Abstract
Background
Vincristine (VCR) is a critical part of treatment in pediatric malignancies and is associated with dose-dependent peripheral neuropathy (vincristine-induced peripheral neuropathy [VIPN]). Our previous findings show VCR metabolism is regulated by the CYP3A5 gene. Individuals who are low CYP3A5 expressers metabolize VCR slower and experience more severe VIPN as compared to high expressers. Preliminary observations suggest that Caucasians experience more severe VIPN as compared to nonCaucasians.
Procedure
Kenyan children with cancer who were undergoing treatment including VCR were recruited for a prospective cohort study. Patients received IV VCR 2 mg/m2/dose with a maximum dose of 2.5 mg as part of standard treatment protocols. VCR pharmacokinetics (PK) sampling was collected via dried blood spot cards and genotyping was conducted for common functional variants in CYP3A5, multi-drug resistance 1 (MDR1), and microtubule-associated protein tau (MAPT). VIPN was assessed using five neuropathy tools.
Results
The majority of subjects (91%) were CYP3A5 high-expresser genotype. CYP3A5 low-expresser genotype subjects had a significantly higher dose and body surface area normalized area under the curve than CYP3A5 high-expresser genotype subjects (0.28 ± 0.15 hr·m2/l vs. 0.15 ± 0.011 hr·m2/l, P = 0.027). Regardless of which assessment tool was utilized, minimal neuropathy was detected in this cohort. There was no difference in the presence or severity of neuropathy assessed between CYP3A5 high- and low-expresser genotype groups.
Conclusion
Genetic factors are associated with VCR PK. Due to the minimal neuropathy observed in this cohort, there was no demonstrable association between genetic factors or VCR PK with development of VIPN. Further studies are needed to determine the role of genetic factors in optimizing dosing of VCR for maximal benefit.
Keywords: cancer, genotyping, neuropathy, pediatrics, vincristine
1 | INTRODUCTION
In resource-limited settings, access to chemotherapeutic agents is confined to a few integral therapies that are available, affordable, and well tolerated. Vincristine (VCR) is a mainstay of therapy in such settings due to its lack of associated myelosuppression and is utilized in the treatment of over half of all pediatric malignancies; however, it is associated with highly variable and cumulative dose-dependent peripheral neuropathy.1 Despite its broad use and utility across a variety of environments, little is known regarding VCR pharmacokinetics (PK) and optimal dosing in relation to genetic factors and observed toxicity. Thus, current dosing strategies for VCR are largely empiric.2
Studies previously conducted by our group in the United States have shown that patients who are high-expresser genotype for cytochrome P450 (CYP) 3A5 metabolize VCR more efficiently.3 Additionally, African American children are more likely to be CYP 3A5 high-expresser genotype and are less likely to develop vincristine-induced peripheral neuropathy (VIPN).4 Based on these findings, it is reasonable to hypothesize that CYP3A5 genotype, VCR PK, and neurotoxicity (VIPN) may be substantially different in Africa. The primary aims of this study are to (1) describe the CYP3A5 genotype and VCR PK in Kenyan children with cancer and (2) assess the presence and severity of VIPN in Kenyan children with cancer. The exploratory aim is the investigation of the association of CYP3A5 genotype and VCR PK with development of neuropathy in the subset of patients with evaluable VIPN.
2 | METHODS
2.1 | Setting
Kenya is a low-income country in sub-Saharan Africa. This study was carried out at Moi Teaching and Referral Hospital in Western Kenya in collaboration with Academic Model for Providing Access to Healthcare (AMPATH) Oncology Institute. Available treatment options include surgery and chemotherapy. Radiotherapy is not currently locally available.
2.2 | Study design
This study was approved by the Moi University Institutional Review Ethics Committee and the Indiana University School of Medicine Institutional Review Board. Patients were recruited prospectively from June 2011 to August 2013 and provided written informed consent and assent (children ≥7 years) for participation in the study. Children aged 1–18 years being treated for any type of cancer in which VCR was part of routine therapy were eligible to participate. Patients with human immunodeficiency virus or underlying baseline neuropathy were excluded. Patients were treated based on standard institutional treatment protocols, in which the VCR dose is 2 mg/m2/dose with a maximum dose of 2.5 mg administered via peripheral venous access by IV push. Subjects were evaluated for development of neuropathy while they were receiving VCR as part of their routine anti-cancer treatment. All patients were followed longitudinally from the time of enrollment until completion of cancer therapy, death from disease, toxicity, or abandonment of care (defined as failure to sustain treatment during four or more successive weeks).5 Genotyping, limited PK sampling, and detailed neuropathy assessments were conducted on each subject as outlined in the following.
2.2.1 | VCR confirmation
Given that counterfeit pharmaceuticals have been noted to be problematic in sub-Saharan Africa, the VCR dispensed at this treatment center was tested for active vinca alkaloid using high performance liquid chromatography-mass spectroscopy/mass spectroscopy. All vials tested had active VCR present and, on average, had 43.4 ± 15.7% more active drug than standard U.S. VCR. No vials were found to have less VCR than U.S. standards.
2.2.2 | Genotyping
DNA sampling for single nucleotide polymorphism (SNP) analysis was obtained using Oragene saliva kits. DNA was extracted and selectively genotyped using an intermediate throughput OpenArray® genotyping platform. Genotyping for CYP3A5*3, *6, and *7 (the most common functionally significant polymorphisms) was performed using Taqman real-time PCR assays.6 Other SNP analyses were performed on candidate genes in the vinca alkaloid pathway, including polymorphisms in multidrug resistance 1 (MDR1) and microtubule-associated protein tau (MAPT).
2.2.3 | Pharmacokinetic analysis
Limited plasma PK sampling was collected on all patients. Up to six samples were collected at the following intervals post-VCR dose: 30 min, 60 min, then daily as long as the patient remained in the hospital. Samples were collected by finger stick onto Whatman protein saver human dried blood spot (DBS) collection paper. DBS cards were stored at room temperature in sealed light-protective bags with desiccant and humidity sensor detector cards until they were transported back to United States for analysis. Stability at room temperature was evaluated and confirmed. VCR was quantified on Whatman protein saver 903 DBS cards using vinorelbine as the internal standard. DBS samples, n = 5 punches of 6 mm diameter, were diluted with water and precipitated with acetonitrile. Chromatographic analysis was performed using an Agilent 1290 series HPLC coupled with a PAL HTC-xt Leap autosampler. All compounds were monitored using an ABSciex 5500 QTRAP triple-quadrupole mass spectrometer equipped with electrospray ionization probe in positive mode. Mass spectrometry settings for the m/z of the parent and daughter ions for VCR and vinorelbine were 413.2/392.2 and 390.1/122.1, respectively. The intraday and interday accuracy and precision (% coefficient of variation) estimates for VCR at four different concentrations were >80% and <20%, respectively. Accuracy and precision were also evaluated at a hematocrit of 30, 45, and 60. VCR accuracy was >80% at all hematocrits tested. The lower limit of quantification for VCR was 0.06 ng/ml. VCR concentration was further normalized by the dose for the follow-up association analysis.
2.2.4 | Neurotoxicity evaluation
The current standard VIPN measurement approach in national cooperative group trials is to utilize the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (NCI-CTCAE). However, this tool lacks sensitivity to detect subtle subclinical peripheral neuropathy due to its coarse scoring criteria.7 Therefore, VIPN phenotype was assessed at the time of each VCR administration using five different neuropathy assessment tools including NCI-CTCAE, Balis Pediatric Scale of Peripheral Neuropathy, Faces Pain Scale, Pediatric Neuropathic Pain Scale, and Total Neuropathy Score (TNS©). All instruments are available as appendices and the most sensitive tool, the TNS©, is summarized in Table 1.8 Because the TNS was the most sensitive tool for detecting neuropathy, this tool was utilized for statistical analysis when comparing CYP3A5 high and low expressers. Each instrument was back translated and forward translated into Swahili. Serial assessments were conducted while patients were receiving VCR.
TABLE 1.
TNS© scoring (reprinted with permission from Smith et al.8)
| 0 | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| Worst subjective symptoma,b (tingling, numbness, neuropathic pain) | None | Limited to fingers or toes | Extension to ankle/wrist | Extension to knee/elbow | Above knees/elbows or functionally disabling |
| Temperature sensibilitya | Normal | Reduced in fingers/toes | Reduced to wrist/ankle | Reduced to elbow/knee | Reduced above elbow/knee |
| Vibration sensibilitya | Normal | Reduced in fingers/toes | Reduced to wrist/ankle | Reduced to elbow/knee | Reduced above elbow/knee |
| Strengtha,c | Normal | Mild weakness, but can overcome resistance | Moderate weakness, can overcome gravity but not resistance | Severe weakness, cannot overcome gravity | Paralysis |
| Tendon reflexesa | Normal | Ankle reflex reduced | Ankle reflex absent | Ankle reflex absent/others reduced | All reflexes absent |
| Autonomic/constipation | Normal | Requiring stool softeners or dietary modification | Requiring laxatives | Obstipation requiring enemas or manual evacuation | Life-threatening consequences (e.g., toxic megacolon, obstruction) including death |
| Laryngeal/hoarseness | Normal voice/cry | Mild or intermittent hoarseness | Persistent hoarseness, but able to vocalize and may have mild to moderate edema | Whispered speech, not able to vocalize and may have marked edema | Marked dyspnea/stridor requiring tracheostomy or intubation |
Score is based on the worst of the three symptoms.
Toe extension/flexion, ankle dorsiflexion, hip flexion, hand grip, thumb abduction, wrist extension, arm abduction; score is based on the weakest muscle group.
TNS© = Total Neuropathy Score A.
2.2.5 | Population PK modeling and statistical data analysis
The dose and body surface area (BSA) normalized VCR was fitted into a one-compartment model using NONMEM. The associations between the clearance and demographic and genetic variables were initially tested using either first-order or conditional first-order approximation algorithms in the NONMEM population PK modeling, but they failed to converge. We decided to use a two-step strategy instead. At step one, a population one-compartment PK model was fitted to the data with only subject-specific clearance parameter. Then these subject specific PK clearances were transformed into subject specific areas under the curve (AUCs), before the second step association analysis was performed between dose normalized AUC for VCR and the demographic variables (age, gender, height, weight, and BSA) and genetic variables (CYP3A5, MDR1, and MAPT). The second step analysis was analyzed in linear regression using R package (lm). SNPs in CYP3A5, MDR1, and MAPT were coded by dominant, recessive, and gene dose models. Haplotypes were estimated by the R package, haplo.stats. Maximum NCI-CTCAE scale score and maximum TNS© score during treatment were used as the neuropathy phenotype. All the nonassessable evaluations were discarded. The associations between neuropathy score and genetics and demographic variables were analyzed similarly using R package lm. Means and standard deviations were reported for the continuous variables, and frequencies were reported for the categorical variables. The association between the genetics and time to the first TNS score ≥ 2 was analyzed using the Cox proportional hazard model. In this analysis, patients who abandoned treatment or died prior to completion of therapy were considered lost to follow-up. In addition, we also compared maximum TNS scores between two groups of patients: patients who completed treatment and patients who were lost to follow-up.
3 | RESULTS
Seventy-eight patients were recruited and all subjects enrolled in the study. Nine patients (11.5%) abandoned treatment prior to completion of therapy. Demographic characteristics are summarized in Table 2. The ethnic background for patients in this study was reflective of the normal population of patients treated at AMPATH Oncology Institute.9,10
TABLE 2.
Patient demographics and CYP 3A5 genotyping
| Demographic variables | CYP 3A5 genotypea | P-value | ||
|---|---|---|---|---|
| Homozygous variant (low expresser) (n = 7) | Heterozygous (n = 50) | Homozygous wild type (high expresser) (n = 21) | ||
| Age (years), mean (SD) | 6.14 (5.21) | 6.54 (3.98) | 6.10 (4.60) | 0.91b |
| Sex | ||||
| – Male, n (%) | 2 (4.8) | 28 (66.7) | 12 (28.6) | 0.46c |
| – Female, n (%) | 5 (13.9) | 22 (61.1) | 9 (25.0) | |
| Body surface area (m2), mean (SD) | 0.81 (0.40) | 0.81 (0.26) | 0.75 (0.30) | 0.77b |
| Height (cm), mean (SD) | 113.06 (31.40) | 113.55 (23.90) | 108.34 (26.03) | 0.73b |
| Weight (kg), mean (SD) | 21.56 (15.49) | 21.01 (9.57) | 19.42 (11.66) | 0.83b |
| Tribe | ||||
| – Luhya, n (%) | 3 (10.7) | 18 (64.3) | 7 (25.0) | 0.96c |
| – Kalenjin, n (%) | 3 (10.7) | 17 (60.7) | 8 (28.6) | |
| – Other, n (%) | 1 (4.6) | 15 (68.2) | 6 (27.3) | |
| Oncology diagnosis | ||||
| – Solid tumor, n (%) | 3 (8.8) | 21 (61.8) | 10 (29.4) | 0.94c |
| –Leukemia/lymphoma, n (%) | 4 (9.1) | 29 (65.9) | 11 (25.0) | |
Homozygous variant = *3, 6, or 7/*3, 6, or 7; Heterozygous = *1/*3, 6, or 7; Homozygous wild type = *1/*1.
ANOVA.
Fisher’s exact test.
3.1 | Genotyping
Seventy-one of the 78 subjects (91%) are CYP3A5 high-expresser genotype (homozygous or heterozygous for the *1 allele, which are phenotypically identical). There were no statistically significant differences in CYP3A5, MDR1, or MAPT genotypes across the demographic variables in this cohort.
3.2 | VCR PK
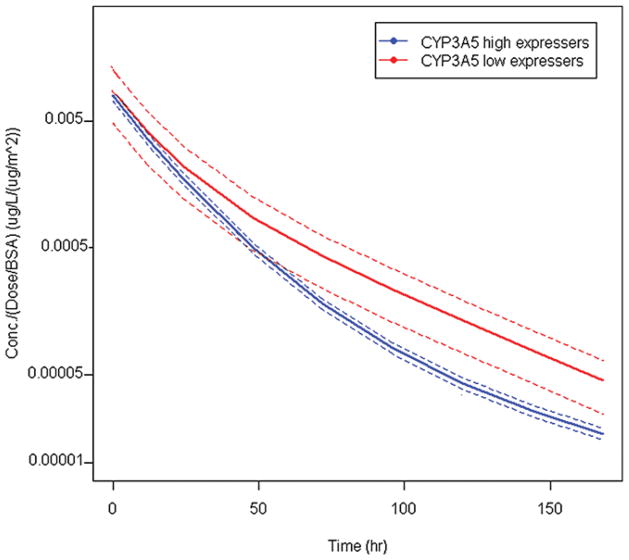
Plasma VCR concentrations obtained from DBSs demonstrated that CYP3A5 low-expresser genotype subjects had a significantly higher dose and BSA normalized AUC than CYP3A5 high-expresser genotype subjects (0.28 ± 0.15 hr·m2/l vs. 0.15 ± 0.011 hr·m2/l, P = 0.027; Figure 1). The average number of VCR measurements among 77 patients is 3.4 with a standard deviation of 1.0.
FIGURE 1.
VCR pharmacokinetic profile of CYP 3A5 low-expresser versus high-expresser genotype groups. The blue curve is the population-average PK model for CYP3A5 high expressers, and the red curve is the population-average PK model for CYP3A5 low expressers. Dotted lines reflect 95% confidence intervals. VCR dose normalized AUC of CYP3A5 high-expresser genotype = 0.28 ± 0.15 hr·m2/l. AUC of CYP3A5 low-expresser genotype = 0.15 ± 0.011 hr·m2/l, P = 0.027
3.3 | VCR-Induced peripheral neuropathy
A cohort of 78 subjects completed a median of 10.2 weeks of VCR-containing therapy. They were followed prospectively for a median time of 6.5 weeks from the time of study enrollment and received a median cumulative VCR dose of 8.5 mg/m2. A total of 1,166 complete neuropathy assessments were conducted and the median score of all assessments was 0. Using the NCI-CTCAE scale for neuropathies (motor, sensory, and autonomic), 57 of 72 evaluable subjects (79.2%) experienced no detectable neuropathy and only two of 72 subjects (2.8%) experienced Grade 2 motor neuropathy. No patients experienced Grade 2 sensory neuropathy, ≥Grade 3 motor or sensory neuropathy. Using the much more sensitive TNS©, only 46 subjects were able to be evaluated due to developmental inability of younger children to complete the full assessment (118 neuropathy assessments on 36 subjects were excluded). Of the 46 evaluable subjects, 13 (28.3%) experienced no detectable neuropathy and only two of 46 subjects (4.3%) experienced clinically significant neuropathy11 with a TNS of ≥5 (scale range 0–28, max score in any subject = 8). Further neuropathy assessments were conducted using the Balis Scale and Pediatric Neuropathic Pain Scale. Cumulative results are summarized in Table 3. Regardless of which assessment tool was used, very minimal neuropathy was detected in this cohort. There was no difference in the presence or severity of neuropathy assessed via TNS between CYP3A5 high- and low-expresser genotype groups, as well as between MDR1 and MAPT haplotypes. The CYP3A5 genotype is not associated with the time to the first TNS score ≥ 2 (P = 0.73). Likewise, there was no statistically significant association between VCR AUC and development of neuropathy. No subjects required VCR dose reduction for the development of VIPN. There was no statistically significant difference in the presence or severity of neuropathy between subjects who completed treatment and patients who were lost to follow-up (P = 0.56).
TABLE 3.
Neuropathy assessment cumulative descriptive statistics
| Grading scale | Number of evaluable subjects | Number of assessments per patient (mean ±SD) | Possible range of scores | Actual range of scores | Percentage of 0 scores | Median score | Clinically significant neuropathy present (%) | Mean score ±SD |
|---|---|---|---|---|---|---|---|---|
| Balis Motor | 72 | 2.63 ± 1.74 | 0–4 | 0–3 | 96.3 | 0 | 1.4 (grade ≥ 2) | 0.08 ± 0.41 |
| Balis Sensory | 72 | 2.63 ± 1.74 | 0–4 | 0–2 | 94.7 | 0 | 1.4 (grade ≥ 2) | 0.06 ± 0.26 |
| CTC Motor | 72 | 2.63 ± 1.74 | 0–5 | 0–2 | 94.7 | 0 | 2.8 (grade ≥ 2) | 0.07 ± 0.33 |
| CTC Sensory | 72 | 2.63 ± 1.74 | 0–5 | 0–1 | 95.8 | 0 | 0 (grade ≥ 2) | 0.04 ± 0.20 |
| Pediatric Neuropathic Pain Scale | 78 | 3.17 ± 1.85 | 0–25 | 0–4 | 94.3 | 0 | 1.3 (total score ≥ 4) | 0.12 ± 0.52 |
| TNS© Total Score A | 46 | 3.11 ± 1.93 | 0–28 | 0–8 | 53.5 | 0 | 4.3 (total score ≥ 5) | 1.09 ± 1.44 |
Data reflect the total number of completed neuropathy or pain assessments. CTC, Common Terminology Criteria for Adverse Events; TNS© –PV, Total Neuropathy Score–Pediatric Vincristine.
4 | DISCUSSION
Our results demonstrate that the majority of Kenyan children (91%) have a CYP3A5 high-expresser genotype and that those with CYP3A5 high-expresser genotype have 58% less VCR exposure (dose- and BSA-normalized VCR AUC) compared to Kenyan children with CYP3A5 low-expresser genotype. Furthermore, Kenyan children experience negligible clinically significant VIPN11 compared to U.S. children despite receiving at least 33% more VCR at baseline due to protocol-based dosing differences between the U.S. and European-derived protocols utilized in Kenya.4,12 Interestingly, the genotype and toxicity phenotype findings in this cohort of Kenyan children are drastically different than a cohort of n = 148 U.S. (primarily Caucasian) children with ALL. In our Kenyan cohort of predominantly CYP3A5 high-expresser genotype, only 4.3% developed clinically significant neuropathy as defined by a TNS score ≥5. By contrast, the U.S. cohort in which only 14% were CYP3A5 high-expresser genotype, 64% developed clinically significant neuropathy using the same assessment tool (data not yet published; personal communication with Dr. Jamie Renbarger).
After ruling out counterfeit VCR as a possible explanation for the negligible VIPN observed in this cohort, we hypothesize that the minimal toxicity observed in Kenyan children is due in part to lower VCR exposure (dose-normalized and BSA-normalized VCR AUC) compared to U.S. children. There are two significant challenges in optimizing VCR exposure in children and adults: (1) there is currently no consensus on what constitutes an appropriate therapeutic VCR AUC and (2) there are significant differences in the methodology of VCR PK measurement. To this end, it is really only currently possible to compare VCR dose- and BSA-normalized AUCs within a given cohort of subjects. Because PK specimens in this study were collected by DBS samples for feasibility in this low-resource setting, it is not possible to directly compare VCR dose-normalized AUC from Kenyan subjects to that of U.S. subjects whose PK specimens were obtained from plasma samples. One potential reason for the suspected differential VCR exposure is increased expression of the drug-metabolizing enzyme CYP3A5, resulting in significantly lower dose- and BSA-normalized VCR AUC than is observed in subjects with CYP3A5 low-expresser genotype. Despite this finding, there was no demonstrated association between VCR exposure and the presence or severity of VIPN within our cohort of Kenyan children. This finding is most likely secondary to the very low incidence of clinically significant VIPN in this population, making a strong association between genotype and toxicity phenotype difficult to demonstrate despite the use of the extensively validated and exceptionally sensitive TNS©.7,8,11,13–20
Previous studies evaluating genotype and toxicity phenotype have documented conflicting results. Egbelakin et al. demonstrated that CYP3A5 genotype is associated with VCR exposure and toxicity phenotype in a cohort of U.S. (primarily Caucasian) subjects.4 Several other publications have reported no association between CYP3A5 genotype and development of VIPN21,22; however, the assessment tools utilized for evaluating neuropathy in those studies (NCI-CTCAE and the Movement Assessment Battery for Children) were suboptimal. The NCI-CTCAE has been demonstrated to have a significant floor effect that lacks adequate sensitivity to detect slight changes in neuropathy.7 The Movement Assessment Battery for Children assesses motor function only, which is likely confounded by steroid myopathy in children being treated for leukemia. Ultimately, although we hypothesize that CYP3A5 genotype and toxicity phenotype are linked, it is possible that there are also other genetic factors or pharmacokinetic factors that have not yet been elucidated that may make Kenyan children less susceptible to the development of VIPN. Despite evaluating for associations between MAPT and MDR1 genotype and VCR exposure, no statistically significant associations were apparent in this cohort.
Interestingly, a recently published genome-wide association study of n = 222 U.S. subjects demonstrated that a SNP in the promoter region of CEP72, which encodes a protein involved in microtubule formation, had a significant association with development of VCR neuropathy. Furthermore, the frequency of the CEP72 risk allele (T) differed by ancestry with a lower frequency in patients with African ancestry.23 A genome-wide association study is being planned in this cohort of patients to further evaluate possible genetic variants, including CEP72 that may contribute to the lack of neuropathy observed in Kenyan children.
VCR is one of the core chemotherapeutic agents in the treatment of over half of all treatment regimens for both adults and children. On the one hand, it is exceptional to have identified a population of children who may not be as susceptible to development of neuropathy and who, therefore, experience less treatment-related morbidity. On the other hand, the question remains what effect, if any, this finding has on disease response and treatment outcomes. One recently published study in a U.S. cohort demonstrated that faster VCR metabolism was associated with a five times increased risk of disease relapse.24 Because the children enrolled in this study have only recently completed treatment, it is too early to determine whether CYP3A5 genotype has an impact on disease outcomes in this cohort. Disease response to treatment, event-free survival, and overall survival outcomes in this cohort of Kenyan children will be evaluated for an association with CYP3A5 genotype when all subjects have been off-treatment for a minimum of 1 year. Despite this, the significantly lower VCR dose normalized AUC of Kenyan children who are CYP3A5 high-expresser genotype leads us to hypothesize that the majority of Kenyan children may be receiving subtherapeutic VCR dosing and could possibly tolerate and potentially benefit from VCR dose escalation to achieve more optimal VCR exposure.
Kenya, like many low-income countries, lacks the resources and infrastructure to provide the basic supportive care measures that are inherent in most high-income settings.25–27 Consequently, complications of myelosuppression are a major cause of treatment-related morbidity and mortality in low- and middle-income countries.25,28 Because of this, several international cooperative oncology groups are now advocating for utilization of reduced intensity chemotherapy protocols in an effort to avoid unacceptable treatment-related morbidity and mortality.25–30 Since VCR is one of the few chemotherapeutic agents that does not cause myelosuppression, it is possible that carefully monitored dose escalation of VCR may allow for safer deescalation of other myelosuppressive therapies and improved disease outcomes for this vulnerable population of children with limited treatment options.
Findings of this study must be interpreted with caution due to the small sample size of this cohort with a lack of significant variation in the studied genotype. Additionally, in this study, VCR was administered by peripheral intravenous access, thereby introducing the possibility of extravasation of VCR resulting in skewed pharmacokinetic data. When extravasation was clinically suspected (pain at infusion site, tissue necrosis following infusion), pharmacokinetic samples were repeated with the next administered VCR dose. Furthermore, patients in this study were enrolled at any point in their therapy and followed longitudinally as long as the patient was available for follow-up. Because many children in Kenya die of treatment-related complications early in therapy or abandon care secondary to financial constraints, conducting ongoing longitudinal assessments is difficult in this setting, which is a large contributor to the low average number of neuropathy assessments per child. Since VIPN is believed to be a cumulative dose-dependent phenomenon, having children enrolled at any point in therapy allows for neuropathy evaluation across a variety of different time points in treatment, thereby increasing the likelihood of detecting neuropathy if it, in fact, exists. Despite this approach, negligible neuropathy was observed even at later time points in treatment when higher cumulative doses of VCR had been administered. Lastly, CYP3A5 low expressers had limited PK samples available for analysis beyond 24 hr. Using only the one-compartment model precludes differentiation of the metabolism process from the distribution process. Hence, the clearance is a combination of metabolism and distribution. Utilizing a longer sampling window for low expressers, we anticipate a better power to investigate the associations between VCR metabolism and CYP3A genetic and/or other PK genetics.
Because we hypothesize that Kenyan children are likely to be CYP3A5 high expressers who could possibly tolerate and potentially benefit from more therapeutic VCR exposure, future studies will be aimed at VCR dose escalation in Kenyan children paired with frequent detailed neuropathy assessments to establish the maximum tolerated dose in this population. Additionally, more extensive genotyping or GWAS analysis may be beneficial in Kenyan children to establish other potential genetic changes that contribute to variability in VCR metabolism and/or susceptibility to toxicity. Once the maximum tolerated dose of VCR is established for this population, we will aim to validate our findings and potentially establish other biomarkers of VCR exposure and possibly subsequent toxicity in addition to CYP3A5 genotype.
Acknowledgments
The authors wish to thank Cellestine Tallam, Sandra Langat, Joyce Musimbi, David Barney, Christine Stone, and Abigail Huskins for their assistance in conducting neuropathy assessments and collecting biospecimens. J.S. also gratefully acknowledges funding from the St. Baldrick’s Foundation, the Indiana Institute of Personalized Medicine, NIH grant 1U54HD071598-01, and the Walther Cancer Foundation.
Abbreviations
- AMPATH
Academic Model for Providing Access to Healthcare
- AUC
area under the curve
- BSA
body surface area
- DBS
dried blood spot
- MAPT
microtubule-associated protein tau
- MDR1
multidrug esistance 1
- NCI-CTCAE
National Cancer Institute Common Terminology Criteria for Adverse Events
- PK
pharmacokinetics
- SNP
single nucleotide polymorphism
- TNS©
total neuropathy score
- VCR
vincristine
- VIPN
vincristine-induced peripheral neuropathy
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- 1.Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 2012;14(Suppl 4):iv45–iv54. doi: 10.1093/neuonc/nos203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCune JS, Lindley C. Appropriateness of maximum-dose guidelines for vincristine. Am J Health Syst Pharm. 1997;54(15):1755–1758. doi: 10.1093/ajhp/54.15.1755. [DOI] [PubMed] [Google Scholar]
- 3.Dennison JB, Jones DR, Renbarger JL, Hall SD. Effect of CYP3A5 expression on vincristine metabolism with human liver microsomes. J Pharmacol Exp Ther. 2007;321(2):553–563. doi: 10.1124/jpet.106.118471. [DOI] [PubMed] [Google Scholar]
- 4.Egbelakin A, Ferguson MJ, MacGill EA, et al. Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56(3):361–367. doi: 10.1002/pbc.22845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mostert S, Arora RS, Arreola M, et al. Abandonment of treatment for childhood cancer: position statement of a SIOP PODC Working Group. Lancet Oncol. 2011;12(8):719–720. doi: 10.1016/S1470-2045(11)70128-0. [DOI] [PubMed] [Google Scholar]
- 6.Eap CB, Buclin T, Hustert E, et al. Pharmacokinetics of midazolam in CYP3A4- and CYP3A5-genotyped subjects. Eur J Clin Pharmacol. 2004;60(4):231–236. doi: 10.1007/s00228-004-0767-7. [DOI] [PubMed] [Google Scholar]
- 7.Smith EM, Beck SL, Cohen J. The Total Neuropathy Score: a tool for measuring chemotherapy-induced peripheral neuropathy. Oncol Nurs Forum. 2008;35(1):96–102. doi: 10.1188/08.ONF.96-102. [DOI] [PubMed] [Google Scholar]
- 8.Smith EM, Li L, Hutchinson RJ, et al. Measuring vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. Cancer Nurs. 2013;36(5):E49–60. doi: 10.1097/NCC.0b013e318299ad23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostert S, Njuguna F, Kemps L, et al. Epidemiology of diagnosed childhood cancer in Western Kenya. Arch Dis Child. 2012;97(6):508–512. doi: 10.1136/archdischild-2011-300829. [DOI] [PubMed] [Google Scholar]
- 10.Strother RM, Busakhala NB, Njiru E, et al. The evolution of comprehensive cancer care in Western Kenya. J Cancer Policy. 2013;1(1–2):e25–e30. [Google Scholar]
- 11.Gilchrist LS, Tanner L. The pediatric-modified Total Neuropathy Score: a reliable and valid measure of chemotherapy-induced peripheral neuropathy in children with non-CNS cancers. Support Care Cancer. 2013;21(3):847–856. doi: 10.1007/s00520-012-1591-8. [DOI] [PubMed] [Google Scholar]
- 12.Renbarger JL, McCammack KC, Rouse CE, Hall SD. Effect of race on vincristine-associated neurotoxicity in pediatric acute lymphoblastic leukemia patients. Pediatr Blood Cancer. 2008;50(4):769–771. doi: 10.1002/pbc.21435. [DOI] [PubMed] [Google Scholar]
- 13.Cavaletti G, Bogliun G, Marzorati L, et al. Grading of chemotherapy-induced peripheral neurotoxicity using the total neuropathy scale. Neurology. 2003;61(9):1297–1300. doi: 10.1212/01.wnl.0000092015.03923.19. [DOI] [PubMed] [Google Scholar]
- 14.Cavaletti G, Frigeni B, Lanzani F, et al. Chemotherapy-induced peripheral neurotoxicity assessment: a critical revision of the currently available tools. Eur J Cancer. 2010;46(3):479–494. doi: 10.1016/j.ejca.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Cavaletti G, Frigeni B, Lanzani F, et al. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst. 2007;12(3):210–215. doi: 10.1111/j.1529-8027.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 16.Cornblath DR, Chaudhry V, Carter K, et al. Total Neuropathy Score: validation and reliability study. Neurology. 1999;53(8):1660–1664. doi: 10.1212/wnl.53.8.1660. [DOI] [PubMed] [Google Scholar]
- 17.Gilchrist LS. Measuring chemotherapy-induced peripheral neuropathy in children: development of the ped-mTNS and pilot study results. Rehabil Oncol. 2009;27(3):7–15. [Google Scholar]
- 18.Griffith KA, Merkies IS, Hill EE, Cornblath DR. Measures of chemotherapy-induced peripheral neuropathy: a systematic review of psychometric properties. J Peripher Nerv Syst. 2010;15(4):314–325. doi: 10.1111/j.1529-8027.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 19.Lavoie Smith EM, Cohen JA, Pett MA, Beck SL. The validity of neuropathy and neuropathic pain measures in patients with cancer receiving taxanes and platinums. Oncol Nurs Forum. 2011;38(2):133–142. doi: 10.1188/11.ONF.133-142. [DOI] [PubMed] [Google Scholar]
- 20.Smith EM, Cohen JA, Pett MA, Beck SL. The reliability and validity of a modified Total Neuropathy Score-reduced and neuropathic pain severity items when used to measure chemotherapy-induced peripheral neuropathy in patients receiving taxanes and platinums. Cancer Nurs. 2010;33(3):173–183. doi: 10.1097/NCC.0b013e3181c989a3. [DOI] [PubMed] [Google Scholar]
- 21.Guilhaumou R, Simon N, Quaranta S, et al. Population pharmacokinetics and pharmacogenetics of vincristine in paediatric patients treated for solid tumour diseases. Cancer Chemother Pharmacol. 2011;68(5):1191–1198. doi: 10.1007/s00280-010-1541-4. [DOI] [PubMed] [Google Scholar]
- 22.Hartman A, van Schaik RH, van der Heiden IP, et al. Polymorphisms in genes involved in vincristine pharmacokinetics or pharmacodynamics are not related to impaired motor performance in children with leukemia. Leuk Res. 2010;34(2):154–159. doi: 10.1016/j.leukres.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Diouf B, Crews KR, Lew G, et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA. 2015;313(8):815–823. doi: 10.1001/jama.2015.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lonnerholm G, Frost BM, Abrahamsson J, et al. Vincristine pharmacokinetics is related to clinical outcome in children with standard risk acute lymphoblastic leukemia. Br J Haematol. 2008;142(4):616–621. doi: 10.1111/j.1365-2141.2008.07235.x. [DOI] [PubMed] [Google Scholar]
- 25.Howard SC, Pedrosa M, Lins M, et al. Establishment of a pediatric oncology program and outcomes of childhood acute lymphoblastic leukemia in a resource-poor area. JAMA. 2004;291(20):2471–2475. doi: 10.1001/jama.291.20.2471. [DOI] [PubMed] [Google Scholar]
- 26.Lemerle J. Management of cancer in children in Africa. Arch Pediatr. 2003;10(Suppl 1):247s–249s. doi: 10.1016/s0929-693x(03)90458-1. [DOI] [PubMed] [Google Scholar]
- 27.Usmani GN. Pediatric oncology in the third world. Curr Opin Pediatr. 2001;13(1):1–9. doi: 10.1097/00008480-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Howard SC, Ribeiro RC, Pui CH. Strategies to improve outcomes of children with cancer in low-income countries. Eur J Cancer. 2005;41(11):1584–1587. doi: 10.1016/j.ejca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Hesseling P, Israels T, Harif M, Chantada G, Molyneux E. Practical recommendations for the management of children with endemic Burkitt lymphoma (BL) in a resource limited setting. Pediatr Blood Cancer. 2013;60(3):357–362. doi: 10.1002/pbc.24407. [DOI] [PubMed] [Google Scholar]
- 30.Israels T, van de Wetering MD, Hesseling P, van Geloven N, Caron HN, Molyneux EM. Malnutrition and neutropenia in children treated for Burkitt lymphoma in Malawi. Pediatr Blood Cancer. 2009;53(1):47–52. doi: 10.1002/pbc.22032. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination therapy: clinical and electrophysiological studies. Ann Neurol. 1994 Mar;35(3):304–11. doi: 10.1002/ana.410350310. [DOI] [PubMed] [Google Scholar]