Abstract
Background
Since 1975, childhood cancer incidence rates have gradually increased in the United States; however, few studies have conducted analyses across time to unpack this temporal rise. The aim of this study was to test the hypothesis that increasing cancer incidence rates are due to secular trends in pregnancy characteristics that are established risk factors for childhood cancer incidence including older maternal age, higher birthweight, and lower birth order. We also considered temporal trends in sociodemographic characteristics including race/ethnicity and poverty.
Procedure
We conducted a time series county-level ecologic analysis using linked population-based data from SEER cancer registries (1975–2013), birth data from the NCHS (1970–2013), and sociodemographic data from the US Census (1970–2010). We estimated unadjusted and adjusted average annual percent changes (AAPCs) in incidence of combined (all diagnoses) and individual types of cancer among children, ages 0–4 years, from Poisson mixed models.
Results
There was a statistically significant unadjusted temporal rise in incidence of combined childhood cancers (AAPC=0.71%; 95% CI=0.55, 0.86), ALL (0.78%; 0.49, 1.07), AML (1.86%; 1.13, 2.59), CNS tumors (1.31%; 0.94, 1.67), and hepatoblastoma (2.70%; 1.68, 3.72). Adjustment for county-level maternal age reduced estimated AAPCs by between 8% (hepatoblastoma) and 55% (combined). However, adjustment for other county characteristics did not attenuate AAPCs, and AAPCs remained significantly above 0% in models fully adjusted for county-level characteristics.
Conclusion
Although rising maternal age may account for some of the increase in childhood cancer incidence over time, other factors, not considered in this analysis, may also contribute to temporal trends.
Keywords: childhood cancer, county-level incidence rates, ecologic time series analysis, maternal age, birthweight, birth order
Introduction
Since 1975, the overall childhood cancer incidence rate has gradually increased in the United States at an annual rate of approximately 0.6% per year,1 with increasing rates documented for several cancers including leukemias, central nervous system (CNS) tumors, and hepatoblastoma.1 However, the underlying cause of rising rates is not known. Some researchers speculate that rising rates may reflect changes over time in diagnostic technology, disease classification, and registry completeness.2–5 For example, it is thought that the sharp increase in CNS tumor diagnoses in the 1980s was due to the introduction of magnetic resonance imaging and stereotactic biopsy.2 However, there are also plausible explanations for why the documented rise may reflect a true increase in disease. We hypothesize that rising incidence rates are attributed to secular trends in pregnancy characteristics, including older maternal age, higher birthweight, and lower birth order, which are established risk factors of many childhood cancers.6 A population-level shift in recent decades towards delayed childbearing and smaller family size is well documented in the United States.7 Further, rising overweight and obesity rates may have influenced birthweight trends,8,9 though prior research suggests that the association may not be strictly linear.10,11 Given mounting evidence that links these pregnancy factors to many, if not most, childhood cancers,6,12–14 it is reasonable to suppose that some proportion of the rise in childhood cancer incidence is due to population-level secular trends in these established risk factors.
Time trend and ecologic studies can be valuable as one piece of evidence among many to triangulate causation, including ruling out alternate explanations.15 For example, time trend studies were central for supporting the hypothesis that smoking caused lung cancer, and ruling out a prevailing hypothesis that genetic constitution was a confounder of this association.15 In the case of childhood cancer, using individual data to answer what underlies the secular rise in childhood cancer has been difficult or infeasible, given the lack of population-based longitudinal data on the population at risk, and the fact that childhood cancer is a rare disease. In the absence of prior studies, an ecologic time series study can be important for first-round evidence vetting of promising explanations, and/or ruling out a common alternate explanation. Yet few studies have undertaken time series analyses to understand what is driving the increase in childhood cancer over the past four decades. To our knowledge, only one prior study has empirically tested temporal associations in the childhood cancer literature, and this study was confined to testing the temporal association between maternal age and acute lymphoblastic leukemia (ALL) incidence, diagnosed 1980–1997, in Piedmont, Italy.16 Additional research is thus needed to understand what factors contribute to secular trends in childhood cancer incidence. The aim of this study was to use time series methods to test the hypothesis that the rise in childhood cancer incidence is due to secular trends in pregnancy characteristics including maternal age, birthweight, and birth order. We also considered temporal associations between sociodemographic characteristics (race/ethnicity and poverty) and childhood cancer incidence rates.
Methods
Study Design
We implemented a county-level ecologic time series analysis to test temporal associations between county-level pregnancy and sociodemographic characteristics (collectively referred to as county-level characteristics) and childhood cancer incidence rates over a 39-year period in the United States (1975–2013). This innovative approach was feasible through linkage of population-based data from three sources: the Surveillance, Epidemiology, and End Results (SEER) Program, the National Center for Health Statistics (NCHS), and the US Census Bureau. Data were merged at the county-level using Federal Information Processing Standards (FIPS) code identifiers. Linkage was not conducted at finer levels of geography (e.g. census tracts) due to confidentiality data agreements and lack of data availability at lower levels. SEER has an estimated case ascertainment rate of 98%, and the SEER 9 database captures 9.4% of the US population.17 NCHS, in collaboration with states, oversees the compilation and provision of all US birth certificate data.18 Prior to 1972, natality data were based on 50% samples from states. Beginning in 1972, some states provided complete natality data, and by 1985, all states provided 100% samples.18
Study Population
Our study sample consisted of 194 counties from eight cancer registries included in the SEER 9 database: Atlanta, Connecticut, Detroit, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah.19 We linked county-level aggregated cancer data, based on addresses at time of diagnosis, to county-level aggregated birth data with the assumption that county of residence was stable from birth to diagnosis. To minimize bias from this assumption, we restricted county incidence rates to younger children, ages 0 to 4 years, given that older children may be more residentially mobile.20 Between 1975 and 2013, average county population size of children, ages 0 to 4 years, in our sample was 8,769 (standard deviation (SD)=20,139; range=22 to 201,142).
Measures
Cancer Incidence Rates
Annual county-level cancer incidence rates, 1975 to 2013, for children, aged 0 to 4 years, were obtained from the SEER 9 database. Denominators were based on US Census Bureau annual population estimates. Incidence rates were estimated for combined childhood cancers (all diagnoses) and for individual types of cancer occurring among younger children including ALL, acute myeloid leukemia (AML), combined CNS tumors, neuroblastoma, retinoblastoma, Wilms tumor, and hepatoblastoma. Cancers were classified using the International Classification of Childhood Cancer, third edition.21
Pregnancy Characteristics
We aggregated maternal age at her child’s birth (14–49 years), birthweight (grams), and birth order (based on number of prior live births; 0–8+) from birth records and estimated county-level means, averaged over the 6-year birth window corresponding to year of diagnosis. For example, 1975 cancer incidence rates for children, ages 0 to 4 years, include cases born between 1970 and 1975; therefore 1975 incidence rates were linked to birth data averaged 1970–1975. Missing or implausible maternal age values were previously imputed by NCHS.18 A small proportion of birth records were missing data on birthweight and/or live birth order (0.2%).
Sociodemographic Characteristics
We linked county-level demographic and socioeconomic data, based on year of diagnosis, from the US Census including proportion of residents, ages 0–4 years, classified as white race (%white) and Hispanic ethnicity (%Hispanic); and proportion of residents, all ages, below the federal poverty line (%poverty). Data on race were available from the Census’s annual population estimates, while data on ethnicity (1980–2010) and poverty (1970–2010) were only available from decennial censuses. We linearly interpolated intercensal years so that, for example, a county with 10% Hispanic children in 1990 and 20% in 2000 would be assigned a value of 11% Hispanic in 1991. Because ethnicity was not available from the 1970 census, we used 1980 values to impute %Hispanic for years 1975–1979.
Statistical Analysis
Among our sample of 194 counties, we descriptively assessed overall trends in cancer incidence and county-level characteristics by graphing annual means and 95% confidence intervals (CI) between 1975 and 2013. We estimated linear time trends in county-level characteristics from linear mixed models with a random intercept for repeated county-level measures over time; a quadratic term for time was included in the model predicting birthweight. We estimated the crude average annual percent change (AAPC) in incidence of combined and individual childhood cancers from Poisson mixed models specifying only year of diagnosis predicting cancer incidence [AAPC = (eβyeardx − 1)*100)]. We also tested models specifying a random slope for time, which was non-significant and dropped from final models. The Poisson model implicitly controls for heterogeneity in population size by specifying rate denominators (county population of children aged 0–4) in the offset term.
We then tested associations between each county-level characteristic and county-level cancer incidence in Poisson mixed models, first in models adjusted only for year of diagnosis, and then in models further adjusted for all other county-level characteristics. Rate ratios (RR) and CIs were estimated for a 5-year change in county-level average maternal age, a 500-gram change in county-level average birthweight, a 1-unit change in county-level average birth order, and a 10 percentage point change in county-level %white, %Hispanic, and %poverty. We tested for interactions between county-level characteristics and year of diagnosis, but found none were significant in adjusted models.
Finally, for cancers with a significant crude AAPC, we estimated the percent change between the crude AAPC and AAPCs adjusted for county-level characteristics [%change = ((βyeardxadjusted−βyeardxcrude)/ βyeardxcrude)*100]. We estimated AAPCs from models adjusted for each county-level characteristic one at a time (e.g. a model specifying year of diagnosis and maternal age predicting incidence), and from models fully adjusted for all county-level characteristics simultaneously. In sensitivity analysis, we estimated crude and adjusted AAPCs within subsets of our sample: (1) restricted to white children within counties, and (2) restricted to counties of smaller childhood populations including <10,000, <5,000, and <1,000 children aged 0–4 years (averaged over 1975–2013). We tested the null hypothesis (AAPC=0%) using a two-sided test; statistical significance was determined as p<0.05. Analyses were performed using Stata 14.2 (Stata Corporation, College Station, Texas).22
Results
Descriptive Analysis of County-Level Characteristics
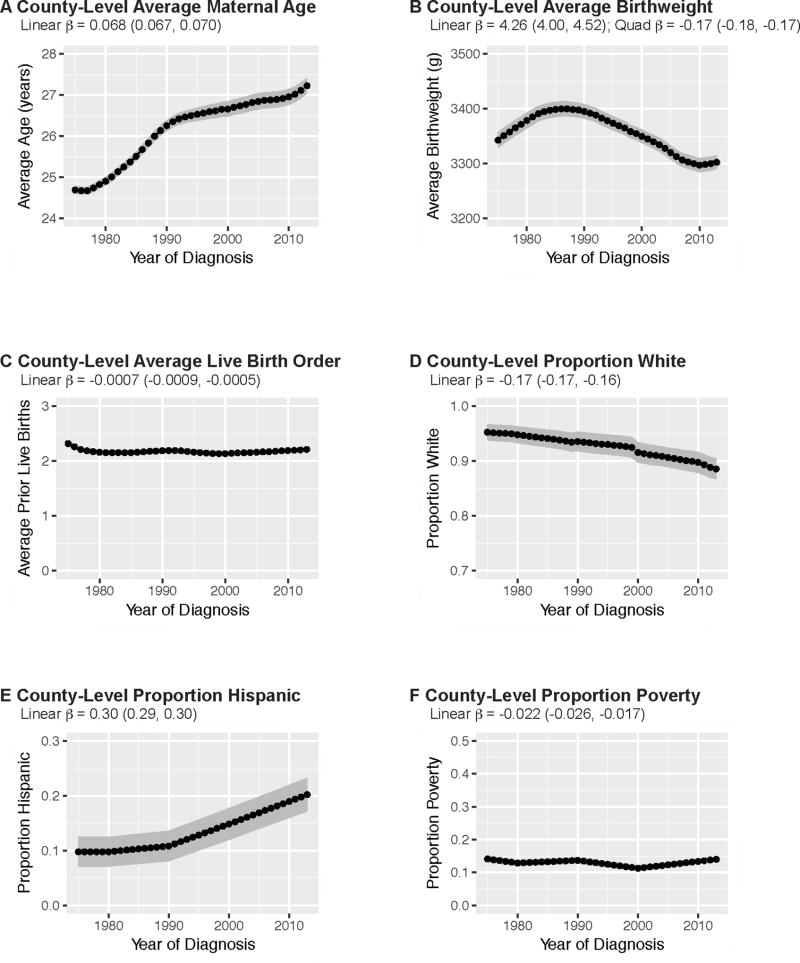
In Table 1, we present county-level characteristics averaged over the first (1975–1980) and last (2008–2013) six years of the study period. Over the study period, average maternal age increased from 24.8 years (SD=0.7) in 1975–1980 to 27.0 years (SD=1.4) in 2008–2013. Average birthweight slightly decreased from 3,361 grams (SD=102) to 3,300 grams (SD=89). Average %white decreased from 95.0% (SD=10.5) in 1975–1980 to 89.4% (SD=13.3) in 2008–2013, while average %Hispanic increased from 9.8% (SD=19.0) to 19.2% (SD=21.2). Average live birth order and %poverty remained stable over the study period.
TABLE 1.
County-level pregnancy and sociodemographic characteristics of SEER 9 counties (N=194)
| 1975–1980 | 2008–2013 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| County-Level Characteristic | Mean | SD | 25th Pct | Median | 75th Pct | Mean | SD | 25th Pct | Median | 75th Pct | Pa |
| Average maternal age (years) | 24.8 | 0.7 | 24.3 | 24.7 | 25.1 | 27.0 | 1.4 | 26.2 | 26.9 | 27.6 | <0.001 |
| Average birthweight (grams) | 3361 | 102 | 3312 | 3388 | 3434 | 3300 | 89 | 3238 | 3320 | 3366 | <0.001 |
| Average live birth order | 2.2 | 0.3 | 2.0 | 2.1 | 2.4 | 2.2 | 0.2 | 2.1 | 2.1 | 2.3 | 0.08 |
| % White | 95.0 | 10.5 | 95.6 | 98.8 | 99.5 | 89.4 | 13.3 | 87.0 | 94.5 | 97.1 | <0.001 |
| % Hispanic | 9.8 | 19.0 | 0.4 | 1.5 | 5.1 | 19.2 | 21.2 | 4.5 | 10.8 | 24.0 | <0.001 |
| % Poverty | 13.5 | 6.4 | 9.4 | 12.1 | 15.3 | 13.5 | 5.1 | 9.9 | 12.5 | 16.2 | 0.99 |
Abbreviations: SD, standard deviation; Pct., percentile; %white, proportion of children, ages 0–4 years, classified as white within county; %Hispanic, proportion of children, ages 0–4 years, classified as Hispanic within county; %poverty, proportion of residents, all ages, classified below the federal poverty line within county
P-value compares county-level means between first and last six years of the study period; estimated from paired t-test.
Annual trends in county-level characteristics are depicted in Figure 1. Temporal trends further demonstrate that, over the study period, average county-level maternal age (β=0.068, CI=0.067, 0.070) and %Hispanic (β=0.30, CI=0.29, 0.30) increased, average %white (β= −0.17, CI= −0.17, −0.16) decreased, and average birth order (β= −0.0007, CI= −0.0009, −0.0005) and %poverty (β= −0.022, 95% CI= −0.026, −0.017) remained relatively stable. Though there was an overall decrease in average birthweight between 1975 and 2013, a steady increase occurred in earlier years before reversing direction in the late 1980s (linear β=4.26, CI=4.00, 4.52; quadratic β= −0.17, CI= −0.18, −0.17). Several county-level characteristics were strongly correlated (Supplementary Table S1), though standard errors remained stable in fully adjusted models.
FIGURE 1.
Temporal trends in county-level pregnancy and sociodemographic characteristics, SEER 9 counties (N=194), 1975–2013. Annual county-level estimates are averaged across the sample of 194 counties (i.e. points represent the annual average of count-level averages/proportions). Linear and quadratic time trends estimated from linear mixed models specifying year of diagnosis predicting the specified county-level characteristic. Birthweight measured in grams (g). County-level proportion white and proportion Hispanic refer to the childhood population, ages 0 to 4 years, within counties. County-level proportion poverty refers to the proportion of individuals, all ages, within counties classified as below the federal poverty line.
Associations between County-level Characteristics and Cancer Incidence
We present associations between county-level characteristics and combined county-level childhood cancer incidence in Table 2. After adjusting for year of diagnosis, county-level average maternal age (RR=1.22, CI=1.11, 1.34), average birthweight (RR=1.57, CI=1.37, 1.80), and %white (RR=1.03, CI=1.01, 1.04) were positively associated with county-level combined childhood cancer incidence over the 39-year study period. County-level average birth order (RR=0.86, CI=0.76, 0.97), %Hispanic (RR=0.97, CI= 0.95, 0.98), and %poverty (RR=0.87, CI=0.83, 0.90) were inversely associated with county-level combined childhood cancer incidence. Associations were attenuated towards the null, and no longer statistically significant, in fully adjusted models. Similar patterns of association were observed among individual types of childhood cancer, though to varying magnitudes and with some differences in directionality (Supplementary Table S2). For example, %Hispanic was positively associated with incidence of ALL (fully adjusted RR=1.05, CI=1.02, 1.08).
TABLE 2.
Associations between county-level characteristics and combined childhood cancer incidence, ages 0 to 4 years, SEER 9 counties (N=194), 1975–2013
| Adjusted Only for Year of Diagnosis |
Fully Adjusted for All Characteristicsa |
|||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| County-Level Characteristic | RR | 95% CI | Pb | RR | 95% CI | Pb |
| Maternal Age (per 5-year increase) | 1.22 | (1.11, 1.34) | <0.01 | 1.08 | (0.97, 1.20) | 0.14 |
| Birthweight (per 500-gram increase) | 1.57 | (1.37, 1.80) | <0.01 | 1.17 | (0.97, 1.41) | 0.10 |
| Birth Order (per 1-unit increase) | 0.86 | (0.76, 0.97) | 0.02 | 0.93 | (0.83, 1.05) | 0.26 |
| % White (per 10% increase) | 1.03 | (1.01, 1.04) | <0.01 | 1.02 | (1.00, 1.03) | 0.08 |
| % Hispanic (per 10% increase) | 0.97 | (0.95, 0.98) | <0.01 | 0.98 | (0.97, 1.00) | 0.08 |
| % Poverty (per 10% increase) | 0.87 | (0.83, 0.90) | <0.01 | 0.95 | (0.89, 1.02) | 0.16 |
Abbreviations: RR, rate ratio; CI, confidence interval; %white, proportion of children, ages 0–4 years, classified as white within county; %Hispanic, proportion of children, ages 0–4 years, classified as Hispanic within county; %poverty, proportion of residents, all ages, classified below the federal poverty line within county
Adjusted for year of diagnosis, maternal age, birthweight, birth order, %white, %Hispanic, and %poverty
P-values estimated from Poisson mixed models with a random intercept for repeated county-level measures over time.
Average Annual Percent Change in Cancer Incidence
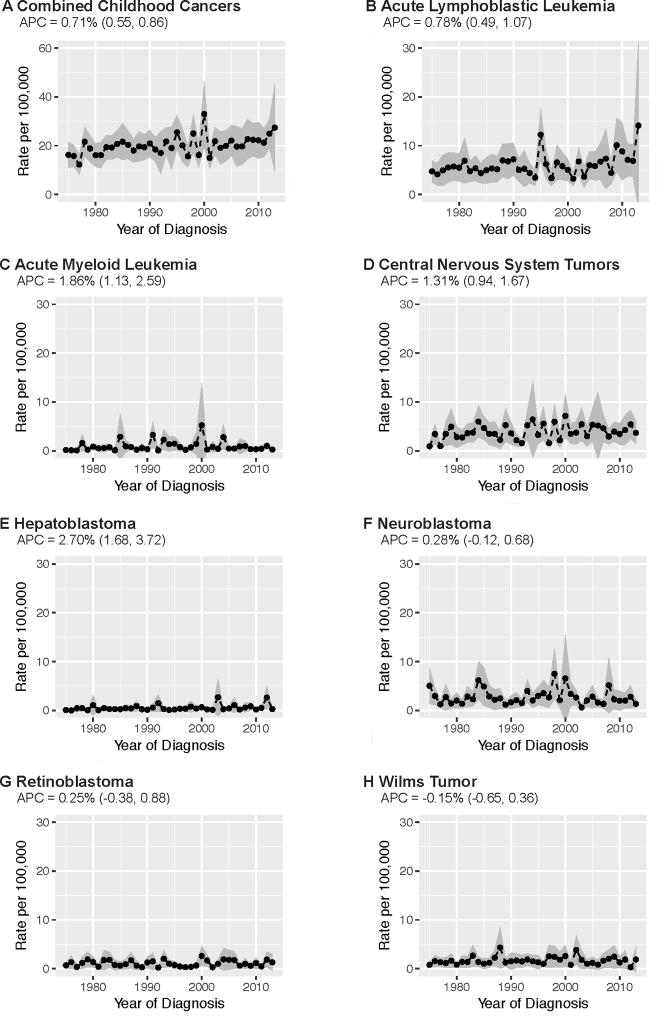
As illustrated in Figure 2, the incidence of combined childhood cancers significantly increased between 1975 and 2013 at an AAPC of 0.71% per year (CI=0.55, 0.86). Between 1975 and 2013, a statistically significant increase in incidence was also observed for ALL (AAPC=0.78%; CI=0.49, 1.07), AML (AAPC=1.86%; CI=1.13, 2.59), CNS tumors (AAPC=1.31%; CI=0.94, 1.67), and hepatoblastoma (2.70%; CI=1.68, 3.72). There was no statistically significant change over time in incidence of neuroblastoma (AAPC=0.28%; CI= −0.12, 0.68), retinoblastoma (AAPC=0.25%; CI= −0.38, 0.88), or Wilms tumor (AAPC= −0.15%; CI= −0.65, 0.36). Average county-level case counts and incidence rates by cancer type are provided in the supplementary materials (Supplementary Table S3).
FIGURE 2.
Temporal trends in childhood cancer incidence rates, ages 0 to 4 years, SEER 9 counties (N=194), 1975–2013. Annual incidence rates represent the average cancer incidence rate among children, ages 0 to 4 years, across the sample of 194 counties (i.e. points represent the annual average of count-level rates). Average annual percent change (AAPC) estimated from Poisson mixed models specifying year of diagnosis predicting cancer incidence rate with a random intercept for repeated county-level measures over time.
In Table 3, we compare crude and adjusted AAPCs in incidence of childhood cancers with significant crude temporal trends (combined, ALL, AML, CNS tumors, hepatoblastoma). Across all the county-level characteristics and all the cancers tested, the most notable reduction in the AAPC in cancer incidence occurred after adjustment for county trends in maternal age. Adjustment for county-level average maternal age reduced the AAPC in combined childhood cancer incidence to 0.32% per year (CI=0.08, 0.56), a 55% reduction from the crude; AAPCs in incidence of individual cancers were reduced by between 8% for hepatoblastoma and 40% for ALL. However, even after adjustment for maternal age, AAPCs remained significant from zero. Adjustment for other county-level characteristics either had no effect on county-level cancer incidence rates over time (e.g. birth order), or increased the AAPC from the crude. For example, adjustment for county-level average birthweight increased the AAPC in combined childhood cancer incidence by 21% from the crude (adjusted AAPC=0.86%; CI=0.69, 1.02), suggesting a masking effect.
TABLE 3.
Average annual percent change (AAPC) in childhood cancer incidence, ages 0 to 4 years, SEER 9 counties (N=194), 1975–2013
| Combined Childhood Cancers |
Acute Lymphoblastic Leukemia |
Acute Myeloid Leukemia |
Central Nervous System Tumors |
Hepatoblastoma | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | AAPC | 95% CI | Pa | %b | AAPC | 95% CI | Pa | %b | AAPC | 95% CI | Pa | %b | AAPC | 95% CI | Pa | %b | AAPC | 95% CI | Pa | %b |
| Crude | 0.71 | 0.55, 0.86 | <0.01 | 0.78 | 0.49, 1.07 | <0.01 | 1.86 | 1.13, 2.59 | <0.01 | 1.31 | 0.94, 1.67 | <0.01 | 2.70 | 1.68, 3.72 | <0.01 | |||||
| adjusted for: | ||||||||||||||||||||
| Maternal Age | 0.32 | 0.08, 0.56 | 0.01 | −55 | 0.47 | 0.02, 0.91 | 0.04 | −40 | 1.48 | 0.47, 2.49 | <0.01 | −20 | 1.02 | 0.50, 1.55 | <0.01 | −22 | 2.48 | 1.19, 3.78 | <0.01 | −8 |
| Birthweight | 0.86 | 0.69, 1.02 | <0.01 | 21 | 0.92 | 0.61, 1.23 | <0.01 | 18 | 1.94 | 1.17, 2.72 | <0.01 | 4 | 1.56 | 1.18, 1.95 | <0.01 | 20 | 2.85 | 1.80, 3.92 | <0.01 | 6 |
| Birth Order | 0.71 | 0.55, 0.86 | <0.01 | 0 | 0.77 | 0.48, 1.06 | <0.01 | 0 | 1.86 | 1.12, 2.59 | <0.01 | 0 | 1.31 | 0.94, 1.67 | <0.01 | 0 | 2.70 | 1.68, 3.72 | <0.01 | 0 |
| % White | 0.80 | 0.63, 0.96 | <0.01 | 12 | 0.98 | 0.68, 1.28 | <0.01 | 26 | 1.80 | 1.05, 2.56 | <0.01 | −3 | 1.47 | 1.09, 1.85 | <0.01 | 12 | 2.79 | 1.76, 3.83 | <0.01 | 3 |
| % Hispanic | 0.86 | 0.69, 1.03 | <0.01 | 21 | 0.72 | 0.40, 1.03 | <0.01 | −8 | 2.28 | 1.49, 3.08 | <0.01 | 23 | 1.51 | 1.11, 1.91 | <0.01 | 15 | 2.57 | 1.50, 3.65 | <0.01 | −5 |
| % Poverty | 0.83 | 0.67, 0.99 | <0.01 | 17 | 0.89 | 0.59, 1.19 | <0.01 | 15 | 1.90 | 1.14, 2.66 | <0.01 | 2 | 1.47 | 1.09, 1.85 | <0.01 | 12 | 2.74 | 1.71, 3.78 | <0.01 | 2 |
| Fully Adjustedc | 0.75 | 0.48, 1.03 | <0.01 | 6 | 0.81 | 0.31, 1.31 | <0.01 | 4 | 1.62 | 0.38, 2.87 | 0.01 | −13 | 1.69 | 1.03, 2.35 | <0.01 | 29 | 2.36 | 0.71, 4.04 | 0.01 | −12 |
Abbreviations: AAPC, average annual percent change; CI, confidence interval; %white, proportion of children, ages 0–4 years, classified as white within county; %Hispanic, proportion of children, ages 0–4 years, classified as Hispanic within county; %poverty, proportion of residents, all ages, classified below the federal poverty line within county
P-values compare a 1-year change in cancer incidence; estimated from Poisson mixed models with a random intercept for repeated county-level measures over time
Percent change between crude and adjusted AAPCs (βyeardxadjusted−βyeardxcrude)/ βyeardxcrude)
Adjusted for year of diagnosis, maternal age, birthweight, birth order, %white, %Hispanic, and %poverty
AAPCs in incidence of combined and individual childhood cancers remained statistically significantly above 0% in models fully adjusted for all county-level characteristics, with only an attenuation towards the null observed for AML (adjusted AAPC=1.62%, CI=0.38, 2.87, 13% reduction) and hepatoblastoma (adjusted AAPC=2.36%, CI=0.71, 4.04, 12%). Fully adjusted AAPCs increased from the crude for combined childhood cancers (0.75%; CI=0.48, 1.03; 6% increase), ALL (0.81%; CI=0.31, 1.31; 4%), and CNS tumors (1.69%; CI=1.03, 2.35; 29%).
Sensitivity Analysis Findings
Similar overall patterns of association were observed when the sample was restricted to white children within counties; only adjustment for maternal age attenuated AAPCs towards the null (Supplemental Table S4). When restricted by county population size, maternal age was again the only county-level characteristic that reduced AAPCs towards the null (Supplemental Table S5). We observed an inverse association between county size and the percent reduction in the AAPC in combined childhood cancer incidence attributed to maternal age, with a 55% reduction among all counties (N=194); a 73% reduction among counties <10,000 children (N=162); and >90% reduction among counties <5,000 (N=144) and <1,000 children (N=70).
Discussion
This is the first study to use time series methods to test the association between population-level secular trends in pregnancy characteristics and cancer incidence rates in the US pediatric population. Using linked population-based registry data over almost four decades, we confirmed that cancer incidence rates among children, 0 to 4 years of age, have been gradually increasing in the United States for several types of cancer including ALL, AML, CNS tumors, and hepatoblastoma.1 For these cancers, we found that population-level trends towards older maternal age may contribute to some of the increase in cancer incidence over time. Adjustment for maternal age reduced the crude AAPC in combined childhood cancers by over half, and by between 8% (hepatoblastoma) and 40% (ALL) for individual cancers. We note that AAPCs still remained significant from the null of 0% change after adjustment for county-level maternal age, suggesting that other factors may also contribute to temporal trends in childhood cancer incidence. However, besides maternal age, the other county-level characteristics examined in this analysis (birthweight, birth order, and sociodemographic factors) were not associated with increasing cancer incidence rates. Adjustment for these factors resulted in estimated AAPCs that were either higher than or no different than the crude. This suggests that, if anything, trends in these county-level characteristics counteract rising childhood cancer incidence rates over time in the United States. Thus, additional childhood cancer risk factors, beyond those included in this study, should be considered in future time series research.
Given the paucity of research on the rise in childhood cancer incidence over time, this study is an important contribution to the literature with several notable strengths. By implementing an ecologic time series study design, we were able to evaluate secular trends in childhood cancer incidence, as well as pregnancy and sociodemographic characteristics, over several decades of data within geographically stable units of analysis. Through this approach, we were thus able to overcome the lack of population-based longitudinal data available at the individual-level. By linking population-based data from national cancer and birth registries, as well as the US Census, we were able to obtain valid and reliable exposure and outcome measures for a large sample of the US pediatric population. Further, by accounting for a number of pregnancy and sociodemographic characteristics, we were able to test whether multiple exposures simultaneously contribute to, or counteract, the rise in childhood cancer incidence rates over time. Finally, by including several individual types of childhood cancer, we were able to assess whether temporal associations differ by tumor type.
There are also limitations to our analysis. First, as with any ecologic study, group-level risk factors may not be associated with incidence at the individual-level (the ecologic fallacy).23 This may be especially true for larger counties in which greater heterogeneity in pregnancy and sociodemographic characteristics is expected. To assess this limitation, we conducted a sensitivity analysis in which we estimated crude and adjusted AAPCs in incidence of combined childhood cancers within subsets of our sample restricted to counties of smaller childhood populations. This revealed that maternal age accounted for a much greater proportion of the annual trend in combined childhood cancer incidence for smaller counties than what we observed in our analysis of all counties with available data (Supplementary Table S5). This is consistent with results we would expect from measurement error of area-level maternal age, which could be higher in larger counties (given larger variation), that serves to minimize the percent reduction after adjustment. However, we note that estimates lost precision upon restriction to smaller counties.
Second, because county of birth is not available in SEER, we made the assumption that county of residence was stable from birth to diagnosis. To minimize potential bias due to this assumption, we restricted our sample to younger children, ages 0 to 4 years, given that residential mobility increases with age.20 We acknowledge that a California-based study reported that 38.5% of leukemia cases, ages 0 to 4 years, had moved away from county of birth by time of diagnosis, indicating that residential mobility is relatively common among younger children, at least in California.20 However, this study found no significant differences in sociodemographic characteristics of counties at birth and diagnosis among residentially mobile cases.20 Further, a recent case-cohort study in Minnesota reported high correlation between the socioeconomic status of residential census tracts at birth and diagnosis among cancer cases, ages 0 to 14 years.24 Therefore, even if our assumption of stable residency is incorrect, it may be appropriate to assume stable county-level characteristics throughout early childhood.
Third, there were limitations to our county-level measures. For example, our measure of county-level poverty was not specific to the early childhood population (0 to 4 years). We were unable to account for other potentially important county-level factors in our analysis, such as region-specific readiness to adopt new diagnostic technology. Further, though we considered pregnancy characteristics for which there is currently strong evidence of an association with childhood cancer risk (i.e. maternal age, birthweight, and birth order), it is possible that other, less established, risk factors, such as parental smoking status or size-for-gestational age, may also contribute to incidence trends. Given that we were not able to fully account for the temporal rise in childhood cancer incidence, future time series research should consider such alternative exposures. We also note potential concerns of a few strong correlations among the county-level characteristics included in our analysis (Supplementary Table S1), which may have hindered our ability to fully disentangle associations, especially in ecologic data.23 To further tease apart county-level characteristics, we conducted a sensitivity analysis in which we tested the temporal relationship between pregnancy characteristics and cancer incidence among only white children within counties, which produced similar overall patterns of association (Supplementary Table S4). Finally, this study was limited by sample size, both in terms of observational units (N=194 counties) and small case counts within counties (Supplementary Table S3). The rarity of childhood cancers required us to assess rates for combined ages 0 to 4 years, rather than assess rates separately for each birth year. This resulted in less precise exposure measures aggregated over the six-year birth window.
In conclusion, we did not find definitive evidence to support our hypothesis that secular trends in pregnancy characteristics account for the documented rise in childhood cancer incidence over time in the United States. While we found some evidence of an association between rising county-level maternal age and childhood cancer incidence over time, comprehensive adjustment for all county-level characteristics had little impact on estimated incidence trends in combined and individual childhood cancers. Therefore, we cannot rule out alternative explanations for increasing trends, including the possibility that rising childhood cancer incidence rates are an artifact of changes over time in diagnostic technology, disease classification, and registry completeness. We also cannot rule out the possibility that rising childhood cancer incidence rates may be attributed to changes over time in other, potentially unidentified, risk factors, such as environmental exposures.25–27 However, we note that there are currently few environmental exposures firmly linked to childhood cancer risk.6 Rising incidence rates remain a topic of debate in the childhood cancer literature, and thus further time series research is warranted that can build upon our findings and ultimately pinpoint the underlying cause of temporal trends.
Supplementary Material
SUPPLEMENTARY TABLE S1 Correlation matrix of county-level characteristics, SEER 9 counties (N=194), 1975–2013
SUPPLEMENTARY TABLE S2 Associations between county-level characteristics and childhood cancer incidence, ages 0 to 4 years, by cancer type, SEER 9 counties (N=194), 1975–2013
SUPPLEMENTARY TABLE S3 County-level case counts and incidence rates among children, ages 0 to 4 years, averaged over the sample of SEER 9 counties (N=194), 1975–2013
SUPPLEMENTARY TABLE S4 Sensitivity analysis restricted to white children: crude and adjusted average annual percent change (AAPC) in childhood cancer incidence, ages 0 to 4 years, SEER 9 counties (N=194), 1975–2013
SUPPLEMENTARY TABLE S5 Sensitivity analysis restricted by county size: crude and adjusted average annual percent change (AAPC) in childhood cancer incidence, ages 0 to 4 years, SEER 9 counties (N=194), 1975–2013
Acknowledgments
This work was supported by the National Institutes of Health Translational Pediatric Cancer Epidemiology Training Grant (T32CA099936). The authors thank the National Center for Health Statistics for their support with data acquisition.
Abbreviations Key
- AAPC
Average annual percent change
- ALL
Acute lymphoblastic leukemia
- AML
Acute myeloid leukemia
- CNS
Central nervous system
- SEER
Surveillance Epidemiology and End Results
- NCHS
National Center for Health Statistics
- FIPS
Federal Information Processing Standards
- %white
Proportion of county residents, ages 0–4 years, classified as white race
- %Hispanic
Proportion of county residents, ages 0–4 years, classified as Hispanic ethnicity
- %poverty
Proportion of county residents, all ages, classified below the federal poverty line
- RR
Rate ratio
- CI
95% confidence interval
- SD
Standard deviation
Footnotes
Conflicts of Interest Statement
The authors have no conflicts of interest to disclose.
References
- 1.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 2.Linet MS, Ries LA, Smith MA, Tarone RE, Devesa SS. Cancer surveillance series: recent trends in childhood cancer incidence and mortality in the United States. J Natl Cancer Inst. 1999;91(12):1051–1058. doi: 10.1093/jnci/91.12.1051. [DOI] [PubMed] [Google Scholar]
- 3.Kroll M, Stiller C, Richards S, Mitchell C, Carpenter L. Evidence for under-diagnosis of childhood acute lymphoblastic leukaemia in poorer communities within Great Britain. Br J Cancer. 2012;106(9):1556–1559. doi: 10.1038/bjc.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adamson P, Law G, Roman E. Assessment of trends in childhood cancer incidence. Lancet. 2005;365(9461):753. doi: 10.1016/S0140-6736(05)17979-3. [DOI] [PubMed] [Google Scholar]
- 5.Bukowski JA. Critical assessment of opposing views on trends in childhood cancer. Int J Health Serv. 2000;30(2):373–377. doi: 10.2190/QQFF-86CM-8UVX-QMJW. discussion 379–386. [DOI] [PubMed] [Google Scholar]
- 6.Spector LG, Pankratz N, Marcotte EL. Genetic and nongenetic risk factors for childhood cancer. Pediatr Clin North Am. 2015;62(1):11–25. doi: 10.1016/j.pcl.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton BE, Martin JA, Osterman MJK, Curtin SC, Mathews TJ. National vital statistics reports. 12. Vol. 64. Hyattsville, MD: National Center for Health Statistics; Births: Final Data for 2014. 2105. [PubMed] [Google Scholar]
- 8.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oken E. Secular trends in birthweight. Nestle Nutr Inst Workshop Ser. 2013;71:103–14. doi: 10.1159/000342576. pmid:23502144. [DOI] [PubMed] [Google Scholar]
- 11.Morisaki N, Esplin MS, Varner MW, Henry E, Oken E. Declines in birth weight and fetal growth independent of gestational length. Obstet Gynecol. 2013;121(1):51–58. doi: 10.1097/aog.0b013e318278d014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson KJ, Carozza SE, Chow EJ, et al. Parental age and risk of childhood cancer: a pooled analysis. Epidemiology. 2009;20(4):475–483. doi: 10.1097/EDE.0b013e3181a5a332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuelsen SO, Bakketeig LS, Tretli S, Johannesen TB, Magnus P. Birth weight and childhood cancer. Epidemiology. 2009;20(4):484–487. doi: 10.1097/EDE.0b013e3181a7786d. [DOI] [PubMed] [Google Scholar]
- 14.Von Behren J, Spector LG, Mueller BA, et al. Birth order and risk of childhood cancer: a pooled analysis from five US States. Int J Cancer. 2011;128(11):2709–2716. doi: 10.1002/ijc.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandenbroucke JP, Broadbent A, Pearce N. Causality and causal inference in epidemiology: the need for a pluralistic approach. Int J Epidemiol. 2016;45(6):1776–1786. doi: 10.1093/ije/dyv341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maule MM, Merletti F, Pastore G, Magnani C, Richiardi L. Effects of maternal age and cohort of birth on incidence time trends of childhood acute lymphoblastic leukemia. Cancer Epidemiology Biomarkers & Prevention. 2007;16(2):347–351. doi: 10.1158/1055-9965.EPI-06-0425. [DOI] [PubMed] [Google Scholar]
- 17.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2013. National Cnacer Institute; Bethesda, MD: [Accessed June 2016]. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 18.Centers for Disease Control and Prevention. VitalStats. Hyattsville, MD: National Center for Health Statistics; [Accessed June 2016]. http://www.cdc.gov/nchs/vitalstats.htm. [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2015 Sub (1973–2013) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. released April 2016, based on the November 2015 submission. [Google Scholar]
- 20.Urayama KY, Von Behren J, Reynolds P, Hertz A, Does M, Buffler PA. Factors associated with residential mobility in children with leukemia: implications for assigning exposures. Ann Epidemiol. 2009;19(11):834–840. doi: 10.1016/j.annepidem.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer, third edition. Cancer. 2005;103(7):1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 22.StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 23.Morgenstern H. Ecologic Studies. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. Lippincott Williams & Wilkins; 2008. pp. 511–531. [Google Scholar]
- 24.Kehm RD, Spector LG, Poynter JN, Vock DM, Osypuk TL. Socioeconomic status and childhood cancer incidence: a population-based multilevel analysis. Am J Epidemiol. 2017:kwx322. doi: 10.1093/aje/kwx322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangano JJ. A rise in the incidence of childhood cancer in the United States. Int J Health Serv. 1999;29(2):393–408. doi: 10.2190/TGRR-L4MV-JMXC-HJKP. [DOI] [PubMed] [Google Scholar]
- 26.Linabery AM, Ross JA. Trends in childhood cancer incidence in the US (1992–2004) Cancer. 2008;112(2):416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 27.Bandi P, Dranger E, Hampton JM, Trentham-Dietz A. Trends in childhood cancer incidence in Wisconsin, 1980–1999. WMJ. 2006;105(7):30–37. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY TABLE S1 Correlation matrix of county-level characteristics, SEER 9 counties (N=194), 1975–2013
SUPPLEMENTARY TABLE S2 Associations between county-level characteristics and childhood cancer incidence, ages 0 to 4 years, by cancer type, SEER 9 counties (N=194), 1975–2013
SUPPLEMENTARY TABLE S3 County-level case counts and incidence rates among children, ages 0 to 4 years, averaged over the sample of SEER 9 counties (N=194), 1975–2013
SUPPLEMENTARY TABLE S4 Sensitivity analysis restricted to white children: crude and adjusted average annual percent change (AAPC) in childhood cancer incidence, ages 0 to 4 years, SEER 9 counties (N=194), 1975–2013
SUPPLEMENTARY TABLE S5 Sensitivity analysis restricted by county size: crude and adjusted average annual percent change (AAPC) in childhood cancer incidence, ages 0 to 4 years, SEER 9 counties (N=194), 1975–2013