Abstract
Rationale
Neutrophilic airway inflammation plays a role in early structural lung disease in cystic fibrosis (CF), but the mechanisms underlying this pathway are incompletely understood.
Methods
Metabolites associated with neutrophilic inflammation were identified by discovery metabolomics on bronchoalveolar lavage fluid (BALF) supernatant from 20 preschool children (2.9±1.3 years) with CF. Targeted MS detection of relevant metabolites was then applied to 34 children (3.5±1.5 years) enrolled in AREST CF who underwent chest CT and BAL from two separate lobes during 42 visits. Relationships between metabolites and localized structural lung disease were assessed using multivariate analyses.
Results
Discovery metabolomics identified 93 metabolites associated with neutrophilic inflammation, including pathways involved in metabolism of adenyl purines, amino acids and small peptides, cellular energy, and lipids. In targeted MS, products of adenosine metabolism, protein catabolism, and oxidative stress were associated with structural lung disease and predicted future bronchiectasis, and activities of enzymes associated with adenosine metabolism were elevated in the samples with early disease.
Conclusions
Metabolomics analyses revealed metabolites and pathways altered with neutrophilic inflammation and destructive lung disease. These pathways can serve as biomarkers and potential therapeutic targets for early CF lung disease.
Keywords: bronchoalveolar lavage, preschool, purines, amino acids
Introduction
Although lungs from children with cystic fibrosis (CF) appear anatomically normal at birth [1], progressive airways disease can begin very early in life [2, 3]. Markers associated with neutrophilic inflammation, including IL-8 and neutrophil elastase, are correlated with the extent and progression of lung disease as quantified by CT [4], and the presence of detectable neutrophil elastase in airways of infants is predictive of later bronchiectasis [5]. These findings strongly suggest that neutrophilic inflammation plays a role in early disease, but the pathophysiological events involved in disease initiation and progression remain ill-defined. Identifying these events is complicated by variable and heterogeneous nature of early CF lung disease that typically involves only a relatively small fraction of the lung [6].
The airway processes involved in early CF lung disease alter cell trafficking and metabolism, including airway surface metabolic pathways and accumulation of inflammatory cells within airway lumens that generate biologically active molecules [7–9]. This scenario suggests that metabolomics approaches, which attempt to characterize the full range of metabolites within biologic samples, can elucidate the pathophysiologic changes related to inflammation and structural disease. Indeed, previous metabolomics studies of airway secretions have demonstrated that neutrophilic airway inflammation in CF patients with established disease is characterized by increased concentrations of metabolites from several pathways, including those involved in cellular energy [10], protein catabolism [10, 11], adenyl purine metabolism [11], and lipid signaling molecules [12]. However, the relevance of these pathways to early disease has not been evaluated.
We hypothesized that metabolomics studies on airway secretions from young children with CF would identify the metabolic pathways associated with neutrophilic airway inflammation, and that these pathways would be predictive of early structural lung disease. To test these hypotheses, we performed mass spectrometric (MS) metabolomics on supernatants of bronchoalveolar lavage fluid (BALF) obtained during clinically indicated bronchoscopy in preschool children with CF to identify pathways associated with neutrophilic inflammation. We then studied relationships between identified metabolic pathways and early localized structural disease in a separate cohort of preschool children enrolled in AREST CF who underwent chest CT and lavage at a time of clinical stability. To address disease heterogeneity, BAL samples were obtained from two separate lobes and analyzed using statistical modeling to examine relationships between BALF metabolite concentrations and both current and future lobe specific CT scores.
Methods
Subjects and samples
For discovery metabolomics, BALF from 20 preschool children with CF was collected during clinically indicated bronchoscopy at the University of North Carolina at Chapel Hill (UNC-CH) via standardized protocols [9] using one to three 10 cc/kg aliquots of sterile saline lavaged into the most visually affected lobe on each side. Return from both sides was combined and averaged 44±15% of lavaged volume. BALF aliquots were centrifuged at 11,000×g for 5 minutes, and the supernatant stored at −80°C. Clinical data were abstracted from medical and research records. All children were fasted for >6 hours at the time of collection.
For comparison to structural lung disease, chest CT and BAL were performed on 34 children enrolled in AREST CF as described [2, 6] during 42 study visits (8 subjects were studied during 2 annual study visits). For BAL, the right middle lobe and the lingula were lavaged and the first aliquot from each side was processed separately, yielding two BAL aliquots per subject visit. BAL samples were centrifuged to remove cellular debris, and supernatants frozen at −80°C and shipped to UNC-CH on dry ice.
From the chest CT, each lung lobe was assessed for lobe specific bronchial wall thickening (BWT) and bronchiectasis (BE) using the modified CF-CT scoring system [2, 13] as well as PRAGMA-CF [14] to give continuous, lobe specific structural lung disease scores. Twenty nine CT images obtained from individual children one year following the BALF samples were also assessed to determine the predictive value of identified biomarkers.
Studies were approved by the UNC IRB (IRB#s 07-0787, 12-1538) and the Princess Margaret Hospital for Children, Perth and Royal Children’s Hospital, Melbourne, Ethics Committees (registration number 1762/EP)
MS Metabolomics
Metabolomic profiling was performed by Metabolon, Inc (Durham, NC). as previously described [15] using three independent platforms (ultra-high-performance liquid chromatography/tandem mass spectrometry for acidic and basic metabolites as well as gas chromatography/mass spectrometry). Metabolites were identified by automated comparison of ion features to a reference library. Values below limits of detection were imputed from the minimum detectable value. The average time between sample collection and analysis was 290 days, with a range of 110–483 days.
Targeted MS
Targeted MS utilized a Quantum-Ultra triple quadrupole mass spectrometer (Thermo-Finnigan, San Jose, CA) with chromatographic conditions similar to those previously described (UPLC T3 HSS C18 column, methanol/formic acid gradients [16]). BALF samples were spiked with isotopically labeled internal standard [17] and filtered through a 10kDa size selection filter (EMD Millipore, Billerica, MA). Biomarker signals were defined as ratios to the internal standard with the closest column run time. The average time between sample collection and analysis was 268 days, with a range of 39–473 days.
Adenosine metabolism
Adenosine metabolism in BALF was assessed by measuring hypoxanthine generated after incubating 5 µL BALF supernatant with 200 µM adenosine in 50 µL Tris pH 7.5 at 37°C for one hour. Resulting hypoxanthine was assessed using a reaction mix containing Amplex red, horseradish peroxidase, and xanthine oxidase from the Xanthine Oxidase Fluorometric Assay Kit (Cayman Chemicals, Ann Arbor, MI). Signal was measured on a fluorometric plate reader with excitation 530 nM/absorbance 570 nM.
Statistical Analysis
MS signals from metabolomic data were analyzed using linear regression as well as Student’s T-test, with the false-discovery rate q-value used to correct for multiple comparisons. MS data were not normally distributed (by D’Agostino and Pearson omnibus normality test) and were log transformed prior to analysis. Categorical comparisons for demographic balance in discovery vs. validation samples were made using Fisher’s exact test. CF-CT scores were analyzed as binary outcomes of no disease (no BWT or BE) vs. disease (BWT or BE present). General estimating equations models (GEE) were fitted for each metabolite with Binomial family, logit link, robust standard errors and were adjusted for sex and batch/lung lobe interactions where appropriate. PRAGMA continuous CT outcomes (% Disease and % Bronchiectasis) were analyzed using hierarchical mixed effects models with random intercepts for each participant and random intercepts and slopes for each batch (as a function of metabolite), adjusted for sex. Predictive ability of metabolites was investigated by hierarchical mixed effects models described above and by plotting Receiver Operating Curves (ROCs) for presence of bronchiectasis at 12 months CT follow up. Area under the curves (AUCs) estimates and 95% confidence intervals were calculated using ROC regression adjusted for the lung lobe (right middle lobe or lingual) and PRAGMA % Bronchiectasis at baseline. Statistical analyses were performed using GraphPad Prism v5.0 (San Diego, CA) and Stata (version 13.0; StataCorp, College Station, TX, USA). Tukey boxplots are used for error bars and outliers.
Results
Discovery metabolomics was performed on BALF supernatants from 20 preschool children with CF undergoing clinically indicated bronchoscopy (Table 1). Persistent cough was the indication for most subjects (14/20), with evaluation of new Pseudomonas infection (3/20) or surveillance in conjunction with another procedure (3/20) less common indications. Respiratory pathogens were recovered from culture in 60% of samples, with Staphylococcus aureus and Pseudomonas aeruginosa most commonly identified (Table 1). Samples for metabolomics were chosen to reflect a range of airway inflammation, with 6 samples having neutrophilic bronchitis (% neutrophils ≥60%, >50,000 pathogens/mL on culture), 9 having no or mild bronchitis (% neutrophils <40%, ≤10,000 pathogens/mL on culture), and 5 samples with intermediate values. A total of 152 metabolites were detected in at least one sample. Samples with greater airway neutrophilia had more overall MS signal, particularly those with % neutrophils >60% (Figure 1).
Table 1.
Subject Demographics
| Discovery Set | AREST CF Set | |
|---|---|---|
| n (subjects) = | 20 | 34 |
| n (BALF) = | 20 | 84 |
| Age (years) | 2.9±1.3 (0.48–4.95) | 3.5±1.5 (0.96–5.83) |
| % Male | 55% | 43% |
| BMI (Z-score) | 0.1±1.1 | 0.0±1.8 |
| Treatment, hypertonic saline | 6% | 15% |
| Treatment, dornase alpha | 3% | 35% |
| Treatment, amoxicillin/clavulanate | 48% | 0% |
| Cell count (×106 cells/ml) | 2.8±3.7 (0.18–12.94) | 0.7±0.3 (0.06–1.67) |
| % PMNs | 48.8±27.2 (2.5–93) | 20.2±23.9 (0.67–92) |
| % with Pathogens | 60% | 17% |
| % with Staphylococcus | 25% | 7% |
| % with Pseudomonas | 25% | 2% |
BALF = bronchoalveolar lavage fluid; PMNs = neutrophils
Figure 1.
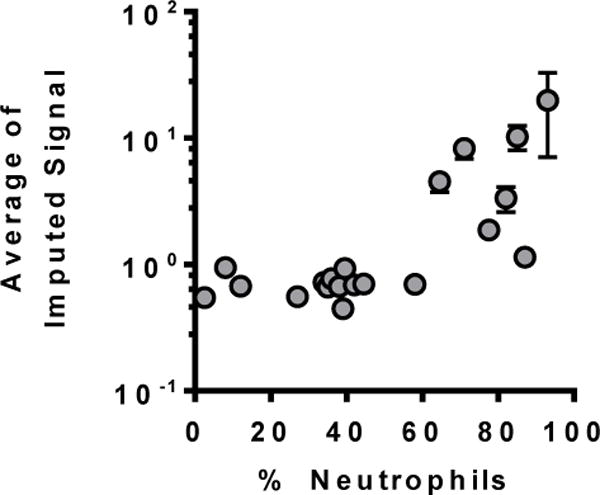
Average MS metabolomics signal in BALF from 20 preschool children with CF plotted relative to % neutrophils on cell counts from these samples. Samples with >60% neutrophils had more overall MS signal.
Metabolites associated with neutrophilic inflammation were defined as those correlated with percent neutrophils at r>0.5 or that had >2-fold increases in signal in samples with bronchitis vs. those with no or mild bronchitis at a false discovery rate <0.05. This included 93 metabolites that fell into four broad metabolic pathways (Table 2 and Supplemental Table 1):
Table 2.
Selected metabolites related to neutrophilic airway inflammation in discovery metabolomics
| Pathway | Metabolite | Corr % PMNs |
Ratio* | Q-value |
|---|---|---|---|---|
| Adenyl purine metabolism | ||||
| adenosine 5′-monophosphate (AMP) | 0.60 | 4.7 | 0.040 | |
| adenosine | −0.69 | 0.2 | 0.002 | |
| hypoxanthine | 0.54 | 4.8 | 0.002 | |
| xanthine | 0.76 | 15.1 | 0.000 | |
| urate | 0.34 | 2.4 | 0.031 | |
| nicotinamide | 0.45 | 3.2 | 0.006 | |
| nicotinamide adenine dinucleotide (NAD+) | 0.63 | 3.5 | 0.022 | |
| kynurenine | 0.65 | 2.5 | 0.016 | |
| Amino acids, dipeptides, and related metabolites | ||||
| alanine | 0.68 | 36.9 | 0.000 | |
| phenylalanine | 0.74 | 36.2 | 0.000 | |
| tyrosine | 0.75 | 44.1 | 0.000 | |
| leucine | 0.37 | 5.4 | 0.058 | |
| isoleucine | 0.61 | 6.6 | 0.002 | |
| methionine | 0.60 | 2.7 | 0.005 | |
| serine | 0.76 | 20.0 | 0.000 | |
| lysine | 0.75 | 141.8 | 0.000 | |
| valine | 0.76 | 43.6 | 0.000 | |
| aspartylleucine | 0.78 | 29.6 | 0.000 | |
| aspartylphenylalanine | 0.73 | 15.0 | 0.000 | |
| glycylleucine | 0.73 | 33.9 | 0.000 | |
| lysylleucine | 0.79 | 5.4 | 0.000 | |
| N-acetylmethionine | 0.45 | 2.5 | 0.032 | |
| N-acetylserine | 0.59 | 2.5 | 0.008 | |
| N6-acetyllysine | 0.76 | 12.0 | 0.000 | |
| cysteine-glutathione disulfide | 0.69 | 9.0 | 0.000 | |
| glutathione, oxidized (GSSG) | 0.34 | 2.5 | 0.022 | |
| arginine | 0.63 | 29.2 | 0.000 | |
| citrulline | 0.79 | 24.3 | 0.000 | |
| fumarate | 0.43 | 2.2 | 0.009 | |
| ornithine | 0.75 | 71.7 | 0.000 | |
| putrescine | 0.35 | 6.5 | 0.061 | |
| adenine | 0.40 | 2.9 | 0.011 | |
| Cellular energy | ||||
| glucose | 0.65 | 9.2 | 0.002 | |
| citrate | 0.66 | 4.1 | 0.009 | |
| malate | 0.66 | 2.9 | 0.004 | |
| lactate | 0.71 | 4.1 | 0.000 | |
| carnitine | 0.43 | 3.2 | 0.009 | |
| acetylcarnitine | 0.50 | 3.6 | 0.003 | |
| 1,5-anhydroglucitol | 0.81 | 7.0 | 0.000 | |
| Lipids | ||||
| phosphoethanolamine | 0.52 | 4.4 | 0.003 | |
| cholesterol | 0.67 | 2.1 | 0.009 | |
| arachidonate (20:4n6) | 0.64 | 9.2 | 0.003 | |
| myo-inositol | 0.43 | 2.3 | 0.023 | |
| 1-stearoylGPE1 | 0.68 | 2.5 | 0.030 | |
| 2-arachidonoylGPE1 | 0.65 | 4.1 | 0.022 | |
| 2-docosahexaenoylGPE1 | 0.66 | 2.7 | 0.009 | |
| 2-oleoylGPE1 | 0.73 | 5.4 | 0.005 | |
GPE = glycerphosphoethanolamine
Ratio = ratio of metabolite concentrations in samples with bronchitis to those with no/mild bronchitis
Adenyl purines and related metabolites
AMP was 4.7-fold elevated in airways with bronchitis (Table 2), but adenosine was reduced in bronchitic airways—the only metabolite in discovery metabolomics significantly decreased in the presence of bronchitis. In addition, the adenosine metabolites hypoxanthine and xanthine were 4.8 and 15.1 fold increased in BALF from bronchitic airways, respectively. The purine containing compound nicotinamide adenine dinucleotide (NAD) was also elevated in bronchitis, as were free nicotinamide and kynurenine, a tryptophan metabolite that serves as an intermediate in NAD synthesis.
Amino acids, small peptides, and related pathways
A large number of amino acids, dipeptides, and tripeptides were highly elevated (>10-fold) in airways with bronchitis, with correlation coefficients to % neutrophils often exceeding 0.7 (Table 2). Similarly, bronchitic airways had higher concentrations of several metabolic products of amino acids, including N-acetylated derivatives of methionine, serine, and lysine. Higher concentrations oxidative products of the antioxidant peptide glutathione, including glutathione disulfide (GSSG) (2.5 fold increased) and cysteine-glutathione disulfide (9.0 fold increased), were also observed.
Arginine related signaling pathways were also implicated in the metabolomics analysis. Arginine, citrulline, and fumarate are involved in generating the signaling molecule nitric oxide [18], and all of these metabolites were elevated in bronchitic samples. Arginine can also be metabolized to ornithine, the initial substrate in synthesis of polyamines [19]. Both ornithine and the polyamine putrescine were elevated in bronchitis, as was free adenine generated primarily within the polyamine synthesis pathway [20].
Cellular energy metabolism
Several metabolites directly related to energy metabolism were found at higher concentrations in samples from bronchitic airways, including glycolytic and Kreb’s cycle metabolites (glucose, citrate, malate). Lactate was 4.1 fold increased, suggestive of anaerobic metabolism. Metabolites involved in fatty acid oxidation, including carnitine and acylcarnitine, were also elevated. Metabolomics also revealed elevated concentrations of 1,5-anhydroglucitol in bronchitis, a compound that regulates glycemic control and has anti-inflammatory properties [21].
Lipids
Several lipid metabolites were elevated in the presence of bronchitis, including both common cell membrane lipids (phosphoethanolamine, cholesterol) and those involved in signaling pathways (arachidonate, myo-inositol). Several lysolipids were also elevated in bronchitis.
Metabolomic biomarkers and early structural lung disease
To determine relationships to early structural lung disease, we developed targeted MS methods for a subset of 28 metabolomic biomarkers amenable to MS detection using previously established methods [11, 16] including amino acids, dipeptides, adenyl purines, nicotinamide, polyamines, glutathione and glutathione disulfide (oxidized glutathione), as well as urea as a potential dilution marker (Supplemental Table 1). This biomarker panel was then applied to a validation set of samples from 34 preschool children enrolled in AREST CF during 42 study visits (8 longitudinally sampled subjects). In contrast to the subjects in the discovery set, bronchoscopy and chest CT in AREST CF are performed at a time of clinical stability, as evidenced by lower neutrophil counts and fewer recovered pathogens compared to our discovery dataset (Table 1). Heterogeneity of early CF lung disease was addressed by obtaining BALF independently from two separate lobes (right middle lobe and lingua) at each study visit and using lobe specific chest CT scores for analysis.
Metabolomic biomarkers and inflammatory markers
Conventional inflammatory markers including cell counts and IL-8 were measured in samples from the right middle lobe in all subjects (n=42). Analyses revealed significant negative associations (coefficients [95% confidence interval]) between BAL neutrophil counts (% of total cell count) and adenosine (−0.376 [−0.569, −0.184]) and glutathione (−0.285 [−0.419, −0.150]). Significant positive associations were found between neutrophils and inosine (0.220 [0.042, 0.399]) and ornithine (0.017 [0.008, 0.025]). For IL-8, a significant negative association was found to glutathione (−0.137 [−0.266, −0.007]), with significant positive associations to inosine (0.365 [0.227, 0.503]), leucine (0.071 [0.004, 0.139]), ornithine (0.012 [0.004, 0.020]) and spermidine (0.010 [0.005, 0.016]). No significant associations were observed between any metabolomic biomarker and neutrophil elastase, though these analyses were limited by the fact that neutrophil elastase was detected in only 5 of 42 samples.
Metabolomic biomarkers and structural lung disease
Statistical modeling was utilized to assess relationships between various metabolites and lobe specific structural lung disease using both dichotomous variables (presence/absence of disease) and the PRAGMA continuous scoring system. Lobes with structural lung disease (bronchial wall thickening [BWT] as a marker of early disease or bronchiectasis [BE] as a marker of later disease) had lower concentrations of adenosine; odds ratio (OR = 0.32, p<0.001, Figure 2, Table 3) than samples from lobes without BWT or BE. Trends towards increases in the downstream adenosine metabolites hypoxanthine (OR = 2.32, p=0.203) and xanthine (OR = 2.30, p=0.197) were also observed. Glutathione was significantly reduced in lobes with structural disease (OR = 0.40, p<0.01), as was oxidized glutathione (OR = 0.16, p<0.01). Associations between amino acids and structural lung disease were not statistically significant, but the Leu-Pro dipeptide was strongly elevated in lobes with structural lung disease (OR>100, p<0.001).
Figure 2.
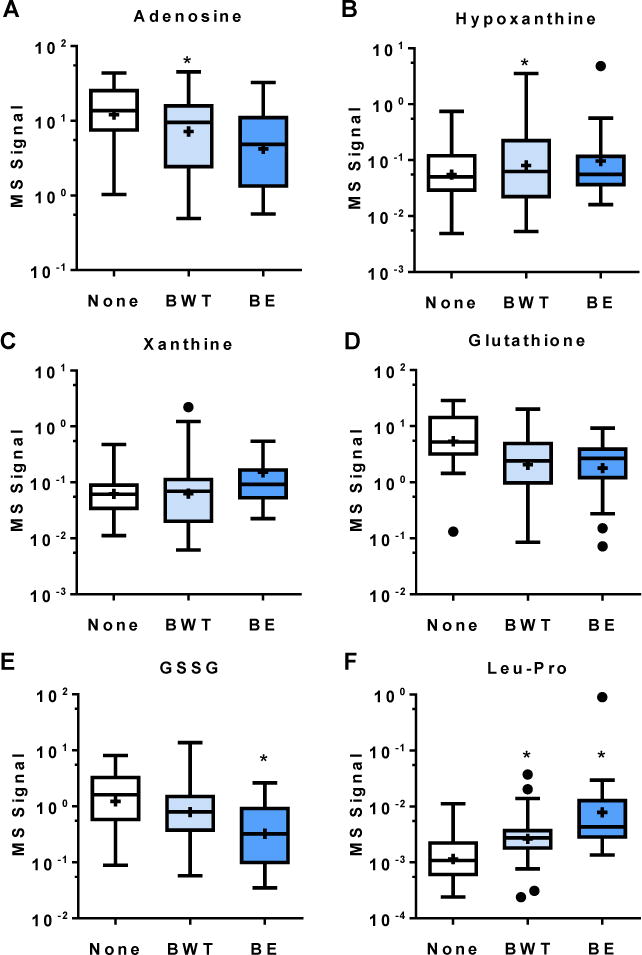
Metabolite concentrations for various metabolites in lobes with bronchial wall thickening (BWT), bronchiectasis (BE), or neither BWT or BE (None) by CF-CT scoring system. *=p<0.05 by multivariate analysis.
Table 3.
Multivariate analysis of metabolite signal and localized structural lung disease
| Disease Presence (CF-CT Scores) |
PRAGMA-CF % Disease |
PRAGMA-CF % Bronchiectasis |
||||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) |
p−value | β (95% CI) R2 |
p−value | β (95% CI) R2 |
p−value | |
| Adenosine | 0.32 (0.18, 0.59) |
<0.001 | −0.74 (−1.34, −0.15) 0.13 |
0.014 | −0.84 (−1.27, −0.40) 0.18 |
<0.001 |
| Hypoxanthine | 2.32 (0.53, 10.14) |
0.203 | 0.93 (0.32, 1.54) 0.20 |
0.003 | 0.76 (0.30, 1.23) 0.19 |
0.001 |
| Xanthine | 2.30 (0.65, 8.20) |
0.197 | 1.46 (0.82, 2.10) 0.32 |
<0.001 | 1.2 (0.70, 1.70) 0.31 |
<0.001 |
| Glutathione | 0.40 (0.22, 0.74) |
0.003 | −0.82 (−1.41, −0.21) 0.14 |
0.008 | −0.55 (−1.10, −0.00) 0.11 |
0.048 |
| Phenylalanine | 3.08 (0.44, 21.71) |
0.259 | 2.14 (1.44, 2.85) 0.44 |
<0.001 | 1.65 (1.07, 2.24) 0.38 |
0.001 |
| Tyrosine | 1.60 (0.78, 3.27) |
0.203 | 2.13 (1.43, 2.83) 0.41 |
<0.001 | 1.5 (0.83, 2.18) 0.39 |
0.001 |
| Leu-Pro | 2.80e+70 (2.01e+17, 3.9e+123) |
0.009 | 2.16 (1.61, 2.71) 0.52 |
<0.001 | 1.65 (1.25, 2.06) 0.51 |
<0.001 |
Using lobe specific PRAGMA disease scores, we observed negative correlations for both adenosine (β = −0.74, p=0.014) and glutathione (β = −0.82, p=0.008) (Table 3). In contrast, positive correlations were observed for the adenosine metabolites hypoxanthine (β = 0.93, p=0.003) and xanthine (β = 1.46, p<0.001) as well as the amino acids phenylalanine, tyrosine, and the Leu-Pro dipeptide (β = 2.14, 2.13, 2.16 respectively, p<0.001 for all). Similar findings were observed for bronchiectasis specific PRAGMA scores (Table 3).
Predictive power of metabolomic biomarkers
Hierarchical mixed effects models and ROCs were utilized to assess the ability of metabolites to predict development of new bronchiectasis at the next annual visit, using data available in 29 children. Metabolites predictive of future bronchiectasis included hypoxanthine (β1 = 0.19 [0.06, 0.31]; p=0.003), phenylalanine (β1 = 0.27 [0.11, 0.43]) and Leu-Pro (β1 = 0.17 [0.01, 0.33]). Each of these metabolites was a better predictor of bronchiectasis than neutrophil elastase (β1 = 0.08 [0.00, 0.16]), a known biomarker of early CF lung disease (Figure 3) [5].
Figure 3.
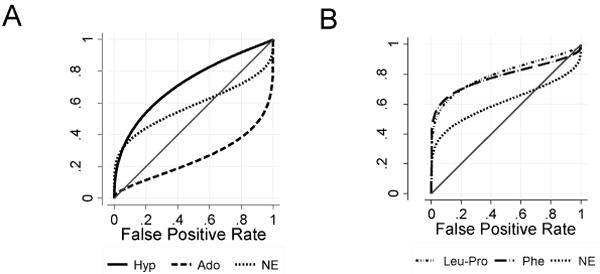
ROC curves demonstrating the sensitivity and specificity for various metabolites relative to neutrophil elastase (NE, marked as ‘X’) in predicting future bronchiectasis. A. Purine metabolites adenosine (Ado, open circles) and hypoxanthine (Hyp, open triangles). B. Protein catabolism metabolites phenylalanine (Phe, filled circles) and the dipeptide Leu-Pro (filled triangles).
Early structural lung disease and adenosine metabolism
These analyses suggested an association between early lung disease and activity of the adenosine metabolic pathway, in which adenosine is converted to uric acid through the actions of adenosine deaminase, purine nucleotide phosphorylases, and xanthine oxidase. To assess these activities directly, we measured adenosine metabolism in BALF supernatants. BALF samples from children with significant bronchitis, but not those without bronchitis, metabolized adenosine to hypoxanthine (Figure 4), indicating elevated activities of adenosine deaminase and purine phosphorylase. AREST CF samples from lobes with bronchiectasis also had significant adenosine metabolic activity. Xanthine oxidase activity in these samples was not detected (not shown).
Figure 4.
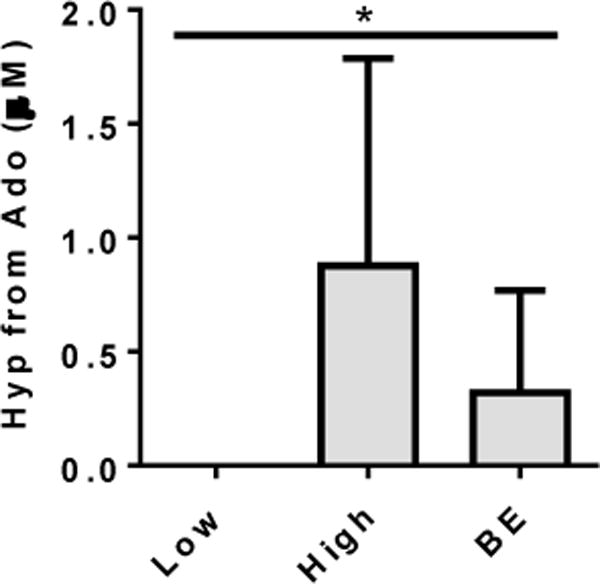
Adenosine metabolic activity in BALF from preschool children with CF was assessed as the ability to generate hypoxanthine (Hyp) from exogenously added adenosine (Ado). Samples with no significant bronchitis (<40% neutrophils) had no significant activity, whereas activity was readily detected in samples with bronchitis (>60% neutrophils). BALF from clinically stable preschoolers with bronchiectasis (BE) on chest CT showed moderate levels of adenosine metabolic activity. N=3–4 per group. * = p<0.01 by Mann-Whitney, with significant post tests for bronchitis and BE groups relative to no bronchitis.
Discussion
Using metabolomics, we identified several metabolites and metabolic pathways altered in the presence of neutrophilic airway inflammation in young children with CF, including those involving adenyl purines, amino acids and peptides, cellular energy, and lipids. Several of these pathways, including those related to adenosine metabolism, oxidative stress, and protein catabolism, were strongly associated with structural lung disease. Although many of these pathways are also altered in older children [10, 11], this study demonstrates that these metabolic changes occur early in the disease process before the onset of permanent structural lung damage. These pathways represent potential therapeutic targets, and the relevant metabolites are biomarkers of patients at risk for developing bronchiectasis.
The adenosine metabolic pathway in particular appears altered early and predictive of future disease. Adenosine plays an important and complex role in modulating signaling responses to inflammation [22], with both pro and anti-inflammatory properties, though normal airway adenosine concentrations are thought to be anti-inflammatory [22, 23]. The decreased adenosine and elevated adenosine metabolic activity likely increase airway inflammatory responses, and the metabolic products hypoxanthine and xanthine contribute to oxidative stress through metabolism by xanthine oxidase, which generates oxygen superoxides [24, 25].
Increased oxidative stress in early disease is consistent with the observed reduction in glutathione, the primary antioxidant in the airway [26]. Decreased lower airway glutathione concentrations have been observed in preschool children with CF [27], although our study is the first to demonstrate a relationship to structural lung disease. Our ability to detect these relationships likely reflects our use of lobe specific lavages and CT scores to account for disease heterogeneity. Somewhat surprisingly, the concentrations of oxidized glutathione were also reduced in the AREST CF samples, though we suspect this may reflect an inability to preserve the oxidative state on storage and transport of these samples.
These findings suggest that drugs that affect the adenosine metabolism are potential therapeutic targets in CF. Such drugs could represent “low hanging fruit,” since several relevant pharmaceuticals are approved or in late stage clinical trials. For example, inhibitors of purine nucleoside phosphorylase that block hypoxanthine formation [28] are in clinical trials for gout. Also, the xanthine oxidase inhibitor febuxostat was recently approved for gout [29] but has also been shown to reduce airway inflammation in an animal model of acute lung injury [30]. Although we did not detect xanthine oxidase activity in BALF supernatant, the high concentrations of xanthine and uric acid (metabolic products of xanthine oxidase) in the airway samples indicate that this enzyme is active in vivo, likely restricted to the airway epithelial cell surface. Indeed, our adenosine metabolism studies may underestimate total activity since we could only assess soluble activities and not the contribution from enzymes found on airway surfaces.
The strong associations between amino acids and dipeptides with early disease are consistent with increased activity of proteases such as neutrophil elastase. These findings are supportive of previous studies identifying airway proteases as potential therapeutic targets in early CF lung disease [5]. Notably, the Leu-Pro dipeptide was more readily detected and more predictive than neutrophil elastase, suggesting that it and similar metabolomics biomarkers may be more sensitive indicators of protease activity.
Discovery metabolomics suggested that several arginine related signaling pathways are upregulated in the presence of airways inflammation. Increased concentrations of ornithine imply greater arginase activity in inflamed airways and are consistent with previously reported increases in CF sputum polyamines [19]. Since urea is another product of arginase activity, this relationship could impact the utility of urea as an airway dilution marker. Increased concentrations of citrulline and fumarate were also observed, suggesting greater flux through the nitric oxide (NO) synthesis pathway. However, exhaled NO is reportedly low in CF [31]. The reasons for this discrepancy are not clear.
In fact, several metabolites identified in our study have potential as biomarkers of early lung disease. Many of these metabolites are detectable by conventional methods and could potentially serve as indicators of at risk children that are more sensitive than the current gold standard of neutrophil elastase [5]. In addition, some of the metabolic signatures observed in BALF are detectable in exhaled breath condensate (EBC) from young children [32, 33], offering the potential to develop a relatively non-invasive technique to identify children with early structural lung disease.
There were several limitations that could affect our findings. All subjects were fasting at the time of bronchoscopy, and it is possible that metabolic patterns would differ in non-fasting individuals. We also did not assess the impact of treatment beyond an indirect effect of altering airway inflammation. Similarly, we were not able to determine the direct contribution of bacterial pathogens to the metabolomic signal. However, given the complexity of the CF airway microbiome [34], assessing the relative contributions of host vs. bacterial pathogens poses considerable challenges.
Another limitation is that we utilized percent neutrophils to define airway inflammation in our discovery metabolomic analysis. While airway neutrophils are a well-accepted marker of inflammation [8], this approach does have some shortcomings since increases in other cell counts (such as lymphocytes during an acute viral infection) or sampling issues could alter the relationship between percent neutrophils and airway inflammation. Similarly, neutrophil counts alone may not perfectly reflect their activity or propensity to cause airway damage. Nevertheless, we did observe associations between many of the metabolites and metabolic pathways identified by discovery metabolomics and other inflammatory markers as well as structural lung disease. These associations raise our confidence that our findings reflect early CF airways disease pathophysiology.
This study focused on metabolites amenable to our established MS methods, and we have not yet analyzed other metabolites identified by discovery metabolomics such as those involved in cellular energy and lipid metabolism. These metabolites will require different MS approaches for detection, but they offer fertile ground for further investigation.
In conclusion, MS metabolomics demonstrate that neutrophilic airway inflammation in young children with CF is associated with increased concentrations of metabolites from many pathways, several of which are predictive of current and future early structural lung disease. In particular, alterations in adenosine metabolism and resulting oxidative stress are linked to both bronchitis and structural lung disease and offer opportunities for noninvasive biomarker detection and serve as promising targets for therapeutic intervention.
Supplementary Material
Acknowledgments
The authors would like to thank Pheris Karanja and Luke Berry for their assistance with these studies.
Grant Support: CRE, LT, MM, and SMS were supported by NIH/NHLBI R01-HL116228. CRE was also supported by NC TraCS 50KR51009, NIH/NIEHS P30-ES10126, and NIH/NHLBI K23-HL089708. LT, TR, and SMS were supported by NHMRC GNT1000896. RCB was supported by NIH/NHLBI HL34322, HL107168, P01-HL08808, P30-DK065988, P01-HL110873, and P50-HL107168.
Footnotes
Take home message: Metabolomics in preschool children with cystic fibrosis reveals biomarkers that correlate with and predict early disease
This article has an online data supplement.
Summary of findings: Increased activity of adenosine, amino acid, and other metabolic pathways predicts early cystic fibrosis lung disease
References
- 1.Davis PB. Cystic Fibrosis Since 1938. Am J Respir Crit Care Med. 2006;173(5):475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 2.Mott LS, Park J, Murray CP, Gangell CL, de Klerk NH, Robinson PJ, Robertson CF, Ranganathan SC, Sly PD, Stick SM, on behalf of AC Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax. 2011 doi: 10.1136/thoraxjnl-2011-200912. [DOI] [PubMed] [Google Scholar]
- 3.Stick SM, Brennan S, Murray C, Douglas T, von Ungern-Sternberg BS, Garratt LW, Gangell CL, De Klerk N, Linnane B, Ranganathan S, Robinson P, Robertson C, Sly PD, Australian Respiratory Early Surveillance Team for Cystic F Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009;155(5):623–628 e621. doi: 10.1016/j.jpeds.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Gangell C, Gard S, Douglas T, Park J, de Klerk N, Keil T, Brennan S, Ranganathan S, Robins-Browne R, Sly PD, Arest CF. Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clin Infect Dis. 2011;53(5):425–432. doi: 10.1093/cid/cir399. [DOI] [PubMed] [Google Scholar]
- 5.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM, Investigators AC Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368(21):1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 6.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF, Ranganathan SC, Australian Respiratory Early Surveillance Team for Cystic F Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180(2):146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 7.Alton EW, Davies JC, Geddes DM. Biomarkers for cystic fibrosis: are we progressing? Am J Respir Crit Care Med. 2007;175(8):750–751. doi: 10.1164/rccm.200702-226ED. [DOI] [PubMed] [Google Scholar]
- 8.Mayer-Hamblett N, Aitken ML, Accurso FJ, Kronmal RA, Konstan MW, Burns JL, Sagel SD, Ramsey BW. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med. 2007;175(8):822–828. doi: 10.1164/rccm.200609-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esther CR, Jr, Alexis NE, Clas ML, Lazarowski ER, Donaldson SH, Ribeiro CM, Moore CG, Davis SD, Boucher RC. Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur Respir J. 2008;31(5):949–956. doi: 10.1183/09031936.00089807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolak JE, Esther CR, Jr, O’Connell TM. Metabolomic analysis of bronchoalveolar lavage fluid from cystic fibrosis patients. Biomarkers. 2009;14(1):55–60. doi: 10.1080/13547500802688194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esther CR, Jr, Coakley RD, Henderson AG, Zhou YH, Wright FA, Boucher RC. Metabolomic evaluation of neutrophilic airway inflammation in cystic fibrosis. Chest. 2015;148(2):507–515. doi: 10.1378/chest.14-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Eiserich JP, Cross CE, Morrissey BM, Hammock BD. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic Biol Med. 2012;53(1):160–171. doi: 10.1016/j.freeradbiomed.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brody AS, Tiddens HA, Castile RG, Coxson HO, de Jong PA, Goldin J, Huda W, Long FR, McNitt-Gray M, Rock M, Robinson TE, Sagel SD, Group CTSiCFSI Computed tomography in the evaluation of cystic fibrosis lung disease. Am J Respir Crit Care Med. 2005;172(10):1246–1252. doi: 10.1164/rccm.200503-401PP. [DOI] [PubMed] [Google Scholar]
- 14.Rosenow T, Oudraad MC, Murray CP, Turkovic L, Kuo W, de Bruijne M, Ranganathan SC, Tiddens HA, Stick SM, Australian Respiratory Early Surveillance Team for Cystic F. PRAGMA-CF A Quantitative Structural Lung Disease Computed Tomography Outcome in Young Children with Cystic Fibrosis. Am J Respir Crit Care Med. 2015;191(10):1158–1165. doi: 10.1164/rccm.201501-0061OC. [DOI] [PubMed] [Google Scholar]
- 15.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81(16):6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 16.Esther CR, Jr, Jasin HM, Collins LB, Swenberg JA, Boysen G. A mass spectrometric method to simultaneously measure a biomarker and dilution marker in exhaled breath condensate. Rapid Commun Mass Spectrom. 2008;22(5):701–705. doi: 10.1002/rcm.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esther CR, Jr, Lazaar AL, Bordonali E, Qaqish B, Boucher RC. Elevated airway purines in chronic obstructive pulmonary disease. Chest. 2011;140(4):954–960. doi: 10.1378/chest.10-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes PJ, Dweik RA, Gelb AF, Gibson PG, George SC, Grasemann H, Pavord ID, Ratjen F, Silkoff PE, Taylor DR, Zamel N. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138(3):682–692. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- 19.Grasemann H, Shehnaz D, Enomoto M, Leadley M, Belik J, Ratjen F. L-ornithine derived polyamines in cystic fibrosis airways. PLoS One. 2012;7(10):e46618. doi: 10.1371/journal.pone.0046618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamatani N, Carson DA. Dependence of adenine production upon polyamine synthesis in cultured human lymphoblasts. Biochimica et Biophysica Acta (BBA) - General Subjects. 1981;675(3–4):344–350. doi: 10.1016/0304-4165(81)90024-6. [DOI] [PubMed] [Google Scholar]
- 21.Meng X, Tancharoen S, Kawahara KI, Nawa Y, Taniguchi S, Hashiguchi T, Maruyama I. 1,5-Anhydroglucitol attenuates cytokine release and protects mice with type 2 diabetes from inflammatory reactions. Int J Immunopathol Pharmacol. 2010;23(1):105–119. doi: 10.1177/039463201002300110. [DOI] [PubMed] [Google Scholar]
- 22.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367(24):2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koeppen M, Di Virgilio F, Clambey ET, Eltzschig HK. Purinergic regulation of airway inflammation. Subcell Biochem. 2011;55:159–193. doi: 10.1007/978-94-007-1217-1_7. [DOI] [PubMed] [Google Scholar]
- 24.Boda D. Role of hyperuricaemia in critically ill patients especially newborns. Acta Paediatr Hung. 1984;25(1–2):23–32. [PubMed] [Google Scholar]
- 25.Quinlan GJ, Lamb NJ, Tilley R, Evans TW, Gutteridge JM. Plasma hypoxanthine levels in ARDS: implications for oxidative stress, morbidity, and mortality. Am J Respir Crit Care Med. 1997;155(2):479–484. doi: 10.1164/ajrccm.155.2.9032182. [DOI] [PubMed] [Google Scholar]
- 26.Dauletbaev N, Viel K, Buhl R, Wagner TO, Bargon J. Glutathione and glutathione peroxidase in sputum samples of adult patients with cystic fibrosis. J Cyst Fibros. 2004;3(2):119–124. doi: 10.1016/j.jcf.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Kettle AJ, Turner R, Gangell CL, Harwood DT, Khalilova IS, Chapman AL, Winterbourn CC, Sly PD, Arest CF. Oxidation contributes to low glutathione in the airways of children with cystic fibrosis. Eur Respir J. 2014;44(1):122–129. doi: 10.1183/09031936.00170213. [DOI] [PubMed] [Google Scholar]
- 28.Gandhi V, Balakrishnan K. Pharmacology and mechanism of action of forodesine, a T-cell targeted agent. Seminars in oncology. 2007;34(6 Suppl 5):S8–12. doi: 10.1053/j.seminoncol.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Pascual E, Sivera F, Yasothan U, Kirkpatrick P. Febuxostat. Nat Rev Drug Discov. 2009;8(3):191–192. doi: 10.1038/nrd2831. [DOI] [PubMed] [Google Scholar]
- 30.Fahmi AN, Shehatou GS, Shebl AM, Salem HA. Febuxostat protects rats against lipopolysaccharide-induced lung inflammation in a dose-dependent manner. Naunyn-Schmiedeberg’s archives of pharmacology. 2016;389(3):269–278. doi: 10.1007/s00210-015-1202-6. [DOI] [PubMed] [Google Scholar]
- 31.Malinovschi A, Ludviksdottir D, Tufvesson E, Rolla G, Bjermer L, Alving K, Diamant Z. Application of nitric oxide measurements in clinical conditions beyond asthma. European clinical respiratory journal. 2015;2:28517. doi: 10.3402/ecrj.v2.28517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel K, Davis SD, Johnson R, Esther CR., Jr Exhaled breath condensate purines correlate with lung function in infants and preschoolers. Pediatr Pulmonol. 2013;48(2):182–187. doi: 10.1002/ppul.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esther CR, Jr, Boysen G, Olsen BM, Collins LB, Ghio AJ, Swenberg JW, Boucher RC. Mass spectrometric analysis of biomarkers and dilution markers in exhaled breath condensate reveals elevated purines in asthma and cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L987–993. doi: 10.1152/ajplung.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zemanick ET, Sagel SD, Harris JK. The airway microbiome in cystic fibrosis and implications for treatment. Curr Opin Pediatr. 2011;23(3):319–324. doi: 10.1097/MOP.0b013e32834604f2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


