Key Points
Question
Would a new strategy of biomarker-based individualized adaptive stereotactic body radiotherapy maximize safety and preserve efficacy for patients with liver tumors?
Findings
In this phase 2 clinical trial that included 90 patients, treatment was well tolerated, with a lower complication rate than expected without adaptation. The 1-year local control rate was 99%, surpassing hypothesized treatment efficacy; the 2-year local control rate was 95%.
Meaning
This strategy of Individualized adaptive radiotherapy may represent a new treatment paradigm in which dose is based on individual, rather than population-based, tolerance to treatment.
Abstract
Importance
Patients with preexisting liver dysfunction could benefit the most from personalized therapy for liver tumors to balance maximal tumor control and minimal risk of liver failure. We designed an individualized adaptive trial testing the hypothesis that adapting treatment based on change in liver function could optimize the therapeutic index for each patient.
Objective
To characterize the safety and efficacy of individualized adaptive stereotactic body radiotherapy (SRBT) for liver tumors in patients who have preexisting liver dysfunction.
Design, Setting, and Participants
From 2010 to 2014, 90 patients with intrahepatic cancer treated with prior liver-directed therapy were enrolled in this large phase 2, single-arm, clinical trial at an academic medical center. All patients had at least 1 year of potential follow-up.
Interventions
Using indocyanine green retention at 15 minutes (ICGR15) as a direct biomarker of liver function and a Bayesian adaptive model, planned SBRT was individually modified midway through the course of therapy to maintain liver function after the complete course.
Main Outcomes and Measures
The primary outcome was local control; the secondary outcome was safety and overall survival.
Results
Patients were 34 to 85 years of age, and 70% (63) were male. Ninety patients (69 [77%] with hepatocellular carcinoma, 4 [4%] with intrahepatic cholangiocarcinoma, and 17 [19%] with metastatic) received treatment to 116 tumors. Sixty-two patients (69%) had cirrhosis, 21 (23%) were Child-Pugh (CP) grade B. The median tumor size was 3 cm; 16 patients (18%) had portal vein involvement. Sixty-two (69%) received all 5 fractions (47 full dose, 15 dose-reduced owing to rising ICGR15). Treatment was well tolerated, with a lower than expected complication rate without adaptation: 6 (7%) experienced a 2-point decline in CP 6 months post-SBRT. The 1- and 2-year local control rates were 99% (95% CI, 97%-100%) and 95% (95% CI, 91%-99%), respectively.
Conclusions and Relevance
We demonstrated that the treatment strategy of individualized adaptive therapy based on a direct biomarker of liver function can be used to achieve both high rates of local control and a high degree of safety without sacrificing either. Individualized adaptive radiotherapy may represent a new treatment paradigm in which dose is based on individual, rather than population-based, tolerance to treatment.
Trial Registration
clinicaltrials.gov Identifier: NCT01522937
This phase 2 clinical trial evaluated the safety and efficacy of individualized adaptive stereotactic body radiotherapy for liver tumors in patients who have preexisting liver dysfunction.
Introduction
Hepatocellular carcinoma (HCC) is a major cause of mortality, and the incidence rate is on the rise due to viral hepatitis, alcohol use, and nonalcoholic fatty liver disease. Only a minority of patients are eligible for liver transplant or resection. For remaining patients, available therapies include stereotactic body radiation therapy (SBRT), radiofrequency or microwave ablation, catheter-based therapy (transarterial chemo-embolization or radio-embolization), and systemic therapy. Most patients require sequential therapies, emphasizing the need to balance tumor control and toxic effects of each liver-directed therapy.
For patients with normal liver function, SBRT is relatively safe. However, most patients with HCC have underlying cirrhosis, so that liver-directed therapies can cause liver decompensation. After SBRT, both classical and nonclassical radiation-induced liver disease can produce substantial morbidity and up to 7% mortality. Patients with advanced liver disease are excluded from many trials and treatment algorithms owing to reports of toxic effects. Alternatively, they are treated with low doses of radiation to maintain safety, potentially at the expense of therapeutic efficacy.
We aimed to develop a strategy of biomarker-based individualized adaptive radiotherapy to allow delivery of the maximally aggressive safe treatment for patients with intrahepatic cancers. We found previously that subclinical decline in a patient's liver function after radiation therapy could be estimated by assessing indocyanine green (ICG) extraction, which is removed from the circulation only by the liver and thus is a direct measurement of dynamic liver function. Furthermore, these changes occur as early as 1 month after completion of therapy and are patient-specific, consistent with individual liver sensitivity to radiation. With this biomarker of liver function, we hypothesized that we could optimize both treatment safety and effectiveness through an individualized adaptive therapy strategy that incorporates a patient’s tolerance to the first portion of SBRT into a model for individualizing the second portion of SBRT. This approach, if successful, would represent a new paradigm in radiation therapy in which, instead of relying on population-based estimates, each patient’s treatment would be modified according to the individual patient’s tolerance.
Methods
Patients
The trial protocol (Supplement 1) and consent forms were approved by the University of Michigan institutional review board. Eligible patients had HCC established with biopsy or American Association of Liver Disease imaging criteria or liver metastases with prior liver-directed therapy. Patients could not be eligible for curative liver resection but had to have adequate performance status (Eastern Cooperative Oncology Group score ≤2) and organ function: platelet count of at least 300 × 103 μL, a blood urea nitrogen level of 40 mg/dL or less, a creatinine level of 2.0 mg/dL or less , international normalized ratio of 1.3 or less or correctable by vitamin K unless anticoagulated for another reason, and bilirubin level of less than 3 mg/dL (in the absence of obstruction or preexisting disease of the biliary tract, eg, primary sclerosing cholangitis). (To convert blood urea nitrogen to millimoles per liter, multiply by 0.357; to convert creatinine to micromoles per liter, multiply by 88.4; to convert bilirubin micromoles per liter multiply by 17.104.) There were no limitations on tumor size, vascular invasion, pretreatment Child-Pugh (CP) score, or pretreatment ICG retention. Patients could not have an iodine allergy (contained in ICG). Written informed consent was obtained from study participants prior to any study-related procedures. They were not compensated for their participation.
Treatment Schema
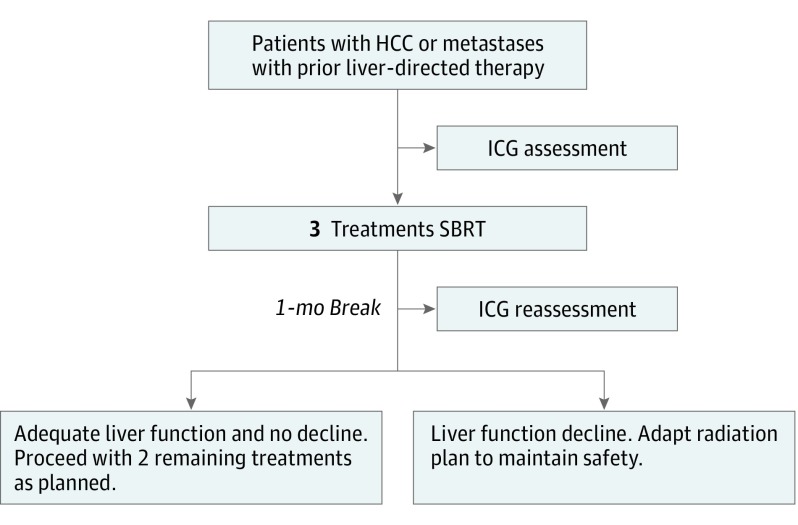
Prior to initiation of SBRT, patients underwent testing with ICG in our clinical research unit. Following intravenous administration, ICG is rapidly bound to plasma proteins and then selectively taken up by hepatic parenchymal cells and secreted into the bile. Blood was collected prior to ICG infusion, through 20 minutes afterward and processed in triplicate in a Clinical Laboratory Improvement Amendments–certified laboratory, as described previously. The ICG retention rate at 15 minutes (ICGR15) was calculated. Patients then received 3 of the 5 planned SBRT treatments, waited 4 weeks for potential subclinical liver function change and underwent repeat assessment of the ICGR15. The dose for the final 2 treatments was adjusted, from 0% to 100% of initial (Figure 1). If the ICGR15 was too high at 1 month to allow for treatment, patients were retested 1 month later, with the opportunity to receive further radiation if the ICGR15 decreased sufficiently.
Figure 1. Study Schema.
HCC indicates hepatocellular carcinoma; ICG, indocyanine green; SBRT, stereotactic body radiation therapy.
The goal of the mid-treatment adaptation was to ensure, with high probability, that the final (1 month after the end of SBRT) ICGR15 was less than 39%, which had been associated with liver failure after wedge resection. During the initial part of the trial, we did not see significant toxic effects in our study patients, which we felt was because our patients were not subjected to the major systemic insults of general anesthesia and surgery. Therefore, we amended the protocol to increase the threshold from 39% to 44%. The adaptation was performed using a statistical model that predicted the final ICGR15 for an individual patient based on the current ICGR15, change in ICGR15 from baseline to mid-treatment, dose during the first course of treatment and the dose (yet to be given) during the second course of treatment. From this model, we calculated the required limit on dose for the second course of treatment so that the expected final ICGR15 would not exceed 44%. If this limit was below the planned dose, the planned dose was reduced. If this limit was greater than the planned dose, the planned dose was given. To learn from previously treated patients, the model incorporated a parameter capturing the ratio of change in the ICGR15 during the first course of treatment to the change in the ICGR15 during the last course of treatment. This parameter was updated throughout the trial as data were accumulated. Patients with a baseline ICGR15 level greater than 44% were also enrolled but were eligible to receive only the first 3 fractions unless their mid-treatment ICGR15 level was below 44%. A full mathematical description of the method is given in eMethods in Supplement 2.
Treatment Planning
The specifics of treatment planning and delivery have been described elsewhere. Briefly, after implantation of fiducial markers as clinically indicated, patients underwent contrast-enhanced computed tomographic (CT) simulation while immobilized in a customized vacuum body mold. Active breathing control was used to eliminate respiratory motion as tolerated, with 4-dimensional CT used for the remaining patients. Tumors (gross tumor volumes) were defined on the simulation CT, with registration of magnetic resonance imaging (MRI) as necessary. For patients treated free-breathing, an internal target volume (ITV) was generated to encompass the range of motion. The gross tumor volume or internal target volume was set equal to the clinical target volume, and a standard margin of 5 mm axially and 8 mm superiorly and inferiorly was added for the planning target volume. SBRT was typically forward-planned, although intensity-modulated radiotherapy was used when targets and normal tissues were in close proximity and tradeoffs between them were required. The treatment course was initially planned for 5 fractions to a maximum predicted rate of radiation-induced liver disease of 15% based on a prior model, or a maximum total dose of 60 Gy. For this plan, liver function was not considered, although it would was in the mid-treatment adaptation. The dose limits to 0.55 cc of the duodenum, stomach, and heart were 30.0, 27.5, and 52.5 Gy, respectively. The chest wall dose to 30.0 cc was kept below 35.0 Gy early in the trial and relaxed to 70.0 cc later on. Dose was prescribed to the isodose surface covering 99.95% of the planning target volume , except in cases in which sparing of adjacent organs was given priority. Cone-beam CT was used for image guidance prior to every treatment.
Evaluation
Patients underwent ICG clearance testing, clinical evaluation with adverse event (CTCAE, version 4) and performance status assessment, and liver function testing 1 month after completion of SBRT. Except for the ICG, these were all repeated every 3 to 6 months for 2 years, along with evaluation with contrast-enhanced liver MRI, except in patients who could not have MRI (eg, owing to a pacemaker), who were followed with contrast-enhanced CT. Freedom from local progression was defined using the Response Evaluation Criteria in Solid Tumors criteria.
Statistical Methods
The primary aim of the trial was to characterize efficacy of individualized SBRT in this patient population. The primary end point was local control (LC). Secondary end points included safety, overall survival (OS), and biomarkers for predicting safety and efficacy. Local control was defined as the time from start of SBRT until progression of the treated lesion. Patients without progression were censored at the earlier of last scan, liver transplant, or initiation of systemic therapy. Analyses for LC were conducted at the lesion level with robust standard errors used to account for the correlation between multiple lesions within the same patient. Local control was estimated at 1 and 2 years using the cumulative incidence function, with death as a competing risk. Overall survival was measured from start of SBRT until death or loss to follow-up. Ten patients enrolled twice in this protocol after developing new lesions and were counted separately in estimating OS. Robust standard errors were also used in OS analysis to account for intrapatient correlation among the patients enrolled twice. Cox proportional hazards regression models were used to assess potential predictors for their relation to LC and OS. Stepwise procedures were used to build multivariate models with an α = .10 significance threshold for inclusion in the model. Local progression was included as a time-dependent indicator variable in the OS analysis. The proportions of patients with at least a 1- or 2-point increase in CP at any point within 6 months of treatment were calculated. Statistical significance was evaluated at P ≤ .05, and all analyses were implemented using R (R Foundation) or SAS (version 9.4: SAS Inc) statistical software. The trial was designed to rule out 1-year LC rates of 65% or less. With 90 patients, the trial had greater than 80% power, based on a 1-sided α = .05 level test and a hypothesized 1-year LC rate of 80%.
Results
Patients and Treatment
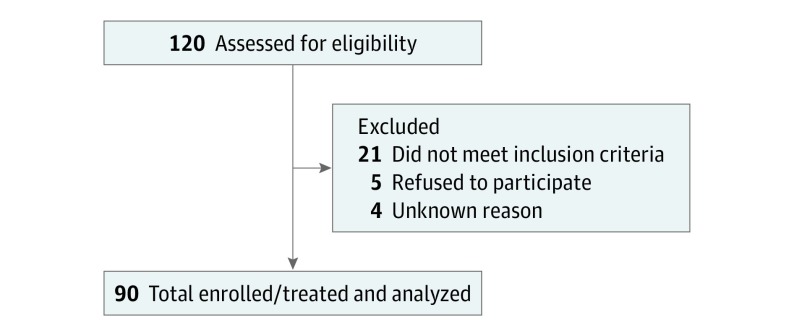
Patients were 34 to 85 years of age, and 70% (63) were male. Between May 2010 and October 2014, 120 patients consented to participate in the trial (Figure 2). Twenty-one patients were screen failure, and 5 patients withdrew consent prior to treatment owing to perceived difficulties traveling for treatment. Ninety patients with 116 tumors received treatment and were evaluable. Eighteen patients had more than 1 lesion treated at once (12 patients had 2, 4 had 3, and 2 had 4). Six patients were enrolled twice, and 2 patients enrolled 3 times. Baseline patient and tumor characteristics are displayed in Table 1. Most patients had HCC and cirrhosis, typically in the setting of hepatitis C virus and/or alcohol use. The median pretreatment CP score was 6 (range, 5-9); 23% of patients were CP grade B. Median pretreatment total bilirubin level was 0.9 mg/dL (range, 0.3-3.5 mg/dL).
Figure 2. CONSORT Diagram.
Table 1. Patient and Tumor Characteristics.
| Characteristic | Value |
|---|---|
| Age, median (range), y | 62 (34-85) |
| Sex ratio, male: female | 70:30 |
| Patients with cirrhosis, No. (%) | 62 (69) |
| Hepatitis C virus | 36 (40) |
| Alcohol use | 14 (16) |
| Othera | 40 (44) |
| Pretreatment Child Pugh score, median (range) | 6 (5-9) |
| Pretreatment ICGR15, median (range) | 22 (4-75) [normal 4-10] |
| Previous liver-directed therapies, No. median (range) | 1.5 (0-6) |
| Tumor histologic findings, No. (%) | |
| HCC | 69 (77) |
| Intrahepatic cholangiocarcinoma | 4 (4) |
| Metastases | 17 (19) |
| Tumor diameter, median (range), cm | 3 (0-13) |
| Portal vein tumor thrombus, No. (%) | 16 (18) |
| Pretreatment AFP for patients with HCC, median (range) | 13 (0.2-25 284) |
Abbreviations: AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; ICGR15, indocyanine green retention at 15 minutes.
Hepatitis B virus, nonalcoholic fatty liver disease, autoimmune, hemochromatosis, primary biliary cirrhosis, sarcoid, congenital heart defect.
The median pretreatment ICGR15 was 22%, over twice the upper limit of normal. Patients had received a median of 2 liver-directed therapies (range, 0-6) prior to SBRT. Seventy had received prior transcatheter arterial chemoembolization; 36, prior radiation therapy; and 13, prior radiofrequency ablation. The median tumor diameter was 3 cm, with a maximum of 13 cm; 16 (18%) were associated with portal vein tumor thrombus. The mean liver dose was a median of 13 Gy (range, 3-30 Gy).
Treatment and Adaptation
Treatment was adapted for safety for 52 (45%) of 116 tumors. Twenty-six tumors were treated with only 3 fractions owing to an elevated pretreatment ICGR15 above the threshold for further treatment that did not decrease below 44% at the midtreatment assessment. For 26 tumors (22%), treatment was adapted based on the patient’s change in ICGR15 after the initial phase of treatment, resulting in a lower dose of SBRT for the last 2 treatments. Sixty-four tumors (55%) received the full planned 5 fraction course of SBRT and did not require adaptation. The median delivered prescription dose was 49 Gy (range 23-60 Gy) (eFigure 1 in Supplement 2).
Treatment adaptation significantly altered the course of predicted decline in liver function (eFigure 2 in Supplement 2). For the patients treated with reduced dose for the final 2 of the 5 therapies, there was a significantly smaller mean increase in ICGR15 2 months posttherapy than would have been predicted with no adaptation (ICGR15 change, 9.2% vs 19.0%; P = .03).
Toxic Effects
Treatment was well tolerated with no classical radiation-induced liver disease and a lower complication rate than expected without adaptation. Grade 3 elevations in aspartate aminotransferase, alanine aminotransferase, and total bilirubin levels occurred in 1, 2, and 1 patient within 6 months of SBRT, respectively. Thirteen (14%) and 6 (7%) of patients experienced a 1- or 2-point increase in CP score within 6 months of treatment. All but 1 of these patients had cirrhosis, and all had a primary liver tumor rather than metastatic disease to the liver. Thus, in the subset of 73 patients with primary liver tumors, 13 (18%) had a 1-point increase in CP score, and 6 (8%) had a 2-point increase in CP score. One patient developed grade 2 ascites, and another developed grade 3 ascites without any other signs of radiation-induced liver disease. One patient had grade 3 duodenal bleeding adjacent to her tumor 7 months after completing therapy. All of these patients had primary liver tumors. The most common toxic effect was grade 2 fatigue in 18%. Patients who had received prior liver-directed therapy did not have a higher rate of toxic effects than those who had not.
Tumor Control and Survival
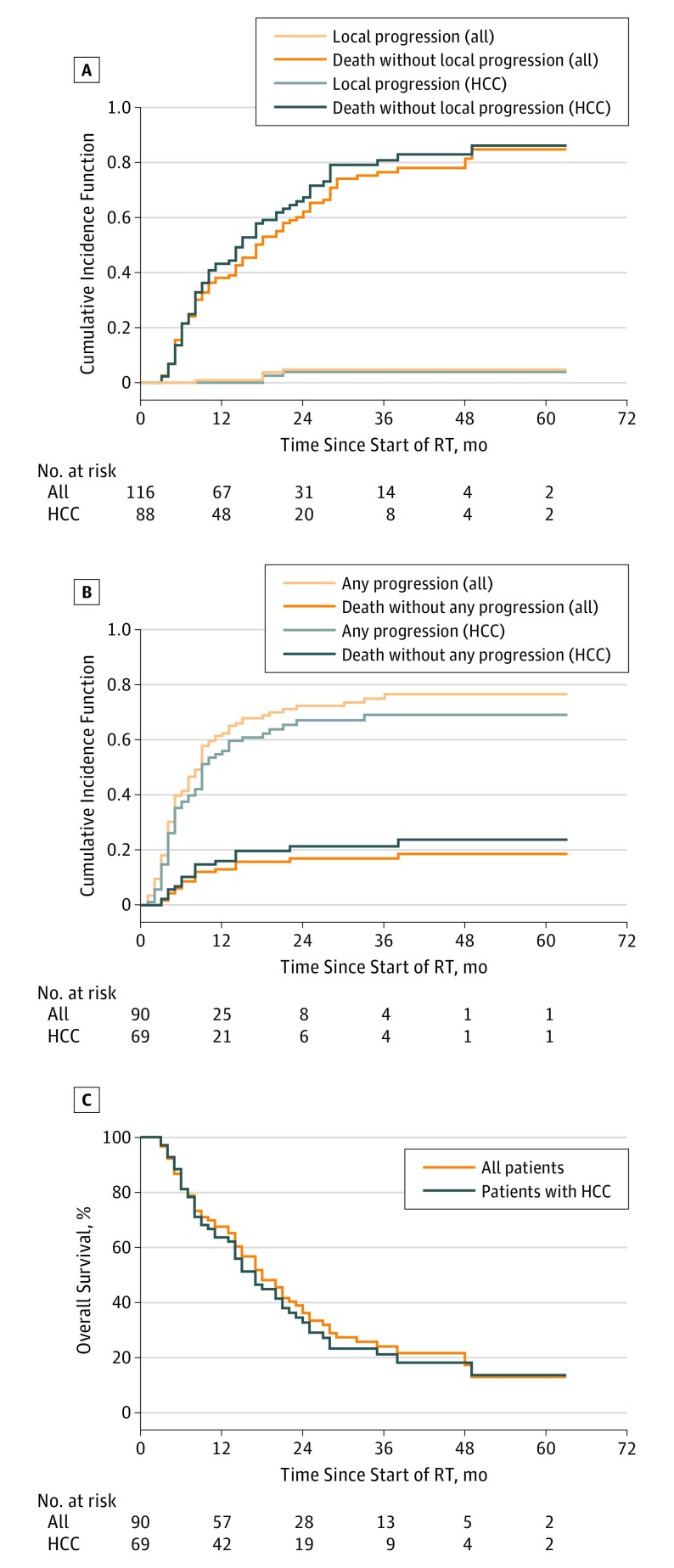
The estimated local control at 1 year was 99% (95% CI, 97%-100%) and was significantly greater than 65% (P < .001), thus achieving our primary study aim. With a median follow-up of 37 months, local control at 2 years for both HCC and metastatic disease was 95% (95% CI, 91%-99%) (Figure 3A). The 5 recurrent tumors were in patients without portal vein tumor thrombus. Three had HCC, 1 had metastases, and 1 had intrahepatic cholangiocarcinoma. Tumor sizes were 12, 19, 26, 30, and 38 mm. Tumors were located in the left lobe in 2, right lobe in 1, caudate in 1, and dome in 1 patient. Pretreatment alpha-fetoprotein tests for patients with HCC were 2.0, 5.7, and 69.7. The number of prior liver-directed therapies in these 5 patients were 4 in 1, 3 in 1, 2 in 2, and 0 in 1. Radiation doses were 30, 33, 50, 50, and 60 Gy. Local recurrences were diagnosed 8, 18, 18, 18, and 21 months after initiation of SBRT.
Figure 3. Outcomes.
A, Local progression from time of radiotherapy start, with death as a competing risk. B, Any progression from time of radiotherapy start, with death as a competing risk. C, Overall survival. HCC indicates hepatocellular carcinoma; RT, radiotherapy.
The median time to progression was 9 months (Figure 3B). These included progression of the treated tumor in 5 patients (<1%) , new tumor(s) elsewhere in the liver in 78 patients (87%), and extrahepatic progression in 40 patients (44%). At the time of progression, subsequent therapy consisted of systemic therapy in 29 patients, transcatheter arterial chemoembolization in 5 patients (<1%), additional radiotherapy in 5 patients (<1%), and radioembolization in 3 patients (<1%).
Overall survival at 1 and 2 years was 67% (95% CI, 58%-78%) and 36% (95% CI, 27%-48%) (Figure 3C). In univariate analysis, sex, age, number of prior liver-directed therapies, tumor histologic findings, and type of liver disease were not associated with local control or survival. Smaller tumor size, no portal vein tumor thrombus, no cirrhosis, lower baseline CP, and higher dose were associated with a longer OS. In a multivariable analysis for LC with histologic findings and total dose, higher dose was associated with better local control; however, this was not statistically significant (hazard ratio, 0.9; 95% CI, 0.8-1.01; P = .08). For OS, multivariable analysis identified fewer prior liver-directed therapies, smaller tumor size, lower baseline CP, and higher dose to be associated with longer survival (Table 2). Local progression of the treated tumor was not associated with shorter survival.
Table 2. Univariate and Multivariate Analysis for Local Control and Overall Survival.
| Variable | Local Progression | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariable Analysis | Univariate Analysis | Multivariable Analysis | |||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Female sex (vs male) | 0.62 (0.06-5.98) | .68 | NA | NA | 0.84 (0.47-1.49) | .54 | NA | NA |
| Age at start of RT | 0.99 (0.94-1.05) | .82 | NA | NA | 1.01 (0.98-1.03) | .70 | NA | NA |
| Prior liver-directed therapies, No. | 0.99 (0.63-1.54) | .95 | NA | NA | 0.99 (0.85-1.16) | .93 | 1.16 (1.02-1.31) | .03 |
| Tumor size, cm | 0.85 (0.57-1.28) | .44 | NA | NA | 1.13 (1.00-1.27) | .04 | 1.18 (1.04-1.35) | .01 |
| Portal vein thrombosis | 0.52 (0.00-4.58) | .16 | NA | NA | 1.71 (0.99-2.94) | .05 | NA | NA |
| Tumor histologic findings (vs HCC) | ||||||||
| Intrahepatic cholangiocarcinoma | 6.65 (0.89-49.9) | .07 | 31.9 (2.51-406.0) | .008 | 0.31 (0.03-3.08) | .32 | NA | NA |
| Metastases | 1.00 (0.08-12.0) | .99 | 2.68 (0.12-59.2) | .53 | 0.77 (0.38-1.55) | .46 | NA | NA |
| Cirrhosis | 0.93 (0.14-6.04) | .94 | NA | NA | 1.97 (1.07-3.62) | .03 | NA | NA |
| Type of liver disease (hepatitis C virus vs other) | 1.26 (0.49-3.24) | .64 | NA | NA | 0.96 (0.56-1.66) | .89 | NA | NA |
| Baseline CTP | NA | NA | NA | NA | 1.42 (1.10-1.84) | .007 | 1.43 (1.13-1.80) | .002 |
| Local progression of tumor | NA | NA | NA | NA | 1.33 (0.38-4.62) | .65 | NA | NA |
| Liver transplant | NA | NA | NA | NA | 0.08 (0.01-1.50) | .09 | NA | NA |
| Total dose, mean (range), Gy | 0.95 (0.85-1.07) | .43 | 0.90 (0.80-1.01) | .08 | 0.96 (0.93-0.98) | .001 | 0.96 (0.94-0.99) | .007 |
| BED | 0.98 (0.92-1.04) | .55 | NA | NA | 0.98 (0.97-0.99) | <.001 | NA | NA |
Abbreviations: BED, biologically effective dose; CTP, Child-Turcotte-Pugh; HCC, hepatocellular carcinoma; HR, hazard ratio; NA, not applicable; RT, radiation therapy.
Discussion
This large phase 2 trial of 90 patients at increased risk for liver damage after local therapy demonstrates that the strategy of biomarker-based individualized adaptive radiotherapy can be used to achieve both high rates of local control and a high degree of safety, rather than sacrificing one for the other. Standard radiation therapy relies solely on population-based toxicity models that limit the aggressiveness of therapy for 95% of patients based on the risk of toxic effects to the most sensitive 5%. In contrast, we have taken the approach of determining the radiation sensitivity of each patient’s liver during treatment, in time to modify the remainder of the course, so that treatment can be deintensified for radiation-sensitive patients. This approach may represent a new paradigm in radiation therapy in which treatment is modified according to the individual patient’s response rather than relying on population-based metrics.
Our results are favorable for a relatively unselected group of patients. In carefully selected patients with minimal liver dysfunction, local tumor control after SBRT has approached 90% in a recent study and 95% in a multi-institutional hypofractionated proton therapy study. However, local control rates have generally been suboptimal, particularly for larger tumors and those recurrent after other therapies—despite using radiation regimens associated with substantial toxic effects. Patients with preexisting liver dysfunction, particularly those with CP grade B or C cirrhosis, have had rates of radiation-induced liver disease of up to 27% and rates of CP decline of 2 or more points up to 34%, despite careful radiation treatment planning using data on population-based, dose-volume toxic effects.
Local control was not associated with improved survival on multivariable analysis, and OS at 2 years was only 36%. This is due to the late stage at which most of our patients were treated, with a median of 2 prior treatments, which exacerbates the competing risk of progressive cirrhosis and the development of additional primary tumors through field cancerization. Thus, we would view our results as proof of principle that radiation can safely control intrahepatic cancers, and we would anticipate improved survival if radiation therapy were used earlier in the course of disease. Adjuvant treatment with sorafenib after surgery or ablation has not proven successful, indicating a need for improved systemic therapies.
In this trial, we used the change in ICG clearance as a biomarker of liver function. This test has been used extensively in Asia to assess the safety of liver resections for HCC and to predict survival in critically ill patients. Other biomarkers are being evaluated for early detection of liver damage, including cytokines and microRNAs, although these are yet to be validated. In addition to biologic markers that provide a global assessment of liver function and sensitivity to radiation, imaging markers are being developed for spatial assessment of liver function. In particular, portal venous perfusion correlates with global liver function and changes in a dose-dependent manner after radiotherapy.
Limitations
This study has several limitations. First, there was slight heterogeneity of the patient population. When designing the trial, we hypothesized that patients who had previous liver-directed therapy and resulting subclinical liver damage would be at high risk for additional dysfunction caused by radiotherapy. During the course of the trial, it became apparent that patients with metastatic tumors were at lower risk than patients with primary liver tumors and/or cirrhosis. Thus, data are presented for all patients as well as specifically those with HCC. In addition, although this is a large phase 2 trial, it has the limitation of being a single-arm, single-institution study.
Besides careful treatment planning and image-guided delivery, another potential way to further minimize liver toxic effects is to deintensify treatment for selected patients, yet not sacrifice local control. Early changes in arterial perfusion on dynamic contrast-enhanced MRI have been demonstrated to predict for tumor control, while the spatial map of portal venous perfusion parallels the distribution of liver function. It is likely that a subset of patients in the trial described herein had radiosensitive tumors that could have been controlled with lower doses of radiation. Determining the minimum dose required for tumor control is a current focus of our research; in our new clinical trial (NCT02460835), patients who have a complete response after three-fifths of planned therapy are spared additional treatment and thus spared additional subclinical liver damage. Radiotherapy is also adapted based on the spatial distribution of liver function as determined with portal venous perfusion dynamic contrast-enhanced MRI, so that high-functioning portions of the liver are preferentially spared from radiation, maximizing the functional reserve. Thus, treatment is being adapted based on each patient’s change in viable tumor as well as liver function to simultaneously deintensify therapy for patients who are predicted to respond, intensify therapy for patients who have more refractory tumors, and maximize posttherapy liver function, reserving function for future interventions.
Conclusions
This study demonstrates that biomarker-based individualized adaptive radiotherapy can be used to achieve both high rates of control and safety in patients with liver tumors.
Clinical Trial Protocol
eMethods. Description of radiation dose adaptation.
eFigure 1. Tumor dose, with the first part of treatment in blue and the second part of treatment in gold.
eFigure 2. Predicted and observed change in liver function for all patients after treatment adaptation.
References
- 1.Theise N. Liver cancer. In: Stewart BW, Wild CP, ed. World Cancer Report 2014 Geneva, Switzerland: World Health Organization; 2014: 576-592. [Google Scholar]
- 2.Guha C, Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol. 2011;21(4):256-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson LA, Brock KK, Kazanjian S, et al. . The reproducibility of organ position using active breathing control (ABC) during liver radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51(5):1410-1421. [DOI] [PubMed] [Google Scholar]
- 4.Méndez Romero A, Wunderink W, Hussain SM, et al. . Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase I-II study. Acta Oncol. 2006;45(7):831-837. [DOI] [PubMed] [Google Scholar]
- 5.Cárdenes HR, Price TR, Perkins SM, et al. . Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12(3):218-225. [DOI] [PubMed] [Google Scholar]
- 6.Culleton S, Jiang H, Haddad CR, et al. . Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014;111(3):412-417. [DOI] [PubMed] [Google Scholar]
- 7.Stenmark MH, Cao Y, Wang H, et al. . Estimating functional liver reserve following hepatic irradiation: adaptive normal tissue response models. Radiother Oncol. 2014;111(3):418-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakka SG, Reinhart K, Meier-Hellmann A. Prognostic value of the indocyanine green plasma disappearance rate in critically ill patients. Chest. 2002;122(5):1715-1720. [DOI] [PubMed] [Google Scholar]
- 9.Normolle D, Pan C, Ben-Josef E, Lawrence T. Adaptive trial of personalized radiotherapy for intrahepatic cancer. Per Med. 2010;7(2):197-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12(1):16-22. [DOI] [PubMed] [Google Scholar]
- 11.Wahl DR, Stenmark MH, Tao Y, et al. . Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34(5):452-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu E, Stenmark MH, Lee OE, et al. . SBRT as an alternative to RFA for the treatment of primary and metastatic liver tumors. J Clin Oncol. 2012;30:158. doi: 10.1200/jco.2012.30.4_suppl.15822162585 [DOI] [Google Scholar]
- 13.Roberson PL, McLaughlin PW, Narayana V, Troyer S, Hixson GV, Kessler ML. Use and uncertainties of mutual information for computed tomography/magnetic resonance (CT/MR) registration post permanent implant of the prostate. Med Phys. 2005;32(2):473-482. [DOI] [PubMed] [Google Scholar]
- 14.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53(4):810-821. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 16.Lin DY, Wei J. The robust inference for the cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074-1078. [Google Scholar]
- 17.Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med. 1994;13(21):2233-2247. [DOI] [PubMed] [Google Scholar]
- 18.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer Science & Business Media; 2013. [Google Scholar]
- 19.Bujold A, Massey CA, Kim JJ, et al. . Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31(13):1631-1639. [DOI] [PubMed] [Google Scholar]
- 20.Hong TS, Wo JY, Yeap BY, et al. . Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016;34(5):460-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tse RV, Hawkins M, Lockwood G, et al. . Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26(4):657-664. [DOI] [PubMed] [Google Scholar]
- 22.Ibarra RA, Rojas D, Snyder L, et al. . Multicenter results of stereotactic body radiotherapy (SBRT) for non-resectable primary liver tumors. Acta Oncol. 2012;51(5):575-583. [DOI] [PubMed] [Google Scholar]
- 23.Abdalla EK, Vauthey JN, Ellis LM, et al. . Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang WY, Jen YM, Lee MS, et al. . Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84(2):355-361. [DOI] [PubMed] [Google Scholar]
- 25.Bruix J, Takayama T, Mazzaferro V, et al. ; STORM Investigators . Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344-1354. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto Y, Ikoma H, Morimura R, et al. . Clinical analysis of anatomical resection for the treatment of hepatocellular carcinoma based on the stratification of liver function. World J Surg. 2014;38(5):1154-1163. [DOI] [PubMed] [Google Scholar]
- 27.Cuneo KC, Davis MA, Schipper M, et al. . High-serum HGF and low-serum CDL40 are associated with liver toxicity after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93:S114. [Google Scholar]
- 28.Cuneo KC, Sun Y, Schipper MJ, et al. . The use of plasma microRNAs to predict toxicity following liver stereotactic body radiation therapy. J Clin Oncol. 2016;34(5)(suppl):245. doi: 10.1200/jco.2016.34.4_suppl.245 [DOI] [Google Scholar]
- 29.Dreher C, Høyer KI, Fode MM, Habermehl D, Combs SE, Høyer M. Metabolic liver function after stereotactic body radiation therapy for hepatocellular carcinoma. Acta Oncol. 2016;55(7):886-891. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, Wang H, Johnson TD, et al. . Prediction of liver function by using magnetic resonance-based portal venous perfusion imaging. Int J Radiat Oncol Biol Phys. 2013;85(1):258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanuki N, Takeda A, Oku Y, et al. . Threshold doses for focal liver reaction after stereotactic ablative body radiation therapy for small hepatocellular carcinoma depend on liver function: evaluation on magnetic resonance imaging with Gd-EOB-DTPA. Int J Radiat Oncol Biol Phys. 2014;88(2):306-311. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Farjam R, Feng M, et al. . Arterial perfusion imaging-defined subvolume of intrahepatic cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical Trial Protocol
eMethods. Description of radiation dose adaptation.
eFigure 1. Tumor dose, with the first part of treatment in blue and the second part of treatment in gold.
eFigure 2. Predicted and observed change in liver function for all patients after treatment adaptation.