Abstract
Mesenchymal stem cells (MSCs) have the ability to self-renew and differentiate into multiple lineages making them an appropriate candidate for stem cell therapy. In spite of achieving considerable success in preclinical models, limited success has been achieved in clinical settings with MSCs. A major impediment that is faced is low survival of MSCs in injured tissues following implantation. In order to enhance the reparative properties of MSCs, it is vital to understand the molecular signals that regulate MSC survival and self-renewal. This review assimilates information that characterizes MSCs and mentions their utilization in myocardial infarction therapy. Additionally, our attempt herein is to gather pertinent published information regarding the role of canonical Wnt and BMP signaling in regulating the potential of MSCs to self-renew, proliferate, differentiate, and survive.
I. Introduction
Stem cell therapy is an exciting new field that shows a lot of promise. The efficacy of mesenchymal stem cells (MSCs) as treatment for regeneration and/or repair is currently being clinically tested. However, preclinical models of MSC-directed wound repair show disparate degrees of effectiveness. The greatest barrier faced in these models is the low levels of engraftment of the transplanted cells. Therefore, increasing MSC maintenance in the wound would increase their reparative capabilities. Understanding MSC biology would allow for clinicians to harness the potential these cells have to offer. Several published reports have looked at molecular mediators of MSC biology and this review aims to assemble such data. Particularly, data pertaining to the canonical Wnt and bone morphogenetic protein (BMP) signaling cascades, which have been implicated in regulating MSC differentiation, proliferation, and survival, will be discussed. Signals which amplify the MSC pool, that is, enhance their self-renewal, are largely unknown. This review will also discuss the effects of secreted Frizzled-related protein 2 (sFRP2) on self-renewal and ultimately try to elucidate important molecules involved in MSC biology.
II. Mesenchymal Stem Cells
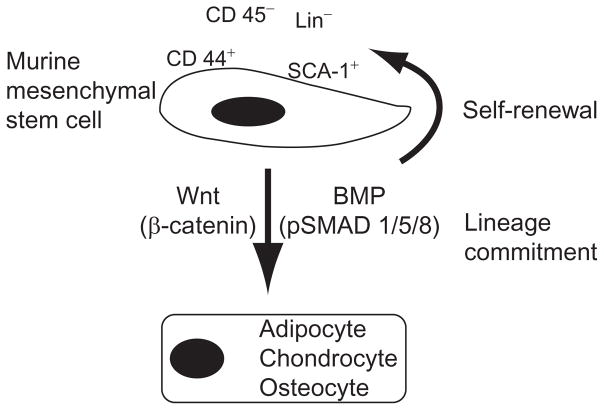
Stem cells are defined by their ability to self-renew and differentiate into multiple cell types (Fuchs and Segre, 2000). MSCs, traditionally defined as bone marrow-derived fibroblast-like cells, can also be isolated from adipose tissue (Madonna et al., 2009), umbilical cord blood (Harris, 2008; Zhang et al., 2011), and fetal tissues (Pozzobon et al., 2010). MSCs can differentiate into osteogenic, chondrogenic, and adipogenic lineages and give rise to any tissue derived from mesenchyme including bone, muscle, fibroblast, tendon, ligament, and adipose tissue (Pittenger et al., 1999; Tropel et al., 2004). Murine MSCs have been characterized to be negative for CD11b, CD14, CD31, CD34, and CD45 but positive for CD44, CD29, CD73, CD105, CD106, CD166, and Stem Cell Antigen 1 (SCA1) (Baddoo et al., 2003; Rombouts and Ploemacher, 2003; Short et al., 2003). However, the minimal criteria for antigenic identification is shown in Fig. 2.1, as murine MSCs must be lineage negative (Lin−), CD45−, CD44+, and SCA-1+.
Figure 2.1.
Definition and molecular mediators of murine mesenchymal stem cells. Murine MSCs are CD44+ and SCA-1+, but CD45−and Lin−. As stem cells, they must give rise to self (self-renewal) and undergo trilineage differentiation toward the adipogenic, chondrogenic, and osteogenic lineages. The cascades involved in the lineage commitment are labeled with their effector molecules in parentheses.
III. Differentiation of MSCs
The trilineage in vitro differentiation of MSCs can be confirmed in different manners. Molecular markers of adipogenic (i.e., peroxisome proliferator-activated receptor gamma, PPAR-γ), chondrogenic (i.e., Collagen XI or Runx2), and osteogenic (i.e., Osteocalcin or Runx2) differentiation may be quantified (Takada et al., 2009). Staining techniques exist for the visual confirmation of lineage commitment. Adipogenesis can be verified with Oil Red-O staining (Delorme and Charbord, 2007). Matrix calcification, which is present following osteogenesis, can be visualized with Alizarin Red or Von Kossa stains (Delorme and Charbord, 2007). Finally, glycosaminoglycans can be stained with Alcian blue (Denker et al., 1999) or dimethylmethylene blue (Farndale et al., 1986) as confirmation of chondrogenic differentiation. Several cytokines, growth factors, adhesion molecules, and extracellular matrix components have been identified as cues that signal MSCs to differentiate (Kratchmarova et al., 2005; Mannello et al., 2006). Included among these are canonical Wnt and BMP signaling which will be discussed in more detail in the following sections.
IV. Self-Renewal
The self-renewal process allows a stem cell to perform symmetric cell division to give rise to two nondifferentiated daughter cells. For self-renewal to occur, stem cells must proliferate in such a way that apoptosis and differentiation are avoided (Satija et al., 2007; Schofield, 1983). Few reports have documented the self-renewal capability of MSCs (Song et al., 2006), and therefore, the signaling involved in this process is largely unknown. On the other hand, self-renewal has been widely documented in the hematopoietic stem cell (HSC) field, and the Wnt, Notch, and BMP signaling cascades are accepted as mediators of the maintenance of a non-differentiated HSC pool (McReynolds et al., 2007; Reya and Clevers, 2005; Suzuki and Chiba, 2005). Canonical Wnt signaling together with Notch and BMP signaling have also been shown to modulate stem cell self-renewal in the intestinal stem cell niche (Haramis et al., 2004; He et al., 2004; van Es et al., 2005). Additionally, Wnt signaling has been shown to direct self-renewal and differentiation of Islet1-expressing precursor cells in neo- and postnatal hearts (Klaus et al., 2007; Qyang et al., 2007; reviewed in reference Klaus and Birchmeier, 2008). Building on this knowledge, our laboratory has recently demonstrated that inhibition of BMP and Wnt by sFRP2 increases MSC self-renewal.
V. MSC Therapy
Although preclinical studies have recognized significant benefits of stem cell therapy, its translation for clinical application is still in its infancy. MSCs have been able to repair infarcted myocardium, bone, and soft tissue (Horwitz et al., 1999; Orlic et al., 2001). MSCs have been reported to induce angiogenesis and secrete paracrine and mitogenic growth factors (Iyer and Rojas, 2008; Kinnaird et al., 2004; Silva et al., 2005; Tang et al., 2004). Two major clinical approaches are utilized for stem cell therapy: endogenous mobilization of progenitor stem cells and exogenous transplantation of culture-expanded stem cells. Presently, there are around 100 clinical trials that involve exogenous human MSCs (http://clinicaltrials.gov/ct2/home; U.S. National Library of Medicine, accessed on 04/28/11). They are at various stages and target a wide variety of pathological conditions such as Crohn’s disease and graft versus host disease (GVHD), cardiovascular disease and myocardial infarction (MI), brain and spinal cord injury, ischemic stroke, diabetes, cartilage and bone injury (Phinney and Prockop, 2007). Mixed results have been obtained from these trials, and strategies to enhance MSC engraftment and survival in the regenerating tissues are being developed.
MSC therapy has demonstrated a few promising results. One particular trial documented that intracoronary administration of MSC following MI initially showed significant improvement in one parameter assessing left ventricular function; however, this difference was no longer significant when analyzed after 18 months (Meyer et al., 2006). A phase II clinical trial documented a 2-year reduction in the mortality rate in the GVHD patients treated with MSCs (Le Blanc et al., 2008). Clinically, MSCs homed to the site of injury when injected intravenously into irradiated osteogenesis imperfecta patients, engrafting into bones, skin, and marrow stroma, ultimately stimulating growth (Horwitz et al., 1999, 2002). There have been no adverse events identified in the past and ongoing trials, this being the most advantageous outcome from these trials.
VI. Immunomodulatory Properties
The majority of the clinical trials, approximately 44%, are utilizing MSCs for their immunomodulatory properties ((http://clinicaltrials.gov/ct2/home; U.S. National Library of Medicine, accessed on 04/28/11)). In conditions like GVHD, Crohn’s disease, primary Sjogren’s syndrome, organ transplantation and rejection, systematic sclerosis, type I diabetes, systemic lupus erythematosus, multiple sclerosis, neuroblastoma, and non-malignant red blood cell disorders, MSCs are being transplanted as treatment by themselves or as adjunct therapy.
The biology behind the effects of MSCs on the immune system is mostly unknown; however, a few experimental models have elucidated some of the key molecular players involved in the anti-inflammatory role of MSCs. One of these models is a rat renal transplantation model where MSC injections increased overall survival of the recipient animals due, in part, to a decrease in interleukin-1α (IL-1), tumor necrosis factor-α (TNF-α), and transforming growth factor (TGF)-β1 (Zhang et al., 2007). The authors of this work remind us that there are still unknowns, as they note that adjunct immunosuppresion (cyclosporine A) treatment that inhibits IL-2 signaling further increases the survival of the rats and further diminishes the levels of the inflammatory cytokines (Laupacis et al., 1982).
Other models have shown a similar effect of MSCs on the downregulation of the immune function. Such is a rodent model of interstitial lung disease, where bleomycin (a cytotoxic glycopeptide antibiotic; Mitchell et al., 1989) treatment induces pulmonary fibrosis and concomitant inflammation (Iyer and Rojas, 2008). Following induction of the disease, Ortiz et al., 2007 treated the mice with MSCs and demonstrated a decrease in the levels of two important proinflammatory cytokines: TNF-α and IL-1α. This group demonstrated the anti-inflammatory capacity of MSCs in this setting due to the expression of interleukin-1 receptor antagonist (IL-1RN). These results were confirmed by in vitro assays in which MSC-conditioned media decreased the proliferation of an IL-1 responsive T-cell population (Ortiz et al., 2007).
Studies using an experimental autoimmune encephalomyelitis model demonstrated that MSCs were able to suppress T-cell activation in vitro and in vivo, and this effect was partially reversible by the addition of IL-2 (Zappia et al., 2005). In this case, the authors suggest that the limited expression of MHC class II molecules as well as lack of costimulatory molecules, such as CD80, CD86, and CD40, on MSCs may be the reason behind the observed suppression. A more detailed study on secreted factors in MSC-conditioned media demonstrated that IL-10, TGF-β1, and prostaglandin E2 (PGE2) were not responsible for the T-cell inhibition (Shi et al., 2000).
In summary, the roles of MSCs in immune suppression have been partially described and the molecular mechanism behind this capacity remains elusive. Further studies on the effects of IL-1RN on T-cells might give insight into how MSCs inhibit inflammation and prevent T-cell activation.
VII. MI Therapy
Treatment for myocardial repair and ventricular dysfunction is the second most common application for MSCs in the clinic (U.S. National Library of Medicine). Recently, Osiris Therapeutics published the safety of utilizing MSCs in the setting of MI. Moreover, they demonstrated that MSC transplantation, compared to placebo control, increased left ventricular ejection fraction and reversed adverse remodeling (Hare et al., 2009). Although the data was only available after a 6-month follow-up, all MSC-treated patients had a statistical improvement in heart function.
In spite of having considerable potential, the clinical application of MSC-based therapy in the context of myocardial repair faces many challenges. Those challenges include poor tissue engraftment, low potency, and low survival of transplanted MSCs. In clinical trials, in spite of MSCs positively affecting myocyte regeneration, poor short-term survival with modest improvement in heart function together with inconsistent outcomes were identified (Lunde et al., 2006; Schachinger et al., 2006; Tendera et al., 2009). In the light of these observations, it is evident that the therapeutic effects of MSCs could only be seen if transplanted MSCs could exert the long-term benefits to the patients. It is therefore very important to understand the biology and the tissue repair mechanism of MSCs in order to overcome the limitations of cell engraftment and survival.
VIII. Molecular Mediators of MSC Biology
A. Wnt pathway
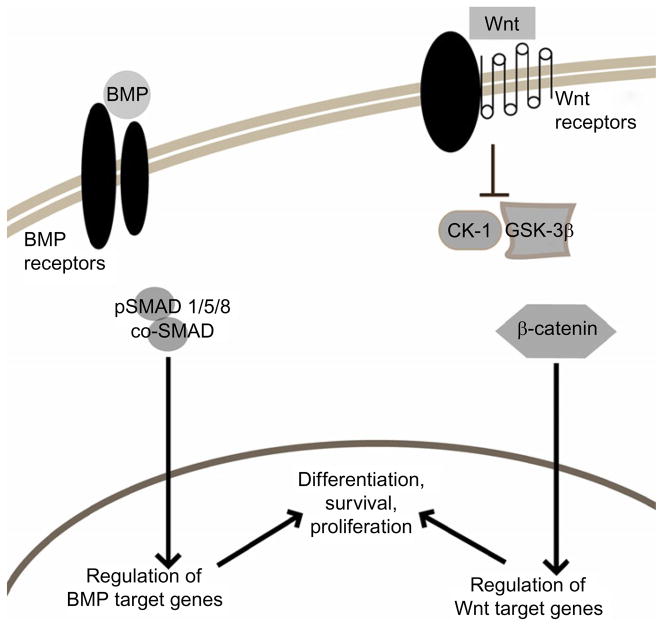
Figure 2.2 depicts a simplified model of the canonical cascade through which secreted Wnt glycoproteins initiate signal transduction upon receptor binding. In the absence of Wnt signaling, β-catenin is in a complex with Axin, APC, Dsh, GSK3-β, and CK-1 (Reya and Clevers, 2005). The latter two phosphorylate β-catenin, marking it for ubiquitination and subsequent proteosomal degradation. Occupancy of the transmembrane receptors LRP5/6 and Frizzled by Wnt family members block the kinase activity of the β-catenin destruction complex, allowing the accumulation of β-catenin in the cytoplasm and its translocation to the nucleus. Within the nucleus, this signaling molecule engages TCF/LEF DNA binding proteins to help drive expression of target genes (Moon et al., 2004).
Figure 2.2.
Simplified schematic of BMP and Wnt signaling. Activation of BMP and Wnt signaling can lead to the regulation of specific target genes which affect MSC biology. Transcriptional control is exerted on the cell upon the nuclear translocation of signaling molecules: phosphorylated Smads (pSMAD 1/5/8) in the case of BMP signaling and β-catenin in the case of Wnt signaling.
Inhibition of the Wnt pathway can occur at different stages of the cascade. For example, Dikkopf-1 (Dkk-1) antagonizes the signaling cascade when it binds LRP5/6, forms a ternary complex with Kremen, and promotes endocytosis and degradation of the receptor (Mao et al., 2001, 2002). The sFRP family members are capable to act as inhibitors by binding to and sequestering the Wnt ligand from its receptor (Kawano and Kypta, 2003; Uren et al., 2000).
The noncanonical and canonical Wnt signaling pathways are involved during embryonic development (Eisenberg and Eisenberg, 2006; Pandur et al., 2002). Wnt plays a role in cellular proliferation (Kioussi et al., 2002), differentiation, and self-renewal (Reya and Clevers, 2005; Wang and Wynshaw-Boris, 2004). More on the specific role of the Wnt cascade on MSC biology is included in subsequent sections.
B. Bone morphogenetic pathway
Members of the TGF superfamily include TGF-β, activins, and BMPs. These members play important and distinct roles in directing differentiation of stem cells during development and in the adult tissue (Korchynskyi and ten Dijke, 2002; Shi et al., 2000). As seen in Fig. 2.2, this superfamily signals through activation of distinct types of serine/threonine kinase receptors and subsequent activation of signaling molecules called Smads. Upon ligand binding to type II receptors, type I receptors are cross-phosphorylated by the activated type II receptor. The now activated type I receptor phosphorylates its receptor-associated Smad protein (pSMAD). Phosphorylated R-Smads must form a complex with Smad 4 (co-SMAD) to translocate to the nucleus and exert a transcriptional effect on the cell (Miyazono et al., 2005). The specificity of receptor activation and R-Smad activation is such that BMP activity uses only Smads 1, 5, and 8 as signaling molecules and thus directs transcription of target genes responsive to these Smads only. The role of BMP signaling in MSC biology will be expanded upon in the following sections.
C. Canonical Wnt and BMP signaling and MSC differentiation
MSCs possess an extensive potential to self-renew and differentiate into multiple lineages (Beresford, 1989; Caplan, 1991). MSCs can be induced to give rise to osteoblasts, chondrocytes, adipocytes, and myoblasts under suitable culture conditions (Caplan, 1991; Prockop, 1997). The ability to self-renew and differentiate into multiple lineages, easy isolation, and accessibility make MSCs a suitable candidate for therapeutic purposes. To enhance the beneficial effects of MSCs, it is necessary to identify the molecular mechanisms that regulate the self-renewal and differentiation of MSCs (Caplan, 2000). In this section, we will discuss the involvement of Wnt and BMP signaling in MSC differentiation.
1. Osteogenesis
The canonical Wnt signaling is involved in the lineage specification of MSCs (Etheridge et al., 2004). Typically, for osteogenic differentiation in culture conditions, MSCs are treated with ascorbic acid, β-glycerophosphate, and dexamethasone in fetal bovine serum (FBS)-containing medium (Jaiswal et al., 1997; Pittenger et al., 1999) that results in the increase in calcium deposition and alkaline phosphatase activity. The effect of canonical Wnt signaling on osteogenesis is context dependent. It varies from differences in the level of Wnt, type of cell, the species, type and timing of stimulus, and other experimental conditions. Enhanced Wnt signaling either by the addition of high levels of exogenous Wnt3a or through overexpression of LRP5 or stabilization of the mutant form of β-catenin augments osteogenesis in human MSCs (De Boer et al., 2004a; Gong et al., 2001; Qiu et al., 2007). On the other hand, some reports have identified that enhanced activity of Wnt signaling via exogenous addition of Wnt3a or lithium, which is a GSK3β inhibitor, diminishes osteogenesis in dexamethasone-induced human MSCs (de Boer et al., 2004b). In murine pluripotent mesenchymal and osteoprogenitor cells, canonical Wnt signaling promotes the osteoblastogenesis via upregulating RUNX2, Dlx5, or osterix (Bennett et al., 2005; Gaur et al., 2005). It is interesting to observe that the stage of targeted cells also determine the effect of canonical Wnt signaling on osteogenesis in some cases. For example, Wnt signaling enhances the differentiation of MSCs that are committed to osteogenic lineage, on the other hand, inhibiting the terminal differentiation of mature osteoblasts (Eijken et al., 2008; Kahler and Westendorf, 2003; Kahler et al., 2006, 2008).
The BMP cascade, as the name implies, is more clearly implicated in osteogenic commitment of MSCs. For example, adenoviral expression of BMP2, 6, and 9 significantly induced alkaline phosphatase activity in pluripotent C3H10T1/2 cells (Cheng et al., 2003). Similarly the preosteoblastic C2C12 cell line had increased alkaline phosphatase activity in the presence of BMP2, 4, 6, 7, and 9 (Cheng et al., 2003). This data translated to the human MSC field as adenoviral gene expression of BMP2 increased their osteogenic commitment as demonstrated by increased expression of Runx2 and Type I collagen and alkaline phosphatase activity (Koch et al., 2005). Human MSCs derived from osteoporotic patients responded beneficially to BMP2 and 7 as they increased alkaline phosphatase activity and total calcium production (Pountos et al., 2010).
2. Chondrogenesis
MSCs are treated with TGF-β for chondrogenic differentiation induction because it has been shown to be a key regulator of early stages of MSC chondrogenesis (Tuli et al., 2003). The chondrogenic differentiation results in the generation of cartilage-specific highly sulfated proteoglycans and type II collagen (Hwang et al., 2007). The expression of β-catenin is different in different stages of chondrogenesis, indicating a differential role of Wnt signaling during various stages of chondrogenesis (Ling et al., 2009). Canonical Wnt signaling has also been shown to regulate chondrocyte differentiation of MSCs in a Sox9-dependent manner (Yano et al., 2005). A dual role of Wnt signaling has been observed in the process of chondrogenesis, which depends on the particular Wnt ligand and also on the developmental stage. In the chicken limb, during the process of chondrogenesis, Wnt4a promotes the maturation of chondrocytes, whereas Wnt5a negatively affects chondrocyte maturation (Hartmann and Tabin, 2000). The observed opposing effect could be due to the involvement of distinct pathways. The activating affect of Wnt4a on chondrogenesis was possibly due to activation of β-catenin and Wnt receptors FZD1 and 7 (Hartmann and Tabin, 2000). Trabecular bone-derived MSCs induced for TGF-β-mediated chondrogenesis involve a cross talk between mitogen-activated protein kinase and Wnt signaling (Tuli et al., 2003). In the process, the canonical Wnt signaling regulates N-cadherin expression during cellular condensation and chondrogenesis. Recently, Maruyama et al. (2010) indicated that Wnt pathway regulates the fate of MSC lineage specification during skeletal development by modulating the balance of the fibroblast growth factor and BMP pathways.
Although BMP2 was implicated in directing osteogenesis of multipotential murine C3H10T1/2 cells, it was also implicated in their chondrogenic lineage commitment (Alcian blue stained cartilage-like matrix and type II collagen; Denker et al., 1999). BMP2-directed chondrogenesis in these cells is presumably due to the induced expression of the cell cycle inhibitory protein/differentiation factor p21/WAF1 (Carlberg et al., 2001). Although involved in noncanonical BMP signaling, BMP13 has been implicated in the chondrogenesis of these cells (Nochi et al., 2004).
3. Adipogenesis
In cell culture conditions, MSCs are induced toward adipogenic differentiation by treatment with dexamethasone, insulin, isobutyl methyl xanthine, and indomethacin in complete media containing serum (Janderova et al., 2003). The differentiation leads to the formation of the lipid vacuoles which are detected by Oil Red-O staining. The commitment of pluripotent MSCs toward adipogenic lineage leads to the formation of preadipocytes before terminally differentiating into mature adipocytes (Bowers and Lane, 2007). Inhibition of Wnt signaling is necessary for the adipogenic differentiation of MSCs (Moldes et al., 2003). The canonical Wnt signaling has been shown to inhibit the expression of adipogenic transcription factor PPARγ in MSCs (Rawadi et al., 2003). Overexpression of Axin2 or dominant-negative TCF4 leads to the inhibition of Wnt signaling which drives MSCs toward adipogenic differentiation (Ross et al., 2000). On the other hand, canonical Wnt1 and Wnt10b and an activated mutant β-catenin averts adipocyte differentiation via reducing the expression of adipogenic transcription factors C/EBPα and PPARγ (Bennett et al., 2005; Ross et al., 2000). It has been demonstrated that in addition to regulating MSC proliferation, cyclin D1 and c-Myc directly inactivate PPARγ and C/EBPα, respectively (Fu et al., 2005; Tetsu and McCormick, 1999). One can speculate that cyclin D1 and c-Myc might be involved in regulating the inhibitory effects of Wnt signaling on adipogenic transcription factors. Additionally, Wnt10b has also been demonstrated to act as a molecular switch from adipogenesis toward osteogenesis of bipotential mesenchymal precursors (Bennett et al., 2005).
Two separate cell lines (growth-arrested 10T1/2 and preadipocyte cell line, A33 cells, derived from 10T1/2 cells) were utilized to demonstrate the involvement of BMP4 in adipocyte development (Bowers and Lane, 2007). This cascade is deemed necessary for this as disruption of BMP4 signaling by noggin blocks the preadipocyte phenotype (Bowers et al., 2006). BMP2 was also implicated in adipogenesis, and overexpression of constitutively active BMP receptor 1A or 1B induced commitment (Huang et al., 2009).
D. Involvement of Wnt on survival and proliferation of MSCs
Many studies have suggested the role of canonical Wnt signaling in self-renewal and maintenance. Exogenous application of Wnt3a in cell culture enhanced MSC proliferation due to both increased proliferation and inhibition of apoptosis (Boland et al., 2004; Cho et al., 2006). The effect of Wnt3a on MSC proliferation is possibly due to its effect on cell cycle regulators, cyclin D1 and c-Myc (Baek et al., 2003). Additionally, the overexpression of LRP5, a coreceptor involved in Wnt signaling, has been demonstrated to enhance proliferation of MSCs (Baksh et al., 2007). On the other hand, canonical Wnt signaling has also been accounted for the inhibition of human MSC proliferation (Qiu et al., 2007). In addition, inhibition of Wnt signaling through Dkk-1 and LRP inhibitors was necessary for human MSCs to reenter cell cycle and proliferate (Gregory et al., 2003). Our studies also indicated that Wnt inhibition is a key factor required for better proliferation, engraftment, and survival of MSCs (Alfaro et al., 2008). One study has reported inhibition and stimulation of human MSC proliferation by Wnt3a is dose dependent (De Boer et al., 2004a). The differences in the findings about the role of Wnt signaling in MSC survival and proliferation may arise from differences in culture conditions, dose and type of Wnt ligands, and handling of the cells. These studies also indicate that Wnt signaling regulation of MSC biology (with specific regard to self-renewal) is a very complex mechanism and further studies are required to understand this mystery.
E. Effects of the BMP cascade on MSC survival and proliferation
BMPs are widely known to affect the differentiation of MSCs and therefore not much is documented on their roles on survival and proliferation, particularly on murine MSCs. Regardless, a few reports have emerged. Noncanonical BMP3 activated TGF-β signaling in C3H10T1/2 MSCs and 3T3-L1 preadipocytes to cause a threefold increase in their proliferation (Stewart et al., 2010). Human adipose-derived MSCs treated with low-dose BMP4 are less apoptotic and more proliferative (Vicente Lopez et al., 2010). Also, human MSCs derived from osteoporotic bones had increased proliferation after addition of high-dose BMP7 (Pountos et al., 2010). In essence, the effects of BMPs (canonical and noncanonical) on MSC survival and proliferation are largely context dependent and no solid data were found on murine bone marrow-derived MSCs.
IX. Enhancing MSC Survival in the Wound
As was briefly touched upon, the survival of MSCs within the wound microenvironment is a limiting factor on their reparative capabilities (Freyman et al., 2006; Hofmann et al., 2005). Thus, several groups have tried to alter MSCs genetically to increase their survival. Although Wnt and BMP signaling have not been directly tied to this effect, several other molecules which might be involved in such cascades will be addressed briefly. Retroviral expression of the prosurvival gene Akt-1 decreased the apoptotic index of MSCs in vitro, an effect also observed in vivo following transplantation into infarcted rat myocardium (Jiang et al., 2006; Mangi et al., 2003). A similar effect was observed with retroviral overexpression of erythropoietin by MSCs: increased survival both in vitro and in vivo in a murine subcutaneous implantation model of matrigel-embedded MSCs (Copland et al., 2008). Our group has shown that overexpression of sFRP2 by MSCs enhances their in vitro proliferative index and increases their engraftment in two separate in vivo wound models (Alfaro et al., 2008).
X. Secreted Frizzled-Related Proteins
The sFRP family was originally identified by their high homology to the Frizzled Wnt receptor (Rattner et al., 1997; Shirozu et al., 1996). There are eight family members; five of these are mammalian (Bovolenta et al., 2008). The function of the five mammalian members, sFRP1 to sFRP5, has been identified as Wnt inhibition (Chang et al., 1999; Finch et al., 1997; Schumann et al., 2000). sFRPs have a N-terminal conserved cysteine-rich domain (CRD) which shares 30–50% similarity with Frizzled proteins (Melkonyan et al., 1997). The C-terminal domain of sFRPs shares a sequence similarity with the axon guidance protein netrin (NTR). The NTR domain has also been found in a few complement proteins and in the tissue inhibitors of metalloproteases (Banyai and Patthy, 1999). sFRPs inhibit Wnt signaling via interacting through their CRD with Wnt ligands (Lin et al., 1997), by interaction of NTR-like domain with the Wnt ligands (Uren et al., 2000) or binding to itself and the Frizzled protein (Bafico et al., 1999). The contradiction in the mode of binding of sFRPs to Wnt and other ligands might be due to different affinity of sFRPs towards their binding partners. sFRPs are secreted proteins; however, in cultured cells, they have been found to be associated with the cell and are released into the culture media upon addition of heparin (Finch et al., 1997).
Further documented roles for the sFRP family members include cytoprotection and proliferation. sFRP2 seems to have a positive function in wound repair. This molecule was implicated in skeletal muscle repair in mice and has been identified as a key factor in MSC-based therapy for myocardial repair (Mirotsou et al., 2007; Zhao and Hoffman, 2004). Melkonyan et al., 1997 identified sFRP2 as a prosurvival protein and showed that its overexpression in human breast adenocarcinoma cell line, MCF7, conferred an antiapoptotic effect. Recently, Sfrp2 was identified as one of the key paracrine factors released by Akt-overexpressing MSCs found to play a critical role in the survival of ischemic cardiac myocytes (Mirotsou et al., 2007). Sfrp2 increases the proliferative index of both murine and human MSCs (Alfaro et al., 2008). Identifying a cytoprotective role for sFRP2 in the context of wound repair will provide insight into improving MSC tissue repair. Recently, a new hypothesis for an alternative role for the sFRP family has emerged; the mammalian members may inhibit the BMP signaling cascade much like the nonmammalian Sizzled members (Lee et al., 2006; Muraoka et al., 2006).
XI. Mediating MSC Self-Renewal
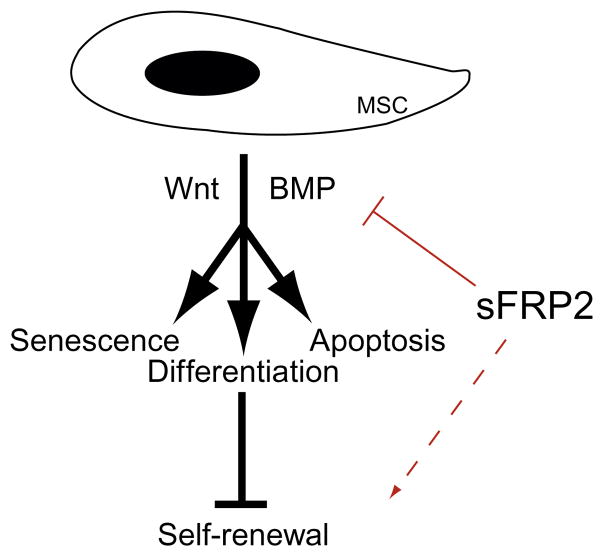
The mechanisms by which MSCs modulate Wnt and BMP signaling events during growth and/or lineage commitment are immensely complex. The molecular interplay between these cascades may modulate MSC self-renewal, a hypothesis based on the self-renewal mechanisms of other stem cells. Recent studies performed by our laboratory demonstrated that over-expression of the Wnt inhibitory protein, sFRP2, by MSCs (sFRP2-MSCs) increases in vitro proliferation (Alfaro et al., 2008) and survival of MSCs by regulating both the BMP and Wnt signaling pathways (Alfaro et al., 2010). The effects of sFRP2, as depicted in Fig. 2.3, indirectly affect MSC self-renewal.
Figure 2.3.
Model of the role of sFRP2 in MSC self-renewal. Self-renewal is evaded by senescence, lineage commitment, and apoptosis of MSCs; Wnt and BMP signaling drive these events. sFRP2 can inhibit these cascades to indirectly drive MSC self-renewal (discontinuous line).
Regardless of their mode of action, direct regeneration, or paracrine effects, MSCs could not impact the repair process until and unless a substantial amount of cells are generated via MSC self-renewal and proliferation within the wounded area. Although encouraging results have been observed in preclinical models (Hung et al., 2007; Shake et al., 2002; Shi et al., 2007), low survival and poor engraftment significantly restrict the efficiency of MSCs for remedial purposes in clinical setting. Some clinical trials address the issue of long-term engraftment of MSCs. For example, the engraftment of donor MSCs in osteogenesis imperfecta patients never exceeded 1% 4–6 weeks following MSC infusion (Horwitz et al., 2002). Enhanced survival of sFRP2-MSCs observed also in vivo in two distinct wound repair models still led to a modest degree of engraftment (Alfaro et al., 2008). Achieving a critical mass of MSCs by improving their survival and self-renewal capacity should increase their numbers in the damaged tissue to ultimately increase their reparative abilities. Figure 2.3 demonstrates one molecule that positively affects MSC self-renewal leading to enhanced wound repair.
XII. Conclusions
The focus of this review was BM-derived murine MSCs, with some discussion of their clinical utilization. The data demonstrated gaps in the knowledge of MSC biology, that if better understood would allow clinicians to improve the potential of MSC-directed wound repair. Particularly, we focused on canonical Wnt and BMP signaling to demonstrate how activation and/or inhibition of these two well-understood cascades could affect MSC activity, including cellular differentiation, survival, and proliferation.
The data gathered demonstrate that the effects of both Wnt and BMP signaling on MSCs are variable. The source of the MSCs (tissue of isolation), the passage number, the quantity of ligand, and the timing are all examples of variables that affect the outcome of these cascades on the MSCs. In general, however, BMP and Wnt signaling are thought to drive senescence, differentiation, and apoptosis of MSCs. We and others have recently identified that sFRP2 is a MSC-derived factor that modulates both Wnt and BMP pathways to improve MSC-mediated cardiovascular and wound repair. sFRP2 increased proliferation, prevented apoptosis, and decreased differentiation of MSCs. As a whole, these data suggest sFRP2 is a potent factor involved in MSC self-renewal. Increasing MSC self-renewal is critical to enhancing their therapeutic efficacy; therefore, other molecules that might have similar effects would be of interest to the field. The significance of this finding can be appreciated by looking at the impact self-renewal factors have had on other stem cell fields. Particularly, the ex vivo expansion of HSCs (CD34+) through addition of recombinant self-renewal factors is being actively pursued and has yielded important results in several clinical trials of a variety of human diseases (Kelly et al., 2009). Manipulation of the MSC self-renewal factors, including sFRP2 or other modulators of both Wnt and BMP signaling, could profoundly control the extent of their senescence, differentiation, and apoptosis enhancing the regeneration of MSC-mediated therapy.
References
- Alfaro MP, et al. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci USA. 2008;105(47):18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro MP, et al. sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. J Biol Chem. 2010;285(46):35645–35653. doi: 10.1074/jbc.M110.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddoo M, et al. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89(6):1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- Baek SH, et al. Regulated subset of G1 growth-control genes in response to derepression by the Wnt pathway. Proc Natl Acad Sci USA. 2003;100(6):3245–3250. doi: 10.1073/pnas.0330217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafico A, et al. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem. 1999;274(23):16180–16187. doi: 10.1074/jbc.274.23.16180. [DOI] [PubMed] [Google Scholar]
- Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- Banyai L, Patthy L. The NTR module: Domains of netrins, secreted frizzled related proteins, and type I procollagen C-proteinase enhancer protein are homologous with tissue inhibitors of metalloproteases. Protein Sci. 1999;8(8):1636–1642. doi: 10.1110/ps.8.8.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CN, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102(9):3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford JN. Osteogenic stem cells and the stromal system of bone and marrow. Clin Orthop Relat Res. 1989;240:270–280. [PubMed] [Google Scholar]
- Boland GM, et al. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93(6):1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, et al. Beyond Wnt inhibition: New functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121(Pt 6):737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- Bowers RR, Lane MD. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle. 2007;6(4):385–389. doi: 10.4161/cc.6.4.3804. [DOI] [PubMed] [Google Scholar]
- Bowers RR, et al. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: Role of the BMP-4 gene. Proc Natl Acad Sci USA. 2006;103(35):13022–13027. doi: 10.1073/pnas.0605789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells and gene therapy. Clin Orthop Relat Res. 2000;379(Suppl):S67–S70. doi: 10.1097/00003086-200010001-00010. [DOI] [PubMed] [Google Scholar]
- Carlberg AL, et al. Efficient chondrogenic differentiation of mesenchymal cells in micromass culture by retroviral gene transfer of BMP2. Differentiation. 2001;67(4–5):128–138. doi: 10.1046/j.1432-0436.2001.670405.x. [DOI] [PubMed] [Google Scholar]
- Chang JT, et al. Cloning and characterization of a secreted frizzled-related protein that is expressed by the retinal pigment epithelium. Hum Mol Genet. 1999;8(4):575–583. doi: 10.1093/hmg/8.4.575. [DOI] [PubMed] [Google Scholar]
- Cheng H, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- Cho HH, et al. Endogenous Wnt signaling promotes proliferation and suppresses osteogenic differentiation in human adipose derived stromal cells. Tissue Eng. 2006;12(1):111–121. doi: 10.1089/ten.2006.12.111. [DOI] [PubMed] [Google Scholar]
- Copland IB, et al. Coupling erythropoietin secretion to mesenchymal stromal cells enhances their regenerative properties. Cardiovasc Res. 2008;79(3):405–415. doi: 10.1093/cvr/cvn090. [DOI] [PubMed] [Google Scholar]
- De Boer J, Wang HJ, Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng. 2004a;10(3–4):393–401. doi: 10.1089/107632704323061753. [DOI] [PubMed] [Google Scholar]
- de Boer J, et al. Wnt signaling inhibits osteogenic differentiation of human mesenchymal stem cells. Bone. 2004b;34(5):818–826. doi: 10.1016/j.bone.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Delorme B, Charbord P. Culture and characterization of human bone marrow mesenchymal stem cells. Methods Mol Med. 2007;140:67–81. doi: 10.1007/978-1-59745-443-8_4. [DOI] [PubMed] [Google Scholar]
- Denker AE, et al. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: I. Stimulation by bone morphogenetic protein-2 in high-density micromass cultures. Differentiation. 1999;64(2):67–76. doi: 10.1046/j.1432-0436.1999.6420067.x. [DOI] [PubMed] [Google Scholar]
- Eijken M, et al. Wnt signaling acts and is regulated in a human osteoblast differentiation dependent manner. J Cell Biochem. 2008;104(2):568–579. doi: 10.1002/jcb.21651. [DOI] [PubMed] [Google Scholar]
- Eisenberg LM, Eisenberg CA. Wnt signal transduction and the formation of the myocardium. Dev Biol. 2006;293(2):305–315. doi: 10.1016/j.ydbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Etheridge SL, et al. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells. 2004;22:849–860. doi: 10.1634/stemcells.22-5-849. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Finch PW, et al. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc Natl Acad Sci USA. 1997;94(13):6770–6775. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyman T, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27(9):1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- Fu M, et al. Cyclin D1 inhibits peroxisome proliferator-activated receptor gamma-mediated adipogenesis through histone deacetylase recruitment. J Biol Chem. 2005;280(17):16934–16941. doi: 10.1074/jbc.M500403200. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Segre JA. Stem cells: A new lease on life. Cell. 2000;100(1):143–155. doi: 10.1016/s0092-8674(00)81691-8. [DOI] [PubMed] [Google Scholar]
- Gaur T, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- Gong Y, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Gregory CA, et al. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278(30):28067–28078. doi: 10.1074/jbc.M300373200. [DOI] [PubMed] [Google Scholar]
- Haramis AP, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303(5664):1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- Hare JM, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DT. Cord blood stem cells: A review of potential neurological applications. Stem Cell Rev. 2008;4(4):269–274. doi: 10.1007/s12015-008-9039-8. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127(14):3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- He XC, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36(10):1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Hofmann M, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111(17):2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99(13):8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, et al. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA. 2009;106(31):12670–12675. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SC, et al. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007;2(5):e416. doi: 10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang NS, et al. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2007;212(2):281–284. doi: 10.1002/jcp.21052. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: Novel concept for future therapies. Expert Opin Biol Ther. 2008;8(5):569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- Jaiswal N, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64(2):295–312. [PubMed] [Google Scholar]
- Janderova L, et al. Human mesenchymal stem cells as an in vitro model for human adipogenesis. Obes Res. 2003;11(1):65–74. doi: 10.1038/oby.2003.11. [DOI] [PubMed] [Google Scholar]
- Jiang S, et al. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99(7):776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- Kahler RA, Westendorf JJ. Lymphoid enhancer factor-1 and beta-catenin inhibit Runx2-dependent transcriptional activation of the osteocalcin promoter. J Biol Chem. 2003;278(14):11937–11944. doi: 10.1074/jbc.M211443200. [DOI] [PubMed] [Google Scholar]
- Kahler RA, et al. Lymphocyte enhancer-binding factor 1 (Lef1) inhibits terminal differentiation of osteoblasts. J Cell Biochem. 2006;97(5):969–983. doi: 10.1002/jcb.20702. [DOI] [PubMed] [Google Scholar]
- Kahler RA, et al. Collagen 11a1 is indirectly activated by lymphocyte enhancer-binding factor 1 (Lef1) and negatively regulates osteoblast maturation. Matrix Biol. 2008;27(4):330–338. doi: 10.1016/j.matbio.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116(Pt 13):2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Kelly SS, et al. Ex vivo expansion of cord blood. Bone Marrow Transplant. 2009;44(10):673–681. doi: 10.1038/bmt.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaird T, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94(5):678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Kioussi C, et al. Identification of a Wnt/Dvl/beta-Catenin –> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111(5):673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8(5):387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Klaus A, et al. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci USA. 2007;104(47):18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H, et al. Refined adenoviral transduction for controlled gene transfer into human adult mesenchymal stem cells. Z Orthop Ihre Grenzgeb. 2005;143(6):677–683. doi: 10.1055/s-2005-872526. [DOI] [PubMed] [Google Scholar]
- Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277(7):4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- Kratchmarova I, et al. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308(5727):1472–1477. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- Laupacis A, et al. Cyclosporin A: A powerful immunosuppressant. Can Med Assoc J. 1982;126(9):1041–1046. [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Lee HX, et al. Embryonic dorsal-ventral signaling: Secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell. 2006;124(1):147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, et al. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc Natl Acad Sci USA. 1997;94(21):11196–11200. doi: 10.1073/pnas.94.21.11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433(1–2):1–7. doi: 10.1016/j.gene.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Lunde K, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- Madonna R, Geng YJ, De Caterina R. Adipose tissue-derived stem cells: Characterization and potential for cardiovascular repair. Arterioscler Thromb Vasc Biol. 2009;29(11):1723–1729. doi: 10.1161/ATVBAHA.109.187179. [DOI] [PubMed] [Google Scholar]
- Mangi AA, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9(9):1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- Mannello F, et al. Role and function of matrix metalloproteinases in the differentiation and biological characterization of mesenchymal stem cells. Stem Cells. 2006;24(3):475–481. doi: 10.1634/stemcells.2005-0333. [DOI] [PubMed] [Google Scholar]
- Mao B, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411(6835):321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Mao B, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417(6889):664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Maruyama T, et al. The balance of WNT and FGF signaling influences mesenchymal stem cell fate during skeletal development. Sci Signal. 2010;3(123):ra40. doi: 10.1126/scisignal.2000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds LJ, et al. Smad1 and Smad5 differentially regulate embryonic hematopoiesis. Blood. 2007;110(12):3881–3890. doi: 10.1182/blood-2007-04-085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkonyan HS, et al. SARPs: A family of secreted apoptosis-related proteins. Proc Natl Acad Sci USA. 1997;94(25):13636–13641. doi: 10.1073/pnas.94.25.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer GP, et al. Intracoronary bone marrow cell transfer after myocardial infarction: Eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113(10):1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- Mirotsou M, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;104(5):1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, et al. Alpha-smooth muscle actin in parenchymal cells of bleomycin-injured rat lung. Lab Invest. 1989;60(5):643–650. [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16(3):251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Moldes M, et al. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J. 2003;376(Pt 3):607–613. doi: 10.1042/BJ20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, et al. WNT and beta-catenin signalling: Diseases and therapies. Nat Rev Genet. 2004;5(9):691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Muraoka O, et al. Sizzled controls dorsoventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol. 2006;8(4):329–338. doi: 10.1038/ncb1379. [DOI] [PubMed] [Google Scholar]
- Nochi H, et al. Adenovirus mediated BMP-13 gene transfer induces chondrogenic differentiation of murine mesenchymal progenitor cells. J Bone Miner Res. 2004;19(1):111–122. doi: 10.1359/jbmr.2004.19.1.111. [DOI] [PubMed] [Google Scholar]
- Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandur P, et al. Wnt11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418(6898):636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multi-potent stromal cells: The state of transdifferentiation and modesl of tissue repair-current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pountos I, et al. The effect of bone morphogenetic protein-2, bone morphogenetic protein-7, parathyroid hormone, and platelet-derived growth factor on the proliferation and osteogenic differentiation of mesenchymal stem cells derived from osteoporotic bone. J Orthop Trauma. 2010;24(9):552–556. doi: 10.1097/BOT.0b013e3181efa8fe. [DOI] [PubMed] [Google Scholar]
- Pozzobon M, Ghionzoli M, De Coppi P. ES, iPS, MSC, and AFS cells. Stem cells exploitation for pediatric surgery: Current research and perspective. Pediatr Surg Int. 2010;26(1):3–10. doi: 10.1007/s00383-009-2478-8. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Qiu W, et al. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. 2007;22(11):1720–1731. doi: 10.1359/jbmr.070721. [DOI] [PubMed] [Google Scholar]
- Qyang Y, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1(2):165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Rattner A, et al. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94(7):2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawadi G, et al. BMP2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18(10):1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17(1):160–170. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- Ross SE, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Satija NK, et al. Mesenchymal stem cells: Molecular targets for tissue engineering. Stem Cells Dev. 2007;16(1):7–23. doi: 10.1089/scd.2006.9998. [DOI] [PubMed] [Google Scholar]
- Schachinger V, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- Schofield R. The stem cell system. Biomed Pharmacother. 1983;37(8):375–380. [PubMed] [Google Scholar]
- Schumann H, et al. Expression of secreted frizzled related proteins 3 and 4 in human ventricular myocardium correlates with apoptosis related gene expression. Cardiovasc Res. 2000;45(3):720–728. doi: 10.1016/s0008-6363(99)00376-4. [DOI] [PubMed] [Google Scholar]
- Shake JG, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: Engraftment and functional effects. Ann Thorac Surg. 2002;73(6):1919–1925. doi: 10.1016/s0003-4975(02)03517-8. (Discussion 1926) [DOI] [PubMed] [Google Scholar]
- Shi Y, et al. BMP signaling is required for heart formation in vertebrates. Dev Biol. 2000;224(2):226–237. doi: 10.1006/dbio.2000.9802. [DOI] [PubMed] [Google Scholar]
- Shi M, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: Role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92(7):897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- Shirozu M, et al. Characterization of novel secreted and membrane proteins isolated by the signal sequence trap method. Genomics. 1996;37(3):273–280. doi: 10.1006/geno.1996.0560. [DOI] [PubMed] [Google Scholar]
- Short B, et al. Mesenchymal stem cells. Arch Med Res. 2003;34(6):565–571. doi: 10.1016/j.arcmed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Silva GV, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- Song L, et al. Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells. 2006;24(7):1707–1718. doi: 10.1634/stemcells.2005-0604. [DOI] [PubMed] [Google Scholar]
- Stewart A, Guan H, Yang K. BMP-3 promotes mesenchymal stem cell proliferation through the TGF-beta/activin signaling pathway. J Cell Physiol. 2010;223(3):658–666. doi: 10.1002/jcp.22064. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Chiba S. Notch signaling in hematopoietic stem cells. Int J Hematol. 2005;82(4):285–294. doi: 10.1532/IJH97.05115. [DOI] [PubMed] [Google Scholar]
- Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5(8):442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- Tang YL, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117(1):3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Tendera M, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: Results of randomized, multicentre myocardial regeneration by intracoronary infusion of selected population of stem cells in acute myocardial infarction (REGENT) trial. Eur Heart J. 2009;30(11):1313–1321. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tropel P, et al. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295(2):395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Tuli R, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278(42):41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- Uren A, et al. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem. 2000;275(6):4374–4382. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- U.S. National Library of Medicine, U.S.N.I.o.H., U.S. Department of Health & Human Services. Clinicaltrials.gov. Lester Hill National Center for Biomedical Communications; [Google Scholar]
- van Es JH, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435(7044):959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Vicente Lopez MA, et al. Low doses of bone morphogenetic protein 4 increase the survival of human adipose-derived stem cells maintaining their stemness and multi-potency. Stem Cells Dev. 2010;20(6):1011–1019. doi: 10.1089/scd.2010.0355. [DOI] [PubMed] [Google Scholar]
- Wang J, Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Curr Opin Genet Dev. 2004;14(5):533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Yano F, et al. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem Biophys Res Commun. 2005;333(4):1300–1308. doi: 10.1016/j.bbrc.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Zappia E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Zhang W, Qin C, Zhou ZM. Mesenchymal stem cells modulate immune responses combined with cyclosporine in a rat renal transplantation model. Transplant Proc. 2007;39(10):3404–3408. doi: 10.1016/j.transproceed.2007.06.092. [DOI] [PubMed] [Google Scholar]
- Zhang X, et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem. 2011;112(4):1206–1218. doi: 10.1002/jcb.23042. [DOI] [PubMed] [Google Scholar]
- Zhao P, Hoffman EP. Embryonic myogenesis pathways in muscle regeneration. Dev Dyn. 2004;229(2):380–392. doi: 10.1002/dvdy.10457. [DOI] [PubMed] [Google Scholar]