Abstract
Study Objective
To assess the incidence of and risk factors associated with severe adverse events in elderly patients who used angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) after an acute myocardial infarction (AMI).
Design
Retrospective cohort study.
Data Sources
Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse (Medicare service claims database), American Community Survey of the U.S. Census Bureau, and Multum Lexicon Drug database.
Patients
A total of 101,588 eligible Medicare fee-for-service beneficiaries aged 66 years or older, who were hospitalized for AMI between January 1, 2008, and December 31, 2009, and used ACEIs or ARBs within 30 days after discharge.
Measurements and Main Results
Primary outcomes were hospitalizations for acute renal failure (ARF) and hyperkalemia. The secondary outcome was discontinuation or suspension of ACEI/ARB therapy after a visit to a health care provider. The primary risk factors of interest were age, sex, race-ethnicity, and chronic kidney disease (CKD). Cumulative incidence curves and multivariable Fine-Gray proportional hazards models with 95% confidence intervals (CIs) were used with death as a competing risk in both intention-to-treat (ITT) and as-treated (AT) analyses. In the study cohort, 2.8% experienced ARF, 0.5% experienced hyperkalemia, and 63.7% discontinued ACEI/ARB therapy within one year after hospital discharge. Approximately half of the incidence of ARF and hyperkalemia occurred within 6 months after hospital discharge, but the cumulative incidence increased after 6 months. Patients older than 85 years had a higher rate of ARF (ITT hazard ratio [HR] 1.15, 95% CI 1.04–1.28) and hyperkalemia (ITT HR 1.33, 95% CI 1.05–1.68) compared with those aged 65–74 years. Patients with baseline CKD had higher rates of ARF (ITT HR 1.61, 95% CI 1.42–1.82), hyperkalemia (ITT HR 1.41, 95% CI 1.11–1.77), and ACEI/ARB therapy discontinuation or suspension (ITT HR 1.05, 95% CI 1.02–1.09).
Conclusion
We found a low incidence of ARF and hyperkalemia in elderly patients treated with ACEIs or ARBs after AMI hospitalization. However, a high rate of treatment discontinuation might prevent a higher rate of occurrence of these events. Long-term careful monitoring of severe adverse events and timely discontinuation of ACEIs or ARBs among elderly patients with advancing age and CKD after an AMI is warranted in clinical practice.
Keywords: myocardial infarction, secondary prevention, angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, ACEI, ARB, acute renal failure, acute kidney injury, hyperkalemia, treatment discontinuation, elderly
In 2010, approximately 819,000 acute myocardial infarction (AMI) hospitalizations occurred in the United States, with incidence increasing with patient age.1 Clinical guidelines recommend angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) for secondary prevention of AMI, based on randomized controlled trial data.2 Acute renal failure (ARF) and hyperkalemia (HK) are known severe adverse effects after initiating ACEI and ARB treatment.3 However, like many other interventions, the elderly are not well represented in clinical trials for ACEIs/ARBs, especially among patients over the age of 75 years.4–6 Evidence generated in a tightly controlled trial setting may not reflect clinical practice in real-world settings, particularly for severe adverse events such as ARF and HK.7–11 There is a scarcity of knowledge in clinical practice settings on the incidence of ARF and HK and the trajectory of the incidence over time among the elderly who used ACEIs or ARBs after discharge from AMI hospitalization.
Furthermore, identifying factors that increase the risk of ARF and HK in this population is important for therapeutic treatment decision making and monitoring. Several factors such as age, race-ethnicity, sex, and presence of chronic kidney disease (CKD) may be of particular interest. Prior studies showed that increasing age is associated with higher risk of ARF and HK among the elderly, especially when taking medications that inhibit the renin-angiotensin system.12–14 Multiple comorbidities and polypharmacy are common among elderly patients. Differences by sex and racial-ethnic group also exist in the long-term use of secondary prevention therapies for AMI.15 For example, pharmacologic agents that inhibit the renin-angiotensin system have differential effects in black patients.3,16 Studies have suggested that ACEIs may be less effective in black patients than in white patients for blood pressure control and reduction of cardiovascular disease outcomes.17,18 ACEIs and ARBs have both been recommended for patients with diabetes mellitus and nephropathy to slow the progression of CKD.19,20 However, the risks of ARF and HK are also higher among patients with CKD after ACEI/ARB initiation.3,21,22 This presents a dilemma for clinicians regarding the use of ACEIs/ARBs among patients with CKD after AMI.
Therefore, the objective of this study was to assess the incidence of and risk factors associated with severe adverse events in elderly patients who used ACEIs or ARBs after an AMI. Specifically, we aimed to investigate the incidence of ARF and HK hospitalizations among elderly users of ACEIs or ARBs after hospital discharge for AMI and to examine risk factors associated with ARF and HK hospitalization in elderly patients who used ACEIs or ARBs after AMI in real-world settings. It is possible that clinicians who closely monitor their patients may discontinue or suspend therapy before these adverse events occur if patients present with signs or symptoms related to the adverse effects. Thus, we also assessed the secondary outcome of discontinuation or suspension of ACEI/ARB therapy after a visit to a health care provider. These findings will help inform clinicians about which elderly patients should be more closely monitored and situations where ACEI/ARB therapy discontinuation may mitigate the risk of these adverse events.12,23
Methods
Study Design and Data Sources
The primary data source for this retrospective cohort study was the Centers for Medicare & Medicaid Services (CMS) Chronic Conditions Data Warehouse (CCW). We used the CCW beneficiary enrollment summary inpatient, outpatient, skilled-nursing facility, and Part D prescription drug event research files for the years 2007–2010. This data source contains 100% of the Medicare fee-for-service and prescription claims for beneficiaries hospitalized for an AMI from 2008–2009. Other data sources used for these analyses included the 2006–2010 American Community Survey (ACS) of the U.S. Census Bureau 5-year estimates data at the block group level and the Multum Lexicon Drug database. We linked the ACS data to CCW beneficiary files by mapping patients’ 9-digit ZIP code to block groups. Multum’s drug data were linked to CCW Part D prescription drug event data using National Drug Codes (NDCs). The study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill (Chapel Hill, NC).
Cohort Selection
The cohort for this study included elderly Medicare patients who were hospitalized for AMI between January 1, 2008, and December 31, 2009. We included patients who were at least 66 years of age at the time of AMI; were continuously enrolled in the Medicare fee-for-service Parts A and B as well as prescription Part D programs at least 12 month prior to the AMI admission date; and survived for at least 30 days after discharge. Additionally, patients with end-stage renal disease at baseline, patients discharged to a hospice, and patients with residence ZIP codes outside of the United States were excluded. Hospitalization with AMI was defined as having International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes of 410.x1 as the primary or secondary discharge codes in Medicare inpatient claims.15 The first AMI hospitalization in the study period was defined as the index AMI hospitalization for each patient. The consort diagram in Appendix Figure A1 describes the cohort selection process for these analyses.
Figure A1.
Study cohort selection criteria. CMS = Centers for Medicare & Medicaid Services; AMI = acute myocardial infarction; ICD9 = International Classification of Diseases, Ninth Revision; ESRD = end-stage renal disease; ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker.
Use of ACEIs and ARBs
Prescription claims for ACEIs and ARBs were identified in the CCW prescription Part D event files using National Drug Codes (NDCs). Patients were defined as ACEI/ARB users if they had a prescription claim for an ACEI/ARB within 30 days after hospital discharge or had ACEI/ARB medication already on hand from before or during the index AMI hospitalization with sufficient days’ supply to last through 30 days after hospital discharge.
Adverse Event Outcome Measures
The primary outcomes for this study were hospitalizations for ARF and HK. These outcomes were identified based on a published algorithm for identifying HK and ARF based on diagnostic codes in claims files.24,25 The secondary safety outcome included discontinuation or suspension of ACEI/ARB treatment after a visit to health care provider. A gap in therapy was defined as a 14-day grace period with no ACEI/ARB refill at the end of a prior prescription supply; this gap was then applied to approximate discontinuation or suspension of treatment due to provider intervention if a patient had a physician office, outpatient, or hospital visit in the period between the last ACEI/ARB fill and the discontinuation date. Anchoring discontinuation or suspension on a provider visit was used to help differentiate normal adherence and persistence patterns from provider-directed discontinuation. Outcome definitions are presented in Appendix Table A1.
Table A1.
Study Outcome Definitions
| Outcome Type | Outcome | Outcome Algorithm |
|---|---|---|
| Primary | Hyperkalemia | Primary or secondary diagnosis code of 276.7 in inpatient claims |
| Acute Renal Failure | Diagnosis code of 584.x, 586.x or 788.5 in any position in inpatient claims | |
| Secondary | Discontinuation of ACEI/ARB after Health Care Provider Visit | Discontinuation of ACEI/ARB treatment after a visit to a health care provider: identified if a patient had a physician office, outpatient, or hospital visit in the period between last fill and discontinuation date. Discontinuation date was defined as last prescription fill date + no. of days of last prescription supply + 14 days |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker.
The follow-up period for all events started at whichever of the following events occurred later: day after the first ACEI/ARB fill or discharge date (for patients who had prescription for ACEI/ARB filled prior to index AMI hospital admission with at least 30 days supply remaining after hospital discharge). Follow-up ended on either the date of censoring or occurrence of an outcome.
Baseline Characteristics
A select list of baseline characteristics of the study patients is presented in Table 1, and a full list of variables considered in the analyses is shown in Appendix Table A2. Baseline patient characteristics were assessed from the Medicare research files during the 12 months prior to the index hospitalization for AMI. They included comorbidity levels as measured by the Charlson comorbidity index,26 condition-specific comorbidities, proxy measures for functional limitations, measures of health care utilization, and treatment. Additional covariates included conditions and procedures assessed during the index hospital admission. Indicators of socioeconomic status (SES; income measured at the U.S. Census block group level and dual eligibility in Medicare and Medicaid) were also included in the model. The primary predictors of interest were age, sex, race-ethnicity, and CKD. All clinical conditions were identified by using algorithms previously validated in the literature.27–37 The complete list of baseline characteristics is presented in Appendix Table A2.
Table 1.
Select Patient Characteristics at Baseline and During the Index Hospital Admission
| Characteristic | No. (%) of Patients | |
|---|---|---|
| ACEI or ARB use during exposure period | ||
|
| ||
| ARB | 23,022 (22.7) | |
| ACEI | 78,566 | 77.3% |
|
| ||
| Sociodemographic covariates | ||
|
| ||
| Age (yrs) | ||
| 66–74 | 35,536 | 35.0% |
| 75–84 | 40,397 | 39.8% |
| ≥ 85 | 25,655 | 25.3% |
| Sex | ||
| Male | 41,174 | 40.5% |
| Female | 60,414 | 59.5% |
| Race-Ethnicity | ||
| White | 86,662 | 85.3% |
| Black | 8,317 | 8.2% |
| Hispanic | 2,883 | 2.8% |
| Asian | 1,968 | 1.9% |
| Other | 1,758 | 1.7% |
| Prescription benefit gap (Part D Donut hole) | 11,530 | 11.3% |
| Dual eligibility in Medicare and Medicaid | 28,481 | 28.0% |
| Median household income at census block groups level ($) | ||
| ≤ 30,000 | 48,315 | 47.6% |
| 30,001–60,000 | 41,767 | 41.1% |
| 60,001–100,000 | 9,336 | 9.2% |
| 100,001–150,000 | 1,663 | 1.6% |
| ≥ 150,001 | 507 | 0.5% |
|
| ||
| Conditions at index admission | ||
|
| ||
| Angiocardiography | 55,938 | 55.1% |
| Acute renal failure | 12,304 | 12.1% |
| Coronary artery bypass grafting | 6,636 | 6.5% |
| Cardiac catheterization | 56,598 | 55.7% |
| Cardiac dysrhythmias | 32,044 | 31.5% |
| Congestive heart failure | 37,487 | 36.9% |
| Cardiogenic shock | 2,599 | 2.6% |
| Hypotension | 5,166 | 5.1% |
| Subendocardial infarction | 75,782 | 74.6% |
| Platelet inhibitors | 4,616 | 4.5% |
| Percutaneous transluminal coronary angioplasty | 37,806 | 37.2% |
| Stent | 34,683 | 34.1% |
| Length of stay (days) | ||
| 1 | 4,480 | 4.4% |
| 2–5 | 58,577 | 57.7% |
| 6–10 | 27,153 | 26.7% |
| ≥ 11 | 11,378 | 11.2% |
| Days in intensive care unit | ||
| 0 | 47,236 | 46.5% |
| 1–3 | 31,727 | 31.2% |
| 4–10 | 19,822 | 19.5% |
| ≥ 11 | 2,803 | 2.8% |
| Days in coronary care unit | ||
| 0 | 64,435 | 63.4% |
| 1–3 | 22,349 | 22.0% |
| 4–10 | 13,264 | 13.1% |
| ≥ 11 | 1,540 | 1.5% |
|
| ||
| Baseline Charlson comorbidity index score | ||
|
| ||
| 0 | 28,031 | 27.6% |
| 1–2 | 32,183 | 31.7% |
| 3–5 | 27,362 | 26.9% |
| 6–8 | 10,818 | 10.6% |
| ≥ 9 | 3,194 | 3.1% |
|
| ||
| Baseline comorbidities | ||
|
| ||
| Acute myocardial infarction | 5,314 | 5.2% |
| Cancer | 10,732 | 10.6% |
| Cerebrovascular disease | 15,957 | 15.7% |
| Congestive heart failure | 24,841 | 24.5% |
| Chronic obstructive pulmonary disease | 23,784 | 23.4% |
| Dementia | 4,836 | 4.8% |
| Diabetes with complications | 13,844 | 13.6% |
| Diabetes without complications | 26,789 | 26.4% |
| AIDS/HIV | 69 | 0.1% |
| Metastatic carcinoma | 1,274 | 1.3% |
| Mild liver disease | 1,538 | 1.5% |
| Moderate or severe liver disease | 139 | 0.1% |
| Paralysis | 1,164 | 1.1% |
| Peptic ulcer disease | 1,582 | 1.6% |
| Peripheral vascular disease | 18,551 | 18.3% |
| Chronic kidney disease | 20,620 | 20.3% |
| Connective tissue disease/Rheumatic disease | 3,581 | 3.5% |
| Angioedema | 180 | 0.2% |
| Atrial fibrillation | 11,098 | 10.9% |
| Asthma | 5,946 | 5.9% |
| Coronary artery bypass grafting | 530 | 0.5% |
| Hyperkalemia | 2,787 | 2.7% |
| Hyperlipidemia | 56,039 | 55.2% |
| Hypotension | 4,620 | 4.5% |
| Hypertension | 78,314 | 77.1% |
| Ischemic heart disease | 46,271 | 45.5% |
| Osteoporosis | 7,678 | 7.6% |
| Percutaneous transluminal coronary angioplasty | 3,363 | 3.3% |
| Rhabdomyolysis | 454 | 0.4% |
| Sinus bradycardia and heart block | 14,758 | 14.5% |
| Stent | 3,900 | 3.8% |
| Unstable angina | 6,037 | 5.9% |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; AIDS/HIV = acquired immunodeficiency syndrome/human immunodeficiency virus.
Table A2.
List of All Covariates Considered in the Models
| Variable Group | Variable List |
|---|---|
| Key predictors | Age, sex, race-ethnicity, chronic kidney disease |
| Sociodemographics | Average household income at census block group level, status of Medicare Part D benefit gap (donut hole), dual eligibility in Medicare and Medicaid |
| Conditions at index admission | Angiocardiography, acute renal failure, coronary artery bypass grafting, cardiac catheterization, cardiac dysrhythmias, congestive heart failure, cardiogenic shock, hypotension, echocardiography, subendocardial infarction, platelet inhibitors, percutaneous transluminal coronary angioplasty, stent, thrombolytic therapy, length of stay, number of days in intensive care unit, number of days in coronary care unit |
| Treatment after acute myocardial infarction | Angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker |
| Baseline comorbidities | Charlson comorbidity index score, acute myocardial infarction, cancer, cerebrovascular disease, congestive heart failure, renal disease, chronic obstructive pulmonary disease, dementia, diabetes, acquired immunodeficiency syndrome/human immunodeficiency virus, metastatic carcinoma, liver disease, paralysis, peptic ulcer, peripheral vascular disease, connective tissue disease/rheumatic disease |
| Baseline clinical conditions | Angioedema, atrial fibrillation, asthma, coronary artery bypass grafting, hyperkalemia, hyperlipidemia, hypotension, hypertension, ischemic heart disease, osteoporosis, percutaneous transluminal coronary angioplasty, rhabdomyolysis, sinus bradycardia and heart block, stent, unstable angina |
| Baseline frailty indicators | Substance abuse, cute respiratory failure, use of other assistive devices (aids), blood loss and deficiency anemia, coagulation deficiency, decubitus, falls, gastrointestinal bleed, hypothyroidism, bladder dysfunction, nail care, other neurological disorders, osteoarthritis, obesity, Parkinson’s disease, pulmonary circ. disorders, use of rehabilitation, rheumatic heart disease, use of screening tests, septic shock, vertigo, weakness, use of wheelchair, weight loss |
| Baseline health care utilization | Number of visits to emergency department, number of visits to hospital, number of visits to cardiologist, number of visits to primary care physician |
| Baseline treatment | Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, mineralocorticoid receptor antagonists, beta-blockers, thiazide diuretics, calcium channel blockers, other antihypertensives, metformin, sulfonylureas, rosiglitazone, pioglitazone, insulin, other hypoglycemic agents, statins, other lipid-lowering agents, nitrates, clopidogrel, and warfarin, antiarrhythmic agents |
Statistical Analysis
All patient characteristics were measured categorically and summarized using percentages. Patient characteristics were also stratified by age group, sex, race-ethnicity, and CKD. Unadjusted cumulative incidence curves using methods that accommodate the competing risk of mortality were used for the whole study cohort. We used Gray tests to evaluate differences in the cumulative incidence of outcomes between groups of interest selected a priori.38 Sensitivity analysis was applied with stratification by ACEIs and ARBs.
Intention-to-treat (ITT) and as-treated (AT) analyses were used to identify the predictors of the safety outcomes. In the ITT analyses, patients were censored at the end of the study period or when they disenrolled from either Medicare fee-for-service or prescription Part D plans. In the AT analyses, patients were additionally censored for switching from target doses in clinical trials to lower doses (or vice versa) or discontinuation. The date of discontinuation was the date of the end of supply for the last dispensed prescription plus a grace period of 14 days. Fine-Gray proportional hazards models with death as competing risk were used to estimate associations between predictive factors and outcomes.39 To assess unadjusted associations, models where each of the key predictors was the only variable in the model were used. Second, fully adjusted models that estimated the effect of primary factors were run twice, once using the ITT approach and second using the AT method. Additionally, we conducted sensitivity analysis by adjusting for the dose of ACEIs/ARBs as a covariate in the regression models. The dose variable was calculated as the average daily dose from the last prescription filled within the 30 days after index AMI hospital discharge. Last, we also assessed the distribution of provider types (cardiologist, primary care physician, and other) for the last physician office, outpatient, or hospital visit before treatment discontinuation.
We used SAS 9.4 statistical analysis software (SAS Institute Inc., Cary, NC) for all analyses.
Results
Baseline Characteristics
In the final analytical cohort, 101,588 patients met our study protocol eligibility. Overall, 39.8% were aged 75–84 years, and 25.3% of patients were aged 85 years of age or older; 59.5% of patients were female, and the majority was white (85.3%). Approximately 20% of patients had baseline CKD, and 72.4% of patients had a Charlson comorbidity index score of at least one. Table 1 presents data on patient characteristics at baseline and during the index hospital admission. Patient characteristics by age, sex, race-ethnicity and CKD are presented in Appendix Tables A3.1–A3.4.
Table A3.1.
Patient Characteristics by Sex
| No. (%) of Patients | ||
|---|---|---|
| Characteristic | Females (n=60,414) | Males (n=41,174) |
| ACEI or ARB use during exposure period | ||
| ARB | 15,592 (25.8) | 7,430 (18.0) |
| ACEI | 44,822 (74.2) | 33,744 (82.0) |
| Sociodemographic covariates | ||
| Age (yrs) | ||
| 66–74 | 16,838 (27.9) | 18,698 (45.4) |
| 75–84 | 24,601 (40.7) | 15,796 (38.4) |
| ≥ 85 | 18,975 (31.4) | 6,680 (16.2) |
| Race-Ethnicity | ||
| White | 50,905 (84.3) | 35,757 (86.8) |
| Black | 5,779 (9.6) | 2,538 (6.2) |
| Hispanic | 1,715 (2.8) | 1,168 (2.8) |
| Asian | 1,064 (1.8) | 904 (2.2) |
| Other | 951 (1.6) | 807 (2.0) |
| Prescription benefit gap | 7,559 (12.5) | 3,971 (9.6) |
| Dual Eligibility in Medicaid & Medicare | 19,424 (32.2) | 9,057 (22) |
| Median household income at census block group level ($) | ||
| ≤ 30,000 | 29,656 (49.1) | 18,659 (45.3) |
| 30,001–60,000 | 24,418 (40.4) | 17,349 (42.1) |
| 60,001–100,000 | 5,198 (8.6) | 4,138 (10.1) |
| 100,001–150,000 | 886 (1.5) | 777 (1.9) |
| ≥ 150,001 | 256 (0.4) | 251 (0.6) |
| Conditions at index admission | ||
| Angiocardiography | 30,709 (50.8) | 25,229 (61.3) |
| Acute renal failure | 7,073 (11.7) | 5,231 (12.7) |
| Coronary artery bypass grafting | 2,871 (4.8) | 3,765 (9.1) |
| Cardiac catheterization | 31,039 (51.4) | 25,559 (62.1) |
| Cardiac dysrhythmias | 18,129 (30) | 13,915 (33.8) |
| Congestive heart failure | 23,839 (39.5) | 13,648 (33.1) |
| Cardiogenic shock | 1,327 (2.2) | 1,272 (3.1) |
| Hypotension | 3,132 (5.2) | 2,034 (4.9) |
| Subendocardial infarction | 46,169 (76.4) | 29,613 (71.9) |
| Platelet inhibitors | 2,360 (3.9) | 2,256 (5.5) |
| Percutaneous transluminal coronary angioplasty or stent | 19,747 (32.7) | 18,100 (44) |
| Length of stay (days) | ||
| 1 | 2,454 (4.1) | 2,026 (4.9) |
| 2–5 | 34,345 (56.8) | 24,232 (58.9) |
| 6–10 | 17,011 (28.2) | 10,142 (24.6) |
| ≥ 11 | 6,604 (10.9) | 4,774 (11.6) |
| Days in intensive care unit | ||
| 0 | 28,914 (47.9) | 18,322 (44.5) |
| 1–3 | 18,061 (29.9) | 13,666 (33.2) |
| 4–10 | 11,910 (19.7) | 7,912 (19.2) |
| ≥ 11 | 1,529 (2.5) | 1,274 (3.1) |
| Days in coronary care unit | ||
| 0 | 39,254 (65) | 25,181 (61.2) |
| 1–3 | 12,264 (20.3) | 10,085 (24.5) |
| 4–10 | 8,045 (13.3) | 5,219 (12.7) |
| ≥ 11 | 851 (1.4) | 689 (1.7) |
| Baseline Charlson comorbidity index score | ||
| 0 | 15,726 (26) | 12,305 (29.9) |
| 1–2 | 19,703 (32.6) | 12,480 (30.3) |
| 3–5 | 16,681 (27.6) | 10,681 (25.9) |
| 6–8 | 6,529 (10.8) | 4,289 (10.4) |
| 9+ | 1,775 (2.9) | 1,419 (3.4) |
| Baseline comorbidities | ||
| Acute myocardial infarction | 3,291 (5.4) | 2,023 (4.9) |
| Cancer | 4,583 (7.6) | 6,149 (14.9) |
| Cerebrovascular disease | 9,909 (16.4) | 6,048 (14.7) |
| Congestive heart failure | 15,796 (26.1) | 9,045 (22) |
| Chronic obstructive pulmonary disease | 14,585 (24.1) | 9,199 (22.3) |
| Dementia | 3,435 (5.7) | 1,401 (3.4) |
| Diabetes | 24,579 (40.7) | 16,054 (39) |
| AIDS/HIV | 19 (0) | 50 (0.1) |
| Metastatic carcinoma | 675 (1.1) | 599 (1.5) |
| Liver disease | 980 (1.6) | 697 (1.7) |
| Paralysis | 702 (1.2) | 462 (1.1) |
| Peptic ulcer disease | 1,014 (1.7) | 568 (1.4) |
| Peripheral vascular disease | 11,268 (18.7) | 7,283 (17.7) |
| Chronic kidney disease | 11,738 (19.4) | 8,882 (21.6) |
| Connective tissue disease-rheumatic disease | 2,691 (4.5) | 890 (2.2) |
| Other baseline clinical conditions | ||
| Angioedema | 128 (0.2) | 52 (0.1) |
| Atrial fibrillation | 6,743 (11.2) | 4,355 (10.6) |
| Asthma | 4,193 (6.9) | 1,753 (4.3) |
| Coronary artery bypass grafting | 279 (0.5) | 251 (0.6) |
| Hyperkalemia | 1,815 (3) | 972 (2.4) |
| Hyperlipidemia | 33,075 (54.7) | 22,964 (55.8) |
| Hypotension | 2,836 (4.7) | 1,784 (4.3) |
| Hypertension | 48,880 (80.9) | 29,434 (71.5) |
| Ischemic heart disease | 25,950 (43) | 20,321 (49.4) |
| Osteoporosis | 7,011 (11.6) | 667 (1.6) |
| Percutaneous transluminal coronary angioplasty | 1,780 (2.9) | 1,583 (3.8) |
| Rhabdomyolysis | 275 (0.5) | 179 (0.4) |
| Sinus bradycardia and heart block | 8,871 (14.7) | 5,887 (14.3) |
| Stent | 2,057 (3.4) | 1,843 (4.5) |
| Unstable angina | 3,547 (5.9) | 2,490 (6) |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; AIDS/HIV = acquired immunodeficiency syndrome/human immunodeficiency virus.
Table A3.4.
Patient characteristics by presence or absence of chronic kidney disease
| No. (%) of Patients | ||
|---|---|---|
| Characteristic | CKD (n=20,620) | No CKD (n=80,968) |
| ACEI or ARB use during exposure period | ||
| ARB | 5,889 (28.6) | 17,133 (21.2) |
| ACEI | 14,731 (71.4) | 63,835 (78.8) |
| Socio-demographic covariates | ||
| Age (yrs) | ||
| 66–74 | 5,801 (28.1) | 29,735 (36.7) |
| 75–84 | 8,470 (41.1) | 31,927 (39.4) |
| 85+ | 6,349 (30.8) | 19,306 (23.8) |
| Sex | ||
| Male | 8,882 (43.1) | 32,292 (39.9) |
| Female | 11,738 (56.9) | 48,676 (60.1) |
| Race-Ethnicity | ||
| White | 16,675 (80.9) | 69,987 (86.4) |
| Black | 2,407 (11.7) | 5,910 (7.3) |
| Hispanic | 634 (3.1) | 2,249 (2.8) |
| Asian | 500 (2.4) | 1,468 (1.8) |
| Other | 404 (2) | 1,354 (1.7) |
| Prescription benefit gap | 3,131 (15.2) | 8,399 (10.4) |
| Dual Eligibility in Medicaid & Medicare | 7,038 (34.1) | 21,443 (26.5) |
| Median household income at census block group level ($) | ||
| ≤ 30,000 | 10,181 (49.4) | 38,134 (47.1) |
| 30,001–60,000 | 8,275 (40.1) | 33,492 (41.4) |
| 60,001–100,000 | 1,788 (8.7) | 7,548 (9.3) |
| 100,001–150,000 | 289 (1.4) | 1,374 (1.7) |
| ≥ 150,001 | 87 (0.4) | 420 (0.5) |
| Conditions at index admission | ||
| Angiocardiography | 8,965 (43.5) | 46,973 (58) |
| Acute renal failure | 5,861 (28.4) | 6,443 (8) |
| Coronary artery bypass grafting | 918 (4.5) | 5,718 (7.1) |
| Cardiac catheterization | 8,872 (43) | 47,726 (58.9) |
| Cardiac dysrhythmias | 5,997 (29.1) | 26,047 (32.2) |
| Congestive heart failure | 10,268 (49.8) | 27,219 (33.6) |
| Cardiogenic shock | 389 (1.9) | 2,210 (2.7) |
| Hypotension | 956 (4.6) | 4,210 (5.2) |
| Subendocardial infarction | 17,030 (82.6) | 58,752 (72.6) |
| Platelet inhibitors | 676 (3.3) | 3,940 (4.9) |
| Percutaneous transluminal coronary angioplasty or stent | 5,491 (26.6) | 32,356 (40) |
| Length of stay (days) | ||
| 1 | 672 (3.3) | 3,808 (4.7) |
| 2–5 | 10,944 (53.1) | 47,633 (58.8) |
| 6–10 | 6,427 (31.2) | 20,726 (25.6) |
| ≥ 11 | 2,577 (12.5) | 8,801 (10.9) |
| Days in intensive care unit | ||
| 0 | 9,921 (48.1) | 37,315 (46.1) |
| 1–3 | 5,730 (27.8) | 25,997 (32.1) |
| 4–10 | 4,361 (21.1) | 15,461 (19.1) |
| ≥ 11 | 608 (2.9) | 2,195 (2.7) |
| Days in coronary care unit | ||
| 0 | 13,590 (65.9) | 50,845 (62.8) |
| 1–3 | 3,786 (18.4) | 18,563 (22.9) |
| 4–10 | 2,893 (14) | 10,371 (12.8) |
| ≥ 11 | 351 (1.7) | 1,189 (1.5) |
| Baseline Charlson comorbidity index score | ||
| 0 | 2,255 (10.9) | 25,776 (31.8) |
| 1–2 | 4,014 (19.5) | 28,169 (34.8) |
| 3–5 | 7,006 (34) | 20,356 (25.1) |
| 6–8 | 5,378 (26.1) | 5,440 (6.7) |
| ≥ 9 | 1,967 (9.5) | 1,227 (1.5) |
| Baseline comorbidities | ||
| Acute myocardial infarction | 1,955 (9.5) | 3,359 (4.1) |
| Cancer | 2,421 (11.7) | 8,311 (10.3) |
| Cerebrovascular disease | 4,376 (21.2) | 11,581 (14.3) |
| Congestive heart failure | 8,819 (42.8) | 16,022 (19.8) |
| Chronic obstructive pulmonary disease | 5,845 (28.3) | 17,939 (22.2) |
| Dementia | 1,328 (6.4) | 3,508 (4.3) |
| Diabetes | 11,327 (54.9) | 29,306 (36.2) |
| AIDS/HIV | 30 (0.1) | 39 (0) |
| Metastatic carcinoma | 279 (1.4) | 995 (1.2) |
| Liver disease | 440 (2.1) | 1,237 (1.5) |
| Paralysis | 284 (1.4) | 880 (1.1) |
| Peptic ulcer disease | 419 (2) | 1,163 (1.4) |
| Peripheral vascular disease | 5,555 (26.9) | 12,996 (16.1) |
| Connective tissue disease-rheumatic disease | 797 (3.9) | 2,784 (3.4) |
| Other baseline clinical conditions | ||
| Angioedema | 39 (0.2) | 141 (0.2) |
| Atrial fibrillation | 2,922 (14.2) | 8,176 (10.1) |
| Asthma | 1,398 (6.8) | 4,548 (5.6) |
| Coronary artery bypass grafting | 123 (0.6) | 407 (0.5) |
| Hyperkalemia | 1,424 (6.9) | 1,363 (1.7) |
| Hyperlipidemia | 12,998 (63) | 43,041 (53.2) |
| Hypotension | 1,531 (7.4) | 3,089 (3.8) |
| Hypertension | 18,234 (88.4) | 60,080 (74.2) |
| Ischemic heart disease | 12,352 (59.9) | 33,919 (41.9) |
| Osteoporosis | 1,498 (7.3) | 6,180 (7.6) |
| Percutaneous transluminal coronary angioplasty | 957 (4.6) | 2,406 (3) |
| Rhabdomyolysis | 151 (0.7) | 303 (0.4) |
| Sinus bradycardia and heart block | 4,260 (20.7) | 10,498 (13) |
| Stent | 1,031 (5) | 2,869 (3.5) |
| Unstable angina | 1,880 (9.1) | 4,157 (5.1) |
CKD = chronic kidney disease; ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; AIDS/HIV = acquired immunodeficiency syndrome/human immunodeficiency virus.
Adverse Outcome Events
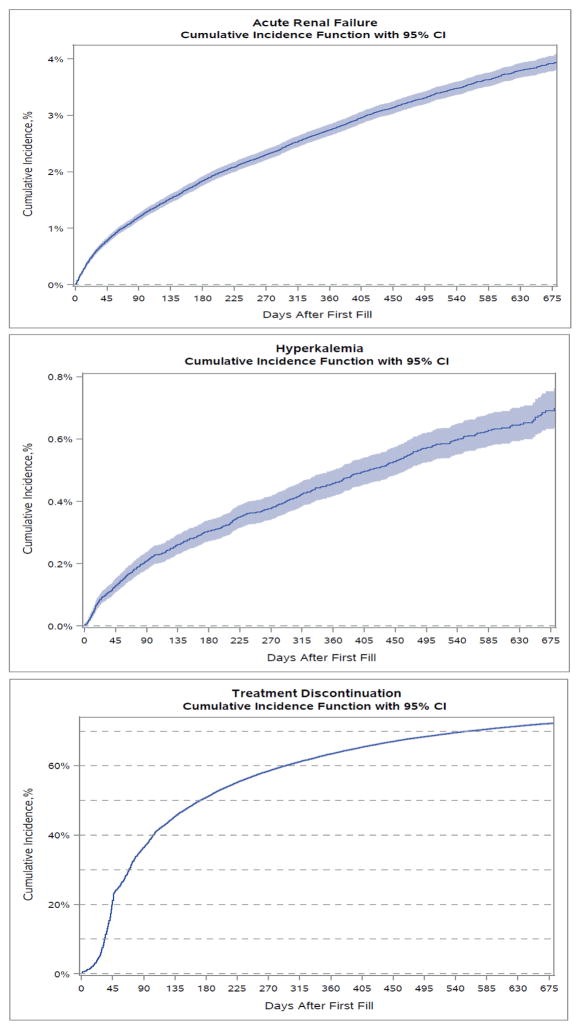
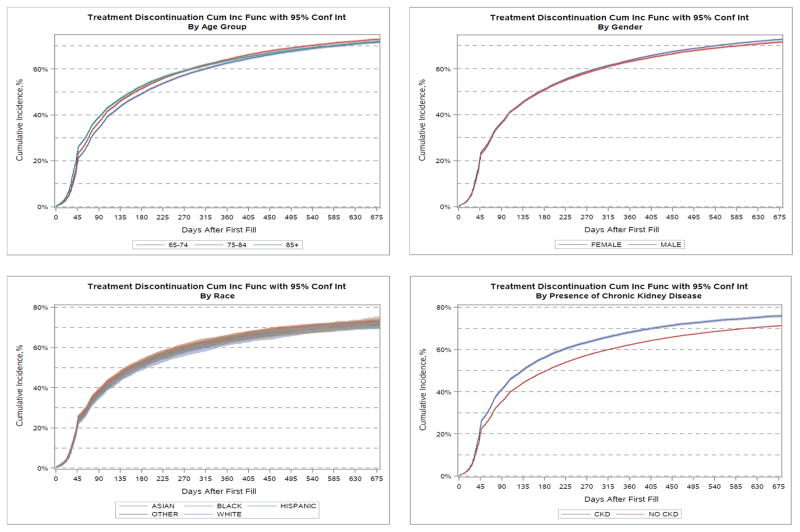
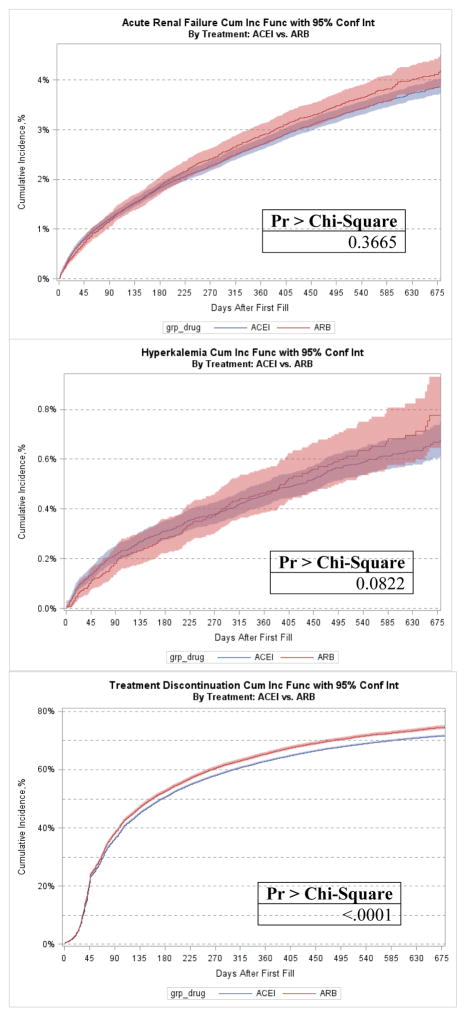
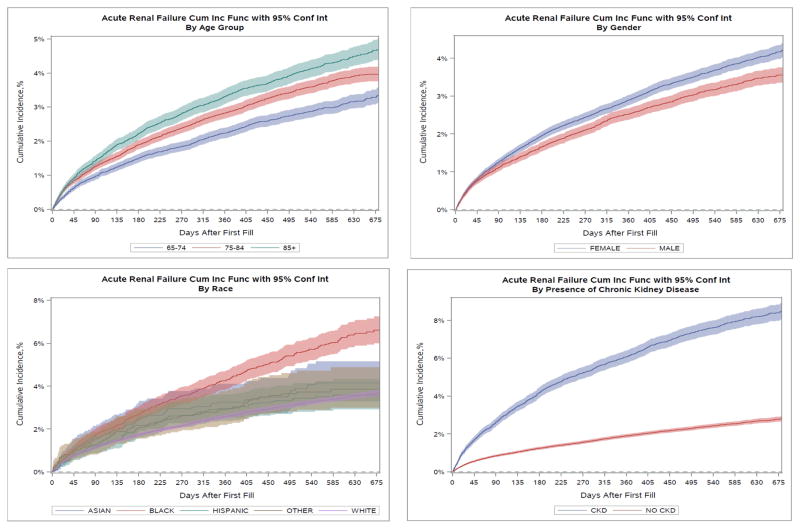
Cumulative incidence rates for all primary and secondary adverse outcome events are described in Figures 1–4 and Table 2. Overall, the cumulative incidence rate at 12 months was 2.8% for ARF, 0.5% for HK, and 63.7% for treatment discontinuation. Among the three age groups, the oldest patients (aged ≥ 85 yrs) had the highest rates of ARF (3.4%) and HK (0.6%). Female patients had slightly but consistently higher incidence rates of ARF (2.9% vs. 2.5% at 12 months), HK (0.49% vs. 0.42%), and treatment discontinuation (63.9% vs. 63.3%) than male patients. Black patients had the highest rate of ARF at 12 months (4.3%) whereas White patients had the lowest incidence (2.6%). Black (0.84%) and Hispanic (0.80%) patients experienced high rates of HK whereas White (0.41%) and Asian (0.41%) patients had the lowest rates. The highest 12-month cumulative incidence of discontinuation was among Black patients (65.5%) and the lowest among Asians (63.4%). Patients with baseline CKD had considerably higher rates of ARF (6.1% vs. 1.9%) at 12 months, HK (0.91% vs. 0.35%) and treatment discontinuation (68.4% vs. 62.5%) compared to patients without CKD. The Gray’s tests comparing the difference in the cumulative incidence of the outcomes among patient subgroups by age, sex, race-ethnicity, and CKD were all statistically significant. In the sensitivity analysis, ARBs only had slightly higher cumulative incidence of therapy discontinuation than ACEIs but similar incidence rates for ARF or HK hospitalization (Appendix Figure A2).
Figure 1.
Cumulative incidence of acute renal failure, hyperkalemia hospitalization, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker treatment discontinuation after AMI hospital discharge. CI = confidence interval.
Figure 4.
Cumulative incidence of angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker treatment discontinuation after AMI hospital discharge. CUM Inc Func = cumulative incidence function; CI = confidence interval; grp = group; CKD = chronic kidney disease.
Table 2.
Cumulative Incidence Rates of Adverse Events at 6 Months at 1 Year by Patient Baseline Characteristicsa
| Characteristic | Group | Acute Renal Failure | Hyperkalemia | Treatment Discontinuation or Suspension | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 6 Months | 1 Year | 6 Months | 1 Year | 6 Months | 1 Year | ||
| All Patients | 1.85% | 2.76% | 0.31% | 0.46% | 51.12% | 63.68% | |
|
| |||||||
| Age Group (yrs) | 66–74 | 1.60% | 2.63% | 0.25% | 0.39% | 49.43% | 62.77% |
| 75–84 | 1.90% | 2.85% | 0.33% | 0.47% | 51.64% | 64.38% | |
| ≥ 85 | 2.24% | 3.35% | 0.35% | 0.55% | 52.65% | 63.85% | |
|
| |||||||
| Sex | Female | 1.97% | 2.91% | 0.30% | 0.49% | 51.27% | 63.94% |
| Male | 1.67% | 2.54% | 0.31% | 0.42% | 50.90% | 63.30% | |
|
| |||||||
| Race-Ethnicity | Asian | 2.54% | 3.25% | 0.25% | 0.41% | 50.92% | 63.40% |
| Black | 2.71% | 4.30% | 0.59% | 0.84% | 53.26% | 65.50% | |
| Hispanic | 2.08% | 2.99% | 0.52% | 0.80% | 51.55% | 64.02% | |
| Other | 2.22% | 2.96% | 0.63% | 0.91% | 52.57% | 64.51% | |
| White | 1.74% | 2.59% | 0.27% | 0.41% | 50.88% | 63.49% | |
|
| |||||||
| Chronic Kidney Disease | Yes | 4.23% | 6.12% | 0.62% | 0.91% | 56.28% | 68.42% |
| No | 1.24% | 1.91% | 0.23% | 0.35% | 49.78% | 62.48% | |
Rates are expressed as cumulative incidence per 100 patients at risk.
Figure A2.
Adverse outcome event by angiotensin-converting enzyme inhibitor (ACEI) and angiotensin II receptor blocker (ARB) after acute myocardial infarction. CUM Inc Func = cumulative incidence function; Conf Int = confidence interval; grp = group.
Table 3 presents estimated associations between patient characteristics of interest and study adverse outcome events. In the unadjusted ITT and AT analyses, advancing age (≥ 75 yrs), sex, race-ethnicity (black), and baseline CKD were all associated with notably higher risk of ARF. In the adjusted ITT and AT analyses, advancing age and CKD remained significant factors associated with ARF. Black race was also associated with higher ARF risk in the adjusted ITT models but it was not statistically significant (hazard ratio [HR] 1.11, 95% confidence interval [CI] 0.94–1.31) in the adjusted AT analysis.
Table 3.
Associations between Patient Characteristics of Interest and Outcomes of Acute Renal Failure, Hyperkalemia, and ACEI or ARB Treatment Discontinuation
| Characteristic | Intention-to-Treat Analysisa | As-Treated Analysisb | ||
|---|---|---|---|---|
| Crude HR (95% CI) |
Adjustedc HR (95% CI) |
Crude HR (95% CI) |
Adjustedc HR (95% CI) |
|
| Acute renal failure | ||||
|
| ||||
| Age (yrs) | ||||
| 66–74 | Reference | Reference | Reference | Reference |
| 75–84 | 1.24 (1.14, 1.34) | 1.10 (1.01, 1.19) | 1.32 (1.17, 1.49) | 1.17 (1.03, 1.32) |
| ≥ 85 | 1.43 (1.32, 1.56) | 1.15 (1.04, 1.28) | 1.54 (1.35, 1.75) | 1.19 (1.02, 1.39) |
| Female (Male as reference) | 1.17 (1.09, 1.25) | 1.04 (0.96, 1.12) | 1.18 (1.06, 1.30) | 1.05 (0.94, 1.18) |
| Race-Ethnicity | ||||
| White | Reference | Reference | Reference | Reference |
| Asian | 1.21 (0.96, 1.51) | 1.05 (0.83, 1.32) | 1.19 (0.84, 1.67) | 0.99 (0.70, 1.41) |
| Black | 1.78 (1.62, 1.96) | 1.30 (1.18, 1.45) | 1.55 (1.33, 1.80) | 1.11 (0.94, 1.31) |
| Hispanic | 1.05 (0.86, 1.28) | 0.84 (0.68, 1.02) | 0.97 (0.71, 1.33) | 0.76 (0.55, 1.04) |
| Other | 1.12 (0.88, 1.44) | 0.99 (0.77, 1.27) | 1.21 (0.85, 1.73) | 1.04 (0.73, 1.49) |
| Baseline CKD | 3.21 (3.01, 3.43) | 1.61 (1.42, 1.82) | 3.18 (2.88, 3.52) | 1.57 (1.30, 1.88) |
|
| ||||
| Hyperkalemia Hospitalization | ||||
|
| ||||
| Age (yrs) | ||||
| 66–74 | Reference | Reference | Reference | Reference |
| 75–84 | 1.21 (1.00, 1.47) | 1.20 (0.98, 1.47) | 1.37 (1.04, 1.81) | 1.40 (1.05, 1.87) |
| ≥ 85 | 1.36 (1.11, 1.67) | 1.33 (1.05, 1.68) | 1.45 (1.07, 1.97) | 1.51 (1.08, 2.11) |
| Female (Male as reference) | 1.19 (1.01, 1.41) | 0.96 (0.80, 1.14) | 1.09 (0.86, 1.38) | 0.88 (0.69, 1.13) |
| Race-Ethnicity | ||||
| White | Reference | Reference | Reference | Reference |
| Asian | 1.07 (0.59, 1.94) | 0.89 (0.49, 1.64) | 0.97 (0.40, 2.35) | 0.71 (0.29, 1.73) |
| Black | 2.29 (1.85, 2.85) | 1.57 (1.24, 1.98) | 1.84 (1.31, 2.57) | 1.25 (0.88, 1.78) |
| Hispanic | 1.87 (1.27, 2.73) | 1.24 (0.83, 1.86) | 1.99 (1.18, 3.36) | 1.32 (0.75, 2.32) |
| Other | 1.97 (1.23, 3.16) | 1.54 (0.96, 2.47) | 1.96 (1.01, 3.81) | 1.52 (0.78, 2.95) |
| Baseline CKD | 2.52 (2.14, 2.97) | 1.41 (1.11, 1.77) | 2.72 (2.16, 3.43) | 1.62 (1.22, 2.16) |
|
| ||||
| ACEI/ARB therapy discontinuation/suspension | ||||
|
| ||||
| Age (yrs) | ||||
| 66–74 | Reference | Reference | Reference | Reference |
| 75–84 | 1.05 (1.04, 1.07) | 1.01 (0.99, 1.03) | 1.06 (1.04, 1.08) | 1.02 (1.00, 1.04) |
| ≥ 85 | 1.06 (1.04, 1.08) | 1.00 (0.98, 1.03) | 1.09 (1.07, 1.11) | 1.02 (1.00, 1.05) |
| Female (Male as reference) | 1.03 (1.01, 1.04) | 1.01 (0.99, 1.02) | 0.99 (0.98, 1.01) | 0.98 (0.96, 1.00) |
| Race-Ethnicity | ||||
| White | Reference | Reference | Reference | Reference |
| Asian | 1.00 (0.94, 1.05) | 1.00 (0.95, 1.06) | 0.97 (0.91, 1.03) | 1.00 (0.94, 1.06) |
| Black | 1.06 (1.03, 1.09) | 1.07 (1.04, 1.10) | 1.01 (0.98, 1.04) | 1.03 (1.00, 1.06) |
| Hispanic | 1.00 (0.96, 1.05) | 0.99 (0.95, 1.04) | 0.91 (0.87, 0.95) | 0.92 (0.87, 0.97) |
| Other | 1.03 (0.97, 1.09) | 1.04 (0.99, 1.11) | 0.98 (0.92, 1.04) | 1.01 (0.94, 1.07) |
| Baseline CKD | 1.17 (1.15, 1.19) | 1.05 (1.02, 1.09) | 1.16 (1.13, 1.18) | 1.05 (1.01, 1.09) |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; HR = hazard ratio; CI = confidence interval; CKD = chronic kidney disease.
In the intention-to-treat analyses, patients were censored at the end of the study period or when they disenrolled from either Medicare fee-for-service or prescription Part D plans.
In the as-treated analyses, patients were additionally censored for switching from an ACEI to ARB (or vice versa) or for treatment discontinuation.
Fully adjusted models were adjusted for all the variables listed in Table 1.
HK incidence rates were significantly higher among Black and patients of other race compared to white patients in both the unadjusted ITT and AT models; however, the effect was attenuated in the adjusted models. Additionally, in multivariable analysis we found that the risk of HK was significantly associated with older age (≥ 75 yrs) and more prominent at 85 years or older, black race (HR 1.57, 95% CI 1.24–1.98 in ITT), and CKD (HR 1.41, 95% CI 1.11–1.77 in ITT, HR 1.62, 95% CI 1.22–2.16 in AT).
Age, sex, race, and baseline CKD were only weakly associated with treatment discontinuation or suspension in unadjusted analyses. These effects were further attenuated after controlling for comorbidities and clinical characteristics. In the adjusted models, ACEI/ARB treatment discontinuation or suspension was significantly associated with black race in the ITT analysis (HR 1.07, 95% CI 1.04–1.10) but was not statistically significant in AT analysis (HR 1.03, 95% CI 1.00–1.06). Treatment discontinuation or suspension was significantly associated with baseline CKD (HR 1.05, 95% CI 1.02–1.09 in ITT, HR 1.05, 95% CI 1.01–1.09 in AT).
Sensitivity analyses showed very similar and consistent results by additionally adjusting for the dose of ACEIs/ARBs (Appendix Table A3). Cardiologist accounted for 17.9%, primary care physician 37.9%, and other provider 44.2% of the last provider a patient visited prior to a treatment discontinuation.
Table A3.3.
Patient characteristics by race-ethnicity group
| No. (%) of Patients | |||||
|---|---|---|---|---|---|
| Characteristic | Asian (n=1,968) | Black (n=8,317) | Hispanic (n=2,883) | Other (n=1,758) | White (n=86,662) |
| ACEI or ARB use during exposure period | |||||
| ARB | 779 (39.6) | 1,931 (23.2) | 787 (27.3) | 465 (26.5) | 19,060 (22) |
| ACEI | 1,189 (60.4) | 6,386 (76.8) | 2,096 (72.7) | 1,293 (73.5) | 67,602 (78) |
| Sociodemographic covariates | |||||
| Age (yrs) | |||||
| 66–74 | 566 (28.8) | 3,359 (40.4) | 818 (28.4) | 738 (42) | 30,055 (34.7) |
| 75–84 | 879 (44.7) | 3,169 (38.1) | 1,294 (44.9) | 657 (37.4) | 34,398 (39.7) |
| ≥ 85 | 523 (26.6) | 1,789 (21.5) | 771 (26.7) | 363 (20.6) | 22,209 (25.6) |
| Sex | |||||
| Male | 904 (45.9) | 2,538 (30.5) | 1,168 (40.5) | 807 (45.9) | 35,757 (41.3) |
| Female | 1,064 (54.1) | 5,779 (69.5) | 1,715 (59.5) | 951 (54.1) | 50,905 (58.7) |
| Prescription benefit gap | 270 (13.7) | 1,053 (12.7) | 429 (14.9) | 206 (11.7) | 9,572 (11) |
| Dual Eligibility in Medicaid & Medicare | 1,573 (79.9) | 4,635 (55.7) | 2,265 (78.6) | 987 (56.1) | 19,021 (21.9) |
| Median household income at census block group level ($) | |||||
| ≤ 30,000 | 814 (41.4) | 5,242 (63) | 1,793 (62.2) | 850 (48.4) | 39,616 (45.7) |
| 30,001–60,000 | 697 (35.4) | 2,425 (29.2) | 830 (28.8) | 632 (35.9) | 37,183 (42.9) |
| 60,001–100,000 | 354 (18) | 522 (6.3) | 223 (7.7) | 211 (12) | 8,026 (9.3) |
| 100,001–150,000 | 79 (4) | 100 (1.2) | 31 (1.1) | 52 (3) | 1,401 (1.6) |
| ≥ 150,001 | 24 (1.2) | 28 (0.3) | 6 (0.2) | 13 (0.7) | 436 (0.5) |
| Conditions at index admission | |||||
| Angiocardiography | 1,006 (51.1) | 4,059 (48.8) | 1,524 (52.9) | 958 (54.5) | 48,391 (55.8) |
| Acute renal failure | 328 (16.7) | 1,433 (17.2) | 404 (14) | 264 (15) | 9,875 (11.4) |
| Coronary artery bypass grafting | 139 (7.1) | 357 (4.3) | 165 (5.7) | 123 (7) | 5,852 (6.8) |
| Cardiac catheterization | 1,001 (50.9) | 4,116 (49.5) | 1,550 (53.8) | 976 (55.5) | 48,955 (56.5) |
| Cardiac dysrhythmias | 546 (27.7) | 2,102 (25.3) | 701 (24.3) | 487 (27.7) | 28,208 (32.5) |
| Congestive heart failure | 766 (38.9) | 3,550 (42.7) | 1,116 (38.7) | 660 (37.5) | 31,395 (36.2) |
| Cardiogenic shock | 84 (4.3) | 146 (1.8) | 85 (2.9) | 74 (4.2) | 2,210 (2.6) |
| Hypotension | 116 (5.9) | 359 (4.3) | 90 (3.1) | 76 (4.3) | 4,525 (5.2) |
| Subendocardial infarction | 1,479 (75.2) | 6,706 (80.6) | 2,246 (77.9) | 1,311 (74.6) | 64,040 (73.9) |
| Platelet inhibitors | 88 (4.5) | 281 (3.4) | 91 (3.2) | 69 (3.9) | 4,087 (4.7) |
| Percutaneous transluminal coronary angioplasty or Stent | 671 (34.1) | 2,311 (27.8) | 994 (34.5) | 624 (35.5) | 33,247 (38.4) |
| Length of stay (days) | |||||
| 1 | 68 (3.5) | 316 (3.8) | 98 (3.4) | 91 (5.2) | 3,907 (4.5) |
| 2–5 | 1,047 (53.2) | 4,511 (54.2) | 1,523 (52.8) | 970 (55.2) | 50,526 (58.3) |
| 6–10 | 569 (28.9) | 2,398 (28.8) | 876 (30.4) | 471 (26.8) | 22,839 (26.4) |
| ≥ 11 | 284 (14.4) | 1,092 (13.1) | 386 (13.4) | 226 (12.9) | 9,390 (10.8) |
| Days in intensive care unit | |||||
| 0 | 737 (37.4) | 4,123 (49.6) | 1,208 (41.9) | 849 (48.3) | 40,319 (46.5) |
| 1–3 | 625 (31.8) | 2,321 (27.9) | 839 (29.1) | 536 (30.5) | 27,406 (31.6) |
| 4–10 | 513 (26.1) | 1,656 (19.9) | 709 (24.6) | 322 (18.3) | 16,622 (19.2) |
| ≥ 11 | 93 (4.7) | 217 (2.6) | 127 (4.4) | 51 (2.9) | 2,315 (2.7) |
| Days in coronary care unit | |||||
| 0 | 1,231 (62.6) | 5,318 (63.9) | 1,672 (58) | 1,103 (62.7) | 55,111 (63.6) |
| 1–3 | 392 (19.9) | 1,679 (20.2) | 645 (22.4) | 389 (22.1) | 19,244 (22.2) |
| 4–10 | 301 (15.3) | 1,180 (14.2) | 503 (17.4) | 237 (13.5) | 11,043 (12.7) |
| ≥ 11 | 44 (2.2) | 140 (1.7) | 63 (2.2) | 29 (1.6) | 1,264 (1.5) |
| Baseline Charlson comorbidity index score | |||||
| 0 | 495 (25.2) | 1,597 (19.2) | 555 (19.3) | 440 (25) | 24,944 (28.8) |
| 1–2 | 673 (34.2) | 2,403 (28.9) | 930 (32.3) | 569 (32.4) | 27,608 (31.9) |
| 3–5 | 554 (28.2) | 2,594 (31.2) | 849 (29.4) | 492 (28) | 22,873 (26.4) |
| 6–8 | 192 (9.8) | 1,262 (15.2) | 411 (14.3) | 208 (11.8) | 8,745 (10.1) |
| ≥ 9 | 54 (2.7) | 461 (5.5) | 138 (4.8) | 49 (2.8) | 2,492 (2.9) |
| Baseline comorbidities | |||||
| Acute myocardial infarction | 106 (5.4) | 526 (6.3) | 175 (6.1) | 94 (5.3) | 4,413 (5.1) |
| Cancer | 148 (7.5) | 853 (10.3) | 243 (8.4) | 136 (7.7) | 9,352 (10.8) |
| Cerebrovascular disease | 298 (15.1) | 1,703 (20.5) | 494 (17.1) | 238 (13.5) | 13,224 (15.3) |
| Congestive heart failure | 458 (23.3) | 2,765 (33.2) | 847 (29.4) | 450 (25.6) | 20,321 (23.4) |
| Chronic obstructive pulmonary disease | 401 (20.4) | 2,033 (24.4) | 736 (25.5) | 370 (21) | 20,244 (23.4) |
| Dementia | 86 (4.4) | 622 (7.5) | 194 (6.7) | 62 (3.5) | 3,872 (4.5) |
| Diabetes | 987 (50.2) | 4,369 (52.5) | 1,619 (56.2) | 888 (50.5) | 32,770 (37.8) |
| AIDS/HIV | * | 27 (0.3) | * | * | 37 (0) |
| Metastatic carcinoma | 20 (1) | 121 (1.5) | 20 (0.7) | 20 (1.1) | 1,093 (1.3) |
| Liver disease | 67 (3.4) | 168 (2) | 81 (2.8) | 59 (3.4) | 1,302 (1.5) |
| Paralysis | 31 (1.6) | 202 (2.4) | 56 (1.9) | 23 (1.3) | 852 (1) |
| Peptic ulcer disease | 60 (3) | 166 (2) | 62 (2.2) | 37 (2.1) | 1,257 (1.5) |
| Peripheral vascular disease | 222 (11.3) | 1,836 (22.1) | 618 (21.4) | 302 (17.2) | 15,573 (18) |
| Chronic kidney disease | 500 (25.4) | 2407 (28.9) | 634 (22.0) | 404 (23.0) | 16,675 (19.2) |
| Connective tissue disease-rheumatic disease | 42 (2.1) | 324 (3.9) | 110 (3.8) | 59 (3.4) | 3,046 (3.5) |
| Other baseline clinical conditions | |||||
| Angioedema | * | 45 (0.5) | * | * | 126 (0.1) |
| Atrial fibrillation | 156 (7.9) | 664 (8) | 231 (8) | 135 (7.7) | 9,912 (11.4) |
| Asthma | 187 (9.5) | 729 (8.8) | 259 (9) | 140 (8) | 4,631 (5.3) |
| Coronary artery bypass grafting | 12 (0.6) | 38 (0.5) | 12 (0.4) | * | 462 (0.5) |
| Hyperkalemia | 59 (3) | 253 (3) | 109 (3.8) | 59 (3.4) | 2,307 (2.7) |
| Hyperlipidemia | 1,160 (58.9) | 4,320 (51.9) | 1,750 (60.7) | 965 (54.9) | 47,844 (55.2) |
| Hypotension | 71 (3.6) | 384 (4.6) | 111 (3.9) | 76 (4.3) | 3,978 (4.6) |
| Hypertension | 1,619 (82.3) | 7,203 (86.6) | 2,414 (83.7) | 1,381 (78.6) | 65,697 (75.8) |
| Ischemic heart disease | 870 (44.2) | 3,854 (46.3) | 1,476 (51.2) | 818 (46.5) | 39,253 (45.3) |
| Osteoporosis | 224 (11.4) | 398 (4.8) | 273 (9.5) | 135 (7.7) | 6,648 (7.7) |
| Percutaneous transluminal coronary angioplasty | 61 (3.1) | 284 (3.4) | 119 (4.1) | 56 (3.2) | 2,843 (3.3) |
| Rhabdomyolysis | * | 51 (0.6) | * | * | 380 (0.4) |
| Sinus bradycardia and heart block | 285 (14.5) | 1,418 (17) | 419 (14.5) | 235 (13.4) | 12,401 (14.3) |
| Stent | 67 (3.4) | 320 (3.8) | 141 (4.9) | 65 (3.7) | 3,307 (3.8) |
| Unstable angina | 116 (5.9) | 555 (6.7) | 232 (8) | 136 (7.7) | 4,998 (5.8) |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; AIDS/HIV = acquired immunodeficiency syndrome/human immunodeficiency virus.
Discussion
Using a large, nationally representative cohort of elderly Medicare patients who were treated with ACEIs or ARBs after an AMI hospitalization, we found that the incidence rates of ARF and HK were relatively low, but discontinuation or suspension of therapy after hospital discharge was high: over 51% within 6 months and 64% at 1 year. The trajectory of the severe adverse events of ARF and HK showed about half the incidence occurred within 6 months after AMI discharge, and the incidence continue to increase in a linear but slower rate thereafter. The trajectory of therapy discontinuation showed a quick increase within 3 months after AMI discharge but plateaued after 6 months. Of all subgroups investigated, patients with CKD prior to their AMI hospitalization had some of the highest rates of ARF, HK, and ACEI/ARB treatment discontinuation or suspension at one year after hospital discharge. Unadjusted ARF rates were also higher among black than white patients and among patients older than 85 years old compared to those aged 66–74 years at 12 months after discharge. HK was also more common among black and Hispanic patients than among white patients at 12 months. The unadjusted treatment discontinuation or suspension rate was higher among black patients at 12 months after hospital discharge, but other racial-ethnic groups had similar rates.
The risk of ARF and HK has been suggested to be elevated in the elderly population.12,13 Several prior studies have indicated that the elderly have a higher risk of ARF and suggest treatment with ACEI/ARBs in this population should be used with caution.13,14 Previous studies have estimated that as high as 38% of all HK events among hospitalized patients are associated with ACEI/ARB use.7 Our study showed much lower incidence rates of ARF and HK in the older adults after discharge from hospital. Our findings are similar to the results from a recent study that also showed low rates of ARF and HK among elderly patients who were treated with aldosterone antagonists.25 Nonetheless, the rates of treatment discontinuation or suspension after health care provider visits were high, reaching over 50% at 6 months and over 60% one year after hospitalization. It is possible that these incidence rates were lower in the noninstitutionalized older population because clinicians were managing and monitoring the risk of ARF and HK associated with ACEI/ARB therapy. However, a 50% discontinuation/suspension rate by 6 month after AMI discharge also indicates a potential marked risk for the severe adverse events. The incidence of ARF and HK appeared to increase the most during 45–90 days after hospital discharge. The rate of ACEI/ARB therapy discontinuation/suspension was also high during this time period. However, when the rate of treatment discontinuation/suspension flattened out after 180 days after discharge, the incidence of ARF and HK continued to increase at a steady rate. Based on these findings, clinicians should continue to monitor ACEI/ARB therapy closely in the long term after AMI hospitalization. Our study also found that the primary care physician accounted for 38% of the physician visits prior to treatment discontinuation. This raises an important question for treatment continuity and coordination, and whether more treatment discontinuation/suspension after primary care physician visit is justified. This question warrants further investigation.
Results from our study suggest that AMI survivors who were 75 years and older or having CKD may have a considerably increased risk of ARF and HK beyond the 3-month postdischarge period and that these patients should continue to be monitored closely for their ACEI/ARB therapy. Previous studies also showed that patients with baseline CKD were more likely to develop ARF.40 Our findings are consistent with these earlier reports and indicate that, despite controlling for variety of other comorbidities and clinical conditions, CKD remains a serious risk factor for ARF. In our adjusted ITT analysis, higher risk of ARF was also found to be associated with black race. However, in the AT analysis, where treatment discontinuation/suspension were incorporated into the analysis, this effect was attenuated and became statistically insignificant. In other words, among users who can tolerate ACEI/ARBs, there is no significant difference in ARF incidence between black and white patients. Among users who cannot tolerate ACE inhibitors/ARBs, black patients may be more likely to have ARF than white patients. This aligns with our finding that black patients have the highest discontinuation/suspension rates. The higher risk of ARF among black patients found in the ITT analysis but not in the AT analysis is worth pondering. One explanation is that among black patients who could not tolerate ACEIs/ARBs, discontinuation or suspension may not have been soon enough or timely enough to prevent their association with events of ARF. Thus, the importance of timely discontinuation/suspension as a prevention of severe adverse outcomes needs to be emphasized.
A number of previous studies linked ACEI/ARB treatment to HK.41–46 Additional investigations showed that elderly patients treated with potassium-altering therapy, such as ACEIs, are particularly predisposed for development of HK. 12,47 Our study concurs with earlier findings and shows that advancing age is a key factor contributing to the elevated risk of HK. The results for the association between race and the rates of HK were similar to our findings for ARF—in the ITT analyses, black patients were more likely to experience HK compared to white patients; however, in analyses adjusted for treatment discontinuation/suspension, this effect was attenuated. Additionally, in line with previous studies, our analyses indicate that patients with CKD are at higher risk of HK. 42,44
A notable secondary finding from our study was the fact that, after adjustment for comorbidities, advancing age was not independently associated with treatment discontinuation or suspension. Similarly, no significant differences in the incidence of discontinuation/suspension were found between men and women. Based on our findings, black patients had a slightly higher chance of discontinuation/suspension – a result that is in concordance with an earlier study that found that elderly black patients had higher odds of suboptimal persistence while treated with statins.48 However, the association between black race and discontinuation/suspension became statistically insignificant in the AT analysis. Our study also pointed out that patients of Hispanic origin have marginally lower rates of therapy discontinuation/suspension than white patients. CKD also found to be weakly associated with treatment discontinuation.
This study has several limitations. First, there was the possibility of variable misclassification using administrative claims data. We followed existing standards using secondary claims data for health services research to mitigate misclassification. Second, time to treatment discontinuation/suspension may have been underestimated if prescriptions were later paid for outside of the Medicare Part D plan after inclusion in the study. However, research has shown that this does not happen often for Medicare beneficiaries, and even when it does, these claims are often adjudicated into Part D records49–51; Medicare patients are also less likely to use medication samples than those in the privately insured population.52 Third, patients may discontinue or suspend therapy without a clinician asking them to stop, leading to misclassification. Additionally, we do not have data on concurrent use of over-the-counter nonsteroidal anti-inflammatory drugs with ACEIs/ARBs, which may increase the risk of ARF and treatment discontinuation. Further study is warranted to investigate key risk factors for treatment discontinuation.
This study possesses several strengths as well. Our study included a nationally representative sample of all Medicare fee-for-service beneficiaries who had an AMI. To our best knowledge, this is the first study that included a comprehensive examination of the incidence of ARF and HK as well as rates of treatment discontinuation among elderly patients treated with ACEI/ARBs after an AMI.
Conclusion
Our study found a low incidence of ARF and HK among elderly patients treated with ACEIs/ARBs after AMI hospitalization. Approximately half of the incidence occurred within 6 months after hospital discharge, but the cumulative incidence continued to increase after 6 months. However, timely treatment discontinuation or suspension might prevent a higher rate of occurrence of these serious adverse events. Advancing age was found to be a strong, independent factor associated with increased incidence of both ARF and HK, whereas CKD was the most important factor affecting these outcomes as well as treatment discontinuation. Long-term careful monitoring of severe adverse events and timely discontinuation of ACEIs or ARBs among high-risk elderly patients after an AMI is warranted in clinical practice.
Figure 2.
Cumulative incidence of acute renal failure stratified by select patient characteristics. CUM Inc Func = cumulative incidence function; CI = confidence interval; grp = group; CKD = chronic kidney disease.
Figure 3.
Cumulative incidence of hyperkalemia hospitalization stratified by select patient characteristics. CUM Inc Func = cumulative incidence function; CI = confidence interval; grp = group; CKD = chronic kidney disease.
Table A3.
Results from Sensitivity Analyses by Additionally Adjusting for ACEI/ARB Dose as Covariate
| Factor | ITT Adjusted HR (95% CI) |
AT Adjusted HR (95% CI) |
|---|---|---|
| Acute renal failure | ||
|
| ||
| Age (yrs) | ||
| 66–74 | 1. | 1. |
| 75–84 | 1.10 (1.01, 1.20) | 1.18 (1.04, 1.33) |
| ≥ 85+ | 1.16 (1.05, 1.28) | 1.21 (1.04, 1.41) |
|
| ||
| Female (male as reference) | 1.04 (0.96, 1.12) | 1.04 (0.93, 1.17) |
|
| ||
| Race-ethnicity | ||
| White | 1. | 1. |
| Asian | 1.05 (0.83, 1.32) | 0.98 (0.69, 1.39) |
| Black | 1.29 (1.17, 1.44) | 1.14 (0.97, 1.35) |
| Hispanic | 0.83 (0.68, 1.02) | 0.79 (0.57, 1.08) |
| Other | 0.99 (0.77, 1.27) | 1.06 (0.74, 1.52) |
|
| ||
| Baseline CKD | 1.61 (1.42, 1.81) | 1.60 (1.32, 1.92) |
|
| ||
| Hyperkalemia hospitalization | ||
|
| ||
| Age (yrs) | ||
| 66–74 | 1. | 1. |
| 75–84 | 1.20 (0.98, 1.47) | 1.41 (1.05, 1.89) |
| > 85 | 1.34 (1.06, 1.69) | 1.51 (1.08, 2.11) |
|
| ||
| Female (male as reference) | 0.96 (0.80, 1.14) | 0.87 (0.68, 1.12) |
|
| ||
| Race-ethnicity | ||
| White | 1. | 1. |
| Asian | 0.89 (0.49, 1.64) | 0.68 (0.28, 1.65) |
| Black | 1.56 (1.23, 1.97) | 1.29 (0.90, 1.84) |
| Hispanic | 1.24 (0.83, 1.86) | 1.39 (0.79, 2.44) |
| Other | 1.54 (0.96, 2.48) | 1.61 (0.83, 3.15) |
| Baseline CKD | 1.40 (1.11, 1.77) | 1.68 (1.26, 2.25) |
|
| ||
| ACEI/ARB therapy discontinuation | ||
|
| ||
| Age (yrs) | ||
| 66–74 | 1. | 1. |
| 75–84 | 1.01 (0.99, 1.03) | 1.02 (1.0, 1.04) |
| > 85 | 1.01 (0.98, 1.03) | 1.01 (0.99, 1.04) |
| Female (male as reference) | 1.0 (0.99, 1.02) | 0.99 (0.98, 1.01) |
| Race-ethnicity | ||
| White | 1. | 1. |
| Asian | 1.0 (0.95, 1.06) | 1.02 (0.96, 1.08) |
| Black | 1.06 (1.03, 1.09) | 1.07 (1.04, 1.11) |
| Hispanic | 0.99 (0.95, 1.04) | 1.0 (0.95, 1.05) |
| Other | 1.04 (0.99, 1.11) | 1.08 (1.01, 1.15) |
| Baseline CKD | 1.05 (1.02, 1.09) | 1.05 (1.02, 1.09) |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; CKD = chronic kidney disease.
Table A3.2.
Patient characteristics by age group
| No. (%) of Patients | |||
|---|---|---|---|
| Characteristic | Age 66–74 Years (n=35,536) | Age 75–84 Years (n=40,397) | Age ≥ 85 Years (n=25,655) |
| ACEI or ARB use during exposure period | |||
| ARB | 7,033 (19.8) | 9,617 (23.8) | 6,372 (24.8) |
| ACEI | 28,503 (80.2) | 30,780 (76.2) | 19,283 (75.2) |
| Sociodemographic covariates | |||
| Sex | |||
| Male | 18,698 (52.6) | 15,796 (39.1) | 6,680 (26) |
| Female | 16,838 (47.4) | 24,601 (60.9) | 18,975 (74) |
| Race-Ethnicity | |||
| White | 30,055 (84.6) | 34,398 (85.1) | 22,209 (86.6) |
| Black | 3,359 (9.5) | 3,169 (7.8) | 1,789 (7) |
| Hispanic | 818 (2.3) | 1,294 (3.2) | 771 (3) |
| Asian | 566 (1.6) | 879 (2.2) | 523 (2) |
| Other | 738 (2.1) | 657 (1.6) | 363 (1.4) |
| Prescription benefit gap | 4,140 (11.7) | 4,638 (11.5) | 2,752 (10.7) |
| Dual Eligibility in Medicaid & Medicare | 9,946 (28) | 11,015 (27.3) | 7,520 (29.3) |
| Median household income at census block group level ($) | |||
| ≤ 30,000 | 17,371 (48.9) | 19,107 (47.3) | 11,837 (46.1) |
| 30,001–60,000 | 14,425 (40.6) | 16,684 (41.3) | 10,658 (41.5) |
| 60,001–100,000 | 3,090 (8.7) | 3,706 (9.2) | 2,540 (9.9) |
| 100,001–150,000 | 500 (1.4) | 687 (1.7) | 476 (1.9) |
| ≥ 150,001 | 150 (0.4) | 213 (0.5) | 144 (0.6) |
| Conditions at index admission | |||
| Angiocardiography | 24,773 (69.7) | 23,623 (58.5) | 7,542 (29.4) |
| Acute renal failure | 3,901 (11) | 5,124 (12.7) | 3,279 (12.8) |
| Coronary artery bypass grafting | 3,524 (9.9) | 2,782 (6.9) | 330 (1.3) |
| Cardiac catheterization | 25,334 (71.3) | 23,906 (59.2) | 7,358 (28.7) |
| Cardiac dysrhythmias | 9,779 (27.5) | 13,224 (32.7) | 9,041 (35.2) |
| Congestive heart failure | 10,464 (29.4) | 14,856 (36.8) | 12,167 (47.4) |
| Cardiogenic shock | 1,205 (3.4) | 1,001 (2.5) | 393 (1.5) |
| Hypotension | 1,771 (5) | 2,083 (5.2) | 1,312 (5.1) |
| Subendocardial infarction | 25,066 (70.5) | 30,628 (75.8) | 20,088 (78.3) |
| Platelet inhibitors | 2,165 (6.1) | 1,874 (4.6) | 577 (2.2) |
| Percutaneous transluminal coronary angioplasty or Stent | 17,205 (48.4) | 15,533 (38.5) | 5,109 (19.9) |
| Length of stay (days) | |||
| 1 | 1,889 (5.3) | 1,730 (4.3) | 861 (3.4) |
| 2–5 | 20,861 (58.7) | 22,507 (55.7) | 15,209 (59.3) |
| 6–10 | 8,593 (24.2) | 11,165 (27.6) | 7,395 (28.8) |
| ≥ 11 | 4,193 (11.8) | 4,995 (12.4) | 2,190 (8.5) |
| Days in intensive care unit | |||
| 0 | 15,316 (43.1) | 18,478 (45.7) | 13,442 (52.4) |
| 1–3 | 12,315 (34.7) | 12,531 (31) | 6,881 (26.8) |
| 4–10 | 6,816 (19.2) | 8,140 (20.2) | 4,866 (19) |
| ≥ 11 | 1,089 (3.1) | 1,248 (3.1) | 466 (1.8) |
| Days in coronary care unit | |||
| 0 | 21,010 (59.1) | 25,446 (63) | 17,979 (70.1) |
| 1–3 | 9,225 (26) | 8,765 (21.7) | 4,359 (17) |
| 4–10 | 4,681 (13.2) | 5,472 (13.5) | 3,111 (12.1) |
| ≥ 11 | 620 (1.7) | 714 (1.8) | 206 (0.8) |
| Baseline Charlson comorbidity index score | |||
| 0 | 10,816 (30.4) | 10,662 (26.4) | 6,553 (25.5) |
| 1–2 | 11,429 (32.2) | 12,681 (31.4) | 8,073 (31.5) |
| 3–5 | 8,558 (24.1) | 11,288 (27.9) | 7,516 (29.3) |
| 6–8 | 3,502 (9.9) | 4,437 (11) | 2,879 (11.2) |
| ≥ 9 | 1,231 (3.5) | 1,329 (3.3) | 634 (2.5) |
| Baseline comorbidities | |||
| Acute myocardial infarction | 1,685 (4.7) | 2,064 (5.1) | 1,565 (6.1) |
| Cancer | 3,478 (9.8) | 4,784 (11.8) | 2,470 (9.6) |
| Cerebrovascular disease | 4,811 (13.5) | 6,740 (16.7) | 4,406 (17.2) |
| Congestive heart failure | 6,856 (19.3) | 9,700 (24) | 8,285 (32.3) |
| Chronic obstructive pulmonary disease | 9,178 (25.8) | 9,596 (23.8) | 5,010 (19.5) |
| Dementia | 610 (1.7) | 1,895 (4.7) | 2,331 (9.1) |
| Diabetes | 15,723 (44.2) | 16,787 (41.6) | 8,123 (31.7) |
| AIDS/HIV | 59 (0.2) | Not reporteda | Not reporteda |
| Metastatic carcinoma | 498 (1.4) | 547 (1.4) | 229 (0.9) |
| Liver disease | 733 (2.1) | 679 (1.7) | 265 (1) |
| Paralysis | 401 (1.1) | 463 (1.1) | 300 (1.2) |
| Peptic ulcer disease | 499 (1.4) | 674 (1.7) | 409 (1.6) |
| Peripheral vascular disease | 5,421 (15.3) | 7,547 (18.7) | 5,583 (21.8) |
| Chronic kidney disease | 5,801 (16.3) | 8,470 (21.0) | 6,349 (24.7) |
| Connective tissue disease-rheumatic disease | 1,256 (3.5) | 1,607 (4) | 718 (2.8) |
| Other baseline clinical conditions | |||
| Angioedema | 63 (0.2) | 73 (0.2) | 44 (0.2) |
| Atrial fibrillation | 2,534 (7.1) | 4,781 (11.8) | 3,783 (14.7) |
| Asthma | 2,284 (6.4) | 2,346 (5.8) | 1,316 (5.1) |
| Coronary artery bypass grafting | 300 (0.8) | 205 (0.5) | 25 (0.1) |
| Hyperkalemia | 818 (2.3) | 1,128 (2.8) | 841 (3.3) |
| Hyperlipidemia | 20,691 (58.2) | 23,382 (57.9) | 11,966 (46.6) |
| Hypotension | 1,341 (3.8) | 1,893 (4.7) | 1,386 (5.4) |
| Hypertension | 25,888 (72.9) | 31,752 (78.6) | 20,674 (80.6) |
| Ischemic heart disease | 15,603 (43.9) | 18,915 (46.8) | 11,753 (45.8) |
| Osteoporosis | 1,602 (4.5) | 3,202 (7.9) | 2,874 (11.2) |
| Percutaneous transluminal coronary angioplasty | 1,466 (4.1) | 1,409 (3.5) | 488 (1.9) |
| Rhabdomyolysis | 146 (0.4) | 175 (0.4) | 133 (0.5) |
| Sinus bradycardia and heart block | 3,854 (10.8) | 6,103 (15.1) | 4,801 (18.7) |
| Stent | 1,748 (4.9) | 1,608 (4) | 544 (2.1) |
| Unstable angina | 2,290 (6.4) | 2,444 (6) | 1,303 (5.1) |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; AIDS/HIV = acquired immunodeficiency syndrome/human immunodeficiency virus.
Data not reported if < 11 patients.
Acknowledgments
This study was supported by a grant from the National Institute on Aging of the National Institutes of Health (R21AG043668 [principal investigator Gang Fang]). The findings and conclusions in this document are those of the authors, who are responsible for its contents, and do not necessarily represent the views of the funding agency.
Footnotes
Conflict of Interest
GF has no conflicts of interest to disclose. JGR reports research grants to institution from Amarin, Amgen, Astra-Zeneca, Eli Lilly, Esai, Esperion, Glaxo-Smith Kline, Merck, Pfizer, Regeneron/Sanofi, Takeda and consultancy for Akcea/Ionis, Amgen, Dr. Reddy’s, Eli Lilly, Esperion, Merck, Pfizer, Regeneron/Sanofi.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011 Nov 29;124(22):2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 3.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) Jama. 2014 Feb 5;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 4.Green P, Maurer MS, Foody JM, Forman DE, Wenger NK. Representation of older adults in the late-breaking clinical trials American Heart Association 2011 Scientific Sessions. Journal of the American College of Cardiology. 2012 Aug 28;60(9):869–871. doi: 10.1016/j.jacc.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurwitz JH, Col NF, Avorn J. The exclusion of the elderly and women from clinical trials in acute myocardial infarction. Jama. 1992 Sep 16;268(11):1417–1422. [PubMed] [Google Scholar]
- 6.Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. Jama. 2001 Aug 8;286(6):708–713. doi: 10.1001/jama.286.6.708. [DOI] [PubMed] [Google Scholar]
- 7.Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. The New England journal of medicine. 2004 Aug 5;351(6):585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 8.Bakris GL, Siomos M, Richardson D, et al. ACE inhibition or angiotensin receptor blockade: impact on potassium in renal failure. VAL-K Study Group. Kidney international. 2000 Nov;58(5):2084–2092. doi: 10.1111/j.1523-1755.2000.00381.x. [DOI] [PubMed] [Google Scholar]
- 9.Speirs C, Wagniart F, Poggi L. Perindopril postmarketing surveillance: a 12 month study in 47,351 hypertensive patients. British journal of clinical pharmacology. 1998 Jul;46(1):63–70. doi: 10.1046/j.1365-2125.1998.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agusti A, Bonet S, Arnau JM, Vidal X, Laporte JR. Adverse effects of ACE inhibitors in patients with chronic heart failure and/or ventricular dysfunction : meta-analysis of randomised clinical trials. Drug safety. 2003;26(12):895–908. doi: 10.2165/00002018-200326120-00004. [DOI] [PubMed] [Google Scholar]
- 11.Izzo JL, Jr, Weir MR. Angiotensin-converting enzyme inhibitors. J Clin Hypertens (Greenwich) 2011 Sep;13(9):667–675. doi: 10.1111/j.1751-7176.2011.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perazella MA, Mahnensmith RL. Hyperkalemia in the elderly: drugs exacerbate impaired potassium homeostasis. Journal of general internal medicine. 1997 Oct;12(10):646–656. doi: 10.1046/j.1525-1497.1997.07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Kader K, Palevsky PM. Acute kidney injury in the elderly. Clinics in geriatric medicine. 2009 Aug;25(3):331–358. doi: 10.1016/j.cger.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akposso K, Hertig A, Couprie R, et al. Acute renal failure in patients over 80 years old: 25-years’ experience. Intensive care medicine. 2000 Apr;26(4):400–406. doi: 10.1007/s001340051173. [DOI] [PubMed] [Google Scholar]
- 15.Lauffenburger JC, Robinson JG, Oramasionwu C, Fang G. Racial/Ethnic and gender gaps in the use of and adherence to evidence-based preventive therapies among elderly Medicare Part D beneficiaries after acute myocardial infarction. Circulation. 2014 Feb 18;129(7):754–763. doi: 10.1161/CIRCULATIONAHA.113.002658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Jama. 2002 Dec 18;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 17.Li JS, Baker-Smith CM, Smith PB, et al. Racial Difference in Blood Pressure Response to Angiotensin-Converting Enzyme Inhibitors in Children: A Meta-Analysis. Clinical pharmacology and therapeutics. 2008;84(3):315–319. doi: 10.1038/clpt.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogedegbe G, Shah NR, Phillips C, et al. Comparative Effectiveness of Angiotensin-Converting Enzyme Inhibitor-Based Treatment on Cardiovascular Outcomes in Hypertensive Blacks Versus Whites. Journal of the American College of Cardiology. 2015;66(11):1224–1233. doi: 10.1016/j.jacc.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012 Jan;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2007 Feb;49(2 Suppl 2):S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. The BMJ. 2013;346:e8525. doi: 10.1136/bmj.e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomlinson LA, Abel GA, Chaudhry AN, et al. ACE Inhibitor and Angiotensin Receptor-II Antagonist Prescribing and Hospital Admissions with Acute Kidney Injury: A Longitudinal Ecological Study. PLOS ONE. 2013;8(11):e78465. doi: 10.1371/journal.pone.0078465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung CM, Ponnusamy A, Anderton JG. Management of acute renal failure in the elderly patient: a clinician’s guide. Drugs & aging. 2008;25(6):455–476. doi: 10.2165/00002512-200825060-00002. [DOI] [PubMed] [Google Scholar]
- 24.Raebel MA, Smith ML, Saylor G, et al. The positive predictive value of a hyperkalemia diagnosis in automated health care data. Pharmacoepidemiology and drug safety. 2010 Nov;19(11):1204–1208. doi: 10.1002/pds.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang TY, Vora AN, Peng SA, et al. Effectiveness and Safety of Aldosterone Antagonist Therapy Use Among Older Patients With Reduced Ejection Fraction After Acute Myocardial Infarction. Journal of the American Heart Association. 2016 Jan 21;5(1) doi: 10.1161/JAHA.115.002612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993 Oct;46(10):1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 27.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. American heart journal. 2004 Jul;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke; a journal of cerebral circulation. 2002 Oct;33(10):2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 29.Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. American heart journal. 2002 Aug;144(2):290–296. doi: 10.1067/mhj.2002.123839. [DOI] [PubMed] [Google Scholar]
- 30.Johnston SS, Conner C, Aagren M, Smith DM, Bouchard J, Brett J. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes care. 2011 May;34(5):1164–1170. doi: 10.2337/dc10-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pakhomov SS, Hemingway H, Weston SA, Jacobsen SJ, Rodeheffer R, Roger VL. Epidemiology of angina pectoris: role of natural language processing of the medical record. American heart journal. 2007 Apr;153(4):666–673. doi: 10.1016/j.ahj.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of clinical epidemiology. 2000 Dec;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 33.Logar CM, Pappas LM, Ramkumar N, Beddhu S. Surgical revascularization versus amputation for peripheral vascular disease in dialysis patients: a cohort study. BMC nephrology. 2005;6:3. doi: 10.1186/1471-2369-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care. 2005 Nov;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 35.Rector TS, Wickstrom SL, Shah M, et al. Specificity and sensitivity of claims-based algorithms for identifying members of Medicare+Choice health plans that have chronic medical conditions. Health services research. 2004 Dec;39(6 Pt 1):1839–1857. doi: 10.1111/j.1475-6773.2004.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronksley PE, Tonelli M, Quan H, et al. Validating a case definition for chronic kidney disease using administrative data. Nephrol Dial Transplant. 2012 May;27(5):1826–1831. doi: 10.1093/ndt/gfr598. [DOI] [PubMed] [Google Scholar]
- 37.Kern EF, Maney M, Miller DR, et al. Failure of ICD-9-CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res. 2006 Apr;41(2):564–580. doi: 10.1111/j.1475-6773.2005.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988 Sep;16(3):1141–1154. [Google Scholar]
- 39.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999 Jun;94(446):496–509. [Google Scholar]
- 40.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. Journal of the American Society of Nephrology : JASN. 2009 Jan;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kostis JB, Shelton B, Gosselin G, et al. Adverse effects of enalapril in the Studies of Left Ventricular Dysfunction (SOLVD). SOLVD Investigators. American heart journal. 1996 Feb;131(2):350–355. doi: 10.1016/s0002-8703(96)90365-8. [DOI] [PubMed] [Google Scholar]
- 42.Ahuja TS, Freeman D, Jr, Mahnken JD, Agraharkar M, Siddiqui M, Memon A. Predictors of the development of hyperkalemia in patients using angiotensin-converting enzyme inhibitors. American journal of nephrology. 2000 Jul-Aug;20(4):268–272. doi: 10.1159/000013599. [DOI] [PubMed] [Google Scholar]
- 43.Ramadan FH, Masoodi N, El-Solh AA. Clinical factors associated with hyperkalemia in patients with congestive heart failure. Journal of clinical pharmacy and therapeutics. 2005 Jun;30(3):233–239. doi: 10.1111/j.1365-2710.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- 44.Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Archives of internal medicine. 2009 Jun 22;169(12):1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raebel MA. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovascular therapeutics. 2012 Jun;30(3):e156–166. doi: 10.1111/j.1755-5922.2010.00258.x. [DOI] [PubMed] [Google Scholar]
- 46.Noize P, Bagheri H, Durrieu G, et al. Life-threatening drug-associated hyperkalemia: a retrospective study from laboratory signals. Pharmacoepidemiology and drug safety. 2011 Jul;20(7):747–753. doi: 10.1002/pds.2128. [DOI] [PubMed] [Google Scholar]
- 47.Amir O, Hassan Y, Sarriff A, Awaisu A, Abd Aziz N, Ismail O. Incidence of risk factors for developing hyperkalemia when using ACE inhibitors in cardiovascular diseases. Pharmacy world & science : PWS. 2009 Jun;31(3):387–393. doi: 10.1007/s11096-009-9288-x. [DOI] [PubMed] [Google Scholar]
- 48.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. Jama. 2002 Jul 24–31;288(4):455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 49.Stuart B, Loh FE. Medicare Part D enrollees’ use of out-of-plan discounted generic drugs. Journal of the American Geriatrics Society. 2012 Feb;60(2):387–388. doi: 10.1111/j.1532-5415.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- 50.Roberto PN, Stuart B. Out-of-plan medication in Medicare Part D. The American journal of managed care. 2014 Sep;20(9):743–748. [PubMed] [Google Scholar]
- 51.Zhou L, Stearns SC, Thudium EM, Alburikan KA, Rodgers JE. Assessing Medicare Part D claim completeness using medication self-reports: the role of veteran status and Generic Drug Discount Programs. Medical care. 2015 May;53(5):463–470. doi: 10.1097/MLR.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown JD, Doshi PA, Talbert JC. Utilization of free medication samples in the United States in a nationally representative sample: 2009–2013. Research in social & administrative pharmacy : RSAP. 2017 Jan-Feb;13(1):193–200. doi: 10.1016/j.sapharm.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]