Summary
Fingolimod is the first oral disease therapy approved for relapsing-remitting multiple sclerosis (MS). Patients can be switched directly from interferon-β or glatiramer acetate to fingolimod without a washout period. Fingolimod's potent efficacy, oral route of administration, and generally good safety and tolerability make it appealing to patients and clinicians. However, several adverse effects have already been identified, overall experience with its use is limited compared to other agents, and its novel and complex mechanism of action make predicting adverse effects challenging. Fingolimod's place in the MS treatment algorithm is evolving. We anticipate that once centers have solved the logistical issues associated with fingolimod initiation and if the experience in clinical practice parallels the efficacy, safety, and tolerability demonstrated in the phase 3 trials, fingolimod's routine use will increase.
 |
Fingolimod (FTY720, Gilenya, Novartis) is the first oral disease therapy approved for relapsing-remitting multiple sclerosis (RRMS). This review summarizes its mechanism of action, clinical trial results for efficacy and safety, and clinical use.
Mechanism of action
The active form of fingolimod, fingolimod-phosphate, is a sphingosine 1-phosphate receptor (S1PR) modulator that renders naive and central memory T-cells and B-cells insensitive to the S1P chemotactic signal necessary for egress from lymph nodes. The resultant interference with lymphocyte recirculation is thought to reduce trafficking of pathogenic lymphocytes into the CNS in multiple sclerosis (MS).1 Peripheral lymphocyte counts decrease to 20%–30% of baseline within the first several weeks of fingolimod administration and recover in most patients 4–8 weeks after discontinuation. T cells are affected more than B cells, with a greater drop in CD4+ than CD8+ T cells. In addition, fingolimod penetrates the CNS, and interaction with S1PRs on neural cells may also contribute to its mechanism of action through neuroprotective or repair-promoting effects.1
Fingolimod should be used as monotherapy. There are no published data concerning safety and efficacy of fingolimod as combination therapy in MS or concerning use in progressive MS or neuromyelitis optica.
Efficacy results in clinical trials
Fingolimod received regulatory approval following 2 phase 3 clinical trials that evaluated fingolimod doses of 0.5 mg and 1.25 mg in RRMS. The FTY720 Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis (FREEDOMS) study2 was a 24-month placebo-controlled study of 1,272 patients. The Trial Assessing Injectable Interferon vs FTY720 Oral in Relapsing-Remitting Multiple Sclerosis (TRANSFORMS) study3 was a 12-month study of 1,292 patients comparing fingolimod to IM interferon β-1a (IFNβ-1a). Both studies demonstrated reduction in annualized relapse rate (the primary endpoint): 0.16–0.18 in the fingolimod groups vs 0.40 in the placebo group in FREEDOMS (p < 0.001); 0.16–0.20 in fingolimod groups vs 0.33 in the IFNβ-1a group in TRANSFORMS (p < 0.001). Fingolimod slowed disability progression relative to placebo in FREEDOMS and reduced MRI lesion activity and brain atrophy progression in both studies. Efficacy was similar for 0.5 mg and 1.25 mg fingolimod doses in both studies, while adverse events (AEs) were more common with 1.25 mg. The better efficacy–safety relationship led to submission of 0.5 mg for regulatory approval.
Adverse events
Known pharmacodynamic effects of fingolimod mediated by S1PRs explain many of its AEs. The mechanisms of others remain uncertain. The risk of any AE in the phase 3 trials was similar across treatment groups and ranged from 86% to 94%.2,3 In FREEDOMS, the study drug discontinuation rates for fingolimod 0.5 mg (7.5%) and placebo (7.7%) were similar, and less than for fingolimod 1.25 mg (14.2%). In TRANSFORMS, the rates of AEs leading to study drug discontinuation were 10% for fingolimod 1.25 mg, 5.6% for fingolimod 0.5 mg, and 3.7% for IFNβ-1a. AEs associated with fingolimod included laboratory abnormalities (lymphopenia or liver enzyme elevations), first-dose bradycardia or slowed atrioventricular (AV) conduction, hypertension, macular edema, possible increased infection risk, cough, dyspnea, back pain, headache, influenza, and diarrhea.
The total number of patients with infections with fingolimod was similar to the comparison groups, aside from mildly increased lower respiratory tract infections. Serious and severe infections were not increased. However, 2 of 3 deaths in patients treated with fingolimod 1.25 mg were related to herpesvirus infections. There was no correlation between lymphocyte count and incidence, type, or severity of infections.1 Because lymphocytes are not depleted but are redistributed from blood to secondary lymphoid organs and their overall function is preserved, the utility of monitoring peripheral blood lymphocyte counts, including lymphocyte subsets, to predict risk of infection is unclear at present.
Use in practice
Beginning in September 2010, the 0.5 mg dose of fingolimod was approved in multiple countries to reduce relapses and delay accumulation of physical disability in relapsing forms of MS (United States) or RRMS elsewhere. Some regulatory agencies, e.g., Health Canada and the European Medicines Agency, approved it as second-line therapy. In other countries, e.g., United States and Switzerland, it was approved as first-line therapy. However, some insurers in the United States have restricted it to second-line use.
Fingolimod is an appropriate option for patients with RRMS with intolerable side effects on IFNβ or glatiramer acetate (GA) or an inadequate therapeutic response (recognizing that precise criteria for this are lacking), particularly in patients who are seropositive for JC virus and, thus, at risk for progressive multifocal leukoencephalopathy with natalizumab.4 The utility of fingolimod relative to natalizumab in JC virus–seronegative patients remains to be defined. Although fingolimod has been approved in the United States as a first-line agent, at present we tend to recommend IFNβ or GA to treatment-naive patients, largely because of the greater experience with their use. Similarly, we do not routinely advise patients with effective disease control on IFNβ or GA and good tolerability to switch to fingolimod. However, we anticipate that if broad use in clinical practice and postmarketing studies confirm good safety, our use of fingolimod as a first-line agent will increase.
Practical aspects related to use of fingolimod are summarized in the table. Pretreatment testing includes complete blood count (CBC) and hepatic panel, pregnancy test in woman of childbearing potential, EKG to rule out bradycardia or an AV conduction abnormality, ophthalmic examination or optical coherence tomography to rule out macular edema, and varicella zoster serology for patients without a clearcut history of chicken pox. Varicella zoster vaccination should be considered prior to fingolimod initiation in patients without prior exposure. An eye examination should be repeated 3–4 months after starting fingolimod treatment. The package insert does not recommend a specific frequency for CBC and hepatic panel monitoring. In general, we repeat a CBC and hepatic panel every 6 months after starting fingolimod, but given the rapid drop in lymphocyte count, including to a level below 0.2 in a small proportion of patients, some practitioners monitor CBC earlier or more frequently.
Table The clinical use of fingolimod
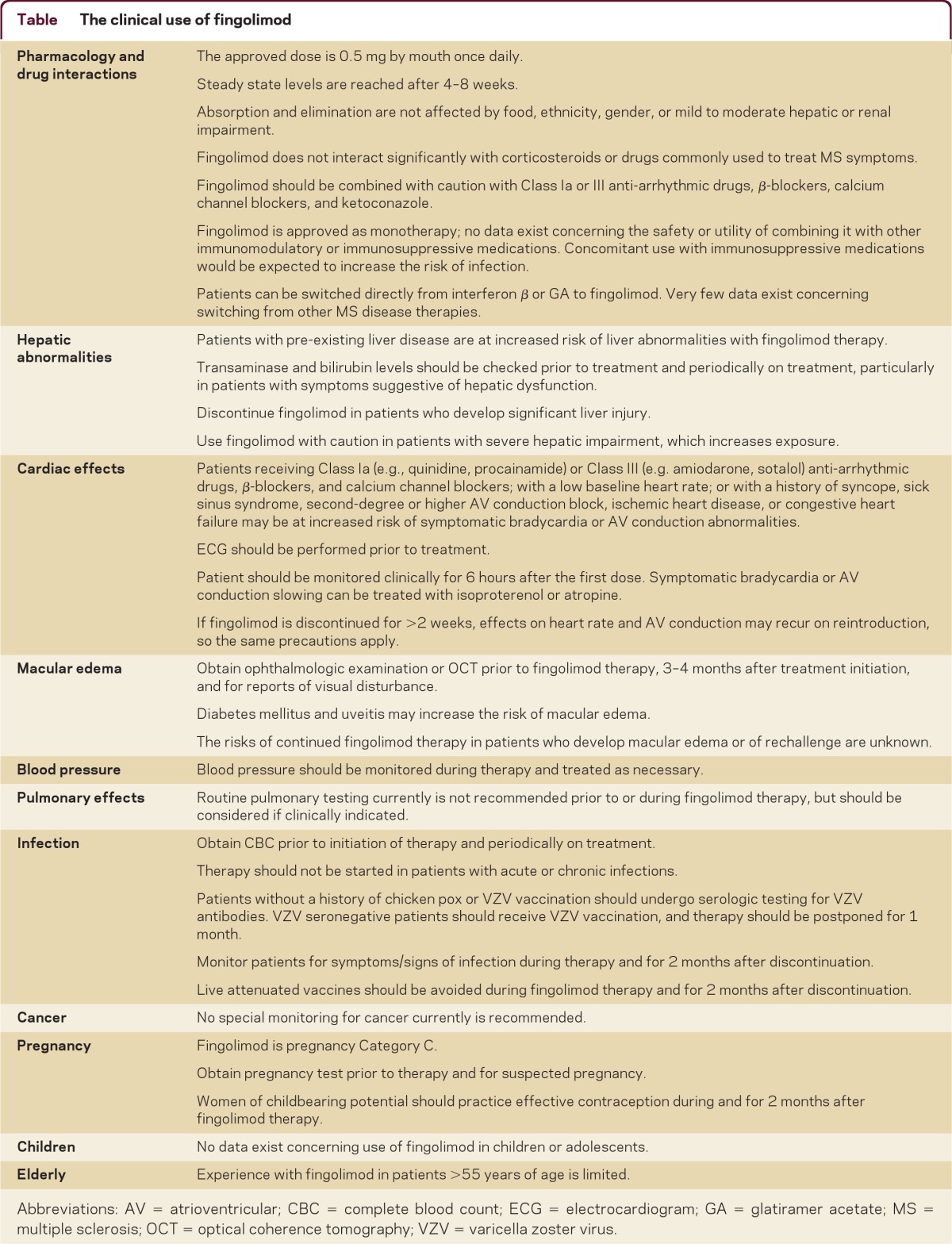
Initial binding of fingolimod-phosphate to S1PRs on atrial myocytes leads to transient bradycardia and rare AV conduction slowing. With receptor desensitization, these effects resolve despite continued treatment. United States Food and Drug Administration guidelines5 recommend that patients be observed for 6 hours following the first fingolimod dose, which creates logistical issues. Some MS centers such as ours have utilized group visits to provide nursing oversight and monitor vital signs after the first dose, and intervene if needed for symptomatic bradycardia or AV conduction block.
Fingolimod should be used as monotherapy. There are no published data concerning safety and efficacy of fingolimod as combination therapy in MS or concerning use in progressive MS or neuromyelitis optica. A phase 3 trial in primary progressive MS is ongoing. The safety and efficacy of fingolimod in pediatric and elderly patients are not established. Fingolimod should not be used during pregnancy. Women of childbearing potential should use effective contraception during and for 2 months after fingolimod therapy.
Although fingolimod has been approved in the United States as a first-line agent, at present we tend to recommend IFNβ or glatiramer acetate to treatment-naïve patients, largely because of the greater experience with their use.
Correspondence to: cohenj@ccf.org
Footnotes
Correspondence to: cohenj@ccf.org
REFERENCES
- 1. Cohen JA, Chun J. Mechanisms of fingolimod's efficacy and adverse effects in multiple sclerosis. Ann Neurol 2011;69:759–777. [DOI] [PubMed]
- 2. Kappos L, Radue E-W, O'Connor P, et al.. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. [DOI] [PubMed]
- 3. Cohen JA, Barkhof F, Comi G, et al.. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402–415. [DOI] [PubMed]
- 4. Gorelik L, Lerner M, Bixler S, et al.. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol 2010;68:295–303. [DOI] [PubMed]
- 5. NDA 02257 FDA-Approved Labeling Text for Gilenya (fingolimod) capsules. Basel: Novartis; 2010.