Abstract
Treatment of refractory, unresectable cutaneous squamous cell carcinoma presents a great challenge in head and neck oncology with poor prognosis. Prior case reports have shown off-label pembrolizumab, a programed cell death receptor antagonist, can be effective in unresectable cutaneous squamous cell carcinoma. Furthermore, prior reports have suggested enhanced efficacy when high mutational burden is present. In this study we present a severe case of unresectable cutaneous squamous cell carcinoma invading the orbit and cavernous sinus with documented tumor MLH1 mutation. The patient had a complete response to palliative, off-label pembrolizumab therapy.
Keywords: PD-1 Inhibitor, Cutaneous Squamous Cell Carcinoma, MLH1, Unresectable
Introduction
Invasive, unresectable cutaneous squamous cell carcinoma (SCC) presents a great challenge to traditional surgical, radiation, and chemotherapy treatment strategies in head and neck oncology with poor survival and poor response to conventional therapy.1–3 Recent reports have suggested that drugs targeting the programed cell death receptor (PD-1) pathway provide great promise as a treatment option for controlling progressive, unresectable, cutaneous SCC.1–5 Pembrolizumab, is a PD-1 pathway antagonist approved by the US Food and Drug administration for advanced melanoma, non-small cell lung cancer, and head and neck squamous cell carcinoma.6,7 The drug has shown activity in a number of cancer types, but data remains limited in advanced cutaneous SCC.
In this study we present a case of unresectable cutaneous SCC with documented tumor MLH1 mutation invading the orbit and cavernous sinus. Treatment with palliative, off-label pembrolizumab therapy resulted in a complete response.
Case Presentation
A 67 year-old male with an initial history of T2, stage II cutaneous squamous cell carcinoma (SCC) of the right forehead presented with recurrent, unresectable SCC and a new lung metastasis. Prior treatment involved definitive radiation to the forehead skin after initial resection with positive margins. After radiation, wide excision was performed with negative margins. Four years later, the patient presented with complaints of an enlarging ulcer near the right eyelid along with worsening blurry vision and diplopia for 2 months.
Physical exam revealed a palpable firm nodularity in the right upper eyelid with limited lateral gaze and intact pupillary reflex. Baseline CT scan demonstrated an extensive primary lesion (39×24×22 mm), Figure 1a, invading the orbit with involvement of the superior and lateral orbital walls and posterior extension into the cavernous sinus. Evidence of periosteal thickening along with a moth-eaten appearance in parts of the orbital roof and apex suggested neoplastic progression. Punch biopsy histology results showed invasive, poorly differentiated, Stage IV skin SCC with perineural invasion. On PET and CT scan there were also identified bilateral spiculated hypermetabolic lesions in the lungs suspicious for metastasis.
Figure 1. Serial CT Monitoring.
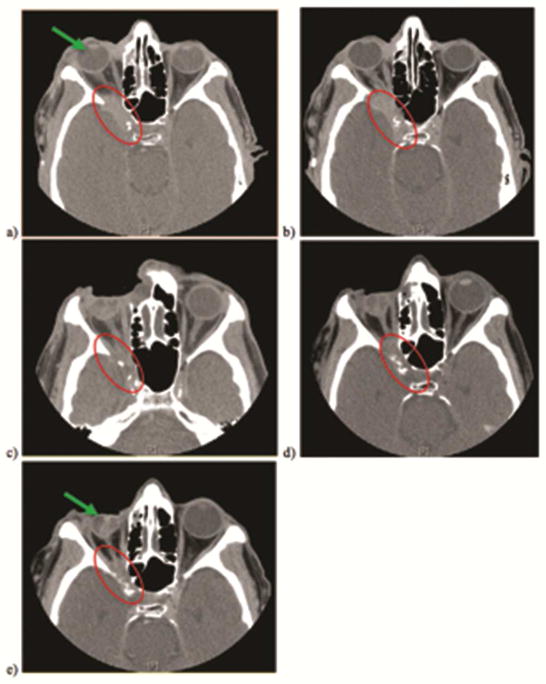
Complete regression of right facial tumor invading right orbit and cavernous sinus (red circles) documented on serial CT examination at baseline (a), 1 ½ months (b), 3 ½ months (c), 8 ½ months (d), and 11 ½ months. Right eye reduction (green arrows) back into orbit demonstrates gross effects of successful tumor shrinkage.
Primary tumor progression into the cavernous sinus along with suspected metastasis, made treatment by further surgery or re-irradiation not preferred. A course of palliative chemotherapy was agreed upon preceded by tumor genomic profiling. Genomic profiling was performed using next-generation sequencing (NGS) with the Foundation One test.8 The results of profiling were significant for a mutation in MLH1 correlating to loss of function (MLH1 100*). DNA mismatch repair (MMR) deficiency was suspected based on the presence of the MLH1 mutation. Tumor mutational burden was estimated at 63.1 mutations per megabase using data from the NGS assay. The patient’s case was reviewed by our institution’s molecular tumor board. Given the reports of excellent responses using PD-1 inhibitors for tumors with DNA MMR,5 and a lack of clinical trial options, a regimen of off-label pembrolizumab 2 mg/kg IV infusion every 3 weeks was initiated. Serial testing of thyroid, liver and renal function was performed on follow-up to monitor for reported adverse outcomes.3 The patient was followed for clinical outcome on an observational study.9
Initial clinical response to therapy was observed after 6 weeks (2 cycles) with reduced protrusion of the right orbital contents and diminished periorbital pain. By 9 weeks, treatment effect became noticeable on serial CT imaging. Continued monitoring over the course of the next 12 months demonstrated a significant treatment response with complete tumor regression. Superficial lesion regression, Figure 2, was also documented on routine clinical visits.
Figure 2. Clinical Progress.
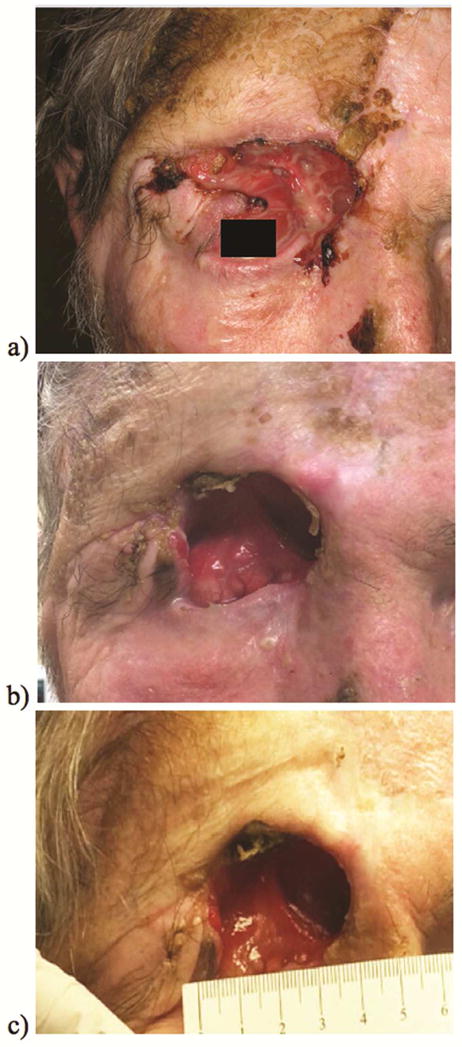
Cutaneous SCC regression changes observed on clinical follow-up from baseline at start of prembolizumab (a), 2 ½ months of treatment (b), and 10 months of treatment (c).
The patient continues with treatment scheduled for a duration of 2 years. Overall, the patient tolerates treatment exceptionally well experiencing only transient, mild fatigue after chemotherapy as the only adverse effect.
Discussion
The head and neck is a region known to exhibit high risk for advanced, invasive cutaneous SCC malignancy trending towards an unfavorable outcome. The 5-year risk of local recurrence and metastasis for invasive cutaneous SCC are indicated at 8% and 5%, respectively.10 The 5-year survival rate is also quite poor at less than 40%.10,11
Situations of progressed, nonresectable, cutaneous SCC represent the predominant circumstance for which chemotherapy strategies are employed either with or without accompanying radiation treatment (RT) in the head and neck.11,12 Traditionally, a platinum-based regimen targeting DNA transcription and replication are selected with an induction agent combination of cisplatin and 5-fluorouracil.11,13,14 The addition of docetaxel, a microtubule disassembly inhibitor, to this treatment strategy has also been evaluated and displayed improved tumor response from the standard 2-drug strategy.11 Investigations of immunochemotherapy using cetuximab, an epidermal growth factor receptor antagonist, either alone or integrated with platinum regimens represent another combination that has demonstrated favorable response results.11,15
Chemotherapeutic regimens employed for treatment of advanced cutaneous SCC tend to be primarily palliative in nature with long-term prognosis being quite poor. The 10-year survival is less than 20% in cases involving regional lymph node involvement and below 10% with distant metastasis.12 Presently only a small number of low-census, prospective controlled studies are available examining systemic therapy for advanced cutaneous SCC disease making oncologic management decisions challenging.12–14 The response rates of advanced cutaneous SCC to various regimens are typically low, being recognized between 17%–78% for platinum-based therapies and around a 28% for cetuximab monotherapy.14 In randomized-controlled studies examining a platinum-based regimen or cetuximab therapy alone the survival rates at one year have been similar, falling around 58% and 52%, respectively.15 However, beyond one-year patient survival tends to decline precipitously being indicated in a phase II study by Shin et al.16 evaluating the efficacy of a platinum-based therapy to progress from 58% to 32% and 21% at 1, 2, and 5-years, respectively. In patients receiving cetuximab monotherapy or cetuximab and a platinum agent combined the overall survival has been found to range between 7.2–8.1 months and 18–64 months, respectively.12,15
In the process of selecting patients appropriate for a chemotherapeutic plan the balance between patient tolerance of an agent’s toxicity profile and susceptibility of the tumor to the chosen treatment must be considered.13,17 For platinum agent combinations there are a number of concerning adverse effects that include the development of febrile neutropenia, mucositis, rash, fatigue, and anorexia. Immunotherapies have been noted for having a lower side effect profile with dermatologic hypersensitivity reaction toxicity being the most remarkable.13–15 Yet, despite the discrepancy in side effects observed between these regimens the clinical significance with regard to agent superiority in treatment remains uncertain.13,14
Off-label pembrolizumab represents a newer immunotherapy that has been observed to produce excellent response in unresectable, cutanous SCC.1,3,5 Preclinical studies and clinical case reports have shown that the high mutation burden20 coupled with observed immunosuppression in instances of cutaneous SCC progression implicates a utility for targeting immune checkpoint elements such as those in the PD-1 pathway.3,5
The present study highlights an application of pembrolizumab in a case of cutaneous SCC with a confirmed gene mutation in MLH1, resulting from loss of DNA mismatch repair (MMR). The incidence of MLH1 and other mismatch repair gene loss of function mutations in cutaneous SCC are presently unknown. Recent analysis from the Cancer Genome Atlas (TCGA) did not reveal a significant rate of mutations of common DNA MMR genes, including MLH1.10 Early efficacy data from studies using DNA MMR deficiency as a biomarker, suggest significant clinical activity when using PD-1 inhibitors.5 Based on previous literature documenting high mutation burden in cutaneous SCC and the findings in this case, an evaluation for the presence of DNA MMR in advanced, cutaneous SCC should be considered during patient work-ups.
This case report describes the observation of a dramatic response by a patient with unresectable SCC having documented tumor genome evidence of a significant loss of function MLH1 mutation in the presence of a high mutational burden. The patient continues to show a sustained response beyond 1 year of treatment. Further research will be needed to confirm the applicable efficacy of PD-1 inhibitors in the greater patient population with advanced, cutaneous SCC. Additionally, the role of DNA MMR deficiency on therapeutic outcomes should be investigated as a predictive biomarker in this disease process.
Clinical Practice Points.
Chemotherapeutic and immunologic agents serve as one of the final therapeutic modalities in addressing unresectable cutaneous SCC of the head and neck. The high mutational burden of cutaneous SCC presents a significant challenge to identifying consistently efficacious agents in diverse patient populations. Prior chemotherapeutic regimens involving platinum-based agents along with newer immunotherapies have provided a means of halting progression along with improving survival in certain instances. Of those agents currently in use, none have been found to provide significantly improved outcomes over the others.
Pembrolizumab, a PD-1 antagonist, represents a newer immunotherapy that has been observed to produce excellent response in unresectable cutaneous SCC. This study discusses the first case of unresectable cutaneous SCC having documented evidence of an MLH1 mutation and showing complete response to treatment with off-label pembrolizumab.
With further research, the potential utility of PD-1 inhibitors in patients with advanced, cutaneous SCC may provide another modality to be considered in addressing this diverse and devastating disease process. Additionally, the role of DNA MMR deficiency on therapeutic outcomes through additional investigation could prove to be a predictive biomarker to guiding the appropriate selection PD-1 inhibitors in disease management.
Acknowledgments
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
- MLH1
MutL Homolog 1 gene
- MMR
Mismatch Repair
- NGS
Next Generation Sequencing
- PD-1
Programmed Cell Death receptor
- SCC
Squamous Cell Carcinoma
- TCGA
The Cancer Genome Atlas
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Resources
- 1.Chang ALS, Kim J, Luciano R, Sullivan-Chang L, Colevas AD. A case report of unresectable cutaneous squamous cell carcinoma responsive to pembrolizumab, a programmed cell death protein 1 inhibitor. JAMA Dermatology. 2016;142:106–108. doi: 10.1001/jamadermatol.2015.2705. [DOI] [PubMed] [Google Scholar]
- 2.Borradori L, Sutton B, Shayesteh P, Daniels GA. Rescue therapy with anti-programmed cell death protein 1 inhibitors (PD-1) of advanced cutaneous squamous cell carcinoma and basosquamous carcinoma: preliminary experience in 5 cases. Br J Dermatol. 2016 doi: 10.1111/bjd.14642. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Jarkowski A, Hare R, Loud P, et al. Systemic therapy in advanced cutaneous squamous cell carcinoma (CSCC): the Rosewell Park experience and review of the literature. Am J Clin Oncol. 2014 doi: 10.1097/COC.0000000000000088. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Improta G, Leone I, Gieri S, Pelosi G, Fraggetta New developments in the management of advanced melanoma – role of pembrolizumab. Onco Targets and Therapy. 2015;8:2535–2543. doi: 10.2147/OTT.S72823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. NEJM. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoja L, Butler MO, Kang SP, Ebbinghaus S, Joshua AM. Pembrolizumab. Journal for ImmunoTherapy of Cancer. 2015 doi: 10.1186/s40425-015-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keytruda (pembrolizumab) [prescribing information] Whitehouse Station, NJ: Merck Sharp & Dohme Corp; 2014. [Google Scholar]
- 8.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanford Health. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Genetic Exploration of the Molecular Basis of Malignancy in Adults (GEMMA) [cited 2016 Aug 25] Available from: https://clinicaltrials.gov/ct2/show/NCT02416518 NLM Identifier: NCT02416518. [Google Scholar]
- 10.Wiser I, Scope A, Azriel D, Zloczower E, Carmel NN, Shalom A. Head and neck cutaneous squamous cell carcinoma clinicopathological risk factors according to age and gender: A population based study. IMAJ. 2016;18(5):275–278. [PubMed] [Google Scholar]
- 11.Bejar C, Maubec E. Therapy of advanced squamous cell carcinoma of the skin. Curr Treat Options Oncol. 2014;15(2):302–320. doi: 10.1007/s11864-014-0280-x. [DOI] [PubMed] [Google Scholar]
- 12.DeConti RC. Chemotherapy of Squamous cell carcinoma of the skin. Semin Oncol. 2012;39(2):145–149. doi: 10.1053/j.seminoncol.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Reigneau M, Robert C, Routier E, et al. Efficacy of neoadjuvant cetuximab alone or with platinum salt for the treatment of unresectable advanced nonmetastatic cutaneous squamous cell carcinomas. British Journal of Dermatology. 2015;173:527–534. doi: 10.1111/bjd.13741. [DOI] [PubMed] [Google Scholar]
- 14.Cranmer LD, Engelhardt C, Morgan SS. Treatment of unresectable and metastatic cutaneous squamous cell carcinoma. Oncologist. 2010;15(12):1320–1328. doi: 10.1634/theoncologist.2009-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maubec E, Ptrow P, Scheer-Senyarich I, et al. Phase II study of cetuximab as a first-line single drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29:3419–3426. doi: 10.1200/JCO.2010.34.1735. [DOI] [PubMed] [Google Scholar]
- 16.Shin DM, Glisson BS, Khuri FR, et al. Phase II and biologic study of interferon alfa, retinoic acid, and cisplatin in advanced squamous skin cancer. J Clin Oncol. 2002;20(2):364–370. doi: 10.1200/JCO.2002.20.2.364. [DOI] [PubMed] [Google Scholar]
- 17.Pickering CR, Zhou JH, Lee JJ, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res. 2014;20(24):6582–6592. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]


