Abstract
Background
Well and poorly differentiated pancreatic neuroendocrine neoplasms are biologically distinct entities with different therapy and prognosis. Well differentiated neoplasms with elevated proliferation (Ki67 >20%) have been shown to have an overlapping histology with poorly differentiated neuroendocrine carcinoma. We compared expert cytomorphologic assessment of differentiation in pancreatic neuroendocrine neoplasms in a multi-institutional study.
Methods
FNAs from pancreatic neuroendocrine neoplasms (WHO 2017 G2 and G3) (n=72) were diagnosed independently by 3 cytopathologists as well (WD) or poorly differentiated (PD), large (PD-L) or small cell type (PD-S) based purely on cytomorphology. Their diagnoses were compared with a final classification supported by immunohistochemistry (RB, DAXX, and ATRX expression), targeted mutation analysis (MSK-IMPACT), prior history of G1–G2 histology, and consensus.
Results
Agreement on differentiation was 38% (15 WD, 12 PD) for the 70 cases included (55 WD (G2 n=19, G3 n=31, cannot be graded n=5) and 15 PD (PD-S n= 6; PD-L n=6; PD-not otherwise specified n=3)). Two cases could not be classified by our methods. PD carcinomas had a higher rate of agreement (10/15 (67%) than WD neoplasms 15/55 (27%). Round nuclei and plasmacytoid cells were associated with agreement in WD while apoptosis and angulated nuclei were associated with disagreement. Necrosis was associated with agreement for PD.
Conclusions
A purely morphological approach to the distinction between G2 and G3 pancreatic neuroendocrine neoplasms based on cytology can be challenging, with disagreement among experienced cytopathologists.
Keywords: Pancreatic neuroendocrine tumor, Pancreatic neuroendocrine carcinoma, pancreas, FNA, Pancreas FNA, pancreas, neuroendocrine differentiation, PanNET, PanNEC
Introduction
Pancreatic neuroendocrine neoplasms are rare, pathologically diverse tumors with varied clinical behavior. The World Health Organization (WHO) 2017 classification of Tumors of the Digestive System defines well differentiated (WD) pancreatic neuroendocrine tumors (PanNET) as morphologically resembling non-neoplastic islet cells while poorly differentiated (PD) pancreatic neuroendocrine carcinomas (PanNEC) are uniformly high grade and exhibit large cell or small cell morphology (large cell neuroendocrine carcinoma or small cell carcinoma).1 The distinction between WD and PD tumors is supported by different underlying genetic alterations, treatment response, and outcome.2–4 Emerging genotypes for WD-PanNET include mutations in DAXX, ATRX, and MEN1 whereas PD-PanNEC shows more similarity to conventional ductal adenocarcinoma and may exhibit mutations of TP53, RB1 and KRAS, among others.4, 5 Approximately 4% of WD-PanNETs have TP53 mutations but they are more likely to occur in PD-NEC. WD-PanNET are often completely asymptomatic and can be followed expectantly for months and sometimes years. In contrast, PD-NEC behave aggressively and are managed with cytotoxic chemotherapy.6
Recent changes in the WHO classification of pancreatic neuroendocrine neoplasms reflect the above data.1 The neoplasms are categorized into four tiers distinguished by morphologic features and mitotic rate or Ki67 proliferation rate: grade 1 PanNET, grade 2 PanNET, grade 3 PanNET, and PanNEC (also grade 3)(WHO 2017, Table 1). Thus, PD-PanNEC is uniformly high grade, whereas WD-PanNET can exhibit a spectrum from low to high grade. Prognosis and therapeutic responses are markedly different between WD-PanNET grade 3 and PanNEC with better median overall survival for WD-PanNET grade 3 (52 months versus 10 months, respectively) and worse response rates to platinum based therapy (10% and 37%, respectively).2
Table 1.
WHO 2017 Classification of Pancreatic Neuroendocrine Neoplasms1
| Classification/grade | Ki67 proliferation index | Mitotic index |
|---|---|---|
| Well-differentiated pancreatic neuroendocrine tumors | ||
| G1 PanNET | <3% | <2 |
| G2 PanNET | 3–20% | 2–20 |
| G3 PanNET | >20% | >20 |
| Poorly differentiated pancreatic neuroendocrine carcinomas | ||
| PanNEC (G3) | >20% | >20 |
| Small cell type | ||
| Large cell type | ||
Adapted from Lloyd R, Osamura RY, Klöppel G, Rosai J. Who classification of tumours of endocrine organs. 4th ed. Lyon IARC Press, 2017.
Ki67 rates >20% do not strictly correlate with WD vs PD neoplasms.7 Furthermore, histologic assessment of differentiation in G3 neuroendocrine neoplasms can be challenging.8 In cytologic specimens, the appearances of various diagnostic parameters used to assess differentiation differs from those used in histology, including apoptotic debris, mitoses, nucleoli, and chromatin pattern. On this premise, 3 cytopathologists from different institutions with a subspecialty interest in pancreatic cytopathology assessed G2–3 neoplasms in an attempt to determine the degree of differentiation using standard morphologic definitions for these entities and no training module. Their responses were compared with each neoplasm’s final classification, which was based on ancillary information reflecting underlying current understanding of tumor biology including tumor genotype, review of prior material, and immunophenotype (DAXX, ATRX, and RB). Cytomorphology was then further reviewed and the association between various cytologic features was assessed in cases with reviewer agreement and those with disagreement regarding tumor differentiation.
Methods
Case selection
With IRB approval, pancreatic neuroendocrine neoplasms previously classified as G2 or above on cytology or histology using the WHO 2010 Classification system were identified retrospectively by one author (C.S.S.) from the pathology database at Memorial Sloan Kettering Cancer Center.9 Neoplasms with a cytology specimen from a primary or metastatic neoplasm were included. All patients were evaluated clinically at the study institution. Patient demographics, radiology findings, and follow up information were available for all patients and were obtained from the electronic medical record.
Morphologic differentiation and assessment of cytology features
Slides were de-identified immediately following retrieval from the slide archive (by T.D.). Representative areas were circled on up to 3 slides (Diff Quik stained, ThinPrep, and Alcohol-fixed smear or cell block with hematoxylin and eosin stain, ThinPrep) by V.W.S. for blinded review by 3 experienced cytopathologists (C.S.S., D.C., M.D.R.) with subspecialty interest in pancreatic cytopathology. For the majority of cases, 2–3 slides were selected which included a DiffQuik stain for 66 patients (92%), Thinprep for 41 patients (57%), cell block for 29 patients (40%), Alcohol fixed smear with hematoxylin and eosin stain 10 patients (14%), and alcohol fixed smear with Papanicolaou stain 3 patients (4%). Fifteen cases consisted of one Diff Quik stained touch imprint slide only. Reviewers were not provided any core biopsies. Reviewers were informed of the biopsy site and that all cases were G2 or G3 pancreatic neuroendocrine neoplasms. They classified each neoplasm using conventional criteria: WD=small to intermediate sized plasmacytoid cells with round to ovoid nuclei, with smooth nuclear membranes and finely granular chromatin as commonly described for PanNETs. PD-S= nuclear molding, hyperchromatic coarse chromatin, minimal to absent cytoplasm, and lack of or inconspicuous nucleoli or PD-L= large nuclei, abundant cytoplasm, large vesicular nuclei, or prominent nucleoli. The Ki67 indices were not provided.
After grading, the same cytology materials from all cases were assessed independently by a cytopathologist and cytology fellow (C.S.S. and V.W.S) to collect specific cytologic features (see below). The cases were reviewed without knowledge of the results of the initial review and final classification, and all cases with disagreements regarding the presence or absence of features were re-reviewed by C.S.S. and V.W.S. at a multi-headed scope for consensus. The following cytologic features were assessed: nuclear size variation (uniform, non-uniform, pleomorphic), nuclear shape (round, ovoid), nuclear contour (smooth, irregular), nuclear angulation, chromatin pattern (fine, coarse), and presence or absence of single prominent nucleoli, plasmacytoid shape, nuclear tangles, nuclear molding, and necrosis. Apoptosis was evaluated in 10 high power fields (Olympus BX43, 40× objective) and graded as present (any), or >5. Mitoses were assessed in 10 high power fields (Olympus BX43, 40× objective) as present (any), or >5.
Ancillary studies
Available paraffin embedded cell blocks and core biopsies were cut into 4 micron sections. Antibodies used included DAXX, ATRX, Rb, and Ki67 (Table 2). IHC for DAXX, ATRX, and Ki67 was performed on BenchMark XT automated equipment (Ventana Medical System Inc., Tucson, AZ). IHC for Rb was performed on Leica BOND automated system (Leica Biosystems, Wetzlar, Germany). Complete loss of DAXX, ATRX, and Rb protein expression, in the presence of positive staining in non-neoplastic cells, was regarded as abnormal. Internal positive controls for nuclear markers included background lymphocytes, hepatocytes, or stromal cells.
Table 2.
Antibodies used for immunohistochemistry
| Antibody (clone) | Dilution | Manufacturer |
|---|---|---|
| Rb (13A10) | 1:50 | Dako, Carpintaria, CA |
| Anti-ATRX | 1:500 | Sigma-Aldrich Corporation, St Louis MO |
| Anti-DAXX | 1:300 | Sigma-Aldrich Corporation, St Louis MO |
| Ki-67 (30-9) | Pre-diluted | Ventana, Tuscon, AZ |
Ki67 proliferation rate was assessed for all cases with available material on a combination of various preparations (core biopsy, cell block, alcohol-fixed cytology smear) as previously described.10 Given the lack of adequate material for formal mitotic counting (50 high power fields are required); the grade was assigned based solely on the Ki67 index, using WHO 2017 criteria. For cases with both a cytology and biopsy specimen obtained concurrently, the higher ki67 rate was used to assign the grade for that biopsy instance.
Results of targeted sequencing for KRAS, DAXX, ATRX, and RB1 were obtained from the institutional pathology database as reported from previous genetic mutation profiling completed by Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT), a custom next-generation sequencing array of 410 cancer-associated genes.11
Final classification
Following blinded categorization, complete clinicopathologic review was performed including review of prior and concurrent biopsy or resection specimens, molecular profiles, and immunohistochemical stains to determine a classification based on current biological understanding of these tumors.12 Final classification of differentiation was assigned based on criteria in Table 3. Feedback was provided to the reviewers on cases where their original diagnosis conflicted with ancillary support for differentiation and representative cases were reviewed. Cases failing to meet criteria for classification were re-reviewed for consensus (C.S.S., M.D.R., D.C.) over a Webex™ (Cisco Systems, Inc., San Jose, California) teleconference. If consensus could not be reached for differentiation (WD versus PD) we called the case “unclassified” (UNC). If consensus for small versus large cell type for PD-PanNEC, could not be reached we called it PD, not otherwise specified (PD-NOS).
Table 3.
Criteria for assignment of final classification of differentiation.
| Well differentiated | Poorly differentiated | |
|---|---|---|
| Excluded by | IHC loss expression of Rb RB or KRAS mutation |
IHC loss expression of for DAXX or ATRX DAXX, ATRX, or MEN1 mutation G1/G2 grade on prior pancreas resection Ki67 <20% on prior specimen |
| Supported by (one of the following) | IHC loss of expression of DAXX or ATRX DAXX, ATRX, or MEN1 mutation G1/G2 neoplasm on prior biopsy or resection* 3 cytologists agreed WD by morphology |
IHC loss of expression of Rb RB or KRAS mutation Adenocarcinoma component 3 cytologists agreed PD by morphology |
Using WHO 2010 criteria.8
Statistical analysis
Interobserver agreement was calculated using unweighted multirater Cohen’s kappa coefficients with corresponding significance testing. The threshold for significance of P value was 0.05. All analyses were performed using Stata 10.0 (Stata Corporation, College Station, TX).
Results
72 pancreatic neuroendocrine neoplasm cytology cases, from 68 patients obtained by EUS-guided FNA of the pancreas (n=11) or CT-guided FNA of a metastatic site (n=61), were included. Biopsies had been collected either for initial diagnosis (n= 35, 49%; liver metastasis n=24, pancreas n=10, other metastasis n=1) or assessment of disease progression in a patient with a known history (n=37, 51%; %; liver metastasis n=36, pancreatic recurrence n=1). Previously performed and clinically reported immunohistochemistry included positive staining for synaptophysin and/or chromogranin (n=71, 99%) and negative staining for at least one marker of acinar differentiation (trypsin or chymotrypsin) (n=41, 57%). MSK-IMPACT testing had been previously performed for 24 patients on material from 18 liver core biopsies, 5 pancreatectomies, and 1 cell pellet from a liver FNA. Of the 24 tested patients, 15 tumors contained mutations contributing to classification as described in Table 4.
Table 4.
The rates ancillary test results were used to support the final classification of differentiation
| Final classification | ||
|---|---|---|
|
| ||
| Well differentiated N=55 (meets criteria/total tested) |
Poorly differentiated N=15 (meets criteria/total tested) |
|
| IHC loss of RB | 0/31 | 6/9 (33%) |
| Mutation of RB or KRAS | 0 | 2/4 (50%) |
| IHC loss of DAXX or ATRX | 6/19 (32%) | 0/4 |
| Mutation of DAXX, ATRX, MEN1 | 13/22 (59%) | 0/4 |
| G1/G2 on prior | 36/36 (100%) | n/a |
| Ki67 <20% on all specimens | 21/50 (42%) | 0/9 |
| 3 cytologists independently agree on morphology | 15/55 (27%) | 10*/15 (67%) |
| Re-review for consensus on morphology** | 4/9 (44%) | 3/9 (33%) |
Does not include the two cases that were agreed to be PD but were super-ceded by ancillary information supporting WD.
9 cases were re-reviewed for consensus agreement on morphology after there was disagreement for differentiation when the cases were reviewed independently.
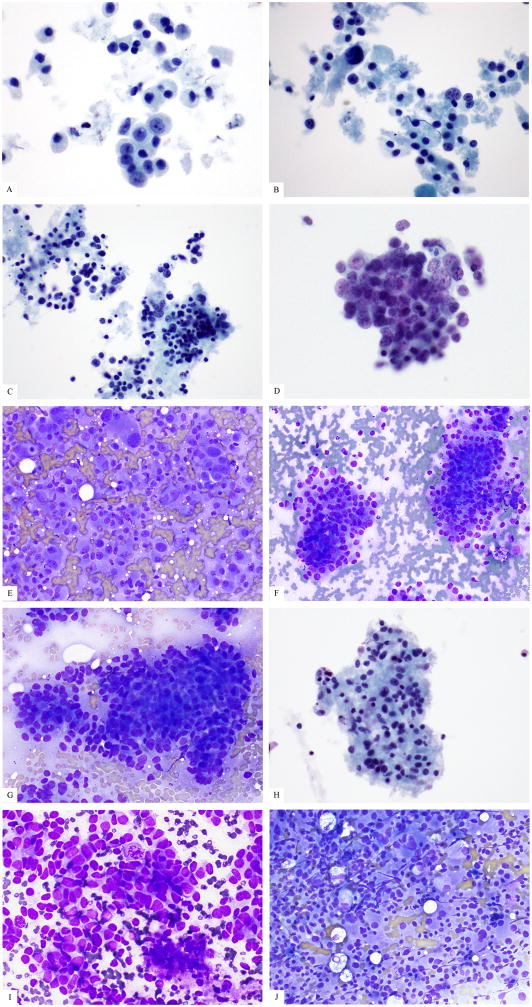
Final classification was established for 70 cases comprising 55 WD (G2 n=19, G3 n=31, cannot be graded n=5), 15 PD (PD-S n= 6; PD-L n=6; PD-NOS n=3). Differentiation was not determined using our methods in 2 cases. A summary of supporting criteria for classification is shown in Table 4. WD classification was supported by meeting 1, 2, 3, or 4 criteria in 39%, 37%, 13%, and 4%, respectively. PD classification was supported by meeting 1 or 2 criteria in 44% and 38%, respectively. A total of 9 cases had insufficient ancillary support for final classification and underwent re-review for consensus agreement by morphology; final classification for these neoplasms was 4 WD, 3 PD, and 2 UNC. Representative images of WD and PD cases with disagreement are shown in figure 2E–G with final classification. The ratio of WD:PD diagnoses for the three reviewers was 58:14, 36:36, and 18:53, respectively. There was complete independent agreement for differentiation among all 3 reviewers in 27/72 (38%) cases (15 WD, 12 PD) with a 0.16 Kappa statistic (p=0.008). The rate of agreement for WD vs PD was 15/55 (27%) vs 10/15 (67%). Individual reviewer diagnoses were the same as the final classification in 87%, 69%, and 49% of cases, respectively. Individual reviewer classification as WD was the same as final classification in 93%, 62%, and 35% of cases, respectively. Individual reviewer classification as PD was the same as final classification in 67%, 93%, and 100%. Complete agreement on classification was confirmed by the final classification except in two cases (#1 and #29, Figure 1 D, E), each with a final classification of WD but complete reviewer agreement of PD. A review of tumor history for Case 1 revealed a prior WD neoplasm with mitotic count of 2.2 per 10 high power fields (G2) (average of 50 fields counted) resected 3 years before the patient underwent liver FNA (Figure 1F). Evidence of underlying tumor biology for Case 29 included a prior FNA with a Ki67 index of 10% which was used as a separate case (#25) where the reviewers uniformly agreed it was WD. We re-reviewed cases 25 and case 29 side-by-side and note the higher Ki67 index of case 25 (29% vs 10%). Cytologically, case 25 had more ovoid, irregular, and angulated nuclei, more nuclear tangles, molding, apoptosis, mitoses, less cytoplasm, and an absence of plasmacytoid cells (Figure 1A–B). The cohort included 2 additional instances of interval biopsies from the same patient but in those other cases all four FNAs from these patients were consistently classified as WD by all reviewers.
Figure 2.
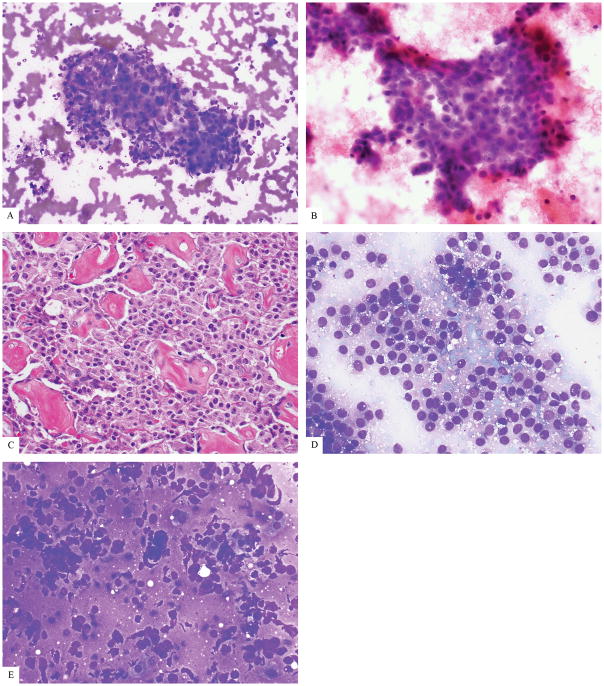
Examples of cases where 3 reviewers agreed (a–d), disagreed (e–g), or could not reach consensus on differentiation (h–j). (a) A G3 PanNET (Ki67 30%) agreed upon as WD (ThinPrep) (b) All three reviewers agreed this was PD-L. There are large cells, abundant cytoplasm, and necrosis (ThinPrep) (c) All three reviewers agreed on PD-S for this neoplasm with small cells, minimal cytoplasm, and apoptosis. (ThinPrep) (d) The reviewers agreed this case was PD but could not agree on small versus large cell type. (ThinPrep) (e) Reviewers did not agree on differentiation for this case but final classification was WD based on DAXX and MEN1 mutations. (f) Reviewers did not agree on differentiation and Rb was retained by immunohistochemistry which was not helpful. Final classification by consensus was PD. (g) Reviewers did not agree on this case, but final classification was WD based on prior a history of resected PanNET G1/G2. (h–j). Consensus was not reached for differentiation on these cases which had supporting data for tumor biology, so they were not classified. ((h) Case 5, ThinPrep) ((i) Case 5, Diff Quik 40x) ((j) Case 9, Diff Quik).
Figure 1.
High grade well differentiated pancreatic neuroendocrine neoplasms can appear poorly differentiated. (a–b) Smears of liver FNA of a WD-PanNET called PD by 3 reviewers can show prominent nucleoli, nuclear enlargement, and hyperchromasia. (c) The patient’s pancreatic resection 3 years prior to the FNA showed WD G2 histology (by mitotic count). (d–e). Liver FNAs from a WD-PanNET from the same patient (cases 25 and 29, respectively) taken one year apart show disparate morphology. (d) The patient’s first biopsy (case 25, (d)) was called WD by all 3 reviewers while case 29 (e) was reviewed as PD by all 3 reviewers.
Two cases were not classifiable because there was no ancillary information and the reviewers could not reach consensus on morphologic differentiation (Figure 2H–J). The neoplastic cells from one of these cases were small with round nuclei, small nucleoli and variable cytoplasm arranged in tight clusters with nuclear tangles, nuclear angulation, molding, apoptoses, and mitoses (Figure 2H–I). The other unclassified case had moderately sized plasmacytoid cells with round, smoothly contoured nuclei, but abundant cytoplasm, nuclear tangles, and mitoses made it difficult to decide on WD versus PD (Figure 2J).
PD-PanNECs (n=16) were sub-classified independently with full agreement into 5 PD-S and 5 PD-L (Figure 2A–C) After consensus review, an additional PD-S and PD-L each were agreed upon, but reviewers did not reach consensus for PD-S versus PD-L for 3 PD cases (PD-UNC)(Figure 2D). The genotypes of two PD-UNC included KRAS and RB1 mutations, among others, while lacking mutations associated with WD-PanNET (DAXX, ATRX, and MEN1).
Results of testing the association of cytologic features with presence or absence of reviewer agreement for WD or PD are shown in Tables 5 and 6. Features associated with agreement for WD included round nuclear shape (P=.03) and plasmacytoid cells (P=.03) whereas apoptosis ((P=0.009) and angulated nuclei (P=0.02) when present in WD-PanNETs tended to cause disagreement. Necrosis was the only factor significantly associated with agreement on poor differentiation (P=0.03).
Table 5.
Analysis for which cytologic features correlated with reviewer agreement in the poorly differentiated neoplasms
| Final classification* | Poorly differentiated N=15 |
P value | |
|---|---|---|---|
| Consensus agreement by all 3 reviewers | Yes | No | |
| N (%) | 10 (67) | 5 (33) | |
| Ki67 median (range) | 56.5 (25–95) | 63.5(22–80) | 0.9 |
| Large nuclear size | 2 | 1 | 0.9 |
| Nuclear size variation | 0.9 | ||
| Uniform | 2 | 1 | |
| Non-uniform | 2 | 2 | |
| Pleomorphic | 5 | 3 | |
| Nucleus shape | 0.2 | ||
| Round | 1 | 3 | |
| Ovoid | 8 | 3 | |
| Nucleus contour | 0.9 | ||
| Smooth | 5 | 4 | |
| Irregular | 3 | 2 | |
| Angulated nuclei | 8 | 5 | 0.9 |
| Chromatin | 0.9 | ||
| Fine | 0 | 1 | |
| Coarse | 8 | 5 | |
| Single prominent nucleolus | 1 | 2 | 0.5 |
| Plasmacytoid cells | 1 | 1 | 0.9 |
| Nuclear tangles | 8 | 5 | 0.9 |
| Molding | 7 | 4 | 0.9 |
| Apoptosis | |||
| Any | 9 | 5 | 0.4 |
| >5/10 hpf | 8 | 3 | 0.9 |
| Mitoses | |||
| Any | 5 | 4 | 0.5 |
| >5/10 hpf | 5 | 1 | 0.3 |
| Necrosis | 6 | 0 | 0.03 |
Two unclassified cases are not included.
Material for Ki67 proliferation was not available for 6 cases (5 WD, 1 PD)
Table 6.
Analysis for which cytologic features correlated with reviewer agreement in the well differentiated neoplasms
| Final classification* | Well differentiated N=55 |
P value | |
|---|---|---|---|
| Consensus agreement by all 3 reviewers | Yes | No | |
| N (%) | 16 (30) | 39 (70) | |
| Grade | |||
| G2 ** | 5 | 8 | |
| G3** | 3 | 9 | |
| Ki67 median (range) | 20.5 (3.2–68) | 32.5 (5–89) | 0.1 |
| Large nuclear size | 0 | 2 | 0.9 |
| Nuclear size variation | 0.06 | ||
| Uniform | 9 | 10 | |
| Non-uniform | 7 | 25 | |
| Pleomorphic | 0 | 4 | |
| Nucleus shape | 0.03 | ||
| Round | 14 | 21 | |
| Ovoid | 2 | 18 | |
| Nucleus contour | 0.9 | ||
| Smooth | 15 | 36 | |
| Irregular | 1 | 3 | |
| Angulated nuclei | 1 | 15 | 0.02 |
| Chromatin | 0.7 | ||
| Fine | 1 | 5 | |
| Coarse | 13 | 27 | |
| Single prominent nucleolus | 0 | 5 | 0.3 |
| Plasmacytoid cells | 15 | 26 | 0.03 |
| Nuclear tangles | 8 | 27 | 0.2 |
| Molding | 1 | 6 | 0.7 |
| Apoptosis | |||
| Any | 9 | 35 | 0.009 |
| >5/10 hpf | 2 | 19 | 0.01 |
| Mitoses | |||
| Any | 6 | 25 | 0.1 |
| >5/10 hpf | 0 | 9 | *** |
| Necrosis | 0 | 6 | 0.2 |
G2 cases were included in this study to investigate if morphological ambiguity exists below the threshold of 20% Ki67 rate. None of the 19 G2 neoplasms was agreed to be PD by all 3 reviewers, however not all G2 neoplasms were clearly WD because 58% (11/19) of G2 were called PD by at least one reviewer.
The median and range of ki67 index were WD G2 14% (5–17%), WD G3 40% (21–89%), and PD 61 (22–95%).
Discussion
Pancreatic neuroendocrine neoplasms with Ki67 proliferation rates exceeding 20% (WHO G3) are rare. It has recently been recognized that these high grade neoplasms comprise both well differentiated neuroendocrine tumors and poorly differentiated neuroendocrine carcinomas which have distinct biology, therapeutic management, and outcome.2 These findings are reflected in the new 2017 WHO classification of pancreatic neuroendocrine neoplasms, which includes both WD-PanNET and PD-PanNEC within the G3 category. Unlike the more commonly encountered G1 neoplasms, which usually appear clearly well differentiated, the distinction between WD and PD high grade neuroendocrine neoplasms can be difficult even when biopsy or resection specimens are available. In the present study we have shown that using only cytologic specimens to assess the morphology, the determination of differentiation in neuroendocrine neoplasms with elevated proliferative rates is quite challenging, even for experienced pancreatic cytopathologists.
Reviewers were provided with conventional descriptions of the entities only; a training set of neoplasms defined on a clinical or genetic basis was not used. Differentiation was independently agreed upon by all three reviewers in only 38% of cases, with a low Kappa of 0.16, comprising 15 WD (8 G2, 7 G3) and 11 PD (5 PD-S, 5 PD-L, 1 PD-NOS). This rate exceeds the 33% agreement rate achieved by gastrointestinal pathologists in a similarly designed study focused on histologic assessment of high grade NEN differentiation, although that study included only G3 neoplasms.8 In the present study, the agreement rate for PD was higher than for WD, 67% versus 27%, suggesting WD–PanNETs in the G2–G3 category can be more ambiguous than PD-PanNECs. We note that 58% of G2 neoplasms were called PD by at least one of the 3 reviewers (not consistently the same reviewer) suggesting morphological ambiguity in WD-PanNETs can be present even in G2 cases. The ratio of WD: PD diagnoses for each reviewer indicated that they were clearly using different thresholds to distinguish WD from PD. Notably, one reviewer’s diagnoses agreed with the final classification in 87% of cases which raises the possibility that morphologic differentiation can be assigned if we do more work to understand the morphologic differences between WD G2 and WD G3 neoplasms. Reviewers agreed on WD in cases with the conventional low grade features of round nuclear shape and plasmacytoid cells. In contrast, WD that showed apoptosis and angulated nuclei tended to result in disagreement. Necrosis was the only factor significantly associated with agreement on poor differentiation.
While a third of cases (24/72) were advanced disease at initial biopsy and without treatment, approximately half of the cases included in this study represented FNA biopsies of progressed disease, which has implications for our results. Management for these rare neoplasms is heterogenous, ranging from observation to alkylating agents and/or platinum-based chemotherapy, therefore many neoplasms could have seen treatment possibly influencing morphology. The reviewers were not informed if a specimen was from the initial diagnosis or progression. Because current concepts of neuroendocrine neoplasm biology do not support the transformation of a WD-PanNET to a PD-PanNEC, in practice, knowledge of a prior WD-PanNET diagnosis can inform the assessment of a presumed metastasis and should help avoid misclassification of a WD-PanNET G3 with worrisome morphological features as PD-PanNEC.4, 8 Second, it may be somewhat reassuring to note that advanced and progressive WD-PanNETs may be much less frequently encountered outside of institutions that routinely re-sample progressive or recurrent disease. Nevertheless, the phenomenon of WD-PanNET with grade transformation from G1/G2 to G3 may be underrecognized.
Prior to this study, aside from Ki67 staining, we had limited experience with applying ancillary immunohistochemical studies to cytology for this differential diagnosis. Ki67 staining certainly can be helpful in a morphologically ambiguous G2 neoplasm when the proliferation rate is ≤20%. But, Ki67 in most cases cannot be used as sole support to assign differentiation in G3 neoplasms, with the caveat that proliferation rates over 90% are more likely to occur in poorly differentiated neoplasms. We found that Rb immunohistochemistry on cell blocks was challenging to interpret because hepatocyte nuclei were often the only background cell to use as potential positive internal controls, but often they showed poor nuclear staining compared with other nuclei (e.g. lymphocytes) during antibody optimization. We also noted that retained Rb staining could not be interpreted as support for WD-PanNET since it is an inconsistent abnormality in PD-PanNEC, particularly in large cell variant that is more common than small cell carcinoma in pancreatic primaries.4. We did not perform DAXX and ATRX immunohistochemistry on cytology specimens because we were unsuccessful at optimizing these antibodies on our cell block material which was initially fixed in ethanol.
A drawback of this study is the limited ancillary support for true differentiation status in the PD-PanNECs, but final classification for the ambiguous WD-PanNETs was robust as only 1 (2%) was classified as such solely by reviewer agreement without other evidence supporting WD.
In summary, the distinction between well differentiated and poorly differentiated pancreatic neuroendocrine neoplasms is clinically relevant for therapeutic management and it is crucial for the diagnosis to be accurate. In this study, we show that experienced cytopathologists show disagreement on differentiation of these neoplasms in a cohort enriched for patients with advanced disease. Our results suggest a need for further comparison between well characterized G2 and G3 neuroendocrine neoplasms in order to enhance our morphologic diagnosis.
Acknowledgments
Funding: Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: Authors have received no funding that would constitute a conflict of interest with the information presented.
Author Contributions:
Carlie S. Sigel: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review and editing, Visualization
Vitor Werneck Kraus Silva: Investigation
Michelle D. Reid: Conceptualization, Investigation, Writing – review and editing
David Chhieng: Conceptualization, Investigation, Writing – review and editing
Olca Basturk: Writing – review and editing
Keith M. Sigel: Formal analysis,
Tanisha D. Daniel: Investigation
David S. Klimstra: Methodology, Writing – review and editing, Supervision
Laura H. Tang: Methodology, Writing – review and editing, Funding acquisition
References
- 1.Lloyd R, Osamura RY, Klöppel G, Rosai J. Who classification of tumours of endocrine organs. 4. Lyon: IARC Press; 2017. [Google Scholar]
- 2.Raj N, Valentino E, Capanu M, et al. Treatment response and outcomes of grade 3 pancreatic neuroendocrine neoplasms based on morphology: Well differentiated versus poorly differentiated. Pancreas. 2017;46:296–301. doi: 10.1097/MPA.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basturk O, Tang L, Hruban RH, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: A clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437–447. doi: 10.1097/PAS.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–184. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiao Y, Shi C, Edil BH, et al. Daxx/atrx, men1, and mtor pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moertel CG, Kvols LK, O’Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–232. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Basturk O, Yang Z, Tang LH, et al. The high-grade (who g3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39:683–690. doi: 10.1097/PAS.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang LH, Untch BR, Reidy DL, et al. Well-differentiated neuroendocrine tumors with a morphologically apparent high-grade component: A pathway distinct from poorly differentiated neuroendocrine carcinomas. Clin Cancer Res. 2016;22:1011–1017. doi: 10.1158/1078-0432.CCR-15-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosman FT. Who classification of tumours of the digestive system. 4. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 10.Sigel CS, Guo H, Sigel KM, et al. Cytology assessment can predict survival for patients with metastatic pancreatic neuroendocrine neoplasms. Cancer. 2017;125:188–196. doi: 10.1002/cncy.21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng DT, Mitchell TN, Zehir A, et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (msk-impact): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang LH, Basturk O, Sue JJ, Klimstra DS. A practical approach to the classification of who grade 3 (g3) well-differentiated neuroendocrine tumor (wd-net) and poorly differentiated neuroendocrine carcinoma (pd-nec) of the pancreas. Am J Surg Pathol. 2016 doi: 10.1097/PAS.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]