Abstract
Objective
In 2016, non-invasive, well-circumscribed and encapsulated, follicular variant of papillary thyroid carcinoma (NI-EFV PTC) was reclassified as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in order to reduce overtreatment of this indolent tumor. However, the study cohort did not include subcentimeter tumors, i.e. papillary thyroid microcarcinoma (mPTC) with NI-EFV morphology, and such lesions are still regarded and staged by most pathologists as microcarcinomas. It is therefore crucial to evaluate the clinical outcome of subcentimeter NI-EFVs.
Methods
A total of 52 patients with unifocal mPTC, NI-EFV from five tertiary hospitals who had at least one year clinical follow up (FU) without post-operative RAI administration were included in the study. A control group of 57 invasive mPTC follicular variant was also included.
Results
The median tumor size was 0.44 cm (range: 0.1 – 0.9 cm). There were no distant or lymph node metastases at diagnosis in all patients. Twenty-three patients (44%) underwent lobectomy alone, while the remaining received total thyroidectomy. No recurrence was observed in the entire cohort (n=52) including all 38 patients with at least 2 years of FU (median FU: 6.3 years). Among 25 patients with ≥ 5 years of FU, none recurred with a median FU of 9.6 years (range 5.2 – 18.1 years). In contrast, in the control group with invasive mPTC follicular variant, there were 5 (9%) patients with nodal metastasis at presentation and 1 (2%) who displayed nodal recurrence.
Conclusion
Papillary thyroid microcarcinoma, NI-EFV, when stringently selected for, lacks metastasis at presentation and follows an extremely indolent clinical course, even when treated conservatively without RAI therapy. Provided stringent inclusion criteria are met, classification of subcentimeter mPTC, NI-EFV as NIFTP should be considered in order to avoid overtreatment of these biologically indolent lesions.
Keywords: non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), follicular variant, papillary thyroid carcinoma, papillary microcarcinoma
Introduction
The incidence of thyroid carcinoma has increased more than that of any other cancer in the United States, with an annual increase of 3.6% per year [1] and 62,980 new cases diagnosed annually [2]. The increase is in large part attributed to a sharp rise in the incidental diagnosis of papillary thyroid microcarcinoma (mPTC) and the follicular variant of papillary thyroid carcinoma (FVPTC), diagnoses made with some degree of subjectivity [3]. The frequency of papillary microcarcinoma among all papillary thyroid carcinoma (PTC) in one tertiary hospital, for example, has increased from 33% in the 1970s to 51% in 2009 [3]. Regardless of tumor size, the proportion of papillary thyroid carcinomas with a follicular growth pattern among all papillary thyroid carcinomas (PTC) has tripled from 18% to 57% for the past four decades, surpassing the classical type, and becoming the most common architectural pattern encountered in PTC [3].
Histologically, the term papillary microcarcinoma is applied to any PTC that is less than or equal to 1 cm in size. FVPTC is characterized by an exclusively follicular growth pattern. Both are considered as variants of PTC and the diagnosis requires the presence of typical nuclear features of PTC [4, 5]. Recently, compelling clinical outcome data and molecular evidence, including The Cancer Genomic Atlas (TCGA) of PTC, have demonstrated that noninvasive FVPTC follows a highly indolent clinical course with negligible risk of recurrence and carries a molecular signature resembling follicular adenoma/follicular carcinoma with RAS mutation as the most frequently encountered alteration [4, 6–9]. Realizing the epidemic in the diagnosis of NI-EFV, as well as its molecular signature and highly indolent nature, a working group of 28 endocrine experts critically reexamined this entity in 2014, advocating for a nomenclature revision to non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), in an effort to reduce overtreatment of this highly indolent tumor by eliminating the term “carcinoma” [4].
As the cohort of 109 patients with NIFTP studied by the consensus conference [4] and in previous reports [10, 8] included only tumors which were equal or more than 1 cm in size, the consensus diagnostic criteria of NIFTP did not explicitly address subcentimeter lesions, i.e. mPTC with NI-EFV morphology. Although it may be surprising that a subcentimeter non-invasive circumscribed follicular-patterned lesion may be classified as a microcarcinoma while a similar histologic lesion that is > 1 cm in size is classified as NIFTP, there is currently no study with long-term follow up that addresses the outcome of mPTC NI-EFV. Additionally, the larger lesions are typically evaluated or noted preoperatively while the smaller lesions are typically identified incidentally, and therefore, often not particularly well-characterized or noted grossly. Thus, such lesions are labelled and staged by most pathologists as microcarcinomas. Of greatest concern, a substantial number of physicians routinely offer radioactive iodine (RAI) therapy to patients with unifocal and especially multifocal mPTC [11] contrary to the recommendations of various medical societies [12, 13]. Therefore, it is important to evaluate the clinical behavior and outcome of papillary thyroid microcarcinoma, NI-EFV.
In the current study, we gathered and studied the clinical outcome of 52 patients with unifocal mPTC NI-EFV from five tertiary hospitals who had at least one year of clinical follow up and were not treated with post-operative RAI.
Material and methods
Study cohort
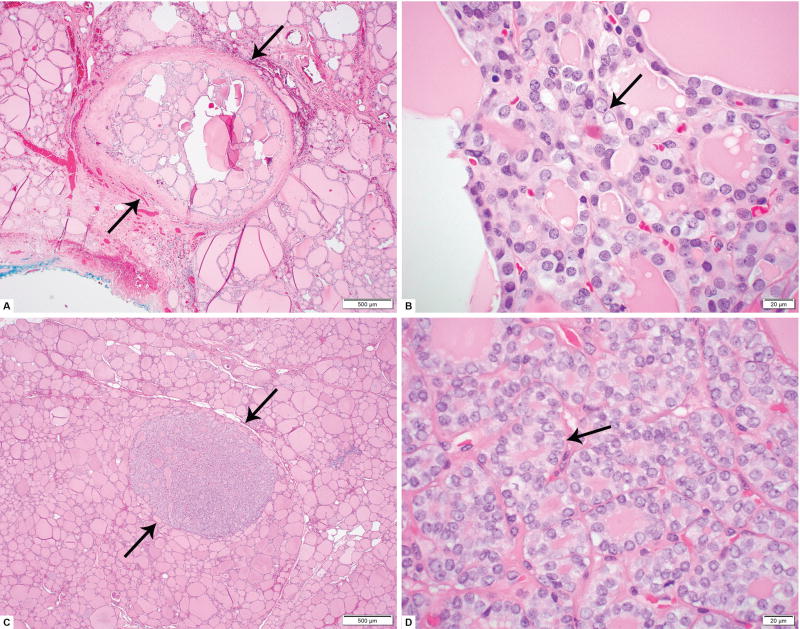
After obtaining approval from the various institutional review boards, the pathology database of five tertiary hospitals (Memorial Sloan Kettering Cancer center, (MSKCC), New York, NY, USA; Bologna University School of Medicine, Bologna, Italy; Massachusetts General Hospital (MGH), Boston, MA, USA; Brigham and Women’s Hospital (BWH), Boston, MA, USA; and Sunnybrook Health Sciences Centre (SHSC), Toronto, ON, Canada were searched for candidate cases of unifocal mPTC NI-EFV. All cases were reviewed independently by nine endocrine pathologists (BX, NF, NK, VN, PS, JB, GT, WF, and RG) to confirm the diagnosis using the criteria proposed by Nikiforov et al. In brief, mPTC NI-EFV was diagnosed when a mPTC that was less than 1 cm in size and fulfilled all of the following criteria: 1) encapsulation or clear demarcation; 2) exclusive/predominant follicular growth pattern lacking psammoma bodies and with < 1% true papillae and < 30% solid growth pattern; 3) nuclear atypia in the form of nuclear enlargement, nuclear membrane irregularity and/or chromatin clearing with a nuclear score of 2–3; 4) absence of invasion (vascular or capsular); 5) no tumor necrosis; and 6) mitotic index < 3 per 10 high power fields (400×) (Figure 1). In addition, patients with separate foci of carcinoma, less than one-year of follow up, or who received post-operative RAI were excluded. A total of 52 cases operated between 1987 and 2015 fulfilled the above inclusion criteria.
Figure 1.
Microscopic pictures of papillary microcarcinoma (mPTC), noninvasive encapsulated/well circumscribed follicular variant. A: Low power view (40×) of a 0.2 cm encapsulated mPTC non-invasive follicular variant. Arrows indicate the capsule of the lesion. B: High power (600×) view of same lesion as in A. Enlarged, clear and irregular nuclei (typical nuclear features of papillary thyroid carcinoma) are indicated by an arrow. Nuclear overlapping and grooves also present. C: Low power view (40×) of a 0.2 cm well circumscribed mPTC non-invasive follicular variant from a different patient. Arrows indicate the well demarcated contours of the lesion with no capsule as well as no invasion in adjacent non-neoplastic thyroid parenchyma. D: High power (600×) view of same lesion as in C. Nuclear features of papillary thyroid carcinoma, are present in the form of clear, irregular, overlapping nuclei with scattered grooves (arrow).
Fifty-seven patients with unifocal invasive papillary microcarcinoma follicular variant were retrieved from the databases of MSKCC and SHSC and were included in this study as the control group.
Clinical review
The patients’ charts were reviewed to record the following clinical parameters: age at diagnosis, gender, clinical presentation, indication for surgery, surgery type (total thyroidectomy vs. lobectomy/hemithyroidectomy), duration of clinical follow up (FU), and clinical outcome.
BRAF mutation analysis
BRAFV600E mutation status was examined in five patients from MGH by immunohistochemistry using the VE1 primary antibodies against BRAF V600E (clone: VE1) as previously described [14], and in six cases from Bologna University using Allele Specific Locked Nucleic Acid Polymerase Chain Reaction (PCR)[15].
Results
Patient cohort
A total of 2229 patients with papillary microcarcinoma as the largest focus in the thyroid resection operated between 1989 and 2014 were reviewed. After excluding cases with multicentric mPTCs and mPTC composed of histologic variant(s) other than NI-EFV, 109 patients (4.9%) were diagnosed with unifocal mPTC NI-EFV. Fifty-seven patients with unifocal mPTC NI-EFV were subsequently excluded as they did not have sufficient follow up (> 1 year) or had received post-operative RAI. Fifty-two patients (2.3%) mPTC NI-EFV fulfilling the inclusion criteria were included in this study. The number of included cases according to the institutions was as follows: MSKCC n = 3; Bologna University n = 8; MGH n = 5; BWH n = 5; and SHSC n = 2.
Clinico-pathologic findings of mPTC NI-EFV
The clinico-pathologic characteristics are summarized in Table 1. Subcentimeter NI-EFV predominantly affected female patients with a male to female ratio of 1:6.4. The median age of diagnosis was 52 (range: 27 – 81). The median size of these mPTC NI-EFV was 0.44 cm (range 0.1 – 0.9 cm). Seven tumors (13%) showed oncocytic features. Twenty-three patients (44%) underwent lobectomy, while the remaining 29 (56%) had total thyroidectomy. Thirteen mPTC NI-EFV (25%) were detected pre-operatively by imaging study (non-incidental), while in the remaining 39 patients (75%), the mPTC NI-EFVs were incidental findings in thyroid resection specimens for other benign etiologies, including follicular adenoma (n = 8), nodular hyperplasia (n = 25), chronic lymphocytic thyroiditis (n = 4), diffuse hyperplasia (n = 1), and a benign thyroid nodule detected incidentally during work up for lacrimal gland carcinoma (n = 1). The preoperative fine needle aspiration (FNA) were available in 12 of the 13 patients with non-incidental mPTC NI-EFV, and the FNA diagnoses were suspicious for PTC (n = 7), suspicious for follicular neoplasm (n = 1), and atypia of undetermined significance (n = 4). The median size of non-incidental mPTC NI-EFV was 0.7 cm (range: 0.4 to 0.9 cm). In 23 (44%) cases, the thyroid was submitted entirely for histologic examination, while in the remaining 29 patients (56%), the thyroid glands were sampled representatively. In cases with representative sampling, a mean of 12 thyroid sections were exanimated per specimen (median = 10, range = 2 to 26). All patients had no prior history of thyroid carcinoma, and lacked evidence of distant metastasis at the initial presentation. All tumors were confined to the thyroid, and resected completely with negative surgical margins during the initial surgery. Lymph node(s) were sampled in 20 (38%) patients. No lymph node metastases were detected at diagnosis. All eleven cases tested were negative for BRAFV600E mutation.
Table 1.
Clinico-pathologic characteristics of patients with unifocal papillary microcarcinoma, noninvasive encapsulated follicular variant (mPTC, NI-EFV) included in the study
| All patients (n = 52) | |||
| Gender | Female | 45 | 87% |
| Male | 7 | 13% | |
| Age, median (range) | 52 (27 – 81) | ||
| Clinical presentation | Incidental finding | 39 | 75% |
| Non-incidental | 13 | 25% | |
| Size, cm, median of all tumors (range) | 0.44 (0.1 – 0.9) | ||
| Size of non-incidental tumor, cm, median (range) | 0.7 (0.4 – 0.9) | ||
| Sampling of the thyroid gland | Entirely sampled | 23 | 44% |
| Representatively Sampled | 29 | 56% | |
| Sections of thyroid sampled, mean, median (range) | 12, 10 (2 – 26) | ||
| Sampling and status of lymph nodes | pNx: Not sampled | 32 | 62% |
| pN0: Benign lymph node(s) N0 | 20 | 38% | |
| Surgical procedure | Lobectomy | 23 | 44% |
| Total thyroidectomy | 29 | 56% | |
| Encapsulation | Encapsulated | 22 | 42% |
| Well circumscribed | 30 | 58% | |
| Other benign tumors | Adenoma | 9 | 17% |
| None | 45 | 83% | |
| Follow up duration, years, median (range) | 4.3 (1.0–18.1) | ||
| Disease status at last follow up (FU) | No evidence of disease (NED) | 52 | 100% |
| Patients with at least 2 year follow up (n = 38) | |||
| Surgical procedure | Lobectomy/hemithyroidectomy | 15 | 39% |
| Total thyroidectomy | 23 | 61% | |
| Follow up duration, years, median (range) | 6.3 (2.0 –18.1) | ||
| Disease status at last FU | NED | 38 | 100% |
| Patients with at least 5 year follow up (n = 25) | |||
| Surgical procedure | Lobectomy | 12 | 48% |
| Total/subtotal thyroidectomy | 13 | 52% | |
| Follow up duration, years, median (range) | 9.6 (5.2–18.1) | ||
| Disease status at last FU | NED | 25 | 100% |
All values are expressed as N and % of column total unless otherwise specified.
Outcome of mPTC NI-EFV
The median follow up in our cohort was 4.3 years (range 1.0 – 18.1). Among them, 38 (73%) had at least 2 years with a median follow up of 6.3 years, and 25 (48%) had at least 5 years of follow up with a median follow up of 9.6 years. No recurrence or disease specific death was observed in the entire cohort.
Clinico-pathologic characteristics and outcome of the control group with unifocal invasive forms of papillary microcarcinoma, follicular variant (n = 57)
The control group included 9 (16%) patients with encapsulated follicular variants with invasion and 48 (84%) with infiltrative follicular variant (Table 2). The median tumor size was 0.2 cm (range: 0.03 to 0.9 cm). Thirty-three patients (58%) underwent lobectomy; while the remaining 24 (42%) had total thyroidectomy. Ten patients (17%) had additional non-carcinomatous lesions within the resection specimens, e.g. adenomas (n = 8) and supracentimeter NIFTP (n = 3). Five patients (9%) harboring papillary microcarcinoma infiltrative follicular variant had nodal metastases at initial presentation: three in the central (AJCC N1a) and two in the lateral neck (AJCC N1b) compartment. The two patients with lateral neck metastatic disease first presented with enlarged lateral neck masses and were found to have multiple lymph nodes containing metastatic papillary thyroid carcinoma, largest ones being 7.0 cm in one patient and 2.0 cm in the other one. The patients subsequently underwent total thyroidectomy. A single focus of infiltrative follicular variant was found in the entirely submitted thyroidectomy specimen with a tumor size of 0.2 and 0.7 cm respectively.
Table 2.
Clinico-pathologic features and outcome of patients with unifocal mPTC, NI-EFV compared to those with unifocal invasive forms of mPTC, follicular variant
| mPTC, NI-EFV | mPTC, follicular variant, invasive |
||
|---|---|---|---|
| Number of patients | 52 | 57 | |
| Gender | Female | 45 (87%) | 48 (84%) |
| Male | 7 (13%) | 9 (16%) | |
| Age, median (range) | 52 (27 – 81) | 51 (18 – 77) | |
| Size, cm, median (range) | 0.44 (0.1 – 0.9) | 0.2 (0.03 – 0.9) | |
| Surgical procedure | Lobectomy | 23 (44%) | 33 (58%) |
| Total thyroidectomy | 29 (56%) | 24 (42%) | |
| Other non-malignant tumors | Adenoma/NIFTP | 9 (17%) | 10 (17%) |
| None | 45 (83%) | 47 (83%) | |
| Lymph node status | N1 | 0 (0%) | 5 (9%) |
| N0 | 20 (38%) | 19 (33%) | |
| Nx | 32 (62%) | 33 (58%) | |
| Follow up duration, years, median (range) | 4.3 (1.0–18.1) | 6.5 (0.4 – 23.3) | |
| Disease status at last FU | NED | 52 (100%) | 39 (95%) |
| Neck recurrence | 0 (0%) | 1 (2%) | |
Forty patients within this group had follow up information available. Two (5%) patients had received post-operative RAI. The median follow up period was 6.5 years (range: 0.4 to 23.3 years). One patient (2%) with a 0.6 cm infiltrative follicular variant of papillary microcarcinoma had neck recurrence 1.3 years after the initial surgery. The remaining patients were disease free at the last follow up.
Discussion
Although papillary thyroid microcarcinomas are considered to be indolent neoplasms, especially when discovered incidentally, they are not infrequently overtreated [11, 16]. In 2003, some suggested renaming the incidental papillary thyroid microcarcinomas as papillary thyroid microtumors [17]. This proposed nomenclature was never adopted, likely due to the fact that rarely, papillary thyroid microcarcinomas metastasize regionally or even distantly [18, 19]. Isolated cases of fatal mPTC have also been described in the literature [19]. These previous studies on mPTC selected cases purely based on size without taking into consideration the architectural, cytologic or invasive features of this tumor. Although papillary mPTC is currently considered to be a variant of PTC, it is the only variant of PTC that is defined by size rather than its architecture and cytomorphology [5]. Theoretically, mPTC could be classified according to PTC subtype/variant. A noninvasive encapsulated or well-circumscribed mPTC with exclusive follicular growth pattern, for example, could be classified as mPTC, NI-EFV, and many do subtype mPTC. As these subcentimeter NI-EFVs have not been included in the NIFTP consensus cohort, and as no previous studies have specifically reported the outcome of subcentimeter NI-EFVs, many pathologists, if they choose to specifically comment on these lesions, especially in the context of a multinodular gland, diagnose them as mPTC rather than NIFTP. Although a recent study by Thompson has included a few (no more than 5) subcentimeter NI-EFV as NIFTPs [20], the current study is the first to focus specifically on the clinical behavior and outcome of these subcentimeter mPTC, NI-EFV, in order to determine if such lesions share a similar indolent clinical course as NIFTP.
All cases were stringently reviewed and selected by multiple expert endocrine pathologists using the same diagnostic criteria of NIFTP. A meticulous histopathologic examination is of paramount importance since a deviation from these criteria may affect behavior. For example, the presence of a minor but significant amount of papillae formation (>1%) may increase the risk of lymph node metastasis despite a total lack of invasion [21]. It is also crucial to carefully scrutinize the border of the lesion for any infiltration into adjacent thyroid tissue, and in fact, there is the caveat of assessing definitive circumscription in such small lesions. Indeed, in very small tumors (e.g. less than 0.2 cm in size), deciding whether the tumor is non-invasive can be a challenging exercise since they are rarely encapsulated or well circumscribed. Unless there is clear demarcation from adjacent follicles such as in the 0.2 cm lesion depicted in figure 1C–D, it is preferable not to label these minute tumors as non-invasive. Similarly, the presence of incipient papillae may be difficult to appreciate in very small tumors. Again, only tumors that can be confidently assessed as having an almost entirely follicular architecture should be included in the NI-EFV category. A BRAF V600E immunostain could be helpful in these difficult situations. NI-EFV has been shown to lack BRAFV600E at the molecular and immunohistochemical levels in the majority of studies [4, 6, 22, 7], including the current one with rare exceptions [23, 24]. Another requirement for an accurate assessment of invasion is a well sampled tumor capsule. Due to their subcentimeter nature, the majority of the lesions likely have the entire capsule submitted, but as many of them are found incidentally and lack gross identification, stating this as a certainty is not possible and a caveat for these lesions.
Only unifocally identified mPTC were included in this study to avoid sequelae associated with multifocal disease, although not all cases had the entire lobe or thyroid submitted for histological review, precluding the possibility of other occult, unsampled lesions. Because of this study design, we cannot address the clinical behavior of multicentric mPTC NI-EFV. Future studies are needed to evaluate the outcome of multifocal mPTC NI-EFV. Additionally, we only included patients that were treated with surgery alone (lobectomy or total thyroidectomy) without post-operative RAI, as recommended by the American Thyroid Association [12] which enables us to follow the natural history of resected mPTC NI-EFV. In regard to follow up, we relied on structural rather than biochemical recurrence to assess patients' disease status. This was in part due to the fact that some cases were old and did not have adequate serum thyroglobulin data. Although a long follow up time was not available in all cases, this study comprised 25 patients, each followed for at least 5 years, treated by surgery without RAI therapy who did not recur (median follow up of 9.6 years). Hence, these tumors seem to have a very indolent behavior similar to their supracentimeter counterparts [20, 25]. Indeed, most differentiated thyroid carcinomas recur during the first decade [26] although late recurrences and distant spread are documented [27]. Additional studies with longer follow up are required to fully address the long-term outcome of these tumors. The indolent behavior of these subcentimeter NI-EFVs further reinforces the fact that invasion rather than nuclear features or tumor size drives outcome in encapsulated follicular patterned tumors [28, 29]. The lack of nodal metastasis in our cohort is also consistent with the fact that NI-EFV and NIFTP are mainly RAS and PAX8-PPARG driven tumors, behaving similarly to other encapsulated follicular neoplasms [7], and in contrast to BRAFV600E-like tumors that typically demonstrate avidity for local lymph nodes [21, 30].
A control group of 57 patients with unifocal invasive forms of follicular variant of papillary microcarcinoma were included in this study. Although the clinical and pathologic characteristics, e.g. age, gender, size of the tumor, and procedure, were similar among patients with noninvasive and invasive forms of mPTC follicular variant, it is clear that the invasive form is associated with a nontrivial risk of nodal metastasis (9%) and disease recurrence (2%). Two patients presented with bulky lateral neck metastases and were found to have unifocal infiltrative follicular variant of papillary microcarcinoma in the entirely-submitted total thyroidectomy specimen. This further highlights the biological difference between noninvasive and invasive forms of papillary microcarcinoma and confirm our previous observation that the histotype of papillary microcarcinoma predicts outcome [18].
The above data strongly suggest that subcentimeter NI-EFV are similar to larger NIFTP at the histologic and behavior levels. Classifying these subcentimeter lesions as NIFTP is not a pure academic exercise and may have important clinical consequences. Despite the past and recent recommendations of several medical societies [12, 13], a significant proportion of clinicians proceed to completion thyroidectomy and RAI adjuvant therapy for unicentric mPTC throughout the world [11]. Although the proportion of unicentric mPTC NI-EFV among papillary microcarcinoma is not high (4.9% in the current study), they are probably in the thousands worldwide each year. Due to increased incidence of mPTC (51% of all PTC in 2009) [3], the estimated universal annual incidence of thyroid carcinomas is approximately 300,000 cases [31]. If subcentimeter lesions meeting stringent criteria are rebranded as NIFTP instead of mPTC NI-EFV, a truly significant decrease in unnecessary treatment might be observed.
Conclusion
In conclusion, our study suggests that papillary thyroid microcarcinoma, noninvasive follicular variant, when stringently selected for, lacks metastasis at presentation and follows an indolent clinical course, even when treated conservatively without RAI therapy. These tumors should be distinguished from infiltrative mPTC and other variants of mPTC which can give rise to nodal metastases and, rarely, distant metastatic disease. The presence of nuclear features of PTC alone should not be sufficient to categorize these tumors as carcinoma. In one of Dr. Rosai's articles on the subject of FVPTC, he quoted Dr. Julian Huxley's remarks from a lecture given in the 1950s: ‘Cancer (malignancy) must be defined operatively in terms of what the tumor cells do, not what they look like; otherwise the term ceases to have biological meaning’ [32]. Because the histology and clinical outcome of mPTC, NI-EFV is similar to that of NIFTP, we recommend including PTC NI-EFV in the spectrum of NIFTP in order to avoid overtreatment and its side effects.
Acknowledgments
Funding: Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Research reported in this publication was supported in part by an Italian Government-Ministero della Salute Grant No. RF-2011-02350857 to G.T.
Footnotes
Conflict of interests: No competing financial interests exist for all contributory authors.
References
- 1.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. Jama. 2017;317(13):1338–48. doi: 10.1001/jama.2017.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Jung CK, Little MP, Lubin JH, Brenner AV, Wells SA, Jr, Sigurdson AJ, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. The Journal of clinical endocrinology and metabolism. 2014;99(2):E276–85. doi: 10.1210/jc.2013-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA oncology. 2016 doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLellis RA, Lloyd RV, Heitz PU, Eng C. Pathology and Genetics of Tumours of the Endocrine Organs. Third. Lyon, France: IARC Press; 2004. [Google Scholar]
- 6.Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–90. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23(9):1191–200. doi: 10.1038/modpathol.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, et al. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107(6):1255–64. doi: 10.1002/cncr.22138. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. American journal of clinical pathology. 2003;120(1):71–7. doi: 10.1309/ND8D-9LAJ-TRCT-G6QD. [DOI] [PubMed] [Google Scholar]
- 10.Rosario PW, Penna GC, Calsolari MR. Noninvasive encapsulated follicular variant of papillary thyroid carcinoma: is lobectomy sufficient for tumours >/=1 cm? Clin Endocrinol (Oxf) 2014;81(4):630–2. doi: 10.1111/cen.12387. [DOI] [PubMed] [Google Scholar]
- 11.Cecoli F, Ceresola EM, Altrinetti V, Cabria M, Cappagli M, Montepagani A, et al. Therapeutic Strategies and Clinical Outcome in Papillary Thyroid Microcarcinoma: A Multicenter Observational Study. Eur Thyroid J. 2016;5(3):180–6. doi: 10.1159/000446746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haugen BRM, Alexander EK, Bible KC, Doherty G, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddad RI, Lydiatt WM, Bischoff L, Busaidy NL, Byrd DR, Callender G, et al. NCCN clinical practive guidelines in oncology (NCCN guidelines): thyroid carcinoma. Version 1.2017. National Comprehensive Cancer Network. 2017 https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf.
- 14.Routhier CA, Mochel MC, Lynch K, Dias-Santagata D, Louis DN, Hoang MP. Comparison of 2 monoclonal antibodies for immunohistochemical detection of BRAF V600E mutation in malignant melanoma, pulmonary carcinoma, gastrointestinal carcinoma, thyroid carcinoma, and gliomas. Human pathology. 2013;44(11):2563–70. doi: 10.1016/j.humpath.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Tallini G, Tuttle RM, Ghossein RA. The History of the Follicular Variant of Papillary Thyroid Carcinoma. The Journal of clinical endocrinology and metabolism. 2016 doi: 10.1210/jc.2016-2976. jc20162976. [DOI] [PubMed] [Google Scholar]
- 16.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. The New England journal of medicine. 2016;375(7):614–7. doi: 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- 17.Rosai J, LiVolsi VA, Sobrinho-Simoes M, Williams ED. Renaming papillary microcarcinoma of the thyroid gland: the Porto proposal. International journal of surgical pathology. 2003;11(4):249–51. doi: 10.1177/106689690301100401. [DOI] [PubMed] [Google Scholar]
- 18.Ghossein R, Ganly I, Biagini A, Robenshtok E, Rivera M, Tuttle RM. Prognostic factors in papillary microcarcinoma with emphasis on histologic subtyping: a clinicopathologic study of 148 cases. Thyroid : official journal of the American Thyroid Association. 2014;24(2):245–53. doi: 10.1089/thy.2012.0645. [DOI] [PubMed] [Google Scholar]
- 19.Piana S, Ragazzi M, Tallini G, de Biase D, Ciarrocchi A, Frasoldati A, et al. Papillary thyroid microcarcinoma with fatal outcome: evidence of tumor progression in lymph node metastases: report of 3 cases, with morphological and molecular analysis. Human pathology. 2013;44(4):556–65. doi: 10.1016/j.humpath.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: A name change to Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features would help prevent overtreatment. Mod Pathol. 2016;29(7):698–707. doi: 10.1038/modpathol.2016.65. [DOI] [PubMed] [Google Scholar]
- 21.Rivera M, Tuttle RM, Patel S, Shaha A, Shah JP, Ghossein RA. Encapsulated papillary thyroid carcinoma: a clinico-pathologic study of 106 cases with emphasis on its morphologic subtypes (histologic growth pattern) Thyroid : official journal of the American Thyroid Association. 2009;19(2):119–27. doi: 10.1089/thy.2008.0303. [DOI] [PubMed] [Google Scholar]
- 22.Howitt BE, Paulson VA, Barletta JA. Absence of BRAF V600E in non-infiltrative, non-invasive follicular variant of papillary thyroid carcinoma. Histopathology. 2015;67(4):579–82. doi: 10.1111/his.12680. [DOI] [PubMed] [Google Scholar]
- 23.Cho U, Mete O, Kim MH, Bae JS, Jung CK. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol. 2017 doi: 10.1038/modpathol.2017.9. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L, Dias-Santagata D, Sadow PM, Faquin WC. Cytological, molecular, and clinical features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features versus invasive forms of follicular variant of papillary thyroid carcinoma. Cancer. 2017 doi: 10.1002/cncy.21839. [DOI] [PubMed] [Google Scholar]
- 25.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA oncology. 2016;2(8):1023–9. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. The American journal of medicine. 1994;97(5):418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 27.Nwatsock JF, Taieb D, Zok FD, Mundler O. Late Recurrences of Thyroid Carcinoma 24 Years after a Complete Remission: When Monitoring Should be Stopped? World J Nucl Med. 2012;11(1):42–3. doi: 10.4103/1450-1147.98749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganly I, Wang L, Tuttle RM, Katabi N, Ceballos GA, Harach HR, et al. Invasion rather than nuclear features correlates with outcome in encapsulated follicular tumors: further evidence for the reclassification of the encapsulated papillary thyroid carcinoma follicular variant. Human pathology. 2015;46(5):657–64. doi: 10.1016/j.humpath.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu B, Tallini G, Scognamiglio T, Roman BR, Tuttle RM, Ghossein RA. Outcome of Large Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid : official journal of the American Thyroid Association. 2017;27(4):512–7. doi: 10.1089/thy.2016.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosai J, DeLellis RA, Carcangiu ML, Frable WJ, G T. Tumor of the thyroid and parathyroid gland (AFIP atlas of tumor pathology series 4) Silver Spring, MD: American Registry of Pathology Press; 2015. [Google Scholar]
- 31.La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. International journal of cancer Journal international du cancer. 2015;136(9):2187–95. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 32.Rosai J. Papillary thyroid carcinoma: a root-and-branch rethink. American journal of clinical pathology. 2008;130(5):683–6. doi: 10.1309/AJCPBF63BWMCYSLW. [DOI] [PubMed] [Google Scholar]