Abstract
Synaptic scaling represents a homeostatic adjustment in synaptic strength that was first identified as a cell-wide mechanism to achieve firing rate homeostasis after perturbations to spiking activity levels. In this review, we consider a form of synaptic scaling that is triggered by changes in action potential-independent neurotransmitter release. This plasticity appears to be both triggered and expressed locally at the dendritic site of the synapse that experiences a perturbation. A discussion of different forms of scaling triggered by different perturbations is presented. We consider work supporting this form of scaling, which we will call neurotransmission-based scaling from multiple groups. This class of homeostatic synaptic plasticity is compared in studies using hippocampal and cortical cultures, as well as in vivo work in the embryonic chick spinal cord. Despite differences in the tissues examined there are clear similarities in neurotransmission-based scaling, which appear to be molecularly distinct from the originally described spike-based scaling.
Keywords: miniature postsynaptic currents, spontaneous release, hippocampal cultures, cortical cultures, embryonic spinal cord
Introduction
Action potential-independent spontaneous release was once thought to represent a functionally unimportant release of synaptic vesicles, whose actual role was limited to spike dependent release. In the last few years it has become clear that spontaneous release and action potential dependent release are two different forms of transmission (Kavalali 2015). Recent work has demonstrated that spontaneous release is important for several aspects of nervous system development, including setting synaptic strength through a form of plasticity known as homeostatic synaptic scaling. Almost 20 years ago, a fundamentally important series of observations were published. They demonstrated that when spike activity was blocked for days in cultured cortical networks, neurons responded in what appeared to be a compensatory direction by increasing the strength of all their excitatory synaptic inputs, as measured by miniature excitatory postsynaptic current (mEPSC) amplitude (Turrigiano 2012; Turrigiano et al. 1998). This increase occurred across the entire distribution of mEPSC amplitudes in a multiplicative or “scaled” manner (e.g. all amplitudes became twice as large); in this way relative differences in synaptic strength achieved through Hebbian means were maintained. This initial study showed that 2-day block of either action potentials with TTX or AMPA receptor activation (AMPAergic transmission) with CNQX triggered upward scaling which was mediated by an increase in the postsynaptic sensitivity to glutamate. These initial results have proven extremely robust, as they have been identified in hippocampal primary cultures and slices, spinal cultures, as well as many other tissues in vitro and in vivo (Deeg and Aizenman 2011; Goel et al. 2006; Gonzalez-Islas and Wenner 2006; O’Brien et al. 1998; Thiagarajan et al. 2002). Scaling has been proposed to act to contribute to the homeostatic maintenance of spiking levels, which is thought to be triggered by reduced spiking in order to restore the spike rate to a set point. However, the picture has become more complicated as recent observations demonstrate that scaling can be triggered by altering action potential (AP)-independent spontaneous release. We will now discuss these reports as they relate to divergent experimental systems.
Hippocampal neurons demonstrate transmission-based scaling
Blockade of NMDA miniature currents trigger scaling
By far the most work on scaling triggered by reducing action potential-independent miniature postsynaptic currents (miniature transmission) has been carried out in hippocampal cultures. Three different labs have demonstrated that reducing NMDA receptor (NMDAR) activation due to AP-independent spontaneous release of glutamatergic vesicles triggers scaling (Aoto et al. 2008; Reese and Kavalali 2015; Sutton et al. 2006). The studies consistently suggested that spontaneous release of glutamate opened NMDARs leading to local calcium elevations which normally inhibited translation of GluA1 through eEF2K (Eukaryotic Elongation Factor 2 Kinase) and prevented synaptic upscaling. When this signaling cascade was interrupted GluA2-lacking (GluA1-containing) AMPARs were inserted into the synaptic membrane and mediated upscaling.
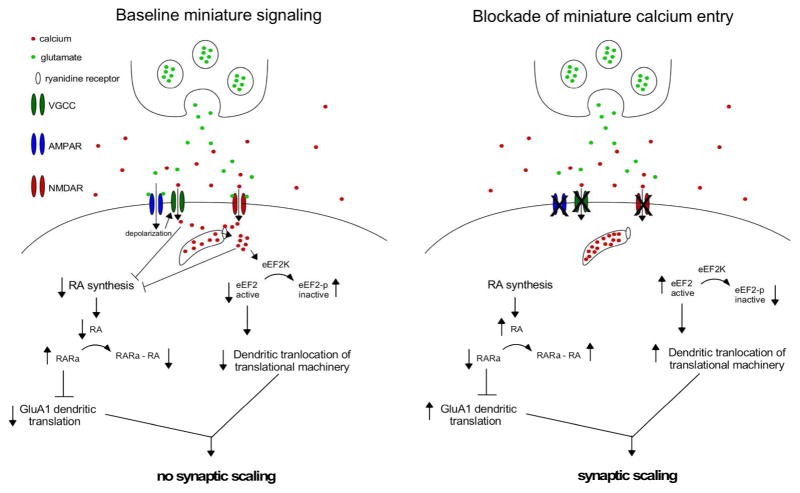
Initial studies by Erin Schuman and Michael Sutton showed that scaling could be triggered in just 4 hrs by blocking spiking activity and NMDAR’s with TTX and an NMDAR antagonist (APV) or just APV, while 4hr blockade of spiking alone (TTX) did not trigger scaling (Jakawich et al. 2010; Sutton et al. 2006; Sutton et al. 2007). Therefore, scaling was only triggered when NMDAergic miniature postsynaptic currents (mPSCs) were blocked. By puffing different drugs locally on a part of the dendrite the authors went on to show that this fast form of synaptic compensation was actually a process that occurred locally in the dendrite. When NMDA neurotransmission was blocked calcium entry was reduced, subsequently reducing Ca2+-calmodulin activation of eEF2K leading to the activation of eEF2 and thereby enhancing dendritic translation (Figure 1) (Sutton et al. 2007). Therefore, blocking miniature-induced calcium entry enhanced local translation leading to an increase in synaptic strength. The studies demonstrated that this GluA1-mediated scaling could also be triggered in primary hippocampal slices, and that such scaling was also observed over longer incubations of TTX/APV (24+ hrs). The results were particularly important because they suggested that synaptic compensations could be triggered by blocking NMDA miniature events, and that this local synaptic homeostasis could take the form of global cell-wide scaling if all NMDA miniature events in a cell or cultured network were blocked.
Figure 1.
Models describing the possible molecular pathways that mediate neurotransmission-based scaling in hippocampal cultures. The normal signaling cascades that operate when miniature transmission is intact and AMPAergic upscaling is not triggered are shown on the left. When calcium entry due to miniature neurotransmission is prevented from entering the cell AMPAergic upscaling is triggered and the molecular pathways are shown on the right.
Lu Chen’s group has also demonstrated upward scaling in hippocampal primary cultures and organotypic hippocampal slices following 24–36hr blockade of NMDA miniature transmission (TTX + APV, (Aoto et al. 2008)). Several advances were made in these studies as a previously unrecognized pathway was found to be responsible for the upward scaling (Figure 1). The work suggested that reductions in calcium entry due to NMDAergic mPSCs disinhibited retinoic acid (RA) synthesis through calcineurin (Aoto et al. 2008; Arendt et al. 2015). Increases in RA lead to scaling by binding to its receptor, RARα that normally acts to prevent translation of GluA1 mRNA (Poon and Chen 2008). Another critical insight from these studies was the demonstration that FMRP (Fragile X mental retardation protein) was necessary for this form of AMPAergic upward scaling (Soden and Chen 2010).
Reducing spontaneous glutamate vesicle release triggers scaling
The studies above assume that this form of upward scaling is mediated by the blockade of calcium entry through NMDARs that occur due to spontaneous glutamatergic vesicle release. These would be rather small calcium signals, and it was not completely clear how such small signals could impact calcium signaling in the cell. A third group (Ege Kavalali) has elegantly demonstrated how such a signaling cascade could occur mechanistically. This group was able to show that NMDA miniature transmission produced discernible calcium signals which were dependent on ryanodine-sensitive calcium-induced calcium release (Reese and Kavalali 2015), which amplified the calcium signal (Figure 1). Normally such signaling suppressed scaling through activation of the eEF2K. Blockade of ryanodine receptors and the associated calcium transients triggered scaling, and this was prevented in eEF2K KO neurons (Reese and Kavalali 2015). In addition, the earlier work argues that glutamatergic spontaneous release is critical to the triggering of upward scaling, at least when activity is coincidentally blocked. This idea was consistent with the possibility that reducing spontaneous glutamatergic release alone (without changes in evoked release or spiking) could trigger scaling. It has now been demonstrated in 2 recent reports that AMPAergic upward scaling can be triggered by specifically reducing the frequency of glutamate spontaneous release, while leaving evoked release intact (Crawford et al. 2017; Ramirez et al. 2017). These studies used a genetic knock down strategy where the expression of 2 different sets of synaptic vesicle proteins (VAMP7/vti1a or Doc2-like protein family) were reduced selectively lowering mEPSC frequency for days and triggering an upward scaling. Further, upscaling triggered in this way was dependent on eEF2K.
AMPAergic upscaling triggered by different methods
The discussion thus far has focused on upward scaling triggered by blockade of NMDA miniature transmission. However, upward AMPAergic scaling has also been triggered by blocking AMPAergic miniature transmission (TTX + AMPAR antagonists CNQX or NBQX). Like NMDA miniature transmission, AMPAergic miniature blockade triggered scaling at 3 and 24+ hours of drug treatment, and was again mediated by GluA2-lacking AMPARs (Jakawich et al. 2010; Sutton et al. 2006). It is not completely clear how reduced AMPA miniature transmission would signal upward scaling since it is not thought that significant calcium entry would occur through basal AMPARs. However, it is possible that blocking the depolarizations associated with quantal activation of AMPARs is responsible; such depolarizations could be important in NMDAR activation, or by activating L-type voltage gated calcium channels as two separate studies have shown that blocking these channels triggered upward AMPAergic scaling (Thiagarajan et al. 2005; Wang et al. 2011). Therefore, it is possible that calcium entry directly through NMDARs and L-type calcium channels activated by AMPAR depolarizations could normally act to prevent upward scaling, and reductions in calcium entry through either route could trigger scaling (Figure 1).
In addition, several studies have demonstrated that simply blocking AMPARs with antagonists in hippocampal cultures, without spike blockade, also triggered scaling. AMPAR antagonists by themselves triggered what appears to be the same form of scaling as triggered by blocking AMPA/NMDA miniature transmission or by reducing glutamate vesicle release, since AMPAR antagonist application also triggered scaling at 3 and 24+ hours of treatment; likewise, all of these treatments led to scaling that was mediated by GluA2-lacking AMPARs, and was dependent on RA synthesis (Jakawich et al. 2010; Thiagarajan et al. 2005; Thiagarajan et al. 2002; Wang et al. 2011).
While there are differences in these studies across labs, it is more impressive how well the experimental results agree. Taken together, the most parsimonious explanation for upward AMPAergic synaptic scaling in hippocampal tissue is that reductions in calcium entry due to reduced miniature NMDAR or AMPAR transmission trigger a cascade in the postsynaptic cell that is proposed in Figure 1. In this model upward AMPAergic scaling is triggered by reductions in miniature calcium entry, which would occur following chronic reductions in glutamate vesicle release or reductions in miniature activation of NMDARs or AMPARs. Reduced dendritic calcium would lead to activation of eEF2 which is important for elongation during protein translation (Ryazanov et al. 1988) and also would be necessary to disinhibit translation of GluA1 mRNA in the dendrite (Poon and Chen 2008). In this way, preventing either protein elongation or disinhibition of GluA1 translation would inhibit scaling. We are proposing that any of these perturbations to miniature transmission trigger a similar form of transmission-based scaling. On the other hand, AMPAergic upscaling that is triggered by spike blockade alone (TTX) appears to be a different form of scaling (Turrigiano 2012). TTX-based scaling typically takes 24–48 hours to be expressed, is not dependent on FMRP or RA synthesis, is mediated by changes in transcription rather than translation, and involves increases in GluA2-containing AMPARs (Ibata et al. 2008; Soden and Chen 2010; Sutton et al. 2006; Turrigiano 2011; Wang et al. 2011). The findings raise the probability that there will be many different molecular components and pathways that are necessary for the appropriate expression of scaling.
Cortical primary cultures
Thus far all the work on miniature transmission-based scaling that we have discussed has been observed in hippocampal cultures. How widespread is this phenomenon? There is a multitude of evidence that upward AMPAergic scaling can be triggered by chronic blockade of AMPAergic transmission. This has been shown in the original study when 2-day CNQX treatment triggered scaling of cortical cultured neurons (Turrigiano et al. 1998), but has also been seen in several other studies (O’Brien et al. 1998; Stellwagen and Malenka 2006). Because scaling that is triggered by glutamatergic receptor blockade appears to be similar to scaling triggered by blocking miniature transmission in the hippocampus, it is very possible that this similarity carries over to other parts of the nervous system. In fact, we have recently shown in cortical cultures that the scaling up produced by 24hr spike blockade can be attenuated by increasing AP-independent spontaneous neurotransmission (Fong et al. 2015). We did this by co-applying TTX and cyclothiazide, which produced an acute increase of mEPSC amplitude and frequency in the presence of spike blockade. This result suggested that miniature transmission influences AMPAergic scaling in cortical cultures as well as hippocampal cultures.
In vivo embryonic spinal cord
Is there evidence that reductions in spontaneous neurotransmission can produce scaling in vivo? We have just published results demonstrating that altering miniature GABAA transmission can trigger both up and down scaling in the embryonic spinal cord in vivo (Gonzalez-Islas et al. 2016). GABA is depolarizing and excitatory in embryonic spinal neurons and appears to assume the role of glutamate in scaling at later stages of development when GABA is not excitatory (Ben-Ari et al. 2012; Chub et al. 2006; Chub and O’Donovan 1998; Gonzalez-Islas et al. 2009). We had initially shown that infusing a voltage gated Na+ channel blocker (lidocaine) was able to reduce spinally driven embryonic movements, and when done over a 2 day period, spinal motoneurons experienced a scaling up of AMPAergic mPSCs, as well as depolarizing GABAergic mPSCs (Gonzalez-Islas and Wenner 2006). AMPAergic scaling up was achieved through the insertion of GluA2-lacking AMPARs, similar to the neurotransmission dependent scaling described in cultured networks (Garcia-Bereguiain et al. 2013). On the other hand GABAergic upscaling was mediated by altering the driving force for these currents through intracellular chloride accumulation (Gonzalez-Islas et al. 2010).
Lidocaine treatment blocked embryonic movements, which did not recover over the 2-day infusion. However, blockade of either GABARs or glutamate receptors only initially blocked embryonic movements, which then homeostatically recovered to control levels after 12 hours of drug treatment. Consistent with the idea that scaling in the embryonic spinal cord was a transmission-based plasticity, we found that blocking GABAergic, but not glutamatergic transmission for 2 days in vivo was also capable of triggering upward scaling of AMPA and GABA mPSCs (Wilhelm and Wenner 2008). This form of plasticity appeared to be similar to the lidocaine-triggered scaling up as the mechanisms of scaling were the same: insertion of GluA2-lacking AMPARs (Garcia-Bereguiain et al. 2013) and chloride accumulation (Lindsly et al. 2014).
We had assumed that GABAergic transmission associated with the bouts of spiking activity were key to the scaling process, however this turned out to be incorrect. We made the striking discovery that we could trigger upward scaling to the same extent (amplitude) as complete GABAA receptor blockade by mildly reducing the frequency of action potential independent GABA vesicle release (Garcia-Bereguiain et al. 2016; Gonzalez-Islas et al. 2015). This was done in vivo by presynaptically manipulating spontaneous GABA release with nicotinic agents. Further arguing the importance of miniature GABAergic neurotransmission, we found that increasing spontaneous GABA vesicle release was capable of triggering a downscaling of AMPA and GABA mPSC amplitude. In fact, when we blocked spiking with lidocaine while increasing GABA spontaneous release, we converted the upscaling observed with lidocaine alone, to downscaling. These results demonstrated the importance of GABAergic spontaneous release in triggering upscaling and downscaling of AMPA and GABA mPSCs in the living system.
The studies in the embryonic chick spinal cord represents the first demonstration that reducing miniature transmission can trigger an upscaling of GABAergic mPSCs. However, previous work has shown that blocking the miniature NMDAergic signaling cascade mediated by retinoic acid can trigger GABAergic downscaling (Sarti et al. 2013). While these 2 studies may appear to be distinct, reducing miniature transmission functionally leads to an increase in excitability in both systems as one increases quantal GABAergic depolarizations (embryo) and the other decreases quantal GABAergic inhibition (hippocampal culture). It should be noted that a third study has shown that reducing spontaneous release of both GABA and glutamate did not lead to GABAergic scaling in hippocampal cultures (Ramirez et al. 2017).
In embryonic spinal neurons GABA is depolarizing and so it will be important to determine if GABAergic mPSCs produce depolarizations that are capable of opening voltage-gated calcium channels which then trigger similar cascades to those that mediate transmission-based scaling in cultured networks. It is possible that GABA mPSCs can signal these pathways early in development when they are depolarizing, but that as the system matures and GABA becomes hyperpolarizing, glutamatergic mPSCs may take over the role of GABAergic mPSCs in triggering scaling. The results also provide a cautionary tale in terms of the potential impact of any genetic disorders or pharmacological exposures to the developing nervous system that may alter GABAergic spontaneous release (e.g. nicotine).
Conclusions
The recent findings that link spontaneous release to transmission-based synaptic scaling are robust and convincing. It is perhaps surprising that miniature transmission, once thought to be functionally unimportant, can have such a profound influence on shaping synaptic strength and maturation. Complete blockade of miniature transmission would not have been thought of as a significant perturbation several years ago, but what is even more striking is that these profound changes in the basic unit of synaptic strength can be triggered by merely altering the frequency of miniature events. Together with earlier studies showing that transmission-based scaling can be a synapse specific process, we consider the possibility that this form of plasticity functions more for a homeostatic maintenance of synaptic strength than for a homeostatic maintenance of spiking activity, as may be the case for TTX-based scaling. Neurotransmission-based scaling might be a process that homeostatically maintains mPSC frequency as a measure of postsynaptic sensitivity, such that when the frequency is reduced the postsynaptic cell interprets this as a sign that amplitudes are too small and are dropping below detection. To correct this and ensure an appropriate postsynaptic sensitivity the synaptic inputs are scaled up until the detected frequency is restored. This process might be particularly important during development when cells and dendrites get larger and leakier, such that the same currents will be less effective at depolarizing the postsynaptic dendrite. Regardless, it is becoming clear that moderate changes in miniature transmission have an important influence on synaptic strength, and this has been described in preparations as diverse as rodent hippocampal primary cultures and chick embryonic spinal motoneurons in vivo.
Moving forward it will be important to determine if neurotransmission-based scaling is observed in other in vivo systems and to identify whether the molecular mechanisms that have been described in vitro are also involved in vivo. It is clear that there are differences in the embryonic spinal cord and cultured cortical/hippocampal networks as the triggering neurotransmitter is different, but are there differences in the signaling pathways downstream to neurotransmitter receptor activation? It appears that TTX-dependent scaling and neurotransmission-dependent scaling are distinct phenomena and it will be important to determine if neurotransmission-dependent scaling could also represent multiple classes of scaling. Finally, whatever different forms of scaling exist, including up and down scaling, the most critical advances will likely come in terms of identifying the functional goals of each of these distinct forms of scaling.
Significance Statement.
Homeostatic synaptic scaling is thought to prevent inappropriate levels of spiking activity through compensatory adjustments in the strength of synaptic inputs. Therefore, it is thought that scaling is triggered by perturbations in spiking. In this review, we discuss a form of synaptic scaling that is triggered in an action potential-independent manner, through alterations in the frequency of miniature postsynaptic currents once thought to be functionally unimportant. The results raise the possibility that profound changes in synaptic strength, and therefore network excitability, could be influenced by any chronic disruption of spontaneous neurotransmitter release.
Acknowledgments
Grant support - NIH, NINDS - R01NS065992, NIH, NINDS - R21NS084358
Footnotes
The authors declare no conflict of interest.
All Authors contributed to the writing of the manuscript.
Bibliography
- Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60(2):308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt KL, Zhang Z, Ganesan S, Hintze M, Shin MM, Tang Y, Cho A, Graef IA, Chen L. Calcineurin mediates homeostatic synaptic plasticity by regulating retinoic acid synthesis. Proc Natl Acad Sci U S A. 2015;112(42):E5744–5752. doi: 10.1073/pnas.1510239112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Woodin MA, Sernagor E, Cancedda L, Vinay L, Rivera C, Legendre P, Luhmann HJ, Bordey A, Wenner P, Fukuda A, van den Pol AN, Gaiarsa JL, Cherubini E. Refuting the challenges of the developmental shift of polarity of GABA actions: GABA more exciting than ever! Front Cell Neurosci. 2012;6:35. doi: 10.3389/fncel.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chub N, Mentis GZ, O’Donovan MJ. Chloride-sensitive MEQ fluorescence in chick embryo motoneurons following manipulations of chloride and during spontaneous network activity. J Neurophysiol. 2006;95(1):323–330. doi: 10.1152/jn.00162.2005. [DOI] [PubMed] [Google Scholar]
- Chub N, O’Donovan MJ. Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. J Neurosci. 1998;18(1):294–306. doi: 10.1523/JNEUROSCI.18-01-00294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Ramirez DM, Trauterman B, Monteggia LM, Kavalali ET. Selective molecular impairment of spontaneous neurotransmission modulates synaptic efficacy. Nat Commun. 2017;8:14436. doi: 10.1038/ncomms14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg KE, Aizenman CD. Sensory modality-specific homeostatic plasticity in the developing optic tectum. Nat Neurosci. 2011;14(5):548–550. doi: 10.1038/nn.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong M-f, Newman JP, Potter SM, Wenner P. Upward synaptic scaling is dependent on neurotransmission rather than spiking. Nature Communications. 2015 doi: 10.1038/ncomms7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bereguiain MA, Gonzalez-Islas C, Lindsly C, Butler E, Hill AW, Wenner P. In Vivo Synaptic Scaling Is Mediated by GluA2-Lacking AMPA Receptors in the Embryonic Spinal Cord. J Neurosci. 2013;33(16):6791–6799. doi: 10.1523/JNEUROSCI.4025-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bereguiain MA, Gonzalez-Islas C, Lindsly C, Wenner P. Spontaneous Release Regulates Synaptic Scaling in the Embryonic Spinal Network In Vivo. J Neurosci. 2016;36(27):7268–7282. doi: 10.1523/JNEUROSCI.4066-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci. 2006;9(8):1001–1003. doi: 10.1038/nn1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Chub N, Garcia-Bereguiain MA, Wenner P. GABAergic synaptic scaling in embryonic motoneurons is mediated by a shift in the chloride reversal potential. J Neurosci. 2010;30(39):13016–13020. doi: 10.1523/JNEUROSCI.1659-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Chub N, Wenner P. NKCC1 and AE3 appear to accumulate chloride in embryonic motoneurons. J Neurophysiol. 2009;101(2):507–518. doi: 10.1152/jn.90986.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Garcia-Bereguiain MA, O’Flaherty B, Wenner P. Tonic nicotinic transmission enhances spinal GABAergic presynaptic release and the frequency of spontaneous network activity. Dev Neurobiol. 2015 doi: 10.1002/dneu.22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Garcia-Bereguiain MA, O’Flaherty B, Wenner P. Tonic nicotinic transmission enhances spinal GABAergic presynaptic release and the frequency of spontaneous network activity. Dev Neurobiol. 2016;76(3):298–312. doi: 10.1002/dneu.22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Wenner P. Spontaneous Network Activity in the Embryonic Spinal Cord Regulates AMPAergic and GABAergic Synaptic Strength. Neuron. 2006;49(4):563–575. doi: 10.1016/j.neuron.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57(6):819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Jakawich SK, Nasser HB, Strong MJ, McCartney AJ, Perez AS, Rakesh N, Carruthers CJ, Sutton MA. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron. 2010;68(6):1143–1158. doi: 10.1016/j.neuron.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci. 2015;16(1):5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- Lindsly C, Gonzalez-Islas C, Wenner P. Activity Blockade and GABAA Receptor Blockade Produce Synaptic Scaling through Chloride Accumulation in Embryonic Spinal Motoneurons and Interneurons. PLoS One. 2014;9(4):e94559. doi: 10.1371/journal.pone.0094559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21(5):1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Poon MM, Chen L. Retinoic acid-gated sequence-specific translational control by RARalpha. Proc Natl Acad Sci U S A. 2008;105(51):20303–20308. doi: 10.1073/pnas.0807740105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DMO, Crawford DC, Chanaday NL, Trauterman B, Monteggia LM, Kavalali ET. Loss of Doc2-Dependent Spontaneous Neurotransmission Augments Glutamatergic Synaptic Strength. J Neurosci. 2017;37(26):6224–6230. doi: 10.1523/JNEUROSCI.0418-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese AL, Kavalali ET. Spontaneous neurotransmission signals through store-driven Ca transients to maintain synaptic homeostasis. eLife. 2015:4. doi: 10.7554/eLife.09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryazanov AG, Shestakova EA, Natapov PG. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988;334(6178):170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- Sarti F, Zhang Z, Schroeder J, Chen L. Rapid suppression of inhibitory synaptic transmission by retinoic acid. J Neurosci. 2013;33(28):11440–11450. doi: 10.1523/JNEUROSCI.1710-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci. 2010;30(50):16910–16921. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125(4):785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic Decoding of Neural Activity: eEF2 as a Biochemical Sensor Coupling Miniature Synaptic Transmission to Local Protein Synthesis. Neuron. 2007;55(4):648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47(5):725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Piedras-Renteria ES, Tsien RW. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36(6):1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4(1):a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391(6670):892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Wang HL, Zhang Z, Hintze M, Chen L. Decrease in calcium concentration triggers neuronal retinoic acid synthesis during homeostatic synaptic plasticity. J Neurosci. 2011;31(49):17764–17771. doi: 10.1523/JNEUROSCI.3964-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JC, Wenner P. GABAA transmission is a critical step in the process of triggering homeostatic increases in quantal amplitude. Proc Natl Acad Sci U S A. 2008;105(32):11412–11417. doi: 10.1073/pnas.0806037105. [DOI] [PMC free article] [PubMed] [Google Scholar]