Abstract
Background
Expanding latent tuberculosis treatment is important to decrease active disease globally. Once-weekly isoniazid and rifapentine for 12 doses is effective but limited by requiring direct observation.
Objective
To compare treatment completion and safety of once-weekly isoniazid and rifapentine by self-administration versus direct observation.
Design
An open-label, phase 4 randomized clinical trial designed as a noninferiority study with a 15% margin. Seventy-five percent or more of study patients were enrolled from the United States for a prespecified subgroup analysis. (ClinicalTrials.gov: NCT01582711)
Setting
Outpatient tuberculosis clinics in the United States, Spain, Hong Kong, and South Africa.
Participants
1002 adults (aged ≥18 years) recommended for treatment of latent tuberculosis infection.
Intervention
Participants received once-weekly isoniazid and rifapentine by direct observation, self-administration with monthly monitoring, or self-administration with weekly text message reminders and monthly monitoring.
Measurements
The primary outcome was treatment completion, defined as 11 or more doses within 16 weeks and measured using clinical documentation and pill counts for direct observation, and self-reports, pill counts, and medication event–monitoring devices for self-administration. The main secondary outcome was adverse events.
Results
Median age was 36 years, 48% of participants were women, and 77% were enrolled at the U.S. sites. Treatment completion was 87.2% (95% CI, 83.1% to 90.5%) in the direct-observation group, 74.0% (CI, 68.9% to 78.6%) in the self-administration group, and 76.4% (CI, 71.3% to 80.8%) in the self-administration–with–reminders group. In the United States, treatment completion was 85.4% (CI, 80.4% to 89.4%), 77.9% (CI, 72.7% to 82.6%), and 76.7% (CI, 70.9% to 81.7%), respectively. Self-administered therapy without reminders was noninferior to direct observation in the United States; no other comparisons met noninferiority criteria. A few drug-related adverse events occurred and were similar across groups.
Limitation
Persons with latent tuberculosis infection enrolled in South Africa would not routinely be treated programmatically.
Conclusion
These results support using self-administered, once-weekly isoniazid and rifapentine to treat latent tuberculosis infection in the United States, and such treatment could be considered in similar settings when direct observation is not feasible.
Primary Funding Source
Centers for Disease Control and Prevention.
Most patients with active tuberculosis (TB) develop disease after a period of asymptomatic infection. The transition from latent TB infection (LTBI) to active disease, termed “reactivation,” accounts for an estimated 86% of active TB in the United States (1). This provides an opportunity for prevention through diagnosis and treatment of LTBI. Targeted testing and treatment have long been recommended in high-risk populations in the United States (2). In 2016, the U.S. Preventive Services Task Force recommended that all adults at risk for TB infection be tested (3). This recommendation reflects the fact that active TB is most commonly diagnosed in foreign-born persons who have lived in the United States for 5 or more years (4). The World Health Organization also recently endorsed expanding LTBI treatment to reduce TB globally (5). Most patients with LTBI are diagnosed and treated through public health TB programs (6). Strategies that make treatment easier for patients and primary care providers are needed to expand TB prevention beyond public health and accelerate progress toward elimination (7–10).
Early clinical trials showed that isoniazid (INH) daily for 6 to 12 months could prevent 60% to 90% of active TB disease (11, 12), but effectiveness has been limited by poor tolerability, long duration of treatment, and low adherence (13–16). The PREVENT TB study showed that 3 months of once-weekly INH and rifapentine by directly observed therapy (DOT) was effective and safe compared with 9 months of daily INH by self-administered therapy (SAT) (17–19). Treatment completion with the combination regimen was significantly higher than with INH (82% vs. 69%; P < 0.001) (17). However, wide implementation of once-weekly therapy has been limited because DOT for LTBI is often unacceptable to patients, prohibitively expensive for TB programs, and unavailable through primary care providers.
Cost-effectiveness modeling found that self-administered, once-weekly INH and rifapentine had an advantage over other regimens if adherence remained high and toxicity did not increase (20). However, adherence to the once-weekly regimen in the SAT groups was unknown, and the frequency and severity of adverse events in patients monitored at monthly visits rather than weekly during DOT had not been evaluated (19, 21). In addition, the role of text message reminders, which have shown some benefit in adherence to other treatments, had not been studied in LTBI (22–25).
The primary objective of the iAdhere Study was to compare treatment completion between participants randomly assigned to DOT versus SAT with and without text message reminders. Assessing safety and tolerability by treatment group was the main secondary objective. Additional objectives described here include evaluations of access to text message reminders and follow-up for progression to active TB disease. Ongoing secondary analyses not presented here include cost-effectiveness, motivations to enroll, and an in-depth evaluation of the text messaging performance.
METHODS
Design and Population
The study was an open-label, phase 4 randomized controlled clinical trial funded by the Centers for Disease Control and Prevention (CDC) and done by the CDC’s Tuberculosis Trials Consortium. Nine sites in the United States, 1 in Spain, and 1 in Hong Kong were selected on the basis of experience with once-weekly INH and rifapentine by DOT, as part of the PREVENT TB study, and 1 site in South Africa that had conducted an independent study. Investigators screened adults (aged ≥18 years) who were diagnosed with LTBI, recommended for treatment, and considered candidates for SAT by local standards.
Enrollment, Randomization, and Study Groups
Men and nonpregnant, nonbreastfeeding women were eligible. Participants had to weigh at least 45 kg because fixed doses of 900 mg each of INH and rifapentine were used. We excluded persons with confirmed or suspected active TB, known contact with someone with INH- or rifampin-resistant TB, prior intolerance to INH or any rifamycin, or prior treatment of active or latent TB lasting more than 1 week. Persons with a baseline serum alanine aminotransferase level more than 5 times the upper limit of normal were ineligible. All participants were offered HIV testing unless they were known to be HIV-positive or had documented negative test results from the past year. Patients receiving antiretroviral therapy, HIV-positive persons with a CD4 count less than 0.350 × 109 cells/L, and those planning to start antiretroviral treatment within 4 months of enrollment were ineligible because of the uncertain potential for drug–drug interactions with rifapentine.
Participants were randomly assigned to receive once-weekly INH and rifapentine by DOT, SAT without reminders, or SAT with weekly text message reminders (Appendix 2, available at Annals.org). Patients were enrolled and randomly assigned centrally at the Tuberculosis Trials Consortium’s Data Coordinating Center through a Web-based program that used the big stick design (26), with a maximum imbalance of 3 participants across treatment groups within sites. A maximum of 3 persons from the same household could participate in the study. The first person enrolled was randomly assigned, and the others were assigned to the same treatment group.
A secondary objective of the trial was to evaluate the performance of text message reminders in improving adherence to SAT. Access to text messaging among persons treated for LTBI was unknown before the trial and therefore was not required for enrollment. To maintain the integrity of the randomization for the primary objective, comparing SAT with DOT, participants were randomly assigned to a group regardless of whether they had access to a cell phone that could receive text messages. Cell phones were not supplied by the study, so participants randomly assigned to SAT with text message reminders who did not have a cell phone received SAT without the reminders. They were included in the SAT-with-reminders group for analyses on the basis of the randomization and intention to treat.
Sanofi supplied INH and rifapentine in prepackaged boxes with a 1-month supply. Vitamin B6 (pyridoxine), 50 mg, was recommended with each dose and supplied locally. Participants were assigned 3 medication boxes after randomization. For persons randomly assigned to DOT, doses were prepared from the assigned medication box and administered in the clinic or community by health care personnel. Participants randomly assigned to SAT were given 1 medication box each month to take home and instructed to bring it to their follow-up visit. An educational flip chart was used during initial visits to standardize education about correct pill-taking and symptoms of drug toxicity.
Text message reminders with the message “Remember, iAdhere today,” in which “remember” and “today” were translated into the participant’s preferred language, were sent by a central service once weekly on the day and time chosen by the patient. Participants were instructed to recognize this message as a reminder to take the study medications but were told not to respond.
Follow-up and End Points
All participants had monthly follow-up visits to assess treatment adherence and monitor for toxicity. The primary end point was treatment completion, defined as receiving at least 11 doses within 16 weeks. Doses had to be at least 3 days apart, with no more than 3 doses in any 18-day period. Participants who missed doses could make them up later if time allowed before 16 weeks. To approximate clinical practice, participants randomly assigned to SAT could receive up to 4 DOT doses during the study (at their initial clinic visit, during monthly follow-up visits, or during a drug rechallenge).
Countable DOT doses were determined using the clinics’ drug administration records and pill counts from the assigned medication boxes; countable SAT doses were determined by self-reports and pill counts during the monthly visits. In addition, medication event monitoring system (MEMS) caps were attached to the INH bottles in the SAT group to record pill bottle openings (27). The participants, site staff, and investigators were blinded to the MEMS data, which were collected electronically and held by a third party (medAmigo AARDEX cloud-based platform) until study completion. The final determination of countable doses and treatment completion in the SAT groups was based on the lowest possible result using a stepwise approach beginning with self-reports, then pill counts, and finally MEMS data (Appendix 3, available at Annals.org).
Participants were assessed for adverse events monthly during treatment and for 28 days after the last dose. Baseline evaluation included a symptom review for the 28 days before enrollment for comparison of symptoms reported later. All events were graded using the U.S. National Cancer Institute Common Toxicity Criteria, version 2.0. Local site investigators categorized adverse events as related (definitely, probably, or possibly) or not related (unlikely, not related, or unclassifiable) to study drugs (Appendix Table 1, available at Annals.org). Systemic drug reactions, which included possible hypersensitivity reactions, were of special interest. Therefore, all adverse events were reviewed centrally regardless of how the local site categorized them. As in the PREVENT TB study, criteria for systemic drug reactions were hypotension, urticarial rash (hives), angioedema, acute bronchospasm, conjunctivitis, or at least 4 of the following symptoms occurring concurrently (1 of which had to be grade 2 or higher): weakness, fatigue, nausea, vomiting, headache, fever, aches, sweats, dizziness, shortness of breath, flushing, or chills (19). Isolated hepatotoxicity or rash, events with a known nondrug cause, grade 1 events, and events in participants able to complete treatment were not categorized as systemic drug reactions.
Statistical Analysis
The sample size was calculated assuming 80% treatment completion by DOT based on the PREVENT TB study. Previous modeling showed that adherence to the once-weekly regimen by SAT could be as much as 23% lower than that by DOT and remain more cost-effective (28). However, a margin that large would put adherence below the threshold at which 9 months of INH would be preferred (Figure 3 of reference 28). Therefore, the protocol team chose a margin of 15% to be within the bounds of our modeling and clinically acceptable. The study was designed and powered as a noninferiority trial with a maximum allowable decrease in treatment completion (noninferiority margin) of 15%. With a significance level of 2.5% (or a 2-sided level of 5%) and a power of 90%, a sample size of 216 per group was required. Sample size was increased to 333 per group to allow up to 30% cluster enrollment within households. We analyzed the data by including only randomly assigned persons (excluding household members who were not randomly assigned) and by including all study participants. The study design and implementation plan ensured that at least 75% of participants would be from U.S. sites to allow for a preplanned subgroup analysis using the same noninferiority criteria.
The primary analysis evaluated the differences in treatment completion between the DOT and SAT groups and used a proportional weighted average based on the number of patients enrolled at each site to account for differences by site and heterogeneity in completion (Appendix 4, available at Annals.org). This did not affect the point estimates but yielded broader, more conservative CIs. If the upper bound of the CI for the weighted difference in completion was less than 15%, treatment completion by the SAT groups was deemed noninferior to that by the DOT groups. Participants who were randomly assigned but never started treatment, were lost to follow-up, or withdrew consent before completing treatment were included in the intention-to-treat analysis.
Univariate and multivariate logistic regression were done on the DOT and combined SAT groups to evaluate possible predictors of treatment noncompletion. Variables included demographic characteristics known or suspected to affect adherence from the literature. Enrollment as part of a household and known liver disease were included in the multivariate analysis as possible confounders. Adverse events were analyzed in all participants randomly assigned to a group. Data were analyzed using SAS software, version 9.3 (SAS Institute).
The institutional review boards or ethics committees of the CDC and all sites reviewed and approved the study. All participants gave written informed consent. An independent data safety monitoring board met annually to review progress and safety.
Role of the Funding Source
The Tuberculosis Trials Consortium, funded by the CDC, designed, conducted, and reported the results of the study. Sanofi provided the INH and rifapentine but did not have any role in the design or conduct of the study, data analyses, or decision to publish the results.
RESULTS
Participants
Between September 2012 and April 2014, a total of 2176 persons were screened. Of these, 408 were ineligible, 218 declined LTBI treatment, 480 chose not to enroll, and 68 were not enrolled on the basis of the local investigator’s decision (Figure 1). Overall, 1002 participants were enrolled, including 774 (77.2%) in the United States (Figure 1 and Table 1). Participants were demographically similar by study group, both overall and for the first person enrolled in a household cluster (Table 1 and Appendix Table 2, available at Annals.org). Median age was 36 years (interquartile range, 27 to 49), and 482 participants (48.1%) were women. Of enrolled participants, 552 (55.1%) were diagnosed with LTBI during routine screening, 344 (34.3%) were known contacts to a person with active TB disease, and 97 (9.7%) converted on LTBI testing. After enrollment, 4 participants were found to be contacts to a person with drug-resistant TB. They were included in the safety analysis but excluded from the completion analysis. All other randomly assigned patients were included in the analyses as intention-to-treat, including 14 participants who never started therapy (3 in the DOT group, 7 in the SAT group, and 4 in the SAT-with-reminders group). The trial ended with completion of the target enrollment and final follow-up visits in August 2014.
Figure 1.
Study flow diagram
DOT = directly observed therapy; LTBI = latent tuberculosis infection; SAT = self-administered therapy; TB = tuberculosis.
*Common reasons: did not understand a language available for translation, 41%; history of ≥1 wk of treatment of active TB or LTBI, 19%; HIV-positive with CD4 count <0.350 × 109 cells/L or antiretroviral medications contraindicated with rifapentine, 8%.
†Common reasons: preferred regular treatment, 13%; considered DOT to be too inconvenient, 9%; not interested in study medications, 9%; worried about pill burden with once-weekly treatment, 7%.
‡ Common reasons: active, symptomatic comorbidities, 28%; concern for poor adherence, 20%; decision to treat with different regimen, 18%.
§ The first participant enrolled in each household was randomly assigned to a treatment group. Subsequent participants enrolled from the same household were automatically assigned to the same group as the first participant.
|| 4 participants (2 in each of the SAT groups) were enrolled as contacts of a person with active TB before susceptibility results had returned showing resistance to isoniazid or rifampin in the source patient. Treatment was stopped, and these participants were included in the safety analysis but excluded from treatment completion.
Table 1.
Demographic and Clinical Characteristics of All Participants, by Study Group*
| Characteristic | Overall (n = 1002) | DOT (n = 337) | SAT (n = 337) | SAT With Reminders (n = 328) |
|---|---|---|---|---|
| Median age (IQR), y | 36 (27–49) | 36 (27–48) | 36 (27–48) | 38 (27–49) |
|
| ||||
| Female | 482 (48.1) | 153 (45.4) | 161 (47.8) | 168 (51.2) |
| Race | ||||
|
| ||||
| White | 518 (51.7) | 171 (50.7) | 175 (51.9) | 172 (52.4) |
|
| ||||
| Black/African American | 250 (25.0) | 84 (24.9) | 91 (27.0) | 75 (22.9) |
|
| ||||
| Asian | 200 (20.0) | 68 (20.2) | 62 (18.4) | 70 (21.3) |
|
| ||||
| Other | 34 (3.4)† | 14 (4.2) | 9 (2.7) | 11 (3.4) |
| Country of enrollment | ||||
|
| ||||
| United States | 774 (77.2) | 261 (77.4) | 262 (77.7) | 251 (76.5) |
|
| ||||
| Spain | 100 (10.0) | 35 (10.4) | 32 (9.5) | 33 (10.1) |
|
| ||||
| Hong Kong | 45 (4.5) | 15 (4.5) | 14 (4.2) | 16 (4.9) |
|
| ||||
| South Africa | 83 (8.3) | 26 (7.7) | 29 (8.6) | 28 (8.5) |
|
| ||||
| Born outside country of enrollment | 603 (60.2) | 208 (61.7) | 207 (61.4) | 188 (57.3) |
|
| ||||
| Completed high school | 640 (63.9)‡ | 218 (64.7) | 218 (64.7) | 204 (62.2) |
|
| ||||
| Homeless within the past year | 51 (5.1) | 16 (4.7) | 16 (4.7) | 19 (5.8) |
|
| ||||
| Primary occupation during the past year§ | ||||
| Health care worker | 136 (13.6) | 43 (12.8) | 48 (14.3) | 45 (13.8) |
|
| ||||
| Non–health care worker | 472 (47.3) | 158 (47.2) | 150 (44.8) | 164 (50.2) |
|
| ||||
| Unemployed, not seeking employment | 221 (22.2) | 78 (23.3) | 81 (24.2) | 62 (19.0) |
|
| ||||
| Unemployed, seeking employment | 168 (16.9) | 56 (16.7) | 56 (16.7) | 56 (17.1) |
|
| ||||
| Indication for LTBI treatment|| | ||||
| Contact to a person with infectious TB | 344 (34.3) | 109 (32.3) | 111 (32.9) | 124 (37.8) |
|
| ||||
| Recent LTBI screening test conversion | 97 (9.7) | 32 (9.5) | 34 (10.1) | 31 (9.5) |
|
| ||||
| HIV infection | 5 (0.5) | 1 (0.3) | 2 (0.6) | 2 (0.6) |
|
| ||||
| Fibrosis on chest radiography | 4 (0.4) | 3 (0.9) | 0 (0) | 1 (0.3) |
|
| ||||
| Positive results on LTBI screening test | 552 (55.1) | 192 (57.0) | 190 (56.4) | 170 (51.8) |
|
| ||||
| Diabetes | 84 (8.4)¶ | 32 (9.5) | 24 (7.1) | 28 (8.5) |
|
| ||||
| Liver disease** | 42 (4.2) | 11 (3.3) | 15 (4.5) | 16 (4.9) |
| HIV status | ||||
|
| ||||
| Positive | 11 (1.1) | 3 (0.9) | 3 (0.9) | 5 (1.5) |
|
| ||||
| Negative | 776 (77.4) | 264 (78.3) | 258 (76.6) | 254 (77.4) |
|
| ||||
| Declined testing | 215 (21.5) | 70 (20.8) | 76 (22.6) | 69 (21.0) |
|
| ||||
| History of alcohol use | 531 (53.0) | 183 (54.3) | 170 (50.4) | 178 (54.3) |
|
| ||||
| History of alcohol abuse (CAGE score ≥2) | 70 (7.0) | 25 (7.4) | 22 (6.5) | 23 (7.0) |
|
| ||||
| Current cigarette smoker | 250 (25.0) | 89 (26.4) | 82 (24.3) | 79 (24.1) |
|
| ||||
| Drug use within the past year | 53 (5.3) | 22 (6.5) | 14 (4.2) | 17 (5.2) |
|
| ||||
| Methadone maintenance therapy | 8 (0.8) | 4 (1.2) | 2 (0.6) | 2 (0.6) |
|
| ||||
| Cell phone with text capability | 911 (90.9) | 309 (91.7) | 303 (89.9) | 299 (91.2) |
|
| ||||
| Willing to receive text message reminders (percentage of those whose cell phone has text capability) | 885 (97.2) | 295 (95.5) | 296 (97.7) | 294 (98.3) |
CAGE = Cut Down, Annoyed, Guilty, and Eye Opener; DOT = directly observed therapy; IQR = interquartile range; LTBI = latent tuberculosis infection; SAT = self-administered therapy; TB = tuberculosis.
Values are numbers (percentages) unless otherwise indicated.
22 participants did not report race and are included here.
52 participants did not report education level.
5 participants did not report primary occupation.
Participants were counted only once in the order presented. The total number of HIV-infected persons who were enrolled in the study is listed elsewhere in the table.
Whether 2 participants had been diagnosed with diabetes or high blood sugar was unknown.
Defined as the presence of any of the following chronic liver conditions: hepatitis B virus infection (n = 9), hepatitis C virus infection (n = 19), hepatitis type unknown (n = 13), hepatitis due to alcohol use (n = 1), or cirrhosis (n = 3). Two participants reported hepatitis C virus infection and cirrhosis. One participant reported hepatitis B and C virus infection.
Treatment Completion and Text Message Reminders
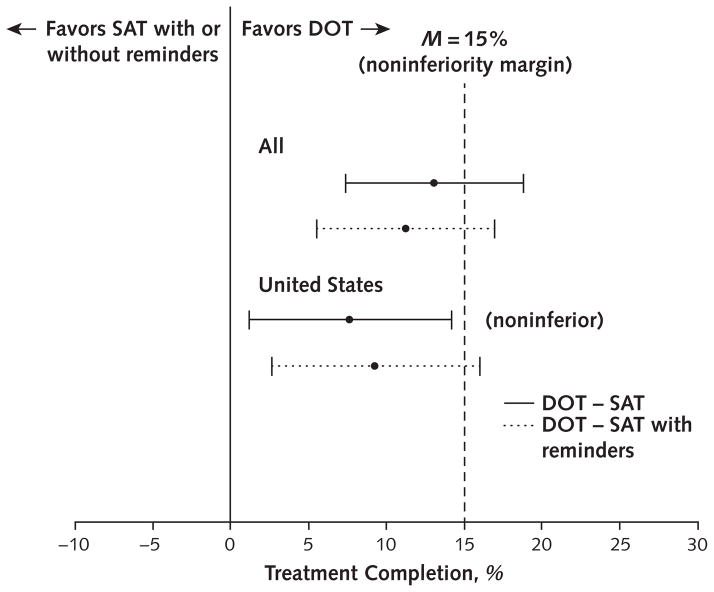
Treatment completion was 87.2% (95% CI, 83.1% to 90.5%) in the DOT group, 74.0% (CI, 68.9% to 78.6%) in the SAT group, and 76.4% (CI, 71.3% to 80.8%) in the SAT-with-reminders group. The weighted difference in treatment completion between DOT and SAT was 13.1% (upper bound, 18.8%); between DOT and SAT with reminders, it was 11.2% (upper bound, 16.9%). Because the upper bounds of the CIs were more than 15%, neither SAT group was noninferior to DOT by the study definition (Figure 2 and Appendix Table 3, available at Annals.org).
Figure 2.
Weighted treatment completion for all participants, by study group.
DOT = directly observed therapy; SAT = self-administered therapy.
In the prespecified subgroup analysis of U.S. participants, treatment completion was 85.4% (CI, 80.4% to 89.4%) in the DOT group, 77.9% (CI, 72.7% to 82.6%) in the SAT group, and 76.7% (CI, 70.9% to 81.7%) in the SAT-with-reminders group. The weighted difference between DOT and SAT was 7.7% (upper bound, 14.2%), meeting the study definition for noninferiority. Between DOT and SAT with reminders, it was 9.3% (upper bound, 16.0%) (Figure 2). The findings were similar when analyses were restricted to the first person enrolled as part of a cluster (Appendix Table 3) and when analyses were repeated without applying the weighting method.
Of 1002 participants enrolled, 911 (90.9%) had a cell phone with text messaging and 885 (88.3%) were willing to receive weekly reminders (Table 1). Overall treatment completion was similar for participants randomly assigned to SAT with and without reminders (Appendix Table 4, available at Annals.org). Treatment completion in the SAT-with-reminders groups was higher than in the SAT groups in Spain (84.8% vs. 73.3%), Hong Kong (100% vs. 78.6%), and South Africa (50.0% vs. 37.9%); however, the study was not powered to evaluate this statistically.
By using multivariate logistic regression, factors associated with noncompletion in the combined SAT groups were enrollment in South Africa compared with the United States (odds ratio, 4.19 [CI, 2.35 to 7.48]), current smoking (odds ratio, 1.85 [CI, 1.21 to 2.83]), and female sex (odds ratio, 1.48 [CI, 1.01 to 2.16]) (Appendix Table 5, available at Annals.org). None of the variables evaluated were significantly associated with noncompletion of treatment in the DOT group (not shown).
Adverse Events
Overall, 208 adverse events were reported in 174 participants, with similar proportions by study group (Table 2 and Appendix Table 6, available at Annals.org). One participant, who was randomly assigned to SAT with reminders but did not start treatment, developed active TB. Seventy-eight participants (7.8%) had drug-related adverse events; 5 of these were serious. Site investigators determined that 45 participants discontinued treatment because of an adverse event: 12 of 337 (3.6%) receiving DOT, 19 of 337 (5.6%) receiving SAT, and 14 of 328 (4.3%) receiving SAT with reminders (P = 0.34). Forty-three systemic drug reactions were identified in 42 participants (4.2%), with similar distribution by group (Table 2). Four participants who required hospitalization recovered with no sequelae. The median number of doses received before the event was 3 (interquartile range, 2.0 to 5.0), and the median time to onset after medication ingestion was 5 hours (inter-quartile range, 2.0 to 10.0). Two participants reported syncope that was possibly related to study drugs, but neither was hospitalized (Table 2, tenth footnote). One death, determined to be unrelated to study drugs, occurred in a participant randomly assigned to SAT with reminders who committed suicide 5 days after receiving the initial dose by DOT (Table 2, sixth footnote).
Table 2.
AEs by Participants Enrolled, Stratified by Study Group*
| Variable | Overall (n = 1002) | DOT (n = 337) | SAT (n = 337) | SAT With Reminders (n = 328) |
|---|---|---|---|---|
| Participants with AE, n | 174 | 53 | 59 | 62 |
|
| ||||
| Attribution | ||||
| Drug-related† | 78 (7.8) | 24 (7.1) | 28 (8.3) | 26 (7.9) |
|
| ||||
| Not drug-related‡ | 96 (9.6) | 29 (8.6) | 31 (9.2) | 36 (11.0) |
|
| ||||
| Discontinuation due to AE | 45 (4.5) | 12 (3.6) | 19 (5.6) | 14 (4.3) |
|
| ||||
| Grade 1 or 2 or not applicable | 25 (2.5) | 9 (2.7) | 9 (2.7) | 7 (2.1) |
|
| ||||
| Grade 3 or 4 | 20 (2.0)§ | 3 (0.9) | 10 (3.0)|| | 7 (2.1) |
| Toxicity grade | ||||
|
| ||||
| Grade 1 or 2 or not applicable | 99 (9.9) | 30 (8.9) | 36 (10.7) | 33 (10.1) |
|
| ||||
| Drug-related† | 46 (4.6) | 16 (4.7) | 18 (5.3) | 12 (3.7) |
|
| ||||
| Not drug-related‡ | 53 (5.3) | 14 (4.2) | 18 (5.3) | 21 (6.4) |
|
| ||||
| Grade 3 or 4 | 74 (7.4) | 23 (6.8) | 23 (6.8) | 28 (8.5) |
|
| ||||
| Drug-related† | 32 (3.2) | 8 (2.4) | 10 (3.0) | 14 (4.3) |
|
| ||||
| Not drug-related‡ | 42 (4.2) | 15 (4.5) | 13 (3.9) | 14 (4.3) |
|
| ||||
| Death (grade 5) | 1 (0.1) | 0 (0) | 0 (0) | 1 (0.3)¶ |
|
| ||||
| SAE** | 22 (2.2) | 11 (3.3) | 5 (1.5) | 6 (1.8) |
|
| ||||
| Drug-related† | 5 (0.5)†† | 3 (0.9) | 0 (0) | 2 (0.6) |
|
| ||||
| Not drug-related‡ | 17 (1.7) | 8 (2.4) | 5 (1.5) | 4 (1.2) |
| AE of special interest | ||||
|
| ||||
| Systemic drug reaction | 42 (4.2)§§ | 13 (3.9) | 15 (4.5) | 14 (4.3) |
|
| ||||
| Hepatitis | 9 (0.9) | 1 (0.3) | 3 (0.9) | 5 (1.5) |
|
| ||||
| Isolated hepatitis | 4 (0.4) | 0 (0) | 1 (0.3) | 3 (0.9) |
|
| ||||
| Drug-related† | 2 (0.2) | 0 (0) | 1 (0.3) | 1 (0.3) |
|
| ||||
| Not drug-related‡‡ | 2 (0.2) | 0 (0) | 0 (0) | 2 (0.6) |
|
| ||||
| Hepatitis associated with systemic drug reaction | 5 (0.5) | 1 (0.3) | 2 (0.6) | 2 (0.6) |
|
| ||||
| Completed treatment | 1 (0.1) | 1 (0.3) | 0 (0) | 0 (0) |
|
| ||||
| Did not complete treatment | 4 (0.4) | 0 (0) | 2 (0.6) | 2 (0.6) |
|
| ||||
| Thrombocytopenia|| || | 1 (0.1) | 0 (0) | 0 (0) | 1 (0.3) |
|
| ||||
| Neutropenia¶¶ | 1 (0.1) | 1 (0.3) | 0 (0) | 0 (0) |
AE = adverse event; DOT = directly observed therapy; SAE = severe adverse event; SAT = self-administered therapy.
Values are numbers (percentages) unless otherwise indicated. Percentages were calculated based on the total participants enrolled by study group.
Considered to be definitely, probably, or possibly related to the study drugs.
Considered to be an unlikely relation to the study drugs, not related, or unclassifiable.
Considered to be related to the study drugs (n = 19).
Grade 4 (n = 2).
A 25-year-old patient born in South Africa with a social history of alcohol consumption, cigarette smoking, and unemployment in the DOT group committed suicide 5 d after the first study dose. The patient had no history of psychiatric disorders or suicidal ideation and no signs of depression or anxiety before or after the first study dose.
Death, any life-threatening experience, any inpatient hospitalization or prolongation of any hospitalization, any persistently or severely disabling event, a congenital anomaly or birth defect, an overdose of study drugs, or any grade 4 toxicity event.
Four patients required hospitalization. One patient in the DOT group and 1 patient in the SAT-with-reminders group had gastrointestinal symptoms, 1 patient in the DOT group had a systemic drug reaction, and 1 patient in the DOT group had a rheumatoid arthritis flare related to reduced efficacy of prednisone due to rifapentine. One patient in the SAT-with-reminders group had grade 4 fatigue but did not require hospitalization.
For patients with >1 AE, the AE was chosen in hierarchical order according to severity, grade, and attribution.
Includes 2 episodes of syncope and 1 episode of hypotension without syncope that were possibly drug-related. A 35-year-old white Hispanic woman developed fever, nausea, vomiting, diarrhea, and syncope along with leukocytosis approximately 2 h after receiving the third study dose. Bacterial gastrointestinal infection was suspected, and the patient recovered after receiving fluid replacement and antibiotic treatment. A 28-year-old woman developed headache, myalgia, and bone pain. The patient reported 2 episodes of syncope approximately 30 h after receiving the second study dose and recovered without treatment or sequelae. A 51-year-old man receiving amlodipine and hydrochlorothiazide for hypertension developed presyncope 1 h after his seventh study dose. The symptoms recurred after the eighth dose and resolved after approximately 6 h without treatment. The patient completed 4 mo of rifampin without incident.
The only event was considered to be related to the study drugs.
The only event was not considered to be related to the study drugs.
Discussion
At the U.S. sites, the iAdhere study found that once-weekly INH and rifapentine by SAT without reminders was noninferior to DOT; neither SAT group in the overall study population nor the SAT-with-reminders group at U.S. sites achieved noninferiority compared with the DOT group. This may have been because DOT had higher-than-expected treatment completion in all countries. Also, treatment completion in the SAT groups was similarly high in the United States, Spain, and Hong Kong; however, it was low in South Africa (Appendix Table 7, available at Annals.org).
In South Africa, SAT completion by self-reports and pill counts was similar to that at other sites. The MEMS data revealed that some participants who reported good adherence were not opening their INH bottles. We ruled out MEMS failure because the caps successfully recorded openings during follow-up visits. Participants in South Africa were younger, more likely to be unemployed, and more likely to report alcohol abuse than those at other sites (Appendix Table 8, available at Annals.org). They were also primarily HIV-negative household contacts of a person with active TB disease.
As designed, the study could not determine the reasons for the low treatment completion in South African participants. Of note, LTBI treatment is not the standard of care for HIV-negative adult contacts in South Africa. Patients in other prevention studies have also reported good adherence despite objective evidence to the contrary (29, 30). Finally, although the local institutional review boards approved reimbursement for study-related expenses as appropriate, this could have influenced patients to report good adherence (31).
If South Africa is excluded, treatment completion with 3 months of once-weekly INH and rifapentine by SAT with or without reminders was higher than reported with 9 months of INH and similar to reports with 4 months of rifampin. Completion with 9 months of INH was 69% in the PREVENT TB trial and has been less than 50% in some programmatic settings (13–15, 17). Between 70% and 80% of patients completed treatment with 4 months of daily self-administered rifampin (32–35). Of note, those studies did not assess rifampin completion with MEMS caps or another highly conservative method and may have overestimated the true completion with self-administered rifampin.
Safety and tolerability are critical for LTBI regimens and affect whether providers will recommend treatment and whether patients will accept and complete it. Weekly INH and rifapentine was less hepatotoxic than daily INH but caused more systemic drug reactions in the PREVENT TB trial. We hypothesized that more adverse events might be reported in participants receiving DOT (because of more frequent contact with health care workers) or that more severe adverse events could occur in the SAT groups (if participants receiving less supervision continued to take medication despite symptoms). However, the total, drug-related, and severe adverse events in iAdhere were similar in the DOT and SAT groups and similar to those reported previously.
Strengths of the study include that it was a large, multisite randomized clinical trial designed to reflect clinical practice. The broad inclusion criteria facilitated enrollment of a representative population of patients with LTBI treated through public health clinics. Enrollment at 9 U.S. sites in 7 states and Washington, DC, suggests good generalizability within the United States. Enrollment in 4 countries increased the diversity of clinical settings but also identified important differences. Adherence to SAT was measured by using a composite end point that included objective MEMS data, which are considered the gold standard for adherence studies (36). Finally, safety evaluations included active assessments of symptoms before treatment was started and at regular intervals during and after treatment.
The study also had several limitations. Although the trial was designed to reflect clinical practice, the MEMS caps and medication boxes required more complex packaging than would be used clinically and may have contributed to lower treatment completion in the SAT groups. Not all participants randomly assigned to receive weekly reminders had access to text messages, and the study did not require confirmation that the messages were received. Only 1 study site was in a country with a high TB burden, and the low completion in the SAT groups may not be generalizable to other high-burden settings. Lastly, the study did not evaluate SAT in adolescents or children, whose adherence would likely reflect parental behavior.
Once-weekly INH and rifapentine for LTBI continues to be well-tolerated in diverse populations, and no new safety concerns have emerged to date. Although text message reminders did not have an effect in the United States, treatment completion in the SAT-with-reminders groups was higher than that in the SAT-alone groups in Spain, Hong Kong, and South Africa. This could be evaluated in subsequent studies.
In conclusion, the iAdhere study found that once-weekly INH and rifapentine by SAT had high treatment completion in the United States, Spain, and Hong Kong. This self-administered regimen with monthly monitoring may be an acceptable strategy for treating LTBI in the United States and could be considered in countries with similar approaches to TB prevention when DOT is not feasible.
Acknowledgments
Financial Support: By the Centers for Disease Control and Prevention’s Tuberculosis Trials Consortium.
Appendix 1: Members of the TB Trials Consortium iAdhere Study Team
Study team members who are not named authors are listed below by their TB Trials Consortium (TBTC) site affiliation. The TBTC sites and locations are listed in numerical order by site number. Individual contributions to the study are in parentheses. (OL = oversight and leadership responsibility for the research activity planning and execution at research location; MC = management and coordination responsibility for the research activity at research location; CR = conducted research and investigation process, specifically performing data collection; PM = provision of study materials, patients, laboratory tests, computing resources, or other analysis tools.)
TBTC Site 20 (North Texas): Philip Slocum (OL, MC, PM), John K. Podgore (MC, CR, PM), Sandra D. Small (OL, MC, CR, PM), and Mauricio Vecino (OL, MC, CR, PM).
TBTC Site 22 (Colorado): Edward M. Gardner (study design), Jacqueline D. Moore (OL, MC, CR, PM), Randall Reves (OL, PM), and Laurie Luna (OL, MC, CR, PM).
TBTC Site 24 (New York): Neil Schluger (OL, MC, CR), Joseph Burzynski (OL, MC, CR, PM), Vilma L. Montero (OL, MC, CR), and Mascha Elskamp (OL, MC, CR, PM).
TBTC Site 28 (San Francisco): Payam Nahid (OL, PM), Cindy Merrifield (MC, CR, PM), Irina Rudoy (CR), Julie Higashi (OL), Phil Hopewell (OL).
TBTC Site 31 (Barcelona, Spain): Jose Antonio Martinez (OL, CR), M. Carmen Ligero (PM), Laura Garcia (PM), Marta Sala (PM), Emma Fernández (PM), Anna Vilella (OL, CR), Amparo Tricas (PM), Virginia Pomar (OL, CR), M. Antonia Sambeat (OL, CR), Jéssica Muñoz (PM), Angels Fontanet (PM), Antonio Moreno (MC, PM), M. Llanos Roldán (MC, PM), Arancha Romero (MC, PM), Luciá del Baño (MC, PM), Laia Fina (MC, PM), Pilar Gorrindo (PM), Angels Orcau (MC), Jesús Ospina (PM), Adrià Curran (MC, CR), Israel Molina (MC, CR), Fernando Salvador (MC, CR), Adrian Sanchez-Montalva (MC, CR), Nuria Saborit (PM), Jose Angel Rodrigo (MC, CR), Xavier Martinez (MC, CR), Sonia Uriona (MC, CR), Xavier Martinezlacasa (OL, PM), Roser Font (PM), Carles Fernàndez (PM), Hernando Knobel (OL, CR), Neus Jové (PM), Maria Angeles Jimenez (OL, CR), Maria Luiza da Souza (OL, CR), and Adela Cantos (PM).
TBTC Site 34 (Soweto, South Africa): Richard Chaisson (OL).
TBTC Site 36 (Hong Kong): Chi Chiu Leung (OL, MC, CR, PM), Kwok Chiu Chang (OL, MC, CR, PM), Kalin Fong (MC, CR), and Lai Chu Kan (OL, MC, CR, PM).
TBTC Site 40 (South Texas): Diane D. Wing (MC, CR, PM), Narciso Lopez (MC, CR, PM), and Juan J. Uribe (OL, MC, CR, PM).
TBTC Site 53 (Washington, DC): Fred M. Gordin (MC), S. Sonia Qasba (MC), Debra Ann Benator (OL, MC), and Shirley Cummins (MC, CR, PM).
TBTC Site 54 (North Carolina): Carol D. Hamilton, MD (OL); Jason E. Stout (OL, MC); Elizabeth Ellen Tolley (study design); and Emily J. Hecker (MC, CR, PM).
TBTC Site 63 (South Texas): Melissa Engle (OL, MC, CR, PM), Polo Pavon (MC, CR, PM), and Rogelio Duque (MC, CR, PM).
TBTC Site 70 (Tennessee): Timothy R. Sterling, MD (OL, PM); Amy Kerrigan (MC, CR, PM); and Diedra Lynnette Freeman (CR, PM).
Appendix 2: Methods: Randomization Schema
Randomization used the big stick approach with a maximum imbalance of 3 participants allowed across groups within each study site. To reduce the predictability of the next assignment, details of the randomization schema were not disclosed to study sites. Before study initialization, randomization schedules were computer-generated for each study site. It was verified that these schedules maintained the big stick structure so that the maximum imbalance held within sites. The randomization schedules for each study site were loaded into an online Web application used for enrolling participants. When an eligible participant was enrolled, the online system would select the next available assignment from the randomization schedule at the specified study site. In the event that a household (maximum of 3 participants) was being enrolled, only 1 participant would be randomly assigned and all other members of the household would be assigned the same group.
The following procedure describes the big stick design used to make sure the maximum difference in sample sizes among the 3 study groups was 3 or less: 1) The maximum difference among the 3 study groups was calculated after each new randomly assigned participant was enrolled. 2) If the difference was less than 3, the next participant was randomly assigned with equal chance to 1 of the 3 study groups. 3) If the difference was equal to 3 and if only 1 group had the highest number of enrollments, the next participant was assigned to 1 of the other 2 groups randomly with equal chance. 4) If the difference was equal to 3 and if 2 groups had the same highest number of enrollments, the next participant was assigned to the third group deterministically.
Appendix 3: Methods: Procedures and Algorithms for Assessing Countable Doses and Treatment Completion in DOT and SAT
Countable Doses
All participants were assigned monthly medication boxes that contained a bottle with 30 INH pills (300 mg/pill) and 4 individually wrapped foil packs containing 8 rifapentine tablets (150 g/tablet). A full treatment dose was defined as 3 INH pills (900 mg) and 6 rifapentine tablets (900 mg). Doses had to be taken at least 3 days apart, and no more than 3 doses could be taken in 18 days. Doses taken less than 3 days apart were counted as a single dose toward treatment completion. If more than 3 doses were taken in 18 days, a maximum of 3 were counted toward treatment completion.
Each month, 4 weekly doses were expected to be taken from each medication box (12 INH pills and 24 rifapentine tablets). If more than 18 INH pills or 8 rifapentine tablets remained at the monthly follow-up, the number of countable doses for that month was fewer than 4. A new medication box was assigned at each follow-up visit. The countable doses from each month were added to those from previous months to determine the total doses counted toward completion.
DOT
Countable doses for DOT were determined by the clinic dose record and pill counts at the monthly follow-up visits.
SAT
For the SAT groups, countable doses were determined by combining data from self-reports, pill counts, and MEMS devices attached to the INH bottles. Participants were instructed that the MEMS device would record the date and time when the bottle was opened and that they should open the bottle only on the day of their dose. At monthly follow-up visits, participants were asked how many pills they took per dose, the number of doses they had taken since the last visit, and the dates of those doses. If a participant reported taking fewer than 3 INH pills or 6 rifapentine tablets with each weekly dose, no countable doses were credited toward completion. After determining the number of self-reported countable doses, pill counts were done and assessed as described. On the basis of the self-report and pill counts, local study staff determined the number of countable doses for the month.
After study completion, the MEMS data were analyzed (number and times of bottle openings were counted) to determine the number of countable doses each month based on the INH bottle openings.
If the monthly countable doses by MEMS differed from the countable doses based on self-report and pill count, the lower total was used in the final determination of doses counted toward treatment completion.
Treatment Completion
Treatment completion was based on the number of countable doses and defined as taking at least 11 once-weekly doses within 16 weeks. The primary analysis was based on a dichotomous evaluation of the primary end point, completion versus noncompletion of therapy.
Appendix 4: Methods: Statistical Considerations for Primary Analysis and Justification for Using the Weighted Averages
For the primary comparisons, we analyzed the data separately by only participants who were randomly assigned (that is, the first participant from each of the enrolled households) and by all persons enrolled in the study. Individual statistical hypothesis testing was applied for each of the 2 primary comparisons. For study site i, i = 1,2, … 12, the true completion rates for the 2 comparing study groups were pia (DOT) and pib (SAT), respectively. Then, the loss-of-completion rate from subtracting SAT from DOT in site i was δi = pia – pib. We assumed that the site effects (δi) were a random sample from a population with mean δ. The mean was considered as the overall (true) loss-of-completion rate from replacing DOT with SAT in the target population. The statistical test can be expressed as H0: δ ≥ δ0 versus H1: δ < δ0. Here, δ0 is the maximum allowable loss-of-completion rate (15%) chosen for the study.
The loss-of-completion rate in each site was estimated directly by the difference between the observed completion frequencies: δ̂i = p̂ia-whereas the overall loss-of-completion rate (δ) was estimated by a weighted average of the , with weights (wi). The weights were defined as the proportion of enrolled participants at each site relative to the total enrollment. The standard error of δ̂ was also calculated. From there, a 95% 2-sided CI of δ was constructed. If the upper bound was less than the prechosen maximum allowable loss in completion rate (15%), then we rejected the null hypothesis and concluded that the completion rate in the SAT group was similar to that in the DOT group. Finite sample adjustments for small sites and continuity correction for small event rates were applied when necessary.
In practice, it is often more natural to use the overall observed difference of completion rates between the 2 study groups to estimate the true overall difference rather than using the weighted average approach for the site effects. Such practice is reasonable if the strata effects δi are constant across strata (study sites). If that is true (with future justifications from the trial practice and data), then the site effects may not be significant and the Mantel–Haenszel approach can be applied to obtain the variance and CI.
In the statistical analysis of this trial’s data, we used weighted averages as our primary method. We did un-weighted analysis as a secondary ad hoc analysis, which was not shown because it did not change the results.
Appendix Table 1.
Classification of AEs by Drug-Relatedness
| Category | Definition |
|---|---|
| Definite | Any event occurring in a timely manner after administration of the study drug(s) that is a known sequela to the administration of the study drug(s) and follows a previously documented pattern of reaction but for which no other explanation is known. This category applies to AEs that the PI believes are incontrovertibly related to the study drug(s). |
| Probable | Any event occurring in a timely manner after administration of the study drug(s) that follows a known pattern of reaction to the study drug(s) and for which no other explanation is known. This category applies to AEs that, after careful medical consideration at the time they are evaluated, are believed with a high degree of certainty to be related to the study drug(s). |
| Possible | Any event occurring in a timely manner after administration of the study drug(s) that does not follow a known pattern of reaction and for which no other explanation is known. This category applies to AEs that, after careful medical consideration at the time they are evaluated, are considered unlikely to be related but cannot be ruled out with certainty. |
| Unlikely | In general, this category can be considered applicable to those AEs that, after careful medical consideration at the time they are evaluated, are considered to be unrelated to administration of the study drug(s). |
| Not related | Any event for which there is evidence that an alternative etiology exists or for which no timely relationship exists to the administration of the study drug(s) and the AE does not follow any previously documented pattern. This category applies to those AEs that, after careful medical consideration, are clearly and incontrovertibly due to causes other than the study drug(s). |
| Unclassifiable | There is insufficient information about the AE to allow an assessment of causality. |
AE = adverse event; PI = principal investigator.
Appendix Table 2.
Demographic Characteristics of Randomly Assigned Participants, by Study Group*
| Characteristic | Overall (n = 964) | DOT (n = 328) | SAT (n = 321) | SAT With Reminders (n = 315) |
|---|---|---|---|---|
| Median age (IQR), y | 36 (27–49) | 36 (27–48) | 36 (27–48) | 38 (27–49) |
|
| ||||
| Female | 460 (47.7) | 148 (45.1) | 152 (47.4) | 160 (50.8) |
| Race | ||||
|
| ||||
| White | 510 (52.9) | 169 (51.5) | 170 (53.0) | 171 (54.3) |
|
| ||||
| Black/African American | 239 (24.8) | 83 (25.3) | 84 (26.2) | 72 (22.9) |
|
| ||||
| Asian | 181 (18.8) | 62 (18.9) | 58 (18.1) | 61 (19.4) |
|
| ||||
| Other | 34 (3.5)† | 14 (4.3) | 9 (2.8) | 11 (3.5) |
| Country of enrollment | ||||
|
| ||||
| United States | 747 (77.5) | 254 (77.4) | 249 (77.6) | 244 (77.5) |
|
| ||||
| Spain | 99 (10.3) | 35 (10.7) | 31 (9.7) | 33 (10.5) |
|
| ||||
| Hong Kong | 38 (3.9) | 13 (4.0) | 13 (4.1) | 12 (3.8) |
|
| ||||
| South Africa | 80 (8.3) | 26 (7.9) | 28 (8.7) | 26 (8.3) |
|
| ||||
| Born outside country of enrollment | 574 (59.5) | 202 (61.6) | 194 (60.4) | 178 (56.5) |
|
| ||||
| Completed high school | 623 (64.6)‡ | 215 (65.6) | 213 (66.4) | 195 (61.9) |
|
| ||||
| Homeless within the past year | 51 (5.3) | 16 (4.9) | 16 (5.0) | 19 (6.0) |
|
| ||||
| Primary occupation during the past year§ | ||||
| Health care worker | 134 (13.9) | 43 (13.1) | 48 (15.0) | 43 (13.7) |
|
| ||||
| Non–health care worker | 455 (47.5) | 155 (47.6) | 144 (45.1) | 156 (49.7) |
|
| ||||
| Unemployed, not seeking employment | 207 (21.6) | 72 (22.1) | 74 (23.2) | 61 (19.4) |
|
| ||||
| Unemployed, seeking employment | 163 (17.0) | 56 (17.2) | 53 (16.6) | 54 (17.2) |
|
| ||||
| Indication for LTBI treatment|| | ||||
| Contact of a person with infectious TB | 321 (33.3) | 105 (32.0) | 100 (31.2) | 116 (36.8) |
|
| ||||
| Recent LTBI screening test conversion | 96 (9.96) | 30 (9.2) | 35 (10.9) | 31 (9.8) |
|
| ||||
| HIV infection | 5 (0.5) | 1 (0.3) | 2 (0.6) | 2 (0.6) |
|
| ||||
| Fibrosis on chest radiography | 4 (0.4) | 3 (0.9) | 0 (0.0) | 1 (0.3) |
|
| ||||
| Diabetes/high blood sugar | 81 (8.4)¶ | 32 (9.8) | 22 (6.9) | 27 (8.6) |
|
| ||||
| Liver disease** | 40 (4.2) | 11 (3.4) | 14 (4.4) | 15 (4.8) |
|
| ||||
| HIV status | ||||
| Positive | 10 (1.0) | 3 (0.9) | 3 (0.9) | 4 (1.3) |
|
| ||||
| Negative | 741 (76.9) | 255 (77.7) | 243 (75.7) | 243 (77.1) |
|
| ||||
| Declined testing | 213 (22.1) | 70 (21.3) | 75 (23.4) | 68 (21.6) |
|
| ||||
| History of alcohol use | 512 (53.1) | 179 (54.6) | 164 (51.1) | 169 (53.7) |
|
| ||||
| History of alcohol abuse (CAGE score ≥2) | 68 (7.1) | 25 (7.6) | 22 (6.9) | 21 (6.7) |
|
| ||||
| Current cigarette smoker | 246 (25.5) | 88 (26.8) | 80 (24.9) | 78 (24.8) |
|
| ||||
| Drug use within the past year | 52 (5.4) | 22 (6.7) | 13 (4.1) | 17 (5.4) |
|
| ||||
| Methadone maintenance therapy | 8 (0.8) | 4 (1.2) | 2 (0.6) | 2 (0.6) |
|
| ||||
| Cell phone with text capability | 884 (91.7) | 303 (92.4) | 292 (91.0) | 289 (91.8) |
|
| ||||
| Willing to receive text message reminders (percentage of those whose cell phone has text capability) | 858 (97.1) | 289 (95.4) | 285 (97.6) | 284 (98.3) |
CAGE = Cut Down, Annoyed, Guilty, and Eye Opener; DOT = directly observed therapy; IQR = interquartile range; LTBI = latent tuberculosis infection; SAT = self-administered therapy; TB = tuberculosis.
Values are numbers (percentages) unless otherwise indicated. Excludes persons enrolled in a household cluster who were not randomly assigned.
22 participants did not report race and are included here.
48 participants did not report education level.
5 participants did not report occupation.
Participants were counted only once in the order presented. The total number of HIV-infected persons who were enrolled in the study is listed elsewhere in the table.
Whether 2 participants had been diagnosed with diabetes or high blood sugar was unknown.
Defined as the presence of any of the following chronic liver conditions: hepatitis B virus infection (n = 8), hepatitis C virus infection (n = 19), hepatitis type unknown (n = 12), hepatitis due to alcohol use (n = 1), or cirrhosis (n = 3). Two participants reported hepatitis C virus infection and cirrhosis. One participant reported hepatitis B and C virus infection.
Appendix Table 3.
Treatment Completion, by Group, DOT vs. SAT and DOT vs. SAT With Reminders, in Eligible Participants
| Participants | Treatment Completion in the DOT Group, n (%) | SAT | SAT With Reminders | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Treatment Completion, n (%) | Weighted Difference vs. DOT, % | Upper Limit for 95% CI, %* | Treatment Completion, n (%) | Weighted Difference vs. DOT, % | Upper Limit for 95% CI, %* | ||
| All sites | |||||||
|
| |||||||
| First enrolled | 328 (86.9) | 320 (74.4) | 12.4 | 18.2 | 313 (75.4) | 11.8 | 17.6 |
|
| |||||||
| All | 337 (87.2) | 335 (74.0) | 13.1 | 18.8 | 326 (76.4) | 11.2 | 16.9 |
| United States | |||||||
|
| |||||||
| First enrolled | 254 (85.0) | 249 (78.3) | 6.8 | 13.4† | 242 (76.0) | 9.5 | 16.4 |
|
| |||||||
| All | 261 (85.4) | 262 (77.9) | 7.7 | 14.2† | 249 (76.7) | 9.3 | 16.0 |
DOT = directly observed therapy; SAT = self-administered therapy.
The completion rate of the treatment group is noninferior if the upper limit of the CI is <15%.
Noninferior to DOT.
Appendix Table 4.
Treatment Completion, SAT vs. SAT With Reminders, in Eligible Participants
| Participants | Treatment Completion in the SAT Group, n (%) | SAT With Reminders | ||
|---|---|---|---|---|
|
| ||||
| Treatment Completion, n (%) | Weighted Difference, % | Upper Limit for 95% CI, % | ||
| All sites | ||||
|
| ||||
| First enrolled | 320 (74.4) | 313 (75.4) | −0.7 | 5.9 |
|
| ||||
| All | 335 (74.0) | 326 (76.4) | −2.0 | 4.4 |
| United States | ||||
|
| ||||
| First enrolled | 249 (78.3) | 242 (76.0) | 2.6 | 9.9 |
|
| ||||
| All | 262 (77.9) | 249 (76.7) | 1.4 | 8.6 |
SAT = self-administered therapy.
Appendix Table 5.
Univariate and Multivariate Analyses of Factors Associated With Treatment Noncompletion of the Combined SAT Groups
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Part of household cluster | 0.70 (0.35–1.44) | 0.33 | 0.74 (0.35–1.59) | 0.44 |
|
| ||||
| Female | 1.20 (0.84–1.71) | 0.31 | 1.48 (1.01–2.16) | 0.045 |
|
| ||||
| Age in years | 1.00 (0.98–1.01) | 0.61 | – | – |
|
| ||||
| Race | <0.001 | – | ||
|
| ||||
| White | Reference (1.00) | – | – | – |
|
| ||||
| Black/African American | 2.11 (1.41–3.17) | <0.001 | – | – |
|
| ||||
| Asian | 0.70 (0.41–1.19) | 0.191 | – | – |
|
| ||||
| Other | 2.08 (0.84–5.14) | 0.114 | – | – |
|
| ||||
| Country of enrollment | <0.001 | – | <0.001 | |
|
| ||||
| United States | Reference (1.00) | – | Reference (1.00) | – |
|
| ||||
| Spain | 0.89 (0.47–1.69) | 0.71 | 0.81 (0.42–1.57) | 0.53 |
|
| ||||
| South Africa | 4.36 (2.48–7.65) | <0.001 | 4.19 (2.35–7.48) | <0.001 |
|
| ||||
| Hong Kong | 0.38 (0.11–1.27) | 0.116 | 0.41 (0.12–1.39) | 0.150 |
|
| ||||
| Born outside country of enrollment | 0.64 (0.45–0.91) | 0.014 | – | – |
|
| ||||
| Indication for LTBI treatment | 0.44 | – | ||
|
| ||||
| Contact of a person with infectious TB | Reference (1.00) | – | – | – |
|
| ||||
| Recent LTBI screening test conversion | 0.67 (0.35–1.29) | 0.23 | – | – |
|
| ||||
| HIV infection | 0.81 (0.08–7.94) | 0.86 | – | – |
|
| ||||
| Positive results on LTBI screening test | 0.70 (0.48–1.03) | 0.068 | – | – |
|
| ||||
| Occupation | 0.003 | – | ||
|
| ||||
| Employed, non-health care worker | Reference (1.00) | – | – | – |
|
| ||||
| Employed, health care worker | 0.95 (0.53–1.70) | 0.85 | – | – |
|
| ||||
| Unemployed, seeking employment | 2.46 (1.54–3.94) | <0.001 | – | – |
|
| ||||
| Unemployed, not seeking employment | 1.47 (0.93–2.33) | 0.099 | – | – |
|
| ||||
| Did not complete high school | 1.60 (1.09–2.33) | 0.016 | – | – |
|
| ||||
| Homeless | 1.05 (0.48–2.29) | 0.90 | – | – |
|
| ||||
| Drug use | 1.25 (0.57–2.78) | 0.58 | – | – |
|
| ||||
| Resident of correctional facility at time of diagnosis | 0.60 (0.07–5.19) | 0.64 | – | – |
|
| ||||
| Alcohol use | 0.75 (0.53–1.07) | 0.111 | – | – |
|
| ||||
| Alcohol abuse | 2.62 (1.42–4.86) | 0.002 | – | – |
|
| ||||
| Current smoker | 1.98 (1.34–2.93) | <0.001 | 1.85 (1.21–2.83) | 0.005 |
|
| ||||
| Diabetes | 1.01 (0.53–1.95) | 0.97 | – | – |
|
| ||||
| Liver disease | 1.47 (0.68–3.19) | 0.33 | 1.61 (0.72–3.60) | 0.25 |
|
| ||||
| HIV positive | 1.91 (0.45–8.09) | 0.38 | – | – |
|
| ||||
| Concomitant medications at baseline | 0.88 (0.66–1.16) | 0.36 | – | – |
|
| ||||
| Text messaging capability | 0.88 (0.62–1.26) | 0.48 | – | – |
LTBI = latent tuberculosis infection; OR = odds ratio; SAT = self-administered therapy; TB = tuberculosis.
Appendix Table 6.
All Reported AEs*
| Variable | Overall (n = 1002) | DOT (n = 337) | SAT (n = 337) | SAT With Reminders (n = 328) |
|---|---|---|---|---|
| All AEs | 208 | 64 | 72 | 72 |
|
| ||||
| Toxicity grade | ||||
| Grade 1 or 2 or not applicable | 118 | 38 | 42 | 38 |
|
| ||||
| Drug-related† | 47 | 16 | 19 | 12 |
|
| ||||
| Not drug-related‡ | 71 | 22 | 23 | 26 |
|
| ||||
| Grade 3 or 4 | 89 | 26 | 30 | 33 |
|
| ||||
| Drug-related† | 33 | 8 | 10 | 15 |
|
| ||||
| Not drug-related‡ | 56 | 18 | 20 | 18 |
|
| ||||
| Death (grade 5) | 1 | 0 | 0 | 1§ |
|
| ||||
| SAE|| | 29 | 14 | 9 | 6 |
|
| ||||
| Drug-related† | 5¶ | 3 | 0 | 2 |
|
| ||||
| Not drug-related‡ | 24 | 11 | 9 | 4 |
|
| ||||
| Attribution | ||||
| Drug-related† | 80 | 24 | 29 | 27 |
|
| ||||
| Not drug-related‡ | 128 | 40 | 43 | 45 |
|
| ||||
| AE of special interest | ||||
| Systemic drug reaction | 43 | 13 | 16 | 14 |
|
| ||||
| Hepatitis | 9 | 1 | 3 | 5 |
|
| ||||
| Isolated hepatitis | 4 | 0 | 1 | 3 |
|
| ||||
| Drug-related† | 2 | 0 | 1 | 1 |
|
| ||||
| Not drug-related‡ | 2 | 0 | 0 | 2 |
|
| ||||
| Hepatitis associated with systemic drug reaction | 5 | 1 | 2 | 2 |
|
| ||||
| Completed treatment | 1 | 1 | 0 | 0 |
|
| ||||
| Did not complete treatment | 4 | 0 | 2 | 2 |
|
| ||||
| Thrombocytopenia | 1** | 0 | 0 | 1 |
|
| ||||
| Neutropenia | 1** | 1 | 0 | 0 |
AE = adverse event; DOT = directly observed therapy; SAE = severe adverse event; SAT = self-administered therapy.
Values are numbers of AEs.
Considered to be definitely, probably, or possibly related to the study drugs.
Considered to be an unlikely relation to the study drugs, not related, or unclassifiable.
A 25-year-old patient born in South Africa with a social history of alcohol consumption, cigarette smoking, and unemployment committed suicide 5 d after the first study dose given by DOT. The patient had no history of psychiatric disorders or suicidal ideation and no signs of depression or anxiety before or after the first study dose.
Death, any life-threatening experience, any inpatient hospitalization or prolongation of any hospitalization, any persistently or severely disabling event, a congenital anomaly or birth defect, an overdose of study drugs, or any grade 4 toxicity event.
Four hospitalizations (1 patient in the DOT group and 1 patient in the SAT-with-reminders group had gastrointestinal symptoms, 1 patient in the DOT group had an event of rheumatoid arthritis related to a drug interaction, and 1 patient in the DOT group had a systemic drug reaction). One patient in the SAT-with-reminders group had grade 4 fatigue without hospitalization.
The only event of thrombocytopenia was considered to be related to the study drugs, whereas the only event of neutropenia was not considered to be related to the study drugs.
Appendix Table 7.
Treatment Completion, by Study Group and Country of Enrollment, in Eligible Participants
| Variable | DOT (95% CI), % | SAT (95% CI), % | SAT With Reminders (95% CI), % |
|---|---|---|---|
| All participants | |||
|
| |||
| United States | 85.4 (80.4–89.4) | 77.9 (72.2–82.6) | 76.7 (70.9–81.7) |
|
| |||
| Spain | 94.3 (79.5–99.0) | 73.3 (53.8–87.0) | 84.8 (67.3–94.3) |
|
| |||
| Hong Kong | 93.3 (66.0–99.7) | 78.6 (48.8–94.3) | 100.0 (75.9–100.0) |
|
| |||
| South Africa | 92.3 (73.4–98.7) | 37.9 (21.3–57.6) | 50.0 (32.6–67.4) |
| First enrolled | |||
|
| |||
| United States | 85.0 (79.9–89.1) | 78.3 (72.6–83.2) | 76.0 (70.1–81.2) |
|
| |||
| Spain | 94.3 (79.5–99.0) | 73.3 (53.8–87.0) | 84.8 (67.3–94.3) |
|
| |||
| Hong Kong | 92.3 (62.1–99.6) | 76.9 (46.0–93.8) | 100.0 (69.9–100.0) |
|
| |||
| South Africa | 92.3 (73.4–98.7) | 39.3 (22.1–59.3) | 46.2 (27.1–66.3) |
DOT = directly observed therapy; SAT = self-administered therapy.
Appendix Table 8.
Demographic Characteristics of All Participants, by Country of Enrollment*
| Characteristic | Overall (n = 1002) | United States (n = 774) | Spain (n = 100) | South Africa (n = 83) | Hong Kong (n = 45) |
|---|---|---|---|---|---|
| Treatment group | |||||
|
| |||||
| DOT | 337 (33.6) | 261 (33.7) | 35 (35.0) | 26 (31.3) | 15 (33.3) |
|
| |||||
| SAT | 337 (33.6) | 262 (33.9) | 32 (32.0) | 29 (34.9) | 14 (31.1) |
|
| |||||
| SAT with reminders | 328 (32.7) | 251 (32.4) | 33 (33.0) | 28 (33.7) | 16 (35.6) |
|
| |||||
| Median age (IQR), y | 36 (27–49) | 39 (27–50) | 35 (28–43) | 29 (23–33) | 38 (30–50) |
|
| |||||
| Female | 482 (48.1) | 391 (50.5) | 35 (35.0) | 32 (38.6) | 24 (53.3) |
|
| |||||
| Race | |||||
| White | 518 (51.7) | 453 (58.5) | 65 (65.0) | 0 (0.0) | 0 (0.0) |
|
| |||||
| Black/African American | 250 (25.0) | 162 (20.9) | 5 (5.0) | 83 (100.0) | 0 (0.0) |
|
| |||||
| Asian | 200 (20.0) | 130 (16.8) | 25 (25.0) | 0 (0.0) | 45 (100.0) |
|
| |||||
| Other | 34 (3.4)† | 29 (3.8) | 5 (5.0) | 0 (0.0) | 0 (0.0) |
|
| |||||
| Born outside country of enrollment | 603 (60.2) | 518 (66.9) | 69 (69.0) | 1 (1.2) | 15 (33.3) |
|
| |||||
| Completed high school | 640 (63.9)‡ | 505 (65.3) | 52 (52.0) | 46 (55.4) | 37 (82.2) |
|
| |||||
| Homeless within past year | 51 (5.1) | 48 (6.2) | 3 (3.0) | 0 (0.0) | 0 (0.0) |
| Primary occupation during past year§ | |||||
|
| |||||
| Health care worker | 136 (13.6) | 128 (16.6) | 6 (6.0) | 1 (1.2) | 1 (2.2) |
|
| |||||
| Non–health care worker | 472 (47.3) | 362 (47.1) | 56 (56.0) | 20 (24.1) | 34 (75.6) |
|
| |||||
| Unemployed, not seeking employment | 221 (22.2) | 190 (24.7) | 14 (14.0) | 8 (9.6) | 9 (20.0) |
|
| |||||
| Unemployed, seeking employment | 168 (16.9) | 89 (11.6) | 24 (24.0) | 54 (65.1) | 1 (2.2) |
| Indication for LTBI treatment|| | |||||
|
| |||||
| Contact of a person with infectious TB | 344 (34.3) | 164 (21.2) | 69 (69.0) | 68 (81.9) | 43 (95.6) |
|
| |||||
| Recent LTBI screening test conversion | 98 (9.8) | 98 (12.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||||
| HIV infection | 5 (0.5) | 0 (0.0) | 3 (3.0) | 1 (1.2) | 1 (2.2) |
|
| |||||
| Fibrosis on chest radiography | 4 (0.4) | 4 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||||
| Positive results on LTBI screening test | 551 (55.0) | 508 (65.6) | 28 (28.0) | 14 (16.9) | 1 (2.2) |
|
| |||||
| Diabetes/high blood sugar | 84 (8.4)¶ | 79 (10.2) | 3 (3.0) | 0 (0.0) | 2 (4.4) |
|
| |||||
| Liver disease** | 42 (4.2) | 37 (4.8) | 3 (3.0) | 0 (0.0) | 2 (4.4) |
|
| |||||
| HIV status | |||||
| Positive | 11 (1.1) | 0 (0.0) | 4 (4.0) | 6 (7.2) | 1 (2.2) |
|
| |||||
| Negative | 776 (77.5) | 589 (76.1) | 66 (66.0) | 77 (92.8) | 44 (97.8) |
|
| |||||
| Declined testing | 215 (21.5) | 185 (23.9) | 30 (30.0) | 0 (0.0) | 0 (0.0) |
|
| |||||
| History of alcohol use | 531 (53.0) | 413 (53.4) | 33 (33.0) | 50 (60.2) | 35 (77.8) |
|
| |||||
| History of alcohol abuse (CAGE score ≥2) | 70 (7.0) | 34 (4.4) | 4 (4.0) | 32 (38.6) | 0 (0.0) |
|
| |||||
| Current cigarette smoker | 250 (25.0) | 164 (21.2) | 38 (38.0) | 43 (51.8) | 5 (11.1) |
|
| |||||
| Drug use within the past year | 53 (5.3) | 40 (5.2) | 8 (8.0) | 5 (6.0) | 0 (0.0) |
|
| |||||
| Methadone maintenance therapy | 8 (0.8) | 8 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||||
| Cell phone with text capability | 911 (90.9) | 691 (89.3) | 95 (95.0) | 80 (96.4) | 45 (100.0) |
|
| |||||
| Willing to receive text message reminders (percentage of those whose cell phone has text capability) | 885 (97.2) | 665 (96.2) | 95 (100.0) | 80 (100.0) | 45 (100.0) |
CAGE = Cut Down, Annoyed, Guilty, and Eye Opener; DOT = directly observed therapy; IQR = interquartile range; LTBI = latent tuberculosis infection; SAT = self-administered therapy; TB = tuberculosis.
Values are numbers (percentages) unless otherwise indicated.
22 participants did not report race and are included here.
52 participants did not report education level.
5 participants did not report primary occupation.
Participants were counted only once in the order presented. The total number of HIV-infected persons who were enrolled in the study is listed elsewhere in the table.
Whether 2 participants had been diagnosed with diabetes or high blood sugar was unknown.
Defined as the presence of any of the following chronic liver conditions: hepatitis B virus infection (n = 9), hepatitis C virus infection (n = 19), hepatitis type unknown (n = 13), hepatitis due to alcohol use (n = 1), or cirrhosis (n = 3). Two participants reported hepatitis C virus infection and cirrhosis. One participant reported hepatitis B and C virus infection.
Footnotes
Disclosures: Dr. Belknap reports grants from the Centers for Disease Control and Prevention (CDC) during the conduct of the study. Dr. Martinson reports that his institution has been contracted to contribute samples to studies of novel tuberculosis assays by 3 companies. Ms. Wright reports grants from the CDC during the conduct of the study. Dr. Moro reports that Sanofi donated rifapentine and rifampin and since 2007 has donated over $2.9 million to the CDC Foundation to supplement available U.S. federal funding for rifapentine research; these funds included salary support for Dr. Moro while this study was conducted. Mr. Scott reports that Sanofi donated rifapentine and since 2007 has donated over $2.9 million to the CDC Foundation to supplement available U.S. federal funding for rifapentine research; in the past, these funds included salary support for Mr. Scott. Dr. Miró reports grants and personal fees from AbbVie, Angelini, Bristol-Myers Squibb, Genentech, Gilead Sciences, Medtronic, Merck/MSD, Novartis, and ViiV Healthcare outside the submitted work. Dr. Weiner reports grants from Veterans Administration during the conduct of the study. Dr. Borisov reports that Sanofi, manufacturer of Priftin (rifapentine) evaluated in this research, donated study drugs to this clinical trial. Also, since 2007 Sanofi has donated over $2.9 million to the CDC Foundation (non-government organization) to supplement available U.S. federal funding for CDC’s rifapentine research; in the past, these funds included salary support for the Foundation’s contractors working at the CDC. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M17-1150.
Author Contributions: Conception and design: R. Belknap, D. Holland, J.P. Millet, J.A. Caylà, A. Wright, M.P. Chen, J.M. Miró, M.E. Villarino, A.S. Borisov.
Analysis and interpretation of the data: R. Belknap, D. Holland, P.J. Feng, J.P. Millet, J.A. Caylà, M.P. Chen, R.N. Moro, N.A. Scott, J.M. Miró, M.E. Villarino, A.S. Borisov.
Drafting of the article: R. Belknap, D. Holland, J.P. Millet, J.A. Caylà, A. Wright, A.S. Borisov.
Critical revision of the article for important intellectual content: R. Belknap, D. Holland, J.P. Millet, J.A. Caylà, A. Wright, M.P. Chen, R.N. Moro, J.M. Miró, M.E. Villarino, A.S. Borisov.
Final approval of the article: R. Belknap, D. Holland, P.J. Feng, J.P. Millet, J.A. Caylà, N.A. Martinson, A. Wright, M.P. Chen, R.N. Moro, N.A. Scott, B. Arevalo, J.M. Miró, M.E. Villarino, M. Weiner, A.S. Borisov.
Provision of study materials or patients: R. Belknap, J.P. Millet, J.A. Caylà, N.A. Martinson, A. Wright, J.M. Miró, M. Weiner.
Statistical expertise: J.P. Millet, J.A. Caylà, M.P. Chen, R.N. Moro.
Obtaining of funding: M.E. Villarino.
Administrative, technical, or logistic support: D. Holland, J.A. Caylà, B. Arevalo, A.S. Borisov.
Collection and assembly of data: R. Belknap, D. Holland, P.J. Feng, J.P. Millet, J.A. Caylà, N.A. Martinson, A. Wright, R.N. Moro, N.A. Scott, J.M. Miró, M. Weiner, A.S. Borisov.
References
- 1.Yuen CM, Kammerer JS, Marks K, Navin TR, France AM. Recent transmission of tuberculosis—United States, 2011–2014. PLoS One. 2016;11:e0153728. doi: 10.1371/journal.pone.0153728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–47. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K, Grossman DC, Curry SJ, Bauman L, Davidson KW, Epling JW, Jr, et al. US Preventive Services Task Force. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962–9. doi: 10.1001/jama.2016.11046. [DOI] [PubMed] [Google Scholar]
- 4.Schmit KM, Wansaula Z, Pratt R, Price SF, Langer AJ. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289–94. doi: 10.15585/mmwr.mm6611a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Getahun H, Matteelli A, Abubakar I, Aziz MA, Baddeley A, Barreira D, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563–76. doi: 10.1183/13993003.01245-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterling TR, Bethel J, Goldberg S, Weinfurter P, Yun L, Horsburgh CR Tuberculosis Epidemiologic Studies Consortium. The scope and impact of treatment of latent tuberculosis infection in the United States and Canada. Am J Respir Crit Care Med. 2006;173:927–31. doi: 10.1164/rccm.200510-1563OC. [DOI] [PubMed] [Google Scholar]
- 7.Horsburgh CR, Jr, Rubin EJ. Clinical practice. Latent tuberculosis infection in the United States. N Engl J Med. 2011;364:1441–8. doi: 10.1056/NEJMcp1005750. [DOI] [PubMed] [Google Scholar]
- 8.Hill AN, Becerra J, Castro KG. Modelling tuberculosis trends in the USA. Epidemiol Infect. 2012;140:1862–72. doi: 10.1017/S095026881100286X. [DOI] [PubMed] [Google Scholar]
- 9.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34:271–86. doi: 10.1146/annurev-publhealth-031912-114431. [DOI] [PubMed] [Google Scholar]
- 10.Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:1269–78. doi: 10.1016/S1473-3099(16)30216-X. [DOI] [PubMed] [Google Scholar]
- 11.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 12.International Union Against Tuberculosis Committee on Prophylaxis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ. 1982;60:555–64. [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Munsiff SS, Tarantino T, Dorsinville M. Adherence to treatment of latent tuberculosis infection in a clinical population in New York City. Int J Infect Dis. 2010;14:e292–7. doi: 10.1016/j.ijid.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Rubinowicz A, Bartlett G, MacGibbon B, Greenaway C, Ronald L, Munoz M, et al. Evaluating the role of primary care physicians in the treatment of latent tuberculosis: a population study. Int J Tuberc Lung Dis. 2014;18:1449–54. doi: 10.5588/ijtld.14.0166. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch-Moverman Y, Shrestha-Kuwahara R, Bethel J, Blumberg HM, Venkatappa TK, Horsburgh CR, et al. Tuberculosis Epidemiologic Studies Consortium (TBESC) Latent tuberculous infection in the United States and Canada: who completes treatment and why? Int J Tuberc Lung Dis. 2015;19:31–8. doi: 10.5588/ijtld.14.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stagg HR, Zenner D, Harris RJ, Muñoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med. 2014;161:419–28. doi: 10.7326/M14-1019. [DOI] [PubMed] [Google Scholar]
- 17.Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–66. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 18.Villarino ME, Scott NA, Weis SE, Weiner M, Conde MB, Jones B, et al. International Maternal Pediatric and Adolescents AIDS Clinical Trials Group. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr. 2015;169:247–55. doi: 10.1001/jamapediatrics.2014.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterling TR, Moro RN, Borisov AS, Phillips E, Shepherd G, Adkin-son NF, et al. Tuberculosis Trials Consortium. Flu-like and other systemic drug reactions among persons receiving weekly rifapentine plus isoniazid or daily isoniazid for treatment of latent tuberculosis infection in the PREVENT Tuberculosis Study. Clin Infect Dis. 2015;61:527–35. doi: 10.1093/cid/civ323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland DP, Sanders GD, Hamilton CD, Stout JE. Costs and cost-effectiveness of four treatment regimens for latent tuberculosis infection. Am J Respir Crit Care Med. 2009;179:1055–60. doi: 10.1164/rccm.200901-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moro RN, Borisov AS, Saukkonen J, Khan A, Sterling TR, Villarino ME, et al. Factors associated with noncompletion of latent tuberculosis infection treatment: experience from the PREVENT TB Trial in the United States and Canada. Clin Infect Dis. 2016;62:1390–1400. doi: 10.1093/cid/ciw126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strandbygaard U, Thomsen SF, Backer V. A daily SMS reminder increases adherence to asthma treatment: a three-month follow-up study. RespirMed. 2010;104:166–71. doi: 10.1016/j.rmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, de Walque D, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25:825–34. doi: 10.1097/QAD.0b013e32834380c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–45. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 25.Nglazi MD, Bekker LG, Wood R, Hussey GD, Wiysonge CS. Mobile phone text messaging for promoting adherence to anti-tuberculosis treatment: a systematic review. BMC Infect Dis. 2013;13:566. doi: 10.1186/1471-2334-13-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soares JF, Jeff Wu CF. Some restricted randomization rules in sequential designs. Comm Stat Theory Methods. 1983;12:2017–34. [Google Scholar]
- 27.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21:1074–90. doi: 10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 28.Holland DP, Sanders GD, Hamilton CD, Stout JE. Potential economic viability of two proposed rifapentine-based regimens for treatment of latent tuberculosis infection. PLoS One. 2011;6:e22276. doi: 10.1371/journal.pone.0022276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. FEM-PrEP Study Group. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26:F13–9. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery ET, Mensch B, Musara P, Hartmann M, Woeber K, Etima J, et al. Misreporting of product adherence in the MTN-003/VOICE trial for HIV prevention in Africa: participants’ explanations for dishonesty. AIDS Behav. 2017;21:481–91. doi: 10.1007/s10461-016-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menzies D, Dion MJ, Rabinovitch B, Mannix S, Brassard P, Schwartzman K. Treatment completion and costs of a randomized trial of rifampin for 4 months versus isoniazid for 9 months. Am J Respir Crit Care Med. 2004;170:445–9. doi: 10.1164/rccm.200404-478OC. [DOI] [PubMed] [Google Scholar]
- 33.Page KR, Sifakis F, Montes de Oca R, Cronin WA, Doherty MC, Federline L, et al. Improved adherence and less toxicity with rifampin vs isoniazid for treatment of latent tuberculosis: a retrospective study. Arch Intern Med. 2006;166:1863–70. doi: 10.1001/archinte.166.17.1863. [DOI] [PubMed] [Google Scholar]
- 34.Menzies D, Long R, Trajman A, Dion MJ, Yang J, Al Jahdali H, et al. Adverse events with 4 months of rifampin therapy or 9 months of isoniazid therapy for latent tuberculosis infection: a randomized trial. Ann Intern Med. 2008;149:689–97. doi: 10.7326/0003-4819-149-10-200811180-00003. [DOI] [PubMed] [Google Scholar]
- 35.Chan PC, Yang CH, Chang LY, Wang KF, Lu BY, Lu CY, et al. Latent tuberculosis infection treatment for prison inmates: a randomised controlled trial. Int J Tuberc Lung Dis. 2012;16:633–8. doi: 10.5588/ijtld.11.0504. [DOI] [PubMed] [Google Scholar]
- 36.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]