Abstract
To investigate the phenotypic evolution of West Nile virus (WNV) in California, we competed sixteen isolates made during 2007-08 against COAV997-5nt, a genetically marked clone from the founding 2003 California isolate COAV997-2003. Using in vivo fitness competitions in House Finches (HOFI) and Culex tarsalis mosquitoes, we found that the majority of WNV WN02 and SW03 genotype isolates exhibited elevated replicative fitness in both hosts compared to COAV997-5nt. Increased replicative capacity in HOFIs was not associated with increased mortality, indicating that these isolates had not gained avian virulence. One WN02 isolate from Coachella Valley, a region geographically close to the isolation of COAV997, showed neutral fitness in HOFIs and reduced fitness in Cx. tarsalis. Two isolates from Kern County and Sacramento/Yolo County out-competed COAV997-nt in HOFIs, but were transmitted less efficiently by Cx. tarsalis. Competition demonstrated neutral or increased fitness that appeared independent of both WN02 and SW03 genotypes.
Keywords: West Nile virus, Competitive fitness, Invasion, Evolution, California, Genotype, Phenotype
Introduction
West Nile virus (WNV; Flavivirus, Flaviviridae) was first detected in California when infectious virus was isolated from a Culex tarsalis Coquillett mosquito pool (COAV997-2003)collected in Imperial County in 2003 (Reisen, Lothrop et al., 2004). In subsequent years, WNV became endemic, with recurring outbreaks of disease in humans and equines throughout the state (Hom, Marcus et al., 2005; Hom, Bonilla et al., 2006). An enzootic transmission cycle between passeriform birds and ornithophagic Culex mosquitoes was shown to maintain WNV in nature, with transmission peaking during the warm summer and fall months. Sequences of WNV isolates made from mosquitoes collected from throughout California indicated that all viruses contained the E-V159A mutation defining the WN02 genotype (Duggal, Reisen et al., 2015). In addition, some isolates also had the NS4A-A85T mutation defining the SW03 clade. Based on phylodynamic studies of time-stamped genomes, WN02 apparently invaded California once during 2002.3 (time interval range 2001.9 – 2002.7) (Duggal, Reisen et al., 2015), agreeing with previous estimates (Pybus, Suchard et al., 2012). Subsequently, there were three introductions of the SW03 clade between 2003.3 and 2006, and by 2008 both genotypes were co-circulating throughout California (Duggal, Reisen et al., 2015). Emergence of an additional SW03 clade was evident from mutation at the NS4B locus from a WN02 virus from the CA-introduced clade (Fig. 1).
Figure 1. Phylogenetic comparison of WNV strains.
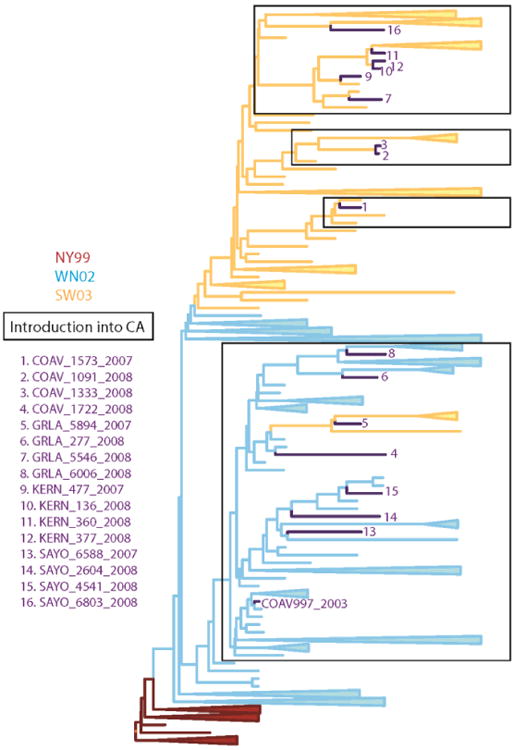
Phylogenetic relationships among WNV isolates used as competition strains. A MCC phylogeny constructed from the open reading frame of 112 WNV isolates is shown. The two emergent genotypes of WNV are indicated on the phylogeny by color: WN02 in blue lines and SW03 in orange lines. The 16 WN02 and SW03 competing strains are numbered and indicated by strain designation (See Table 1 for details).
What remains unclear is how the establishment of different genotypes of WNV in the varied biomes of California and their persistence during winter has altered the replicative phenotypes of the genetic variants detected through sequencing studies. The distribution of WNV in California encompasses very diverse biomes, including hot and dry desert habitats in the southeast, cool maritime locales in the Los Angeles basin and coastal regions, mixed agricultural areas in the southern San Joaquin Valley, and comparatively wet conditions in the northern Sacramento Valley rice production area. Enzootic transmission cycles in these regions differ by vector and host species composition, with Cx. tarsalis being the primary vector in rural areas and Culex pipiens quinquefasciatus Say and Culex pipiens intergrades predominating in urban habitats in the south and north, respectively (Kothera, Nelms et al., 2013). American crows (Corvus brachyrhynchos) are important amplification hosts in the Los Angeles and Sacramento areas, but not in Coachella Valley and Kern County where they are less abundant (http://www.mbr-pwrc.usgs.gov/bbs/ra2013/ra04880.htm).
The current study was originally designed to determine if the phenotype of the invading WN02 genotype, as defined by competitive fitness, changed over a five year period as the virus dispersed and adapted to different hosts and vectors in California. However, subsequent sequencing studies revealed repeated invasions by the SW03 genotype, emergence of the SW03 variant from the CA-introduced WN02 genotype, and dispersal by both the WN02 and SW03 genotypes from southern to northern California (Duggal et al. 2015), confounding our adaptive hypothesis and resulting in the current investigation describing how different isolates from different biomes varied in competitive fitness compared to the founding virus from Imperial Valley. Although the evolution of WNV can be tracked by sequencing studies, these documented genetic changes may not necessarily be linked directly to infection phenotype, virulence or epidemic potential.
To study changes in the phenotype of WNV, we previously developed an in vivo fitness competition model that compared the concurrent competitive replication of two viruses within the same host (Worwa, Wheeler et al., 2015). We selected the House Finch (Haemorhous mexicanus [Muller], HOFI) and the mosquito Cx. tarsalis as experimental hosts, because they were considered to be moderately susceptible to WNV infection (Reisen, Fang et al., 2005) and should therefore best express differences in viral fitness. An infectious clone derived from the California founding isolate, COAV997-2003, was genetically marked with five synonymous nucleotide substitutions in the envelope gene (COAV997-5nt), and an allele-specific qRT-PCR assay was developed to quantify viral RNA from samples containing both wildtype WNV and COAV997-5nt viruses (Worwa, Andradeet al., 2014). COAV997-5nt retained equal fitness compared to COAV997 in both in vitro and in vivo evaluations in single and dual infections (Worwa, Andrade et al., 2014; Worwa, Wheeler et al.,2015). To determine whether WNV had changed phenotypically over time and space, we characterized the fitness of sixteen WNV isolates made during 2007 and 2008 by competing them against COAV997-5nt in HOFIs and Cx. tarsalis.
Results and Discussion
Genetic characterization of viral strains
Complete genome sequences of all 16 WNV isolates were determined and the consensus sequences compared to the COAV997-2003 strain (Supplemental Table 1). All viruses contained the NS3-249 mutation imparting elevated virulence in American crows (Brault, Huang et al., 2007) and the E-V159A mutation defining the WN02 genotype (Davis, Ebel et al., 2005). The NS4A-F92L mutation associated with the temperature-sensitive phenotype of COAV997 (Andrade, Maharaj et al., 2011) was not retained in any of the wildtype isolates, which was expected because elevated temperatures such as those recorded in febrile American crows have been shown in vitro to preclude replication (Andrade, Maharaj et al., 2011). Based on the presence of the NS4A-A85T mutation that defines the SW03 genotype (McMullen, May et al., 2011), ten of the viruses were considered to be the SW03 genotype and six were the WN02 genotype (Table 1). Therefore, ten fitness evaluations compared the founding WN02 strain in California to the SW03 strain, and six evaluations compared the founding WN02 strain to WN02 viruses collected five years after adapting and dispersing in California. The phylogenetic relatedness of these 16 isolates based on sequence data is shown in Fig. 1 and discussed within a broader genetic context in Duggal, Reisen et al. (2015).
Table 1.
Mosquito pools selected from California study sites for in vivo fitness competitions.
| Isolate | Region | Collection date | Mosquito species | Ct valuea | Titerb | Ratioc | NCBI GenBank accession no. |
|---|---|---|---|---|---|---|---|
| COAV-07-1573 | Mecca, Riverside County, CA, USA | 09/18/07 | Cx. tarsalis | 28.0 | 8.71 | 0.15 | KR348934 |
| COAV-08-1091 | Mecca, Riverside County, CA, USA | 06/12/08 | Cx. tarsalis | 23.1 | 8.52 | 0.17 | KR348926 |
| COAV-08-1333 | Mecca, Riverside County, CA, USA | 07/10/08 | Cx. tarsalis | 22.8 | 8.87 | 0.67 | KR348929 |
| COAV-08-1722 | Palm Springs, Riverside County, CA, USA | 09/25/08 | Cx. quinquefasciatus | 29.2 | 9.08 | 0.14 | KR348936 |
| GRLA-07-5894 | Encino, Los Angeles County, CA, USA | 09/18/07 | Cx. quinquefasciatus | 23.4 | 8.65 | 0.20 | KR348947 |
| GRLA-08-277 | Downey, Los Angeles County, CA, USA | 05/22/08 | Cx. quinquefasciatus | 28.5 | 8.70 | 0.22 | KR348950 |
| GRLA-08-5546 | Van Nuys, Los Angeles County, CA, USA | 07/24/08 | Cx. quinquefasciatus | 24.4 | 8.36 | 0.08 | KR348946 |
| GRLA-08-6006 | Los Angeles, Los Angeles County, CA, USA | 09/25/08 | Cx. quinquefasciatus | 25.8 | 8.95 | 0.17 | KR348953 |
| KERN-07-477 | Bakersfield, Kern County, CA, USA | 09/14/07 | Cx. quinquefasciatus | 24.4 | 9.05 | 0.37 | KR348991 |
| KERN-08-136 | Bakersfield, Kern County, CA, USA | 06/27/08 | Cx. quinquefasciatus | 24.2 | 8.80 | 0.26 | KR348978 |
| KERN-08-360 | Lamont, Kern County, CA, USA | 09/10/08 | Cx. tarsalis | 26.2 | 8.88 | 0.23 | KR348987 |
| KERN-08-377 | Bakersfield, Kern County, CA, USA | 09/12/08 | Cx. quinquefasciatus | 34.4 | 8.69 | 0.30 | KR348988 |
| SAYO-07-6588 | Wilton, Sacramento County, CA, USA | 09/25/07 | Cx. tarsalis | 28.8 | 8.94 | 0.27 | KR349013 |
| SAYO-08-2604 | Sacramento, Sacramento County, CA, USA | 06/27/08 | Cx. pipiens | 31.3 | 8.96 | 0.29 | KR348998 |
| SAYO-08-4541 | Courtland, Sacramento County, CA, USA | 07/25/08 | Cx. tarsalis | 31.2 | 8.99 | 0.21 | KR349000 |
| SAYO-08-6803 | Woodland, Yolo County, CA, USA | 08/26/08 | Cx. tarsalis | 33.2 | 8.76 | 0.22 | KR349014 |
Cycle threshold (Ct) value of the mosquito pool tested by qRT-PCR at initial detection.
Infectious virus titer (log10 PFU/mL) after propagation of the mosquito pool homogenate in Vero cell culture.
Ratio refers to the RNA copy-to-PFU ratio of stock virus.
The place and date of virus isolation, the mosquito host, the Ct value [index of RNA copies], titer at first passage on Vero cell culture, and the RNA copy to plaque forming unit ratio are shown in Table 1. The RNAcopy-to-PFU ratio of each virus stock varied markedly, ranging between 0.08 and 0.67 (Table 1). In contrast, the RNA copyto-PFU ratio for COAV997-5nt was 0.004, and therefore considerably greater indicating the generation of a greaternumber of non-infectious RNA copies for this virus. To correct for this bias in RNA copy generation, we determined the ratio of RNA copies from COAV997-5nt and wildtype viruses for all subsequent bird inocula and mosquito blood meals (Tables 3 and 4) and adjusted the RNA copy numbers from the competition samples accordingly, as describedpreviously (Worwa, Wheeler et al., 2015).
Table 3.
Titers, RNA copy numbers and copy number ratios of HOFI inocula.
| Isolate | Birds (n) | Titer (log10 PFU/mL) a | RNA copy wildtype b | RNA copy COAV997-5nt b | RNA ratio c |
|---|---|---|---|---|---|
| COAV-07-1573 | 6 | 3.27±0.14 | 604±597 | 6088±139 | 0.099 |
| COAV-08-1091 | 7 | 3.58±0.35 | 986±245 | 8530±161 | 0.115 |
| COAV-08-1333 | 6 | 2.96±0.38 | 66±85 | 2520±303 | 0.026 |
| COAV-08-1722 | 7 | 3.24±0.21 | 288±0 | 3866±224 | 0.074 |
| GRLA-07-5894 | 6 | 3.40±0.10 | 135±0 | 2660±281 | 0.050 |
| GRLA-08-277 | 6 | 3.75±0.11 | 398±0 | 4187±833 | 0.095 |
| GRLA-08-5546 | 6 | 3.94±0.06 | 2212±1 | 8644±678 | 0.255 |
| GRLA-08-6006 | 6 | 3.34±0.08 | 321±0 | 2912±271 | 0.110 |
| KERN-07-477 | 6 | 3.89±0.43 | 562±219 | 2502±392 | 0.224 |
| KERN-08-136 | 6 | 3.93±0.38 | 1112±0 | 2089±395 | 0.532 |
| KERN-08-360 | 6 | 3.87±0.36 | 468±0 | 2305±337 | 0.203 |
| KERN-08-377 | 6 | 3.95±0.45 | 514±116 | 1075±1444 | 0.478 |
| SAYO-07-6588 | 6 | 4.10±0.57 | 212±0 | 2511±243 | 0.084 |
| SAYO-08-2604 | 6 | 3.94±0.92 | 274±130 | 7493±3789 | 0.036 |
| SAYO-08-4541 | 6 | 3.91±0.61 | 1281±0 | 3864±2483 | 0.331 |
| SAYO-08-6803 | 6 | 3.98±0.42 | 1048±0 | 2893±817 | 0.362 |
| Mock-infected | 13 | 0 | 0 | 0 | N/A |
The number of HOFIs (n) inoculated per group is presented including titers of inocula measured by plaque assay on Vero cell culture and shown as log10 PFU/mL of the mean of duplicate assays including standard deviation.
RNA copy numbers quantified by qRT-PCR are listed as the mean of duplicates including standard deviation.
RNA ratios represent the relative difference of input RNA of the wildtype virus compared to COAV997-5nt within each inoculum.
Table 4.
Cx. tarsalis infectious titers, RNA copy numbers and copy number ratios in mosquito bodies immediately following blood meal ingestion.
| Isolate | Females (n) b | Titer (log10 PFU/mL) a | RNA copy wildtype b | RNA copy COAV997-5nt b | RNA ratio c |
|---|---|---|---|---|---|
| COAV-07-1573 | 50 | 4.70±0.16 | 145±66 | 1675±455 | 0.086 |
| COAV-08-1091 | 50 | 5.19±0.12 | 509±158 | 8930±1378 | 0.056 |
| COAV-08-1333 | 41 | 4.77±0.12 | 23±17 | 2215±678 | 0.010 |
| COAV-08-1722 | 50 | 4.93±0.15 | 8703±1783 | 68497±10056 | 0.127 |
| GRLA-07-5894 | 32 | 5.32±0.22 | 9972±1935 | 64659±6394 | 0.154 |
| GRLA-08-277 | 50 | 5.47±0.13 | 10646±3440 | 72059±13236 | 0.147 |
| GRLA-08-5546 | 50 | 5.40±0.08 | 21494±2764 | 51030±10264 | 0.421 |
| GRLA-08-6006 | 31 | 5.33±0.27 | 15588±3565 | 73228±11090 | 0.212 |
| KERN-07-477 | 57 | 5.09±0.23 | 725±272 | 7964±2402 | 0.091 |
| KERN-08-136 | 40 | 5.20±0.18 | 288±168 | 6831±2387 | 0.042 |
| KERN-08-360 | 42 | 4.93±0.13 | 7580±2845 | 65470±13639 | 0.115 |
| KERN-08-377 | 50 | 4.86±0.12 | 6291±2932 | 52264±17694 | 0.120 |
| SAYO-07-6588 | 50 | 4.83±0.12 | 452±115 | 11312±1396 | 0.039 |
| SAYO-08-2604 | 32 | 5.10±0.22 | 168±54 | 9707±1702 | 0.017 |
| SAYO-08-4541 | 32 (+50) d | 4.87±0.16 | 561±291 | 10303±1660 | 0.054 |
| SAYO-08-6803 | 32 (+17) d | 4.83±0.34 | 285±146 | 8063±4249 | 0.035 |
| Mock-infected | 21 | 0 | 0 | 0 | N/A |
The homogenates of five fully engorged females collected immediately following blood meal ingestion from each group were titered individually by plaque assay and titers presented in log10 PFU/mL as means including standard deviation.
The RNA copy numbers in the homogenates of five fully engorged females collected immediately following blood meal ingestion were measured in duplicate by qRT-PCR and listed for each wildtype isolate and COAV997-5nt.
The RNA ratio represents the relative input of RNA copies from each wildtype virus compared to COAV997-5nt.
For isolates SAYO-08-4541 and SAYO-08-6803 additional mosquitoes (numbers presented in parenthesis) were frozen at experiment termination on 14 dpi and later analyzed to obtain additional infection data.
Recent WNV isolates demonstrate greater competitive fitness in HOFIs
The peak and duration of elevated viremia is a biologically relevant metric for viral fitness, because viral titers in infected avian hosts must reach or exceed levels that effectively infect mosquitoes for transmission to continue (Komar, Langevin et al., 2003; Reisen, Fang et al., 2005). HOFI were dual-inoculated with a mixture of a wildtype WNV isolate and COAV997-nt marked virus. The RNA copy numbers for wildtype and COAV997-5nt viruses in HOFI sera collected between 1 and 7 days post infection (dpi) are presented in Figure 2. Additionally, the relative abundance of viral RNA from wildtype or COAV997-5nt viruses was presented as a percentage of the total virus population present on any given day (Fig. 2). On 1 dpi there were no statistically significant differences in RNA copies between COAV997-5nt and any of the wildtype isolates. Between 2 and 7 dpi all WNV WN02 and SW03 isolates from GRLA, KERN and SAYO demonstrated either neutral or significantly higher competitive fitness in HOFIs compared to COAV997-5nt. Viremias of all wildtype isolates peaked on 3 dpi, except for WN02 isolate COAV-08-1722 that peaked on 4 dpi, concurrent with COAV997-5nt. At peak viremia, higher RNA copy numbers from wildtype isolates were present compared to COAV997-5nt, except for COAV-08-1091 which showed equal replication efficiency on 3 dpi (Fig.2, Table 2). Although isolates COAV-07-1573, COAV-08-1333 and SAYO-08-6803 replicated significantly better in HOFIs than COAV997-5nt early in the infection, their RNA copy numbers decreased on 6 dpi to non-significantly different levels compared to COAV997-5nt (Table 2). Interestingly, isolate COAV-08-1091 of the SW03 clade exhibited neutral replication to COAV997-5nt during 2, 3 and 4 dpi, but ultimately “lost” the competition to COAV997-5nt on 5, 6 and 7 dpi (Fig. 2). By the end of the competitions on 7 dpi, RNA from COAV997-5nt was not detectable in many of the birds that previously showed better replication of wildtype isolates. However, although replicating equally on 3 dpi, isolate COAV-08-1091 disappeared by 6 dpi with only COAV997-5nt RNA being detected on 7 dpi. Similarly, isolate COAV-08-1333 demonstrated equal replication to COAV997-5nt by the end of the competition (Fig 2). This may indicate some degree of attenuation of these isolates compared to contemporary strains that outcompeted COAV997-5nt. These data generally agreed with host competence studies in house sparrows (HOSP; Passer domesticus) that showed recent isolates of WNV exhibited increased viremia and host competence (Duggal, Bosco-Lauth et al., 2014).
Figure 2. RNA copy numbers from fitness competitions in HOFIs.
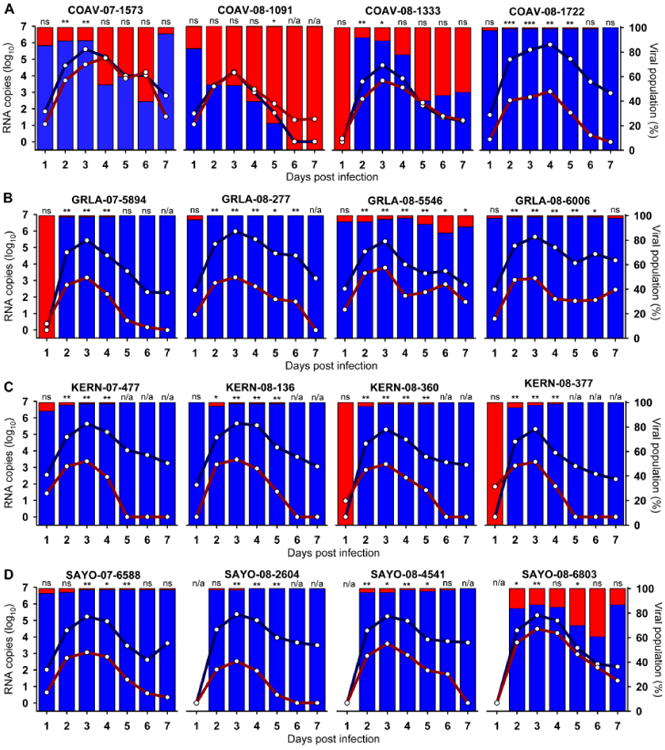
Serum samples from all HOFIs were analyzed by qRT-PCR for the presence of RNA from wildtype or COAV997-5nt viruses. The number of RNA copies for each virus is presented as the arithmetic mean of all birds per group (Table 3) between1 and 7 dpi (x-axis). Lines represent mean viral RNA copy number as log10 RNA copies (left y-axis), and bars show the percentage of the viral population (right y-axis) present on each day for wildtype isolates (blue color) and COAV997-5nt (red color). Panels show results for isolates from COAV (A), GRLA (B), KERN (C) and SAYO (D) study sites.
Table 2.
Relative fitness of WNV isolates after in vivo competition in HOFIs and Cx. tarsalis.
| Isolate | HOFI | Cx. tarsalis | |||
|---|---|---|---|---|---|
|
| |||||
| RNA 3 dpi | RNA 6 dpi | RNA body | Infection | Transmission | |
| COAV-07-1573 | wildtype** a | neutral (ns) | wildtype*** | wildtype (1.41) b | wildtype (3.16) |
| COAV-08-1091 | neutral (ns) | COAV997-5nt | COAV997-5nt * | wildtype (1.05) | COAV997-5nt (0.61) |
| COAV-08-1333 | wildtype * | neutral (ns) | wildtype *** | wildtype (1.24) | wildtype (1.40) |
| COAV-08-1722 | wildtype *** | neutral (ns) | wildtype *** | wildtype (1.17) | wildtype (1.41) |
| GRLA-07-5894 | wildtype ** | neutral (ns) | neutral (ns) | wildtype (1.33) | neutral (1.00) |
| GRLA-08-277 | wildtype ** | wildtype ** | wildtype ** | wildtype (1.23) | wildtype (1.83) |
| GRLA-08-5546 | wildtype ** | wildtype * | neutral (ns) | wildtype (1.62) | wildtype (1.69) |
| GRLA-08-6006 | wildtype ** | wildtype * | neutral (ns) | wildtype (1.30) | wildtype (1.14) |
| KERN-07-477 | wildtype ** | wildtype | wildtype *** | wildtype (1.12) | wildtype (1.40) |
| KERN-08-136 | wildtype ** | neutral (ns) | wildtype *** | wildtype (1.33) | wildtype (1.12) |
| KERN-08-360 | wildtype ** | wildtype | neutral (ns) | neutral (1.00) | COAV997-5nt (0.57) |
| KERN-08-377 | wildtype ** | wildtype | wildtype *** | wildtype (1.83) | wildtype (2.36) |
| SAYO-07-6588 | wildtype ** | neutral (ns) | neutral (ns) | wildtype (1.61) | COAV997-5nt (0.77) |
| SAYO-08-2604 | wildtype ** | wildtype | n/a | wildtype (1.50) | wildtype (3.00) |
| SAYO-08-4541 | wildtype * | neutral (ns) | wildtype *** | wildtype (1.09) | wildtype (2.00) |
| SAYO-08-6803 | wildtype ** | neutral (ns) | neutral (ns) | wildtype (2.50) | wildtype (2.25) |
Statistical significance inferred from RNA copy differences in HOFI sera and mosquito bodies is indicated either as significant (*, P<0.05), very significant (**, P<0.01), extremely significant (***, P<0.001) or not significant (ns, P>0.05).
An additional measure of fitness was derived from mosquito infection and transmission rates, by dividing the number of bodies and expectorants positive for wildtype-RNA by those positive for COAV997-5nt, respectively, and fitness determined as either neutral (1.00), wildtype (>1.00) or COAV997-5nt (<1.00).
Blue and red color highlight either the wildtype isolate or the marked founding California strain, respectively, as winners of the fitness competition, while green color indicates neutral fitness.
Mortality in birds may be associated with WNV virulence due to increased viral replication (Brault, Langevin et al., 2004; Langevin, Brault et al., 2005) and thus impact viral fitness. Previously, we determined that the mortality rate for COAV997-5nt in feral HOFIs from California was less than 17% in single and dual infections (Worwa, Wheeler et al., 2015). Most wildtype WNV isolates in the current study did not cause mortality in HOFIs, similar to COAV997-5nt (Fig. 3). Therefore, although these WNV isolates demonstrated increased replicative fitness in HOFIs compared to COAV997-5nt in dual infections (Fig. 2), virulence as indicated by increased mortality did not appear to be concurrently elevated. These results differed somewhat from recent studies using the HOSP as the experimental host (Duggal, Bosco-Lauth et al., 2014), where recent WNV strains showed high peak viremia and mortality. Interestingly, competition with isolate WN02 GRLA-08-277 resulted in a markedly elevated mortality rate of 67%, with one bird from this group developing overt neurological signs of WNV disease on 11 dpi. When analyzing this HOFI's brain homogenate by qRT-PCR, we found over 6 log10 of RNA copies of isolate GRLA-08-277 and no viral RNA for COAV997-5nt. The GRLA-08-277 isolate was collected in the spring of 2008 in Los Angeles prior to a major WNV outbreak of human disease later that year (Kwan, Kluh et al., 2010), possibly indicating that isolates from outbreaks might be associated with elevated avian virulence in addition to increased replicative fitness.
Figure 3. HOFI mortality rates.
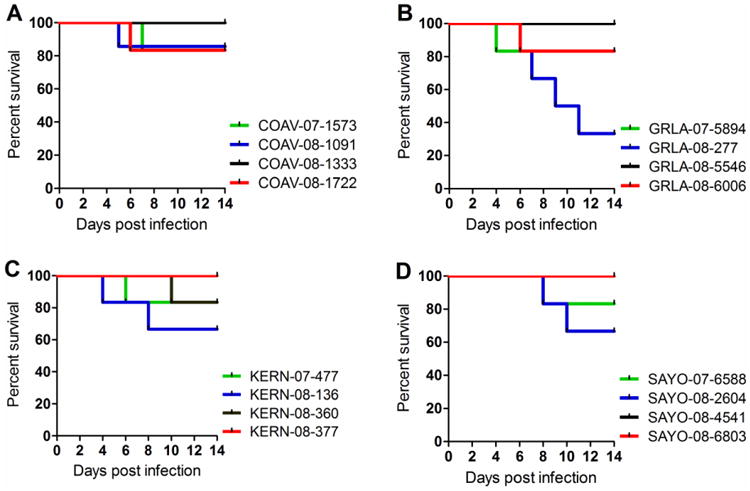
The percent survival (y-axis) is presented over the 7-day course of the fitness competition (x-axis) per experimental group for isolates from COAV (A), GRLA (B), KERN (C) and SAYO (D).
Neutral or elevated replication of recent isolates in Culex tarsalis
To be transmitted effectively by mosquitoes, viruses must infect and replicate in multiple tissues and then be expectorated during the imbibing of subsequent blood meals (Hardy, Houk et al., 1983). Culex tarsalis were fed a bloodmeal containing a mixture of a recent wildtype WNV isolate and the COAV997-5nt marked virus. RNA copy numbers in Cx. tarsalis bodies were assessed at 14 dpi using qRT-PCR, the amount of RNA from wildtype isolates and COAV997-5nt statistically compared (Fig. 4), and the ‘competition winner’ listed in Table 2. Isolate COAV-08-1091 of the SW03 clade was the only isolate that infected and replicated less efficiently compared to COAV997-5nt. Isolates GRLA-07-5894, GRLA-08-5546, GRLA-08-6006, KERN-08-360, SAYO-07-6588 and SAYO-08-6803 from both WN02 and SW03 lineages replicated to equal levels compared to COAV997-5nt, and therefore showed neutral fitness (Fig. 4, Table 2). All other wildtype isolates consisting of both WN02 and SW03 lineages infected and replicated to significantly greater levels of wildtype RNA than COAV997-5nt. There were not sufficient mosquitoes infected with isolate WN02 SAYO-08-2604 to allow statistical comparison.
Figure 4. RNA copy numbers in Cx. tarsalis bodies.
Upon completion of the fitness competitions on 14 dpi, the RNA copy numbers of wildtype and COAV997-5nt viruses were determined by qRT-PCR in the bodies of all surviving females from each isolate (A-D). Dots represent the number of RNA copies as log10 (y-axis) detected in each individual mosquito body positive for wildtype isolates (blue color) and COAV997-5nt (red color) and include results from singly and dually infected mosquitoes. Bars indicate the means and standard deviation and means were compared using Wilcoxon Rank Sum test with statistical significance set to P<0.05.
To assess transmission rates following infection competition in Cx. tarsalis, viral RNA was measured in the expectorants of mosquitoes that had detectable WNV RNA present in their bodies at 14 dpi (Fig. 5). Both wildtype and COAV997-5nt viruses were found in mosquito bodies and expectorants from each group, suggesting that, unlike infections in HOFI, a complete displacement of one of the viruses had not occurred during infection and subsequent dissemination from the midgut to the salivary glands (Fig. 5). Therefore, to determine relative fitness based on infection, the ratio between RNA from wildtype isolates and COAV997-5nt in bodies and expectorants (Table 2) was compared to the ratio of RNA in the blood meals used to infect Cx. tarsalis (Table 4). With a ratio of 1, the isolate SW03 KERN-08-360 was neutrally fit compared to COAV997-5nt based on equal numbers of bodies containing KERN-08-360 and COAV997-5nt viral RNA. For all other groups, the wildtype viruses infected proportionately more mosquitoes, resulting in a ratio greater than 1. Virus-specific infection rates for wildtype or COAV997-5nt viruses were summarized in Fig. 5. Surprisingly, the number of RNA copies found in the mosquito bodies (Fig. 4) did not always agree with the infection rate (or proportion of mosquitoes infected) (Fig. 5); e.g., isolate COAV-08-1091 replicated to lower RNA levels (Fig. 4), but infected a greater number of mosquitoes compared to COAV997-5nt (Fig. 5). Therefore, RNA concentration in the mosquito and the frequency of infection among mosquitoes may represent two different measures of fitness in the vector that should be evaluated separately. We observed lower infection frequencies for mosquitoes infected with SAYO 2008 isolates, especially isolate WN02 SAYO-08-2604. Interestingly, this low infectivity was seen with both wildtype and COAV997-5nt viruses.
Figure 5. Detection of viral RNA in Cx. tarsalis expectorants.

Expectorants from mosquitoes whose bodies tested positive for viral RNA (Fig. 4) were analyzed by qRT-PCR for the presence of viral RNA in the original diluted expectorant (Exp. w/o Vero) and in the supernatant following one passage in Vero cells (Exp. w Vero). The height of the bars shows the total number of expectorants (n) that tested positive for RNA from either wildtype and/or COAV997-5nt viruses. Colors within the bars indicate the number of expectorants that tested positive for wildtype isolates (blue) and COAV997-5nt (red) and include data from singly and dually infected mosquitoes. For reference, the number of mosquito bodies positive for viral RNA was included for each competition. Panels group isolates from COAV (A), GRLA (B), KERN (C) and SAYO (D).
Culex tarsalis predominately transmits recent WNV isolates
Viral RNA in mosquito expectorants was quantified by qRT-PCR directly from the originalmaterial and from Vero cell culture supernatant after one passage (Fig. 5). Passage of thetransmitted viruses did not markedly change the ratio of the abundance of these viruses comparedto the unpassaged expectorant (Fig. 5), but increased the number of positive expectorantsamplesconsiderably as few viral particles were expectorated by many females. We again determined the ratio between RNA from wildtype and COAV997-5nt viruses in the cell culture supernatant of passaged expectorants to assess competitive fitness among the viruses based on transmission (Table 2). Additionally, the percentage of dually and singly infected mosquitoes as well as virus-specific transmission rates were summarized in Fig. 6. SW03 isolates COAV-08-1091, KERN-08-360 and SAYO-07-6588 presented reduced fitness with ratios of 0.61, 0.57 and 0.77, respectively, indicating decreased transmission of these viruses compared to WN02 COAV997-5nt. Isolate SW03 GRLA-07-5894 demonstrated equal fitness compared to COAV997-5nt, with a ratio of 1 and a transmission rate of 71% for both viruses (Fig. 5). In contrast, all other wildtype viruses of both WN02 and SW03 lineages were transmitted more frequently than COAV997-5nt (Table 2, Fig. 5). Similar to Cx. tarsalis body infections, both viruses appeared in the expectorants of each group, yet in varying concentrations. Finally, the transmission rate agreed better with RNA levels found in mosquito bodies than with the body infection frequency data, likely because viruses with high concentrations in the bodies were transmitted more frequently (Table 2).
Figure 6. Infection and transmission rates of singly and dually infected Cx. tarsalis.
Total infection rates (green bars) were calculated based on the number of all bodies that tested positive by qRT-PCR over all blood fed mosquitoes surviving to 14 dpi (n). Similarly, total transmission rates (green bars) were determined based on expectorants with detectable viral RNA over all qRT-PCR positive bodies. Additionally, total infection and transmission rates were split to reflect single (green dotted bars) and dual (green striped bars) infections. Virus-specific infection and transmission rates are shown for wildtype isolates (blue bars) and COAV997-5nt (red bars) determined from all qRT-PCR positive bodies and expectorants, respectively. Transmission results were derived from expectorants passaged in Vero cells.
After analyzing infections and transmissions as a sum of all infected mosquito bodies and expectorant samples from each group, we then examined the frequency of singly and dually infected mosquitoes (Fig. 6). We identified single and dual infections of bodies in all groups, with 288 out of 473 (61%) infected bodies containing both viruses at 14 dpi. In contrast, many expectorants tested positive for only one virus, indicating that dissemination or salivary gland barriers selected for one of the viruses infecting their bodies (Fig. 6). To demonstrate that this observation was not biased by propagation in Vero cells, we showed that transmission of a single virus was also more frequent in the original expectorant (supplemental Fig. 1).
It was unclear how entry of one virus into the midgut might impact or potentially facilitate entry and concurrent replication of a second virus. It is possible that viruses formed individual infection foci and may not have actually competed within the same midgut cells. In this case, replicative fitness would have been determined by the number of infection foci, the amount of virus escaping the midgut and entering the hemocoel, and therefore the amount of virus disseminating to the salivary gland. However, although dual infection of the mosquito enabled direct competition of viral growth within the vector, it is virus transmission that is the biologically relevant outcome measure of fitness. Based on this metric, the majority of wildtype isolates showed greater transmission fitness compared to COAV997-5nt, indicating enhanced vector competence over time for both WN02 and SW03 lineage viruses.
Conclusions
Most of the sixteen WNV isolates representing both the WN02 and SW03 lineages from California showed increased replicative fitness in HOFIs [day 3 viremia: 100% and 90%, respectively] and Cx. tarsalis mosquitoes [transmission ratios: 60% and 83%, respectively] compared to the founding 2003 California isolate COAV997-nt (Table 2). However, mosquitoes frequently were infected with and expectorated both strains of virus. These data suggest that the the avian host may have provided greater selection pressure than the RNAi pathways within the mosquito host that tolerated replication of both viruses.
Virus strains from Coachella Valley adjacent to the Salton Sea and near to Niland in Imperial County where COAV997-2003 was isolated showed the most limited changes in fitness. The isolate COAV-08-1091 demonstrated an attenuated phenotype in Cx. tarsalis and neutral fitness in HOFIs based on peak viremia. The hot and dry climate in Coachella Valley and utilization of the same vector and host species in enzootic transmission as the founding virus may have limited WNV genetic change compared to other regions in California. In addition, several introductions of the WN02 and SW03 genotypes occurred in California during and after the time COAV997-2003 was isolated, which also may have contributed to differences seen in WNV in these other regions over time (Duggal, Reisen et al., 2015). We did not identify phenotypic differences among isolates collected at different time points during the WNV transmission season, including fall and spring of successive years, suggesting that overwintering did not have an immediate and measurable impact on replicative fitness. What remains to be investigated is whether isolates preceding and during outbreaks, such as GRLA-08-277, exhibit higher viremias and more virulent phenotypes in birds, similar to isolates belonging to the East Coast genotype, or more infectious phenotypes for vectors to facilitate explosive amplification to epidemic levels.
Materials and Methods
Ethics
The University of California in Davis is approved for animal experimentation under the National Institutes of Health (NIH) assurance number A3433. All animal experimentation strictly followed the recommendations of the Guide for the Care and Use of Laboratory Animals from the American Veterinary Medical Association (AVMA). House Finches were trapped under US Federal Fish and Game permit MB-082812-1 and State Fish and Game collecting permit SC-002281. Depredation permits allowed trapping and removal of feral House Finches at multiple vineyards near Bakersfield, CA using modified Australian Crow traps and no endangered species were caught by us during this process. The Kern Mosquito and Vector Control District in Bakersfield provided permission to obtain blood samples from chickens for the generation of infectious blood meals approved under IACUC protocol 15892. No collection permits were necessary to operate dry-ice baited traps for the collection of mosquito pools from multiple sites as part of the California Mosquito-Borne Encephalitis Virus Surveillance Program. Husbandry and experimental procedures with House Finches were approved under the University of California Davis Institutional Care and Use Committee (IACUC) protocol number 15895. The animal biosafety level 3 facility housing the aviaries and insectary used for the current study was approved under USDA permit 47901 and Biological Use Authorization 0872 by the Environmental Health and Safety Institutional Biosafety Committee of the University of California, Davis. House Finches were observed daily through cage-side inspection by a veterinarian (G. Worwa) and animals showing clinical signs of disease such as shivering, puffy plumage appearance, lethargy, disorientation and ataxia were assessed individually. Animals exhibiting any of the above signs, or animals expressing visible pain or distress, were humanly euthanized via inhalation of carbon dioxide as specified in IACUC protocol number 15895 and accepted by the American Veterinary Medical Association (AVMA) Guidelines for Euthanasia of Animals.
California study sites and selection of WNV isolates
A total of sixteen mosquito pools were selected from four representative study sites at Sacramento County, Kern County, Los Angeles County and the Coachella Valley in Riverside County. The mosquito pools were comprised of the most common mosquito vector species in each area and had tested positive for WNV RNA by qRT-PCR as part of the California mosquito-borne surveillance program during 2007 and 2008 (Brault, Fang et al., 2015). Although these Culex species feed on different bird species in different areas of California, HOFI were consistently among the most frequently utilized hosts (Thiemann, Lemenager et al., 2012). Pools from each site were selected based on the time of collection to include one from early, mid and late WNV 2007 and early 2008 transmission seasons to investigate seasonal variation in fitness, including overwintering as a potential bottleneck. A summary of the collection dates, locations and mosquito species for each pool is presented in Table 1. Supernatants from the sixteen selected mosquito pool homogenates were propagated once in African Green Monkey kidney cells (Vero cells; ATCC no. CCL-81) maintained in Dulbecco's Modified Eagle Medium (DMEM; Life Technologies, Gibco, Carlsbad, CA) and supplemented with 10% of FBS. The Ct value of the original mosquito pool homogenates and viral titers following one passage in Vero cell culture are listed in Table1 as well as the range of RNA copy to PFU ratios.
Phylogenetic analyses of California strains used as COAV997 competitors
Previously full-length genomes of WNV isolates collected between 1999 and 2012 were sequenced (Duggal, Reisen et al., 2015). The coding regions of all sequences were aligned using Clustal Omega (Sievers, Wilm et al., 2011) and edited manually. jModelTest2 was used to determine the most appropriate nucleotide substitution model, which was a generalized time reversible (GTR) model with a gamma (Γ) distribution of rate variation among sites (Darriba, Taboada et al., 2012; Guindon, Dufayard et al., 2010). Phylogenies were constructed using the Bayesian MCMC method in BEAST v1.8 (Drummond, Suchard et al., 2012) with a GTR + Γ substitution model, a lognormal relaxed molecular clock, and a time-aware smoothed GMRF Bayesian Skyride coalescent model (Drummond, Nicholls et al., 2002; Minin, Bloomquist et al., 2008). Distances were calculated based on great circle distances. Spatial parameters were estimated using relaxed random walks (RRW) and a heterogeneous probability distribution of diffusion to allow for variation in dispersal rate, as previously determined to be most appropriate for WNV in North America (Pybus, Suchard et al., 2012). MCMC chains were run for 200 million states, sampling every 20,000 states. Convergence was evaluated using Tracer v1.6. The maximum clade credibility (MCC) tree was determined using TreeAnnotator and visualized using FigTree v1.4.2 and SPREAD (Bielejec, Rambaut et al., 2011). Computing resources were used from the Cipres Science Gateway (Miller, Pfeiffer et al., 2010).
In vivo fitness competitions in HOFIs
Feral, hatching-year House Finches (HOFI; Haemorhous mexicanus) were captured in Kern County CA during late summer of 2011 using grain-baited traps and transferred to a mosquito-proof outdoor aviary. All birds were administered 0.2mg/mL of chlortetracycline (Fort Dodge, Overland Park, KS) in drinking water for 14 days, and sera collected to screen for the presence of antibodies against WNV, SLEV and WEEV using an immunosorbent assay (Chiles & Reisen, 1998; Ebel, DuPuis et al., 2002). Seronegative birds then were transferred to the ABSL-3 facility at UC Davis and allowed a 14 day acclimation period prior to inoculation.
Each of the sixteen WNV isolates was competed against COAV997-5nt in HOFIs by inoculating approximately an equal concentration of the two viruses (Worwa, Wheeler et al., 2015). Briefly, groups of six to seven birds were injected subcutaneously above the right Musculus pectoralis with a total of 50 μL of each 1:1 virus mixture at 4.3 log10 PFU/mL equaling ca. 500 PFU for each virus. Mean+/-SE WNV titer and number of wildtype and COAV997-5nt RNA copies per inoculum were shown in Table 3. Here and for mosquito infection, COAV997-5nt RNA copies were adjusted to account for non-infectious RNA copies produced during culture. Mock-infected controls were administered 50 μL of DMEM.
Following infection, birds were observed daily for clinical signs of WNV disease until the end of the experiment at 14 days post infection (dpi). On the day of inoculation and between 1 and 7 dpi, a total of 100 μL of blood was collected from each bird from the Vena jugularis using a 28-gauge needle and diluted in 500μL of DMEM to achieve an approximate 1:10 serum dilution. A terminal blood sample was collected from all animals prior to euthanasia. Birds surviving acute WNV infection were euthanized upon project completion at 14 dpi. Bird inocula and serum samples were stored at -80°C until analysis by plaque assay and qRT-PCR for determination of infectious titer and RNA copy numbers, respectively. Infectious titers and RNA copy numbers quantified in bird inocula are presented in Table 3.
In vivo fitness competitions in Culex tarsalis mosquitoes
Culex tarsalis mosquitoes from a laboratory-adapted colony established from the Kern National Wildlife Refuge in Kern County California during 2002 were used for all fitness competitions (Worwa, Wheeler et al., 2015). Uninfected mosquitoes were reared in a BSL-2 insectary at 22°C, a photoperiod of 16:8 (L:D) hours and offered daily a 10% sucrose solution. Prior to blood meal exposure, mosquitoes were transferred to the BSL-3 insectary and held at 26°C, 12:12 (L:D) hours and approximately 60% humidity.
For fitness competitions, virus-spiked infectious blood meals were generated for each of the sixteen WNV isolates by mixing equal concentrations of each wildtype isolate with COAV997-5nt. Groups of female Cx. tarsalis mosquitoes were starved for 24 hours before being exposed orally to a blood meal containing ca. 7 log10 PFU/mL of the 1:1 virus mixture. This concentration was achieved by a 1:10 dilution of the 1:1 virus mixture at 8 log10 PFU/mL in heparinized chicken blood and offered to mosquitoes in the dark for 1 hour at a blood meal temperature of 37°C using the Hemotek membrane system (Discovery Workshops, Accrington, Lancashire, UK). Mock-infected mosquitoes were exposed to blood meals spiked with DMEM. After exposure, mosquitoes were anesthetized using carbon dioxide and fully engorged females transferred to a clean container. From each group, five fully engorged females were frozen at -80°C immediately after exposure to determine the infectious titer and RNA copy numbers for all imbibed blood meals (Table 4).
On 14 dpi, all surviving mosquitoes were anesthetized using triethylamine (Fisher Scientific, USA) and expectorants collected from each female by inserting the proboscis into a glass capillary filled with a 1:1 solution of 50% FBS and 5% sucrose for 10 minutes (Aitken, 1977). The expectorants were then transferred into a cryovial containing 250 μL of DMEM supplemented with 10% FBS, 100 U/mL penicillin, 0.1 g/mL streptomycin and 1.25 μg/mL amphotericin B (Life technologies, Gibco, Carlsbad, CA). After removal of the legs, the mosquito bodies were placed individually in cryovials containing two 5mm glass beads each and the above DMEM diluent, and samples were stored at -80°C. Infectious titers from five fully engorged females collected following blood meal exposure and RNA copy numbers quantified in blood meals are presented in Table 4.
Sample processing and analysis
Five fully engorged mosquitoes from each group frozen immediately post-blood meal and all mosquito bodies were homogenized for a total of four minutes at 24 Hz using a Tissue Lyser (Qiagen, USA) and the homogenate clarified by centrifugation. Expectorants were passaged once in Vero cell monolayers for additional amplification of transmitted viruses, thereby allowing enhanced determination of the competition outcome. Previously we found that few infectious virus particles were present in most mosquito expectorant samples, thus making it difficult to identify differences in RNA copy numbers measured by our qRT-PCR. However, a single passage of expectorants in Vero cell culture did not alter the initial ratio of the competing viruses, but markedly improved the ability to measure them (Worwa, Wheeler et al., 2015). To confirm the dose administered to birds and mosquitoes at the beginning of the competition, viral titers were determined in bird inocula and five fully engorged mosquitoes from immediately after blood ingestion using Vero cell plaque assay (Brault, Langevin et al., 2004) and titers expressed as log10 PFU/mL. Nucleic acids were extracted from all bird and mosquito samples using the MagMAX magnetic particle processor and MagMAX-96 Viral RNA isolation Kit (Applied Biosystems, USA) according to the manufacturer's protocol. The RNA copy number for each virus was quantified from mixed samples using an allele-specific quantitative RT-PCR. We designed this approach specifically to distinguish between COAV997-5nt and wildtype isolates of WNV based on the five nucleotide polymorphism previously introduced into COAV997-5nt (Worwa, Andrade et al., 2014). Resulting RNA copy numbers were normalized to the respective RNA ratios detected in the bird inocula and mosquito blood meals to account for minor differences at the start of the competition.
Genomes of all WNV isolates were sequenced using the Sanger technique and amino acid changes compared to the founding California strain COAV997 [Supplemental Table 1). Cell culture supernatants of all isolates were clarified and viral RNA extracted using Viral RNA Mini kit (Qiagen, USA). The viral genome was amplified in 6 overlapping amplicons as previously described (Duggal, Reisen et al., 2015) with amplicons sequenced directly. 5′ and 3′ genomic termini (5′ UTR and 3′ UTR) were generated and sequenced by 5′ and 3′ RACE amplification (Invitrogen). Primer sequences are available upon request. NCBI GenBank accession numbers are presented in Table 1.
GraphPad Prism version 5 and SigmaPlot version 11.0 were used for data plotting and statistical analysis. For comparisons between two groups, a Mann-Whitney test was utilized and statistical significance set to P<0.05.
Supplementary Material
Supplemental Figure 1. Single and dual transmission of viruses detected with and without Vero cell culture propagation. Side-by-side comparison of singly and dually transmitted viruses detected in expectorants (w/o Vero) and in supernatant following Vero cell culture passage of expectorants (with Vero). The number of infected bodies is presented for reference.
Highlights.
Using a novel replicative fitness assay, 16 strains of West Nile virus isolated in 2007-2008 were competed in vivo against a genetically marked clone from the founding WNV strain isolated in 2003.
Most recent strains of both the WN02 and SW03 genotypes exhibited elevated fitness in both House finch and Culex tarsalis mosquito hosts.
Viremias in House finches were dominated by a single strain.
Although Cx. tarsalis frequently were infected by both competing strains, only one strain was transmitted successfully.
Acknowledgments
We thank Brian Carroll and Amy Jobe from the Arbovirus Field Station in Bakersfield, CA for gathering the House Finches from vineyards, and Dr. Shirley Luckhardt for allocating BSL-2 insectary space for rearing uninfected mosquitoes.
Financial Disclosure: G. Worwa was funded by the Swiss National Science Foundation with grant PBBEP3_128345 and the Swiss Foundation for Grants in Biology and Medicine through grant PASMP3_137034. Funding was provided, in part, by the US NIH National Institute of Allergy and Infectious Diseases grant RO1-AI55607. The funding organizations had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author Contributions: Conceived and designed the experiments: GW ACB WKR. Performed the experiments: GW AAH MF NKD. Analyzed the data: GW WKR ACB. Contributed reagents/materials/analysis tools: GW ACB WKR. Wrote the paper: GW ACB WKR. Obtained animal use and care protocols, fish and game collecting permits, managed the BSL3 laboratory: WKR.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitken THG. An in vitro feeding technique for artificially demonstrating virus transmission by mosquitoes. Mosq News. 1977;37:130–133. [Google Scholar]
- Andrade CC, Maharaj PD, Reisen WK, Brault AC. North American West Nile virus genotype isolates demonstrate differential replicative capacities in response to temperature. J Gen Virol. 2011;92:2523–2533. doi: 10.1099/vir.0.032318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielejec F, Rambaut A, Suchard MA, Lemey P. SPREAD: spatial phylogenetic reconstruction of evolutionary dynamics. Bioinformatics. 2011;27:2910–2912. doi: 10.1093/bioinformatics/btr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC, Fang Y, Reisen WK. Multiplex qRT-PCR for the Detection of Western Equine Encephalomyelitis, St. Louis Encephalitis, and West Nile Viral RNA in Mosquito Pools (Diptera: Culicidae) J Med Entomol. 2015;52:491–499. doi: 10.1093/jme/tjv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC, Huang CY, Langevin SA, Kinney RM, Bowen RA, Ramey WN, Panella NA, Holmes EC, Powers AM, Miller BR. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat Genet. 2007;39:1162–1166. doi: 10.1038/ng2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC, Langevin SA, Bowen R, Panella NA, Biggerstaff BJ, Miller BR, Komar N. Differential virulence of West Nile strains for American crows. Emerg Infect Dis. 2004;10:2161–2168. doi: 10.3201/eid1012.040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiles RE, Reisen WK. A new enzyme immunoassay to detect antibodies to arboviruses in the blood of wild birds. J Vector Ecol. 1998;23:123–135. [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CT, Ebel GD, Lanciotti RS, Brault AC, Guzman H, Siirin M, Lambert A, Parsons RE, Beasley DW, Novak RJ, Elizondo-Quiroga D, Green EN, Young DS, Stark LM, Drebot MA, Artsob H, Tesh RB, Kramer LD, Barrett AD. Phylogenetic analysis of North American West Nile virus isolates, 2001-2004: Evidence for the emergence of a dominant genotype. Virology. 2005;342:252–265. doi: 10.1016/j.virol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Nicholls GK, Rodrigo AG, Solomon W. Estimating mutation parameters, population history and genealogy simultaneously from temporally spaced sequence data. Genetics. 2002;161:1307–1320. doi: 10.1093/genetics/161.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal NK, Bosco-Lauth A, Bowen RA, Wheeler SS, Reisen WK, Felix TA, Mann BR, Romo H, Swetnam DM, Barrett AD, Brault AC. Evidence for co-evolution of West Nile Virus and house sparrows in North America. PLoS Negl Trop Dis. 2014;8:e3262. doi: 10.1371/journal.pntd.0003262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal NK, Reisen WK, Fang Y, Newman RM, Yang X, Ebel GD, Brault AC. Genotype-specific variation in West Nile virus dispersal in California. Virology. 2015;485:79–85. doi: 10.1016/j.virol.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel GD, DuPuis AP, Nicholas D, Young D, Maffei J, Kramer LD. Detection by Enzyme-Linked Immunosorbent Assay of Antibodies to West Nile virus in Birds. Emerg Infect Dis. 2002;8:979–982. doi: 10.3201/eid0809.020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- Hom A, Bonilla D, Kjemtrup A, Kramer VL, Cahoon-Young B, Barker CM, Marcus L, Glaser C, Cossen C, Baylis E, Jean C, Eldridge BF, Carney R, Padgett K, Sun B, Reisen WK, Woods L, Glover J, Erickson C, Barclay C, Husted S. Surveillance for mosquito-borne encephalitis virus activity and human disease, including West Nile virus, in California, 2005. Proc Mosq Vector Control Assoc Calif. 2006;74:43–55. [Google Scholar]
- Hom A, Marcus L, Kramer VL, Cahoon B, Glaser C, Cossen C, Baylis E, Jean C, Tu E, Eldridge BF, Carney R, Padgett K, Sun B, Reisen WK, Woods L, Husted S. Surveillance for mosquito-borne encephalitis virus activity and human disease, including West Nile virus, in California, 2004. Proc Mosq Vector Control Assoc Calif. 2005;73:66–77. [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothera L, Nelms BM, Reisen WK, Savage HM. Population genetic and admixture analyses of Culex pipiens complex (Diptera: Culicidae) populations in California, United States. Am J Trop Med Hyg. 2013;89:1154–1167. doi: 10.4269/ajtmh.13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan JL, Kluh S, Madon MB, Reisen WK. West Nile virus emergence and persistence in Los Angeles, California, 2003-2008. Am J Trop Med Hyg. 2010;83:400–412. doi: 10.4269/ajtmh.2010.10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin SA, Brault AC, Panella NA, Bowen RA, Komar N. Variation in virulence of West Nile virus strains for house sparrows (Passer domesticus) Am J Trop Med Hyg. 2005;72:99–102. [PubMed] [Google Scholar]
- McMullen AR, May FJ, Li L, Guzman H, Bueno R, Jr, Dennett JA, Tesh RB, Barrett AD. Evolution of new genotype of West Nile virus in North America. Emerg Infect Dis. 2011;17:785–793. doi: 10.3201/eid1705.101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proc.Gateway Computing Envirobnments Workshop (GCE); 1-8. 2010; New Orleans, LA. [Google Scholar]
- Minin VN, Bloomquist EW, Suchard MA. Smooth skyride through a rough skyline: Bayesian coalescent-based inference of population dynamics. Mol Biol Evol. 2008;25:1459–1471. doi: 10.1093/molbev/msn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pybus OG, Suchard MA, Lemey P, Bernardin FJ, Rambaut A, Crawford FW, Gray RR, Arinaminpathy N, Stramer SL, Busch MP, Delwart EL. Unifying the spatial epidemiology and molecular evolution of emerging epidemics. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1206598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez VM. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission J Med Entomol. 2005;42:367–375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD, Chiles RE, Madon MB, Cossen C, Woods L, Husted S, Kramer VL, Edman JD. West Nile Virus in California. Emerg Infect Dis. 2004;10:1369–1378. doi: 10.3201/eid1008.040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemann TC, Lemenager DA, Kluh S, Carroll BD, Lothrop HD, Reisen WK. Spatial variation in host feeding patterns of Culex tarsalis and the Culex pipiens complex (Diptera: Culicidae) in California. J Med Entomol. 2012;49:903–916. doi: 10.1603/me11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worwa G, Andrade CC, Thiemann TC, Park B, Maharaj PD, Anishchenko M, Brault AC, Reisen WK. Allele-specific qRT-PCR demonstrates superior detection of single nucleotide polymorphisms as genetic markers for West Nile virus compared to Luminex(R) and quantitative sequencing. J Virol Methods. 2014;195:76–85. doi: 10.1016/j.jviromet.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worwa G, Wheeler SS, Brault AC, Reisen WK. Comparing competitive fitness of West Nile virus strains in avian and mosquito hosts. PLoS One. 2015;10:e0125668. doi: 10.1371/journal.pone.0125668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Single and dual transmission of viruses detected with and without Vero cell culture propagation. Side-by-side comparison of singly and dually transmitted viruses detected in expectorants (w/o Vero) and in supernatant following Vero cell culture passage of expectorants (with Vero). The number of infected bodies is presented for reference.


