Abstract
Mounting evidence suggests that weight management and physical activity (PA) improves overall health and well-being, and reduces the risk of morbidity and mortality among cancer survivors. While many opportunities exist to include weight management and PA into routine cancer care, several barriers remain. This review summarizes key topics addressed in a recent National Academies of Science, Engineering, and Medicine Workshop entitled, “Incorporating Weight Management and Physical Activity throughout the Cancer Care Continuum.” Discussions related to body weight and PA among cancer survivors included: (i) current knowledge and gaps related to health outcomes; (ii) effective intervention approaches; (iii) addressing the needs of diverse populations of cancer survivors; (iv) opportunities and challenges of workforce, care coordination, and technologies for program implementation; (v) models of care; and (vi) program coverage. While more discovery is still needed for the provision of optimal weight management and PA programs for cancer survivors, obesity and inactivity currently jeopardize their overall health and quality-of-life. Actionable future directions are presented for research, practice and policy changes required to assure the availability of effective, affordable, and feasible weight management and PA services for all cancer survivors as a part of their routine cancer care.
Keywords: weight management, physical activity, supportive care, nutrition, survivorship
With growing evidence on the association between obesity, excess weight and cancer, the National Cancer Policy Forum (NCPF) of the National Academies of Sciences, Engineering, and Medicine hosted a workshop in 2011 on the “Role of Obesity in Cancer Survival and Recurrence.” This workshop examined epidemiologic evidence, biological mechanisms, preclinical studies, and a limited number of randomized controlled trials (RCTs) of interventions that promoted weight loss via caloric restriction or increased physical activity (PA) in patients with cancer.1 Two scientific papers emanated from this endeavor – one that was more mechanistic in nature,2 and the other focused on translational research for patient care.3 Both emphasized gaps in knowledge.
Six years later, and after considerable advances in this arena, including a position paper and campaign on obesity and cancer issued by the American Society of Clinical Oncology (ASCO),4 another NCPF workshop on obesity and cancer was convened - this time focusing on translating research findings into clinical practice and community-based programs. This workshop, “Incorporating Weight Management and Physical Activity throughout the Cancer Care Continuum,” also drew international experts, but with greater emphasis on behavioral science, clinical research, public policy, dissemination science, and health economics. Workshop presentations and discussions examined the available evidence regarding the value of promoting weight management and PA across the period of cancer survivorship, from diagnosis to end of life,5, 6 along with evaluation of opportunities and challenges in current approaches to promote PA and weight management. To ensure that discussions would be patient-centered, the opening session of the workshop featured two cancer survivors who shared their experiences with cancer treatment, weight management, and physical activity (see key excerpts Table 1). Also, throughout the workshop, presentations and discussions illustrated the broad spectrum of diversity among cancer survivors in terms of cancer type, stage, molecular subtype, length of survivorship, comorbidity and functional status, age, race/ethnicity, gender, and geographical location that require consideration in tailoring weight management and PA interventions and recognition that “one size does not fit all.” The workshop culminated in a discussion of whether the strength of evidence warranted the provision and coverage of services for weight management and PA that are specifically directed toward cancer survivors, and ways to enhance the delivery of these services to the growing sector of cancer survivors in this nation, who currently number well over 15 million.7 Herein, we report a summary of the workshop presentations, discussions, and conclusions.
Table 1.
Prioritizing Patient Voices
| It is essential to hear and prioritize the voices of cancer survivors. Below are select statements from two patient advocates who, despite differing backgrounds, diagnoses, and points in their survivorship journey, offer statements with common themes. |
|---|
| Karen Cochrane is a white, 53-year old nurse who was recently diagnosed with early-stage breast cancer and is currently receiving chemotherapy. She is concerned about her overall well-being and is working toward reaching a healthy weight and being physically active. Robert Harrison is a black, 72-year old, retired, businessman who was diagnosed with metastatic prostate cancer 14 years ago. Currently, he is actively monitoring his cancer with his urologist. He has played an active role in his care and lost over 60 pounds, and he considers himself a cancer “thriver.” Both voice their thoughts about weight management and physical activity during cancer treatment and beyond: |
|
Body Weight, Physical Activity and Health Outcomes for Cancer Survivors: Knowledge & Gaps
This first section, which provides a foundation for the remainder of the paper, is devoted to critically evaluating the state of knowledge regarding the relationship between body weight or PA, and health outcomes for cancer survivors. It begins with an overview of the evidence on cancer outcomes and then addresses other outcomes, such as quality-of-life (QOL) and fatigue. Lastly, it identifies evidence gaps addressed by ongoing and recent trials, gaps that remain, and the opportunities to fill these gaps.
Overview of Obesity and Cancer Outcomes
Excess weight gain, overweight, and obesity are associated with an increased risk of many cancers; recently, the International Agency for Research on Cancer reported that there is sufficient evidence to conclude that avoidance of excess body fat is associated with a lower risk for cancers of the endometrium, esophagus (adenocarcinoma), gastric cardia, kidney (renal cell), multiple myeloma, meningioma, liver, pancreas, colorectum, gallbladder, breast (postmenopausal), ovary, and thyroid.8 There also is growing observational evidence that obesity is associated with poorer cancer outcomes among individuals with cancer. The largest body of evidence relates to breast cancer. A meta-analysis of 82 studies involving 213,075 women with breast cancer found a 41% relative increase in all-cause mortality for women with obesity vs those of normal weight (relative increases were 75% in premenopausal women and 34% in postmenopausal women).9 This study also found increased all-cause mortality for overweight women, albeit the relative increases were smaller. Another meta-analysis found that the risk of mortality associated with overweight and obesity was similar for patients with estrogen receptor (ER) positive and negative breast cancer, although some (but not all) subsequent individual studies have suggested risk may be present only in women with ER positive disease.10 Among breast cancer survivors treated with anthracyclines, obesity also is associated with greater cardiotoxicity.11 Similarly, adverse associations of obesity with survival are reported for endometrial, prostatic, pancreatic, colorectal and ovarian cancer, as well as some hematologic malignancies.3, 4 In contrast, overweight and obesity are associated with somewhat better outcomes in lung, esophageal and kidney cancer – cancers in which the morbidity of cachexia and advanced stage at diagnosis are more common.12
The association of excess weight gain, overweight, and obesity with cancer is biologically complex. Increased adiposity results in changes in adipose tissue, including death of adipocytes leading to infiltration of inflammatory cells, as well as secretion of cytokines and other factors that stimulate cancer cell growth, invasion, angiogenesis, and metastasis.13 Increased adiposity also is associated with changes in systemic physiology, including insulin resistance, dysglycemia, altered adipokines, and increased inflammation; together these changes enhance signaling through key growth pathways (e.g., PI3K, RAS, JAK-STAT) and alter cellular metabolism.2, 14 These obesity-associated effects at the tissue and physiologic levels invoke changes in many of the hallmarks of cancer,15 including sustained proliferative signaling, activated invasion and metastasis, induced angiogenesis, and resistance to cell death.2 Overweight and obesity also enable deregulation of cellular energetics and tumor-promoting inflammation.2, 15 While observational data, obtained from a multitude of studies, coupled with this strong biologic rationale provide strong support for an association of obesity with poor cancer outcomes, there is insufficient evidence to conclude that this association is causal.
Studies examining weight-related changes in the transcriptome of breast cancers indicate that cancers developing in women with obesity are biologically different from those in women of normal weight in terms of altered gene regulation and expression.16 At this time, it is unclear whether reversal of obesity will lead to reversal of these differences or lead to improved cancer outcomes. RCTs of weight loss or pharmacologic interventions that reverse obesity-associated changes related to overweight and obesity are needed.
Overview of the Evidence on Cancer Outcomes Related to Physical Activity
Evidence linking increased PA to improved cancer outcomes is preliminary, but promising. A recent systematic review and pooled analysis of 26 observational studies found that cancer survivors who engaged in higher levels of PA (>18 MET-hours/week) had a 37% lower risk of dying from cancer, compared to those who engaged in lower levels of PA (<1.5 MET-hours/week) (hazard ratio [HR]=0.63; 95% confidence interval [CI]: 0.54–0.73).17 This risk reduction is remarkably consistent across breast, colorectal and prostate cancer survivors. There also is growing evidence that the association between PA and cancer mortality varies by specific molecular or genetic markers, implying a possible precision medicine approach to exercise oncology (e.g., a strong inverse association between PA and colon cancer mortality is noted for survivors whose tumors express p27 [HR: 0.32, 95%CI=0.12–0.85]). In addition, the link between PA and cancer outcomes has strong biological plausibility related to sex hormones, cell growth regulators, DNA damage repair, inflammatory markers, immune function, and antioxidant pathways.18
RCTs are needed to establish the causal effects of PA on cancer outcomes. The Colon Health and Life-Long Exercise Change (CHALLENGE) Trial is the first phase III trial examining the effects of a 3-year structured PA program on disease-free survival in patients with stage II and III colon cancer who have recently completed chemotherapy.19 To date, the trial has demonstrated feasibility in accrual20 and PA behavior change;21 it has randomized over 590 of the planned 962 patients. The Intense Exercise for Survival (INTERVAL) Trial is another phase III trial examining the effects of a 2-year structured PA program on overall survival in 866 men with metastatic castrate-resistant prostate cancer.22 These trials, and others like them, will provide the first definitive evidence on the role of PA in improving cancer outcomes.
Influence of Weight Management and Physical Activity on Quality of Life Outcomes
While obtaining evidence of the impact of weight management and PA on cancer progression and mortality is critical, many cancer survivors experience significant comorbidities, or cancer- and treatment-related physical and psychosocial problems that compromise their QOL.23 Healthy eating, regular PA, and maintaining a healthy weight have been recommended for cancer survivors to prevent, mitigate, and manage these downstream sequelae.24, 25
Weight gain with concomitant loss of muscle (i.e., sarcopenic obesity) and bone are common after chemo- and hormonal therapy, placing cancer survivors at risk for comorbidities, such as cardiovascular disease [CVD], diabetes, second primary cancers, osteoporosis, and functional decline.25, 26 Research indicates that diet- and exercise-induced weight management interventions can produce clinically-meaningful weight loss in cancer survivors within six months, resulting in improved blood lipids and metabolic health, and reduced inflammation.27–31 Also, several studies have reported positive effects of targeted PA on bone health,32–34 which is important since osteoporosis and the risk of subsequent fractures is increased by 15–20% among cancer survivors who receive hormonal treatment for breast or prostate cancer.35
A growing number of studies have examined the effects of PA on CVD in cancer survivors with a meta-analysis finding PA improves cardiorespiratory fitness - a powerful predictor of mortality.36, 37 Growing evidence also suggests that PA may improve cognitive function,38 and lessen peripheral neuropathy,39 lymphedema,40 and arthralgia41 in patients treated for cancer.
In 2010, the American College of Sports Medicine (ACSM) published PA guidelines for cancer survivors based on 85 PA trials conducted during or post-treatment.24 The systematic review and findings from two more recent meta-analyses,42, 43 show that PA is safe and effective in improving QOL, cancer-related fatigue, and physical function. While overall effect sizes are small, there is consistent empirical evidence to support PA promotion as part of cancer care.42, 43
Evidence Gaps and Ongoing Randomized Weight Management and Physical Activity Trials in Cancer Survivors
While many trials have evaluated the impact of weight management and PA interventions on outcomes, such as body composition, fitness, and QOL in cancer survivors, critical gaps remain. Most notably, evidence from RCTs is not yet available that weight management or increased PA after cancer diagnosis will improve survival or reduce cancer recurrence. The Women’s Intervention Nutrition Study, conducted among 2437 women with early stage breast cancer, provides some of the only RCT data and suggests that a mean non-prescribed weight loss of six pounds, resulting from a fat-restricted diet, was associated with a significant decrease in subsequent breast cancer events (local, regional, and distant recurrence; ipsilateral breast recurrence after lumpectomy; and contralateral breast cancer) compared to a control arm (i.e., 9.8% vs. 12.4%, p=0.034), a finding driven by women diagnosed with ER negative disease.44 However, because the dietary fat intervention also led to weigh loss in this study,45 it is impossible to disentangle whether the low fat diet or the weight loss was most responsible for cancer control. Other questions remain about the biologic pathways that underlie the relationship between weight management and PA and malignancy, the relative contribution of body weight, diet, and/or PA to cancer outcomes, the optimal timing, dose and duration of weight management and PA interventions, and the best ways of implementing weight management and PA interventions in diverse cancer populations.44 Moreover, the science regarding cancer outcomes and sedentary time also needs to be further developed, as well as the evaluation of potential interventions to limit sedentary time.
A number of ongoing trials aim to address these evidence gaps (see Table 2).19, 46–48 Each of the ongoing studies examines the impact of weight loss or increased PA alone or in combination with improvements in diet quality, on cancer recurrence, cancer-related mortality, or overall survival in individuals diagnosed with a single malignancy. None of the studies compares the effects of different weight management or PA interventions or of different doses or durations of intervention. Half of the trials enroll breast cancer survivors, and the majority focus on those with no evidence of active disease.
Table 2.
Ongoing randomized trials of weight management and physical activity interventions in cancer survivors with recurrence and mortality endpoints
| BWEL* (A011410) | SUCCESS C46 | DIANA 547** | LIVES*** (NRG0225)48 | CHALLENGE19# | INTERVAL (GAP4)## | |
|---|---|---|---|---|---|---|
| N | 3136 | ~1400 | 1241 | 1040 | 962 | 866 |
| Cancer site | Breast | Breast | Breast | Ovary | Colon | Prostate |
| Disease Stage | II–III | II–III | I–III | II–IV after optimal debulking | II–III | IV, Castrate resistant |
| Primary Endpoint | Invasive disease free survival | Disease free survival | Invasive disease free survival | Progression free survival | Disease free survival | Overall survival |
| Study Design | Two arm RCT: Weight loss intervention + health education vs. Heath education | Two arm RCT: General lifestyle + intensive lifestyle intervention vs. general lifestyles alone | Two arm RCT: Weight loss intervention vs. general lifestyle guidance | Two arm RCT: Diet and physical activity intervention vs. attention control intervention | Two arm RCT: Structured physical activity intervention vs. general health education materials | Two arm RCT: Supervised exercise intervention vs. supportive care |
| Intervention Target | Weight loss (Diet + increased physical activity) | Mediterranean /macrobiotic dietary plan plus increased physical activity | Weight Loss (Diet + physical activity) | Increased physical activity, low fat, increased intake of vegetables and fiber | Increased physical activity | Increased high-intensity physical activity |
| Intervention Duration | 2-years | 2-years | 4-years | 2-years | 3-years | 2-years |
| Intervention Approach | Telephone-based | Telephone-based | Clinic-based group | Telephone-based | Supervised, mixed clinic and home-based | Supervised, mixed clinic-and home-based |
| Enrollment Setting | Cooperative group | Cooperative group | Individual clinics | Cooperative group | Cooperative group | Individual clinics |
| Correlative Specimens | Blood, tumor and benign tissue | Blood | Blood | Blood | Blood | Blood |
BWEL (Breast Cancer Weight Loss)
DIANA-5 (Diet and Androgens-5)
LIVES (Lifestyle Intervention for Ovarian Cancer Enhanced Survival)
CHALLENGE (Colon Health and Lifestyle Long Exercise)
INTERVAL (Intense Exercise for Survival among Men with Metastatic Castrate Resistant Prostate Cancer)
Although these trials will provide critical information regarding the role of weight management and PA in the management of cancer survivors, a number of gaps will remain. Given that each trial focuses on the effect of a particular weight management or PA intervention in a specific cancer survivor population, it will be difficult to generalize the information gained from these studies across all cancer survivors or to other types of interventions. Moreover, from a feasibility and economic standpoint, it is unlikely that there ever will be trials conducted to evaluate the effect of each type of weight management and PA intervention on every malignancy. So, how do we bridge these evidence gaps and ensure that all cancer survivors have access to weight management and PA interventions that could reduce risk of recurrence and improve survival after cancer diagnosis? Part of the key to expanding knowledge gained from ongoing individual trials comes from the correlative science that is embedded in each and the potential to pool data and samples across smaller studies. By evaluating the effect of weight management and PA interventions on blood-based biomarkers (and extant tumor tissue) and determining the relationship between changes in markers, such as insulin and c-reactive protein, and cancer recurrence and survival, intermediate biomarkers could be established to inform future research,3 akin to research in CVD, where trials are powered to examine changes in blood pressure or lipid levels, rather than on clinical endpoints, such as myocardial infarction.49 Correlative science also could discover predictive markers of response and determine which cancer survivor populations are most likely to derive benefit from specific interventions.
Summary
Ongoing trials will provide vital information on weight management and PA interventions with and without improvements in diet quality - on cancer recurrence and survival, but a number of important knowledge gaps will remain. Biomarker analyses offer the potential to extend the knowledge gained from these trials to other patient populations and could ultimately determine the components of optimal interventions and how they are best applied in a personalized medicine approach to improve cancer outcomes. While more research is needed to elucidate the impact of weight management and PA on cancer-specific outcomes, it is important to note that ASCO now recommends discussion about weight management, including dietary and PA changes among oncology providers and their patients.4 This recommendation stems from solid evidence that diet, PA, and reduced adiposity play critical roles in preventing CVD and diabetes, and exert a positive influence on QOL, physical function, and fatigue.
Effective Approaches for Improving Weight Management and Physical Activity
Large RCTs related to weight loss and the control of chronic conditions, such as diabetes, have provided sufficient evidence to warrant changes in weight management recommendations from the United States Preventive Services Task Force (USPSTF), as well as from professional organizations.4, 50–53 Research evidence on the benefits of PA (including aerobic, resistance training, and flexibility and coordination) has expanded significantly. Currently, the Physical Activity Guidelines Advisory Committee is preparing a scientific report with a scheduled release date of early 2018. This evidence has contributed to the development of weight management and PA interventions for cancer survivors and RCTs to evaluate the impact on important short- and longer-term outcomes in cancer survivors, such as QOL, tolerance for cancer therapy, comorbidity, and disease-free survival. Long-term evidence from previous weight loss and PA trials has demonstrated repeatedly that a high proportion of trial participants have difficulty maintaining behavior changes outside the context of a clinical trial, in part because the current US environment provides little support for being physically active or eating a healthy diet. This recognition has led to an increase in research examining the environmental, policy, and systems changes needed to help individuals adopt and maintain recommended behaviors.
Interventions for Weight Management in Other Populations that are Applicable to Cancer Survivors
Lifestyle modifications to alter eating behaviors and increase PA are the cornerstone of treatment for overweight and obesity, and have been used successfully in several large-scale trials. The American College of Cardiology (ACC), American Heart Association (AHA), and The Obesity Society (TOS) reviewed the results from these RCTs and concluded within the 2013 Guidelines for the Treatment of Obesity that adherence to a calorically-restricted diet predicts weight loss success, independent of the type of diet or macronutrient composition.54 The guidelines also recommend using body mass index (BMI) and waist circumference to advise patients of their risk of developing other comorbidities and to prescribe a set number of calories per day according to the following: 1,200 to 1,500 kcal/d for women and 1,500 to 1,800 kcal/d for men to promote a 1–2 pound weight loss per week.54 A sustained weight loss of as little as 3–5% of initial body weight reduces the risk of type II diabetes and risk factors for CVD. 54
The Look AHEAD (Action for Health in Diabetes)55 and Diabetes Prevention Program (DPP)56 trials are two of the most successful long-term studies to illustrate the ability of lifestyle interventions to reduce and maintain body weight and reduce the risk of chronic diseases; both were instrumental in informing the 2013 AHA/ACC/TOS Obesity treatment guidelines. The lifestyle interventions in these two trials were similar (i.e., low fat, low calorie diet with the use of meal replacements and 150–175 minutes/week of moderate to vigorous intensity PA).
Subsequently, some studies have shown that higher protein diets (1.2 – 1.6 gm protein/kg of body weight/d) provide benefits beyond weight loss and may preserve lean body mass (LBM), especially in older men and women.57, 58 Resistance training also has been shown to be particularly beneficial in older adults, including breast and prostate cancer survivors, to preserve LBM and bone health, maintain a higher resting metabolic rate, preserve physical functioning, and reduce falls and injury).59–61
Successful long-term weight management requires several behavioral strategies.62 Tactics for weight success include: maintaining a low fat, low calorie dietary pattern; limiting dietary variety; eating breakfast most days of the week; daily to weekly weighing; performing 2500 kcal/week of PA (e.g., brisk walking for ~ 1 hour/d); and reducing television watching.
Interventions for Physical Activity in Other Populations that are Applicable to Cancer Survivors
Over the past few decades, numerous studies have clearly shown that PA of sufficient volume and intensity reduces the risk of several chronic diseases and improves physical function.63 More recently, research has started to examine the impact of physical inactivity on overall morbidity and mortality.64–66
Aerobic PA of sufficient volume and intensity (“exercise”) to improve cardiorespiratory fitness, a potent biomarker of morbidity and all-cause mortality, needs to be frontline care in both healthy and cancer survivor populations.67–69 Consistent aerobic exercise can delay the onset of disability by more than 10 years and markedly increases survival among older adults with projected lifespans of at least 20 years (the length of survivorship which burgeoning numbers of cancer survivors are now achieving).7, 70 Among cancer survivors, data indicate that high vs. low cardiorespiratory fitness reduces the risk of mortality by 45%.71 Likewise, resistance PA of sufficient volume and intensity (“exercise”) to increase neuromuscular fitness (i.e., LBM, strength, power, fatigue resistance) is key to frontline care. Low LBM is a major predictor of all-cause mortality and physical disability.72 Resistance exercise has repeatedly shown to improve neuromuscular fitness and skeletal health, and reduce risk of disability.73, 74
While the molecular underpinnings of PA-driven health benefits have not been fully elucidated, significant progress has been made,75 and more information will be gleaned via the NIH-funded Molecular Transducers of Physical Activity Consortium (MoTrPAC). MoTrPAC is charged with mapping molecular responses to aerobic and resistance exercise to more fully understand the cellular and molecular signals that drive potential health benefits. This vital step will enable a precision medicine approach and the individualization of exercise prescriptions.63, 75
Interventions for Weight Management in Cancer Survivors
Data indicate that weight loss can be promoted among cancer survivors who are overweight or have obesity.31, 76 Sentinel studies of weight management are summarized in Table 3, and generally rely on cornerstone elements of weight loss (i.e., dietary modification to promote caloric restriction, increased PA, and behavior modification). Nonetheless, there are acknowledged limitations to this research (e.g., brief study periods, lack of repeated and objective measures [including body composition outcomes], and over-representation of breast cancer survivors who may be “worried, white, and well”). Many questions remain within the context of well-designed and controlled efficacy trials (e.g., intervention timing, inclusion of sleep hygiene or stress management components, and discerning the full impact of weight loss across a broad range of symptoms and conditions). Discovery is needed to inform personalized medicine-based approaches and thereby elucidate molecular and metabolic predictors important for tailoring weight loss regimens for individual patients in terms of dose and optimal macronutrient distribution. In addition, there is a need for pragmatic interventions that can overcome well-known barriers imposed by distance, economics, co-occurring medical conditions, and culture. The diversity of needs among cancer survivors, many of whom are older, increases the urgency of pragmatic trials to test and compare both high-touch/more-effect approaches and lower-touch/less-burden approaches. Well-designed research across the spectrum requires broad representation by cancer-type, age, gender, and race/ethnicity, as well as sufficient sample sizes to conduct subgroup analyses. Ideally, interventions need to be designed with the input of oncologists, dietitians, exercise specialists, behavioral scientists, statisticians, software specialists (if needed), community stakeholders, and most importantly of all, cancer survivors. The input of health economists also is key to developing programs that are sustainable and can be widely disseminated.
Table 3.
Select weight management intervention trials in cancer survivors by mode of delivery
| Lead Author, Study name | Cancer type | N mean age | Intervention | Duration | Weight change (kg) |
|---|---|---|---|---|---|
| Clinically-based / supervised | |||||
|
| |||||
| Thompson 218 CHOICE |
Breast Post-menopausal | N = 249 54.9 y |
RD counseling, 42-day cycle menu, low fat or low carbohydrate, 3500 kcal deficit/week, 10,000 steps/day | 6 months | −8.9, −10.5, −0.3 kg Low fat, Low carbohydrate, control, respectively |
| Swisher 219 GetFit for Fight |
Breast Triple-negative | N = 28 53.7 y |
RD counseling to decrease fat intake by 200 kcal/week; Exercise physiologist-supervised moderate aerobic PA 3×/week/ 2 unsupervised + stretching and resistance training | 12 weeks | −3.0 vs −0.4 kg, Intervention vs Control |
| Thomson 220 Modified-Atkins |
Breast Stage I–II | N = 40 56 y |
RD counseling -low fat or low carbohydrate, 500 kcal deficit/day | 6 months | −6.3, −5.9 kg Low fat vs Low carbohydrate, respectively |
| McCarroll 221 SUCCEED |
Endometrial Stage I–II | N = 75 Age not reported |
Physician-led group and individual counseling; 16 sessions - diet, PA and behavior modification | 6 months | −1.5 kg/m2 vs +0.1 kg/m2, Intervention vs Usual care |
| Travier 222 | Breast Stage I–IIIb | N =42 55.8 y |
12 weekly, 1-hour group sessions with RD; 1200–1500 kcal/day; PA: 24 bi-weekly, 75-min supervised aerobic + strength sessions | 12 weeks | −7.8 kg in completers, Phase II, Single arm |
| Saxton 223 | Breast | N = 85 56 y |
3 small group, supervised exercise sessions/week; aerobic + strength; weight loss on a plate Scottish Dietetic Assoc. program /600 kcal/day deficit; weekly small group nutrition seminars | 6 months | −1.1 vs −0.4 kg, Intervention vs Usual care |
|
| |||||
| Mixed-modalities (Clinic-based + Telephone Counseling) | |||||
|
| |||||
| Rock 27 ENERGY |
Stage I–III Breast | N=692 56 y |
RD-led, 4M weekly group sessions, tapering to biweekly, then monthly; reinforced by 1:1 telephone/email. Deficit 500–1000 kcal/day + 60 minutes/day PA; tailored print materials | 2 years | −3.6 vs −0.9 kg, Group +telephone vs control |
| Von Gruenigan 224 | Stage I–II endometrial cancer | N =45 54.5 y |
RD + MD-led weekly, then bi-weekly, then monthly group sessions (9); telephone or newsletter every non-group meeting week; walking- 5 days/week, > 45 minutes | 6 months | −3.3 vs +2.1 kg, Group + telephone vs usual care |
| Sheppard 115 Stepping Stone |
Breast | N = 22 (analytic) Black | Nutritionist + exercise physiologist-led individually tailored, group sessions twice monthly / alternate week telephone counseling by trained survivor coach; survivor and interventionist toolkit; ACS guidelines for diet (lower fat + fruits and vegetables) and PA (10,000 steps) | 12 weeks | −0.8 vs +0.2 kg, Intervention vs control |
|
| |||||
| Telephone Counseling | |||||
|
| |||||
| Harrigan 30 LEAN |
Breast | N =100 59 y |
RD/exercise physiologist delivered; 1:1 weekly × 4 week…bi-weekly × 8 weeks…monthly × 3 months. DPP adaptation with – 500 kcals /25% fat kcals = 150 minutes PA/week (walking, lower sedentary time) + mindfulness; Self-monitoring | 6 months | 3-arm RCT (in-person, telephone, control), −5.6, −4.8, −1.7 kg; in-person, telephone and usual care |
| Harris 225 CASTLE |
Breast Stage I–IIIa | N = 52 52.8 y |
Health professionals employed by Trestle-Tree, Inc. provided 15–60-minute telephone coaching weekly × 25 weeks…monthly × 6M; behavioral targets -diet and PA | 12 months | −4.0 vs −3.3 kg, telephone vs in-person, respectively |
| Goodwin 28 LISA |
Breast T1-3NO1-3MO on Letrozole | N = 338 61 y |
Trained lifestyle coaches; 19 calls +workbook-directed call content adapted from DPP; −500–1000 kcal/day; 150–200 minutes PA weekly; behavior modification | 24 months | −3.1 vs −0.3 kg telephone vs. print alone (all received general health educational print material) |
| Befort 226 | Breast Stage I–III | N = 91 58.9 y Rural |
Trained group leads; 24 conference calls in groups of 8–14; kcal deficit −500to −1000 kcals/day; meal replacements; 225 minutes PA/week – aerobic and resistance; self-monitoring | 6 months | −12.5 Single arm Group telephone |
| Befort 29 | Breast Stage I–III | N = 210 (N = 172 phase 2) 58 y Rural |
As above (Phase 1) Maintenance: New kcal goal; 2 meal replacements/day; 225 minutes PA/week; continued bi-weekly group conference calls or mailed newsletter | 6 + 12 months | −12.2 vs 13.2 Regain +3.3 vs +4.9 kg Group telephone vs newsletter |
|
| |||||
| Community-based | |||||
|
| |||||
| Greenlee 110 Cocinar Para Su Salud |
Breast Stage 0–III Hispanic | N = 70 56.6 y |
RD, MD, health educators, trialists delivered; 4 2-hour nutrition education roundtables + 3, 3.5 hour cooking sessions + 2 food shopping field trips over 12 weeks (24 hours); culturally-tailored Cook for Your Life curriculum | 12 weeks | −2.5 vs +3.8, intervention vs control |
| Stolley 116 Moving Forward |
Breast Cancer | N = 23 51.4 y African American |
Study lead and certified exercise instructor from the community; 24 weekly classes with pre-determined topics: diet, PA and behavior modification; food shopping field trip, menu planning | 6 months | −2.52, Single arm |
|
| |||||
| Commercial programs | |||||
|
| |||||
| Djuric 227 Weight Watchers (WW) |
Breast Stage I–II | N = 48 36–70 y |
Weekly WW meetings vs 1:1 with RD weekly × 3 months, biweekly × 3 months, monthly × 6 months; both promoted low fat, 500–1000 kcal deficit/day + self-monitoring | 12 months | −2.6, −8.0, −9.4 and −0.85 in control, Weight Watchers alone, Individual (RD counseling), WW+ Individual, respectively |
| Greenlee 109 Curves |
Breast 0–IIIa | N = 42 50.6 y Hispanic, Black |
Curves staff-led Curves Weight management program curriculum; exercise at Curves 30 minutes, 3 x/week + 2 days at home; bi-directional strength training + low-impact aerobic PA; 60 increasing to 75% maximal heart rate; 6, 1-hour weekly nutrition classes 1200 increasing to 1600 kcals/day + weekly MI telephone calls | 6 months | −2.87 vs −1.42, Curves program vs waitlist control |
|
| |||||
| Home-based, print materials | |||||
|
| |||||
| Morey 151 RENEW |
Breast, Prostate, Colorectal | N = 641 Older 73 y |
Health counselor delivered intervention; personally-tailored workbook + quarterly newsletters +15 telephone counseling sessions and 8 prompts; PA: 15 minutes strength training 2/week + 30 minutes aerobic PA/day; high fruit/vegetable + low saturated and total fat; kcal restriction to promote up to 1 lb/week | 12 months | −2.06 vs −0.92, intervention vs control |
| Demark-Wahnefried 228 DAMES |
Breast Stage 0–III | N = 136; 68 mothers, 68 daughters | Tailored print materials; ACS and US Dietary Guidelines; Kcal deficit of 500–1000/day / remove or substitute 3 major diet-recall identified caloric sources; 150 minutes/week PA; self-monitoring | 12 months | −3.77 vs −0.87 in mothers for individual vs control |
|
| |||||
| Technology-based | |||||
|
| |||||
| Huang 149 Fit4Life |
ALL | N= 38 13 y |
Professional, caregiver and parent + survivor -input to material development; 4-month web-based and text and telephone counseling; ACS and Children’s Oncology Group guidelines for healthy weight; kcal deficit, 60 minutes moderate-vigorous PA/day; 15,000 steps/day; self-monitoring | 4 months | −0.1 vs +1.4 web, SMS, telephone vs control |
| McCarroll 229 LoseIt! App |
Stage I–II Endometrial and Breast | N =50 58.4 y |
Study personnel-assisted app orientation (30–60 minutes); LoseIt! App for daily self-monitoring of diet and PA. Low carbohydrate, high fiber; 150 min PA/week, vigorous > 40 min/week | 4 weeks | −2.3 (single arm) |
| Haggerty 230 Text4Diet |
Endometrial Pre and stage 1 | N=20 60.5 y |
Telemedicine adapted DPP delivered by MD and MS clinician; weekly telephone and wifi weigh-ins daily × 6 months; SMS received Text4Diet® with 3–5 personalized SMS daily with monthly themes; 2-way communications weekly wi-fi weigh-ins; all educated to consume 1200–1500 kcals/day; self-monitoring | 6 months | −9.7 vs −3.9. telemed vs SMS |
RD = Registered Dietitian nutritionist; MD = Medical Doctor; PA = physical activity; ACS = American Cancer Society
Interventions to Improve Physical Activity in Cancer Survivors
Various PA interventions have been evaluated in cancer survivors, though the body of evidence is primarily limited to short-term studies of 12–16 weeks in duration, and among breast cancer survivors. Approaches are typically clinic- or home-based (e.g., telephone counseling, print, web, social media). In general, stronger outcomes are associated with clinic-based programs, while greater reach and reduced participant burden are associated with home-based interventions.77 However, this generalization is affected by the motivation of the cancer survivor; as shown by data from the LEAN study that found no differences in effects between the two modes of delivery.30 On-site, clinic-based programs are generally supervised by exercise professionals and tend to have higher exercise intensity dose and closer supervision and monitoring. Home-based programs tend to promote moderate-to-light PA, reach individuals who cannot travel or meet the scheduling requirements of on-site programs, are more likely to be theory-based and are less costly.78 However, supervision for home-based programs may be minimal, so individuals with significant comorbidity or safety issues are generally excluded. More recently, hybrid programs that are able to support sustained PA have emerged. Generally, these programs begin with an on-site supervised phase and then taper to an off-site phase (e.g., CHALLENGE trial).21.
A review of behavior change studies, including a meta-analyses of 14 RCTs among breast cancer survivors,79 found that the key elements of effective interventions are self-monitoring of PA, individualized guidance or coaching, and setting clear goals and expectations. Since PA maintenance may be particularly challenging with long-term (e.g., fatigue) or later effects of cancer treatment (e.g., arthralgia), attention to symptom management (which ideally starts as prehab and continues across the cancer survivorship trajectory) may be an important consideration to optimize PA uptake and adherence long-term.21, 80 Likewise, there is potential for interventions (especially PA) to reduce these symptoms and therefore contribute to the survivor’s ability to maintain healthy behavior changes over time. Given that obesity may adversely affect adherence to PA regimens there is a need to determine the relative timing or sequence of PA in relation to caloric restriction within the context of weight management.73, 81 As indicated in previous sections, research is needed to determine what type of PA works (modality [e.g., aerobic, strength], intensity, frequency and duration) to achieve which outcomes and for whom,82 as well as when in the course of the cancer continuum should programs be offered. Identifying the minimal PA dose for QOL improvements, weight and symptom management, and survival will assist in developing pragmatic programs that target outcomes relevant to patients’ needs (e.g., management of fatigue and pain).
Looking ahead, improvements in the recovery and functioning of the growing numbers of cancer survivors may emerge from use of behavior change theories to inform intervention development, use of IT and mHealth technologies to widen the reach of programs, plans for maintenance of behavior change, and assessment of program costs. There are numerous missed opportunities for healthcare professionals in oncology and primary care settings to promote PA for their patients at various points across the cancer care continuum. Addressing the barriers faced by providers,77, 83 and providing guidelines to help triage patients to effective programs (e.g., clinic-, community-, or home-based) is sorely needed.
Summary
Research in the general population has demonstrated the benefits of weight management and PA for prevention and management of diabetes and other chronic diseases, reducing disability, and delaying mortality. As research on weight management, PA, and cancer survivorship moves beyond small, early clinical trials that evaluate effects of these interventions on biomarkers and QOL, larger trials are needed to test the effects of the interventions on disease-free and overall survival especially in disease-sites other than breast cancer, and to include adequate representation of population subgroups defined by comorbidity/functional status, race, ethnicity, or age. To ensure that clinical trials are appropriately designed to provide more definitive answers, the NIH has recently established new guidance related to funding applications submitted to the NIH for all clinical trials.84 Specific institutes, such as the National Cancer Institute (NCI), have utilized expert working groups to discuss trial design issues within their clinical trial networks, including those related to behavioral interventions.
Within the NIH Obesity Research Task Force, the National Heart Lung and Blood Institute (NHLBI) is leading an effort to identify additional factors that may predict successful response to weight management interventions. The Accumulating Data to Optimally Predict obesity Treatment (ADOPT) Core Measures Project was developed in response to the well-documented individual variability in response.85 ADOPT is designed to provide investigators with tools to generate an evidence base that may advance understanding of the behavioral, psychosocial, environmental, and biological sources of this variability. Working with an expert panel of investigators, a trans-NIH group identified an initial core set of high-priority measures that, when consistently used in trials, may facilitate the prediction of treatment response. The NIH is now exploring approaches for increasing the use of consistent measures across trials so that data can be pooled and used to identify reliable predictors, mediators, and moderators of response. This accumulation of efficacy evidence will likely spur the translation of effective interventions into clinical practice, though research in implementation science also is needed to best adapt interventions to enhance their reach, scope, and uptake among populations and settings that may not be representative of clinical trials.
Addressing the Weight Management and PA Needs of Diverse Populations of Cancer Survivors
Low income, minority populations, particularly African-Americans and Hispanics, as well as those who are older and live in rural areas bear a disproportionate burden of cancer.7, 86–88 Moreover, these populations also are more likely to be overweight or obese, physically inactive, and to manifest health conditions that are affected by these factors, such as metabolic syndrome (MetS) – all of which are associated with greater comorbidity and reduced survival (overall and cancer-specific). This section addresses weight management and PA among diverse populations.
Meeting the Needs of Diverse Survivors in Terms of Race/Ethnicity, Culture, and Language
Among cancer survivors, prevalence of overweight and obesity is high, especially among non-Hispanic blacks and Hispanics compared to non-Hispanic whites.89 Concordantly, cancer survivors who are members of minority groups have lower adherence to diet and PA guidelines and are more likely to report poorer health status compared to non-minority cancer survivors or racial/ethnic minorities without cancer.90–96 Thus, there is a critical need to develop and examine weight management and PA interventions among cancer survivors of racial/ethnic minority status to enhance outcomes and reduce disparities. Consideration of patients’ environmental, cultural, and survivorship context is critical to the success of these efforts.97 Racial and ethnic minority survivors are more likely to live in areas characterized by high segregation, traffic density and crime, and by low neighborhood socioeconomic status and access to full service supermarkets and PA resources. 98–100 Despite this, most communities, including lower socio-economic neighborhoods, also have some assets such as farmers’ markets, public recreation systems, and community gardens that support PA, healthy eating, and reduction of chronic disease. 101–103 Partnering with community organizations to bring interventions to under-resourced neighborhoods provides opportunities to build social capital, reach more cancer survivors, and increase potential for sustainability.104
Consideration of cultural norms is important. Culture varies among and within racial and ethnic groups, influencing beliefs, behaviors and patient-provider interactions related to cancer, obesity, and lifestyle. The conceptual framework of Kreuter et al.105 can inform cultural tailoring and structure formative work, thereby enhancing the relevance of an intervention approach and content to a particular population (see Table 4). Similarly, because cancer survivors report greater interest in programs that acknowledge their cancer journey and concerns, it is important for programs to address these issues.106 In addition to context, biopsychosocial approaches to research are needed to understand and address the multi-level (cells to society) factors that affect weight status and behavior, and influences on cancer control, overall health, and QOL.88
Table 4.
Framework to guide cultural tailoring of behavioral interventions105
| Domain | Action |
|---|---|
| Peripheral | Design study materials to appear culturally appropriate (i.e., logo, recruitment materials) |
| Evidential | Enhance relevance of targeted health issues by presenting evidence of its impact (i.e., cancer disparities, impact of obesity, comorbidity burden) |
| Constituent-involving | Draw directly on the experiences of the target group (i.e., staff represent target group; inform intervention using qualitative data from target population; engage advisory group to provide feedback on study materials and procedures) |
| Sociocultural | Discuss health-related issues in the context of broader social and/or cultural values (i.e., role of God and faith in one’s daily life, woman’s central role in families, cancer fatalism and stigma, body image ideals, and the traditional roles of food) |
| Linguistic | Make health education programs and materials more accessible by providing them in the dominant or native language of target group |
To date, weight management and PA interventions among racial/ethnic minority cancer survivors have established feasibility and safety, and report positive, albeit modest, results including weight loss, behavioral changes, improved QOL and biomarker status, and decreased cancer-related anxiety.107–118 Limitations of many studies include quasi-experimental designs, small sample sizes, a focus on behavioral outcomes, and sole inclusion of breast cancer survivors. Only three studies assessed biomarkers,109–111 and only one targeted a cancer other than breast119 - none included men. Recent efforts address some of these limitations.120, 121 Important steps to advance the science of obesity and lifestyle interventions in diverse populations include using more rigorous methodologies, addressing multi-level mediators and moderators of change, examining biological mechanisms related to energy balance and cancer, and addressing more diverse cancer survivor populations.
Meeting the Needs of Cancer Survivors across the Lifespan
Currently, 62% of cancer survivors are age 65 and older – a subpopulation that will continue to grow with the aging of the population, as well as earlier diagnosis and improvements in treatment.122 However, cancer also affects the young. In the US, there are almost 400,000 childhood cancer survivors and 70,000 adolescent and young adult cancer survivors, many of whom could have long lives ahead of them.122 Lifestyle interventions are sorely needed by survivor populations of all age groups, since suboptimal diets and insufficient PA are noted in 40–70% and 54–84% of younger cancer survivors, respectively,123–126 and in 52–85% and 53–70% of older cancer survivors, respectively.127–129 In addition, up to 71% of older cancer survivors are overweight or have obesity, and these conditions also are prevalent in children, adolescents and young adults diagnosed with acute lymphocytic leukemia and some forms of brain cancer.125, 127
A unifying theme shared by both young and old cancer survivors is that of long-term and late effects of cancer and its treatment, many of which are influenced by nutritional status and PA,130 such as increased risk of CVD, second cancers, osteoporosis, MetS, fatigue, cognitive changes, and sarcopenia. Underlying many of these conditions is the process of accelerated aging and frailty among cancer survivors, which occurs across all age groups.
Frailty, or an “insufficient reserve to recover,” is generally preceded by diminished function,131 both of which increase with age. However, illnesses and injury, as occurs with cancer and its treatment, accelerate this course, especially among females.130 Other factors, such as a poor diet, physical inactivity, and obesity, further exacerbate functional decline and the onset of frailty.132 Current data indicate that the odds of frailty are significantly increased among individuals with BMI’s ≥30 kg/m2 (Odds Ratio: 1.12 [95% CI: 1.01–1.19], p=0.003).133 Weight management and PA interventions can potentially reorient neuromuscular control, increase muscle strength, and reduce frailty. 33, 60, 61, 134
Despite the potential benefits of weight management to forestall frailty and common comorbidities, caution is needed in pursuing weight loss. Until more data are available specifically on cancer survivors, the AHA/ACC/TOS and NHLBI guidelines can inform best practices.54, 135 Among these guidelines is the recommendation for a rate of weight loss of 1–2 pounds per week. Because sarcopenia is a common condition that accompanies cancer and its treatment, and one that accelerates aging, slower rates of weight loss that minimize LBM loss, concurrent with strength training are recommended.136 For older adults, weight loss guidelines suggest an energy deficit of 500–750 kcal/d to promote a weight loss of up to 1.5 lbs./week;137 for childhood and adolescent cancer survivors energy deficits of up to 250 kcal/d to invoke a maximum weight loss of 0.5 lbs/week are recommended.138 Also, behavioral approaches, such as substituting higher- with lower-energy density foods, avoiding distracted eating, and adopting slower rates of eating, are commonly-used tactics to help prevent weight gain in adults and allow children to “grow into their weight.”
Regular PA is important for cancer survivors of any age to achieve optimal health. Thus, the avoidance of inactivity is key and adaptations need to be made to accommodate limitations or comorbidities due to cancer or its treatment.139 Guidelines suggest that children pursue 300 minutes per week of moderate to vigorous PA, whereas the guidelines for adults (including older adults) suggest 150 minutes of moderate or 75 minutes of vigorous PA per week.25, 140 Strength training 2–3 times per week is recommended across the lifespan,25, 140 though for children this recommendation is made within the context of a sports curriculum and with adequate supervision.141 The benefits of low intensity PA have recently been reported as well; however, to date there are no guidelines in this area.142 The means by which weight management and PA are promoted in younger vs. older cancer survivors differs.143–147 For example, children have preferences for game- or play-based interventions, whereas older adults favor holistic interventions (e.g., gardening, dancing) that have personal meaning and/or involve others. Due to prevalent functional and sensory deficits among the young and the old, it is critical that interventions employ large font (and screen size), volume control, module brevity, pre-training, and support for new technologies, especially those that allow for home-based delivery.148 Exemplar interventions are featured in Table 3.149–151 In addition, given the key role that caregivers play in the lives of children and older adults, an unexplored area with potential is the use of dyadic approaches.
Meeting the Needs of Rural Cancer Survivors
Rural cancer survivors, i.e., those residing in non-metropolitan counties as defined by the Office of Management and Budget,152 have higher cancer mortality rates compared to urban residents across all regions of the US. Moreover, death rates from cancer have declined at a slower rate in rural compared to urban counties.153 Among cancer survivors, those from rural areas report poorer health status, more psychological distress,154 higher rates of depression and anxiety,155 and greater knowledge gaps related to their cancer and its treatment effects. Rural cancer survivors also report high levels of unmet support needs.156 These disparities are compounded by higher rates of comorbidities, obesity, and physical inactivity among rural as compared to urban residents.157–160
Rural cancer disparities affect a significant proportion of our population. Nearly 20% of Americans,161 and an estimated 21% of cancer survivors reside in a rural area, representing roughly 2.8 million survivors.154 Rural residents are a diverse group. Nationwide, 78% of rural residents are non-Hispanic White; however, higher proportions of African Americans and Hispanics reside in the south and southwest, respectively. Despite their diversity, rural residents often share common cultural elements, such as conventional attitudes, self-reliance, and orientation toward work, family, and religion.162 Rural residents of all racial/ethnic groups are also older, poorer, and have less education than their urban counterparts.161 These demographic differences, in addition to the contextual, cultural, and access factors stemming from place of residence, contribute to rural cancer disparities.163 All of these factors need to be considered when designing lifestyle interventions for rural cancer survivors.
Effective remote-based interventions are essential to maximize reach into rural communities, due to challenges with access to healthcare services (including specialized services for weight management and PA), travel time, and financial barriers. With ~3% of medical oncologists164 and very few specialized psycho-oncology providers practicing in rural communities, there is a gap in services for supportive care and lifestyle interventions.165 Travel time and transportation costs pose barriers for in-person lifestyle interventions, particularly in frontier regions.
Survey results among rural breast cancer survivors in Kansas and Illinois found that the vast majority did not met PA guidelines,166 but rated PA and weight management programs as a top need.167 To date, there is only one published trial of a full-scale lifestyle intervention done exclusively in a rural setting. Befort and colleagues168 enrolled 210 breast cancer survivors into a 6-month telephone-based intervention delivered via weekly conference calls. The study demonstrated feasibility and achieved a 12.9% weight loss – with a 10.6% net loss maintained at 18 months, via continued, but scaled-back conference calls. Lessons learned from this study are needs for: (1) direct patient recruitment via cancer registries (direct mailing yielded 84% of participants, whereas physician referrals yielded only 4%); (2) clinical integration (due to high levels of comorbid conditions); and (3) group support among rural women.169 While the intervention was exclusively home-based, many participants arranged to meet in person with one another; thus, some face-to-face contact may enhance intervention efficacy for some, and needs to be considered in future programming. Additional research also is needed to better understand environmental determinants of diet and PA in rural areas, and contextual factors influencing successful implementation across various healthcare and community settings.
Summary
Overall, there are limited data available on the effectiveness of weight management and PA interventions for diverse populations, though feasibility and safety have been established. In each unique population, a variety of factors must be addressed to ensure that weight management, PA, and behavioral modification elements address physiologic needs and health issues (e.g., promoting slower weight loss among pediatric and geriatric cancer survivors who are at greater risk for stunting and sarcopenia, respectively), individual preferences (e.g., home-based delivery to overcome travel barriers), and community-based resources (e.g., partnering with community-based organizations for program implementation). Given the higher prevalence of overweight and obesity and suboptimal lifestyle practices among certain subpopulations of cancer survivors (e.g., racial/ethnic minorities, those residing in rural areas, pediatric and older cancer survivors) there is a need to target interventions to these diverse populations that are currently more likely to have poorer outcomes and shorter years of survival.
Opportunities and Challenges for the Workforce, Care Coordination, and Technologies to Support Weight Management and Physical Activity in Cancer Survivors
Several factors currently limit the ability to deliver weight management and PA programs to all cancer survivors who need them. These barriers exist at multiple levels. Barriers at the level of the cancer survivor and family have been covered in forerunning sections and include factors such as high costs, lack of geographic access to these programs, or lack of knowledge or motivation of how to change health behaviors. These may be compounded by barriers at the level of the clinician, such as lack of clinician comfort with discussing weight with patients or lack of knowledge of what intervention to refer or prescribe, as well as competing demands for time in the clinical encounter. Finally, barriers at the level of the healthcare system and the environment present further challenges; for example, a lack of prioritization of PA, weight management or disease prevention in general, a lack of insurance coverage for lifestyle change programs, or the obesogenic environment. These challenges and current strategies to overcome them will be described in this section.
Weight Management and Physical Activity: Clinical Care Opportunities and Challenges
A health care professional’s recommendation to exercise significantly improves PA engagement;77, 170 yet, many providers do not counsel patients who might benefit on the need for PA or weight management. Research shows that providers are more likely to encourage health behavior change if they have established a positive patient-physician relationship, have available referral resources to facilitate health behavior change, and believe that health behavior engagement will benefit cancer outcomes or overall health and well-being.171 Several individual and systems barriers need to be overcome in order to promote PA and weight management in the delivery of survivorship care. Competing time demands during oncology visits dictate that oncology care providers help patients make difficult choices about therapy, monitor side effects, promote adherence to oral medications, administer screening evaluations, and help patients cope with the psychological effects of cancer diagnosis and treatment, presenting significant obstacles to discussions of PA and weight management.171–174 Moreover, in the current age of electronic medical records, health care professionals in general spend only half of their scheduled clinic visit time talking with the patient and over one-third of their time on documentation.175 Furthermore, most health care professionals do not receive adequate training in how to operationalize health behavior recommendations at the level of an individual patient, 173 so a generalized lack of expertise or competency in knowing what and how to recommend changes is a major barrier. Providers also need resources for appropriate services (e.g., dietitians, exercise specialists, or programs),171, 176, 177 but a lack of supportive infrastructure, including lack of access to appropriate referrals or effective strategies, as well as limited insurance coverage, can inhibit provider recommendations.172, 173 Care providers also may be skeptical that a cancer survivor can change behavior or worry that engaging in weight management or PA during or after treatment may be risky to the survivor’s overall health.171 Finally, a lack of knowledge about the benefits of weight management and PA can result in a lack of motivation to focus on this topic.171 Fortunately, there are several resources available for cancer survivors and health care professionals to help promote weight management and PA (see Table 5).
Table 5.
Resources for weight management and physical activity
| Organization | Resources Available | Website | Telephone |
|---|---|---|---|
| American Cancer Society | Survivorship guidelines (nutrition and physical activity; cancer-specific) | www.cancer.org | (800) 227-2345 |
| American College of Sports Medicine | Physical activity guidelines for exercise professionals | www.acsm.org | (317) 637-9200 |
| American Institute for Cancer Research | Health behavior information and recommendations | www.aicr.org | (800) 843-8114 |
| American Physical Therapy Association/The Oncology Section | Physical activity and safety considerations for the cancer survivor | www.apta.org | (800) 999-2782 |
| American Society of Clinical Oncology | Survivorship Compendium, Obesity Toolkit | www.asco.org | (571) 483-1300 |
| Cancer Nutrition Consortium | Nutrition Guidance | www.cancernutrition.org | (857) 301-8495 |
| LIVESTRONG | Health behavior tools, LIVESTRONG at the YMCA | www.livestrong.org | (855) 220-7777 |
| National Cancer Institute/Office of Cancer Survivorship | Facing Forward series, general recommendations, workshops and conferences | www.cancercontrol.cancer.gov/ocs | (800) 422-6237 |
| National Center for Health Promotion & Disease Prevention/VHA | Weight management resources | www.move.va.gov | 1-844-698-2311 |
| National Comprehensive Cancer Network | Survivorship and disease-specific guidelines for health care providers | www.nccn.org | (215) 690-0300 |
| National Heart, Lung, and Blood Institute | Weight management resources | www.nhlbi.nih.gov | (301) 592-8573 |
| Silver Sneakers | Physical activity for older adults | www.silversneakers.com | (866) 584-7389 |
Overcoming Workforce Issues and Establishing Common Competencies
The contribution of excess weight and physical inactivity to cancer and overall health outcomes emphasizes the need for health professional education and adoption of appropriate competencies. The lack of a standard of care for overweight and obesity and its associated lifestyle factors, the mismatch of disease burden with care provider capacity, and the lack of integrated clinical and community services constitute major barriers to effective care. Behavior change is the cornerstone of therapy. For the care of pediatric patients with obesity, the USPSTF recommends moderate to high intensity behavioral interventions, including nutrition, PA, and behavioral counseling for a minimum of 26 contact hours.178 For adults with obesity, the USPSTF recommends obesity behavioral interventions that include self-monitoring and 12–26 visits over the course of a year.51 However, few providers have been trained in the delivery of behavior change therapies, and currently, few major insurance plans provide reimbursement for the duration of care recommended by the USPSTF.
A second gap is the lack of understanding of the most fundamental elements of obesity care. For example, less than 50% of internists, family practitioners, obstetricians/gynecologists, and nurse practitioners surveyed knew the recommended level of PA for adults, and even fewer knew USPSTF guidelines for treatment of obesity. Similar surveys have not been administered to oncology care providers; however, their knowledge in these areas is not likely to be better.
To address these gaps, 24 organizations involved in the care of obesity convened to develop common competencies for the prevention and treatment of obesity.179 This effort, funded by the Robert Wood Johnson Foundation, led to the development of 10 major competencies, which are shown in an abbreviated form in Table 6.180 The consensus-derived competencies are not intended to be an obesity curriculum. Rather, the expectation is that each of the groups involved in their development will adapt them to the needs of their specific profession; a limitation however is that oncology was not represented among these groups.179
Table 6.
Competencies for health care professionals in obesity prevention and control180
| Understanding the framework of obesity as a medical condition |
| Knowledge of epidemiology and key drivers of the epidemic |
| Knowledge of disparities and inequities in obesity prevention and care |
| Providing interprofessional obesity care |
| Integration of clinical and community care for obesity |
| Use of patient-centered communication |
| Recognition and mitigation of weight bias and stigma |
| Accommodation of people with people with obesity |
| Use of strategies for patient care related to obesity |
| Recognition of acute warning signs of obesity complications |
A final issue is the need for providers to be sensitive to the issue of stigma and bias. The stigma associated with obesity may be secondary only to race.181, 182 Because obesity is so highly stigmatized, providers who are uncomfortable with the topic and unaware how to discuss it with their patients often add to this burden.181 As a result, patients with obesity may not receive the care they need. The competencies therefore include understanding terms that are acceptable to patients in discussions about weight, and the need for joint decision making with respect to care.
Opportunities and Challenges posed by New Technologies
Self-monitoring is a strong predictor of weight management success,183 but engagement with self-tracking declines over time.184 New technologies have improved adherence over traditional paper modes, and pairing feedback with tracking optimizes behavior change.184 However, the challenges of maintaining self-monitoring of diet and PA remain. Daily weighing, via an internet-connected scale, paired with text message feedback has been found to promote clinically-meaningful weight loss of 6% within six months in healthy populations.185 These data suggest that self-monitoring strategies that are both discrete and simple achieve high engagement and desired clinical outcomes. However, mobile application abandonment rates have been well documented in commercial and research settings.186, 187 Such data indicate that multiple strategies (including web, email, interactive voice response, and text messaging) are needed to keep users connected to feedback or coaching and are necessary to complement self-monitoring strategies - strategies that may vary by population subgroup. Current research shows that feedback strategies that are real-time responsive (e.g., app messages) are better positioned than those that are delayed (e.g., weekly coaching calls), but there is a need to better understand the reasons users disengage from technology. Moreover, while technology is currently used to address data collection, analytics, and integration with a goal of providing actionable feedback to users, the integration with health care professionals to provide healthcare decision support has yet to be made and is a needed leap.
Summary
The considerable body of research on weight management and PA interventions has documented myriad positive effects during and after cancer care. Despite the numerous challenges in delivering weight management and PA programs for cancer survivors, this is a time of unprecedented opportunity to include these programs as part of standard cancer and follow-up care. Several national trends and changes in healthcare are contributing to this opportunity, while addressing some of the multi-level barriers to delivering these programs. At the level of the survivor and family, it is clear that engaged and activated patients participate more fully in their healthcare. Thus, efforts by clinical and public health groups have focused on patient activation and education about the importance of weight management and PA. Examples of this are patient education materials available on the websites of ASCO, the American Cancer Society (ACS), and the NCI, as well as the Springboard Beyond Cancer mobile health tool (www.survivorship.cancer.gov) and SurvivorSHINE (https://survivorshine.org) websites that help survivors change their lifestyle behaviors along with managing their ongoing symptoms and self-managing their health. The latter are examples of another ongoing trend discussed earlier: using technology to increase the feasibility of delivering interventions to survivors regardless of location. Technological solutions also may help reduce financial barriers to these programs for survivors who have limited economic resources.
At the level of the health care professional and clinical practice, there are now multiple guidelines from the ACS, ACSM, ASCO, NCCN, and others delineating weight management and PA as part of overall cancer care and follow-up care. The work described above, to establish weight management competencies for clinicians, will help increase the ability of the clinical workforce to deliver appropriate care. Additionally, health care professionals need safe and efficacious programs available for patient referral and time during the clinical encounter to discuss these issues and resources with patients and survivors. Interventions in community settings, such as the LIVESTRONG at the YMCA, the availability of ACSM-certified Cancer Exercise Trainers in gyms across the country, and other programs for cancer survivors are helping to provide sources for appropriate referral.
Finally, changes in American healthcare present an opportunity to integrate weight management and PA programs into cancer care and follow-up care. The transition from fee-for-service to value-based payment models is also bringing attention to identifying interventions that are “good buys” (i.e., that result in positive effects in multiple ways, in terms of patient outcomes and cost savings). The multiple positive effects of weight management and PA on overall health, well-being, and QOL are prompting healthcare funders and decision makers look more seriously at these interventions.
Weight Management and Physical Activity Programs to Cancer Survivors: Models of Care
Successful incorporation of PA and weight management services into cancer survivorship care requires effective models of care delivery services. Because survivors come with a range of needs and preferences for services, oncology health care professionals need algorithms for assessing these factors in order to triage survivors and refer them to appropriate programs. Guidelines for providing smoking cessation assistance in primary care settings use a schema that may prove useful in conceptualizing this process; the 5 As (Ask if a patient is smoking, Advise quitting, Assess patient motivation for making a quit attempt, Assist with counseling referral and pharmacotherapy, and Arrange follow-up within a week of quit date).188
Ask/Advise
Referral of cancer survivors to PA and weight management services starts with a conversation between the health care professional and their patient about these issues. A survey of 15,254 cancer survivors in the United Kingdom found that survivors who recalled a conversation with their care provider about exercise were 88% more likely to be physically active and to meet PA recommendations;189 however, only 31% of the respondents recalled such a conversation. In the US, discussion about PA is documented in only 35% of patient-oncologist encounters.190 Therefore, provider prompts may enhance discussions on this topic within the workflow and need to be considered in models of care delivery.
Assess
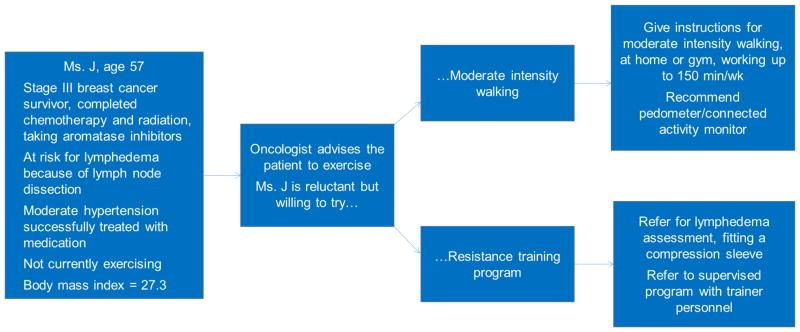
Cancer survivors have a range of needs and limitations regarding their symptoms, physical limitations, and co-morbid health conditions that need to be considered. A process for assessing these conditions is necessary to determine optimal programs for weight loss or PA to ensure appropriate supervision and patient safety. At the same time, the cancer survivors’ goals, preferences, and prior experience with PA and weight management need to be considered. The ACSM health screening guidelines, which take into account the patient’s current activity level, signs and symptoms of disease, and desired intensity of exercise are used to assess capacity for PA in the general population, and may be useful to implement for cancer survivor populations.191 Figure 1 provides an example of how survivor goals and preferences might interact in recommending an exercise program for a breast cancer survivor at risk for lymphedema.
Figure 1.
Example of tailoring exercise program to patient’s physical needs and preferences
Assist (or Refer)
Few clinical practices providing oncology/survivorship care are able to provide PA and weight management services and often require referral to outside programs. There are a growing number of programs available; some are cancer specific (e.g., LIVESTRONG at the YMCA), but programs intended for the general population may also be appropriate (e.g., Silver Sneakers). In addition, home-based or self-directed programs that rely on books and other print material, websites, mobile apps, and wearable devices like pedometers and connected activity monitors and scales can assist cancer survivors with PA and weight management. Cancer survivor-specific versions of such programs have shown efficacy in research studies, but are not widely available outside of the research setting. Cancer survivors who have physical limitations or comorbid medical conditions may need to start with a clinic-based program, such as a cancer rehabilitation program or a medical weight loss program. A challenge for health care professionals is knowing which programs are reliable and of high quality for cancer survivors. There is a need to develop and refine program standards and staff certification to optimize safety and effectiveness.
Connect
Even if effective programs are available, there often is a need to motivate cancer survivors to follow-up on recommendations to access services. Research on smoking cessation shows that placing the onus of contact on the service provider (assuming that the patients’ permission is obtained to share their contact information) can boost enrollment by 13–30 fold.192,193 A similar connection strategy could be employed by an oncology clinic referring patients to programs like LIVESTRONG at the YMCA or cancer rehabilitation.
Cancer survivors need access to a variety of safe and effective programs and clinical services to assist them with increasing PA and managing their weight. In addition, there also is a need to increase provider competencies, develop tools to assist providers, and build capacity in workflow and procedures that increase the likelihood that the patient-provider discussion about weight management and PA transpire, that referral to appropriate programs that are aligned with survivors’ needs and preferences occurs, and that follow-through takes place.
Exemplar Programs
Over the past two decades, and with increasing recognition of the heightened needs of cancer survivors for supportive care to improve both emotional and physical health, a number of PA and weight management interventions have been developed and tested. Brief descriptions of some of these exemplars follow:
MOVE!
In 2006, the Veterans Health Administration (VHA) implemented the MOVE! Weight Management Program for Veterans,194 an evidence-based comprehensive lifestyle intervention (CLI) available to veterans receiving care at all VHA medical centers. MOVE! assists veterans who are overweight or have obesity and an obesity-associated condition, such as cancer or CVD, to achieve clinically significant weight loss.194 The program adheres to evidence-based recommendations from the Department of Veterans Affairs/Department of Defense (VA/DoD) Clinical Practice Guideline for Screening and Management of Overweight and Obesity (CPG),195 which provides flexibility in addressing the unique needs of cancer survivors. VA/DoD CPG recommendations include: yearly screening and documentation of overweight/obesity; pharmacotherapy and bariatric surgery as adjuncts to CLI; shared decision-making among providers and patients to support patient engagement based on individual values and preferences; and repeated assessment of response to treatment, with adjustments as needed to ensure clinically-meaningful weight change. MOVE! and weight management care are embedded within the health care system, while also supporting care coordination across primary and specialty care settings (including oncology), and inpatient and outpatient care.
LIVESTRONG at the YMCA
Over the past decade the YMCA, which consists of a national resource center (YMCA of the USA, or Y-USA) and over 2,700 local YMCAs, has partnered with many public health and health care stakeholders to transform its network to better serve the health of the nation. Local Ys are trained to build their capacity, and to develop competencies needed to become a strong partner. Thereafter they are licensed to deliver standardized programs and services for those with special health needs (e.g., the YMCA’s DPP for people with prediabetes, Healthy Weight and Your Child for families with children who are challenged by obesity, etc.). To address the challenges faced by cancer survivors, Y-USA partnered with the LIVESTRONG Foundation to develop and scale a 12-week PA program.
The program model was nested within a 6-month organizational change process modeled after the Institute for Healthcare Improvement’s “Plan, Do, Study, Act.” Local YMCAs that have an interest in serving cancer survivors within their communities are encouraged to submit a readiness assessment to Y-USA. The LIVESTRONG Foundation, the local YMCA leadership, and more recently the Centers for Disease Control and Prevention, have supported YMCAs that demonstrate the highest levels of commitment to go through the organizational change process and become trained and authorized to deliver the LIVESTRONG at the YMCA program. Through the organizational change process, YMCA staff build competencies, such as those related to understanding cancer survivors’ needs, providing welcoming environments, and developing partnerships with local cancer centers and oncology health care professionals.
Data indicate that the program leads to significant improvements in PA, stamina, and QOL,196 and has been scaled to over 500 locations in 39 states, serving nearly 50,000 cancer survivors. Currently, philanthropy is required to make this program free of charge to all cancer survivors, and wait lists have been established in cases where demand exceeds availability.
In 2016, Y-USA completed a claims-based cost savings demonstration of the YMCA’s DPP that led to Medicare coverage of that program.197 A partnership between Y-USA and the Robert Wood Johnson Foundation was instrumental in transforming the systems behind scaling and sustainability that produced health outcomes and value within the health care system. Connections were made between the electronic medical record system of health care organizations and local YMCA business units. Health care providers are increasingly referring patients to programs like LIVESTRONG at the YMCA under alternative payment models (e.g., Accountable Care Organization [ACO] structures or bundled payment models) in which the value and savings of these programs are re-invested in partnerships with the Y, and payers are providing coverage for the YMCA’s DPP under claims-based reimbursements.
Physical Activity and Lymphedema (PAL) Trial and the Strength after Breast Cancer
The challenge of knowing which cancer survivors can be safely referred to home and community based exercise remains unresolved,198–200 although initiating exercise programming within the context of outpatient rehabilitation clinics is a potential approach. The PAL trial was revised to use this approach among breast cancer survivors after establishing the safety and efficacy of a weight-lifting intervention for lymphedema and other side effects of cancer and its treatment.32, 201–209 The PAL intervention was originally implemented in select YMCAs in which fitness staff received training (including pre-intervention safety evaluations) and ongoing support from PAL investigators. Because of high staff turnover at the YMCAs, concern about implementing safety evaluations, and an unwillingness of participants to continue to pay for YMCA memberships, this program led to creation of “Strength After Breast Cancer” – a dissemination and implementation study to translate PAL into the setting of outpatient rehabilitation.210
As noted in other effective interventions, the “Strength After Breast Cancer” intervention optimally begins with a referral by the oncology/primary care provider to the outpatient rehabilitation clinic, which then contacts the patient. Clinic staff implement a safety evaluation, then deliver an educational session on lymphedema, as well as four sessions of weight lifting instruction prior to release to a home-based program. Favorable comparison of the efficacy of this revised program to the original PAL trial led to an online continuing education course that targets outpatient rehabilitation specialists (http://klosetraining.com/course/online/strength-abc).210 Over 400 outpatient rehabilitation clinicians have completed the course, and no difficulties have been reported in obtaining third party payer reimbursement.
Healthy Living after Cancer
Healthy Living after Cancer (HLaC) is a Partnership Project among four Australian state-based Cancer Councils, funded by the Australian National Health & Medical Research Council. It is evaluating the implementation of an evidence-based, 6-month telephone-delivered lifestyle program, delivered by the Cancer Councils via their national cancer information and support service. HLaC is provided free of charge to cancer survivors with any type cancer, following treatment with curative intent. It provides behaviorally-based support to achieve internationally-agreed recommendations for PA, healthy eating, and healthy weight. In this phase IV dissemination study (single-group, pre-post design with assessments at baseline and six months), primary outcomes relate to program implementation: adoption (referral sources); reach (# of participants) and retention; fidelity of implementation; participant and staff satisfaction; and fixed and recurrent program costs. Secondary outcomes are patient-reported and validated measures of weight, PA, and dietary intake/behavior, QOL, cancer-related side effects, and fear of recurrence. To date, 500 patients have enrolled: 89% female; mean age 55 (SD = 11); average BMI = 29 kg/m2 (SD = 6); with a wide range of cancers. The retention (program completion) rate is 57%. Among the first 200 program completers, significant (p<.05) and clinically-meaningful improvements have been seen in all secondary, patient-reported outcomes. This collaborative undertaking provides an opportunity for national dissemination of an evidence-based intervention to support healthy living among cancer survivors. Rigorous evaluation of service-level and patient-reported outcomes will provide the practice-based evidence needed to achieve sustained support.
Summary
MOVE!, Strength After Breast Cancer, LIVESTRONG at the Y, and HLaC all serve as model programs that effectively address the PA and weight management needs of cancer survivors. These programs, and many others like them, have established feasibility, safety, and efficacy. While successful, common challenges such as referral, training, triage, support, and reimbursement remain as barriers.
Insurance Coverage of Weight Management and Physical Activity in Cancer Care
Obesity presents unique challenges in patients with cancer, but because of the high prevalence of obesity within the US and its association with a constellation of chronic diseases,211 most payers consider it a general concern across all of their health plan membership. While evidence of what works in the treatment of obesity is growing,54, 212, 213 the services that are covered, and how the coverage is implemented in health benefit plans remains highly variable. Private and governmental payers of health insurance/benefit plans consider multiple factors as they decide what to cover, and how to implement coverage. While many consumers and healthcare professionals assume that coverage decisions are based solely on cost, payers generally consider a number of factors in coverage decisions, including consumer/employer demand for a service, evidence for effectiveness and efficiency of the service, the ability to administer the benefit consistently and fairly, presence of state/federal governmental mandates for a service, and how the benefit will affect the marketability/adverse selection of a health plan.
In its role as a fiduciary agent for its members, and for taxpayers in the case of the Centers for Medicare and Medicaid Services (CMS), insurers are faced with balancing their fiscal responsibilities with the mission and values of their organizations. An insurer first determines whether a service is effective, and then assesses the impact of that service on the overall cost of care for the members utilizing that service. For example, coverage of the previously mentioned Y-USA DPP was based on relatively short-term data from a CMS Innovation Center demonstration project that indicated modest weight loss and significant savings in total costs of care. However, because the program was projected to reduce premature mortality, actuarial evaluation suggested that the program could ultimately lead to higher costs for Medicare patients who had care needs for a longer period of time. In response, CMS determined that longer life would not be considered as a cost (i.e., care costs over a longer life span were zeroed-out). As a result, CMS announced that in 2018, Medicare would begin reimbursing all DPP programs that meet CDC requirements.214
Interestingly, as value-based payment systems become more prevalent for both government and private payers, issues historically considered within the insurer’s purview will shift to health care providers who share the financial risks (e.g., Next Generation ACOs).215 These payment models provide payment based on a defined population of consumers and include incentives and potential penalties for both quality and cost metrics. Private payers, including multi-state Anthem Blue Cross and Blue Shield and most national health plans have developed similar value-based payment models. The expectation is that ACOs, or groups of doctors, hospitals, and other health professionals who come together voluntarily to provide coordinated high quality care, will provide services that improve the overall health and well-being for a defined set of patients, including both clinical and community services for the prevention and treatment of obesity.
While ACOs currently account for ~10% of the private health insurance market, insurers such as Anthem indicate that up to 60% of their fully-insured membership may be covered under value-based payment models. The US Department of Health and Human Services has set a goal of tying 90% of all Medicare fee-for-service payments to quality or value metrics by 2018.216
Value-based metrics, however, are evolving and incomplete. The National Quality Forum has only endorsed four screening metrics related to obesity, and has yet to reach consensus on any outcome measures,217 but promising developments have transpired with Medicare coverage of the DPP, which includes an outcomes-based payment tied to both short-term and long-term patient outcomes.
The Bipartisan Policy Center has convened both commercial and government payers to discuss coverage for services related to obesity, including an effort to develop shared benefit design. Their discussions have focused on benchmarks of efficacy and cost effectiveness, in addition to issues related to member retention, return on investment, community partnerships, senior leadership support, and data tracking. Thus, health professionals interested in working with payers to improve coverage for obesity-related services need to understand not only the needs of their patients, but the context in which payers make their coverage determinations.
Advancing Progress in Tertiary Prevention: Stakeholder Insights and Recommendations
As cancer treatment advances and survivors live significantly longer, enhancing health and QOL for cancer survivors has become a major public health goal--one that also has widespread implications for the financial well-being of survivors and families and of the healthcare system. Evidence continues to accumulate that strongly suggests that weight management and PA can improve the management of cancer and comorbid conditions, and QOL. However, there are three urgent challenges that must be overcome to connect cancer survivors with interventions that can ideally help them.
The first challenge is identifying the optimal type of intervention for a given survivor (e.g., specific tumor type, cultural factors, comorbidities, functional status) and a specific goal (e.g., fatigue management or decreasing risk of recurrence). This involves research to test varied types of interventions and capturing multiple types of patient data (tumor subtypes, clinical lab values, patient-reported symptom data) in multiple settings (identifying who needs medically-supervised programs vs. who benefits from community programs vs. home-based interventions).
The second challenge is identifying how to deliver evidence-based interventions to support weight management and PA, not only in medical settings but also in the community or through the use of technology. Effective examples of model programs exist, e.g., MOVE!, Strength After Breast Cancer, LIVESTRONG, and HLaC. Research is needed to continue to test clinic-based, community-based, and technology-delivered interventions, as well as identify how to facilitate referrals from oncologists and other healthcare professionals to these interventions. Educating healthcare providers about the importance of these interventions is a valuable, but insufficient step. Future efforts need to address how to integrate weight management and PA interventions into standard cancer care.
The third challenge is accumulating the right data about weight management and PA programs to inform healthcare payer decisions to cover these interventions. Reimbursement by insurers will help make these interventions more affordable for survivors and drive widespread availability of these services. Part of this evidence will come from ongoing trials that will provide evidence regarding the benefits of post-diagnosis weight management and PA on recurrence and survival. However, even if these interventions do not affect recurrence specifically, coverage decisions may be made based on comorbidity management, or effects on downstream healthcare utilization. Future research needs to test the cost effectiveness of these interventions on these important outcomes to inform healthcare coverage decisions.
The key to our success in implementing weight management and PA programs will be to bridge the silos of expertise as represented in the NCPF workshop in 2017 in cancer biology, epidemiology, survivorship, nutrition, PA, weight management, and economics, as well as health care systems (e.g., ACOs, payers, hospitals, oncology practices), care providers (e.g., oncologists, primary care providers, allied health professionals, cancer rehabilitation, behavioral medicine) and the community (e.g., advocacy organizations, YMCAs). The efforts to implement effective programs will need to address individual, provider/workforce, and systemic barriers that include barriers specific to cancer survivors (symptoms and treatment side effects), as well as individual and cultural differences. The challenge is great. The opportunities and benefits of collaboration across disciplines and key stakeholders has significant potential to enhance outcomes for the growing number of cancer survivors in the United States and beyond.
Acknowledgments
The responsibility for the content of this article rests with the authors and does not necessarily represent the views of the National Academies of Sciences, Engineering, and Medicine, its committees, its sponsors, or its convening activities and does not represent the official position of the Centers for Disease Control and Prevention (CDC), the Veterans Health Administration, the Department of Veterans Affairs or the US Government. The National Cancer Policy Forum workshop was supported by the CDC, National Institutes of Health/National Cancer Institute, American Association for Cancer Research, American Cancer Society (ACS), American College of Lifestyle Medicine, American College of Radiology, American College of Sports Medicine, American Council on Exercise, American Society for Radiation Oncology, American Society of Clinical Oncology, American Society of Hematology, Association of American Cancer Institutes, AstraZeneca, Bristol-Myers Squibb, Cancer Support Community, CEO Roundtable on Cancer, Dana-Farber Cancer Institute, Flatiron Health, Helsinn Healthcare SA, LIVESTRONG Foundation, the Medical Fitness Association, Merck Research Laboratories, National Comprehensive Cancer Network, Novartis Oncology, Oncology Nursing Society, Penn State Cancer Institute, Pfizer Inc., and the University of Texas MD Anderson Cancer Center. Support for individual efforts were as follows: ACS Clinical Research Professor Award (CRP-14-111-01-CPPB) and the NCI (P30 CA13148) for WDW and JRB; Breast Cancer Research Foundation and Hold’Em for Life Charities for PJG; the NCI (P30 CA023074) for CAT; the Australian National Health and Medical Research Council Senior Research Fellowship (APP1041789) for EGE, the NIH (P2CHD086851) for MMB; and the Duncan Family Institute, the Cancer Prevention Research Institute of Texas (PP 130079 and PP170023) and the NCI (P30 CA 16672) for KB-E.
Footnotes
Potential Conflicts of interest: All authors have no conflicts of interest, except Dr. Caroline Apovian (Nutrisystem, Zafgen, Sanofi-Aventis, NovoNordisk, Scientific Intake, Merck, and Johnson and Johnson: Advisor/ Gelesis, Orexigen, GI Dynamics: Research Funding and Advisor/ Takeda: Research Funding and Speaker’s Bureau/Aspire Bariatrics, Myos, Vela Foundation, Dr. Robert C. and Veronica Atkins Foundation, Coherence Lab, and Energesis: Research Funding), Dr. Crystal Denlinger (Advaxis, Genentech, InCyte Corporation, OncoMed, Astex Pharmaceuticals, Bristol Myers Squibb, and Macrogenics: Research Funding/Carevive: Consultant/ Eli Lilly and Company and Merrimack Pharmaceuticals: Research Funding and Advisor/Merck and EMD Serono: Advisor/ MedImmune LLC: Research Funding and Medical Writing Support), Dr. Katherine Schmitz (Strength After Breast Cancer Educational Materials: Copyright), and Ms. Erin Balogh and Dr. Sharyl Nass (AstraZeneca, Bristol-Myers Squibb, Flatiron Health, Helsinn Healthcare SA, Medical Fitness Association, Merck Research Laboratories, Novartis Oncology, Pfizer, Inc: Research Funding).
Contributor Information
Wendy Demark-Wahnefried, Professor of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL, 1675 6th Avenue South, Wallace Tumor Institute, Room 102M, Birmingham, AL 35294.
Kathryn H. Schmitz, Professor of Public Health Sciences, Penn State College of Medicine, Hershey, PA.
Catherine M. Alfano, Vice President, Survivorship, American Cancer Society, Inc., Washington DC
Jennifer R. Bail, Post-doctoral Fellow, Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL.
Pamela J. Goodwin, Professor of Medicine, Mount Sinai Hospital, Lunenfeld-Tanenbaum Research Institute at the University of Toronto.
Cynthia A. Thomson, Professor of Health Promotion Sciences, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, AZ.
Don W. Bradley, Associate Consulting Professor Community & Family Medicine, Duke School of Medicine, Durham, North Carolina.
Kerry S. Courneya, Professor of Physical Education and Recreation, University of Alberta, Edmonton, Canada
Christie A. Befort, Associate Professor of Preventive Medicine, University of Kansas Medical Center, Kansas City, KS.
Crystal S. Denlinger, Associate Professor of Hematology/Oncology, Fox Chase Cancer Center, Philadelphia, PA.
Jennifer A. Ligibel, Associate Professor of Medicine, Harvard Medical School, Boston, MA.
William H. Dietz, Chair, Redstone Global Center for Prevention and Wellness, George Washington University
Melinda R. Stolley, Professor of Medicine, Medical College of Wisconsin, Milwaukee WI.
Melinda L. Irwin, Professor of Epidemiology, Yale School of Public Health, New Haven, CT.
Marcas M. Bamman, Professor of Cell Developmental and Integrative Biology, University of Alabama at Birmingham, Birmingham, AL.
Caroline M. Apovian, Professor of Medicine, Boston University School of Medicine.
Bernardine M. Pinto, Professor of Nursing, University of South Carolina, Columbia SC.
Kathleen Y. Wolin, Coeus Health, Chicago, IL.
Rachel M. Ballard, Director, Prevention Research Coordination, Office of Disease Prevention, Office of the Director, National Institutes of Health, Bethesda, MD.
Andrew J. Dannenberg, Professor of Medicine, Weill Cornell Medicine, New York, NY.
Elizabeth G. Eakin, Professor and Director, Cancer Prevention Research Centre, School of Public Health, Faculty of Medicine, The University of Queensland, Australia.
Matt M. Longjohn, Vice President and National Health Officer, YMCA of the USA, Chicago, IL.
Susan D. Raffa, National Program Director for Weight Management, Veterans Health Administration, Durham, NC.
Lucile L. Adams-Campbell, Professor of Oncology, Georgetown Lombardi Comprehensive Cancer Center, Washington, DC.
Joanne Buzaglo, Research & Training Institute, Cancer Support Community, Philadelphia, PA.
Sharyl J. Nass, National Cancer Policy Forum and Board on Health Care Services, Health & Medicine Division, National Academies of Science, Engineering, and Medicine, Washington, DC.
Greta Massetti, Centers for Disease Control and Prevention, Atlanta, GA.
Erin P. Balogh, Senior Program Officer, National Cancer Policy Forum, Health and Medicine Division, National Academies of Science, Engineering, and Medicine, Washington, DC.
Elizabeth Kraft, Senior Clinical Officer, Anthem Blue Cross Colorado, Denver, CO.
Anand Parekh, Chief Medical Advisor, Bipartisan Policy Center, Washington, DC.
Darshak Sanghavi, Chief Medical Officer, Senior Vice President, Translation OptumLabs, Cambridge, MA.
G. Stephen Morris, Professor of Physical Therapy, Wingate University, Wingate, NC.
Karen Basen-Engquist, Professor of Behavioral Science, The University of Texas - M. D. Anderson Cancer Center, Houston, TX.
References
- 1.Institute of Medicine. The Role of Obesity in Cancer Survival and Recurrence: Workshop Summary. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 2.Hursting SD, DiGiovanni J, Dannenberg AJ, et al. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res (Phila) 2012;5:1260–1272. doi: 10.1158/1940-6207.CAPR-12-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21:1244–1259. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–3574. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Academies of Sciences, Engineering, and Medicine. Incorporating weight management and physical activity throughout the cancer care continuum: Proceedings of a workshop. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 6.National Academies of Sciences Engineering, and Medicine website. Available from: www.nationalacademies.org/hmd/Activities/Disease/NCPF/2017-FEB-13.aspx.
- 7.American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016–2017. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 8.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer: Viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat. 2012;134:769–781. doi: 10.1007/s10549-012-2073-x. [DOI] [PubMed] [Google Scholar]
- 11.Guenancia C, Lefebvre A, Cardinale D, et al. Obesity As a Risk Factor for Anthracyclines and Trastuzumab Cardiotoxicity in Breast Cancer: A Systematic Review and Meta-Analysis. J Clin Oncol. 2016;34:3157–3165. doi: 10.1200/JCO.2016.67.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakimi AA, Furberg H, Zabor EC, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst. 2013;105:1862–1870. doi: 10.1093/jnci/djt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. 2015;66:297–309. doi: 10.1146/annurev-med-050913-022228. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin PJ, Stambolic V. Impact of the obesity epidemic on cancer. Annu Rev Med. 2015;66:281–296. doi: 10.1146/annurev-med-051613-012328. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Fuentes-Mattei E, Velazquez-Torres G, Phan L, et al. Effects of obesity on transcriptomic changes and cancer hallmarks in estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical Activity and Cancer Outcomes: A Precision Medicine Approach. Clin Cancer Res. 2016;22:4766–4775. doi: 10.1158/1078-0432.CCR-16-0067. [DOI] [PubMed] [Google Scholar]
- 18.Thomas RJ, Kenfield SA, Jimenez A. Exercise-induced biochemical changes and their potential influence on cancer: a scientific review. Br J Sports Med. 2017;51:640–644. doi: 10.1136/bjsports-2016-096343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courneya KS, Booth CM, Gill S, et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol. 2008;15:279–285. doi: 10.3747/co.v15i6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courneya KS, Vardy J, Gill S, et al. Update on the Colon Health and Life-Long Exercise Change Trial: A phase III study of the impact of an exercise program on disease-free survival in colon cancer survivors. Curr Colorectal Cancer Rep. 2014;10:321–328. [Google Scholar]
- 21.Courneya KS, Vardy JL, O’Callaghan CJ, et al. Effects of a Structured Exercise Program on Physical Activity and Fitness in Colon Cancer Survivors: One Year Feasibility Results from the CHALLENGE Trial. Cancer Epidemiol Biomarkers Prev. 2016;25:969–977. doi: 10.1158/1055-9965.EPI-15-1267. [DOI] [PubMed] [Google Scholar]
- 22.Saad F, Kenfield SA, Chan JM, et al. Intense exercise for survival among men with metastatic castrate-resistant prostate cancer (INTERVAL–MCRPC): A Movember funded multicenter, randomized, controlled phase III study. J Clin Oncol. 2016;34(Suppl 15):5092. [Google Scholar]
- 23.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 25.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 26.Lustberg MB, Reinbolt RE, Shapiro CL. Bone health in adult cancer survivorship. J Clin Oncol. 2012;30:3665–3674. doi: 10.1200/JCO.2012.42.2097. [DOI] [PubMed] [Google Scholar]
- 27.Rock CL, Flatt SW, Byers TE, et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: A Behavioral Weight Loss Intervention in Overweight or Obese Breast Cancer Survivors. J Clin Oncol. 2015;33:3169–3176. doi: 10.1200/JCO.2015.61.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodwin PJ, Segal RJ, Vallis M, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol. 2014;32:2231–2239. doi: 10.1200/JCO.2013.53.1517. [DOI] [PubMed] [Google Scholar]
- 29.Befort CA, Klemp JR, Sullivan DK, et al. Weight loss maintenance strategies among rural breast cancer survivors: The rural women connecting for better health trial. Obesity (Silver Spring) 2016;24:2070–2077. doi: 10.1002/oby.21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrigan M, Cartmel B, Loftfield E, et al. Randomized Trial Comparing Telephone Versus In-Person Weight Loss Counseling on Body Composition and Circulating Biomarkers in Women Treated for Breast Cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. J Clin Oncol. 2016;34:669–676. doi: 10.1200/JCO.2015.61.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chlebowski RT, Reeves MM. Weight Loss Randomized Intervention Trials in Female Cancer Survivors. J Clin Oncol. 2016;34:4238–4248. doi: 10.1200/JCO.2016.69.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winters-Stone KM, Laudermilk M, Woo K, Brown JC, Schmitz KH. Influence of weight training on skeletal health of breast cancer survivors with or at risk for breast cancer-related lymphedema. J Cancer Surviv. 2014;8:260–268. doi: 10.1007/s11764-013-0337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winters-Stone KM, Dobek J, Nail LM, et al. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporos Int. 2013;24:1637–1646. doi: 10.1007/s00198-012-2143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toriola AT, Liu J, Ganz PA, et al. Effect of weight loss on bone health in overweight/obese postmenopausal breast cancer survivors. Breast Cancer Res Treat. 2015;152:637–643. doi: 10.1007/s10549-015-3496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Poznak CH. Bone health in adults treated with endocrine therapy for early breast or prostate cancer. Am Soc Clin Oncol Educ Book. 2015:e567–574. doi: 10.14694/EdBook_AM.2015.35.e567. [DOI] [PubMed] [Google Scholar]
- 36.Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16:112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams SC, DeLorey DS, Davenport MH, et al. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: a phase 2 randomized controlled trial. Cancer. 2017 doi: 10.1002/cncr.30859. [DOI] [PubMed] [Google Scholar]
- 38.Hartman SJ, Nelson SH, Myers E, et al. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: The memory & motion study. Cancer. 2017 doi: 10.1002/cncr.30987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleckner I, Kamen CS, Peppone LJ, et al. A URCC NCORP nationwide randomized controlled trial investigating the effect of exercise on chemotherapy-induced peripheral neuropathy in 314 cancer patients. J Clin Oncol. 2016;34:10000–10000. [Google Scholar]
- 40.Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer–related lymphedema. N Engl J Med. 2009;361:664–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 41.Irwin ML, Cartmel B, Gross CP, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol. 2015;33:1104–1111. doi: 10.1200/JCO.2014.57.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buffart LM, Kalter J, Sweegers MG, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. doi: 10.1016/j.ctrv.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2015;15:77. doi: 10.1186/s12885-015-1069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 45.Rack B, Andergassen U, Neugebauer J, et al. The German SUCCESS C Study - The First European Lifestyle Study on Breast Cancer. Breast Care (Basel) 2010;5:395–400. doi: 10.1159/000322677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villarini A, Pasanisi P, Traina A, et al. Lifestyle and breast cancer recurrences: the DIANA-5 trial. Tumori. 2012;98:1–18. doi: 10.1177/030089161209800101. [DOI] [PubMed] [Google Scholar]
- 47.Thomson CA, Crane TE, Miller A, Garcia DO, Basen-Engquist K, Alberts DS. A randomized trial of diet and physical activity in women treated for stage II–IV ovarian cancer: Rationale and design of the Lifestyle Intervention for Ovarian Cancer Enhanced Survival (LIVES): An NRG Oncology/Gynecologic Oncology Group (GOG-225) Study. Contemp Clin Trials. 2016;49:181–189. doi: 10.1016/j.cct.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312:923–933. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 49.Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension. 2011;58:950–958. doi: 10.1161/HYPERTENSIONAHA.111.177071. [DOI] [PubMed] [Google Scholar]
- 50.LeBlanc E, O’Connor E, Whitlock EP, Patnode C, Kapka T U.S. Preventive Services Task Force. Screening for and Management of Obesity and Overweight in Adults. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011. Evidence Syntheses, formerly Systematic Evidence Reviews. [PubMed] [Google Scholar]
- 51.Moyer VA. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:373–378. doi: 10.7326/0003-4819-157-5-201209040-00475. [DOI] [PubMed] [Google Scholar]
- 52.Lyznicki JM, Young DC, Riggs JA, Davis RM. Obesity: assessment and management in primary care. Am Fam Physician. 2001;63:2185–2196. [PubMed] [Google Scholar]
- 53.American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. Washington, DC: American College of Obstetricians and Gynecologists; 2007. [Google Scholar]
- 54.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Look ARG, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhasin S, Apovian CM, Travison TG, et al. Design of a randomized trial to determine the optimum protein intake to preserve lean body mass and to optimize response to a promyogenic anabolic agent in older men with physical functional limitation. Contemp Clin Trials. 2017;58:86–93. doi: 10.1016/j.cct.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volpi E, Campbell WW, Dwyer JT, et al. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci. 2013;68:677–681. doi: 10.1093/gerona/gls229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 60.Winters-Stone KM, Nail L, Bennett JA, Schwartz A. Bone Health and Falls: Fracture Risk in Breast Cancer Survivors With Chemotherapy-Induced Amenorrhea. Oncol Nurs Forum. 2009;36:315–325. doi: 10.1188/09.ONF.315-325. [DOI] [PubMed] [Google Scholar]
- 61.Winters-Stone KM, Moe E, Graff JN, et al. Falls and Frailty in Prostate Cancer Survivors: Current, Past, and Never Users of Androgen Deprivation Therapy. J Am Geriatr Soc. 2017;65:1414–1419. doi: 10.1111/jgs.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med. 2014;46:17–23. doi: 10.1016/j.amepre.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 63.Bamman MM, Cooper DM, Booth FW, et al. Exercise biology and medicine: innovative research to improve global health. Mayo Clin Proc. 2014;89:148–153. doi: 10.1016/j.mayocp.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55:2895–2905. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]
- 65.Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol. 2011;111:1497–1504. doi: 10.1152/japplphysiol.00420.2011. [DOI] [PubMed] [Google Scholar]
- 66.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laukkanen JA, Zaccardi F, Khan H, Kurl S, Jae SY, Rauramaa R. Long-term Change in Cardiorespiratory Fitness and All-Cause Mortality: A Population-Based Follow-up Study. Mayo Clin Proc. 2016;91:1183–1188. doi: 10.1016/j.mayocp.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 69.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 70.Chakravarty EF, Hubert HB, Lingala VB, Fries JF. Reduced disability and mortality among aging runners: a 21-year longitudinal study. Arch Intern Med. 2008;168:1638–1646. doi: 10.1001/archinte.168.15.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmid D, Leitzmann MF. Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol. 2015;26:272–278. doi: 10.1093/annonc/mdu250. [DOI] [PubMed] [Google Scholar]
- 72.Kelley GA, Kelley KS. Is sarcopenia associated with an increased risk of all-cause mortality and functional disability? Exp Gerontol. 2017;96:100–103. doi: 10.1016/j.exger.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34:329–348. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- 74.Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016;23:1034–1047. doi: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neufer PD, Bamman MM, Muoio DM, et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015;22:4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 76.Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W. Weight loss intervention trials in women with breast cancer: a systematic review. Obes Rev. 2014;15:749–768. doi: 10.1111/obr.12190. [DOI] [PubMed] [Google Scholar]
- 77.Jones LW, Courneya KS, Fairey AS, Mackey JR. Effects of an oncologist’s recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: a single-blind, randomized controlled trial. Ann Behav Med. 2004;28:105–113. doi: 10.1207/s15324796abm2802_5. [DOI] [PubMed] [Google Scholar]
- 78.Pinto BM, Ciccolo JT. Physical activity motivation and cancer survivorship. Recent Results Cancer Res. 2011;186:367–387. doi: 10.1007/978-3-642-04231-7_16. [DOI] [PubMed] [Google Scholar]
- 79.Bluethmann SM, Vernon SW, Gabriel KP, Murphy CC, Bartholomew LK. Taking the next step: a systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res Treat. 2015;149:331–342. doi: 10.1007/s10549-014-3255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mutrie N, Campbell A, Barry S, et al. Five-year follow-up of participants in a randomised controlled trial showing benefits from exercise for breast cancer survivors during adjuvant treatment. Are there lasting effects? J Cancer Surviv. 2012;6:420–430. doi: 10.1007/s11764-012-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blundell JE, Gibbons C, Caudwell P, Finlayson G, Hopkins M. Appetite control and energy balance: impact of exercise. Obes Rev. 2015;16(Suppl 1):67–76. doi: 10.1111/obr.12257. [DOI] [PubMed] [Google Scholar]
- 82.Courneya KS, McKenzie DC, Mackey JR, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105:1821–1832. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 83.Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Health Psychol. 2013;32:616–626. doi: 10.1037/a0029886. [DOI] [PubMed] [Google Scholar]
- 84.National Institutes of Health. Clinical Trial Requirements for Grants and Contracts. Available from: https://grants.nih.gov/policy/clinical-trials.htm.
- 85.National Heart, Lung, and Blood Institute. Accumulating Data to Optimally Predict Obesity Treatment (ADOPT) Core Measures Working Group Meetings. Available from: https://www.nhlbi.nih.gov/research/reports/accumulating-data-optimally-predict-obesity-treatment-adopt-core-measures-working-group-meetings.
- 86.Bail J, Meneses K, Demark-Wahnefried W. Nutritional Status and Diet in Cancer Prevention. Semin Oncol Nurs. 2016;32:206–214. doi: 10.1016/j.soncn.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 87.King D, Miranda P, Gor B, et al. Addressing cancer health disparities using a global biopsychosocial approach. Cancer. 2010;116:264–269. doi: 10.1002/cncr.24765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98:1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014. J Clin Oncol. 2016;34:3133–3140. doi: 10.1200/JCO.2016.66.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dennis Parker EA, Sheppard VB, Adams-Campbell L. Compliance with national nutrition recommendations among breast cancer survivors in “stepping stone”. Integr Cancer Ther. 2014;13:114–120. doi: 10.1177/1534735413503550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paxton RJ, Phillips KL, Jones LA, et al. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer. 2012;118:4024–4031. doi: 10.1002/cncr.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nayak P, Paxton RJ, Holmes H, Thanh Nguyen H, Elting LS. Racial and ethnic differences in health behaviors among cancer survivors. Am J Prev Med. 2015;48:729–736. doi: 10.1016/j.amepre.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 93.Nichols HB, Trentham-Dietz A, Egan KM, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18:1403–1409. doi: 10.1158/1055-9965.EPI-08-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weaver KE, Foraker RE, Alfano CM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv. 2013;7:253–261. doi: 10.1007/s11764-013-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 96.Ansa B, Yoo W, Whitehead M, Coughlin S, Smith S. Beliefs and Behaviors about Breast Cancer Recurrence Risk Reduction among African American Breast Cancer Survivors. Int J Environ Res Public Health. 2015;13 doi: 10.3390/ijerph13010046. ijerph13010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Golden SH, Ferketich A, Boyington J, et al. Transdisciplinary cardiovascular and cancer health disparities training: experiences of the centers for population health and health disparities. Am J Public Health. 2015;105(Suppl 3):S395–402. doi: 10.2105/AJPH.2014.302489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mellerson J, Landrine H, Hao Y, Corral I, Zhao L, Cooper DL. Residential segregation and exercise among a national sample of Hispanic adults. Health Place. 2010;16:613–615. doi: 10.1016/j.healthplace.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 99.Shariff-Marco S, Von Behren J, Reynolds P, et al. Impact of Social and Built Environment Factors on Body Size among Breast Cancer Survivors: The Pathways Study. Cancer Epidemiol Biomarkers Prev. 2017;26:505–515. doi: 10.1158/1055-9965.EPI-16-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zenk SN, Mentz G, Schulz AJ, Johnson-Lawrence V, Gaines CR. Longitudinal Associations Between Observed and Perceived Neighborhood Food Availability and Body Mass Index in a Multiethnic Urban Sample. Health Educ Behav. 2017;44:41–51. doi: 10.1177/1090198116644150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Litt JS, Soobader MJ, Turbin MS, Hale JW, Buchenau M, Marshall JA. The influence of social involvement, neighborhood aesthetics, and community garden participation on fruit and vegetable consumption. Am J Public Health. 2011;101:1466–1473. doi: 10.2105/AJPH.2010.300111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harris E. The role of community gardens in creating healthy communities. Australian Planner. 2009;46:24–27. [Google Scholar]
- 103.Zick CD, Smith KR, Kowaleski-Jones L, Uno C, Merrill BJ. Harvesting more than vegetables: the potential weight control benefits of community gardening. Am J Public Health. 2013;103:1110–1115. doi: 10.2105/AJPH.2012.301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brown AF, Morris DM, Kahn KL, et al. The Healthy Community Neighborhood Initiative: Rationale and Design. Ethn Dis. 2016;26:123–132. doi: 10.18865/ed.26.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kreuter MW, Lukwago SN, Bucholtz RD, Clark EM, Sanders-Thompson V. Achieving cultural appropriateness in health promotion programs: targeted and tailored approaches. Health Educ Behav. 2003;30:133–146. doi: 10.1177/1090198102251021. [DOI] [PubMed] [Google Scholar]
- 106.Stolley MR, Sharp LK, Wells AM, Simon N, Schiffer L. Health behaviors and breast cancer: experiences of urban African American women. Health Educ Behav. 2006;33:604–624. doi: 10.1177/1090198106290845. [DOI] [PubMed] [Google Scholar]
- 107.Wilson DB, Porter JS, Parker G, Kilpatrick J. Anthropometric changes using a walking intervention in African American breast cancer survivors: a pilot study. Prev Chronic Dis. 2005;2:A16. [PMC free article] [PubMed] [Google Scholar]
- 108.Djuric Z, Mirasolo J, Kimbrough L, et al. A pilot trial of spirituality counseling for weight loss maintenance in African American breast cancer survivors. J Natl Med Assoc. 2009;101:552–564. doi: 10.1016/s0027-9684(15)30940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Greenlee HA, Crew KD, Mata JM, et al. A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity (Silver Spring) 2013;21:65–76. doi: 10.1002/oby.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Greenlee H, Gaffney AO, Aycinena AC, et al. ¡Cocinar Para Su Salud!: Randomized Controlled Trial of a Culturally Based Dietary Intervention among Hispanic Breast Cancer Survivors. J Acad Nutr Diet. 2015;115:709–723. e3. doi: 10.1016/j.jand.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Griffith KA, Royak-Schaler R, Nesbitt K, et al. A culturally specific dietary plan to manage weight gain among African American breast cancer survivors: a feasibility study. Nutr Health. 2012;21:97–105. doi: 10.1177/0260106012459938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nock NL, Owusu C, Kullman EL, et al. A Community-Based Exercise and Support Group Program in African-American Breast Cancer Survivors (ABCs) J Phys Ther Health Promot. 2013;1:15–24. doi: 10.18005/pthp0101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spector D, Deal AM, Amos KD, Yang H, Battaglini CL. A pilot study of a home-based motivational exercise program for African American breast cancer survivors: clinical and quality-of-life outcomes. Integr Cancer Ther. 2014;13:121–132. doi: 10.1177/1534735413503546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Conlon BA, Kahan M, Martinez M, et al. Development and Evaluation of the Curriculum for BOLD (Bronx Oncology Living Daily) Healthy Living: a Diabetes Prevention and Control Program for Underserved Cancer Survivors. J Cancer Educ. 2015;30:535–545. doi: 10.1007/s13187-014-0750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sheppard VB, Hicks J, Makambi K, Hurtado-de-Mendoza A, Demark-Wahnefried W, Adams-Campbell L. The feasibility and acceptability of a diet and exercise trial in overweight and obese black breast cancer survivors: The Stepping STONE study. Contemp Clin Trials. 2016;46:106–113. doi: 10.1016/j.cct.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stolley MR, Sharp LK, Oh A, Schiffer L. A weight loss intervention for African American breast cancer survivors, 2006. Prev Chronic Dis. 2009;6:A22. [PMC free article] [PubMed] [Google Scholar]
- 117.Chung S, Zhu S, Friedmann E, et al. Weight loss with mindful eating in African American women following treatment for breast cancer: a longitudinal study. Support Care Cancer. 2016;24:1875–1881. doi: 10.1007/s00520-015-2984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mama SK, Song J, Ortiz A, et al. Longitudinal social cognitive influences on physical activity and sedentary time in Hispanic breast cancer survivors. Psychooncology. 2017;26:214–221. doi: 10.1002/pon.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rossi A, Garber CE, Ortiz M, Shankar V, Goldberg GL, Nevadunsky NS. Feasibility of a physical activity intervention for obese, socioculturally diverse endometrial cancer survivors. Gynecol Oncol. 2016;142:304–310. doi: 10.1016/j.ygyno.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 120.Stolley MR, Sharp LK, Fantuzzi G, et al. Study design and protocol for moving forward: a weight loss intervention trial for African-American breast cancer survivors. BMC Cancer. 2015;15:1018. doi: 10.1186/s12885-015-2004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stolley M, Sheean P, Gerber B, et al. Efficacy of a Weight Loss Intervention for African American Breast Cancer Survivors. J Clin Oncol. 2017;35:2820–2828. doi: 10.1200/JCO.2016.71.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.National Cancer Institute. Office of Cancer Survivorship. Available from: https://cancercontrol.cancer.gov/ocs/statistics/statistics.html.
- 123.Badr H, Paxton RJ, Ater JL, Urbauer D, Demark-Wahnefried W. Health behaviors and weight status of childhood cancer survivors and their parents: similarities and opportunities for joint interventions. J Am Diet Assoc. 2011;111:1917–1923. doi: 10.1016/j.jada.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Demark-Wahnefried W, Werner C, Clipp EC, et al. Survivors of childhood cancer and their guardians. Cancer. 2005;103:2171–2180. doi: 10.1002/cncr.21009. [DOI] [PubMed] [Google Scholar]
- 125.Zhang FF, Saltzman E, Must A, Parsons SK. Do Childhood Cancer Survivors Meet the Diet and Physical Activity Guidelines? A Review of Guidelines and Literature. Int J Child Health Nutr. 2012;1:44–58. doi: 10.6000/1929-4247.2012.01.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ford JS, Barnett M, Werk R. Health Behaviors of Childhood Cancer Survivors. Children (Basel) 2014;1:355–373. doi: 10.3390/children1030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 128.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 129.Niu C, Eng L, Qiu X, et al. Lifestyle Behaviors in Elderly Cancer Survivors: A Comparison With Middle-Age Cancer Survivors. J Oncol Pract. 2015;11:e450–459. doi: 10.1200/JOP.2014.002287. [DOI] [PubMed] [Google Scholar]
- 130.Henderson TO, Ness KK, Cohen HJ. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am Soc Clin Oncol Educ Book. 2014:e423–430. doi: 10.14694/EdBook_AM.2014.34.e423. [DOI] [PubMed] [Google Scholar]
- 131.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8:1–17. [PubMed] [Google Scholar]
- 132.Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bennett JA, Winters-Stone KM, Dobek J, Nail LM. Frailty in older breast cancer survivors: age, prevalence, and associated factors. Oncol Nurs Forum. 2013;40:E126–134. doi: 10.1188/13.ONF.E126-E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 135.National Heart Lung and Blood Institute. Selecting a Weight Loss Program. Available from: https://www.nhlbi.nih.gov/health/educational/lose_wt/wtl_prog.htm.
- 136.Muscaritoli M, Anker SD, Argiles J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 137.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 138.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 139.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 140.Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2016;3:CD008796. doi: 10.1002/14651858.CD008796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McCambridge TM, Stricker PR. Strength training by children and adolescents. Pediatrics. 2008;121:835–840. doi: 10.1542/peds.2007-3790. [DOI] [PubMed] [Google Scholar]
- 142.Blair CK, Morey MC, Desmond RA, et al. Light-intensity activity attenuates functional decline in older cancer survivors. Med Sci Sports Exerc. 2014;46:1375–1383. doi: 10.1249/MSS.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Badr H, Chandra J, Paxton RJ, et al. Health-related quality of life, lifestyle behaviors, and intervention preferences of survivors of childhood cancer. J Cancer Surviv. 2013;7:523–534. doi: 10.1007/s11764-013-0289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Courneya KS, Vallance JK, McNeely ML, Karvinen KH, Peddle CJ, Mackey JR. Exercise issues in older cancer survivors. Crit Rev Oncol Hematol. 2004;51:249–261. doi: 10.1016/j.critrevonc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 145.Klepin HD, Mohile SG, Mihalko S. Exercise for older cancer patients: feasible and helpful? Interdiscip Top Gerontol. 2013;38:146–157. doi: 10.1159/000343597. [DOI] [PubMed] [Google Scholar]
- 146.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103:323–328. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- 147.Whitehead S, Lavelle K. Older breast cancer survivors’ views and preferences for physical activity. Qual Health Res. 2009;19:894–906. doi: 10.1177/1049732309337523. [DOI] [PubMed] [Google Scholar]
- 148.Hong Y, Dahlke DV, Ory M, et al. Designing iCanFit: A Mobile-Enabled Web Application to Promote Physical Activity for Older Cancer Survivors. JMIR Res Protoc. 2013;2:e12. doi: 10.2196/resprot.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang JS, Dillon L, Terrones L, et al. Fit4Life: a weight loss intervention for children who have survived childhood leukemia. Pediatr Blood Cancer. 2014;61:894–900. doi: 10.1002/pbc.24937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Snyder DC, Morey MC, Sloane R, et al. Reach out to ENhancE Wellness in Older Cancer Survivors (RENEW): design, methods and recruitment challenges of a home-based exercise and diet intervention to improve physical function among long-term survivors of breast, prostate, and colorectal cancer. Psychooncology. 2009;18:429–439. doi: 10.1002/pon.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Office of Management and Budget. Rural Policy Note. Available from: http://www.ruralhome.org/storage/documents/rrbriefs/rpb_omb_outside_metro.pdf.
- 153.Henley SJ, Anderson RN, Thomas CC, Massetti GM, Peaker B, Richardson LC. Invasive Cancer Incidence, 2004–2013, and Deaths, 2006–2015, in Nonmetropolitan and Metropolitan Counties - United States. MMWR Surveill Summ. 2017;66:1–13. doi: 10.15585/mmwr.ss6614a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weaver KE, Geiger AM, Lu L, Case LD. Rural-urban disparities in health status among US cancer survivors. Cancer. 2013;119:1050–1057. doi: 10.1002/cncr.27840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Burris JL, Andrykowski M. Disparities in mental health between rural and nonrural cancer survivors: a preliminary study. Psychooncology. 2010;19:637–645. doi: 10.1002/pon.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wilson SE, Andersen MR, Meischke H. Meeting the needs of rural breast cancer survivors: what still needs to be done? J Womens Health Gend Based Med. 2000;9:667–677. doi: 10.1089/15246090050118198. [DOI] [PubMed] [Google Scholar]
- 157.Weaver KE, Palmer N, Lu L, Case LD, Geiger AM. Rural-urban differences in health behaviors and implications for health status among US cancer survivors. Cancer Causes Control. 2013;24:1481–1490. doi: 10.1007/s10552-013-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Garcia MC, Faul M, Massetti G, et al. Reducing Potentially Excess Deaths from the Five Leading Causes of Death in the Rural United States. MMWR Surveill Summ. 2017;66:1–7. doi: 10.15585/mmwr.ss6602a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Befort CA, Nazir N, Perri MG. Prevalence of obesity among adults from rural and urban areas of the United States: findings from NHANES (2005–2008) J Rural Health. 2012;28:392–397. doi: 10.1111/j.1748-0361.2012.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matthews KA, Croft JB, Liu Y, et al. Health-Related Behaviors by Urban-Rural County Classification - United States, 2013. MMWR Surveill Summ. 2017;66:1–8. doi: 10.15585/mmwr.ss6605a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.U.S Census Bureau. [Accessed August 30, 2017];2010 Census Urban and Rural Classification and Urban Area Criteria. 2013 Available at http://www.census.gov/geo/reference/ua/urban-rural-2010.html.
- 162.Slama K. Minnesota Psychologist. 2004. Rural culture is a diversity issue; pp. 9–11. [Google Scholar]
- 163.Meilleur A, Subramanian SV, Plascak JJ, Fisher JL, Paskett ED, Lamont EB. Rural residence and cancer outcomes in the United States: issues and challenges. Cancer Epidemiol Biomarkers Prev. 2013;22:1657–1667. doi: 10.1158/1055-9965.EPI-13-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kirkwood MK, Bruinooge SS, Goldstein MA, Bajorin DF, Kosty MP. Enhancing the American Society of Clinical Oncology workforce information system with geographic distribution of oncologists and comparison of data sources for the number of practicing oncologists. J Oncol Pract. 2014;10:32–38. doi: 10.1200/JOP.2013.001311. [DOI] [PubMed] [Google Scholar]
- 165.Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of Rural Cancer Care in the United States. Oncology (Williston Park) 2015;29:633–640. [PubMed] [Google Scholar]
- 166.Rogers LQ, Markwell SJ, Verhulst S, McAuley E, Courneya KS. Rural breast cancer survivors: exercise preferences and their determinants. Psychooncology. 2009;18:412–421. doi: 10.1002/pon.1497. [DOI] [PubMed] [Google Scholar]
- 167.Befort CA, Austin H, Klemp JR. Weight control needs and experiences among rural breast cancer survivors. Psychooncology. 2011;20:1069–1075. doi: 10.1002/pon.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Befort CA, Bennett L, Christifano D, Klemp JR, Krebill H. Effective recruitment of rural breast cancer survivors into a lifestyle intervention. Psychooncology. 2015;24:487–490. doi: 10.1002/pon.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fazzino TL, Sporn NJ, Befort CA. A qualitative evaluation of a group phone-based weight loss intervention for rural breast cancer survivors: Themes and mechanisms of success. Support Care Cancer. 2016;24:3165–3173. doi: 10.1007/s00520-016-3149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Park J-H, Lee J, Oh M, et al. The effect of oncologists’ exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: A randomized controlled trial. Cancer. 2015;121:2740–2748. doi: 10.1002/cncr.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Baker AM, Smith KC, Coa KI, et al. Clinical Care Providers’ Perspectives on Body Size and Weight Management Among Long-Term Cancer Survivors. Integr Cancer Ther. 2015;14:240–248. doi: 10.1177/1534735415572882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Denlinger CS, Filchner K, O’Grady M, et al. Adherence to NCCN Survivorship Care Guidelines in Non-Small Cell Lung Cancer and Colorectal Cancer. J Natl Compr Canc Ne. 2015;13:e71. (abstract AB2015–2028) [Google Scholar]
- 173.Puhringer PG, Olsen A, Climstein M, Sargeant S, Jones LM, Keogh JWL. Current nutrition promotion, beliefs and barriers among cancer nurses in Australia and New Zealand. Peer J. 2015;3:e1396. doi: 10.7717/peerj.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sharp L, Deady S, Gallagher P, et al. The magnitude and characteristics of the population of cancer survivors: using population-based estimates of cancer prevalence to inform service planning for survivorship care. BMC Cancer. 2014;14:767. doi: 10.1186/1471-2407-14-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: A time and motion study in 4 specialties. Ann Intern Med. 2016;165:753–760. doi: 10.7326/M16-0961. [DOI] [PubMed] [Google Scholar]
- 176.Beehler GP, Rodriques AE, Kay MA, Kiviniemi MT, Steinbrenner L. Perceptions of barriers and facilitators to health behavior change among veteran cancer survivors. Mil Med. 2014;179:998–1005. doi: 10.7205/MILMED-D-14-00027. [DOI] [PubMed] [Google Scholar]
- 177.Aycinena AC, Valdovinos C, Crew KD, et al. Barriers to Recruitment and Adherence in a Randomized Controlled Diet and Exercise Weight Loss Intervention Among Minority Breast Cancer Survivors. J Immigr Minor Health. 2017;19:120–129. doi: 10.1007/s10903-015-0310-1. [DOI] [PubMed] [Google Scholar]
- 178.Grossman DC, Bibbins-Domingo K, Curry SJ, et al. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:2417–2426. doi: 10.1001/jama.2017.6803. [DOI] [PubMed] [Google Scholar]
- 179.Bipartisan Policy Center. Provider Competencies for the Prevention and Management of Obesity. Available from: https://bipartisanpolicy.org/library/provider-competencies-for-the-prevention-and-management-of-obesity/
- 180.Provider Training and Education Workgroup of the Integrated Clinical and Social Systems for the Prevention and Management of Obesity Innovation Collaborative. Provider Competencies for the Prevention and Management of Obesity. Available from: https://cdn.bipartisanpolicy.org/wp-content/uploads/2017/06/Provider-Competencies-for-the-Prevention-and-Management-of-Obesity.pdf.
- 181.Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009;17:941–964. doi: 10.1038/oby.2008.636. [DOI] [PubMed] [Google Scholar]
- 182.Puhl RM, Andreyeva T, Brownell KD. Perceptions of weight discrimination: prevalence and comparison to race and gender discrimination in America. Int J Obes (Lond) 2008;32:992–1000. doi: 10.1038/ijo.2008.22. [DOI] [PubMed] [Google Scholar]
- 183.Baker RC, Kirschenbaum DS. Self-monitoring may be necessary for successful weight control. Behav Ther. 1993;24:377–394. [Google Scholar]
- 184.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111:92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Steinberg DM, Levine EL, Askew S, Foley P, Bennett GG. Daily text messaging for weight control among racial and ethnic minority women: randomized controlled pilot study. J Med Internet Res. 2013;15:e244. doi: 10.2196/jmir.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.O’Connell C. 23% of Users Abandon an App After One Use. Localytics. Available from: http://info.localytics.com/blog/23-of-users-abandon-an-app-after-one-use.
- 187.Laing B, Mangione C, Tseng C. Encouraging Use of the MyFitnessPal App Does Not Lead to Weight Loss in Primary Care Patients. JCOM. 2015:22. [Google Scholar]
- 188.Siu AL Force USPST. Behavioral and Pharmacotherapy Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622–634. doi: 10.7326/M15-2023. [DOI] [PubMed] [Google Scholar]
- 189.Fisher A, Williams K, Beeken R, Wardle J. Recall of physical activity advice was associated with higher levels of physical activity in colorectal cancer patients. BMJ Open. 2015;5:e006853. doi: 10.1136/bmjopen-2014-006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nyrop KA, Deal AM, Williams GR, Guerard EJ, Pergolotti M, Muss HB. Physical activity communication between oncology providers and patients with early-stage breast, colon, or prostate cancer. Cancer. 2016;122:470–476. doi: 10.1002/cncr.29786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med Sci Sports Exerc. 2015;47:2473–2479. doi: 10.1249/MSS.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 192.Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458–464. doi: 10.1001/jamainternmed.2013.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vidrine JI, Shete S, Li Y, et al. The Ask-Advise-Connect approach for smokers in a safety net healthcare system: a group-randomized trial. Am J Prev Med. 2013;45:737–741. doi: 10.1016/j.amepre.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kinsinger LS, Jones KR, Kahwati L, et al. Design and dissemination of the MOVE! Weight-Management Program for Veterans. Prev Chronic Dis. 2009;6:A98. [PMC free article] [PubMed] [Google Scholar]
- 195.VA/DoD. Clinical Practice Guideline for the Screening and Management of Overweight and Obesity. 2014 Available from URL: http://www.healthquality.va.gov/guidelines/cd/obesity/
- 196.Irwin ML, Cartmel B, Harrigan M, et al. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer. 2017;123:1249–1258. doi: 10.1002/cncr.30456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Spitalnic P. Certification of Medicare Diabetes Prevention Program. Centers for Medicare & Medicaid Services; 2016. [Google Scholar]
- 198.Brown JC, Ko EM, Schmitz KH. Development of a risk-screening tool for cancer survivors to participate in unsupervised moderate- to vigorous-intensity exercise: results from a survey study. PM R. 2015;7:113–122. doi: 10.1016/j.pmrj.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang X, Haggerty AF, Brown JC, et al. The prescription or proscription of exercise in endometrial cancer care. Gynecol oncol. 2015;139:155–159. doi: 10.1016/j.ygyno.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bauml J, Kim J, Zhang X, Aggarwal C, Cohen RB, Schmitz K. Unsupervised exercise in survivors of human papillomavirus related head and neck cancer: how many can go it alone? J Cancer Surviv. 2017;11:462–468. doi: 10.1007/s11764-017-0604-5. [DOI] [PubMed] [Google Scholar]
- 201.Schmitz KH, Troxel AB, Cheville A, et al. Physical activity and lymphedema (the PAL trial): Assessing the safety of progressive strength training in breast cancer survivors. Contemp Clin Trials. 2009;30:233–245. doi: 10.1016/j.cct.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Brown JC, Schmitz KH. Weight Lifting and Physical Function Among Survivors of Breast Cancer: A Post Hoc Analysis of a Randomized Controlled Trial. J Clin Oncol. 2015;33:2184–2189. doi: 10.1200/JCO.2014.57.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Brown JC, Schmitz KH. Weight lifting and appendicular skeletal muscle mass among breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2015;151:385–392. doi: 10.1007/s10549-015-3409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Brown JC, Troxel AB, Schmitz KH. Safety of weightlifting among women with or at risk for breast cancer-related lymphedema: musculoskeletal injuries and health care use in a weightlifting rehabilitation trial. Oncologist. 2012;17:1120–1128. doi: 10.1634/theoncologist.2012-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hayes SC, Speck RM, Reimet E, Stark A, Schmitz KH. Does the effect of weight lifting on lymphedema following breast cancer differ by diagnostic method: results from a randomized controlled trial. Breast Cancer Res Treat. 2011;130:227–234. doi: 10.1007/s10549-011-1547-6. [DOI] [PubMed] [Google Scholar]
- 206.Hormes JM, Bryan C, Lytle LA, et al. Impact of lymphedema and arm symptoms on quality of life in breast cancer survivors. Lymphology. 2010;43:1–13. [PubMed] [Google Scholar]
- 207.Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA. 2010;304:2699–2705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 208.Schmitz K, Ahmed RL, Troxel A, et al. Weight lifting in women with breast cancer-related lymphedema. N Engl J Med. 2009;361:664–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 209.Speck RM, Gross CR, Hormes JM, et al. Changes in the Body Image and Relationship Scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res Treat. 2010;121:421–430. doi: 10.1007/s10549-009-0550-7. [DOI] [PubMed] [Google Scholar]
- 210.Beidas RSPB, Barg F, Branas A, Brown JC, Glanz K, DeMichele A, DiGiovanni L, Salvatore D, Schmitz KH. A hybrid effectiveness-implementation trial of an evidence-based exercise intervention for breast cancer survivors. J Natl Cancer Inst Monogr. 2014;2014:338–345. doi: 10.1093/jncimonographs/lgu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Centers for Disease Control and Prevention. Adult obesity prevalence maps. Available from: https://www.cdc.gov/obesity/data/prevalence-maps.html.
- 212.Preventive Services Task Force. USPSTF A and B Recommendations. Available from: https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/
- 213.Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 214.Centers for Medicare & Medicaid Services. Certification of Medicare Diabetes Prevention Program. Available from: https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/
- 215.Centers for Medicare & Medicaid Services. CMS Next Generation ACO Model. Available from: https://innovation.cms.gov/initiatives/Next-Generation-ACO-Model/
- 216.Muhlestein DMM. Accountable Care Organizations. 2016: Private And Public-Sector Growth And Dispersion. Health Affairs Blog. 2016 [Google Scholar]
- 217.CA Roundtable on obesity management and coverage focuses on quality measures. TOS Connect. 2017 [Google Scholar]
- 218.Thompson HJ, Sedlacek SM, Playdon MC, et al. Weight loss interventions for breast cancer survivors: impact of dietary pattern. PLoS One. 2015;10:e0127366. doi: 10.1371/journal.pone.0127366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Swisher AK, Abraham J, Bonner D, et al. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Support Care Cancer. 2015;23:2995–3003. doi: 10.1007/s00520-015-2667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Thomson CA, Stopeck AT, Bea JW, et al. Changes in body weight and metabolic indexes in overweight breast cancer survivors enrolled in a randomized trial of low-fat vs. reduced carbohydrate diets. Nutr Cancer. 2010;62:1142–1152. doi: 10.1080/01635581.2010.513803. [DOI] [PubMed] [Google Scholar]
- 221.McCarroll ML, Armbruster S, Frasure HE, et al. Self-efficacy, quality of life, and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): a randomized controlled trial. Gynecol Oncol. 2014;132:397–402. doi: 10.1016/j.ygyno.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 222.Travier N, Fonseca-Nunes A, Javierre C, et al. Effect of a diet and physical activity intervention on body weight and nutritional patterns in overweight and obese breast cancer survivors. Med Oncol. 2014;31:783. doi: 10.1007/s12032-013-0783-5. [DOI] [PubMed] [Google Scholar]
- 223.Saxton JM, Scott EJ, Daley AJ, et al. Effects of an exercise and hypocaloric healthy eating intervention on indices of psychological health status, hypothalamic-pituitary-adrenal axis regulation and immune function after early-stage breast cancer: a randomised controlled trial. Breast Cancer Res. 2014;16:R39. doi: 10.1186/bcr3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.von Gruenigen VE, Courneya KS, Gibbons HE, Kavanagh MB, Waggoner SE, Lerner E. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gynecol Oncol. 2008;109:19–26. doi: 10.1016/j.ygyno.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 225.Harris MN, Swift DL, Myers VH, et al. Cancer survival through lifestyle change (CASTLE): a pilot study of weight loss. Int J Behav Med. 2013;20:403–412. doi: 10.1007/s12529-012-9234-5. [DOI] [PubMed] [Google Scholar]
- 226.Befort CA, Klemp JR, Austin HL, et al. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat. 2012;132:631–639. doi: 10.1007/s10549-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Djuric Z, DiLaura NM, Jenkins I, et al. Combining weight-loss counseling with the Weight Watchers plan for obese breast cancer survivors. Obes Res. 2002;10:657–665. doi: 10.1038/oby.2002.89. [DOI] [PubMed] [Google Scholar]
- 228.Demark-Wahnefried W, Jones LW, Snyder DC, et al. Daughters and Mothers Against Breast Cancer (DAMES): main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer. 2014;120:2522–2534. doi: 10.1002/cncr.28761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.McCarroll ML, Armbruster S, Pohle-Krauza RJ, et al. Feasibility of a lifestyle intervention for overweight/obese endometrial and breast cancer survivors using an interactive mobile application. Gynecol Oncol. 2015;137:508–515. doi: 10.1016/j.ygyno.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 230.Haggerty AF, Huepenbecker S, Sarwer DB, et al. The use of novel technology-based weight loss interventions for obese women with endometrial hyperplasia and cancer. Gynecol Oncol. 2016;140:239–244. doi: 10.1016/j.ygyno.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]