Abstract
The suitability of various neurokinin-2 (NK2) receptor agonists and routes of administration to elicit on-demand voiding of the bladder and bowel, as future therapy for individuals with spinal cord injury, was examined using a rat model. The current study examined the feasibility of alternative routes of administration, which are more practical for clinical use than intravenous (IV) administration. Voiding and isovolumetric cystometry were recorded in anesthetized, acutely spinalized, female rats after IV, subcutaneous (SC), intramuscular (IM), intranasal (IN), or sublingual (SL) administration of [Lys5,MeLeu9,Nle10]-NKA(4–10] (LMN-NKA). Administration of LMN-NKA (1–10 μg/kg IV; 10–300 μg/kg SC or IM; 15–1000 μg/kg IN or 300–1,500 μg/kg SL) elicited rapid-onset, short-duration, dose-related increases in bladder pressure and voiding with the rank order for time of both onset and duration being IV < IN < SC = IM < SL. The incidence of voiding was dependent on the dose and route, with all routes resulting in a high voiding efficiency (~70%). Like LMN-NKA, neurokinin A (NKA 1–100 μg/kg IV) and GR 64349 (0.1–30 μg/kg IV or 1–300 μg/kg SC) produced rapid-onset, short-duration increases in bladder pressure, as well as colorectal pressure. Administration of vehicle never produced bladder or rectal contractions or voiding. Transient hypotension was observed after IV injection of LMN-NKA, which was less pronounced after SC injection. Hypotension was not apparent with GR 64349. In conclusion, selective NK2 receptor agonists, administered through various non-IV routes of administration, may provide a safe, convenient, and efficacious method for inducing voiding.
Keywords: Neurokinin-2 receptor, spinal cord injury, voiding efficiency, cystometry, colorectal pressure
1. Introduction
The development of a safe and efficacious pharmaceutical for on-demand micturition and defecation would greatly improve the quality-of-life of individuals with spinal cord injury (SCI). The neurokinin-2 (NK2) receptor is a potential candidate for pharmacological intervention. Agonists can contract the bladder and colon both by directly activating NK2 receptors on the smooth muscle (Burcher et al, 1986; Mussap et al, 1996; Parlani et al, 1996; Warner et al, 2003; Burcher et al, 2000) and indirectly by activation of neural pathways (Maggi et al, 1987; Maggi et al, 1991; Bushfield et al, 1993). NK2 receptor agonists also increase bladder tone, contractility, and distension-evoked responses (Maggi et al, 1987; Tramontana et al, 1998; Kullmann et al, 2017) and increase gastrointestinal (GI) activity (Lecci et al, 1997; Mule et al, 2000) in vivo. However, the ability to contract bladder and GI smooth muscle does not necessarily indicate clinical utility because resistance of the urethral and anal sphincters must be overcome for voiding to occur. It is unclear whether this can be achieved since Palea et al (1996) found that a NK2 receptor agonist contracted human urethral muscle, suggesting that occlusion of the urethra might occur simultaneously with contraction of the bladder to obstruct voiding.
NK2 receptor activation may also cause bronchoconstriction (Evans et al, 1988). Many NK2 receptor agonists also have high affinity for the ‘septide binding site’ of the NK1 receptor (Sagan et al, 1996; Hastrup & Schwartz, 1996; Torrens et al, 2000). Activation of NK1 receptors may cause hypotension and bronchoconstriction (Feldman, 1995; Mutoh et al, 2000). Accordingly, we identified LMN-NKA ([Lys5,MeLeu9,Nle10]-NKA(4–10)) and GR 64349 as compounds of interest based on their >250-fold selectivity for NK2 over NK1 receptors, compared with only 20-fold selectivity for the endogenous NK2 receptor agonist, NKA (Rupniak et al, unpublished results).
Drug-induced voiding should mimic physiological voiding by eliciting a rapid onset, short-duration bladder contraction. Intravenous administration is not practical for daily use. Therefore the present study examined the pharmacodynamic characteristics of subcutaneous and intramuscular dosing, and the feasibility of intranasal and sublingual administration, of NK2 receptor peptide agonists.
2. Materials and Methods
2.1. Animals
Female Sprague-Dawley rats (230–300 g, Charles River, Raleigh, NC) were maintained under standard conditions of laboratory housing with free access to food and water. Experiments conformed with NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Integrated Laboratory Systems Animal Care and Use committee. The number of animals was the minimum needed to determine the pharmacodynamic characteristics of LMN-NKA administered via various routes of administration and compare its characteristics to NKA and GR 63,449 (n = 2–21 per dose group). There was a variation in group size because fewer animals were tested at the lower doses that were ineffective at producing a pharmacodynamic effect and at the higher doses that reached a plateau of response.
2.2. Surgical procedures
Rats were anesthetized with urethane (1.2–1.4 g/kg urethane subcutaneous (SC), supplemented as needed to maintain adequate anesthesia). Body temperature was maintained at 37°C with a heated blanket. For the spinal cord transection, the skin and muscle over the middle thoracic vertebrae were incised, the spinal cord was exposed by a laminectomy, and the cord transected at T9-T10 at least 60 min prior to administration of test compounds. The carotid artery and jugular vein were cannulated for blood pressure recording and intravenous (IV) injections, respectively. The urinary bladder was exposed via a midline abdominal incision, and the ureters were ligated and cut. A flared tipped catheter (PE 50) was inserted into the bladder through a small incision at the dome and tied in place. The catheter was connected to an infusion pump (Harvard Apparatus; Perfusion Pump PhD2000) to allow the bladder to be filled with 0.9% saline, and a pressure transducer (Utah Medical Products; DelTran II) to record bladder pressure. Colorectal pressure was measured in a separate group of animals. The rectum was cleared of feces and a balloon catheter (2.5 cm length, made using the tip of a Trojan ENZ non-lubricated condom attached to PE 50 tubing and a 20 G blunt needle) was inserted 2–4 cm into the rectum from the anus and inflated with 0.1–0.2 ml increments of saline until a stable baseline pressure between 15–20 mmHg was achieved. Bladder and rectal pressures were amplified using a bridge amplifier (Transbridge 4M, World Precision Instruments). At the end of the experiment, rats were euthanized with an overdose of urethane followed by thoracotomy.
2.3. Bladder Pressure and Voiding
2.3.1 Isovolumetric Cystometry
To determine capacity, the bladder was emptied and cystometrograms (CMGs) were performed with the urethra open. The bladder was filled with saline (0.04–0.1 ml/min) until passive leaking of fluid from the bladder was noted; the pump was stopped, the fluid removed from the bladder and the volume recorded as the bladder capacity. Three CMGs were averaged to determine the baseline bladder capacity.
The effect of test compounds on bladder pressure was determined with the urethral meatus clamped and the bladder filled to 70% of bladder capacity, where it remained throughout the procedure. When bladder pressure was stable, vehicle and ascending doses of test compounds were administered, each separated by at least 20 min to allow the bladder to return towards baseline pressure.
2.3.2. Voiding Cystometry
The bladder infusion rate was adjusted (0.04–0.1 ml/min) for each rat in order to achieve leak point capacity within 15 min and remained constant throughout the experiment. The bladder was filled to 70% of capacity and the infusion pump switched off to examine the effects of test compounds on bladder voiding pressure and efficiency. The effect of vehicle or test compounds was recorded for 30 min, and the volume voided (if any) after the first 5 min was measured. The bladder was then emptied to measure the residual volume. The infusion pump was restarted and distension-induced contractions were monitored until the bladder returned to baseline characteristics or at least 40 min had elapsed. The cycle was then repeated with the next dose of test compound.
2.3.3. Bladder Parameters Measured
Bladder pressure was amplified and digitized for off-line analysis. Baseline pressure, change from baseline pressure, peak pressure, pressure area under the curve (AUC, for 0–30 min post dosing), time to onset of bladder contraction/voiding, time to peak pressure, voiding pressure, voided volume (VV), residual volume (RV), and voiding efficiency (VE) calculated as ((VV/(VV + RV)) × 100%). The voiding responder rate was calculated as the number of rats voiding >0.03 ml divided by total number of rats per group × 100%. A test compound-induced contraction was defined as an increase in bladder pressure of ≥ 5 mmHg above baseline within 5 min of dosing.
2.4. Colorectal Pressure
Colorectal manometry was performed using a balloon catheter that was inflated incrementally with 0.1–0.2 ml saline until a steady pressure reading of 15–20 mmHg was obtained (total volume 0.3–0.7 ml). When colorectal pressure was stable, vehicle was administered followed by ascending doses of test compound, each separated by 20–45 min. For colorectal pressures, the AUC was calculated 0–5 min after each dose. Colorectal pressures were amplified and digitized for off-line analysis.
2.5. Other Pharmacodynamic Effects
Blood pressure values were recorded continuously via the carotid artery, and heart rate was calculated off line from the blood pressure signal (LabChart 8, ADInstruments). The effect of test compounds was expressed as a change from baseline values immediately before each dose. Changes in skin color, salivation and lacrimation were recorded if present. Respiration was observed visually for any obvious changes.
2.6. Pharmacokinetics
Pharmacokinetic (PK) studies were performed to define plasma exposure following IV and SC administration of LMN-NKA. In addition, preliminary PK studies were performed with IN and SL dosing. Spinal intact female rats were anesthetized with urethane 1.4 g/kg intraperitoneally and supplemented as needed to maintain adequate anesthesia. A catheter (PE 50) placed in the carotid artery was used for collection of up to 7 blood samples (700 μl each) following IV (1, 2, 3, 4, 6 and 8 min) and SC (1, 3, 5, 10, 20 and 40 min) dosing. Blood was directly collected into EDTA tubes (BD Microtainer Tube 365974, FisherScientific, Pittsburg, PA, USA) prefilled with 70 μl of cold 10% ascorbic acid for stabilization. Tubes were immediately inverted and mixed in a gentle vortex. Samples were placed on ice until centrifugation within 20 min for 5–7 min at 4°C, 2000 rcf. Plasma samples were frozen and stored at −80 °C until bioanlaysis. Samples were analyzed using 100 μl aliquots and a solid-phase extraction procedure followed by liquid chromatography/tandem mass spectrometry (LC/MS/MS). Concentrations of LMN-NKA were calculated with a 1/x2 linear regression over a concentration range of 0.088 to 88.0 ng/ml using [13C; 15N]LMN-NKA as an internal standard. An API 5000 platform was operated under optimized conditions for detection of LMN-NKA positive ions formed by electrospray ionization. The qualified concentration range for this assay was 1–1000 ng/ml.
2.7. Formulation and Administration of Test Compounds
NKA and GR 64349 ([Lys3,Gly8-R-gamma-lactam-Leu9]-NKA(3–10)) were purchased from Tocris (Bristol, UK) as the trifluroacetic acid salts. LMN-NKA was synthetized by Bachem (Torrance, CA, USA) as the acetate salt. Compounds were dissolved in 0.9% saline. For IV injection, compounds were administered in 200 μl followed by a 300 μl saline flush; for SC and intramuscular (IM) administration, the injection volume was 100–300 μl of a 0.01–10 mg/ml solution. The dose range tested was 0.01–1000 μg/kg. For sublingual (SL) administration, a prototype orally disintegrating tablet was developed containing 0.4–5 mg LMN-NKA in a matrix base of 3% gelatin with 2% glycine and 1% sorbitol to increase hydrophilicity (Bae et al, 2016). Aliquots (200 uL) of the formulation were blister packed and frozen at –80°C prior to lyophilization under vacuum (200 Torr) using the step gradient method with a VirTis adVantage Wizard 2.0 desktop lyophilizer (SP Scientific, New York, USA). On the day of the experiment, the rat’s mouth was swabbed and ¼, ½ or a whole tablet (9 × 4 mm) was placed under the tongue, followed by 50–200 μl of water to promote dissolution. The mouth was held closed for the time required for complete tablet dissolution (~20 s in vitro) in order to maintain contact with the sublingual mucosa. For intranasal (IN) administration, LMN-NKA was dissolved in 0.9% saline with or without the following excipients: 1% polysorbate 80 (surfactant) or 0.2% ethylenediaminetetraacetic acid (EDTA, antioxidant). 5–200 μl of a 0.5–3 mg/ml solution was administered into one nostril using a micropipette; each formulation was tested 4–8 times and the data presented are the mean of the 3 formulations as no differences were noted.
2.8. Data Analysis and Statistics
Cystometric and manometry data were recorded using LabChart software (versions 7 and 8) through a PowerLab/8SP data acquisition system (ADInstruments, Australia). Data were analyzed using Excel (Microsoft, Redmond, WA) and Prism 6 (GraphPad Software Inc., San Diego, CA). Data were subjected to ANOVA with Dunnett’s multiple comparison test or Sidak’s multiple comparison test to compare the effect of test compounds with vehicle, or Student’s t-test, as appropriate; P < 0.05 was considered statistically significant. Bioanalytical data were collected using Analyst (MDS Sciex, Framingham, MA, USA). Regression analysis was generated using Watson Laboratory Information Management System (ThermoFisher Scientific, Logan, UT, USA) and Excel (Microsoft, Redmond, WA, USA). Noncompartmental PK analysis was performed using Phoenix WinNonlin 6.3 (Certara, St. Louis, MO, USA) or PK Solver (Zhang et al, 2010). AUC was calculated between the first and last sample time points. All values are expressed as the mean + standard deviation (S.D.), except the cardiovascular data in Fig. 6 which is presented as mean + standard error (S.E.).
Figure 6.
Change in Diastolic Blood Pressure Following IV or SC Injection of LMN-NKA and GR 64349.
LMN-NKA, but not GR 64349, induced hypotension in acute spinalized rats. Data are mean + S.E. from 3–19 rats. Filled circles - LMN-NKA; open circles - GR 64349. One-way ANOVA for LMN-NKA data: F(3,8) = 6.13 for IV and F(4,32) = 4.38 for SC, both p=0.061. *p<0.05 compared with 0. 1 μg/kg IV or 3 μg/kg SC, Dunnett’s multiple comparison test. LMN-NKA was compared with GR 64349 using two-way ANOVA: F(1,57) = 9.394, P=0.003 for SC. # P<0.05 compared with GR 64349, Sidak’s multiple comparison test.
3. Results
3.1. EFFECTS ON LOWER URINARY TRACT
3.1.1. Comparison of alternative routes of administration of LMN-NKA on bladder pressure during isovolumetric cystometry
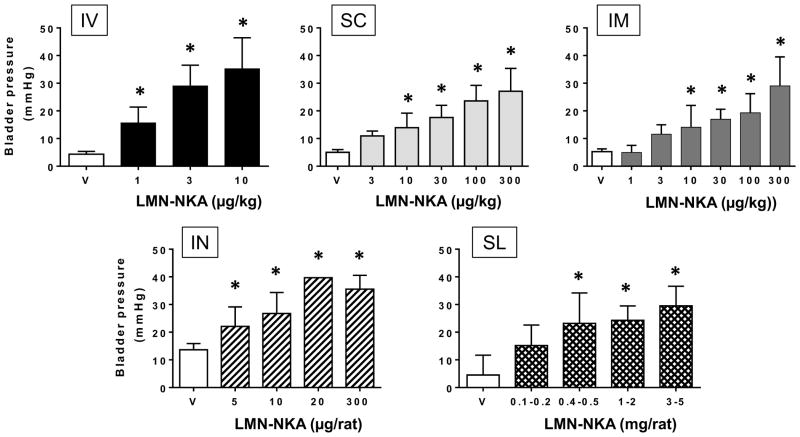
Under isovolumetric conditions, bladder pressure was increased in a dose-dependent manner by up to 35 mmHg following IV injection of LMN-NKA at doses of 1–10 μg/kg (Fig. 1). Dose-related increases in bladder pressure were also recorded after SC or IM administration of doses in the range 10–300 μg/kg, indicating 10 to 30-fold lower potency compared to the IV route (Fig. 1). Following IN administration of three prototype formulations of LMN-NKA (see Methods), bladder pressure increased with doses of 5 to 300 μg per rat (~15–1000 μg/kg), comparable to the increase produced by an IV dose of 3 μg/kg, indicating good efficacy after IN application. Exploratory studies using prototypes of orally disintegrating tablets of different dose strengths (0.1–5 mg per rat, or ~300–1,500 μg/kg), also revealed a dose-related increase in bladder pressure, indicating that there was sufficient absorption of LMN-NKA from the SL mucosa to produce a pharmacodynamic effect of comparable magnitude to that seen with an IV bolus dose (Fig. 1), but requiring much higher doses.
Figure 1.
Increase in Isovolumetric Bladder Pressure Following IV, SC, IM, IN or SL Administration of LMN-NKA. LMN-NKA produced dose-related increases in bladder pressure in acute spinalized rats under isovolumetric conditions with the bladder at 70% capacity. Values represent the increase above baseline pressure and are the mean + S.D. from 4–21 rats per group, except 20 μg IN where N=2. One-way ANOVA: F (3,45) = 25.30, for IV; F (5,41) = 16.48, for SC; F (6,50) = 17.03, for IM; F (4,28) = 19.27, for IN and F (4,35) = 17.08 for SL, all p<0.001. *P<0.05 compared with vehicle, Dunnett’s multiple comparison test.
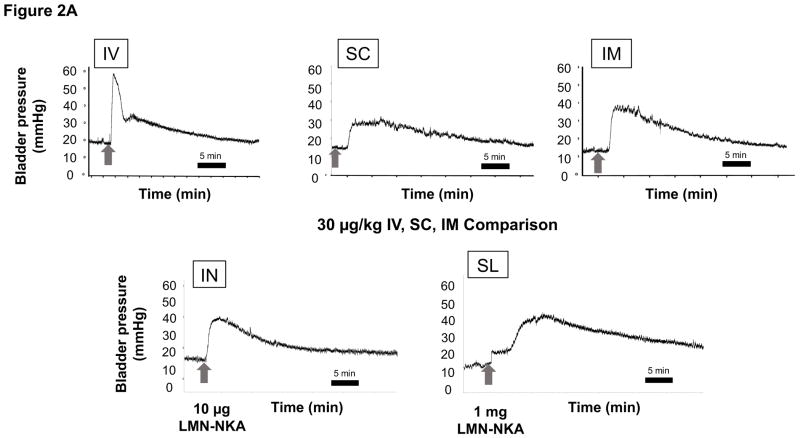
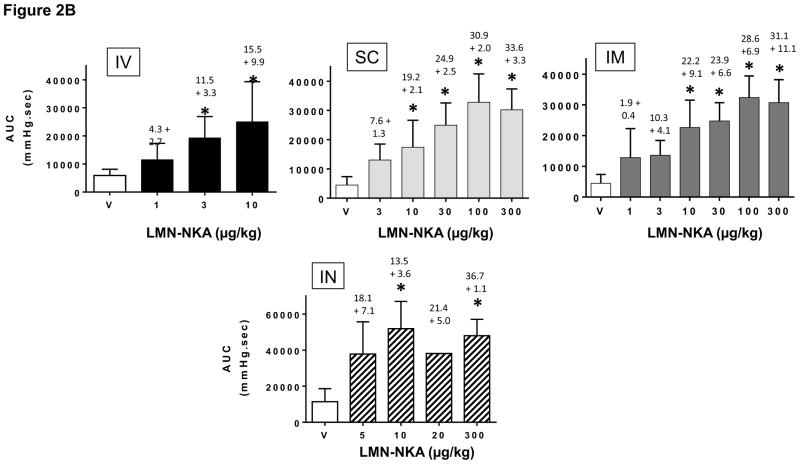
The increase in bladder pressure was rapid, peaking within 1 min after IV, within 2 min after IN, less than 4 min after SC and IM, and within 8 min after SL administration of LMN-NKA (Fig. 2A). Physiograph recordings revealed that after IV doses >10 μg/kg, there was a sharp spike in bladder pressure at the onset of effect that was not present after SC, IM, IN, or SL administration (Fig. 2A). The duration of the bladder pressure response under isovolumetric conditions was shortest after IV injection (Fig. 2A), around 20 min after IN, 20–30 min after SC or IM (Fig. 2B), and 20–40 min after SL administration. There was a dose-related increase in the bladder pressure AUC recorded over 30 min after IV, SC and IM administration, with similar maximal AUCs for each of these routes of administration (2,500–3,000 mmHg*s; Fig. 2B).
Figure 2.
[a] Representative Physiograph Tracings after IV, SC, IM, IN and SL Administration of LMN-NKA and [b] Increase in Isovolumetric Bladder Pressure AUC after IV, SC, IM and IN Administration.
[a] Representative physiograph tracings illustrate the effect of IV, SC and IM (30 μg/kg of LMN-NKA), IN (10 μg/rat LMN-NKA) and SL (1 mg/rat LMN-NKA) on bladder pressure during isovolumetric cystometry.
[b] AUC was measured over 30 min after each dose in acute spinalized rats under isovolumetric conditions. Histograms display the mean + S.D. from 4–11 rats per group after IV, SC, IM and IN administration of LMN-NKA. Numbers above each bar represent the mean + S.D. of the duration of the bladder response. One-way ANOVA: F (3,45) = 9.66, for IV; F (5,40) = 13.2, for SC and F (6,41) = 13.34 for IM, all P<0.001: and F(4,18) = 4.247 for IN, P= 0.138. *P<0.05 compared with vehicle Dunnett’s multiple comparison test.
3.1.2. Bladder voiding pressure and voiding efficiency using transvesical cystometry
At a volume of 70% of leak point capacity, no increase in bladder pressure was observed, and no rats voided within 5 min of administration of vehicle. Voiding was induced dose-dependently by LMN-NKA after IV, SC, IM and IN injection in up to 67–100% of rats, depending on the dose and route (Table 1). Voiding occurred most rapidly after IV (within 30 s) and IM (within 2 min) dosing, followed by SC and IN administration (within 5 min). There was a dose-related increase in VE up to ~70% with all routes of administration. Comparison of the doses that produced >50% VE (>30 μg/kg for IV, >66 μg/kg for IN and >100 μg/kg for SC and IM) indicated a 2–3-fold rightward shift in the dose response curves for SC, IM and IN versus IV administration (Table 1).
Table 1.
Voiding Responder Rates and Voiding Efficiency Following IV, SC, IM and IN Administration of LMN-NKA
| Route of Administration | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| IV | SC | IM | IN # | ||||||
| Dose (μg/kg) | % Voiding | Voiding Efficiency | % Voiding | Voiding Efficiency | % Voiding | Voiding Efficiency | Dose (μg/rat) | % Voiding | Voiding Efficiency |
| Vehicle | 0 (0/12) | 0 | 0 (0/3) | 0 | 0 (0/5) | 0 | Vehicle | 0 (0/4) | 0 (0/4) |
| 3 | ND | ND | ND | ND | 50 (1/2) | 34.9 | ND | ND | ND |
| 10 | 89 (8/9) | 31.4 + 30.8 | 22 (2/7) | 7.6 + 7.7 | 58 (7/12) | 33.4 + 29.4 | 5 | 67 (2/3) | 37.2 + 41.7 |
| 30 | 100 (3/3) | 59.5 + 42.5 | 89 (8/9) | 25.2 + 24.7 | 55 (6/11) | 34.5 + 21.1 | 10 | 75 (6/8) | 45.0 + 42.7 |
| 100 | 80 (4/5) | 71.7 + 22.3 | 86 (6/7) | 58.5 + 35.2 | 75 (9/12) | 47.5 + 30.7 | 20 | 67 (4/6) | 58.8 + 21.6 |
| 300 | ND | ND | 89 (8/9) | 68.7 + 24.8 | 73 (8/11) | 76.1 + 15.7 | 300 | 67 (2/3) | 76.3 + 6.5 |
| 1000 | ND | ND | 100 (2/2) | 77.8 + 31.4 | 100 (3/3) | 66.6 + 13.5 | ND | ND | ND |
% voiding was calculated as the number of rats voiding >0.03 ml divided by total number of rats per group × 100%. Note #: IN doses are expressed as μg/rat and % voiding groups include 1 rat that did not void with any IN dose. Voiding Efficiency (VE) was calculated for rats that voided. Values are mean + S.D. from 2–12 rats per group. ND: not determined.
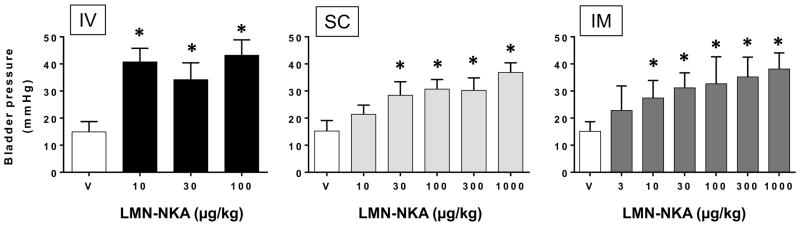
Maximal drug induced bladder voiding pressures were generally <40 mmHg (Fig. 3) regardless of dose or route of administration of LMN-NKA. The voiding pressure appeared to be slightly greater with IV administration of LMN-NKA than the average leak point pressures of 34.7 ± 7.86 mmHg (N=28) that was recorded during constant filling with saline. Maximum voiding pressures after SC and IM administration of LMN-NKA were similar to the average leak point pressures
Figure 3.
Bladder Pressure during Voiding Induced by IV, SC and IM Administration of LMN-NKA.
Values are the mean + S.D. bladder voiding pressure after LMN-NKA from 2–12 rats per group. Note that voiding pressure did not increase with increasing doses of LMN-NKA. This indicates that voiding pressure is set by intrinsic properties of the urethra in acute spinal animals and that LMN-NKA was not influencing urethral resistance. (No voiding occurred with saline vehicle and vehicle bars represent the baseline pressures at 70% bladder capacity) One-way ANOVA: F(3,24) = 68.02, for IV; F(5,41) = 21.19, for SC; F(6,48) = 6.39, for IM, all P<0.0001. *P<0.05 compared with vehicle baseline pressure, Dunnett’s multiple comparison tests.
3.1.3. Comparison of analogs of LMN-NKA under isovolumetric conditions
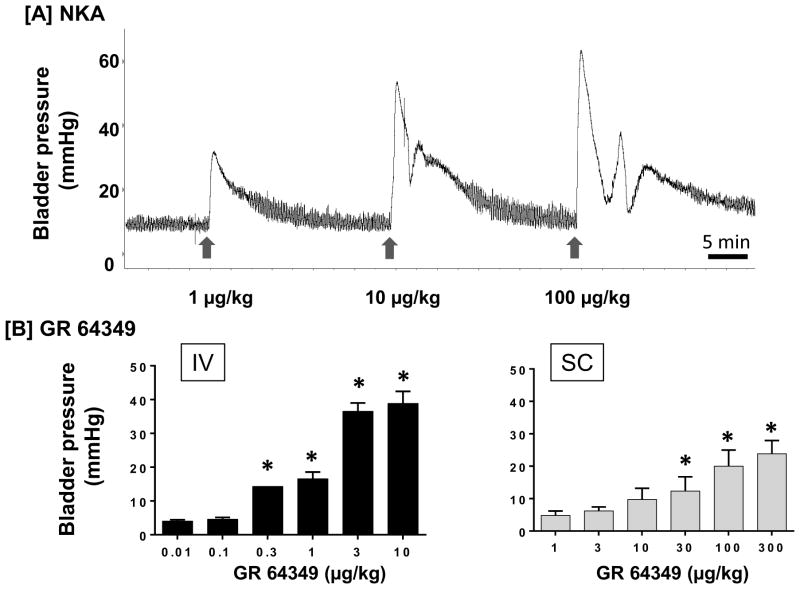
The effects of two additional NK2 receptor agonists were also examined for comparison with LMN-NKA. NKA (1–100 μg/kg IV) produced a dose-related increase in bladder pressure with a rapid onset, peaking within 1 min, similar to the effects seen with IV injection of LMN-NKA (Fig. 4A). Similarly, GR 64349 (0.01–10 μg/kg IV and 1–300 μg/kg SC) caused a dose-related increase in bladder pressure with a rapid onset (<1 min) (Fig. 4B). While the duration of action of NKA was similar to that of LMN-NKA, the duration of GR 64349 was significantly shorter for IV, but not SC, administration (after 1 μg/kg IV, the duration was 1.5 + 0.07 min for GR 64349 versus 8.4 ± 1.65 min for LMN-NKA, p=0.002 paired Student’s t-test, N=3; after 300 μg/kg SC, the duration was 23.9 ± 12.22 min for GR 64349 (N=4) versus 33.5 ± 3.34 min for LMN-NKA (N=11), p=0.39 unpaired Student’s t-test).
Figure 4.
Increase in Isovolumetric Bladder Pressure Following IV or SC Administration of NKA and GR 64349. [a] Physiograph showing a dose-related increase in bladder pressure with rapid onset after IV injection of NKA (1–100 μg/kg) determined under isovolumetric conditions in a representative acute spinalized rat. Arrows indicate the time of each dose. [b] IV and SC administration of GR 64349 produced dose-related increases in bladder pressure over baseline under isovolumetric conditions. Bladder volume was set at 70% capacity. Values represent the increase above baseline pressure. Values are the mean + S.D. from 3–5 rats per group, except 0.3 μg/kg IV, where N=2. One-way ANOVA: F(6,13) = 151.4 for IV; F(5,23) = 21.3 for SC, both P<0.001. *P<0.05 compared with 0.01 μg/kg IV or 1 μg/kg SC, Dunnett’s multiple comparison test.
3.2. EFFECTS ON GASTROINTESTINAL TRACT
3.2.1. Comparison of alternative routes of administration of NK2 agonists on colorectal pressure
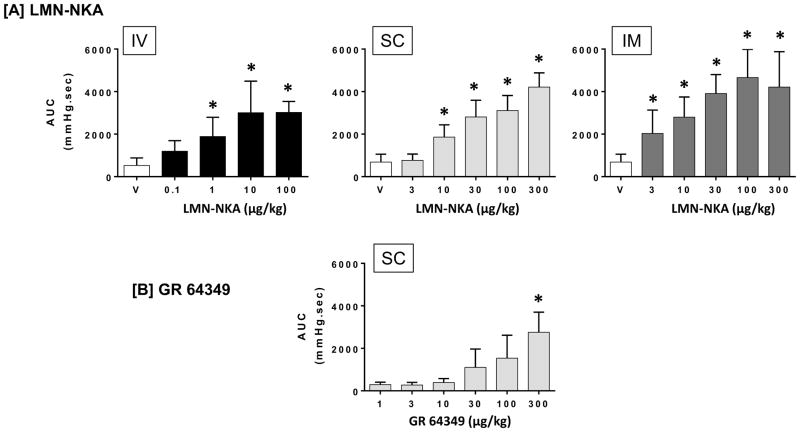
Administration of LMN-NKA produced a dose-dependent increase in colorectal pressure after IV (>1 μg/kg), SC (>10 μg/kg) or IM (>3 μg/kg) administration. The AUCs for colorectal pressures recorded for 5 min after each dose of LMN-NKA are shown in Fig. 5A. Increased colorectal pressure was seen at similar doses to those that activated the bladder. Colorectal activity remained elevated for up to 20 min after 100 μg/kg IV, up to 50 min after 300 μg/kg SC, and 43 min after 300 μg/kg IM. Subcutaneous administration of GR 64349 also increased colorectal pressure, but only at the highest doses tested (100 and 300 μg/kg) and the duration of action at these doses were similar to the effects on the bladder (20–25 min). The AUC for colorectal pressure after SC administration of GR 64349 is shown in Fig. 5B.
Figure 5.
Increase in Colorectal Activity Following IV, SC or IM Administration of LMN-NKA and SC administration of GR 64349.
Graphs show the area under the curve (AUC) for colorectal activity. [a] LMN-NKA increased colorectal activity over baseline in a dose-related manner following IV, SC and IM administration. [b] GR 64349 increased colorectal activity following SC administration of 300 μg/kg. Values are the mean + S.D. from 4–14 rats per group. One-way ANOVA for LMN-NKA: F (4,27) = 10.2, P<0.001 for IV; F (5,36) = 41.8, p<0.001 for SC, and F (5,47) = 21.5, P<0.009 for IM. One-way ANOVA for GR 64349: F (5,16) = 6.632. *P<0.05 compared with vehicle or 1 μg/kg dose for GR 64349, Dunnett’s multiple comparison test.
3.3. OTHER PHARMACODYNAMIC EFFECTS
Transient hypotension was observed after injection of high doses of LMN-NKA (and NKA, data not shown). Diastolic blood pressure was reduced after IV (F (4,41)=4.865, p=0.0026) and SC (F(4,32)=4.384, p=0.0061) administration of LMN-NKA (Fig. 6). The hypotensive effect was significantly greater after IV than SC administration at doses that were equipotent on the bladder and rectum (30% fall from baseline diastolic pressure after 100 μg/kg IV compared to 15% after 300 μg/kg SC; unpaired Student’s t-test p=0.0058). There were small changes in systolic blood pressure and heart rate after IV administration of LMN-NKA that were not significant (<8% decrease from baseline systolic pressure, ANOVA F(4,41) = 0.80, p=0.53; <7% increase from baseline heart rate, ANOVA F(4,42) = 0.50, p=0.74). No significant blood pressure changes were observed after administration of GR 64349 (up to 30 μg/kg IV and 300 μg/kg SC, Fig. 6).
Transient flushing of the ears and paws was observed after administration of LMN-NKA (> 10 μg/kg IV; > 30 μg/kg SC or IM). NKA and GR 64349 also produced transient flushing of the ears and paws at similar or higher doses, respectively, compared to LMN-NKA. Salivation was observed only after extremely high doses of LMN-NKA (1,000 μg/kg, SC or IM). No obvious changes in respiration were observed with doses of LMN-NKA up to 300 μg/kg SC or IM, or with NKA or GR 64349.
3.4. PHARMACOKINETICS
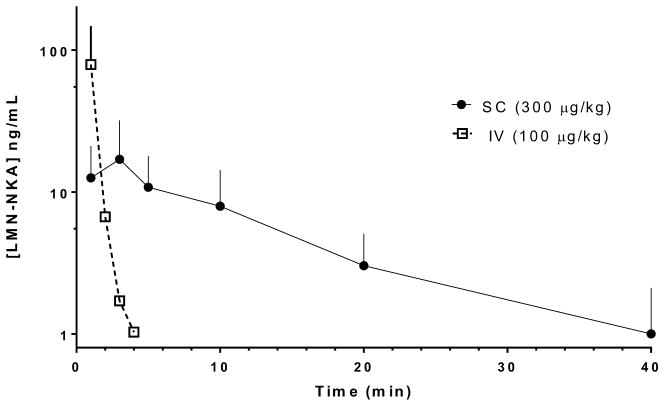
Plasma concentrations of LMN-NKA following IV and SC dose administration are shown in Fig. 7. Following IV dosing at 100 μg/kg the maximal plasma concentration (Cmax, 79 + 68 ng/ml) was observed at the shortest time measured, 1 min. The plasma concentration fell rapidly, with a plasma half-life (t1/2) of 0.7 + 0.6 min and was below the level of quantitation by 6 min post-dose. The extrapolated area under the curve (AUC) was 500 + 682 ng/ml*min. Exposures after 300 μg/kg SC (Cmax 18.9 + 14.0 ng/ml and AUC 183 + 115 ng/ml*min) were considerably lower compared with IV dosing of 100 μg/kg. The peak plasma concentration was observed at 3 min post-dose, and t1/2 was 10.2 + 4.4 min. Preliminary analysis of plasma samples confirmed measurable exposure to LMN-NKA following administration by the IN (~1000 μg/kg dose, N=2, Cmax=14.0+11.3 ng/ml) and SL (~7000 μg/kg dose, N=4, Cmax=59.6+45.6 ng/ml) routes, but detailed PK analysis awaits optimization of a final formulation.
Figure 7.
Comparison of LMN-NKA plasma concentrations following IV and SC dosing.
Individual rats (SC, N=12; IV, N=4) received doses of LMN-NKA at time=0 and plasma samples were collected at the times indicated. Data points represent the mean + S.D. Values below the level of detection (1 ng/ml) were not included in the analysis. Each time point represents N=9–12 (SC) and N=1–4 (IV) detected concentration values.
4. Discussion
The central nervous system regulates voluntary alternation between storage and voiding of urine and feces by coordinating the reciprocal activity of bladder and bowel smooth muscle with urethral and anal sphincter activity. After SCI, there is a loss of coordination that leads to conditions of urinary and fecal retention, as well as involuntary release of urine and feces (incontinence), creating a major impediment to the physical, psychological, occupational, and social rehabilitation of these patients (Krassioukov et al, 2010; Lee et al, 2016). Depending on the site of neural damage, symptoms may include a loss of storage capacity (where the bladder reflexively contracts and leaks urine as it fills); an areflexive bladder (where the detrusor does not contract, becomes distended, and leaks urine); and/or detrusor-sphincter dyssynergia caused by concomitant contraction of the detrusor and sphincter muscles (Han et al, 1998; Miyazato et al, 2013; Stoffel, 2016). Storage and emptying of the bowel is also dysfunctional, often requiring digital stimulation and manual extraction of stools. These conditions, and complications arising from their management, are a leading cause of morbidity and hospitalization following SCI (Manack et al, 2011; DeVivo and Farris 2011).
The objectives of the present studies were to establish 1) whether efficient, low pressure micturition could be elicited by LMN-NKA in rats with acute SCI, 2) whether practical formulations for life-long use are feasible, and 3) whether this property is shared by other NK2 receptor agonists. Following SCI, rats have severely underactive, areflexive bladders that do not void when full; but rather, fluid leaks from the urethra when bladder pressure exceeds urethral resistance. In these rats, various routes of administration of LMN-NKA (IV, SC, IM and IN) produced dose-related increases in bladder pressure and efficient voiding of urine. LMN-NKA dose-dependently increased both the incidence of voiding (up to 100% of rats) and the efficiency of voiding (up to ~70%). Consistent with the lower Cmax plasma exposures, doses ~3-fold higher was needed by the SC route than the IV route to produce equivalent effects on the bladder and colon. The peak bladder pressures recorded during voiding were typically <40 mmHg, below the range that might pose a risk of urine reflux or kidney damage.
As previously observed (Kullmann et al, 2017), bladder voiding pressure following IV administration of LMN-NKA (~40 mmHg) appeared to be slightly higher than leak point pressure (~35 mmHg), but the voiding pressure after IV administration did not increase in a dose dependent manner (dose range, 10–100 μg/kg), suggesting that urethral resistance is set by intrinsic urethral properties in acutely spinalized rats. However, a trend towards a dose-dependent increase in bladder pressure was observed with SC and IM administration which suggests that, in the absence of neural control (acute SCI), increasing drug doses produce an increase in both bladder and urethral pressure. Therefore, further studies are required to address the effects of various routes of administration of LMN-NKA on urethral function in vivo. An in vitro study comparing contraction of the human detrusor with the prostatic urethra showed GR 64349 produced contraction of detrusor muscle strips that were equivalent to maximal KCL-induced contractions with an EC50 74 nM, while contraction of urethral smooth muscle was only 43% of maximal KCL-induced contractions and required higher concentrations (EC50 150 nM; Palea et al, 1996). Regardless, LMN-NKA induced voiding does not generate clinical concern regarding elevated voiding pressure or concerns about renal function. As in the urethra, NK2 receptors are involved in controlling the internal anal sphincter (Tichenor et al, 2002), and their activation may increase resistance to defecation. Further studies are in progress to assess the effects of LMN-NKA on defecation efficiency.
A second objective was to determine if LMN-NKA could contract the bladder and colon using alternative routes of administration that are more feasible for repeated, chronic use than IV. Like IV administration, SC, IM, IN and SL administration of LMN-NKA increased bladder pressure and induced voiding. The rapid onset and efficacy of the IN and SL formulations on bladder contraction pressure, was somewhat unexpected considering that penetration of peptides through the IN and SL mucosa, and rapid enzymatic degradation of the peptides, are obvious concerns for trans-mucosal peptide drug delivery. Considering that the IN and SL formulations used in this study were simple prototypes created to determine feasibility, their efficacy supports optimization of these formulations.
An optimal pharmacotherapy for this indication would not only have a rapid onset, but also a short duration of action so that the bladder and bowel can quickly return to their resting state after voiding is complete. While bladder pressure recorded under isovolumetric conditions indicated pressure remained elevated for 20–30 min, voiding cystometry indicated that voiding was finished in less than 5 min and was accompanied by a more rapid pressure drop after IV, SC, IM, or IN injection of LMN-NKA. The longer time to onset and duration of effects after SC versus IV administration was correlated with the plasma concentrations, which increased slower and remained elevated longer after SC compared to IV administration. This was expected since the SC injection site acts as a depot for absorption. With further formulation refinement, achieving a more rapid absorption and depletion of the peptides from the subcutaneous space is a reasonable expectation.
The 2 other NK2 receptor agonists, NKA and GR 64349, also produced elevations in bladder and rectal pressure that were similar in regards to dose ranges and time to onset of contractions. While NKA exhibited a similar duration of action to LMN-NKA, GR 64349 had a significantly shorter duration of action. Since a short duration of action is a desirable property for drug-induced voiding therapy, GR 64349 may be a better candidate and require less refinement of formulation. ([β-Ala8]-NKA(4–10) also increased bladder pressure (unpublished observations), but a dose-response was not constructed because of limited solubility.
A further objective of this study was to examine the hypotensive effects of LMN-NKA. After IV injection of a high dose of LMN-NKA (100 μg/kg), transient hypotension (30% fall from baseline diastolic blood pressure) was observed. Hypotension was significantly less following SC compared to IV administration of LMN-NKA (Fig. 6) at doses that were equipotent regarding VE (Table 1), suggesting that the high initial plasma exposures resulting from IV injection may cause undesirable cardiovascular effects. The Cmax measured after IV administration was 4 times higher than after SC administration of a 3-fold higher, but equipotent, dose (79 vs 18.9 ng/ml, respectively, after 100 μg/kg IV and 300 μg/kg SC). However, because the half-life of the peptide in plasma is short (0.7 min), substantial metabolism of the peptide occurs immediately after injection, and the Cmax measured at the earliest time point (1 min) is substantially lower than the “real” Cmax. This difference in plasma concentrations after IV versus SC administration is likely responsible for the more prominent hypotensive response after IV injection. Blood pressure effects are especially important to monitor in individuals with SCI above T6 who have impaired ability to regulate blood pressure by cardiac and peripheral vascular mechanisms (Bauman et al, 2012). Selection of a compound that activates NK1 receptors weakly, or not at all, combined with a method of administration that avoids unnecessarily high plasma exposure should provide suitable efficacy and tolerability for chronic human use.
Importantly, hypotension was not observed after administration of GR 64349 (up to 30 μg/kg IV and 300 μg/kg SC), which has markedly lower NK1 receptor activity than LMN-NKA or NKA. Using a functional assay of NK1 receptor activation in human recombinant cell lines, GR 64349 was a very weak partial agonist (EC50 ~5 μM; Rupniak et al, unpublished observations), while LMN-NKA was a full agonist (EC50 15.5 nM). Thus, the absence of hypotension with GR 64349 suggests that the NK1 receptor could mediate the hypotension. This suggestion is supported by our on-going studies in dogs, minipigs, and monkeys showing that transient hypotension after IV injection of NKA and LMN-NKA is blocked by the NK1 receptor antagonists, CP-99,994 and spantide I (unpublished observations). However, further studies are needed to determine whether LMN-NKA and NKA-induced hypotension in the rat is blocked with NK1 receptor antagonists and whether GR 64349 produces hypotension in other species, since intra-arterial GR 64349 was reported to cause a transient drop in blood pressure in guinea pigs (Beattie et al, 1993).
Finally, flushing of the paws and ears observed in the current study with every NK2 receptor agonist tested may be analogous to the dermal flushing reported after IV infusion of low doses of NKA in humans (2 pmol/kg/min). The flushing was not blocked by a selective NK2 receptor antagonist, suggesting that it was not mediated via NK2 receptors (Lordal et al, 2001). Importantly, no obvious respiratory effects were observed with any of the NK2 receptor agonists tested at doses that caused significant contraction of the bladder and colon in vivo.
The present studies in anesthetized, acutely spinalized rats indicate that selective NK2 receptor agonists may be a novel class of potential therapeutics to produce on-demand urinary voiding in a dose range that is free of undesirable cardiovascular or respiratory effects. Moreover, such agents may be effectively administered via multiple routes of administration that are convenient for use by individuals with SCI and other conditions associated with an underactive bladder and bowel.
Acknowledgments
This work was supported by National Institutes of Health [grant numbers DK103392 and NS092178]. The authors thank Dr J. Bae and Dr M. Jay for providing the tablet formulations, and Integrated Laboratory Systems for their collaboration.
Footnotes
Conflict of interest statement
The authors declare they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bae J, Johnston TA, Huckle J, Marson L, Jay M. Formulation and evaluation of lyophilized orally disintegrating tablets of [Lys5,MeLeu9,Nle10]-neurokinin A(4–10).. Presented at Am. Assoc. Pharmaceut. Sci. (AAPS).2016. [Google Scholar]
- Bauman CA, Milligan JD, Lee FJ, Riva JJ. Autonomic dysreflexia in spinal cord injury patients: an overview. J Can Chiropr Assoc. 2012;56:247–250. [PMC free article] [PubMed] [Google Scholar]
- Beattie DT, McNeil DK, Connor HE. The influence of neurokinins and calcitonin gene-related peptide on cerebral blood flow in anaesthetized guinea-pigs. Neuropeptides. 1993;24:343–349. doi: 10.1016/0143-4179(93)90005-u. [DOI] [PubMed] [Google Scholar]
- Burcher E, Buck SH, Lovenberg W, O’Donohue TL. Characterization and autoradiographic localization of multiple tachykinin binding sites in gastrointestinal tract and bladder. J Pharmacol Exp Ther. 1986;236:819–831. [PubMed] [Google Scholar]
- Burcher E, Zeng XP, Strigas J, Shang F, Millard RJ, Moore KH. Autoradiographic localization of tachykinin and calcitonin gene-related peptide receptors in adult urinary bladder. J Urol. 2000;163:331–337. [PubMed] [Google Scholar]
- Bushfield M, Metcalfe M, Naylor AM. Activation of the micturition reflex by NK2 receptor stimulation in the anaesthetized guinea-pig. Br J Pharmacol. 1993;115:875–882. doi: 10.1111/j.1476-5381.1995.tb15891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVivo MJ, Farris V. Causes and costs of unplanned hospitalizations among persons with spinal cord injury. Top Spinal Cord Injury Rehab. 2011;16:53–61. [Google Scholar]
- Evans TW, Dixon CM, Clarke B, Conradson TB, Barnes PJ. Comparison of neurokinin A and Substance P on cardiovascular and airway function in man. Br J Clin Pharmacol. 1988;25:273–275. doi: 10.1111/j.1365-2125.1988.tb03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman PD. Neurokinin1 receptor mediation of the vasodepressor effects of substance P in the nucleus of the tractus solitarius. J Pharmacol Exp Ther. 1995;273:617–623. [PubMed] [Google Scholar]
- Han TR, Kim JH, Kwon BS. Chronic gastrointestinal problems and bowel dysfunction in patients with spinal cord injury. Spinal Cord. 1998;36:485–490. doi: 10.1038/sj.sc.3100616. [DOI] [PubMed] [Google Scholar]
- Hastrup H, Schwartz TW. Septide and Neurokinin A are high-affinity ligands on the NK-1 receptor: evidence from homologous versus heterologous binding analysis. FEBS Letts. 1996;399:264–266. doi: 10.1016/s0014-5793(96)01337-3. [DOI] [PubMed] [Google Scholar]
- Krassioukov A, Eng JJ, Claxton G, Sakakibara BM, Shum S SCIRE Research Team. Neurogenic bowel management after spinal cord injury: A systematic review of the evidence. Spinal Cord. 2010;48:718–733. doi: 10.1038/sc.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann FA, Katofiasc M, Thor KB, Marson L. Pharmacodynamic evaluation of Lys5,MeLeu9, Nle10-NKA(4–10) prokinetic effects on bladder and colon activity in acute spinal cord transected and spinally intact rats. Naunyn Schmied Arch Pharmacol. 2017;390:163–173. doi: 10.1007/s00210-016-1317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecci A, Tramontana M, Giuliani S, Maggi CA. Role of tachykinin NK1 and NK2 receptors on colonic motility in anesthetized rats: effect of agonists. Can J Physiol Pharmacol. 1997;75:582–586. [PubMed] [Google Scholar]
- Lee JS, Kim SW, Jee SH, Kim JC, Choi JB, Cho SY, Kim JH Korea Spinal Cord Injury Association. Factors affecting quality of life among spinal cord injury patients in Korea. Int Neurourol J. 2016;20:316–320. doi: 10.5213/inj.1630540.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordal M, Navalesi G, Theodorsson E, Maggi CA, Hellstrom PM. A novel tachykinin NK2 receptor antagonist prevents motility-stimulating effects of neurokinin A in small intestine. Br J Pharmacol. 2001;134:215–223. doi: 10.1038/sj.bjp.0704217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S, Santicioli P, Abelli L, Giachetti A. Facilitation of reflex micturition by intravesical administration of [β-Ala8]NKA(4–10), a selective NK2 tachykinin receptor agonist. J Urol. 1991;145:184–187. doi: 10.1016/s0022-5347(17)38287-3. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S, Santicioli P, Abelli L, Regoli D, Meli A. Further studies on the mechanism responsible for the tachykinin-induced activation of reflex micturition: evidence for the involvement of the capsaicin-sensitive bladder mechanoreceptors. Eur J Pharmacol. 1987;136:189–205. doi: 10.1016/0014-2999(87)90711-4. [DOI] [PubMed] [Google Scholar]
- Manack A, Motsko SP, Haag-Molkenteller C, Dmochowski RR, Goehring EL, Nguyen-Khoa BA, Jones JK. Epidemiology and healthcare utilization of neurogenic bladder patients in a US claims database. Neurourol Urodyn. 2011;30:395–401. doi: 10.1002/nau.21003. [DOI] [PubMed] [Google Scholar]
- Miyazato M, Yoshimura N, Chancellor MB. The other bladder syndrome: underactive bladder. Rev Urol. 2013;15:11–22. [PMC free article] [PubMed] [Google Scholar]
- Mule F, D’Angelo S, Tabacchi G, Serio R. Involvement of tachykinin NK2 receptors in the modulation of spontaneous motility in rat proximal colon. Neurogastroenterol Motil. 2000;12:459–466. doi: 10.1046/j.1365-2982.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- Mussap CJ, Stamatakos C, Burcher E. Radioligand binding, autoradiographic and functional studies demonstrate tachykinin NK-2 receptors in dog urinary bladder. J Pharmacol Exp Therap. 1996;279:423–434. doi: 10.1163/2211730x96x00225. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Bonham AC, Joad JP. Substance P in the nucleus of the solitary tract augments bronchopulmonary C fiber reflex output. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1215–1223. doi: 10.1152/ajpregu.2000.279.4.R1215. [DOI] [PubMed] [Google Scholar]
- Palea S, Corsi M, Artibani W, Ostardo E, Pietra C. Pharmacological characterization of tachykinin NK2 receptors on isolated human urinary bladder, prostatic urethra and prostate. J Pharmacol Exp Ther. 1996;277:700–705. [PubMed] [Google Scholar]
- Parlani M, Conte B, Cirillo R, Manzini S. Characterization of tachykinin NK2 receptor on dog proximal colon. Antagonism by MEN 10,627 and SR 48,968. Eur J Pharmacol. 1996;318:419–424. doi: 10.1016/s0014-2999(96)00799-6. [DOI] [PubMed] [Google Scholar]
- Sagan S, Chassaing G, Pradier L, Lavielle S. Tachykinin peptides affect differently the second messenger pathways after binding to CHO-expressed human NK-1 receptors. J Pharmacol Exp Therap. 1996;276:1039–1048. [PubMed] [Google Scholar]
- Stoffel JT. Detrusor sphincter dyssynergia: a review of physiology, diagnosis, and treatment strategies. Transl Androl Urol. 2016;5:127–135. doi: 10.3978/j.issn.2223-4683.2016.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichenor SD, Buxton IL, Johnson P, O’Driscoll K, Keef KD. Excitatory motor innervation in the canine rectoanal region: role of changing receptor populations. Br J Pharmacol. 2002;137:1321–1329. doi: 10.1038/sj.bjp.0704987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens Y, Beaujouan JC, Saffroy M, Glowinski J. Further evidence for the presence of “septide-sensitive” tachykinin binding sites in tissues possessing solely NK(1) tachykinin receptors. Biochem Biophys Res Commun. 2000;270:668–772. doi: 10.1006/bbrc.2000.2477. [DOI] [PubMed] [Google Scholar]
- Tramontana M, Patacchini R, Lecci A, Giuliani S, Maggi CA. Tachykinin NK2 receptors in the hamster urinary bladder: in vitro and in vivo characterization. Naunyn Schmied Arch Pharmacol. 1998;358:293–300. doi: 10.1007/pl00005256. [DOI] [PubMed] [Google Scholar]
- Warner FJ, Miller RC, Burcher E. Human tachykinin NK2 receptor: a comparative study of the colon and urinary bladder. Clin Exp Pharmacol Physiol. 2003;30:632–639. doi: 10.1046/j.1440-1681.2003.03887.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huo M, Zhou J, Xie S. PK Solver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Meth Prog Biomed. 2010;99:306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]