Abstract
Purpose
This study compared the relative incidence of treatment-related toxicities and the event-free and overall survival between Hispanic and non-Hispanic children undergoing therapy for acute lymphoblastic leukemia (ALL) on Dana-Farber Cancer Institute ALL Consortium protocol 05-001.
Patients and Methods
Secondary analysis of prospectively collected data from a phase III multi-center study in children and adolescents, 1 – 18 years with previously untreated ALL.
Results
Between 2005 and 2011, 794 eligible patients enrolled on DFCI 05-001, 730 of whom were included in this analysis (19% [N=150] Hispanic, 73% [N=580] non-Hispanic). Hispanic patients were more likely to be ≥10 years of age (32% vs. 24%, p=0.045) at diagnosis. Toxicity analyses revealed that Hispanic patients had significantly lower cumulative incidence of bone fracture (p<0.001) and osteonecrosis (p=0.047). In multivariable risk regression, the risk of osteonecrosis was significantly lower in Hispanic patients ≥10 years (HR 0.23; p=0.006). Hispanic patients had significantly lower 5-year event-free survival (EFS) (79.4%; 95% CI: 71.6% to 85.2%) and overall survival (OS) (89.2%; 95%CI: 82.7%–93.4%) than non-Hispanic patients (EFS: 87.5%; 95%CI: 84.5%–90.0%, p=0.004. OS: 92.7%; 95%CI: 90.2%–94.6%), (p=0.006). Exploratory analyses revealed differences between Hispanic and non-Hispanic patients in the frequency of common variants in genes related to toxicity or ALL outcome.
Conclusion
Hispanic children treated for ALL on DFCI 05-001 had fewer bone-related toxicities and inferior survival than non-Hispanic patients. While disease biology is one explanatory variable for outcome disparities, these findings suggest that biologic and non-biologic mechanisms affecting drug delivery and exposure in this population may be important contributing factors as well.
Keywords: Acute lymphoblastic leukemia, Hispanic, ethnicity, survival, toxicities, outcomes
Introduction
Despite overall cure rates near 90% in childhood acute lymphoblastic leukemia (ALL), survival in Hispanic children and adolescents remains inferior to survival in non-Hispanic patients.1–4 These disparities are particularly striking in light of dramatic improvements in survival for all children with leukemia over the past three decades.5,6 In a large retrospective analysis from 12 Children’s Cancer Study Group (CCSG) ALL trials (1983–1995), 5-year event-free survival (EFS) was significantly lower in Hispanic children (65.9% ± 1.5%) when compared with 5-year EFS in white (72.8% ± 0.6%) and Asian children (75.1% ± 3.5%), (p<0.001).7 More recent studies, including a Surveillance Epidemiology and End Results (SEER) investigation of survival trends in ALL (1995–2012), have revealed a persistent survival difference (5–15 percentage points) between Hispanic and non-Hispanic children.1 The reasons for reduced survival in Hispanic children with ALL in North America are multifactorial and likely include both biologic and non-biologic factors, such as differences in the frequency of high-risk leukemia subtypes, host pharmacogenomics, reduced access-to-care, and non-adherence to oral chemotherapy.8
Differences in survival outcomes between Hispanic and non-Hispanic patients with ALL have been described. 1,9–11 Fewer studies have investigated whether the incidence of TRT during ALL therapy varies by self-reported ethnicity, and none have described both survival and TRT in the same cohort. 9,12,13 The development of serious TRTs may result in an inability to tolerate full-dose chemotherapy, and the consequent interruptions in planned therapy (treatment delays, dose reductions) could theoretically contribute to increased risk of relapse. Conversely, development of very few TRTs might indicate lower overall drug exposure, either due to genetic polymorphisms affecting drug metabolism or to non-biologic factors, such as chemotherapy non-adherence. We conducted an analysis of TRTs and survival in Hispanic and non-Hispanic children and adolescents undergoing treatment for newly diagnosed ALL on the Dana-Farber Cancer Institute ALL Consortium protocol 05-001 (DFCI 05-001).14 We sought to compare the relative incidence of TRTs, EFS and overall survival (OS) between these two patient cohorts. Because common genetic variants are associated with risk of TRT,15,16 we also explored whether the prevalence of these polymorphisms in our patient population differed by ethnicity.
Methods
Patients and eligibility criteria
Children and adolescents aged 1–18 years with newly diagnosed ALL were enrolled on DFCI 05-001 at 11 sites in Canada and the United States, including Puerto Rico. Patients whose ethnicity was documented at the time of study enrollment were eligible for inclusion in this analysis. The Institutional Review Board of each participating institution approved the original treatment protocol and informed consent was obtained from each patient’s guardian. All enrolled patients with known ethnicity (Hispanic and non-Hispanic) were included in the induction toxicity analysis. Patients with a documented complete remission (CR), final risk group, and treatment assignment were included in post-induction treatment analyses. For the investigation of targeted genetic variants, patients who met the above criteria and who also had genomic DNA available for analysis were included.
Ethnicity designation
Patient ethnicity (Hispanic or non-Hispanic) was documented at the time of study enrollment by a clinical research associate and was based on patient/parent report and/or patient’s country of origin, as was the standard during the period in which the clinical trial was conducted.17 Ethnicity designation was guided by the national standards for the classification of federal data on race and ethnicity as defined by the Office of Management and Budget (OMB) Statistical Policy Directive No. 15.18 Patients were categorized as underweight, normal, overweight and obese based on body mass index (BMI). For outcome analyses, patients were categorized as obese, (BMI ≥ the 95th percentile for age and sex) vs. not obese (BMI <95th percentile).
Therapy
Details of the DFCI 05-001 treatment regimen have been previously published.14 In brief, all patients underwent multi-agent remission induction followed by risk-adapted post-induction therapy based on final risk group assignment. Final risk group was based on age, presenting leukocyte count, immunophenotype, presence or absence of leukemia in the cerebrospinal fluid at diagnosis, leukemia-associated cytogenetic abnormalities, and end-induction levels of minimal residual disease (MRD). All patients were scheduled to receive 24 months of post-induction treatment. Patients were eligible to participate in a randomized comparison of intramuscular native E.coli L-asparaginase and intravenous pegaspargase during post-induction treatment. Patients who declined to participate, and those enrolled onto the trial after the randomized comparison had met its target accrual, were directly assigned to receive native E.coli L-asparaginase.
Toxicity assessment
Treatment-related toxicities were defined using Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0 and included: bone fracture (all grades), grade 2 or worse osteonecrosis (ON), grade 3 or worse infection (bacterial, fungal, viral and/or pneumocystis pneumonia), and grade 2 or worse asparaginase-associated toxicities (allergy, pancreatitis, thrombosis or bleeding).14 A diagnosis of bone fracture or ON required both clinical symptoms and radiographic confirmation. Study staff at each participating institution prospectively collected TRT data at the time of CR, every three months subsequently until treatment completion, and annually thereafter.
Analysis of genetic variants
We conducted a secondary analysis of genomic data that were gathered for a separate correlative study looking at toxicities in the same study population. In this study, toxicities were not analyzed by ethnicity. Genomic DNA was isolated from peripheral blood collected after patients achieved CR. Nineteen candidate genetic variants were selected for investigation through a non-exhaustive literature review, with the following criteria: (1) variants present in genes related to pathways presumed to be relevant to TRT; (2) variants known to be associated with altered function of the gene product; and (3) variants with a population prevalence of at least 10%.15,16 Single nucleotide polymorphisms (SNPs) were detected using polymerase chain reaction (PCR)-based allelic discrimination assays (Life Technologies, Grand Island, NY). The number of 28-bp repeats in the 5′ untranslated region of the thymidylate synthase (TS) gene was determined by PCR-product length analysis, as previously described.15
Statistical methods
Toxicity rates during induction and post-induction therapy were compared between groups with the Fisher’s exact test. In patients who were assigned a final risk group after achieving CR, ON and bone fracture with follow-up information were analyzed within age subgroups (<10 years vs. ≥10 years). The cumulative incidences of ON and fracture were estimated with the cuminc utility in the ‘cmprsk’ package in R and were tested using the Gray test, with relapse and death in remission identified as competing risks. Time-to-event was calculated as the time (years) from remission date to the date of first event. If the bone event occurred in induction, it was considered an event at time 0. The cumulative incidence was also modeled in univariate and multivariable analyses using competing risks regression. Multivariable models were adjusted for sex, asparaginase randomization, and final risk group. The grouping used in modeling for final risk group classification varied by age due to the protocol definition of age >10 as high risk.14
Overall survival and EFS were estimated with the Kaplan-Meier method and were compared between groups with the log rank test. Overall survival was defined as the time from registration to death from any cause. Event-free survival was defined as the time from registration to the first event of relapse, death, or second malignancy. Induction events, including death and/or failure to achieve CR, were considered events at time 0. Cox proportional hazards models were used to model OS and EFS by group univariately and were adjusted in multivariable analyses for diagnostic age, immunophenotype, WBC, obesity, and sex. In patients receiving a single full dose of IV pegaspargase, a Wilcoxon rank sum test was used to compare the serum asparaginase activity (SAA) between Hispanic and non-Hispanic patients at days 4, 11, 18, and 25 during induction.
The association between ethnicity group and SNPs were analyzed with the Fisher’s exact test. A false discovery rate (FDR), using the method of Benjamini and Hochberg19, was used to adjust for multiple comparisons. Comparisons padjusted<0.05 were considered significant. Additionally, an exploratory analysis was conducted to assess the univariate association between SNPs and toxicity (overall infection, pancreatitis, thrombosis, and allergy) within ethnicity group. The relationship between EFS and SNPs within these groups was also explored.
Results
Patient characteristics
Between 2005 and 2011, 794 eligible children and adolescents (ages 1 – 18 years) enrolled on DFCI 05-001, 730 of whom had ethnicity documented (150 [19%] Hispanic, 580 [73%] non-Hispanic). When compared with non-Hispanic children, a higher percentage of Hispanic patients were ≥10 years at the time of diagnosis (32% vs. 24%, p=0.045). A higher percentage of Hispanic patients were obese (20% vs. 12%, p=0.024). There was no significant difference in the presence or absence of the following leukemia-associated cytogenetic characteristics: high hyperdiploidy (51–65 chromosomes), BCR-ABL1, KMT2A (MLL)-rearrangement, hypodiploidy, and iAMP21 by ethnicity (Table 1). Hispanic patients were significantly less likely to have the ETV6-RUNX1 fusion (p=0.018). Presenting leukocyte count, immunophenotype, National Cancer Institute (NCI) risk group, final DFCI risk group, or assigned randomized treatment arm (Table 1) did not significantly differ by ethnicity.
TABLE 1.
Demographic and clinical characteristics of study participants on DFCI 05-001
| Entire Cohort (With Ethnicity) | Ethnicity | p-value | |||||
|---|---|---|---|---|---|---|---|
| Hispanic | Non-Hispanic | ||||||
|
| |||||||
| No. | % | No. | % | No. | % | ||
| Cohort size | 730 | 100 | 150 | 100 | 580 | 100 | - |
| Age, years | 0.045 | ||||||
| <10 | 545 | 75 | 102 | 68 | 443 | 76 | |
| ≥10 | 185 | 25 | 48 | 32 | 137 | 24 | |
|
| |||||||
| White blood cell count (cells/μL) | 0.57 | ||||||
| <50,000 | 578 | 79 | 116 | 77 | 462 | 80 | |
| ≥50,000 | 152 | 21 | 34 | 23 | 118 | 20 | |
|
| |||||||
| NCI risk group | 0.19 | ||||||
| Standard risk | 445 | 61 | 84 | 56 | 361 | 62 | |
| High risk | 285 | 39 | 66 | 44 | 219 | 38 | |
| Immunophenotype | 0.58 | ||||||
| T-cell | 89 | 12 | 16 | 11 | 73 | 13 | |
| B-cell | 641 | 88 | 134 | 89 | 507 | 87 | |
|
| |||||||
| Sex | 0.52 | ||||||
| Female | 325 | 45 | 63 | 42 | 262 | 45 | |
| Male | 405 | 55 | 87 | 58 | 318 | 55 | |
|
| |||||||
| Body mass index (n=729) | 0.053 | ||||||
| Underweight | 47 | 6 | 10 | 7 | 37 | 6 | |
| Normal | 468 | 64 | 83 | 55 | 385 | 66 | |
| Overweight | 112 | 15 | 26 | 17 | 86 | 15 | |
| Obese | 102 | 14 | 30 | 20 | 72 | 12 | |
| Cytogenetics* | |||||||
| ETV6-RUNX1 | 136 | 17 | 18 | 12 | 118 | 20 | 0.018 |
| High Hyperdiploidy (51–65 chromosomes) | 184 | 25 | 45 | 30 | 139 | 24 | 0.14 |
| Ph+ (BCR-ABL-1) | 19 | 3 | 4 | 3 | 15 | 3 | 1.00 |
| KMT2A(MLL)-rearrangement | 12 | 2 | 1 | 1 | 11 | 2 | 0.48 |
| Hypodiploidy | 10 | 1 | 1 | 1 | 9 | 2 | 0.70 |
| iAMP21 | 12 | 2 | 1 | 1 | 11 | 2 | 0.48 |
|
| |||||||
| Achieved complete remission | 695 | 95 | 141 | 94 | 554 | 96 | 0.36 |
|
| |||||||
| Final DFCI risk group+ | 0.81 | ||||||
| Standard risk | 370 | 54 | 71 | 51 | 299 | 54 | |
| High risk | 242 | 35 | 54 | 39 | 188 | 34 | |
| Very high risk | 62 | 9 | 12 | 9 | 50 | 9 | |
| Ph+ | 16 | 2 | 3 | 2 | 13 | 2 | |
|
| |||||||
| Asparaginase therapy+ | 0.75 | ||||||
| Directly Assigned to IM E. Coli | 267 | 39 | 52 | 37 | 215 | 39 | |
| Randomized to IM E. Coli | 205 | 30 | 40 | 29 | 165 | 30 | |
| Randomized IV pegaspargase | 218 | 32 | 48 | 34 | 170 | 31 | |
Abbreviations: NCI: National Cancer Institute; B-cell: B-cell acute lymphoblastic leukemia; T-cell: T-cell acute lymphoblastic leukemia; Ph+: Philadelphia chromosome positive ALL; iAMP21: Intrachromosomal amplification of chromosome 21; IM E.Coli: Intramuscular E. Coli asparaginase; IV Peg: IV pegaspargase;
n=12 not screened for cytogenetics including ETV6-RUNX1, high hyperdiploidy, KMT2A (MLL)-rearrangement, hypodiploidy, and iAMP21
Achieved a complete remission and assigned a post induction asparaginase group
NCI risk group: Standard risk (WBC<50,000 and Age <10 years), High Risk (WBC≥50,000 or Age ≥10 years)
Treatment-related toxicities
Infection
The overall rate of infection during the induction treatment phase was not significantly different between Hispanic and non-Hispanic patients (25% vs. 29%, p=0.36). Hispanic patients trended toward having fewer bacterial infections than non-Hispanic patients (19% vs. 27%), but this difference was not statistically significant (p=0.07) (Table 2). Post-induction infections were documented in 31% of Hispanic patients and in 32% of non-Hispanic patients (p=0.92) (Table 2).
TABLE 2.
Treatment-related toxicities by ethnicity during induction and post-induction therapy
| Induction Toxicity | Ethnicity | p-value | |||||
|---|---|---|---|---|---|---|---|
| Entire Cohort | Hispanic | Non-Hispanic | |||||
|
| |||||||
| No. | % | No. | % | No. | % | ||
| All | 730 | - | 150 | - | 580 | - | - |
|
| |||||||
| Infection | 203 | 28 | 37 | 25 | 166 | 29 | 0.36 |
| Bacterial | 183 | 25 | 29 | 19 | 154 | 27 | 0.07 |
| Fungal | 30 | 4 | 8 | 5 | 22 | 4 | 0.36 |
| Viral | 5 | <1 | 1 | 1 | 4 | 1 | 1.00 |
| Opportunistic | 3 | <1 | 1 | 1 | 2 | 0 | - |
|
| |||||||
| Asparaginase toxicity | 47 | 6 | 10 | 7 | 37 | 6 | 0.85 |
| Pancreatitis | 17 | 2 | 6 | 4 | 11 | 2 | 0.13 |
| Allergy | 10 | 1 | 2 | 1 | 8 | 1 | 1.00 |
| Thrombosis | 20 | 3 | 2 | 1 | 18 | 3 | 0.40 |
|
| |||||||
| Bone Event | 3 | <1 | 3 | 1 | 0 | 0 | - |
| Bone Fracture | 3 | <1 | 3 | 1 | 0 | 0 | - |
| Osteonecrosis | 0 | <1 | 0 | 0 | 0 | 0 | - |
|
| |||||||
| Post-Induction Toxicity | Ethnicity | p-value | |||||
| Entire Cohort | Hispanic | Non-Hispanic | |||||
|
| |||||||
| No. | % | No. | % | No. | % | ||
|
| |||||||
| All | 690 | - | 140 | - | 550 | - | - |
|
| |||||||
| Infection | 220 | 32 | 44 | 31 | 176 | 32 | 0.92 |
| Bacterial | 158 | 23 | 30 | 21 | 128 | 23 | 0.74 |
| Fungal | 17 | 2 | 2 | 1 | 15 | 3 | 0.55 |
| Viral | 59 | 9 | 17 | 12 | 42 | 8 | 0.09 |
| Opportunistic | 21 | 3 | 1 | 1 | 20 | 4 | 0.10 |
|
| |||||||
| Asparaginase toxicity | 183 | 27 | 40 | 29 | 143 | 26 | 0.59 |
| Pancreatitis | 75 | 11 | 20 | 14 | 55 | 10 | 0.17 |
| Allergy | 63 | 9 | 14 | 10 | 49 | 9 | 0.74 |
| Thrombosis | 72 | 10 | 11 | 8 | 61 | 11 | 0.35 |
|
| |||||||
| Bone Event* | 163 | 24 | 15 | 11 | 148 | 27 | <0.0001 |
| Bone Fracture | 131 | 19 | 11 | 8 | 120 | 22 | <0.0001 |
| Osteonecrosis | 54 | 8 | 4 | 3 | 50 | 9 | 0.013 |
Only includes bone toxicity on therapy
Asparaginase-associated toxicities
The overall incidence of post-induction asparaginase-associated toxicities including allergy, pancreatitis and thrombosis, was not significantly different between Hispanic and non-Hispanic patients (Table 2). The rate of ON and fracture during post induction therapy was lower in Hispanic patients (p=0.013 and <0.0001 respectively) (Table 2).
Serum asparaginase activity (SAA)
At least one induction SAA level was available in 318 patients. During remission induction, when all patients received a single dose of pegasapargase, we did not observe differences between Hispanic and non-Hispanic patients in median SAA levels at 4, 11, 18, and 25 days after the dose (Supplemental Figure S1).
Osteonecrosis
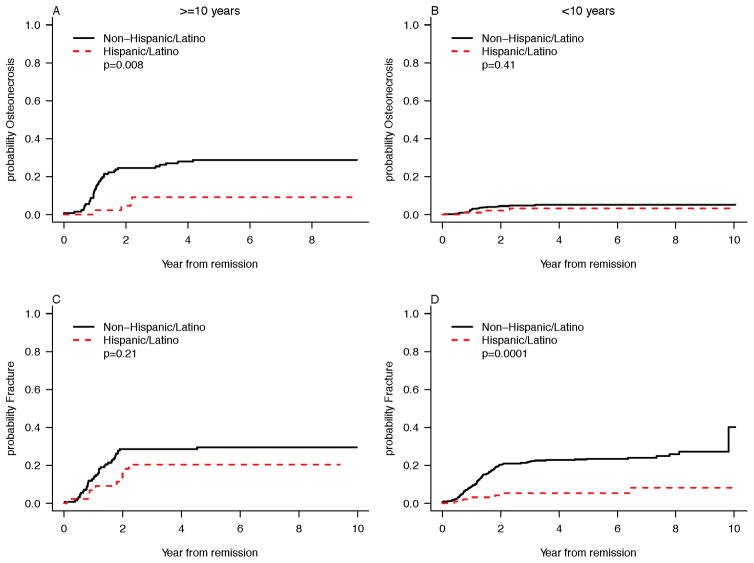
Overall, the incidence of ON differed by age (p<0.0001) with patients ≥10 years having more events. In patients ≥10 years of age, Hispanic ethnicity was associated with a significantly lower cumulative incidence of ON (hazard ratio, HR [95% confidence interval] 0.28 [0.10–0.76]; p=0.013; Fig. 1A). This result remained significant in multivariable modeling (p=0.006; Table 3). In patients <10 years of age there was no statistically significant difference in the rate of ON between Hispanic and non-Hispanic patients (0.61 [0.18–2.02], p=0.41, Fig. 1B). Additionally, in competing risks regression there was no detectable difference in cumulative incidence of ON by obesity for each age group (Table 3). Analysis of SNPs revealed no significant difference between Hispanic and non-Hispanic patients in the frequency of the TS polymorphism, which we have previously shown is associated with risk of bone toxicity in this patient population.20,21
FIGURE 1.
Probability of Osteonecrosis and Probability of Fracture by Age at Diagnosis (<10y vs. ≥10y) in Hispanic and Non-Hispanic Patients: Skeletal toxicity data are shown for (A) Osteonecrosis in patients ≥10 years of age (B) Osteonecrosis in patients <10 years of age (C) Bone fracture in patients ≥10 years of age, and (D) Bone fracture in patients <10 years of age.
TABLE 3.
Competing risks regression for skeletal toxicities by age subgroup and asparaginase treatment arm
| Bone Fracture | Osteonecrosis | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||
| Hazard Ratio [95%CI] | p-value | Hazard Ratio [95%CI] | p-value | Hazard Ratio [95%CI] | p-value | Hazard Ratio [95%CI] | p-value | |
| Age <10 Years | ||||||||
| Hispanic vs. Non-Hispanic | 0.24 [0.10–0.54] | 0.0006 | 0.23 [0.10–0.51] | 0.0003 | 0.61 [0.18–2.02] | 0.41 | 0.59 [0.18–1.95] | 0.39 |
| Female vs. Male | 1.25 [0.86–1.81] | 0.25 | 1.25 [0.86–1.82] | 0.23 | 0.35 [0.14–0.88] | 0.025 | 0.34 [0.13–0.84] | 0.020 |
| Post induction ASP | ||||||||
| Direct assignment vs. Not | 0.96 [0.65–1.42] | 0.86 | 0.97 [0.61–1.52] | 0.88 | 1.92 [0.87–4.22] | 0.11 | 1.79 [0.68–4.69] | 0.23 |
| IM E. Coli vs. Not | 1.09 [0.73–1.63] | 0.67 | 1.03 [0.64–1.67] | 0.90 | 0.58 [0.22–1.56] | 0.28 | 0.90 [0.27–3.01] | 0.86 |
| SR vs. Not | 0.95 [0.63–1.42] | 0.80 | 0.96 [0.64–1.43] | 0.83 | 1.64 [0.62–4.35] | 0.32 | 1.79 [0.68–4.71] | 0.24 |
| Obese vs. Not | 1.18 [0.69–2.03] | 0.55 | 1.42 [0.82–2.47] | 0.21 | 0.56 [0.13–2.4] | 0.43 | ⱡ | ⱡ |
| Age ≥10 years | ||||||||
| Hispanic vs. Non-Hispanic | 0.63 [0.31–1.28] | 0.20 | 0.62 [0.31–1.27] | 0.19 | 0.28 [0.10–0.76] | 0.013 | 0.23 [0.08–0.66] | 0.006 |
| Female vs. Male | 0.99 [0.55–1.79] | 0.98 | 0.91 [0.51–1.64] | 0.76 | 0.61 [0.31–1.22] | 0.16 | 0.49 [0.25–0.97] | 0.042 |
| Post induction ASP | ||||||||
| Direct Assignment vs. not | 0.72 [0.37–1.42] | 0.35 | 0.80 [0.37–1.73] | 0.57 | 0.34 [0.14–0.81] | 0.015 | 0.32 [0.12–0.82] | 0.020 |
| IM E. Coli vs. Not | 1.53 [0.85–2.73] | 0.15 | 1.38 [0.70–2.71] | 0.35 | 1.66 [0.88–3.11] | 0.12 | 1.14 [0.59–2.20] | 0.70 |
| VHR vs. Not | 0.77 [0.28–2.14] | 0.62 | 0.75 [0.27–2.07] | 0.58 | 0.41 [0.10–1.72] | 0.22 | ⱡ | ⱡ |
| Obese vs. not | 1.21 [0.56–2.60] | 0.63 | 1.31 [0.60–2.83] | 0.50 | 0.42 [0.13–1.39] | 0.16 | ⱡ | ⱡ |
Abbreviations: Direct assignment: Directly assigned to receive native E. Coli asparaginase IM E.Coli: Intramuscular E. Coli asparaginase; SR: Standard risk; VHR: Very high risk; ASP: asparaginase
Due to the small number of bone events (n<4) not considered in multivariable modeling
Fracture
In patients ≥10 years of age, there was no significant difference fracture incidence between Hispanic and non-Hispanic patients (HR 0.63 [0.31–1.28], p=0.20 Fig. 1C). In children <10 years, cumulative incidence of fracture was significantly lower in the Hispanic group (0.24 [0.10–0.54], p=0.0006; Fig. 1D). This remained significant in multivariable modeling (p=0.0003; Table 3). In competing risks regression there was no detectable difference in cumulative incidence of fracture by obesity for each age group (Table 3).
Survival
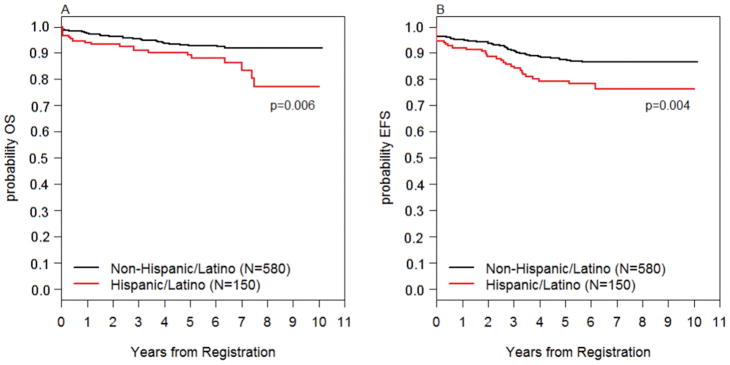
The median follow-up time for those still alive was 6.12 years. Five-year OS was significantly lower in Hispanic patients (89.2% [82.7%–93.4%]) vs. non-Hispanic patients (92.7 [90.2%–94.6%]), (p=0.006; Figure 2A). Five-year EFS was also significantly lower in Hispanic patients (79.4% [71.6%–85.2%]) vs. non-Hispanic patients (87.5% [84.5%–90.0%]), (p=0.004; Figure 2B). While both cohorts had nearly identical CR rates (94–95%), a higher percentage of Hispanic vs. non-Hispanic (13% vs. 9%) patients experienced disease relapse (Supplemental Table S1). There were no detectable differences in the site of relapse between groups (Supplemental Table S2). Of the B-ALL patients with a documented CR, there was no statistically significant difference in the proportion of patients with high end-induction MRD (defined as ≥10–3): Hispanic (11%) vs. non-Hispanic (9%), p=0.55. Additionally, there was no difference in incidence of treatment-related mortality or in incidence of second malignant neoplasm between Hispanic and non-Hispanic patients. Ethnicity retained significance in multivariable Cox modeling for EFS (p=0.030) when adjusting for age, WBC, sex, immunophenotype and obesity, and marginal significance (p=0.07) in multivariable modeling for OS when adjusting for the same variables. In the multivariable models, obesity was significantly associated with OS (p=0.012) but EFS (p=0.27) (Table 4).
FIGURE 2.
Overall Survival and Event-Free Survival by Ethnicity: (A) Overall survival in Hispanic vs. non-Hispanic patients (B) Event-free survival in Hispanic vs. non-Hispanic patients.
TABLE 4.
Cox proportional hazards univariate and multivariable models of overall survival (OS) and event-free survival (EFS) by ethnicity. BMI group was re-grouped to obese vs. not obese to account for overlap between obesity and overweight categories. No differences were seen with EFS. Adjusting for other variables, obesity remains significant and ethnicity is marginally significant.
| Univariate | Multivariable | |||
|---|---|---|---|---|
| Hazard Ratio [95% CI] | p-value | Hazard Ratio [95% CI] | p-value | |
| Overall Survival (OS) | ||||
| Hispanic vs. non-Hispanic | 2.06 [1.21–3.52] | 0.008 | 1.67 [0.95–2.89] | 0.07 |
| Age ≥10y vs. <10y | 1.62 [0.96–2.72] | 0.07 | 1.41 [0.82–2.42] | 0.21 |
| WBC ≥50K vs. <50K | 3.26 [1.97–5.38] | <0.0001 | 3.61 [2.13–6.12] | <0.0001 |
| Female vs. male | 0.84 [0.51–1.40] | 0.51 | 0.95 [0.57–1.60] | 0.85 |
| B-ALL vs. T-ALL | 1.07 [0.49–2.35] | 0.87 | 2.03 [0.88–4.65] | 0.09 |
| Obese vs. not obese | 2.37 [1.34–4.20] | 0.003 | 2.10 [1.18–3.76] | 0.012 |
| Event-Free Survival (EFS) | ||||
| Hispanic vs. non-Hispanic | 1.82 [1.19–2.77] | 0.005 | 1.61 [1.05–2.49] | 0.030 |
| Age ≥10 vs. <10y | 1.65 [1.10–2.46] | 0.015 | 1.45 [0.96–2.20] | 0.08 |
| WBC ≥50K vs. <50K | 2.44 [1.64–3.63] | <0.0001 | 2.52 [1.64–3.85] | <0.0001 |
| Female vs. male | 0.87 [0.59–1.28] | 0.48 | 0.96 [0.65–1.44] | 0.85 |
| B-ALL vs. T-ALL | 0.84 [0.48–1.47] | 0.53 | 1.38 [0.75–2.53] | 0.30 |
| Obese vs. not obese | 1.46 [0.89–2.41] | 0.13 | 1.33 [0.80–2.20] | 0.27 |
Abbreviations: WBC: White blood cell count; B-ALL: B-cell acute lymphoblastic leukemia; T-ALL: T-cell acute lymphoblastic leukemia
Polymorphisms
Genotyping data were available for 587 patients with ethnicity information, 574 of who received a final risk group classification (116 [20%] Hispanic, 458 [80%] non-Hispanic). After noting a difference in bone toxicity between Hispanic and non-Hispanic patients, we tested whether there was also a significant difference in the prevalence of a polymorphism in thymidylate synthase (TS) known to be associated with bone toxicity.16 In addition we tested whether there were disparities associated with ethnicity for 18 other TRT-related polymorphisms previously assessed in this cohort.15 Hispanic and non-Hispanic patients differed significantly in the proportion with the target genotype of four polymorphic genes: MTHFR A1298C (rs1801131; padjusted=0.001), SLCO2A1 (padjusted=0.003), IL1B (padjusted=0.003), and TCN2 (padjusted=0.002) (Supplementary Table S3). Of these four polymorphisms, only TCN2 was associated with both TRT and disease outcome. In Hispanic patients, having (vs. not having) the target TCN2 genotype was associated with increased risk of induction infection (32% vs. 11%, p=0.010). In the Hispanic cohort, the TCN2 polymorphism was univariately associated with EFS within the Hispanic patient cohort. In multivariable modeling, TCN2 was marginally associated with EFS (HR=3.15, p=0.047) (Supplemental Table S4).
Discussion
This analysis of TRTs and survival from DFCI ALL 05-001 demonstrated that overall, Hispanic patients had lower rates of ON and fracture as well as reduced EFS and OS relative to non-Hispanic patients. The observation of both reduced toxicity and decreased survival in the Hispanic cohort suggests that host and/or environmental factors, rather than differences in leukemia biology alone, likely contributed to these outcomes.
In our Hispanic cohort, the lower incidence of skeletal toxicity is suggestive of reduced exposure to dexamethasone, which may be related to variations in medication adherence or to variations in disease biology or host pharmacogenomics. A potential mechanism of reduced dexamethasone exposure is oral chemotherapy adherence. Chemotherapy agents that need to be orally administered at home, including mercaptopurine and corticosteroid, are important components of the treatment regimen for children and adolescents with ALL.17,22 In a 2012 report from the Children’s Oncology Group (COG), Bhatia and colleagues found that patients who were <95% adherent to mercaptopurine during maintenance therapy had a 2.5-fold higher risk of relapse than those who were ≥ 95% adherent.23 Further analyses revealed that Hispanic ethnicity, adolescent age ≥12 years and low socioeconomic status were all associated with lower adherence.23 Of interest, the in patients with high adherence, Hispanic ethnicity was still associated with higher relapse rate. This further emphasizes the possibility that differential findings between Hispanic and non-Hispanic patients are likely driven in large part by biologic differences between groups, rather than only by differences in adherence. In 2012, Kawedia, et al. reported that dexamethasone clearance may be higher in patients with anti-asparaginase antibodies. In that study, the increased clearance and/or the presence of the antibodies were associated with a higher risk of relapse.24 Although we did not prospectively assess asparaginase antibodies on the 05-001 study, we serially measured SAA in patients during treatment,25 and demonstrated no differences in SAA between Hispanic and non-Hispanic patients, indicating similar exposure to this agent by ethnic group.
Having identified reduced rates of ON in Hispanic patients, we were particularly interested in whether there were differences between cohorts in the frequency of an enhancer-repeat genotype (2R/2R) polymorphism in the TS gene.16,26 Our analysis did not identify a difference in prevalence of the 2R/2R TS polymorphism between Hispanic and non-Hispanic patients suggesting that either untested germline genetic factors or other variables beyond genetic polymorphisms may have contributed to differences in skeletal toxicities.27–29 The incidence of ON was significantly different between Hispanic and non-Hispanic patients in the older (≥10 years of age) patients, and the incidence of fracture was significantly different between Hispanic patients and non-Hispanic patients, in the younger (<10 years of age) group. The association between older age and ON in ALL patients has been well-documented, as has the association between fracture and younger age.30,31 To our knowledge, no published study has identified a clear explanation for this phenomenon. Possible mechanisms may include hormonal interactions related to older age, timing of skeletal development, and unidentified genetic predispositions. Further, while obesity is a known predictor of reduced bone mineral density in children without leukemia,32 it was not significantly predictive of either fracture or ON in our patient cohort and would not explain difference by age.
While host genetic variations likely play an important role in determining drug pharmacokinetics and pharmacodynamics, somatic abnormalities in leukemia cells are critical determinants of response and resistance to therapy as well. Differences in the frequency of prognostically significant subtypes of ALL between Hispanic and non-Hispanic patients have been described, some of which could explain some of the outcome differences we observed.11,33 For example, we observed a significantly lower incidence of the favorable ETV6-RUNX1 (TEL/AML1) fusion, in our Hispanic cohort, which may have contributed to a higher risk of relapse.34
We15,16 and others20,35–40 have previously described associations between functional genetic polymorphisms and TRT or survival among children with leukemia and the prevalence of some of these polymorphisms is known to differ between ethnic groups.11,20,33,41–43 In exploratory analyses, we targeted a small subset of polymorphisms that were relatively common (population prevalence of at least 10%) and that could potentially impact either TRT or survival. We observed significant differences between Hispanic and non-Hispanic patients in the prevalence of four of the 19 polymorphisms analyzed (Supplemental Table 3) however, the clinical import of these germline genetic differences remains unclear. The TCN2 rs1801198 polymorphism was more prevalent in Hispanic patients and was associated with inferior EFS within that cohort. This polymorphism was also associated with increased risk of induction infection in the whole study population, but there was no significant difference in infection rates between Hispanic and non-Hispanic patients; in fact, Hispanic patients tended to have fewer bacterial infections overall.
This study has some important limitations. First, the analysis of genetic polymorphisms was not prospectively designed or powered to detect associations between all polymorphisms and uncommon outcomes. Additionally, we did not analyze incidence of poor prognostic indicators, including BCR-ABL1-like subtype and deletions of the Ikaros (IKZF1) gene, both of which have been reported to be more common in Hispanic patients.36–38,41 These two features, which are frequently observed together, are independently associated with adverse outcomes in children with ALL. Thus, the inferior EFS and OS that we observed in Hispanic patients may be due to overrepresentation of these unfavorable biologic features within this population.44 While these alterations may have contributed to survival differences by ethnicity, they would not explain the difference in TRTs.
Also, there was not a standard approach to designating patient ethnicity at the time of study enrollment. Hispanic ethnicity as a single broad category does not delineate between different Hispanic/Latino groups (e.g. Cuban, Mexican, Puerto Rican, South or Central American, Spanish), each of which are known to have unique biologic and non-biologic factors associated with disease outcome.45 Because of sample size limitations we did not analyze outcomes by combined race/ethnicity. We acknowledge there are more objective ways of classifying patients’ ethnicity, for example by using genome-wide ancestry estimates. These methods, while precise in their characterization of genetic and biologic variation, are limited in their ability to account for sociocultural influences.45–48 For future studies, we will define both race and ethnicity using patient report, and will define genetic or biogeographical ancestry using modern genomic techniques.33,44 Comparing self-reported ethnicity to genetic ancestry will be an important part of investigating whether biology, sociocultural influences, or both, are contributing to observed outcome differences between ethnically distinct populations.49
Conclusion
Hispanic children and adolescents enrolled on the DFCI 05-001 had significantly lower rates of skeletal toxicities as well as significantly lower EFS and OS compared to non-Hispanic patients. Hispanic patients were more frequently obese than non-Hispanic patients and obesity was associated with inferior OS, it did not explain differences in ON, fracture or EFS by ethnicity. It is likely that the mechanisms behind our observations are a combination of biogeographical variables (i.e. inherited host genetic factors), gene-environment interactions, and sociocultural variables (i.e. early childhood exposures, baseline nutrition, health beliefs).50–52
Other studies have compared self-defined ethnicity to genetic ancestry in childhood ALL, and have explored how these groups associate with relapse and adverse events.53 Our combined analyses of disease outcomes and toxicity in a homogeneously treated patient population suggests that factors beyond genomics are involved. Considering the observation of both reduced toxicities and inferior survival in the Hispanic cohort, the possibility of sub-optimal drug exposure in these patients likely deserves further inquiry. Thus, while differences in both host and leukemia biology are prognostically important, future studies will focus on host pharmacogenomics, detailed analyses of nutrition status and obesity trends,54 inter-patient differences in biomarkers of drug exposure, frequency of drug interruptions for toxicity, and oral chemotherapy adherence.
Supplementary Material
Supplemental Figure S1: Median (IQR) Serum Asparaginase Activity levels in Hispanic and Non-Hispanic Patients: During remission induction, when all patients received a single dose of pegasapargase, median serum asparaginase activity (SAA) was measured 4 (D4), 11 (D11), 18 (D18), and 25 (D25) days after the dose. At least one induction SAA level was available in 318 patients. At D4, n=289, D11, n=318, D18 n=271, and D25 n=274. We did not observed differences between Hispanic and non-Hispanic patients in median SAA levels 4, 11, 18, and 25 days after a dose.
Supplemental Table S1: Events in Hispanic and non-Hispanic patients.
Both Hispanic and non-Hispanic patients had nearly identical complete remission (CR) rates (94–95%), however, a higher percentage of Hispanic vs. non-Hispanic (13% vs. 9%) patients experienced disease relapse.
Supplemental Table S2: Distribution of sites of relapse by ethnicity. There was no significant difference in sites of relapse between Hispanic and non-Hispanic patients.
Supplemental Table S3: Target polymorphisms by ethnicity.
Hispanic and non-Hispanic patients differed significantly in the proportion with the target genotype of four polymorphic genes: MTHFR A1298C (rs1801131; padjusted=0.001), SLCO2A1 (padjusted=0.003), IL1B (padjusted=0.003), and TCN2 (padjusted=0.002).
Supplemental Table S4: Analyses of polymorphisms vs. disease-free survival (DFS) and event-free survival (EFS) in Hispanic and non-Hispanic patients with nominal p-values, overall and by ethnicity.
In the Hispanic cohort, the TCN2 polymorphism was univariately associated with EFS within the Hispanic patient cohort. In multivariable modeling, TCN2 was marginally associated with EFS (HR=3.15, p=0.05).
Acknowledgments
This work was supported in part by funding from the National Institutes of Health (R25 CA094061) (J.M.K.) and the St. Baldrick’s Foundation (Supportive Care Research Award) (P.D.C.). Clinical trial information for DFCI 05-001: ClinicalTrials.gov, number NCT00400946
We thank the patients, families, physicians, nurses, research coordinators, and all others who participated in the data collection associated with this work. Thank you to the Dana-Farber Cancer Institute ALL Consortium for its contribution to this work.
The patients described in this report were enrolled at the following Dana-Farber Cancer Institute (DFCI) Acute Lymphoblastic Leukemia Consortium sites: DFCI/Boston Children’s Hospital (Boston, MA), Columbia University Medical Center, Morgan Stanley Children’s Hospital of New York-Presbyterian (New York, NY), Hospital Sainte Justine (Montreal, QC, Canada), Le Centre Hospitalier de L’Universite Laval (Quebec City, QC, Canada), McMaster Children’s Hospital (Hamilton, ON, Canada), San Jorge Children’s Hospital (San Juan, PR), University of Rochester Medical Center (Rochester, NY), Hospital Ste. Justine (Montreal, Quebec, Canada), Hasbro Children’s Hospital (Providence, RI), Inova/Fairfax Hospital for Children, (Falls Church, VA).
Abbreviations Table
- ALL
Acute lymphoblastic leukemia
- DFCI
Dana-Farber Cancer Institute
- DFCI 05-001
Dana-Farber Cancer Institute ALL Consortium Protocol 05-001
- CCSG
Children’s Cancer Study Group
- SEER
Surveillance Epidemiology and End Results Program
- TRT
Treatment-related toxicities
- CTCAE 3.0
Common Terminology Criteria for Adverse Events Version 3.0
- CR
Complete remission
- ON
Osteonecrosis
- SNPs
Single nucleotide polymorphisms
- EFS
Event-free survival
- OS
Overall survival
- TS
Thymidylate synthase
- PCR
Polymerase chain reaction
Footnotes
Conflict of Interest Statement: L.B.S. has served on advisory boards for Sigma-Tau Pharmaceuticals and JAZZ Pharmaceuticals. All other authors declare no competing financial interests.
Presented in abstract form at the 56th American Society of Hematology Annual Meeting and Exposition, December 5–8, 2015, Orlando, FL, USA.
Author Contributions:
Concept and design: Justine M. Kahn, Peter D. Cole, Traci M. Blonquist, Kristen E. Stevenson, Lewis B. Silverman and Kara M. Kelly.
Provision of study materials or patients: Lewis B. Silverman, Uma H. Athale, Peter D. Cole, Luis A. Clavell, Kara M. Kelly, Caroline Laverdiere, Jean-Marie Leclerc, Bruno Michon, Marshall A. Schorin, Jennifer J.G. Welch.
Collection and assembly of data: Justine M. Kahn, Peter D. Cole, Traci M. Blonquist, Kristen E. Stevenson, Lewis B. Silverman and Kara M. Kelly.
Data analysis and interpretation: Justine M. Kahn, Peter D. Cole, Traci M. Blonquist, Kristen E. Stevenson, Zhezhen Jin, Donna S. Neuberg, Sergio Barrera, Randy Davila, Emily Roberts, Stephen E. Sallan, Lewis B. Silverman and Kara M. Kelly.
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Kahn JM, Keegan TH, Tao L, Abrahao R, Bleyer A, Viny AD. Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer. 2016;122(17):2723–2730. doi: 10.1002/cncr.30089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahao R, Lichtensztajn DY, Ribeiro RC, et al. Racial/ethnic and socioeconomic disparities in survival among children with acute lymphoblastic leukemia in California, 1988–2011: A population-based observational study. Pediatr Blood Cancer. 2015;62(10):1819–1825. doi: 10.1002/pbc.25544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya S, Hsieh S, Shinohara ET, DeWees T, Frangoul H, Perkins SM. Effects of Race/Ethnicity and Socioeconomic Status on Outcome in Childhood Acute Lymphoblastic Leukemia. J Pediatr Hematol Oncol. 2016;38(5):350–354. doi: 10.1097/MPH.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 4.Goggins WB, Lo FF. Racial and ethnic disparities in survival of US children with acute lymphoblastic leukemia: evidence from the SEER database 1988–2008. Cancer Causes Control. 2012;23(5):737–743. doi: 10.1007/s10552-012-9943-8. [DOI] [PubMed] [Google Scholar]
- 5.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30(14):1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadan-Lottick NS, Ness KK, Bhatia S, Gurney JG. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290(15):2008–2014. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100(6):1957–1964. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia S. Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56(6):994–1002. doi: 10.1002/pbc.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karol SE, Mattano LA, Jr, Yang W, et al. Genetic risk factors for the development of osteonecrosis in children under age 10 treated for acute lymphoblastic leukemia. Blood. 2016;127(5):558–564. doi: 10.1182/blood-2015-10-673848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drachtman RA, Masterson M, Shenkerman A, Vijayanathan V, Cole PD. Long-term outcomes for children with acute lymphoblastic leukemia (ALL) treated on The Cancer Institute of New Jersey ALL trial (CINJALL) Leuk Lymphoma. 2016;57(10):2275–2280. doi: 10.3109/10428194.2016.1141406. [DOI] [PubMed] [Google Scholar]
- 11.Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312–5321. doi: 10.1182/blood-2009-09-245944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Relling MV, Ramsey LB. Pharmacogenomics of acute lymphoid leukemia: new insights into treatment toxicity and efficacy. Hematology Am Soc Hematol Educ Program. 2013;2013:126–130. doi: 10.1182/asheducation-2013.1.126. [DOI] [PubMed] [Google Scholar]
- 13.Karol SE, Yang W, Van Driest SL, et al. Genetics of glucocorticoid-associated osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2015;126(15):1770–1776. doi: 10.1182/blood-2015-05-643601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia colil-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05–001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015;16(16):1677–1690. doi: 10.1016/S1470-2045(15)00363-0. [DOI] [PubMed] [Google Scholar]
- 15.Cole PD, Finkelstein Y, Stevenson KE, et al. Polymorphisms in Genes Related to Oxidative Stress Are Associated With Inferior Cognitive Function After Therapy for Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2015;33(19):2205–2211. doi: 10.1200/JCO.2014.59.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelstein Y, Blonquist TM, Vijayanathan V, et al. A thymidylate synthase polymorphism is associated with increased risk for bone toxicity among children treated for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2016 doi: 10.1002/pbc.26393. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124(15):2345–2353. doi: 10.1182/blood-2014-01-552166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman DJ, Cohen BB, Averbach AR, Norton JM. Race/ethnicity and OMB Directive 15: implications for state public health practice. Am J Public Health. 2000;90(11):1714–1719. doi: 10.2105/ajph.90.11.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamini Y, Cohen R. Weighted false discovery rate controlling procedures for clinical trials. Biostatistics. 2016 doi: 10.1093/biostatistics/kxw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drachtman RA, Masterson M, Shenkerman A, Vijayanathan V, Cole PD. Long-term outcomes for children with acute lymphoblastic leukemia (ALL) treated on The Cancer Institute of New Jersey ALL trial (CINJALL) Leuk Lymphoma. 2016:1–6. doi: 10.3109/10428194.2016.1141406. [DOI] [PubMed] [Google Scholar]
- 21.Finkelstein YBT, Vijayanathan V, Stevenson KE, Neuberg DS, Silverman LB, Vrooman LM, Sallan SE, Cole PD. A Thymidylate Synthase Polymorphism is Associated with Increased Risk for Bone Toxicity Among Children Treated for Acute Lymphoblastic Leukemia. Pediatric Blood and Cancer. 2016 doi: 10.1002/pbc.26393. [DOI] [PubMed] [Google Scholar]
- 22.Koren G, Ferrazini G, Sulh H, et al. Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children. N Engl J Med. 1990;323(1):17–21. doi: 10.1056/NEJM199007053230104. [DOI] [PubMed] [Google Scholar]
- 23.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children’s oncology group. J Clin Oncol. 2012;30(17):2094–2101. doi: 10.1200/JCO.2011.38.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawedia JD, Liu C, Pei D, et al. Dexamethasone exposure and asparaginase antibodies affect relapse risk in acute lymphoblastic leukemia. Blood. 2012;119(7):1658–1664. doi: 10.1182/blood-2011-09-381731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015;16(16):1677–1690. doi: 10.1016/S1470-2045(15)00363-0. [DOI] [PubMed] [Google Scholar]
- 26.Relling MV, Yang W, Das S, et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. J Clin Oncol. 2004;22(19):3930–3936. doi: 10.1200/JCO.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Kunstreich M, Kummer S, Laws HJ, Borkhardt A, Kuhlen M. Osteonecrosis in children with acute lymphoblastic leukemia. Haematologica. 2016;101(11):1295–1305. doi: 10.3324/haematol.2016.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niinimaki RA, Harila-Saari AH, Jartti AE, et al. Osteonecrosis in children treated for lymphoma or solid tumors. J Pediatr Hematol Oncol. 2008;30(11):798–802. doi: 10.1097/MPH.0b013e31818ab29d. [DOI] [PubMed] [Google Scholar]
- 29.Niinimaki RA, Harila-Saari AH, Jartti AE, et al. High body mass index increases the risk for osteonecrosis in children with acute lymphoblastic leukemia. J Clin Oncol. 2007;25(12):1498–1504. doi: 10.1200/JCO.2006.06.2539. [DOI] [PubMed] [Google Scholar]
- 30.Sala A, Mattano LA, Jr, Barr RD. Osteonecrosis in children and adolescents with cancer - an adverse effect of systemic therapy. Eur J Cancer. 2007;43(4):683–689. doi: 10.1016/j.ejca.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Mattano LA, Jr, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. J Clin Oncol. 2000;18(18):3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 32.Rocher E, Chappard C, Jaffre C, Benhamou CL, Courteix D. Bone mineral density in prepubertal obese and control children: relation to body weight, lean mass, and fat mass. J Bone Miner Metab. 2008;26(1):73–78. doi: 10.1007/s00774-007-0786-4. [DOI] [PubMed] [Google Scholar]
- 33.Yang JJ, Cheng C, Devidas M, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet. 2011;43(3):237–241. doi: 10.1038/ng.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhojwani D, Pei D, Sandlund JT, et al. ETV6-RUNX1-positive childhood acute lymphoblastic leukemia: improved outcome with contemporary therapy. Leukemia. 2012;26(2):265–270. doi: 10.1038/leu.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karol SE, Mattano LA, Jr, Yang W, et al. Genetic risk factors for the development of osteonecrosis in children under age 10 treated for acute lymphoblastic leukemia. Blood. 2015 doi: 10.1182/blood-2015-10-673848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gharbi H, Ben Hassine I, Soltani I, et al. Association of genetic variation in IKZF1, ARID5B, CDKN2A, and CEBPE with the risk of acute lymphoblastic leukemia in Tunisian children and their contribution to racial differences in leukemia incidence. Pediatr Hematol Oncol. 2016;33(3):157–167. doi: 10.3109/08880018.2016.1161685. [DOI] [PubMed] [Google Scholar]
- 37.Boer JM, van der Veer A, Rizopoulos D, et al. Prognostic value of rare IKZF1 deletion in childhood B-cell precursor acute lymphoblastic leukemia: an international collaborative study. Leukemia. 2016;30(1):32–38. doi: 10.1038/leu.2015.199. [DOI] [PubMed] [Google Scholar]
- 38.Clappier E, Grardel N, Bakkus M, et al. IKZF1 deletion is an independent prognostic marker in childhood B-cell precursor acute lymphoblastic leukemia, and distinguishes patients benefiting from pulses during maintenance therapy: results of the EORTC Children’s Leukemia Group study 58951. Leukemia. 2015;29(11):2154–2161. doi: 10.1038/leu.2015.134. [DOI] [PubMed] [Google Scholar]
- 39.Kaluzna E, Strauss E, Zajac-Spychala O, et al. Functional variants of gene encoding folate metabolizing enzyme and methotrexate-related toxicity in children with acute lymphoblastic leukemia. Eur J Pharmacol. 2015;769:93–99. doi: 10.1016/j.ejphar.2015.10.058. [DOI] [PubMed] [Google Scholar]
- 40.Vujkovic M, Kershenbaum A, Wray L, et al. Associations between genetic variants in folate and drug metabolizing pathways and relapse risk in pediatric acute lymphoid leukemia on CCG-1952. Leuk Res Rep. 2015;4(2):47–50. doi: 10.1016/j.lrr.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris MMB, TM, Athale U, Clavell LA, Cole PD, Kelly KM, Laverdiere C, Leclerc JM, Michon B, Schorin MA, Welch JJG, Neuberg DS, Sallan SE, Silverman LB. Ikaros Gene Deletion Significantly Predicts Relapse in Pediatric B-ALL Patients with Low End-Induction Minimal Residual Disease. Blood. 2015;126(23):2613. [Google Scholar]
- 42.Xu H, Cheng C, Devidas M, et al. ARID5B genetic polymorphisms contribute to racial disparities in the incidence and treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2012;30(7):751–757. doi: 10.1200/JCO.2011.38.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moriyama T, Yang YL, Nishii R, et al. Novel variants in NUDT15 and thiopurine intolerance in children with acute lymphoblastic leukemia from diverse ancestry. Blood. 2017;130(10):1209–1212. doi: 10.1182/blood-2017-05-782383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karol SE, Larsen E, Cheng C, et al. Genetics of ancestry-specific risk for relapse in acute lymphoblastic leukemia. Leukemia. 2017 doi: 10.1038/leu.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mersha TB, Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum Genomics. 2015;9:1. doi: 10.1186/s40246-014-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banda Y, Kvale MN, Hoffmann TJ, et al. Characterizing Race/Ethnicity and Genetic Ancestry for 100,000 Subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) Cohort. Genetics. 2015;200(4):1285–1295. doi: 10.1534/genetics.115.178616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim JY, Bhatia S, Robison LL, Yang JJ. Genomics of racial and ethnic disparities in childhood acute lymphoblastic leukemia. Cancer. 2014;120(7):955–962. doi: 10.1002/cncr.28531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pui CH, Boyett JM, Hancock ML, Pratt CB, Meyer WH, Crist WM. Outcome of treatment for childhood cancer in black as compared with white children. The St Jude Children’s Research Hospital experience, 1962 through 1992. JAMA. 1995;273(8):633–637. [PubMed] [Google Scholar]
- 49.Perez A. Acculturation, Health Literacy, and Illness Perceptions of Hypertension among Hispanic Adults. J Transcult Nurs. 2014 doi: 10.1177/1043659614524785. [DOI] [PubMed] [Google Scholar]
- 50.Landier W, Hughes CB, Calvillo ER, et al. A grounded theory of the process of adherence to oral chemotherapy in Hispanic and caucasian children and adolescents with acute lymphoblastic leukemia. J Pediatr Oncol Nurs. 2011;28(4):203–223. doi: 10.1177/1043454211409582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez AD, Hirschman C. The Changing Racial and Ethnic Composition of the US Population: Emerging American Identities. Popul Dev Rev. 2009;35(1):1–51. doi: 10.1111/j.1728-4457.2009.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klimentidis YC, Miller GF, Shriver MD. Genetic admixture, self-reported ethnicity, self-estimated admixture, and skin pigmentation among Hispanics and Native Americans. Am J Phys Anthropol. 2009;138(4):375–383. doi: 10.1002/ajpa.20945. [DOI] [PubMed] [Google Scholar]
- 53.Salari K, Burchard EG. Latino populations: a unique opportunity for epidemiological research of asthma. Paediatr Perinat Epidemiol. 2007;21(Suppl 3):15–22. doi: 10.1111/j.1365-3016.2007.00880.x. [DOI] [PubMed] [Google Scholar]
- 54.Ladas EJ, Orjuela M, Stevenson K, et al. Dietary intake and childhood leukemia: The Diet and Acute Lymphoblastic Leukemia Treatment (DALLT) cohort study. Nutrition. 2016;32(10):1103–1109. e1101. doi: 10.1016/j.nut.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1: Median (IQR) Serum Asparaginase Activity levels in Hispanic and Non-Hispanic Patients: During remission induction, when all patients received a single dose of pegasapargase, median serum asparaginase activity (SAA) was measured 4 (D4), 11 (D11), 18 (D18), and 25 (D25) days after the dose. At least one induction SAA level was available in 318 patients. At D4, n=289, D11, n=318, D18 n=271, and D25 n=274. We did not observed differences between Hispanic and non-Hispanic patients in median SAA levels 4, 11, 18, and 25 days after a dose.
Supplemental Table S1: Events in Hispanic and non-Hispanic patients.
Both Hispanic and non-Hispanic patients had nearly identical complete remission (CR) rates (94–95%), however, a higher percentage of Hispanic vs. non-Hispanic (13% vs. 9%) patients experienced disease relapse.
Supplemental Table S2: Distribution of sites of relapse by ethnicity. There was no significant difference in sites of relapse between Hispanic and non-Hispanic patients.
Supplemental Table S3: Target polymorphisms by ethnicity.
Hispanic and non-Hispanic patients differed significantly in the proportion with the target genotype of four polymorphic genes: MTHFR A1298C (rs1801131; padjusted=0.001), SLCO2A1 (padjusted=0.003), IL1B (padjusted=0.003), and TCN2 (padjusted=0.002).
Supplemental Table S4: Analyses of polymorphisms vs. disease-free survival (DFS) and event-free survival (EFS) in Hispanic and non-Hispanic patients with nominal p-values, overall and by ethnicity.
In the Hispanic cohort, the TCN2 polymorphism was univariately associated with EFS within the Hispanic patient cohort. In multivariable modeling, TCN2 was marginally associated with EFS (HR=3.15, p=0.05).