Abstract
Background
Despite several new medications being FDA-approved for overactive bladder (OAB) and new prescription drug payment programs, there are limited population-based data regarding OAB medication use among older adults.
Objectives
To examine (1) impacts of new medications and $4 generic programs on time trends for OAB-related medication dispensing for older adults in the United States, (2) differences by age and gender, and (3) temporal changes in OAB-related medication payments.
Methods
Using Truven Health Analytics’ Medicare Supplemental Database (2000–2015), we analyzed OAB-related medication claims for 9,477,061 Medigap beneficiaries age 65–104. We estimated dispensing rates (per 1000 person-months), assessed dispensing trends using interrupted time-series methods, compared dispensing rates by age and gender, and summarized payment trends.
Results
From 2000–2015, 771,609 individuals filled 13,863,998 OAB-related prescriptions. During 2000–2007, three new extended-release medications became available (tolterodine, darifenacin, solifenacin), leading to increases in overall OAB-related dispensing rates by 19.1 (99% confidence interval, CI: 17.0–21.2), a 92% increase since 2000; overall rates remained stable during 2008–2015. By 2015, the most common medications were oxybutynin (38%), solifenacin (20%), tolterodine (19%), and mirabegron (12%). Dispensing rates peaked at age 90 (rate: 53.4, 99% CI: 53.1–53.7). Women had higher rates than men at all ages (average ratewomen-ratemen: 22.0). The gap between upper and lower percentiles of medication payments widened between 2008–2015; by 2015, 25% of reimbursed dispensed prescriptions had total payments exceeding $250.
Conclusions
Medication-specific dispensing rates for OAB changed when new alternatives became available. Recent changes in utilization and cost of OAB medications have implications for clinical guidelines, pharmaco-epidemiologic studies and payment policies.
Keywords: health services research, databases, incontinence, geriatrics, prescription drugs
INTRODUCTION
Overactive bladder syndrome (OAB) is defined as urinary urgency, which can occur with or without urinary incontinence, and often occurs with frequency or nocturia.1,2 These OAB-related symptoms negatively impact quality of life,3,4 affect both women (13%–17%) and men (11%–16%), and are most prevalent in older adults.3–5
First-line pharmacotherapy for OAB includes antimuscarinics. Two prominent antimuscarinics are oxybutynin and tolterodine, FDA-approved in 1975 and 1998, respectively.6,7 More recently, several new antimuscarinics (trospium, solifenacin, darifenacin, fesoterodine) and one β3-adrenergic-agonist (mirabegron) were FDA-approved for OAB7 (Table S1.1, Supplemental Digital Content 1). These new medications have recently granted patients the possible availability of alternative pharmacotherapeutic options for OAB. Additionally, in 2006, large nationwide retail pharmacy chains introduced $4 generic programs offering $4/$10 prices for 30/90 days’ supply of >200 generic prescription medications, including immediate-release (IR) oxybutynin tablets.8–10 Subsequently, the costs of OAB therapy have changed over time, coinciding with new payment plans designed to increase patients’ access to medication.
Nationally representative cross-sectional data from 2009–2010 showed concentrated OAB-related prescribing in older adults, mostly for oxybutynin, tolterodine, and solifenacin.11,12 However, no published data describe OAB-related time trends for utilization or payments, and no studies are recent enough to have assessed mirabegron. Data are needed regarding the impact of these population-level changes (i.e., newly available medications, payment plans) on OAB-related prescribing and medication payments. Furthermore, the absence of studies in older adults is particularly concerning, given the potential risks due to anticholinergic properties of antimuscarinics (e.g., cognition, constipation).13–18
In this study of older adults in the United States (U.S.), we sought to examine: (1) time trends for dispensing rates of OAB-related prescriptions, potentially brought about by newly emerging OAB medications and $4 generic programs; (2) dispensing rate differences by age and gender; and (3) time trends in beneficiary and insurer payments for OAB medications.
METHODS
Setting and participants
Data for this analysis were drawn from Truven Health Analytics’ Medicare Supplemental Database (©2017 Truven Health Analytics Inc., all rights reserved), which contain de-identified individual-level enrollment and administrative healthcare claims data for inpatient, outpatient, and prescription drug services in the U.S. from 2000–2015, and have been adjudicated and validated by Truven Health.19,20 These data include Medicare- and employer-covered portions of healthcare claims for individuals enrolled in Medicare who receive Medicare Supplemental insurance (henceforth, “Medigap”) from their employer or former employer, aggregated from over 300 large employers across the U.S.19,20 We restricted this analysis to individuals age 65-104 years.
We analyzed enrollment data and claims for dispensed prescriptions for seven different types of IR and extended-release (ER) medications for OAB: oxybutynin, tolterodine, trospium, darifenacin, solifenacin, fesoterodine, and mirabegron. We identified National Drug Codes for these medications by searching their generic names and ATC codes in the National Drug Data File®-NDDF Plus (First Databank, http://www.firstdatabank.com/) and Red Book data (Truven Health Analytics Inc.).
Measures
The primary measure of interest was the dispensing rate (henceforth, “rate”) for each OAB medication. The numerator of the rate was the number of dispensed prescriptions, where we defined one “prescription” as a 1-month (i.e., 30-day) supply of medication; the denominator of the rate was the number of person-months (equal to 30 person-days) of Medigap prescription drug coverage (footnote, Table 2).
TABLE 2.
Population Characteristics, Crude Dispensing Rates, and Payments for Dispensed Medications for Overactive Bladder among Adults Age 65–104 in the United States, 2000–2015
| Person-months* (%) (total = 421,122,388) | Dispensing rates per 1000 person-months† (SE)‡ by medication group
|
Median payment per prescription, $ (IQR) ¶
|
||||
|---|---|---|---|---|---|---|
| All OAB medications | IR§ only | ERǁ only | Beneficiary# | Total** | ||
| Year | ||||||
| 2000–2003 | 54,964,664 (13.1) | 28.6 (0.02) | 10.5 (0.01) | 18.2 (0.02) | 14 (8–24) | 118 (105–123) |
| 2004–2007 | 111,662,629 (26.5) | 33.8 (0.02) | 6.6 (0.01) | 27.2 (0.02) | 14 (7–28) | 119 (106–126) |
| 2008–2011 | 134,665,751 (32.0) | 34.7 (0.02) | 5.1 (0.01) | 29.7 (0.01) | 17 (6–30) | 134 (89–146) |
| 2012–2015 | 119,829,345 (28.5) | 32.1 (0.02) | 5.8 (0.01) | 26.2 (0.01) | 11 (5–32) | 155 (44–202) |
| Gender | ||||||
| Female | 234,209,076 (55.6) | 43.3 (0.01) | 8.5 (0.01) | 34.8 (0.01) | 14 (6–29) | 123 (86–145) |
| Male | 186,913,313 (44.4) | 19.9 (0.01) | 3.7 (0.00) | 16.2 (0.01) | 14 (6–29) | 123 (82–146) |
| Age (years) | ||||||
| 65–69 | 122,804,616 (29.2) | 20.8 (0.01) | 3.7 (0.01) | 17.1 (0.01) | 17 (6–29) | 123 (84–145) |
| 70–74 | 100,060,259 (23.8) | 27.7 (0.02) | 5.2 (0.01) | 22.5 (0.02) | 16 (6–28) | 122 (87–144) |
| 75–79 | 83,311,563 (19.8) | 36.3 (0.02) | 7.0 (0.01) | 29.3 (0.02) | 16 (6–28) | 122 (89–143) |
| 80–84 | 62,677,427 (14.9) | 45.2 (0.03) | 9.0 (0.01) | 36.2 (0.02) | 17 (6–29) | 123 (88–145) |
| 85–89 | 35,650,223 (8.5) | 51.8 (0.04) | 10.6 (0.02) | 41.1 (0.03) | 18 (6–30) | 124 (82–149) |
| 90–94 | 13,311,618 (3.2) | 52.2 (0.06) | 11.8 (0.03) | 40.5 (0.06) | 18 (6–31) | 124 (74–152) |
| 95–99 | 2,923,504 (0.7) | 46.1 (0.13) | 11.1 (0.06) | 34.9 (0.11) | 18 (6–31) | 124 (72–152) |
| 100–104 | 383,179 (0.1) | 32.0 (0.29) | 7.4 (0.14) | 24.6 (0.26) | 20 (6–31) | 125 (79–156) |
| Region | ||||||
| Midwest†† | 141,213,642 (33.5) | 37.9 (0.02) | 6.8 (0.01) | 31.1 (0.01) | 11 (5–25) | 121 (93–140) |
| Northeast‡‡ | 64,934,286 (15.4) | 28.7 (0.02) | 4.6 (0.01) | 24.1 (0.02) | 16 (5–30) | 128 (91–157) |
| South§§ | 122,473,508 (29.1) | 33.0 (0.02) | 5.3 (0.01) | 27.8 (0.02) | 18 (7–33) | 125 (99–147) |
| Westǁǁ | 89,602,283 (21.3) | 28.3 (0.02) | 8.6 (0.01) | 19.7 (0.01) | 14 (6–28) | 119 (30–147) |
| Missing | 2,898,669 (0.7) | |||||
SE, standard error; $, United States dollar; IQR, interquartile range; OAB, overactive bladder; IR, immediate-release mechanism; ER, extended-release mechanism.
Person-months with Medigap prescription drug coverage.
Rate of dispensed prescriptions per 1000 person-months of Medigap prescription drug coverage, calculated as [(n days’ supply)/30]/[(N days’ drug coverage)/30]×1000.
Standard errors calculated assuming a Poisson distribution, as ((n days’ supply)/30)−½.
Immediate-release: includes some formulations of oxybutynin, tolterodine, and trospium (Table, Supplemental Digital Content 1).
Extended-release: includes some formulations of oxybutynin, tolterodine, and trospium, and all formulations of darifenacin, solifenacin, fesoterodine, and mirabegron (Table, Supplemental Digital Content 1).
Payments required for 30 days’ supply of medication, adjusted for inflation to the year 2015.24
Beneficiary payments: deductible, coinsurance, copayment, and coordination of benefits.
Total payments: beneficiary payments plus all post-discount payments by insurer.
Midwest: IL, IN, IA, KS, MI, MN, MO, NE, ND, OH, SD, WI.
Northeast: CT, ME, MA, NH, NJ, NY, PA, RI, VT.
South: AL, AR, DE, DC, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV.
West: AK, AZ, CA, CO, HI, ID, MT, NV, NM, OR, UT, WA, WY.
For each medication, we estimated stratum-specific rates per 1000 person-months by calendar time (2000–2015), gender (male/female), year-specific age (65–104), and geography, with 99% confidence intervals (CI) assuming a Poisson distribution.21,22 Geography was assessed using a 52-level variable (50 states, District of Columbia, Puerto Rico) and a 4-level variable (Midwest, Northeast, South, West Census regions).
In addition to rates, we assessed payments for dispensed OAB medications. For each claim, we calculated payments per prescription (i.e., 1-month supply) in U.S. dollars ($). We measured payments in two ways: (1) beneficiary payments (deductible, coinsurance, copayment, and coordination of benefits); and (2) total payments (beneficiary payments plus all post-discount payments by the insurer). We excluded claims for total payments ≤$0 (0.6%) and negative beneficiary payments (0.02%).23 To account for inflation during 2000–2015, payments were adjusted to dollar amounts in the year 2015, using the “Medical care” expenditure category of the Consumer Price Index.24
Interrupted time-series analysis of dispensing rates
To estimate time trends in dispensing for each medication, we conducted an interrupted time-series analysis,25,26 which is a common quasi-experimental method to assess impacts of policy changes or other population-level changes. Below, we describe our approach in detail.
First, we defined 52 weeks in each year based on 7-day increments. Second, for each week from 2000–2015 (n=832 weeks), we estimated the dispensing rate22 for all medications combined and separately for each medication. To control for year-to-year variation in the geographic distribution and types of insurance plans included in Truven’s Medigap database, we estimated standardized rates22,27 for each week, which rendered time trends independent of sampling artifacts in the database (Text, Supplemental Digital Content 2).
Third, we identified twelve interruptions during 2000–2015 when new medications or payment plans were introduced at the population level in the U.S., which could have altered dispensing of medications for OAB (Table 1). Some pre-specified interruptions were nearly concurrent; to reduce complexity and minimize spurious results, we consolidated interruptions occurring within 20 weeks (footnotes, Table 1). As a result, our primary interrupted time-series analysis explicitly considered seven interruption time points. In sensitivity analysis, we also assessed the additional impacts of Beers Criteria updates in 2003 and 201214,28 and Medicare Part D in 2006;29 we did not assess the most recent Beers criteria update in October 201515 due to insufficient data for modeling.
TABLE 1.
Interruption time points and hypothesized trend deflections for dispensing rates of prescription medications in older adults with overactive bladder in the United States
| Interruption | Date* | Description | Hypothesized trend deflection† |
|---|---|---|---|
| 1 | 01/11/2001 | Market entry: ER tolterodine‡ | ↓ Other IR (i.e., IR tolterodine) ↓ ER oxybutynin |
|
| |||
| 2 | 05/01/2003 | Market entry: ER oxybutynin transdermal patch‡ | ↑ ER oxybutynin ↓ ER tolterodine |
|
| |||
| 3§ | 01/05/2005 | Market entry: IR trospium, ER darifenacin, and ER solifenacin‡ | ↓ ER oxybutynin and ER tolterodine ↓ IR oxybutynin ↑ Other IR |
|
| |||
| 4‖ | 11/27/2006 | Nationwide $4 generic programs8–10 | ↓ IR oxybutynin ↓ Other IR |
|
| |||
| 5 | 12/31/2007 | Market entry: ER trospium‡ | ↓ for all other ER medications |
|
| |||
| 6¶ | 03/18/2009 | Market entry: ER fesoterodine and ER oxybutynin transdermal gel‡ | ↑ ER oxybutynin ↓ for all other ER medications |
|
| |||
| 7# | 10/05/2012 | Market entry: ER mirabegron‡; and FDA approval of over-the-counter oxybutynin transdermal patch for women6 | ↓ for all other ER medications |
| Main sensitivity analyses | |||
| 2s | 12/08/2003 | Beers criteria update28 | ↓ for all medications |
|
| |||
| 3s | 01/01/2006 | Medicare Part D went into effect29 | ↑ for all medications |
|
| |||
| 6s | 02/29/2012 | Beers criteria update15 | ↓ for all medications |
ER, extended-release mechanism; IR, immediate-release mechanism.
MM/DD/YYYY.
Trend deflections for each interruption were defined as the upward or downward change in the trend for the dispensing rate over time.
Market entry based on the earliest observed dispensing in this study based on available claims data from Truven Health Analytics’ Medicare Supplemental databases, 2000–2015.
Near-coincident timing of earliest observed claims for IR trospium (08/17/2004), darifenacin (01/05/2005), and solifenacin (01/09/2005); consolidated to the same interruption date, 01/05/2005.
$4 generic programs were first piloted (09/21/2006)8,9 and then scaled nationwide 10 weeks later (11/27/2006).10 To allow a lag for the completion of nationwide scaling, these interruptions were consolidated to the same interruption date, 11/27/2006.
Near-coincident timing of earliest observed claims for fesoterodine (03/18/2009) and ER oxybutynin transdermal gel (05/12/2009); consolidated to the same interruption date, 03/18/2009.
Near-coincident timing of earliest observed claims for mirabegron (10/05/2012) and FDA approval of over-the-counter oxybutynin transdermal patch for women (01/25/2013);6 They were consolidated to the same interruption date, 10/05/2012.
Finally, using ordinary least squares, we specified medication-specific segmented linear regression models for standardized dispensing rates to estimate the trend (and 99% CI) between every pair of neighboring interruptions. We controlled potential confounding by seasonality using a transformed cosine periodic function.30,31 To account for error autocorrelation over time, we used Durbin-Watson statistical tests32 (α=0.05) to specify autoregressive parameters in our models for lags up to 14 months. Our models did not include parameters for level changes between adjacent segments, based on our hypothesis that interruptions would gradually affect dispensing.33 For each medication that entered the market during the study period, in the week preceding its earliest observed claim in the database, we forced its intercept to zero.
Table 1 enumerates all of our specific a priori hypotheses for the interrupted time series analysis. Broadly, based on principles of innovation diffusion, we first hypothesized that new medications entering the market would lead to downward trend deflections (i.e., gradual changes over time) for other medications they might replace. We expected greater downward deflections for medications sharing the same release mechanism (e.g., solifenacin would induce negative deflections for other ER medications). Second, for new medications belonging to a larger group, we hypothesized upward trend deflections for that medication group. For example, ER-oxybutynin transdermal gel was introduced in 2009 after many prior formulations of ER-oxybutynin had long existed; we expected the addition of the transdermal gel formulation to increase treatment rates for ER-oxybutynin. After the introduction of $4 generic programs, we expected many IR oxybutynin tablets prescriptions to be paid for out-of-pocket by beneficiaries, leading Medicare data to lack complete data on IR oxybutynin dispensing; therefore, our third hypothesis was that $4 generic programs would be associated with decreasing trends in claims data for IR oxybutynin and other IR medications (tolterodine and trospium).
Age and gender differences
We used stratified analysis methods22 to estimate rate differences by age and gender for all medications combined, averaged over the study period. To account for correlation between age and gender, we report rate differences comparing age groups separately for women and men, and rate differences comparing women versus men separately by age. We explicitly did not estimate rate ratios because they depend on the referent rate, which was highly variable across age groups. We estimated 99% CIs using percentiles from 2000 bootstrap resamples.34
Payments analysis
We used percentiles to summarize beneficiary and total payments per prescription, and compared payments across calendar time, gender, age, and geography. Results for age and gender were averaged over the study period. To control for potential confounding by geography, we standardized payment distributions (Text, Supplemental Digital Content 2). Whereas we used interrupted time-series methods to assess explicit hypotheses related to OAB medication dispensing, our analysis of payments lacked hypotheses for specific time-related impacts, and is therefore exploratory in nature.
Data management and statistical analyses were conducted using SAS version 9.4 (SAS Institute, Inc.; Cary, North Carolina USA). Segmented regression models were implemented using SAS PROC AUTOREG and the %AR macro in SAS PROC MODEL, which enable the investigator to identify autoregressive parameters and obtain estimates with standard errors for any linear combination of parameters from a model with autocorrelated errors. Graphics were created using R version 3.3.2 (R Foundation for Statistical Computing; Vienna, Austria). This study was reviewed and exempted from ethics approval by the institutional review board at the University of North Carolina at Chapel Hill (10-0153).
RESULTS
During 2000–2015, we assessed 9,477,061 individuals age 65–104 over 421,122,388 person-months of Medigap prescription drug coverage; the 5th–25th–50th–75th–95th percentiles of years with Medigap coverage were 0.2-1.0-2.7-5.0-11.0. Approximately 1 in 12 older adults in this population filled at least one OAB-related prescription (771,609 individuals, 13,863,998 total OAB-related fills). Oxybutynin and tolterodine, the only OAB medications available until 2004,7 were the most common OAB medications during 2000–2015 (34% and 42% of dispensed prescriptions, respectively). By 2015, after several new medications became available, oxybutynin and tolterodine together accounted for 56% of dispensed prescriptions; solifenacin (20%) and mirabegron (12%) were also common. Total payments for OAB medications during 2000–2015 exceeded $1.6 billion, approximately 2% of all prescription drug spending in this population. Table 2 shows crude subgroup-specific rates and payment summaries.
Interrupted time-series analysis of dispensing rates
Figure 1 shows standardized dispensing rates for each OAB medication group, and seasonality-adjusted segmented trend estimates from our interrupted time-series analysis. Trend estimates were precise at α=0.01 (Table S3.1, Supplementary Digital Content 3). During 2000–2007, the new availability of ER-tolterodine, darifenacin, and solifenacin led to a 92% increase in overall OAB-related rates since 2000, by 19.1 prescriptions per 1000 person-months (99% CI: 17.0–21.2). While overall rates stabilized in later years (range 37–40 during 2008–2015), medication-specific time trends varied dynamically as more new medications became available. By 2015, however, medication-specific rates were more similar than at any prior time during the study period (range of five highest: 5.0–8.8 per 1000 person-months). Below, we briefly summarize segmented regression model results.
FIGURE 1.
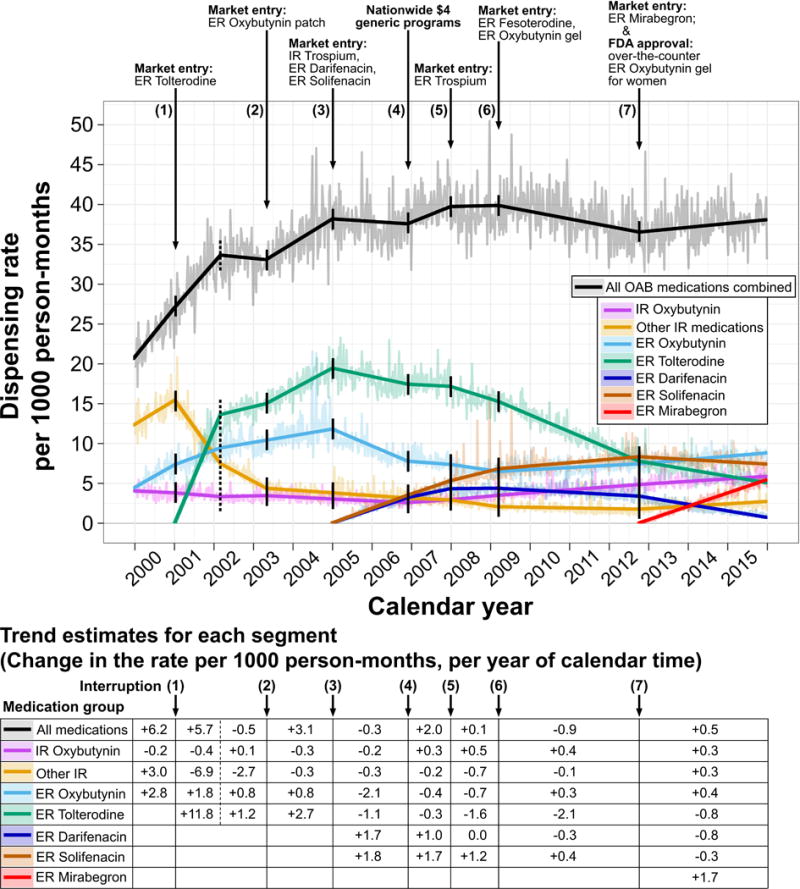
Segmented trends over calendar time for dispensing rates (per 1000 person-months) of prescription medications for overactive bladder (OAB), among adults age 65–104 in the United States, 2000–2015. Trend segments (bold lines) and pointwise rates (faded jagged lines) are shown for all OAB medications combined (black) and each medication separately (colors). Pointwise rates were standardized to control for annual variation in geography and insurance plans included in the database. Trend estimates are listed for each segment below the plot for the change in rate (per 1000 person-months) per year of calendar time; trends are adjusted for seasonality using a transformed cosine periodic function and account for serial error autocorrelation using autoregressive model parameters. Interruptions are indicated by downward arrows and solid vertical lines. To relax linearity assumptions for trends during 2001–2003, a post hoc hinge point was added at week 114, halfway between interruptions 1 and 2 (dotted vertical lines). Rates for extended-release trospium and fesoterodine never exceeded 2.5 per 1000 person-months; therefore, they are not shown separately in the figure but are included in rates for all OAB medications combined. IR, immediate-release; ER, extended-release.
At interruption 1 in 2001, ER-tolterodine market entry resulted in a sharp trend reversal for IR-tolterodine, leading to a rate decrease by 8.0 per 1000 person-months (99% CI: 7.3–8.6) during January 2001–March 2002; the ER-oxybutynin trend was stable through interruption 1. At interruption 3 in 2005 (market entry of darifenacin and solifenacin), prior positive trends became negative for ER-oxybutynin and ER-tolterodine; subsequently, rates (per 1000 person-months) decreased during January 2005–November 2006 by 4.0 (99% CI: 3.0–5.0) for ER-oxybutynin and 2.0 (99% CI: 0.9–3.2) for ER-tolterodine.
Contrary to our hypothesis for interruption 4 (in 2006) that $4 generic programs would negatively deflect trends for IR oxybutynin and other IR medications, trends for IR oxybutynin nominally deflected upward, while other-IR trends were unaffected.
At interruption 6 in 2009 (market entry of ER-oxybutynin transdermal gel), the trend for ER-oxybutynin – which had been negative since 2005 – became positive, as rates increased through 2012 by 1.0 per 1000 person-months (99% CI: 0.3–1.7). Mirabegron market entry occurred at interruption 7 in October 2012; trends deflected downward for solifenacin and darifenacin, while mirabegron rates increased to 5.4 per 1000 person-months (99% CI: 5.3–5.6) by December 2015.
Other hypothesized interruptions (i.e., newly available ER-oxybutynin patches, IR/ER-trospium, ER-fesoterodine) had negligible impacts on dispensing of other medications (Figure 1). A sensitivity analysis, which added three new interruptions for Beers criteria updates and Medicare Part D to the original seven interruptions in the primary analysis, had minor impacts on time trends for each medication group (Figure S4.1, Supplemental Digital Content 4).
Age and gender differences
Age-specific rates ranged from 19.0–53.4 prescriptions per 1000 person-months. For both men and women, dispensing rates for OAB increased with increasing age (Figure 2), peaking at age 90 (53.4 per 1000 person-months; 99% CI: 53.1–53.7). Past age 90, rates declined steadily with increasing age among both women and men. Rate differences between women and men were 22.0 per 1000 person-months on average; however, rate differences varied across the age range, increasing from 19.2 (age 65) to a peak difference of 24.8 (age 85). Over age 90, rates decreased more for women than men, thus reducing rate differences at the highest ages. Rate differences by age and gender were stable over calendar time, and medication-specific rates and trends over time were proportional by age and gender (data not shown).
FIGURE 2.
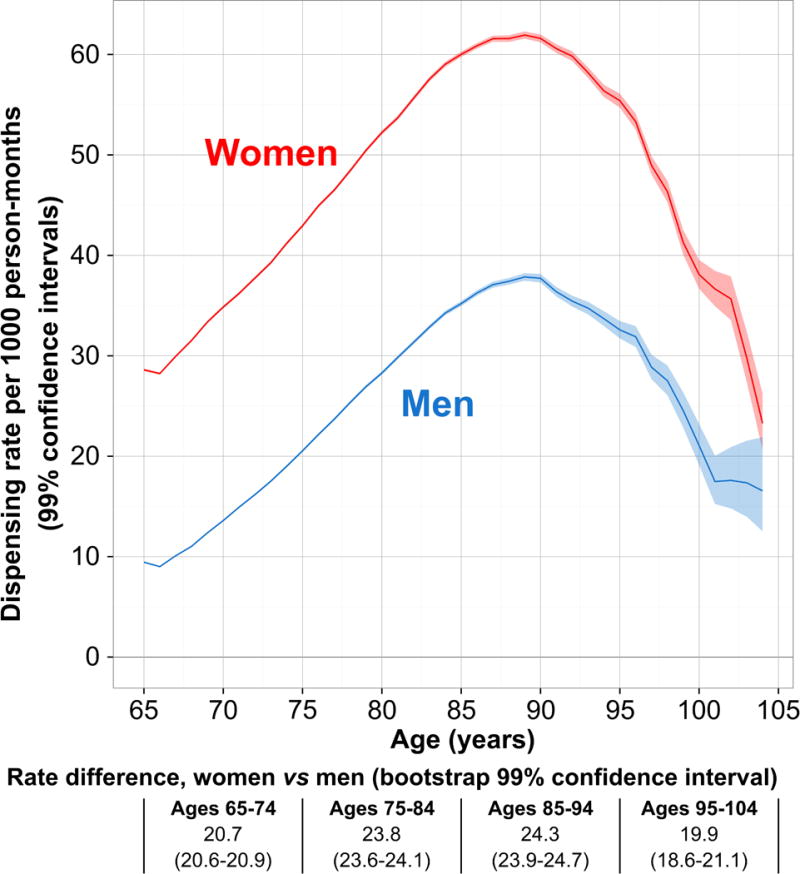
Dispensing rates (per 1000 person-months) with 99% confidence intervals, by gender and age, for all combined prescription medications for overactive bladder, among adults age 65–104 in the United States, 2000–2015. Rate differences are reported beneath the plot for age-group-specific comparisons between women versus men, with 99% bootstrap confidence intervals.
Payments for OAB medications
Figure 3 shows time trends for percentiles of beneficiary and total payments per prescription. Beneficiary payments per prescription were stable during 2000–2011 (Figure 3A). While the vast majority of beneficiary payments remained unchanged through 2015, those in the top 10% more than doubled between 2012–2015 (Figure 3A). During the first half of the study period, total payments per prescription were stable over time, shown by the largely horizontal loess curves during 2000–2007 (Figure 3B). Between 2008–2015, there were marginal increases in median total payments, from $125–$150 per prescription, but the gap between upper and lower percentiles of total payments widened. Beneficiary and total payments remained stable for IR medications across the study period (Figure S5.1, Supplemental Digital Content 5). Beneficiary and total payments increased over time for all ER medications except ER-oxybutynin tablets (Figures S5.2–S5.3, Supplemental Digital Content 5). A sensitivity analysis of payments over time, which used stratified analyses to assess potential confounding by insurance type and data supplier, did not affect our interpretation of results (data not shown).
FIGURE 3.
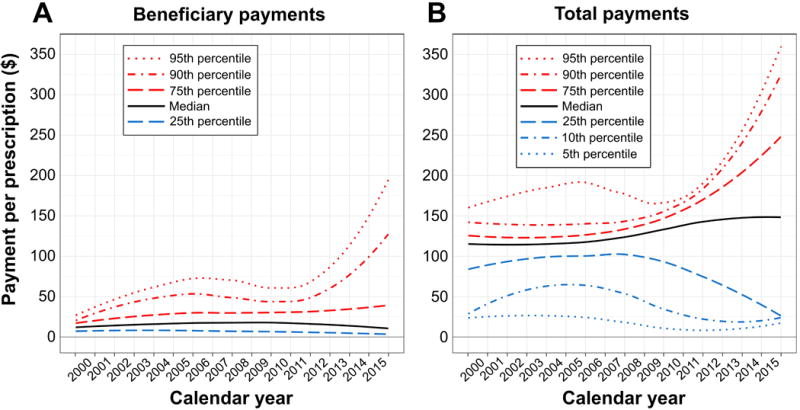
Payments per prescription over calendar time for prescription medications for overactive bladder, among adults age 65–104 in the United States, 2000–2015, adjusted for inflation to United States dollars ($) in 2015. (A) Beneficiary payments include deductible, coinsurance, copayment, and coordination of benefits. (B) Total payments include beneficiary payments and all post-discount payments by the insurer. Payment percentiles were calculated for each week of the study period, standardized by geography, and plotted using loess smoothers.
Beneficiary and total payments were similar between genders and across age groups. The only exception concerned individuals with the highest 5% of beneficiary payments for OAB medications, in whom higher age was associated with higher beneficiary payments per prescription, for women and men. Between ages 65-74, the 95th percentile beneficiary payment ranged $61–$65 per prescription; from ages 75–99, the 95th percentile beneficiary payment per prescription increased monotonically by $13 with each decade of age (99% CI: $11–$15), up to $96 per prescription at age 99.
DISCUSSION
In this population-based study of healthcare claims data during 2000–2015 on older adults in the U.S., we observed increases in prescription drug dispensing for OAB from 2000–2007 as new medications became available (i.e., ER-tolterodine, darifenacin, and solifenacin). Oxybutynin and tolterodine were the most prominent medications in the early years of the study, but their rates decreased over time as new alternatives darifenacin, solifenacin, and mirabegron became more widely dispensed. Compared with the significant impacts of certain emerging medications on time trends, $4 generic programs, Beers criteria, and Medicare Part D had nominal effects on OAB medication dispensing rates in this population. Rates increased with increasing age, were highest between age 85–90 for both women and men, and were higher for women than men at all ages. We observed stable beneficiary and total payment levels until the latter years of the study period, when the most expensive prescriptions became costlier to the beneficiary and insurer over time. Payment increases over time were driven by all ER medications except ER-oxybutynin tablets. By 2015, 25% of total payments for OAB medications exceeded $250 per reimbursed dispensed prescription.
Our novel results provide key information on OAB medication dispensing over time, specifically regarding medication-specific trends being impacted by new alternatives. ER medications accounted for an increasing share of OAB dispensing over time (to 78% by 2015), potentially due in part to their lower adverse event and discontinuation rates versus IR.35,36 Regarding ER medications, darifenacin and solifenacin debuted in 2005; as their rates increased alongside decreasing rates for ER-oxybutynin and tolterodine, overall rates were stable through 2015. These data suggest that ER medications released after 2005 may have served, to some degree, as therapeutic replacements for oxybutynin and tolterodine.
Comprehensive systematic reviews have found that antimuscarinic OAB medications exhibit similar intended treatment effects.36,37 Trospium, despite its unique structural advantage over other antimuscarinics that it cannot cross the blood-brain barrier38 (and thus should induce less anticholinergic effect), never became highly prevalent in this study population.
In contrast to trospium, these new findings about mirabegron have potential implications for clinicians, researchers, and policy-makers; these results underscore the ongoing need for adherence, effectiveness, and safety studies that account for time-related shifts in dissemination of each OAB medication. Given ongoing concerns about antimuscarinic-associated anticholinergic effects and subsequent adverse effects in OAB patients, there is potential for mirabegron to fill a decades-long therapeutic gap, since mirabegron – a β3-adrenergic-agonist – is the only non-antimuscarinic treatment alternative.7,16,39 Uptake of Mirabegron (2012) resembled that of solifenacin (2005–2008), despite mirabegron entering the market later with more contemporary treatment alternatives compared to solifenacin. If real-world treatment effectiveness of mirabegron is equivalent/superior to ER antimuscarinics, patients might benefit more from mirabegron due in part to its non-anticholinergic properties. Recent increased dissemination of mirabegron could be signal of relative treatment benefit for mirabegron in some patients.
To investigate this possibility and further inform clinical guidelines for OAB medications, future research should track utilization patterns of mirabegron (and all other OAB medications) beyond 2015, and assess their impact on patient outcomes. Additionally, future studies should consider the time-varying nature of branded-versus-generic status when comparing medications, as well as the recent introduction of over-the-counter oxybutynin40 and posterior tibial nerve stimulation.41
Our results indicate a negligible impact of $4 generic programs on OAB dispensing in this insured population. Given that data on cash payments for prescription drugs are missing from claims data,42 we consider two hypothetical explanations for this result: (1) $4 generic program did not impact how these insured patients accessed OAB medication, or (2) OAB treatment prevalence increased after 2006, but only among Medigap-insured patients who initiated treatment after $4 generics became available. For explanation #2 to be valid, two necessary conditions apply to those patients: (i) their symptom onset was after 2006 or their physician withheld pharmacotherapy until after 2006, and (ii) they opted to pay $4 in cash despite their ability to acquire equivalent medication through their insurer for $7 (IQR: $4–$14) (Figure S5.1, Supplemental Digital Content 5). We consider explanation #1 more plausible; however, our conclusion would be far less tenable had more medications besides IR oxybutynin been in $4 generics programs. In this light, future research should assess changes over time and across populations in the impact of $4 generics programs.43
Dispensing rates increased with age, likely reflecting the fact that OAB prevalence increases with age.44 Additionally, dispensing rates were higher for women than men, which extends prior symptom-centered evidence that women suffer more than men from “wet” OAB with urgency urinary incontinence.44,45 These data are striking amid concerns over potential adverse events of antimuscarinics, especially among older adults,16–18 and recent updates to Beers criteria for potentially inappropriate prescribing in older adults.14,15,28
These new payment data are important for clinical and policy decision-making concerning OAB medications. Our results demonstrate significant cost differences to Medigap beneficiaries and insurers depending on the specific OAB medication that was prescribed. As evidence emerges concerning comparative effectiveness and safety of these medications, the cost gradient of these medications may become an increasingly critical consideration for clinical treatment decisions and payment policies.
Given that healthcare expenditures for Medigap beneficiaries exceed Medicare-only by 24%,46 it remains unclear whether expenditure differences result from unnecessary healthcare utilization among Medigap beneficiaries.47,48 Future research should consider how drug-specific payment differences manifest across insurance subgroups, and should leverage our novel real-world data covering 16 years of payments for OAB medications in the Medigap population.
Limitations of our study should be considered when interpreting results. First, these data reflect dispensed prescriptions. This study therefore could not examine medication consumption or treatment adherence. Second, we lacked data on free samples and redeemed prescriptions that were not reimbursed by health insurance.42,49,50 As mentioned above, this missing data problem limits our interpretation of $4 generic programs’ impact. Instead of the preferable ability to explicitly enumerate IR oxybutynin prescriptions paid for with cash, we substituted a priori assumptions – which we consider plausible – about how an impact would have occurred for this drug class in the Medigap population. Third, this study assessed older adults with Medigap prescription drug coverage through their employer or former employer; subsequently, due to potentially unique demographic, clinical, and policy-related characteristics in the Medigap population, our results may not be generalizable to the larger Medicare population, those on Medicaid, or the uninsured. Fourth, this study did not exclude patients with dementia or other conditions associated with high anticholinergic load; given that these individuals may have had contraindications for antimuscarinic therapy, our results may underestimate dispensing rates among those considered eligible for treatment. Fifth, these healthcare claims data lacked detailed information on OAB symptoms and severity, which limited our ability to estimate rates in clinically meaningful subgroups.
This study has several strengths. We provide new population-based estimates of OAB medication dispensing for Medigap enrollees across the U.S. with prescription drug coverage. The databases that we linked include accurate individual-level data on Medigap enrollment and Medicare- and employer-covered portions of dispensed prescriptions (date, days supplied, medication, and payments). Our interrupted time-series analysis examined a critical 16-year period of changes in OAB treatment; several new medications became available, nationwide pharmacy chains introduced $4 generic programs, Medicare Part D was introduced, and concern surged regarding anticholinergic effects in older adults. Additionally, to reduce potential confounding or unaccounted co-intervention effects on time trend estimates for rates and payments, we standardized for changes over time in the geographic distribution and types of insurance plan in the databases.26,27
Conclusions
Overall dispensing rates for OAB medications among older adults in the U.S. increased during 2000–2007 as new medications became available, then stabilized through 2015. Medication-specific rates were dynamic, however, and were impacted by several new OAB medications that emerged during the study period. Dispensing rates were highest for women and adults age 85–90. Beneficiary payments for OAB medications were stable during 2000–2015, but large disparities emerged in total payments during the latter half of the study, driven largely by newly available extended-release medications. These new data inform translational research into patient outcomes and payment structures related to OAB medications, which are especially important for the older adult population.
Supplementary Material
SUPPLEMENTAL DIGITAL CONTENT 1. Table of FDA-Approved Medications for OAB.
SUPPLEMENTAL DIGITAL CONTENT 2. Standardized Dispensing Rates and Payments.
SUPPLEMENTAL DIGITAL CONTENT 3. Variability of Segmented Regression Estimates.
SUPPLEMENTAL DIGITAL CONTENT 4. Sensitivity Analysis of Interrupted Time Series.
SUPPLEMENTAL DIGITAL CONTENT 5. Payment Time Trends by Medication
Acknowledgments
The database infrastructure used for this project was funded by the Department of Epidemiology, UNC Gillings School of Global Public Health; the Cecil G. Sheps Center for Health Services Research, UNC; the CER Strategic Initiative of UNC’s Clinical Translational Science Award (UL1 TR001111); and the UNC School of Medicine. A preliminary version of this research was presented at the 2014 American Geriatrics Society Annual Scientific Meeting
Funding disclosure:
A.C.K. was supported in part by a National Service Research Award Post-Doctoral Traineeship from the Agency for Healthcare Research and Quality sponsored by the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill (5T32 HS000032-28); the Eunice Kennedy Shriver National Institute of Child Health & Human Development (T32 HD052468-05); and the Center for Pharmacoepidemiology in the Department of Epidemiology, Gillings School of Global Public Health (current members: GlaxoSmithKline, UCB BioSciences, Merck). M.J.F. was supported in part as Principal Investigator by grant support from the National Heart, Lung, and Blood Institute (R01 HL118255); as a Co-Investigator on grants from the NIH National Institute on Aging (R01 AG023178), the National Center for Advancing Translational Sciences, National Institutes of Health (UL1 TR001111), and AstraZeneca. M.M.C. was supported in part by a student fellowship funded by Amgen, Inc. A.D.M. was supported in part by grant support from the United States Veterans Health Administration (B6126W) and the National Institutes of Health (R21DK096201). J.M.W. was supported in part by grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23 HD068404). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The database infrastructure used for this project was funded by the Department of Epidemiology, UNC Gillings School of Global Public Health; the Cecil G. Sheps Center for Health Services Research, UNC; the CER Strategic Initiative of UNC’s Clinical Translational Science Award (UL1 TR001111); and the UNC School of Medicine.
Footnotes
Conflict of interest:
A.C.K. and M.J.F. do not accept personal compensation of any kind from any pharmaceutical company, though they received training support (A.C.K.) and salary support (M.J.F.) from the Center for Pharmacoepidemiology in the Department of Epidemiology, Gillings School of Global Public Health (current members: GlaxoSmithKline, UCB BioSciences, Merck). M.J.F. is a member of the Scientific Steering Committee for a post-approval safety study of an unrelated drug class funded by GSK. All compensation for services provided on the Scientific Steering Committee is invoiced by and paid to the University of North Carolina at Chapel Hill. J.M.W. reports receiving financial support from Procter and Gamble as a consultant regarding a device for stress urinary incontinence, research funding from Boston Scientific for an investigation of pelvic organ prolapse surgery and research funding from Pelvalon for a device for fecal incontinence.
There are no other disclosures to report.
References
- 1.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: Report from the standardisation sub-committee of the international continence society. Am J Obstet Gynecol. 2002;187(1):116–126. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 2.Ouslander JG. Management of overactive bladder. N Engl J Med. 2004;350(8):786–799. doi: 10.1056/NEJMra032662. [DOI] [PubMed] [Google Scholar]
- 3.Chapple C. Muscarinic receptor antagonists in the treatment of overactive bladder. Urology. 2000;55(Suppl 5A):33–46. doi: 10.1016/s0090-4295(99)00492-6. [DOI] [PubMed] [Google Scholar]
- 4.Athanasopoulos A. The pharmacotherapy of overactive bladder. Expert Opin Pharmacother. 2011;12(7):1003–1005. doi: 10.1517/14656566.2011.554397. [DOI] [PubMed] [Google Scholar]
- 5.Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306–14-5. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Merck Consumer Care. US Food and Drug Administration Pharmacology/Toxicology New Drug Application Review and Evaluation - Oxytrol for Women. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/202211Orig1s000Pharm.pdf. Published 2010. Accessed January 14, 2015.
- 7.U.S. Food and Drug Administration. Drug Approvals and Databases Orange Book Data Files. http://www.fda.gov/drugs/informationondrugs/ucm129689.htm. Published 2014. Accessed July 10, 2014.
- 8.Wal-Mart Stores Inc. Wal-Mart Cuts Generic Prescription Medicines to $4. http://corporate.walmart.com/_news_/news-archive/2006/09/21/wal-mart-cuts-generic-prescription-medicines-to-4. Published 2006. Accessed March 10, 2017.
- 9.Appleby J. Target says it will match Wal-Mart’s $4 generic drug price. USA Today. http://usatoday30.usatoday.com/money/industries/retail/2006-09-21-walmart-drugs_x.htm. Published 2006. Accessed March 10, 2017.
- 10.Wal-Mart Stores Inc. Wal-Mart’s $4 Generics Program Launched in Final 11 States. http://corporate.walmart.com/_news_/news-archive/2006/11/27/wal-marts-4-generics-program-launched-in-final-11-states. Published 2006. Accessed March 10, 2017.
- 11.Ju R, Garrett J, Wu JM. Anticholinergic medication use for female overactive bladder in the ambulatory setting in the United States. Int Urogynecol J. 2014;25(4):479–484. doi: 10.1007/s00192-013-2246-0. [DOI] [PubMed] [Google Scholar]
- 12.Kachru N, Sura S, Chatterjee S, Aparasu R. Antimuscarinic Medication Use in Elderly Patients with Overactive Bladder. Drugs & Aging2. 2016;33(10):755–763. doi: 10.1007/s40266-016-0399-5. [DOI] [PubMed] [Google Scholar]
- 13.Maman K, Aballea S, Nazir J, et al. Comparative Efficacy and Safety of Medical Treatments for the Management of Overactive Bladder: A Systematic Literature Review and Mixed Treatment Comparison. Eur Urol. 2013;65(4):755–765. doi: 10.1016/j.eururo.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 14.The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 16.Gray SL, Anderson ML, Dublin S, et al. Cumulative Use of Strong Anticholinergics and Incident Dementia. JAMA Intern Med. 2015;175(3):401–407. doi: 10.1001/jamainternmed.2014.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrams P, Andersson K-E, Buccafusco JJ, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148(5):565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481–1486. doi: 10.1177/0091270006292126. [DOI] [PubMed] [Google Scholar]
- 19.Truven Health Analytics. MarketScan Research Databases and Online Tools. http://truvenhealth.com/your-healthcare-focus/life-sciences/data-databases-and-online-tools. Published 2015. Accessed January 21, 2017.
- 20.Danielson E. White Paper; Health Research Data for the Real World: The MarketScan Databases. 2014 http://truvenhealth.com/your-healthcare-focus/life-sciences/data-databases-and-online-tools.
- 21.Greenland S, Rothman KJ. Introduction to Categorical Analysis. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 238–257. [Google Scholar]
- 22.Greenland S, Rothman KJ. Introduction to Stratified Analysis. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 258–282. [Google Scholar]
- 23.Peterson C, Xu L, Florence C, Grosse SD, Annest JL. Professional Fee Ratios for US Hospital Discharge Data. Med Care. 2015;53(10):840–849. doi: 10.1097/MLR.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bureau of Labor Statistics. Archived Consumer Price Index Detailed Report Information. https://www.bls.gov/cpi/cpi_dr.htm#2015. Published 2017. Accessed April 7, 2017.
- 25.Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. 2nd. Boston: Houghton Mifflin; 2001. [Google Scholar]
- 26.Wagner A, Soumerai S, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 27.Freeman DH, Jr, Holford TR. Summary rates. Biometrics. 1980;36(2):195–205. [PubMed] [Google Scholar]
- 28.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 29.Kaiser Family Foundation. Medicare Timeline. https://kff.org/medicare/timeline/medicare-timeline/. Published 2015. Accessed March 28, 2017.
- 30.Nam J. Efficient method for identification of cyclic trends in incidence. Commun Stat - Theory Methods. 1983;12(9):1053–1068. doi: 10.1080/03610928308828515. [DOI] [Google Scholar]
- 31.Brookhart MA, Rothman KJ. Simple estimators of the intensity of seasonal occurrence. BMC Med Res Methodol. 2008;8(67):1–9. doi: 10.1186/1471-2288-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durbin J, Watson G. Testing for serial correlation in least squares regression. I. Biometrika. 1950;37(3-4):409–428. doi: 10.2307/2332325. [DOI] [PubMed] [Google Scholar]
- 33.Gillings D, Makuc D, Siegel E. Analysis of interrupted time series mortality trends: An example to evaluate regionalized perinatal care. Am J Public Health. 1981;71(1):38–46. doi: 10.2105/AJPH.71.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson DB, Kinlaw AC, MacLehose RF, Cole SR. Standardized binomial models for risk or prevalence ratios and differences. Int J Epidemiol. 2015;44(5):1660–1672. doi: 10.1093/ije/dyv137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Souza AO, Smith MJ, Miller L-A, Doyle J, Ariely R. Persistence, Adherence, and Switch Rates Among Extended-Release and Immediate-Release Overactive Bladder Medications in a Regional Managed Care Plan. J Manag Care Pharm. 2008;14(3):291–301. doi: 10.18553/jmcp.2008.14.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartmann KEK, McPheeters MML, Biller DDH, et al. Treatment of Overactive Bladder in Women. Vol. 187. Rockville, MD: 2009. [PMC free article] [PubMed] [Google Scholar]
- 37.Shamliyan TA, Kane RL, Wyman J, Wilt TJ. Systematic Review: Randomized, Controlled Trials of Nonsurgical Treatments for Urinary Incontinence in Women. Ann Intern Med. 2008;148:459–473. doi: 10.7326/0003-4819-148-6-200803180-00211. [DOI] [PubMed] [Google Scholar]
- 38.Abrams P, Andersson K-E. Muscarinic receptor antagonists for overactive bladder. BJU Int. 2007;100(5):987–1006. doi: 10.1111/j.1464-410X.2007.07205.x. [DOI] [PubMed] [Google Scholar]
- 39.Suehs BT, Davis C, Franks B, et al. Effect of Potentially Inappropriate Use of Antimuscarinic Medications on Healthcare Use and Cost in Individuals with Overactive Bladder. J Am Geriatr Soc. 2016;64(4):779–787. doi: 10.1111/jgs.14030. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Food and Drug Administration. FDA News Release: FDA approves over-the-counter Oxytrol for Women to treat overactive bladder. Press Announcements. https://wayback.archive-it.org/7993/20170112223110/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm336815.htm. Published 2013. Accessed January 25, 2013.
- 41.Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597. doi: 10.1007/s00192-012-1712-4. [DOI] [PubMed] [Google Scholar]
- 42.Choudhry NK, Shrank WH. Four-Dollar Generics — Increased Accessibility, Impaired Quality Assurance. N Engl J Med. 2010;363(20):1885–1887. doi: 10.1056/NEJMp1006189. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Gellad WF, Zhou L, Lin YJ, Lave JR. Access to and use of $4 generic programs in Medicare. J Gen Intern Med. 2012;27(10):1251–1257. doi: 10.1007/s11606-012-1993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 45.Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology. 2011;77(5):1081–1087. doi: 10.1016/j.urology.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 46.Keane M, Stavrunova O. Adverse selection, moral hazard and the demand for Medigap insurance. J Econom. 2016;190(1):62–78. doi: 10.1016/j.jeconom.2015.08.002. [DOI] [Google Scholar]
- 47.Ettner SL. Adverse selection and the purchase of medigap insurance by the elderly. J Health Econ. 1997;16(5):543–562. doi: 10.1016/S0167-6296(97)00011-8. [DOI] [PubMed] [Google Scholar]
- 48.Letter from Kevin McCarty, Adam Hamm, Sandy Praeger, James Donelon, and Monica Lindeen of the National Association of Insurance Commissioners (NIAC), to Kathleen Sebelius, Secretary of the U.S. Department of Health and Human Services, December 19, 2012. http://www.naic.org/documents/committees_b_senior_issues_related_docs_draft_letter_sebelius_121219.pdf. Published 2012
- 49.Sears CLG, Lewis C, Noel K, Albright TS, Fischer JR. Overactive bladder medication adherence when medication is free to patients. J Urol. 2010;183(3):1077–1081. doi: 10.1016/j.juro.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 50.Kornfield R, Donohue J, Berndt ER, Alexander GC. Promotion of Prescription Drugs to Consumers and Providers, 2001-2010. PLoS One. 2013;8(3):1–7. doi: 10.1371/journal.pone.0055504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL DIGITAL CONTENT 1. Table of FDA-Approved Medications for OAB.
SUPPLEMENTAL DIGITAL CONTENT 2. Standardized Dispensing Rates and Payments.
SUPPLEMENTAL DIGITAL CONTENT 3. Variability of Segmented Regression Estimates.
SUPPLEMENTAL DIGITAL CONTENT 4. Sensitivity Analysis of Interrupted Time Series.
SUPPLEMENTAL DIGITAL CONTENT 5. Payment Time Trends by Medication


