Abstract
Background
The association between new-onset left ventricular (LV) dysfunction during sepsis with long-term heart failure outcomes is lesser understood.
Methods
Retrospective cohort study of all adult patients with severe sepsis and septic shock between 2007 and 2014 that underwent echocardiography within 72 hours admitted to the intensive care unit. Patients with prior heart failure, LV dysfunction, and structural heart disease were excluded. LV systolic dysfunction was defined as LV ejection fraction <50% and LV diastolic dysfunction as ≥grade II. Primary composite outcome included new hospitalization for acute decompensated heart failure and all-cause mortality at two-year follow-up. Secondary outcomes included persistent LV dysfunction, and hospital mortality and length of stay.
Results
During this 8-year period, 434 patients with 206 (48%) patients having LV dysfunction were included. The two groups had similar baseline characteristics, but those with LV dysfunction had worse function as demonstrated by worse LV ejection fraction, cardiac index, and LV diastolic dysfunction. In the 331 hospital survivors, new-onset acute decompensated heart failure hospitalization did not differ between the two cohorts (15% vs. 11%). The primary composite outcome was comparable at two-year follow-up between the groups with and without LV dysfunction (p=0.24). Persistent LV dysfunction was noted in 28% hospital survivors on follow-up echocardiography. Other secondary outcomes were similar between the two groups.
Conclusions
In patients with severe sepsis and septic shock, the presence of new-onset LV dysfunction did not increase the risk of long-term adverse heart failure outcomes.
Keywords: Left ventricular dysfunction, sepsis, heart failure, echocardiography
INTRODUCTION
Sepsis is a leading cause of death and disability worldwide and in the United States is associated with health care costs estimated at $17 billion annually.(1) The onset of septic shock in a septic patient frequently heralds the development of multi-organ failure and has been associated with higher short- and long-term mortality.(1, 2) Cardiac dysfunction in sepsis is driven primarily by release of cytokines, mitochondrial dysfunction, and tissue hypoxia that leads to cardiac myocyte injury and death.(3, 4) New-onset left ventricular (LV) dysfunction is estimated to occur in between 20-60% of septic patients; however its impact on long-term outcomes remains unclear.(1, 2, 5) Persistent cardiovascular dysfunction after sepsis is hypothesized to be due to multiple mechanisms including extra-cardiac organ dysfunction, immune system dysregulation, coagulation abnormalities and destabilization of atherosclerotic plaques.(6) Prior literature has demonstrated an increase in the incidence of long-term atrial fibrillation, stroke, transient ischemic attacks and coronary revascularization after sepsis hospitalization.(7, 8) As heart disease continues to occupy the largest share of United States health care spending, there is a growing need to understand the long-term cardiovascular disease burden in sepsis survivors.(7–10)
The association of sepsis with the development of new-onset heart failure during long-term follow-up has been studied infrequently. Heart failure is a leading cause of cardiovascular mortality and morbidity that currently affects 5.7 million American adults and is projected to increase by 46% in the next 15 years.(9) In this study, we hypothesized that in sepsis survivors without prior clinical heart failure and LV dysfunction, those who developed new-onset LV dysfunction during sepsis hospitalization, had a higher incidence of hospitalization for new-onset acute decompensated heart failure (ADHF) and all-cause mortality during two-year follow-up.
MATERIAL AND METHODS
This study was an eight-year retrospective cohort study from January 1, 2007, through December 31, 2014, performed at the Mayo Clinic Rochester. This study was conducted in accordance with the amended Declaration of Helsinki and the need for informed consent was waived by the Mayo Clinic Institutional Review Board. All adult patients admitted to the intensive care units (ICU) with severe sepsis and septic shock that underwent an echocardiogram within 72 hours of ICU admission were included in the study. These patients were admitted to three ICUs (medical, mixed medical-surgical and surgical) with a total of 65 beds that are continually staffed by critical care physicians. All echocardiograms were ordered for standard clinical indications by the treating intensivist. Patients with denial of Minnesota research authorization, prior moderate or greater valvular stenosis or regurgitation, prior documented heart failure or asymptomatic LV dysfunction, prior congenital heart disease, and recent acute coronary syndrome (<1 week) without follow-up echo were excluded from the study. Data from a prior prospective study at our institution that recruited 106 patients were also included in this study population.(2)
Data: Definitions, Sources, and Management
The 2001 American College of Chest Physicians/Society of Critical Care Medicine consensus criteria were used to define sepsis.(11) Severe sepsis was defined as consequent organ dysfunction, hypoperfusion or hypotension, and septic shock defined as hypotension refractory to the fluid resuscitation of 30 mL/kg body weight. Hypoperfusion was defined as lactate level ≥2.3 mmol/L, organ dysfunction as Sequential Organ Failure Assessment score ≥2 at the time of echocardiography, and hypotension was defined as systolic blood pressure ≤90 mm Hg or ≤40 mm Hg from baseline.(2, 12) Sepsis and septic shock are detected using previously validated automated search algorithms and they were manually reviewed by two independent reviewers for inconsistencies (SV, MK).(13, 14) Demographic and clinical information was automatically abstracted from the electronic health records saved in the integrated Multidisciplinary Epidemiology and Translational Research in Intensive Care Laboratory DataMart.(15, 16) Prior acute and chronic heart failure were evaluated using a combination of International Classification of Diseases, Clinical Modification version diagnostic codes, pre-hospitalization echocardiogram and a customized electronic search algorithm using natural language processing software. Laboratory, imaging and physiological parameters closest to ICU admission were abstracted. We excluded patients with evidence of LVEF <50% or LV diastolic dysfunction (grade II or greater) on an echocardiogram within the prior one-year, or with evidence of congenital heart disease or moderate or greater valvular heart disease on prior or in-hospital echocardiogram.
LV systolic dysfunction was defined as left ventricular ejection fraction (LVEF) <50%.(17) LV diastolic dysfunction was classified using standard American Society of Echocardiography criteria, and grades II-IV were considered as LV diastolic dysfunction.(18, 19) Persistent myocardial dysfunction was defined as the presence of continued LV dysfunction on follow-up echocardiography. Two-dimensional, M-mode techniques and Doppler data were used to calculate LVEF, relative wall thickness, stroke volume and pulmonary artery systolic pressure using American Society of Echocardiography criteria.(17)
ADHF was defined using the Framingham criteria and was obtained from the electronic record using manual chart review by three independent reviewers (SV, MK, ASi).(20) Major criteria are acute pulmonary edema, cardiomegaly, hepatojugular reflux, neck vein distension, orthopnea or paroxysmal nocturnal dyspnea, pulmonary rales, S3 gallop, weight loss of > 4.5 kg in five days after treatment. Minor criteria include ankle edema, exertional dyspnea, hepatomegaly, nocturnal cough, pleural effusion and tachycardia (>120/min). The diagnosis of ADHF is confirmed by two major or one major and two minor criteria.
Clinical Outcomes
The primary outcome was a composite of new-onset ADHF and all-cause mortality at two-year follow-up in all severe sepsis and septic shock survivors. Mortality data was abstracted from the Mayo Clinic databases, the State of Minnesota electronic death certificates and the Rochester Epidemiology Project death data system.(21) Secondary outcomes included in-hospital mortality, ICU and hospital lengths of stay for index sepsis admission and persistent LV dysfunction on follow-up echocardiograms. These follow-up echocardiograms were performed as a part of routine clinical care and were at varied time points during follow-up.
Statistical Analysis
For an anticipated difference in proportions of 7.5% in the primary outcome and exposure rate of 0.35 from previous literature for the progression of asymptomatic LV dysfunction to clinical heart failure,(22, 23) with a two-sided alpha of 0.05 and power of 0.8, the calculated minimum sample size was 263 in each cohort. Continuous data are presented as median (interquartile range [IQR]) and categorical data as totals (percentages). Unpaired t-test and chi-square test were used to evaluate continuous and categorical outcomes. Kaplan-Meier failure curves were used to assess for primary outcome over two-year follow-up. Two-tailed p <0.05 was considered statistically significant. All statistical analyses were performed with JMP version 10.0.1 (SAS Institute, Cary, NC).
RESULTS
During this 8-year period, a total of 1757 adult patients were admitted to the ICU with severe sepsis and septic shock, of which a total of 434 (24.7%) met our inclusion criteria (Figure 1). Baseline characteristics of the cohorts with and without echocardiography performed within 72 hours are presented in Supplementary Table 1. Compared to patients who did not receive formal echocardiography, patients who underwent echocardiography had greater severity of illness, higher vasopressor requirements and longer duration of mechanical ventilation. Of the 434 included patients, new-onset LV dysfunction was noted in 206 (47.5%) patients with 43, 123 and 40 patients having LV systolic, LV diastolic and combined LV systolic and diastolic dysfunction respectively. Baseline characteristics of the cohorts with and without LV dysfunction are presented in Table 1. The cohorts were similar at baseline without any differences in comorbidity, the severity of illness or ICU management. Echocardiographic parameters of patients with and without LV dysfunction are presented in Table 2. Patients with LV dysfunction had worse LV function as demonstrated by lower LVEF, lower cardiac index, and mitral valve tissue Doppler velocities. Concomitant right ventricular dysfunction was noted more commonly in patients with LV dysfunction.
Figure 1. Study population.
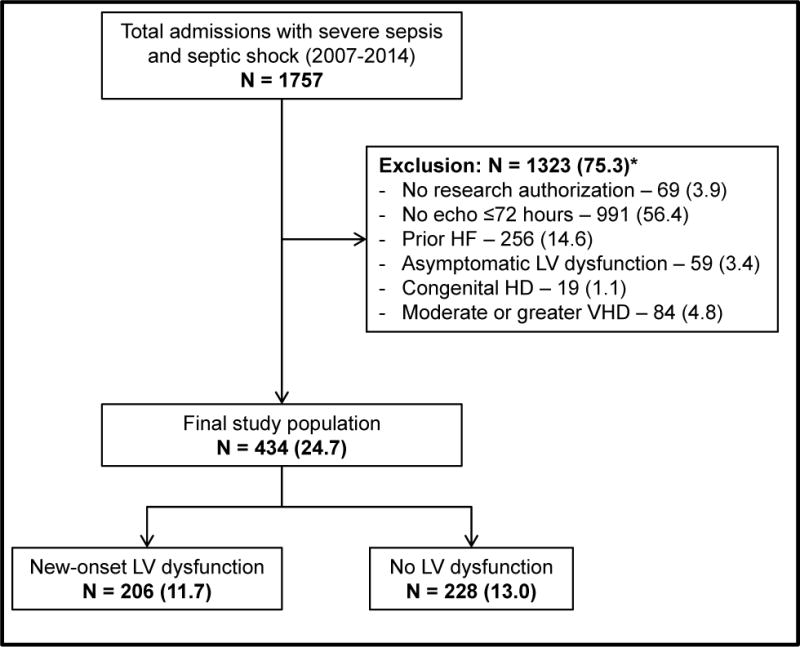
*Individual percentages are not additive due to multiplicity of exclusion criteria
Represented as: Number (Percentage)
Abbreviations: HD: heart disease; HF: heart failure; LV: left ventricular; VHD: valvular heart disease
Table 1.
Baseline characteristics of cohorts
| Parameter | LV Dysfunction (N=206) | No LV Dysfunction (N=228) | P |
|---|---|---|---|
| Age (years) | 68 (5.7–78.1) | 65.6 (55.3–77.1) | 0.48 |
| Male sex | 113 (54.9) | 116 (50.9) | 0.44 |
| Body mass index (kg/m2) | 28.8 (25.1–33.9) | 29.4 (24.9–35.3) | 0.55 |
| Coronary artery disease | 42 (20.4) | 34 (14.9) | 0.16 |
| Prior myocardial infarction | 22 (10.7) | 16 (7) | 0.23 |
| Atrial fibrillation | 20 (9.7) | 21 (9.2) | 0.87 |
| Charlson comorbidity index | 6 (4–8) | 5 (3–8) | 0.43 |
| APACHE-III score | 85 (70–109) | 82 (68–103) | 0.15 |
| SOFA score | 9 (7–12) | 8 (6–12) | 0.11 |
| Septic shock | 150 (72.8) | 161 (70.6) | 0.67 |
| Acute respiratory distress syndrome | 61 (29.6) | 65 (28.5) | 0.83 |
| Acute kidney injury | 140 (68) | 142 (62.3) | 0.23 |
| Admission troponin-T (ng/mL) | 0.07 (0.03–0.16) | 0.06 (0.02–0.14) | 0.22 |
| Highest lactate (mmol/L) | 3.4 (1.8–5.5) | 2.7 (1.7–5) | 0.10 |
| Total norepinephrine (mg) | 12 (4–37.9) | 17.5 (3.9–44.5) | 0.52 |
| Total crystalloid in 24 hours (L) | 4.2 (1.9–6.7) | 4.2 (2.3–6.8) | 0.74 |
| Mechanical ventilation | 108 (52.4) | 127 (55.7) | 0.50 |
Represented as: Total (percentage) or median (interquartile range)
Abbreviations: APACHE-III: Acute Physiology and Chronic Health Evaluation III; LV: left ventricular; SOFA: Sequential Organ Failure Assessment
Table 2.
Echocardiographic parameters of cohorts
| Parameter | LV Dysfunction (N=206) | No LV Dysfunction (N=228) | P | ||
|---|---|---|---|---|---|
| Individual n | Value | Individual n | Value | ||
| LV ejection fraction (%) | 206 | 54 (44–61) | 228 | 61 (56–67) | <0.001 |
| LV end-systolic diameter (mm) | 163 | 32 (28–37) | 164 | 28 (25–32) | <0.001 |
| LV end-diastolic diameter (mm) | 179 | 48 (44–52) | 183 | 46 (41–50) | <0.001 |
| LV mass index (g/m2) | 164 | 89 (75–103) | 153 | 86 (72–101) | 0.24 |
| LV stroke volume index (mL/m2) | 171 | 39 (32–46) | 152 | 41.5 (35–50) | 0.02 |
| Cardiac index (L/min/m2) | 169 | 3.5 (2.9–4.1) | 152 | 3.8 (3.2–4.5) | 0.002 |
| Left atrial volume index (mL/m2) | 102 | 36 (29–43) | 84 | 31.5 (25.3–38) | 0.005 |
| Mitral E velocity (m/s) | 146 | 0.9 (0.7–1) | 151 | 0.8 (0.7–1) | 0.42 |
| Mitral A velocity (m/s) | 121 | 0.8 (0.6–1) | 134 | 0.8 (0.6–1) | 0.60 |
| Mitral E/A ratio | 121 | 1 (0.8–1.5) | 134 | 1 (0.8–1.3) | 0.25 |
| Mitral e` velocity (medial) (m/s) | 154 | 0.07 (0.05–0.08) | 142 | 0.08 (0.06–0.1) | <0.001 |
| Mitral e` velocity (lateral) (m/s) | 111 | 0.09 (0.07–0.11) | 109 | 0.1 (0.08–0.13) | <0.001 |
| Mitral E/e` ratio (medial) | 139 | 12.9 (10–16) | 139 | 11 (8.3–13.8) | <0.001 |
| Mitral E/e` ratio (lateral) | 99 | 9 (7.7–12.5) | 106 | 8 (6.3–10.3) | 0.002 |
| Tricuspid regurgitant jet velocity (m/s) | 88 | 2.7 (2.4–3) | 60 | 2.8 (2.4–3.1) | 0.29 |
| Estimated RA pressure (mm Hg) | 178 | 10 (5–14) | 173 | 10 (5–14) | 0.23 |
| RV systolic pressure (mm Hg) | 173 | 41 (33–49) | 165 | 41 (34–52) | 0.29 |
| TAPSE (mm) | 32 | 18 (15–21) | 19 | 22 (15–25) | 0.07 |
| Tricuspid valve systolic velocity TDI (m/s) | 79 | 0.13 (0.1–0.14) | 56 | 0.14 (0.13–0.17) | 0.006 |
Represented as: Total (percentage) or median (interquartile range)
Abbreviations: LV: left ventricle; RA: right atrial; RV: right ventricular; TAPSE: tricuspid annular plane systolic excursion; TDI: tissue Doppler imaging
Clinical Outcomes
In the 331 hospital survivors, the primary composite outcome of new-onset ADHF and all-cause mortality during two-year follow-up was comparable between the cohorts with and without LV dysfunction (p=0.07 by log-rank test) (Figure 2). The individual outcomes of new-onset ADHF requiring hospitalization at two-year follow-up did not differ between patients with new LV dysfunction (24 [14.6%]) and those without (19 [11.4%]); p=0.42. Two-year mortality between the two cohorts was not different (LV dysfunction 17.7% vs. no LV dysfunction 16%, p=0.74). Of the total cohort of 434 patients, in-hospital mortality during index admission was recorded in 103 (23.7%) with no differences between groups with and without LV dysfunction (20.4% vs. 26.8%; p=0.14). Lengths of ICU (3 [IQR 1.6–6.1] days vs. 3 [1.7–6.2] days; p=0.73) and hospital (8.7 [IQR 6–15] days vs. 9.1 [6–18.8] days; p=0.53) stay were not significantly different between the groups with and without LV dysfunction for the index sepsis admission. For the 331 hospital survivors, follow-up echocardiography was available in 69 (20.9%) patients with LV dysfunction at a median time of 285 (IQR 72–799) days after hospital discharge. Persistent LV dysfunction was noted in 18 (28.2%) patients with a median change in LVEF of 4% (interquartile range 0–11).
Figure 2. Primary outcome for hospital survivors with and without LV dysfunction*.
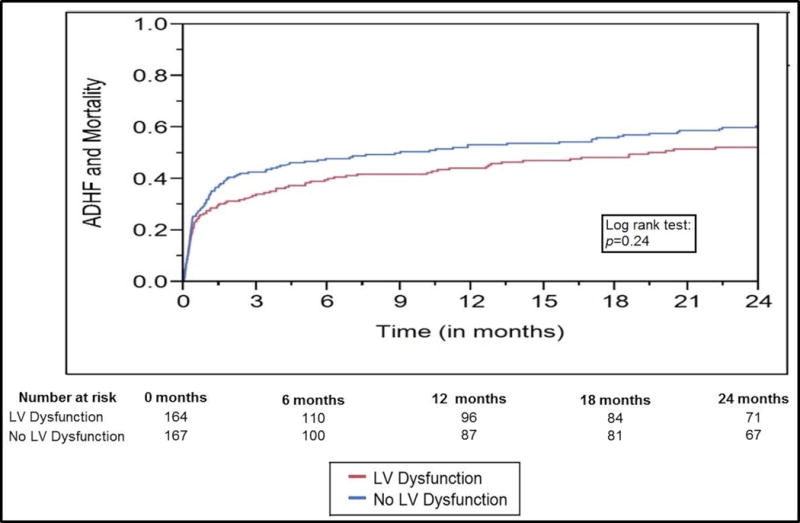
p=0.24 by log-rank test
*Composite outcome of new-onset acute decompensated heart failure and all-cause mortality
Abbreviations: LV: left ventricular
DISCUSSION
This eight-year retrospective cohort study on sepsis and septic shock patients sought to evaluate the association of new-onset LV dysfunction with all-cause mortality and new hospitalization for ADHF. The composite primary outcome of all-cause mortality and ADHF-hospitalization was not different between sepsis-survivors with and without LV dysfunction at two-year follow-up. Hospital outcomes of mortality, ICU length of stay and hospital length of stay were comparable between patients with and without LV dysfunction. Persistent LV dysfunction was noted in 28% of the population that underwent follow-up echocardiography.
Sepsis is associated with worse long-term cardiovascular outcomes. Employing a Medicare claims database, Yende et al.(7) established that severe sepsis survivors had a 13-fold higher risk of cardiovascular events compared to unmatched controls. Cardiovascular events, defined by stroke, myocardial infarction, transient ischemic attacks and coronary revascularization, occurred in nearly 30% of survivors. Walkey and colleagues evaluated sepsis survivors and noted that new-onset atrial fibrillation during the acute sepsis episode was associated with long-term atrial fibrillation recurrence and higher risks of hospitalization for heart failure, ischemic stroke, and death.(8) As in our study, LV dysfunction is noted in nearly half of all patients admitted with sepsis, but prior studies have infrequently evaluated the long-term clinical consequences.(1, 2)
This study sought to capture only new-onset LV dysfunction in our population to understand its correlation with long-term outcomes on subsequent follow-up. In patients with sepsis, LV systolic dysfunction is believed to be a reversible modulation of cardiac function with an uncertain prognostic impact.(5) However, LV systolic dysfunction is frequently associated with right ventricular dysfunction and LV diastolic dysfunction, both of which have been shown to have worse long-term outcomes.(1, 24) In contrast to the common understanding that LV dysfunction is reversible in 7-10 days, persistent LV dysfunction was noted in a little over a quarter of this cohort with follow-up echocardiography, the majority of whom were asymptomatic. Asymptomatic LV dysfunction is 3-4 times more common in the community than symptomatic heart failure, with a significant risk of progression to clinical heart failure over long-term follow-up.(22)
In our population, contrary to existing literature, there were no differences in short- and long-term outcomes between the cohorts with and without LV systolic or diastolic dysfunction. This could be explained by multiple hypotheses. First, literature has suggested that LV dysfunction is an adaptive mechanism in patients with sepsis, explaining the better survival in patients with concomitant LV systolic dysfunction with or without LV dilatation.(25, 26) Although this finding did not reach statistical significance in our population, the composite outcome Kaplan-Meier curves did appear to suggest better outcomes in patients with LV dysfunction. The timing of echocardiography is crucial since adequate fluid resuscitation and hemodynamic restoration can result in ‘unmasking’ of LV systolic dysfunction as manifested by a decrease in LVEF within the first 72 hours.(27) Second, the sensitivity and specificity of the current definitions of LV systolic and diastolic definitions have demonstrated poor applicability to patients with septic cardiomyopathy.(5, 28) Use of definitions developed by advanced echocardiographic techniques has demonstrated greater correlation to outcomes; however, these technologies are not readily usable in critical illness.(24) Other authors have presented simplified definitions of diastolic dysfunction that demonstrate greater applicability to clinical outcomes; however, these need further validation in independent cohorts prior to adoption.(28) Third, despite noting numerically higher ADHF hospitalizations in survivors with LV dysfunction (14.6% vs. 11.4%); this did not achieve statistical significance, likely due to under-powered cohort sizes and/or due to decreasing incidence of heart failure in the community with comparable demographics.(29)
This study has certain limitations. Use of a historical database carries inherent selection and informational bias; this was mitigated by the use of holistic and validated search algorithms.(12, 13) Prior echocardiography within the last one year was available for only 29% of the patients; it is certainly possible that prior asymptomatic LV dysfunction could have been missed in our study population despite the low incidence of 2.2–6%.(23) Echocardiographic data within 72 hours was available for 44% of the initial population, affecting the generalizability of these results to all severe sepsis and septic shock patients. Furthermore, echocardiography parameters were not uniformly documented in all patients in this cohort. Due to the retrospective nature of the study, only 21% of these patients had follow-up echocardiography that was obtained per routine clinical care. The development of the sepsis-3 criteria could influence the interpretation of the results of this study.(30) However, this cohort of severe sepsis and septic shock are less likely to be missed with either definition due to them comprising the extreme spectrum of illness.(31) Apart from the study definition, echocardiographic parameters did not differ significantly between patients with and without LV dysfunction potentially explaining the lack of differences in outcomes in this population. The study duration also correlated with the evolution of critical care ultrasonography and changes in health care delivery at the Mayo Clinic, both of which conceivably could have influenced the study results. Finally the single-center, single-region and referral center nature of the institution could impact the generalizability to other populations.
Future directions for research include development and application of standardized definitions of LV dysfunction in patients with sepsis and septic shock. Use of strain imaging for detection and prognostication of patients with LV dysfunction has shown promising results.(24) Novel approaches to management of LV dysfunction such as use of beta-blockers for decreasing adrenergic overdrive, describing the best single or combination vasoactive medications, and individualizing fluid resuscitation by advanced imaging parameters are potential avenues for future clinical and translational research to understand the impact of LV dysfunction on long-term outcomes.(32–34)
CONCLUSIONS
In this eight-year study of echocardiography in severe sepsis/septic shock patients, LV dysfunction was noted in nearly 48% of the patients. Patients with LV dysfunction did not differ in short- and long-term outcomes in comparison to patients without LV dysfunction. More than a quarter of the patients with a follow-up echocardiogram demonstrated persistent LV dysfunction. Further dedicated prospective trials are needed to evaluate the relationship of LV dysfunction with long-term adverse cardiovascular events, specifically heart failure-related clinical and quality-of-life outcomes.
Supplementary Material
Supplementary Table 1. Cohorts with and without echocardiography within 72 hours
Acknowledgments
Sources of Funding: This research was supported by funding from the below listed sources. These sources did not have any role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Supported, in part, by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Supported, in part, by intramural funding from the Critical Care Research Committee, Critical Care Independent Multidisciplinary Program, Mayo Clinic, Rochester MN
ABBREVIATIONS
- ADHF
acute decompensated heart failure
- ICU
intensive care unit
- IQR
interquartile range
- LV
left ventricular
- LVEF
left ventricular ejection fraction
Footnotes
CONFLICTS OF INTEREST
All authors report no financial or intellectual conflicts of interest related to this manuscript.
Author Contributions:
Study design, literature review, and statistical analysis: SV, JCJ, JBG, KK, JGM, OG, RK
Data management, data analysis, drafting manuscript: SV, JCJ, MK, ASa, ASi, JTP, RK
Manuscript revision, intellectual revisions, mentorship: JCJ, JBG, KK, JGM, OG, RK
Final approval: SV, JCJ, JBG, MK, ASa, ASi, JTP, KK, JGM, OG, RK
Prior presentation: Slide presentation, 46th Critical Care Congress, Society of Critical Care Medicine, Honolulu HI (January 2017)
- METRIC Laboratory, Anesthesia Clinical Research Unit, Echocardiography and Vascular Physiology Research Unit and Cardiac Catheterization Laboratory Interventional Research Database Unit
- Juan N Pulido, MD, Shane M Gillespie, DO, Gregory Wilson, RRT and Kumar Sarvottam, MBBS for generously sharing their prior work and expertise.
References
- 1.Sanfilippo F, Corredor C, Fletcher N, Landesberg G, Benedetto U, Foex P, Cecconi M. Diastolic dysfunction and mortality in septic patients: a systematic review and meta-analysis. Intensive Care Med. 2015;41(6):1004–1013. doi: 10.1007/s00134-015-3748-7. [DOI] [PubMed] [Google Scholar]
- 2.Pulido JN, Afessa B, Masaki M, Yuasa T, Gillespie S, Herasevich V, Brown DR, Oh JK. Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clin Proc. 2012;87(7):620–628. doi: 10.1016/j.mayocp.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reilly JM, Cunnion RE, Burch-Whitman C, Parker MM, Shelhamer JH, Parrillo JE. A circulating myocardial depressant substance is associated with cardiac dysfunction and peripheral hypoperfusion (lactic acidemia) in patients with septic shock. Chest. 1989;95(5):1072–1080. doi: 10.1378/chest.95.5.1072. [DOI] [PubMed] [Google Scholar]
- 4.Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, Parrillo JE. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989;321(5):280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- 5.Sevilla Berrios RA, O’Horo JC, Velagapudi V, Pulido JN. Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30-day mortality in patients with severe sepsis and septic shock: a systematic review and meta-analysis. J Crit Care. 2014;29(4):495–499. doi: 10.1016/j.jcrc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116(7):793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 7.Yende S, Linde-Zwirble W, Mayr F, Weissfeld LA, Reis S, Angus DC. Risk of cardiovascular events in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;189(9):1065–1074. doi: 10.1164/rccm.201307-1321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146(5):1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 10.Chao PW, Chu H, Chen YT, Shih YN, Kuo SC, Li SY, Ou SM, Shih CJ. Long-Term Outcomes in Critically Ill Septic Patients Who Survived Cardiopulmonary Resuscitation. Crit Care Med. 2016;44(6):1067–1074. doi: 10.1097/CCM.0000000000001608. [DOI] [PubMed] [Google Scholar]
- 11.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 12.Schramm GE, Kashyap R, Mullon JJ, Gajic O, Afessa B. Septic shock: a multidisciplinary response team and weekly feedback to clinicians improve the process of care and mortality. Crit Care Med. 2011;39(2):252–258. doi: 10.1097/CCM.0b013e3181ffde08. [DOI] [PubMed] [Google Scholar]
- 13.Harrison AM, Thongprayoon C, Kashyap R, Chute CG, Gajic O, Pickering BW, Herasevich V. Developing the surveillance algorithm for detection of failure to recognize and treat severe sepsis. Mayo Clin Proc. 2015;90(2):166–175. doi: 10.1016/j.mayocp.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herasevich V, Pieper MS, Pulido J, Gajic O. Enrollment into a time sensitive clinical study in the critical care setting: results from computerized septic shock sniffer implementation. J Am Med Inform Assoc. 2011;18(5):639–644. doi: 10.1136/amiajnl-2011-000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herasevich V, Pickering BW, Dong Y, Peters SG, Gajic O. Informatics infrastructure for syndrome surveillance, decision support, reporting, and modeling of critical illness. Mayo Clin Proc. 2010;85(3):247–254. doi: 10.4065/mcp.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh B, Singh A, Ahmed A, Wilson GA, Pickering BW, Herasevich V, Gajic O, Li G. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc. 2012;87(9):817–824. doi: 10.1016/j.mayocp.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 20.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 21.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1013. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echouffo-Tcheugui JB, Erqou S, Butler J, Yancy CW, Fonarow GC. Assessing the risk of progression from asymptomatic left ventricular dysfunction to overt heart failure: A systematic overview and meta-analysis. JACC Heart Fail. 2016;4(4):237–248. doi: 10.1016/j.jchf.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 24.Landesberg G, Jaffe AS, Gilon D, Levin PD, Goodman S, Abu-Baih A, Beeri R, Weissman C, Sprung CL, Landesberg A. Troponin elevation in severe sepsis and septic shock: the role of left ventricular diastolic dysfunction and right ventricular dilatation. Crit Care Med. 2014;42(4):790–800. doi: 10.1097/CCM.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 25.Antonucci E, Fiaccadori E, Donadello K, Taccone FS, Franchi F, Scolletta S. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care. 2014;29(4):500–511. doi: 10.1016/j.jcrc.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Jardin F, Fourme T, Page B, Loubieres Y, Vieillard-Baron A, Beauchet A, Bourdarias JP. Persistent preload defect in severe sepsis despite fluid loading: A longitudinal echocardiographic study in patients with septic shock. Chest. 1999;116(5):1354–1359. doi: 10.1378/chest.116.5.1354. [DOI] [PubMed] [Google Scholar]
- 27.Jardin F, Brun-Ney D, Auvert B, Beauchet A, Bourdarias JP. Sepsis-related cardiogenic shock. Crit Care Med. 1990;18(10):1055–1060. doi: 10.1097/00003246-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Lanspa MJ, Gutsche AR, Wilson EL, Olsen TD, Hirshberg EL, Knox DB, Brown SM, Grissom CK. Application of a simplified definition of diastolic function in severe sepsis and septic shock. Crit Care. 2016;20(1):243. doi: 10.1186/s13054-016-1421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175(6):996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Ciche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent JL, Martin GS, Levy MM. qSOFA does not replace SIRS in the definition of sepsis. Crit Care. 2016;20(1):210. doi: 10.1186/s13054-016-1389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanfilippo F, Santonocito C, Morelli A, Foex P. Beta-blocker use in severe sepsis and septic shock: a systematic review. Curr Med Res Opin. 2015;31(10):1817–1825. doi: 10.1185/03007995.2015.1062357. [DOI] [PubMed] [Google Scholar]
- 33.Mehta S, Granton J, Gordon AC, Cook DJ, Lapinsky S, Newton G, Bandayrel K, Little A, Siau C, Ayers D, et al. Cardiac ischemia in patients with septic shock randomized to vasopressin or norepinephrine. Crit Care. 2013;17(3):R117. doi: 10.1186/cc12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanspa MJ, Grissom CK, Hirshberg EL, Jones JP, Brown SM. Applying dynamic parameters to predict hemodynamic response to volume expansion in spontaneously breathing patients with septic shock. Shock. 2013;39(2):155–160. doi: 10.1097/SHK.0b013e31827f1c6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Cohorts with and without echocardiography within 72 hours


