of Garcia et al. 1 and the GeneAtlas database indicates that the expression of GPR75 at the mRNA and protein levels is relatively low in the kidney. Therefore, it will be important to establish that GPR75 is expressed in the PT and TALH, and mediates the natriuretic actions of 20-HETE. Similarly, GPR75 is highly expressed in the lung which produces 20-HETE. However, 20-HETE dilates, rather than constricts, bronchiole smooth muscle and pulmonary arteries 4,5 and it will be interesting to determine if GPR75 plays a role in the vasodilatory responses.
Overall, the discovery that GPR75 serves as a 20-HETE receptor is a scientific breakthrough in understanding the role of CYP450 eicosanoids in the regulation of cardiovascular function. Since 20-HETE plays a critical role in the pathogenesis of hypertension, stroke, myocardial infarction, vascular hypertrophy, renal ischemia/reperfusion injury, angiogenesis, cell proliferation and cancer, 4,5 it is likely that the identification of this first 20-HETE receptor will herald the discovery of additional receptors for CYP metabolites of AA and other fatty acids, and lead to the development of drugs targeting these pathways for the treatment of cardiovascular disease.
Acknowledgments
Sources of Funding
This study was supported by grants HL36279 (Roman) and DK104184 (Roman), AG050049 (Fan) and P20GM104357 (Roman and Fan) from the NIH and a grant16GRNT31200036 (Fan) from the American Heart Association.
Non-standard Abbreviations and Acronyms
- 20-HETE
20-Hydroxyeicosatetraenoic acid
- AA
Arachidonic acid
- ADP
Adenosine diphosphate
- Akt
Protein Kinase B
- ATP
Adenosine Tri-Phosphate
- BK
Calcium-activated potassium channel
- CaM
Calmodulin
- CCL5
Chemokine (C-C motif) ligand 5
- c-Src
Proto-oncogene tyrosine-protein kinase
- CYP
Cytochrome P450
- DAG
Diacylglycerol
- DHT
Dihydrotestosterone
- EGFR
Epidermal growth factor receptor
- eNOS
Endothelial nitric oxide synthase
- G1T1
GPCR-kinase interacting protein 1
- GPR
G protein-coupled receptor
- HIC-5
Hydrogen peroxide-inducible clone 5 protein
- Hsp90
Heat shock protein 90
- IKK-β
inhibitor of nuclear factor kappa-B kinase subunit beta
- IP3
Inositol triphosphate
- IP3R
Inositol triphosphatereceptor
- MAPK
Mitogen-activated protein kinases
- MEK
Mitogen-activated protein kinase kinase
- MLCK
Myosin light-chain kinase
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NHE3
Sodium–hydrogen antiporter 3
- NKCC2
Na-K-2Cl cotransporter
- PI3K
Phosphatidylinositol 3-kinase
- PIP2
Phosphatidylinositol 4, 5-bisphosphate
- PKC
Protein kinase C
- PLC
Phospholipase C
- PT
Proximal tubule
- RANTES
Regulated on activation, normal T cell expressed and secreted
- ROMK
Renal outer medullary potassium channel
- ROS
Reactive oxygen species
- SK
Small conductance calcium-activated potassium channels
- TALH
Thick ascending loop of Henle
- TRP
Transient receptor potential channels
- VGCC
Voltage gated calcium channel
- VSMC
Vascular smooth muscle cell
Footnotes
Disclosures
None.
References
- 1.Garcia V, Gilani A, Shkolnik B, Pandey V, Zhang FF, Dakarapu R, Gandham SK, Reddy NR, Graves JP, Gruzdev A, Zeldin DC, Capdevila JH, Falck JR, Schwartzman ML. 20-HETE Signals Through G Protein-Coupled Receptor GPR75 (Gq) to Affect Vascular Function and Trigger Hypertension. Circ Res. 2017 doi: 10.1161/CIRCRESAHA.116.310525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ignatov A, Robert J, Gregory-Evans C, Schaller HC. RANTES stimulates Ca2+ mobilization and inositol trisphosphate (IP3) formation in cells transfected with G protein-coupled receptor 75. Br J Pharmacol. 2006;149:490–497. doi: 10.1038/sj.bjp.0706909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu B, Hassan Z, Amisten S, King AJ, Bowe JE, Huang GC, Jones PM, Persaud SJ. The novel chemokine receptor, G-protein-coupled receptor 75, is expressed by islets and is coupled to stimulation of insulin secretion and improved glucose homeostasis. Diabetologia. 2013;56:2467–2476. doi: 10.1007/s00125-013-3022-x. [DOI] [PubMed] [Google Scholar]
- 4.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 5.Fan F, Ge Y, Lv W, Elliott MR, Muroya Y, Hirata T, Booz GW, Roman RJ. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci (Landmark Ed) 2016;21:1427–1463. doi: 10.2741/4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol. 1999;277:F790–796. doi: 10.1152/ajprenal.1999.277.5.F790. [DOI] [PubMed] [Google Scholar]
- 7.Akbulut T, Regner KR, Roman RJ, Avner ED, Falck JR, Park F. 20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR-and c-Src-dependent mechanism. Am J Physiol. 2009;297:F662–F670. doi: 10.1152/ajprenal.00146.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoopes SL, Garcia V, Edin ML, Schwartzman ML, Zeldin DC. Vascular actions of 20-HETE. Prostaglandins Other Lipid Mediat. 2015;120:9–16. doi: 10.1016/j.prostaglandins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu-Zhang AX, Newton AC. Protein kinase C pharmacology: refining the toolbox. Biochem J. 2013;452:195–209. doi: 10.1042/BJ20130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, Henriksen FH, Salomonsson M, Morita H, Kawarabayashi Y, Mori M, Mori Y, Ito Y. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res. 2009;104:1399–1409. doi: 10.1161/CIRCRESAHA.108.193227. [DOI] [PubMed] [Google Scholar]
- 11.Fan F, Muroya Y, Roman RJ. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr Opin Nephrol Hypertens. 2015;24:37–46. doi: 10.1097/MNH.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia V, Schwartzman ML. Recent developments on the vascular effects of 20-hydroxyeicosatetraenoic acid. Curr Opin Nephrol Hypertens. 2017;26:74–82. doi: 10.1097/MNH.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 13.Renic M, Klaus JA, Omura T, Kawashima N, Onishi M, Miyata N, Koehler RC, Harder DR, Roman RJ. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2009;29:629–639. doi: 10.1038/jcbfm.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowicki S, Chen SL, Aizman O, Cheng XJ, Li D, Nowicki C, Nairn A, Greengard P, Aperia A. 20-Hydroxyeicosa-tetraenoic acid (20 HETE) activates protein kinase C. Role in regulation of rat renal Na+, K+-ATPase. J Clin Invest 1997. 1997 Mar 15;99:1224–1230. doi: 10.1172/JCI119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu RM, Wei Y, Falck JR, Krishna UM, Wang WH. Effects of protein tyrosine kinase and protein tyrosine phosphatase on apical K(+) channels in the TAL. Am J Physiol Cell Physiol. 2001;281:C1188–1195. doi: 10.1152/ajpcell.2001.281.4.C1188. [DOI] [PubMed] [Google Scholar]