Abstract
Many neuropsychiatric disorders are associated with abnormal decision making involving risk of punishment, but the underlying molecular basis remains poorly understood. Methyl CpG-binding Protein 2 (MeCP2) is an epigenetic factor that regulates transcription by directly binding to methylated DNA. Here, we evaluated MeCP2 expression in the context of risk-taking behaviors using the Risky Decision-making Task (RDT), in which rats make discrete choices between a small “safe” food reward and a large “risky” food reward accompanied by varying probabilities of punishment. In Experiment 1, expression of MeCP2 as assessed by immunoblotting in the medial prefrontal cortex (mPFC), but not the striatum, was inversely correlated with the degree of preference for the large, risky reward (risk taking) seven days after the last RDT test. In Experiment 2, MeCP2 expression 90 min after RDT testing, assessed using immunohistochemistry, was suppressed in both the dorsal mPFC (dmPFC) and nucleus accumbens compared to home cage controls, indicating that MeCP2 expression is modulated by RDT performance. Additional experiments revealed that RDT performance increased expression of MeCP2 phosphorylation at Ser421 (associated with neuronal activity and activation of gene expression) in dmPFC principal neurons. Finally, as in Experiment 1, lower expression of MeCP2 in the ventral mPFC was associated with greater risk taking under baseline conditions. Together, these findings indicate a complex regulatory role of MeCP2 in risky decision making, and suggest that epigenetic factors may be an important component of the molecular mechanisms underlying such decision-making processes.
Introduction
Decision making involves weighing the consequences of different options before selecting the most beneficial. Oftentimes, however, the most beneficial option can be accompanied by the risk of adverse or negative consequences. Although many individuals can weigh these benefits and risks and decide adaptively, decision making in neuropsychiatric disorders may skew toward elevated risk taking (e.g., in substance use disorders; Gowin et al., 2013) or pathological risk aversion (e.g., in anorexia nervosa; Kaye et al., 2013). In several of these disorders, maladaptive risk-based decision making may contribute substantially to their core features (e.g., in the case of substance use disorders, continued substance use despite the risk of adverse consequences). Hence, a better understanding of the mechanisms underlying risk-based decision making may yield novel insights into potential therapeutic approaches for these conditions.
Accumulating evidence suggests that epigenetic mechanisms of gene transcription can play a significant role in brain circuit maladaptations underlying neuropsychiatric disorders (Tsankova et al., 2007; Pena et al., 2014; Ibi and Gonzalez-Maeso, 2015). DNA methylation is one such mechanism that is linked to long-lasting changes in neural circuitry associated with psychiatric disorders (Holliday, 1999, Jaffe et al, 2016; Houtepen et al., 2016). Methyl CpG–binding protein-2 (MeCP2) is a DNA binding protein that serves as a reader for DNA methylation, where it can activate or repress gene transcription (Kishi and Macklis, 2004). MeCP2 is predominantly expressed in neurons in the brain, and mutations in MeCP2 are linked to the autism spectrum disorder Rett syndrome (Chahrour and Zoghbi, 2007; Skene et al., 2010). Further studies reveal that MeCP2 also plays important roles in aspects of behaviors linked to multiple psychiatric disorders, including anxiety, social behavior, and sensitivity to psychostimulant drugs of abuse (Cohen et al, 2011; Deng et al., 2014); however, its role in more complex behaviors (e.g., decision making) that may be altered in psychiatric disorders remains unknown.
The goal of the experiments in the current study was to investigate the role of MeCP2 in risk-based decision making using a rat model. To do so, we employed a Risky Decision-making Task (RDT) used previously in our laboratory, in which rats make discrete trial choices between a small “safe” food reward and a large “risky” food reward that is accompanied by variable probabilities of a mild foot shock (Simon et al. 2009). Prior work in our laboratory showed that individual differences in rats’ preference for the large, risky reward are stable from adolescence through adulthood, and predict acquisition of cocaine self-administration (with greater risk taking associated with greater cocaine intake; Mitchell et al. 2014). In addition, chronic cocaine self-administration causes an increase in risk taking behavior that persists through at least 6 weeks of abstinence (Mitchell et al. 2014; Ferland and Winstanley 2017). Given the stability of the risk-taking behavioral phenotype, it is likely that epigenetic factors contribute to its establishment and/or its modification by chronic cocaine. As with other forms of risk-based decision making, performance on the RDT engages a network of brain systems, including the medial prefrontal cortex, striatum (particularly ventral striatum), and amygdala (Simon et al. 2011; Mitchell et al. 2014; Orsini et al. 2015a; Orsini et al. 2015b; Winstanley and Floresco 2016). Hence, we investigated relationships between risk-taking behavior and expression of MeCP2 in these brain regions.
Materials and Methods
Subjects
Male Long–Evans rats (weighing 250-275 g upon arrival; Charles River Laboratories; n=36) were housed individually and kept on a 12 h light/dark cycle with free access to food and water. During behavioral testing, rats were food restricted to 85% of their free-feeding weight, with their weights adjusted upward by 5 g/week. Experiments were done in accordance with the University of Florida Institutional Animal Care and Use Committee, and followed guidelines established by the National Institutes of Health.
Apparatus
Behavioral testing occurred in eight behavioral test chambers (Coulbourn Instruments). Each chamber was housed in a sound-attenuating cubicle and contained a recessed food pellet delivery trough equipped with a photobeam to detect nose pokes into the trough and a 1.12 W lamp to provide illumination. The trough was located 2 cm above the floor in the center of the front wall of the chamber. Retractable levers were located to the left and right of the trough, 11 cm above the chamber floor. A 1.12 W house light was mounted on the rear wall of the sound-attenuating cubicle. The floor of the test chamber was made of stainless steel bars connected to a shock generator that delivered scrambled footshocks. An infrared activity monitor was positioned on top of each test chamber to measure locomotor activity. The chambers were connected to a computer running Graphic State 3.0 software, which controlled task event delivery and data collection.
Behavioral procedures
Overall Experiment 1 Design
Rats (n=12) were trained in the RDT for 26 daily sessions until stable performance was obtained (see Risky Decision-making Task section below for details). Following training, rats were returned to ad lib feeding and left undisturbed for 7 days, after which they were euthanized directly from their home cage.
Overall Experiment 2 Design
Rats (n=24) were trained in two cohorts in the RDT for 15-33 days, at which point performance had stabilized. Upon reaching stability, rats were divided into two groups matched for RDT performance. One group of rats (n=12) was tested in a final RDT session, after which they were returned to their home cage for 90 min prior to euthanasia. The other group of rats (n=12) did not receive this final test session, but were instead euthanized directly from their home cage.
Risky decision-making task
Before training on the RDT, rats were shaped to perform the various task components (nosepoking into the food trough, lever pressing) as described in previous publications (Simon et al., 2009; Mitchell et al., 2011). The food rewards used in the task were 45 mg grain-based food pellets (5TUM, TestDiet). Each RDT test session was 60 min in duration and consisted of five blocks of 18 trials each. Each 40 s trial started with illumination of the house and food trough lights. A nosepoke into the food trough extinguished the trough light and triggered extension of either a single lever (on forced choice trials) or both levers simultaneously (on free choice trials). If rats failed to nosepoke within 10 s, both lights were extinguished and the trial was counted as an omission. A press on one lever always resulted in delivery of a small food reward (1 pellet; small “safe” reward), whereas a press on the other lever always resulted in delivery of a large food reward (3 pellets) accompanied by a possible footshock (1 s, 0.4–0.5 mA; large “risky” reward). The probability of footshock was set at 0% in the first block and increased across five successive blocks of trials (25, 50, 75, and 100%). The large food reward was delivered upon every choice of the risky lever, regardless of shock delivery. The association of reward type (small safe or large risky) with the position of the lever (left or right) was counterbalanced across rats, but for each rat the association of reward type and lever position remained the same throughout testing. Each block of trials began with eight forced choice trials (only one lever was present) in which the punishment contingencies in effect for that block were established (4 presentations of each lever, randomly presented) and was followed by 10 free choice trials (both levers were present). If rats did not press a lever within 10 s, the levers were retracted and the lights extinguished, and the trial was scored as an omission. Food delivery was accompanied by re-illumination of the house light and food trough light, which were extinguished after rats retrieved the food pellets. On the forced choice trials, the probability of shock following a press on the large reward lever was dependent across the four trials in each block (i.e., in the 25% risk block, one and only one of the four forced choice trials (randomly selected) always resulted in shock, and in the 75% risk block, three and only three of the four forced choice trials always resulted in shock). In contrast, the probability of shock on the free choice trials was independent, such that the shock probability on each trial was the same regardless of shocks delivered on previous trials in that block.
Western Blot and Quantification
To harvest tissue for western blotting in Experiment 1, rats were deeply anesthetized with isofluorane, followed by rapid decapitation. The brains were rapidly dissected and placed in OCT for snap-freezing in an isopentane/dry ice bath, then stored at -80°. Coronal sections from frozen brains were cut on a freezing microtome, and mPFC and striatum were dissected by hand, weighed, and lysed in SDS sample buffer to a final concentration of 100 mg/mL. One mg total cell lysate/sample was run on SDS-PAGE and transferred to nitrocellulose for western blotting with the following antibodies: mouse anti-MeCP2 1:1,000 (Ab50005, Abcam), rabbit anti-GAPDH 1:2,000 (sc-25778, Santa Cruz), goat anti-rabbit 770 and goat anti-mouse 680 1:1,000 (Biotium). Fluorescent immunoreactivity was imaged on a LI-COR Odyssey system and quantified using ImageJ. MeCP2 expression was normalized to GAPDH expression in the same lane.
Immunohistochemistry and Quantification
To collect tissue for immunohistochemistry in Experiment 2, rats were given an overdose of Euthasol and perfused transcardially with PBS (0.1M) followed by 4% paraformaldehyde in 0.1M PBS. Brains were extracted and postfixed in 4% paraformaldehyde for 24 h, followed by 20% sucrose in 0.1M PBS. Brains were sectioned (40 μm) on a cryostat maintained at −19°, and sections were kept in cryo-preservative in -20° C until use. To evaluate expression of MeCP2 and pMeCP2, a previously established immunolabeling protocol was used (Deng et al. 2010). Brain sections through the areas of the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), basolateral amygdala (BLA) and central amygdala (CeA) were placed in a petri dish filled with PBS and photographed prior to immunolabeling. Sections from rats in different treatment groups were then mixed and underwent immunolabeling procedures within the same staining chamber (this approach achieves a high degree of comparability of labeling among tissue sections from different groups by exposing them to the same reagents and conditions). The photographic images collected before immunolabeling were used to identify the rats from which the sections were collected after immunolabeling. For immunolabeling, sections were incubated in mouse monoclonal anti-MeCP2 1:1,000 (AB5062P-100; Abcam), rabbit polyclonal anti-phospho-Ser421 MeCP2 1:15,000 (self-raised as in Deng et al., 2010), chicken polyclonal anti-parvalbumin (CPCA-Pavlb, EnCor Biotechnology), chicken polyclonal anti-calbindin (CPCA-Calb, EnCor Biotechnology), chicken polyclonal anti-calretinin (CPCA-Calretinin, EnCor Biotechnology) and a rabbit anti-DARPP-32 (Cat# SAB4503329; Sigma), overnight at 4°C. After washing 3 times in 0.1M PBS, the sections were incubated in goat anti-mouse AlexaFluor 488 and goat anti-rabbit AlexFluor 594 for 1 h at room temperature. Nuclei were labeled with DAPI to facilitate anatomical localization. To evaluate MeCP2 and pMeCP2 expression, fluorescent images of the designated brain regions (one section per animal) were collected on an Olympus Fluoview 300 microscope (MBF Bioscience, Williston, VT) and a Zeiss Axioskop 2 Plus microscope using fixed exposure conditions (i.e., exposure time and aperture) across all sections within an experiment. To quantify MeCP2, pMeCP2 and DAPI expression, we used ImageJ, using the standard protocol for the “Analyze Particles” function. For all images within the same experiment, we applied fixed settings for Threshold prior to Analyze, and multiplied the Total Area and the Mean Intensity to obtain the Relative Intensity of each marker within a region of interest. For percentages of pMeCP2-positive cells in dmPFC and NAc, numbers (Count) of pMeCP2-positive cell signals were divided by numbers of DAPI-positive cell signals.
Data analysis
Choice performance on the RDT was measured as the percentage of free choice trials in each block on which rats chose the large reward. To determine stable behavioral performance in the RDT, a repeated-measures ANOVA (session × trial block) was conducted on free choice trials from five consecutive sessions. A Greenhouse-Geisser correction was applied in cases in which sphericity was violated. As in previous work (Winstanley et al., 2004; Simon and Setlow, 2012), stable performance was defined as the absence of either a main effect of session or an interaction between session and trial block in this analysis. In Experiment 1, to evaluate relationships between RDT performance and MeCP2 expression, we conducted Spearman’s correlations between the western blotting quantitative values and the mean percent choice of the large reward, averaged across all blocks of trials on the RDT during stable baseline behavior. In Experiment 2, expression of MeCP2 and pMeCP2 in RDT-tested and home cage control rats was compared using unpaired t-tests (two-tailed). P-values were adjusted in cases in which the data violated a Levene’s test for equality of variances. Relationships between expression and individual RDT performance were evaluated in two ways due to non-normal distributions of the behavioral data. First, Spearman’s correlations were used to compare behavioral and MeCP2 expression data. Second, rats’ baseline performance was used to divide them into high and low risk taking groups (above and below 30% mean choice of the large, risky reward, which was a natural break in the distribution), and MeCP2 expression was compared between the two groups using unpaired t-tests (two-tailed). In all cases, p-values less than or equal to 0.05 were considered significant. For all analyses, outlying data points (points which fell beyond 2 SD from the group mean) were excluded. Such outliers were only evident for immunohistochemical measures in Experiment 2, resulting in different sample sizes across measures.
Results
Experiment 1
Relationships between individual differences in risk taking and MeCP2 expression in the medial prefrontal cortex and striatum
To evaluate potential contributions of MeCP2 to risk-taking behavior, rats were trained in the RDT until stable performance was achieved. Rats were then left undisturbed for 7 days, after which they were sacrificed for analysis of MeCP2 expression via western blotting. Figures 1A and B show the group mean performance on the RDT (expressed as the percentage of choices of the large reward plotted against risk of punishment, averaged across 5 consecutive sessions of stable task performance), as well as the individual performance of each rat in the group (averaged across the same 5 days of performance). Figures 1C and 1E show Western blots of brain tissue from mPFC and striatum collected from these same rats. Figures 1D and 1F show MeCP2 expression (normalized to GAPDH expression) in the mPFC and striatum, respectively, plotted as a function of mean RDT performance (averaged across all five trial blocks as in Simon et al. 2011; Mitchell et al. 2014). MeCP2 expression in the mPFC was significantly and negatively correlated with choice of the large, risky reward (ρ = -0.87, p < 0.001, n=11), such that less MeCP2 expression was associated with greater risk taking. In contrast, there was neither a relationship between MeCP2 expression in the striatum and RDT performance (ρ = -0.30, p = 0.34, n=12), nor was there a relationship between MeCP2 expression across the two brain regions (ρ = 0.23, p = 0.50).
Figure 1. MeCP2 expression in the medial prefrontal cortex correlates with RDT performance.
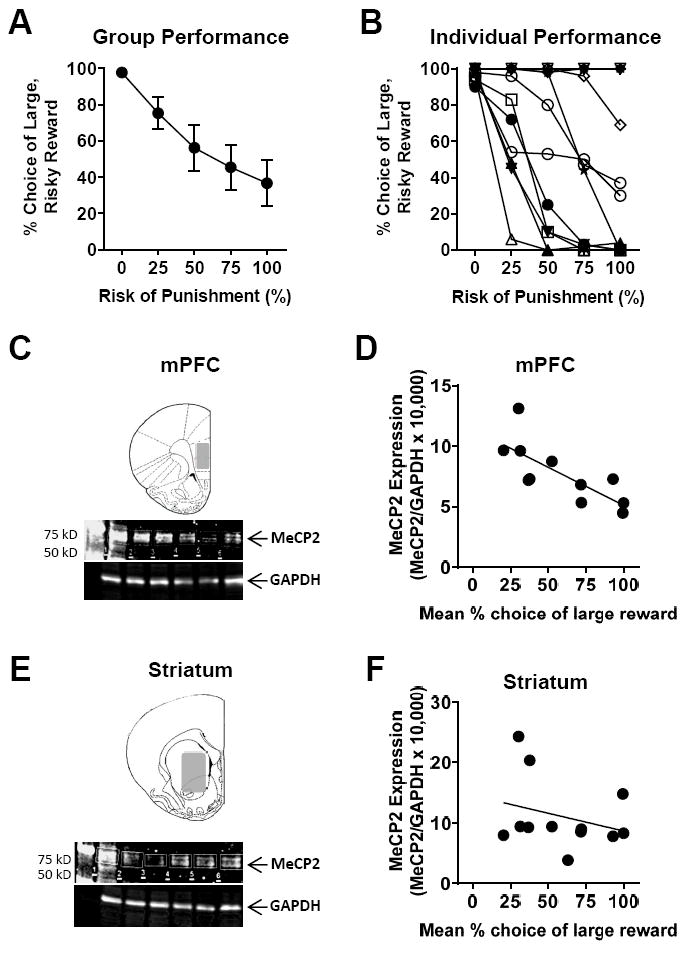
A, B. Stable behavioral performance in the RDT (mean % choice of the large, risky reward, n=12). A. Group mean performance (± standard error of the mean). B. Performance of each rat in the group (each line represents data from a single rat). C, E. Western blot for MeCP2 and GAPDH expression using tissue collected from mPFC (C) and striatum (E) as indicated in the shaded areas of each schematic. D, F. Scatterplots show individual mean percent choice of large, risky reward vs. MeCP2 expression (MeCP2/GAPDH x 10,000). There was a significant negative correlation between choice of the large, risky reward and MeCP2 expression in the mPFC (D; n=11), but not in the striatum (F; n=12).
Experiment 2
Acute effects of RDT performance on MeCP2 expression in the medial prefrontal cortex, nucleus accumbens, and amygdala
The results of Experiment 1 suggest either that constitutive MeCP2 expression in the mPFC predicts risk-taking behavior, or that experience with different patterns of performance in the RDT (risk seeking vs. risk averse) causes lasting changes in mPFC MeCP2 expression. Experiment 2 was designed to expand upon Experiment 1 in several ways. First, immunohistochemistry was used to detect MeCP2 expression, to allow a greater degree of anatomical specificity. Second, expression of both MeCP2 and phospho-MeCP2 (pMeCP2, which is implicated in transcriptional activation; Chen et al. 2003) was evaluated. Third, changes in MeCP2 expression resulting from acute performance in the RDT were compared with home cage control conditions.
Rats were first trained in the RDT until stable performance was achieved. They were then divided into two groups (RDT testing, n=12 and home cage; HC, n=12) matched for RDT performance (a two factor repeated measures ANOVA comparing the two groups showed a main effect of block, F(4,88)=38.68, p<0.001, but no main effect of group, F(1, 22)=0.02, p=0.90 or block × group interaction, F(4,88)=0.18, p=0.67; Figure 2A). On the following day, rats in the RDT group underwent a single RDT session and were euthanized 90 min after the start of the session (i.e., 30 min after completion of the session). Home cage controls were euthanized on the same day but in the absence of behavioral testing. Figures 2B, 2D, 2F and 2H show representative images of MeCP2 immunolabeling in the dmPFC, vmPFC, NAc, BLA, and CeA in rats in the HC and RDT groups respectively. Figures 2C, 2E, 2G and 2I show the group means of MeCP2 expression in the respective brain regions. In the dmPFC, MeCP2 expression was significantly decreased in the RDT compared to the HC group (t(18)=2.32, p=0.03, n=10,10; Fig. 2C), whereas there was no group difference in the vmPFC (t(22)=-0.53, p=0.60, n=12,12; Fig. 2E). MeCP2 expression was also significantly decreased in the RDT group compared to the HC group in the NAc (t(16)=2.94, p=0.01, n=9,9; Fig. 2G). Finally, MeCP2 expression was decreased in the BLA (t(18)=2.18, p=0.04, n=10,10; Fig. 2I), but was unchanged in the CeA (t(14) =-0.24, p=0.81, n=8,8; Fig. 2I). Considered together, these data show that acute behavioral testing in the RDT causes a decrease in MeCP2 expression across limbic cortico-striatal brain systems.
Figure 2. MeCP2 expression in the mPFC, NAc, and amygdala after acute RDT testing.
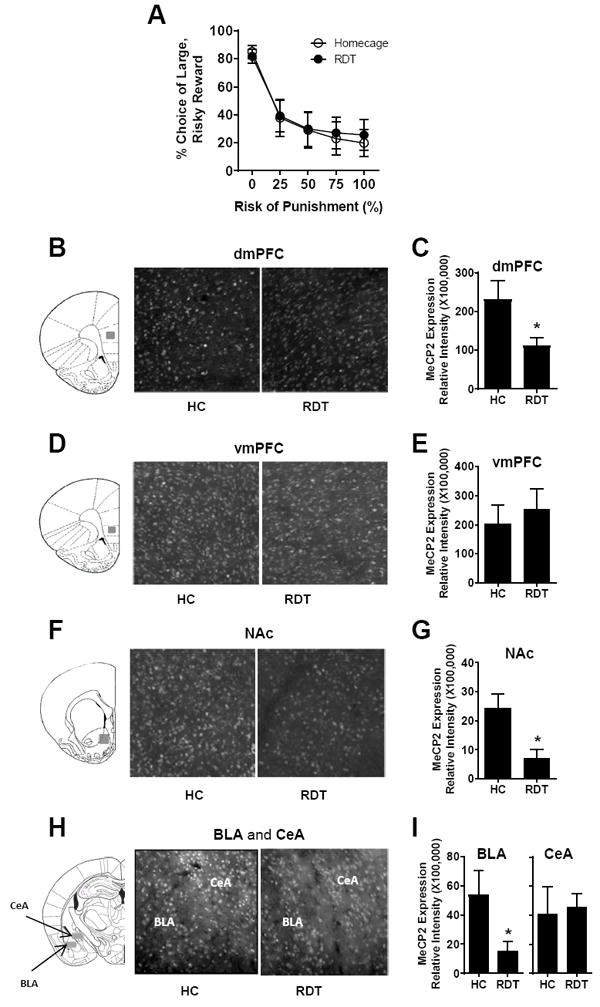
A. Both RDT (n=12) and home cage (HC; n=12) control groups decreased their choice of the large, risky reward as the risk of punishment increased across the session. Graph represents mean % choice of the large, risky reward. B, D, F, H. Tissue sections across dorsal medial prefrontal cortex (dmPFC), ventral medial prefrontal cortex (vmPFC), nucleus accumbens (NAc), basolateral amygadala (BLA) and central amygdala (CeA), respectively, were processed with immunohistochemistry to evaluate MeCP2 expression in the regions indicated by gray shaded areas in the coronal section schematics (Paxinos and Watson, 2007). C, E, G, I. Quantification of MeCP2 expression in the dmPFC (n=10,10), vmPFC (n=12,12), NAc (n=9,9), BLA (n=10,10) and CeA (n=8,8), respectively, as represented by relative fluorescence intensity. * denotes p<0.05, RDT group compared to HC group. Error bars represent standard error of the mean.
MeCP2 immunolabeling was also evaluated in comparison to RDT performance (both under baseline conditions and in the test session just prior to sacrifice). Because of the strongly non-normal distribution of RDT performance, this comparison was conducted in two ways. First, a non-parametric correlation (Spearman’s rho) was used to compare individual values for each measure. There were no significant relationships between MeCP2 immunolabeling and either measure of RDT performance using this analysis. Second, rats’ baseline performance was used to divide them into high and low risk taking groups, and MeCP2 expression was compared between the two groups. MeCP2 expression in vmPFC showed a robust difference between high and low risk taking groups (t(22)=-3.15, p=.005), with expression being significantly lower in the high vs. the low risk taking group. No other differences reached statistical significance (ts<1.32, ps>0.20).
Acute effects of RDT performance on expression of Ser421-phosphorylated MeCP2 in the mPFC, NAc, and amygdala
MeCP2 phosphorylation at Ser421 (pMeCP2) is a post-translational modification associated with neuronal activity-dependent gene transcription (Chen et al., 2003, Zhou et al, 2006). More importantly, phosphorylation at this site can switch MeCP2 from a transcriptional repressor to a transcriptional activator, which may in part explain observations that MeCP2 can have opposite effects on gene expression (Zhou et al., 2006; Chahrour et al., 2008). To evaluate how pMeCP2 expression changes in response to RDT testing, sections from the same brains used for MeCP2 expression were probed with an antibody against pMeCP2. Figures 3A, 3C, 3E and 3G show representative images of pMeCP2 immunolabeling in the dmPFC, vmPFC, NAc, BLA, and CeA in rats in the HC and RDT groups. Figures 3B, 3D, 3F and 3H show group means for quantification in the respective brain regions. In the dmPFC, pMeCP2 expression was significantly increased in the RDT compared to the HC group (t(18)=-2.76, p=0.01, n=10,10; Fig. 3B), whereas there was no group difference in the vmPFC (t(22)=-0.30, p=0.77, n=12,12; Fig. 3D). In the NAc, there was a trend toward a significant decrease in pMeCP2 expression in the RDT compared to the HC group (t(16)=1.84, p=0.08, n=9,9; Fig. 3F). There were no group differences in expression in either the BLA (t(20)=0.76, p=0.46, n=11,11) or CeA (t(16)=0.50, p=0.62, n=9,9; Fig. 3H). It is noteworthy that the expression profile of pMeCP2 was very different between the dmPFC and NAc. Although significant numbers of cells expressed pMeCP2 in the dmPFC (Fig. 3A), only a few cells expressed pMeCP2 in the NAc (Fig. 3E). To quantitatively evaluate this difference, we obtained the ratios of cells expressing pMeCP2 over the total number of cells in each region as indicated by DAPI staining. We found that 52.6% of the cells in the dmPFC were pMeCP2-positive after the RDT test, whereas only 1.6% of the cells in the NAc cells were pMeCP2-positive.
Figure 3. MeCP2 phosphorylation at Ser421 (pMeCP2) in the mPFC, NAc and amygdala after acute RDT testing.
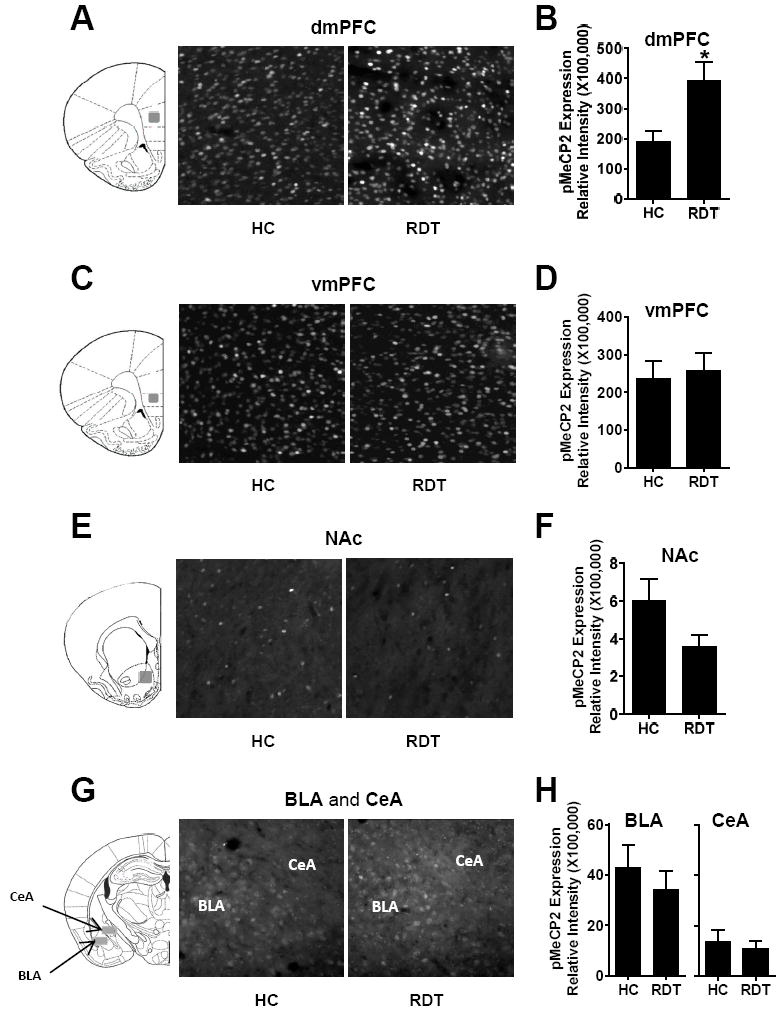
A, C, E, G. Tissue sections across dorsal medial prefrontal cortex (dmPFC), ventral medial prefrontal cortex (vmPFC), nucleus accumbens (NAc), basolateral amygadala (BLA) and central amygdala (CeA), respectively, were processed with immunohistochemistry to evaluate pMeCP2 expression in the regions indicated by gray shaded areas in the coronal section schematics (Paxinos and Watson, 2007). B, D, F, H. Quantification of pMeCP2 expression in the dmPFC (n=10,10), vmPFC (n=12,12), NAc (n=9,9), BLA (n=11,11) and CeA (n=9,9), respectively, as represented by relative fluorescence intensity. * denotes p<0.05, RDT group compared to HC group. Error bars represent standard error of the mean.
Finally, pMeCP2 immunolabeling was evaluated in comparison to RDT performance (both under baseline conditions and in the test session just prior to sacrifice). As for the analysis of MeCP2 expression above, this comparison was conducted in two ways. First, a non-parametric correlation (Spearman’s rho) was used to compare individual values for each measure as in Experiment 1. There were no significant relationships between pMeCP2 immunolabeling and either measure of RDT performance. Second, rats’ baseline performance was used to divide them into high and low risk taking groups, and pMeCP2 expression was compared between the two groups. There were no differences in pMeCP2 expression in any of the brain regions sampled (ts<1.39, ps>0.18).
Cell type specificity of pMeCP2 expression in dmPFC and NAc
As described above, MeCP2 is linked to gene suppression (Jones et al., 1998), whereas pMeCP2 is linked to gene activation (Chen et al. 2003). In the dmPFC, the findings of MeCP2 down-regulation (Fig. 2C) and pMeCP2 up-regulation (Fig. 3B) suggest an overall effect of gene activation following RDT performance. In the NAc however, a distinct pattern of expression was observed, such that MeCP2 (Fig. 2G) was down-regulated but pMeCP2 (Fig. 3F) was unchanged. To better understand these changes in MeCP2 and pMeCP2 expression between the dmPFC and NAc, we evaluated the cell types associated with pMeCP2 expression following RDT testing.
We used antibodies against dopamine- and cAMP-regulated phosphoprotein 32 kDa (DARPP-32), which is a specific marker for striatal medium spiny neurons (MSNs), and a panel of markers for GABAergic interneurons (parvalbumin, calbindin and calretinin) in brain sections through the dmPFC and NAc of rats from Experiment 2. As illustrated in Figure 4, pMeCP2-expressing cells did not express any of the interneuron markers in dmPFC (Fig 4A). As roughly half of the cells expressed pMeCP2 in the dmPFC, these data suggest that pMeCP2 expression is likely limited to glutamatergic pyramidal neurons. In the NAc, pMeCP2-expressing cells did not express DARPP-32, suggesting that pMeCP2 was not induced in MSNs (Fig 4B). Instead, pMeCP2 was co-expressed with parvalbumin (found in so-called “fast-spiking” interneurons in the striatum (Kawaguchi et al. 1995)), but not with other interneuron markers (Fig 4B). These results indicate that changes in pMeCP2 expression are selective to specific neuron populations in the dmPFC and NAc, suggesting a potential function of pMeCP2 in modifying specific circuits selectively in each brain region in response to RDT testing.
Figure 4. pMeCP2 expression in specific cell types in the dmPFC and NAc.
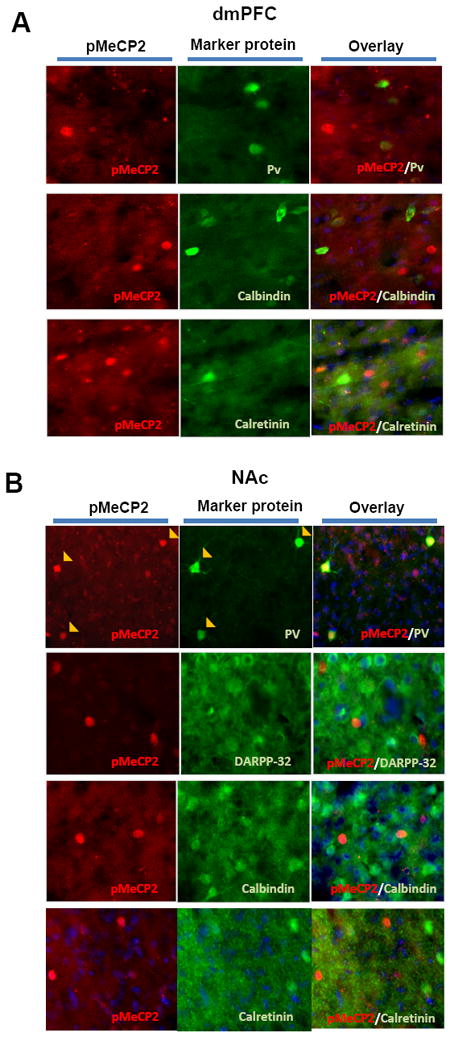
A. In the dorsal medial prefrontal cortex (dmPFC), pMeCP2 is not expressed in parvalbumin (PV)-, calbindin- or calretinin-expressing cells. B. In the nucleus accumbens (NAc), pMeCP2 is expressed in PV-expressing cells, but not in dopamine- and cAMP-regulated phosphoprotein 32 kDa (DARPP-32)-, calbindin- or calretinin-expressing cells. Left and middle columns in both panels show immunolabeling for pMeCP2 or marker proteins as indicated; right columns are merged images. Yellow filled arrowheads indicate cells that were double-labeled.
Discussion
Alterations in propensity for engaging in risky behavior are associated with numerous psychiatric conditions. The molecular mechanisms underlying these propensities are poorly understood, but likely involve epigenetic factors that can dynamically regulate gene expression. The results reported here show that stable individual differences in risk-taking behavior in a rat model are correlated with expression of the epigenetic factor MeCP2 in the mPFC, and that risky decision-making task performance induces rapid changes in MeCP2 expression and phosphorylation in brain systems implicated in risky decision making. Specifically, MeCP2 expression in the mPFC, but not the NAc, is negatively associated with preference for the large, risky reward following stable RDT performance in well-trained rats (Fig 1D and Experiment 2). In addition, MeCP2 expression in the dmPFC, NAc and BLA is rapidly down-regulated by acute RDT testing compared to untested home cage controls (Fig 2). As down-regulation of MeCP2 is believed to be associated with active gene expression (Jones, et al, 1998), these results suggest that risk-taking behavior in the context of the RDT may involve a role for MeCP2 in key brain regions important for this form of decision making. In addition, MeCP2 phosphorylation at S421 is up-regulated in the major population of neurons in the dmPFC (Fig 3), but trends toward down-regulation in parvalbumin-positive interneurons in the NAc (Figs. 3, 4). As S421 phosphorylation of MeCP2 is associated with activity-dependent de-repression of gene transcription (Chen et al. 2003), these results suggest that risk-taking behavior also engages pMeCP2-mediated gene expression in selected neuron populations that function in specific circuits involving the dmPFC and NAc.
Because of its long-lasting and global regulatory effects on gene expression, DNA methylation-mediated epigenetic mechanisms of gene transcription have been proposed as an important component of the molecular basis of stable behavior (Holliday 1999). MeCP2 is a key protein factor in the brain that serves as a “reader” of methyl DNA by binding directly to DNA at methylated cytosine and serving as a docking site for histone modifier proteins to repress gene transcription. Hence, reduced MeCP2 expression is linked to active gene transcription (Jones et al., 1998). Since MeCP2 is constitutively expressed in almost all neurons in the brain, and it binds densely to DNA at methylated cytosine (Skene et al, 2010), the finding that MeCP2 in the mPFC is inversely related to the degree of risk taking in the RDT suggests that the expression level of MeCP2 in the mPFC could be a predisposing factor for different risk-taking phenotypes. Interestingly, Experiment 2 showed that this inverse relationship was evident only in the vmPFC, whereas only in the dmPFC was MeCP2 expression significantly altered by RDT testing. This dissociation suggests that acute changes in MeCP2 expression in the dmPFC are important for behavioral adaptation to RDT experience, whereas more constitutive levels of MeCP2 expression in the vmPFC may regulate RDT performance. Further investigation of the function of MeCP2 in these two PFC subregions may reveal the specific role each plays in risky decision-making processes.
It is possible that individual risk tendencies directed by other factors could cause lasting alterations in mPFC MeCP2 expression (e.g., that frequent choices of the risky option and the consequences thereof cause lasting MeCP2 down-regulation in the mPFC). Moreover, the exact role of the mPFC in risk taking under conditions of explicit punishment has not yet been well-defined (and some evidence suggests that its role in other forms of decision making is to allow flexible adaptations of choice strategy in response to changing outcome contingencies, rather than regulating choice behavior per se (St. Onge and Floresco 2010)). Nevertheless, the fact that MeCP2 expression in the mPFC was associated with RDT performance across different conditions (both 7 days and 90 min after the last test session, and across both acutely tested and home cage control groups) lends support to a role for this epigenetic factor in the decision making process. Future experiments in which MeCP2 expression or function is manipulated directly will be required to distinguish among these possibilities.
Intense research over the past two decades has revealed multiple roles for MeCP2 in regulating gene transcription, most surprising among which is that MeCP2 can mediate not only gene repression but also gene activation (Chahrour 2008). Further studies find that post-translational modifications of MeCP2, which include phosphorylation, sumoylation, and others (Zhou et al, 2006; Tao et al., 2009; Ebert et al., 2013), significantly enhance the ability of MeCP2 to regulate gene transcription to achieve temporal and spatial specificity, as well as determine the direction of gene expression (activation or repression). In particular, MeCP2 phosphorylation at S421 can activate gene transcription in a neuronal activity-dependent manner (Zhou et al, 2006). We found previously that pMeCP2 is induced in fast-spiking (parvalbumin-positive) NAc interneurons following repeated amphetamine administration in a behavioral sensitization paradigm (Deng et al. 2010). Our current finding that pMeCP2 expression in the NAc and dmPFC is altered by RDT performance (Figs. 3, 4) further supports a potentially important role of pMeCP2 in modulating circuit function serving specific behaviors.
It is notable that, unlike in the dmPFC where MeCP2 down-regulation is accompanied by up-regulation of pMeCP2, pMeCP2 expression in the NAc trends toward down-regulation (Fig 3C), which suggests two possibly opposing directions of gene regulation—gene activation by MeCP2 down-regulation and gene suppression by lower pMeCP2 expression—concurrently in the same brain region. This difference between the dmPFC and NAc in the patterns of MeCP2 and pMeCP2 expression likely coincides with the different circuits upon which pMeCP2 may act to modulate these two brain regions after RDT performance. As a cortical structure, the dmPFC primarily contains pyramidal excitatory neurons. As 52.6% of the cells in the dmPFC express pMeCP2 after RDT testing, these data suggest that pMeCP2 is primarily induced in glutamatergic pyramidal neurons in the dmPFC. In contrast, we found that pMeCP2 is restricted to 1.6% of the cell population in the NAc, which we further identified as parvalbumin-positive (Fig 4B). These results suggest that whereas both MeCP2- and pMeCP2-mediated mechanisms may act coherently to drive gene transcription in pyramidal neurons in the dmPFC, they act independently to drive gene transcription in MSNs and parvalbumin-positive interneurons in the NAc during RDT performance. Interestingly, as we observed in our previous work that pMeCP2 induction is also restricted to parvalbumin-positive interneurons in the NAc after activating dopamine or serotonin signaling by administering amphetamine (Deng et al 2010) or imipramine (Hutchinson et al., 2012), such targeted pMeCP2 regulation in NAc may represent an intrinsic regulatory apparatus for circuit adaptations.
Even though RDT performance in Experiment 2 was associated with changes in MeCP2 and pMeCP2 expression in several brain regions, we should be cautious in concluding that MeCP2 is linked to risk taking performance per se. For example, in Experiment 2, other factors may have contributed to the differences observed between the RDT and HC groups. In contrast to the HC group, rats tested in the RDT were exposed to both aversive (footshock) and appetitive (food reward) stimuli prior to sacrifice, which, independent of the decision-making process, could have driven the changes in MeCP2 expression. Indeed, MeCP2 is required for normal behavioral responses to aversive stimuli in fear conditioning (Adachi et al., 2009). Similarly, MeCP2 expression is induced after rats undergo operant food conditioning (but not passive food delivery; Bodetto et al., 2014). Thus, mere exposure to the footshock or the food reward may have driven changes in MeCP2 expression in the current experiment. Handling by the experimenters, as well as other enriching components of behavioral testing, could also account for the differences in MeCP2 expression between the two groups. Since MeCP2 is found in almost all neurons to potentially act as a global regulator of gene transcription in response to neuronal activity, it is not surprising that MeCP2 expression changes following various forms of behavioral testing. It should be noted, however, that the current results do show that MeCP2 and pMeCP2 are dynamically regulated by acute behavioral experience in a regionally- and cell-specific manner. A key task for future studies will be to identity the specific neural circuits in which MeCP2 may act to regulate specific behaviors. Thus, to directly test whether risk taking alters MeCP2 expression, more targeted methods, such as virally-mediated genetic deletion, are required.
In conclusion, the results of Experiments 1 and 2 suggest a potentially important role for the epigenetic factor MeCP2 in regulating risky decision making (and, to our knowledge, provide the first evidence for potential epigenetic contributions to decision making). As maladaptive risk taking is a feature of several psychiatric disorders, further research in this area could reveal novel mechanisms and therapeutic targets for restoring and maintaining adaptive decision making.
Highlights.
MeCP2 Expression in the mPFC, not in the striatum, inversely correlates with the preference for risky reward after RDT.
MeCP2 expression was suppressed in both dorsal mPFC (dmPFC) and nucleus accumbens (NAc) 90 min after RDT testing.
RDT performance increased expression of MeCP2 phosphorylation at Ser421 (pMeCP2), in the dmPFC principal neurons.
Acknowledgments
This work was supported by a NARSAD Young Investigator Award 19279 (JVD), DA029421 and DA036534 (BS) and a McKnight Brain Institute Fellowship (CAO), and a Thomas H. Maren Postdoctoral Fellowship (CAO). We thank Dr. Jennifer Bizon (University of Florida College of Medicine) for her assistance with this project, and EnCor Biotechnology, Inc. (Gainesville, FL) for antibody supplies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi M, Autry AE, Covington HE, 3rd, Monteggia LM. MeCP2-mediated transcription repression in the basolateral amygdala may underlie heightened anxiety in a mouse model of Rett syndrome. J Neurosci. 2009;29:4218–27. doi: 10.1523/JNEUROSCI.4225-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodetto SP, Romieu P, Sartori M, Tesone-Coelho C, Majchrzak M, Barbelivien A, Zwiller J, Anglard P. Differential regulation of MeCP2 and PP1 in passive or voluntary administration of cocaine or food. Int J Neuropsychopharmacology. 2014;17:2031–44. doi: 10.1017/S1461145714000972. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–9. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Cohen S, Gabel HW, Hemberg M, Hutchinson AN, Sadacca LA, Ebert DH, Harmin DA, Greenberg RS, Verdine VK, Zhou Z, Wetsel WC, West AE, Greenberg ME. Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron. 2011;72:72–85. doi: 10.1016/j.neuron.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JV, Rodriguiz RM, Hutchinson AN, Kim IH, Wetsel WC, West AE. MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat Neurosci. 2010;13:1128–36. doi: 10.1038/nn.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JV, Wan Y, Wang X, Cohen S, Wetsel WC, Greenberg ME, Kenny PJ, Calakos N, West AE. MeCP2 phosphorylation limits psychostimulant-induced behavioral and neuronal plasticity. J Neurosci. 2014;34:4519–27. doi: 10.1523/JNEUROSCI.2821-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert DH, Gabel HW, Robinson ND, Kastan NR, Hu LS, Cohen S, Navarro AJ, Lyst MJ, Ekiert R, Bird AP, Greenberg ME. Activity-dependent phosphorylation of MeCP2 threonine 308 regulates interaction with NCoR. Nature. 2013;499:341–345. doi: 10.1038/nature12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland JN, Winstanley CA. Risk-preferring rats make worse decisions and show increased incubation of craving after cocaine self-administration. Addict Biol. 2017;22:991–1001. doi: 10.1111/adb.12388. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Mackey S, Paulus MP. Altered risk-related processing in substance users: imbalance of pain and gain. Drug Alcohol Depend. 2013;132:13–21. doi: 10.1016/j.drugalcdep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R. Is there an epigenetic component in long-term memory? J Theor Biol. 1999;200:339–41. doi: 10.1006/jtbi.1999.0995. [DOI] [PubMed] [Google Scholar]
- Houtepen LC, van Bergen AH, Vinkers CH, Boks MP. DNA methylation signatures of mood stabilizers and antipsychotics in bipolar disorder. Epigenomics. 2016;8:197–208. doi: 10.2217/epi.15.98. [DOI] [PubMed] [Google Scholar]
- Hutchinson AN, Deng JV, Aryal DK, Wetsel WC, West AE. Differential regulation of MeCP2 phosphorylation in the CNS by dopamine and serotonin. Neuropsychopharmacology. 2012;37:321–337. doi: 10.1038/npp.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, González-Maeso J. Epigenetic signaling in schizophrenia. Cell Signal. 2015;27:2131–6. doi: 10.1016/j.cellsig.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AE, Gao Y, Deep-Soboslay A, Tao R, Hyde TM, Weinberger DR, Kleinman JE. Mapping DNA methylation across development, genotype and schizophrenia in the human frontal cortex. Nat Neurosci. 2016;19:40–7. doi: 10.1038/nn.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–91. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–35. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 2013;36:110–120. doi: 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Mitchell MR, Vokes CM, Blankenship AL, Simon NW, Setlow B. Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology (Berl) 2011;218:703–12. doi: 10.1007/s00213-011-2363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Weiss VG, Beas BS, Morgan D, Bizon JL, Setlow B. Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling. Neuropsychopharmacology. 2014;39:955–62. doi: 10.1038/npp.2013.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Trotta RT, Bizon JL, Setlow B. Dissociable roles for the basolateral amygdala and orbitofrontal cortex in decision-making under risk of punishment. J Neurosci. 2015a;35:1368–79. doi: 10.1523/JNEUROSCI.3586-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB. Neural mechanisms regulating different forms of risk-related decision-making: Insights from animal models. Neurosci Biobehav Rev. 2015b;58:147–67. doi: 10.1016/j.neubiorev.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press; London: 2007. p. 456. [Google Scholar]
- Peña CJ, Bagot RC, Labonté B, Nestler EJ. Epigenetic signaling in psychiatric disorders. J Mol Biol. 2014;426:3389–412. doi: 10.1016/j.jmb.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34:2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, Bañuelos C, Vokes CM, Taylor AB, Haberman RP, Bizon JL, Setlow B. Dopaminergic modulation of risky decision-making. J Neurosci. 2011;31:17460–70. doi: 10.1523/JNEUROSCI.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Setlow B. Modeling risky decision making in rodents. Methods Mol Biol. 2012;829:165–75. doi: 10.1007/978-1-61779-458-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, Andrews R, Bird AP. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell. 2010;37:457–68. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Prefrontal cortical contribution to risk-based decision making. Cereb Cortex. 2010;20:1816–28. doi: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- Tao J, Hu K, Chang Q, Wu H, Sherman NE, Martinowich K, Klose RJ, Schanen C, Jaenisch R, Wang W, Sun YE. Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function. Proc Natl Acad Sci USA. 2009;106:4882–4887. doi: 10.1073/pnas.0811648106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–67. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


