Abstract
OBJECTIVES
Unplanned 30-day readmission rates contribute significantly to growing national healthcare expenditures. Drivers of unplanned 30-day readmission after spinal cord stimulator (SCS) implantation are relatively unknown. The aim of this study was to determine drivers of 30-day unplanned readmission following SCS implantation.
METHODS
The National Readmission Database (NRD) was queried to identify all patients who underwent SCS implantation for the 2013 calendar year. Patients were grouped by readmission status,“No Readmission” and “Unplanned 30-day Readmission.” Patient demographics and comorbidities were collected for each patient. The primary outcome of interest was the rate of unplanned 30-day readmissions and associated driving factors. A multivariate analysis was used to determine independent predictors of unplanned 30-day readmission after SCS implantation.
RESULTS
We identified 1,521 patients who underwent SCS implantation, with 113 (7.4%) experiencing an unplanned readmission within 30 days. Baseline patient demographics, comorbidities and hospital characteristics were similar between both cohorts. The 3 main drivers for 30-day readmission after SCS implantation include: [1] infection (not related to SCS device), [2] infection due to device (limited to only hardware infection), and [3] mechanical complication of SCS device. Furthermore, obesity was found to be an independent predictor of 30-day readmission (OR: 1.86, p=0.008).
CONCLUSION
Our study suggests that infectious and mechanical complications are the primary drivers of unplanned 30-day readmission after SCS implantation, with obesity as an independent predictor of unplanned readmission. Given the technological advancements in SCS, repeated studies are necessary to identify factors associated with unplanned 30-day readmission rates after SCS implantation to improve patient outcomes and reduce associated costs.
Keywords: Infection, Low Back Pain, Obesity, Readmission, Spinal Cord Stimulator
INTRODUCTION
Reducing unplanned readmission has become a major goal of health care reform initiatives in the United States. Unplanned readmissions are costly, resulting in a burden of over $40 billion annually to the US health care system.1 Furthermore, high readmission rates negatively impact patient outcomes, and high 30-day readmission rates are linked to increased risk of post-surgical mortality.2–4 Accordingly, recent health care reform efforts have been initiated to reduce 30-day readmission rates and improve patient outcomes accordingly.5 For example, the Centers for Medicare and Medicaid Services (CMS) have implemented initiatives to penalize hospitals financially for high 30-day readmission rates.5
The spine is the most common location for chronic pain, and chronic back pain has been reported to affect 54–80% of the population.6 Spinal cord stimulation (SCS) is a treatment modality that has dramatically risen in popularity to treat chronic, medically refractory back and leg pain.7 Nearly 14,000 patients undergo implantation of SCS devices annually for conditions such as complex regional pain syndrome (CRPS), failed back surgery syndrome (FBSS), and ischemic leg pain.8 SCS has been shown to reduce overall healthcare costs otherwise associated with neuropathic pain due to decreased requirement for narcotic consumption and hospital visits.9 Recent studies have explored the complication profile associated with SCS, reporting mechanical complications (i.e. electrode migration, electrode breaks) to be the most common complications that led to SCS failure.10–13 However, to date, the drivers of unplanned 30-day readmission after SCS implantation remain relatively unknown. The aim of this study was to determine the drivers of 30-day unplanned readmissions following SCS implantation.
METHODS
Data Source and Patient Population
We utilized the Healthcare Cost and Utilization Project Nationwide Readmissions Database (HCUP-NRD), containing more than 49.0% of the U.S. population and hospitalizations reported to the American Hospital Association (AHA) in 2013. This database contains more than 100 clinical and non-clinical variables, including patient demographics, diagnoses, procedures performed, expected source of payment, total hospital charges, length of stay (LOS), and hospital readmission. We performed a retrospective review of patients undergoing primary SCS implantation during the 2013 calendar year, and the all-cause reason for 30-day hospital readmission.
International Classification of Diseases, Ninth Revision [ICD-9] procedural code 03.93 was used to select patients undergoing an SCS implantation. A total of 1,521 patients were identified and grouped by readmission status (No Readmission: 1,408 patients; Unplanned 30-Day Readmission: 113 patients).
Data Collection
Patient demographics, hospital characteristics, comorbidities, and discharge dispositions were collected for each patient and compared between the cohorts. Patient demographics included age, gender, median household income percentile, and primary expected payer (Medicare, Medicaid, private insurer, self-pay, other). Hospital characteristics included teaching status (metropolitan teaching, metropolitan non-teaching, and non-metropolitan) and bed size (small, medium, large). Comorbidities assessed included alcohol abuse, congestive heart failure (CHF), chronic pulmonary disease, coagulopathy, deficiency anemias, depression, diabetes, drug abuse, hypertension (HTN), hypothyroidism, other neurological disorders, obesity, peripheral vascular disorders (PVD), pulmonary circulation disorders, and rheumatoid arthritis/collagen vascular diseases. Obesity was defined as a BMI ≥30 kg/m2, as indexed in NRD.
The primary outcome investigated in this study was the rate of unplanned 30-day readmission and the most prevalent drivers.
Statistical Analysis
Descriptive statistics were used to for patients’ demographics, hospital characteristics, and comorbidities, of the study population grouped by those with unplanned 30–day readmission and no readmission after SCS implantation. Continuous variables were summarized by readmission groups using means and standard deviations if normally distributed and medians with interquartile range (IQR) in addition if skewed. Univariate comparisons between readmission groups were made by t-test if normally distributed or Wilcoxon rank sum test if skewed. Categorical variables were described using counts and percentages. Univariate comparisons between groups were made using the chi-square test or Fisher’s exact test in the presence of expected cell counts smaller than 5, due to increased accuracy for small counts. For the most frequent principle diagnoses among the study population, proportions of unplanned 30-day readmission were described by counts and percentages. Logistic regression was used to evaluate the effect of each risk factor on unplanned 30-day readmission adjusting for other potential risk factors. Potential risk factors were selected among all risk factors that were both most clinically relevant with sufficient sample size under each of risk factor category. Bonferroni correction on p-value was also used in identifying risk factors using multivariate logistic regression. Significance cutoff was chosen at 0.05. All analyses and data processing were conducted using SAS software, V9.4, SAS Institute Inc., Cary, NC, USA.
RESULTS
In our study cohort a total of 1,521 patients underwent SCS implantation, with 7.4% having an unplanned readmission within 30 days (Unplanned readmission: n=113, No readmission: n=1,408). Mean ages of the cohorts were similar with the readmitted cohort being 59.9 ± 14.7 years and 57.3 ± 14.3 years for the non-readmitted cohort (p=0.0893). Between both groups, no significant differences in gender (p=0.7177), median household income percentile (p=0.8594), or primary expected payer (p=0.3029) were observed. Hospital characteristics were similar between both groups, with no differences observed between teaching status (p=0.8113) and hospital bed size (p=0.8919), Table 1.
Table 1.
Patient Demographics and Hospital Characteristics
| Demographic Variables | No Readmission (N=1408) |
Unplanned Readmission (N=113) |
P-Value |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 57.3 ± 14.3 | 59.9 ± 14.7 | 0.0894 |
| Median [IQR] | 56.0 [47.0, 68.0] | 61.0 [50.0, 71.0] | |
| Gender N(%) | |||
| Male | 586 (41.6%) | 49 (43.4%) | 0.7177 |
| Female | 822 (58.4%) | 64 (56.6%) | |
| Median Household Income Percentile N(%) | |||
| 0–25th | 294 (21.1%) | 25 (22.1%) | 0.8594 |
| 26–50th | 322 (23.1%) | 29 (25.7%) | |
| 51–75th | 389 (27.9%) | 28 (24.8%) | |
| 76–100th | 387 (27.8%) | 31 (27.4%) | |
| Primary Expected Payer | |||
| Medicare | 717 (51.0%) | 59 (52.2%) | 0.3029 |
| Medicaid | 59 (4.2%) | 3 (2.7%) | |
| Private insurance | 362 (25.7%) | 22 (19.5%) | |
| Self-pay | 5 (0.4%) | 0 (0.0%) | |
| Other | 264 (18.8%) | 29 (25.7%) | |
| Teaching Status of Hospitals | |||
| Metropolitan Non-teaching | 592 (42.0%) | 43 (38.1%) | 0.5961 |
| Metropolitan Teaching | 786 (55.8%) | 67 (59.3%) | |
| Non-Metropolitan hospital | 30 (2.1%) | 3 (2.7%) | |
| Hospital Bed Size | |||
| Small | 95 (6.7%) | 7 (6.2%) | 0.8919 |
| Medium | 283 (20.1%) | 21 (18.6%) | |
| Large | 1030 (73.2%) | 85 (75.2%) | |
There were no significant differences between both groups in prevalence of other co-morbidities such as alcohol abuse, CHF, chronic pulmonary disease, coagulopathy, deficiency anemias, depression, diabetes (uncomplicated), drug abuse, HTN, hypothyroidism, other neurological disorders, obesity, PVD, pulmonary circulation disorders, and rheumatoid arthritis/collagen vascular diseases, Table 2.
Table 2.
Patient Comorbidities
| Comorbidity | No Readmission (N=1408) |
Unplanned Readmission (N=113) |
P-Value |
|---|---|---|---|
| Alcohol abuse | 10 (0.7%) | 0 (0.0%) | 1.000 |
| Congestive heart failure | 40 (2.8%) | 4 (3.5%) | 0.5634 |
| Chronic pulmonary disease | 282 (20.0%) | 28 (24.8%) | 0.2278 |
| Coagulopathy | 22 (1.6%) | 3 (2.7%) | 0.4255 |
| Deficiency Anemias | 64 (4.5%) | 7 (6.2%) | 0.4239 |
| Depression | 277 (19.7%) | 30 (26.5%) | 0.0798 |
| Diabetes | 313 (22.2%) | 21 (18.6%) | 0.4093 |
| Drug abuse | 37 (2.6%) | 4 (3.5%) | 0.5404 |
| Hypertension | 726 (51.6%) | 66 (58.4%) | 0.1611 |
| Hypothyroidism | 172 (12.2%) | 12 (10.6%) | 0.6166 |
| Other neurological disorders | 45 (3.2%) | 5 (4.4%) | 0.4809 |
| Obesity | 244 (17.3%) | 31 (27.4%) | 0.0072 |
| Peripheral vascular disorders | 35 (2.5%) | 6 (5.3%) | 0.0745 |
| Pulmonary circulation disorders | 10 (0.7%) | 2 (1.8%) | 0.2224 |
| Rheumatoid arthritis/Collagen vascular diseases | 53 (3.8%) | 4 (3.5%) | 1.000 |
The most prevalent driver of unplanned 30-day readmissions were due to infection, with 26.54% due to other infections not related to device (including systemic, pneumonia, and wound infections) and 12.39% due to infection of the SCS device (limited to only hardware infection), Figure 1. These rates are proportions of the 7.4% of patients readmitted, therefore the non-device-related and device-related infection rates of the entire 1,521 cohort are 1.97% and 0.92%, respectively.
FIGURE 1.
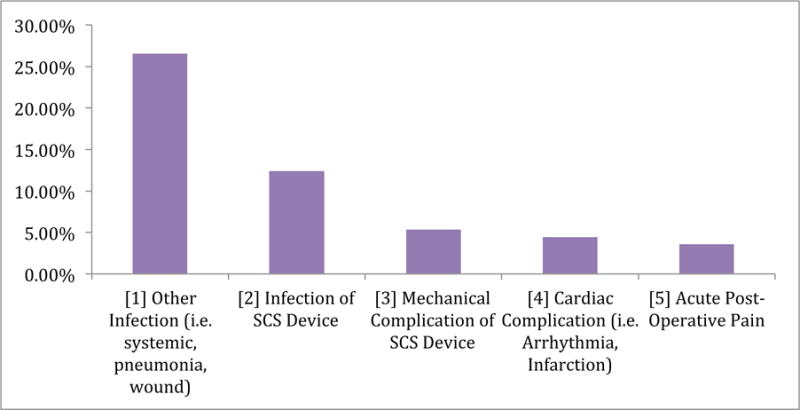
5 Most Common Reasons for Unplanned 30-Day Readmission following SCS implantation.
Obesity was an independent predictor of unplanned 30-day readmission while adjusting for age, gender, income status, chronic pulmonary disease, depression, diabetes, and hypertension without Bonferroni correction on p-value, Table 3. Patients with obesity were found to have a 1.86 higher odds of having an unplanned 30-day readmission, compared to non-obese patients (OR: 1.86, p=0.008).
Table 3.
Multivariate-Regression Analysis Predicting Unplanned 30-Day Readmission
| Factor | Odds-Ratio | 95% CI | P-Value |
|---|---|---|---|
|
| |||
| Age Group | |||
|
|
|||
| ≥65 | 1.63 | (0.72, 3.69) | 0.242 |
|
|
|||
| 40 – 64 | 1.37 | (0.63, 2.97) | 0.426 |
|
|
|||
| <40 | reference | ||
|
| |||
| Gender | |||
|
|
|||
| Female | 0.86 | (0.58, 1.29) | 0.470 |
|
|
|||
| Male | reference | ||
|
| |||
| Income | |||
|
|
|||
| 76–100th percentile | 0.95 | (0.54, 1.66) | 0.854 |
|
|
|||
| 51–75th percentile | 0.86 | (0.49, 1.51) | 0.597 |
|
|
|||
| 26–50th percentile | 1.08 | (0.62, 1.91) | 0.778 |
|
|
|||
| 0–25th percentitle | reference | ||
|
| |||
| Comorbidity | |||
|
|
|||
| Chronic pulmonary disease | 1.19 | (0.75, 1.89) | 0.455 |
|
|
|||
| Depression | 1.44 | (0.92, 2.26) | 0.114 |
|
|
|||
| Diabetes | 0.60 | (0.36, 1.02) | 0.059 |
|
|
|||
| Hypertension | 1.19 | (0.78, 1.82) | 0.415 |
|
|
|||
| Obesity | 1.86 | (1.18, 2.95) | 0.008* |
DISCUSSION
In this retrospective study, we found that infection and mechanical complications were the major drivers of unplanned 30-day readmission after SCS implantation, with obesity as an independent predictor of readmission.
Infection is one of the most costly and preventable complications associated with SCS implantation.14 Infections are typically one of the earliest appearing complications following SCS implantation surgery,15 and occur in the subcutaneous pocket where the device was placed or on the device surface between the extension and the electrode.14 According to the Neuromodulation Appropriateness Consensus Committee (NACC), infection is considered one of the major complications of SCS implantation and a prevalent driver towards removal or failure of the device, with a reported infection rate between 4% and 10%.7,16 Adherence to infection control guidelines regarding antibiotic prophylaxis and surgical techniques have lowered the reported incidence of infections following SCS implantation.7,10–13 In a retrospective study of 260 patients undergoing SCS implantation, Reig et al. reported an infection incidence of 5% with the majority of complications instead attributable to hardware complications.10 Similarly, Cameron et al. reported infection-related complications to be significantly less common than technical complications following SCS implantation in a retrospective literature review of 68 studies reporting SCS outcomes.11 In this review, an infection rate of 3.4% was reported among the overall population of 3,679 patients receiving SCS devices.11 In comparison, we identified a non-device infection rate of approximately 26%, with device-related infection of approximately 12% in patients readmitted within 30-days of SCS implantation. However, while these infection rates only represent those within 30-days of SCS implantation, there may be more infections after 30-days.
Previous studies have reported a high incidence of mechanical complications after SCS implantation. In a retrospective, 20-year-long study of 260 patients with SCS implants, Reig et al. found that hardware complication due to lead migration, lead breakage, or generator failure accounted for 39% of the observed complications.10 Similarly, in a systematic review of available literature studying the use of SCS to treat FBSS, Turner et al. reported that the most common complications that afflicted 30% of patients were related to hardware malfunction.13 Zan et al. also reported prominent technological problems that led to a 16.7% revision rate in a retrospective study of 24 patients with implantable SCS devices.12 In a retrospective literature review of 68 articles totaling 3,679 patients undergoing SCS for chronic pain conducted by Cameron et al., lead migration and hardware malfunction were the two most common complications.11
Since the conception of SCS implantation in 1967, the hardware used for SCS has advanced considerably in an attempt to lower the high rate of technical complications.8 Lead migration is a common problem leading to early complications, as recent studies have identified a prevalence of 13–22% of migrations occuring.11,17 Early interventions to reduce this complication included the advent of multi-contact electrode arrays, along with percutaneous and laminotomy-guided lead placement; while migration rates lowered after these advances, the problem still persists.18 In a comparison study of 13,774 patients who underwent SCS implantation with paddle versus percutaneous leads, Babu et al. demonstrated that patients receiving paddle leads were more likely to have greater initial postoperative complications, but were associated with a significantly lower long-term reoperation rate when compared to patients who received the percutaneous leads.19 More recently, North et al. found that injection of adhesive into the silicone elastomer lead anchor as well as use of stronger suture and a fascial incision to secure the anchor eliminated this complication in a retrospective study of 142 patients undergoing SCS from 2007–2013.18 Lead breakage is another common hardware complication of SCS; to circumvent this problem, Henderson et al. developed a computer model to simulate SCS movement and found that paramedian placement of leads as well as abdominal pulse generator placement reduced the incidence of breakage and increased SCS hardware longevity.20 Despite these advances, we found that hardware complications remain a common cause of unplanned readmission after SCS. Given the recent technological evolution of SCS devices, future studies are necessary to monitor whether more recent hardware improvements lead to better patient outcomes and lower readmission rates.
While the potential complications associated with SCS have been reported in the aforementioned studies, readmission rates and drivers after SCS implantation remain relatively understudied. Zan et al. reported a readmission rate of 8.3% due to irritation/discomfort at the implantable pulse generator site.12 In a prospective, randomized study of 10 patients with heart failure undergoing SCS implantation conducted by Torre-Amione et al., one patient was readmitted due to infection around the implantable pulse generator site.21 This finding is consistent with the predominance of infectious etiologies for unplanned 30-day readmissions in our cohort. However, we also reported non-infectious causes of readmission including mechanical complications and medical problems including cardiac complications and post-operative pain. Further studies are needed to examine the prevalence of infectious, hardware-related and medical causes of 30-day readmission after SCS implantation in an effort to maximize healthcare savings and improve patient outcomes.
We found that obesity was an independent predictor of unplanned 30-day readmission while adjusting for age, gender, income status, chronic pulmonary disease, depression, diabetes, and hypertension. Obesity poses unique risks both from a technical and infectious standpoint during implantation of spinal cord stimulators. Accordingly, a link between obesity and post-operative complications after spinal cord stimulator implantation has been established in the literature.22,23 Marola et al. reported a higher complication rate among patients with BMI > 36.5 in a retrospective review of 77 patients undergoing SCS implantation.23 Similarly, in a retrospective study of 141 patients undergoing SCS implantation for chronic pain syndrome, Bir et al. found that BMI > 30 kg/m2 was predictive of need for early revision after surgery due to mechanical complications such as hardware malfunction or lead migration.22 These mechanical complications are likely attributable to technical challenges inherent to operating on obese patients, as patient positioning and tissue depth can interfere with lead and generator placement.23,24 In addition to mechanical complications, high BMI has been linked with increased post-operative infections after spinal surgery.25–28 Both of these complications contribute to the association between obesity and increased 30-day readmission rates seen both in the spine surgery literature29–31 and in our study. Owing to the increasing number of obese patients suffering from chronic pain syndrome and undergoing SCS implantation, these risks warrant consideration in the pre-operative setting.23
This study has limitations, which has implications for its interpretation. First, our sample size is small, which limits our ability to make any firm conclusions. Our data is only representative of readmission rates in 2013 and variables such as the electrode placement location, technique and stimulator parameters, and whether the leads were paddle or cylindrical were not collected. Additionally, the nature of national databases limits the ability to provide details regarding the reason and medical and/or surgical management for patients with complications and 30-day readmissions, such as hardware infections or mechanical complications. Moreover, the data collected were only for patients readmitted to a hospital, whereas patients could have been seen in an outpatient setting, thus preventing hospital readmission and may have implications on the results. Although pre- and perioperative variables were prospectively recorded into the study registry at the time of surgery, these variables were retrospectively analyzed for the purposes of this study and as such are subject to the pitfalls associated with all retrospective reviews. Furthermore, NRD indexes obesity as a BMI ≥30 kg/m2, therefore the BMI range of the readmitted patients is unavailable and may have implications on the impact obesity has on 30-day readmission rates. Despite these limitations, this study demonstrates that infection and mechanical complications are the major drivers of unplanned 30-day readmission after SCS implantation.
CONCLUSION
Our study suggests that infectious and mechanical complications are major drivers of unplanned 30-day readmission after SCS implantation, with obesity as an independent predictor of unplanned readmission. Given the technological advancement of SCS, repeated studies are necessary to identify risk factors associated with unplanned 30-day readmission rates after SCS implantation in order improve patient outcomes and reduce associated healthcare costs. Future solutions that focus on reducing preventable readmissions to evaluation in the clinic setting can increase patient quality of care and reduce healthcare costs.
Acknowledgments
Source of financial support: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001117
Shivanand Lad, MD, PhD, has received fees for serving as a speaker and consultant for Medtronic Inc., Boston Scientific, and St. Jude Medical. He serves as the Director of the Duke Neuro-outcomes Center, which has received research funding from NIH KM1 CA 156687, Medtronic Inc. and St. Jude Medical.
Footnotes
Conflict of Interest: The remaining authors report no conflicts of interest or financial disclosures.
Authorship statements: Mr. Elsamadicy, Ms. Sergesketter, Mr. Hussaini, Dr. Laarakker, Dr. Rahimpour, and Ms. Ejikeme prepared the manuscript draft and results with important input from Drs. Lad, Pagadala, and Ms. Parente. Mr. Ren, Dr. Xie and Ms. Yang conducted the study and provided the statistical analysis for analyzing the data and had complete access to the study data. Mr. Elsamadicy, Ms. Sergesketter, Mr. Hussaini, Dr. Laarakker, Dr. Rahimpour, and Ms. Ejikeme provided interpretation of the data. All authors approved the final manuscript.
References
- 1.Hines AL, Barrett ML, Jiang HJ, Steiner CA. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): 2006. Conditions With the Largest Number of Adult Hospital Readmissions by Payer, 2011: Statistical Brief #172. [PubMed] [Google Scholar]
- 2.Dickinson H, Carico C, Nuno M, et al. Unplanned readmissions and survival following brain tumor surgery. J Neurosurg. 2015 Jan;122(1):61–68. doi: 10.3171/2014.8.JNS1498. [DOI] [PubMed] [Google Scholar]
- 3.Nuno M, Ly D, Ortega A, et al. Does 30-day readmission affect long-term outcome among glioblastoma patients? Neurosurgery. 2014 Feb;74(2):196–204. doi: 10.1227/NEU.0000000000000243. discussion 204–195. [DOI] [PubMed] [Google Scholar]
- 4.Tsai TC, Joynt KE, Orav EJ, Gawande AA, Jha AK. Variation in surgical-readmission rates and quality of hospital care. N Engl J Med. 2013 Sep 19;369(12):1134–1142. doi: 10.1056/NEJMsa1303118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein AM. Revisiting readmissions–changing the incentives for shared accountability. N Engl J Med. 2009 Apr 2;360(14):1457–1459. doi: 10.1056/NEJMe0901006. [DOI] [PubMed] [Google Scholar]
- 6.Manchukonda R, Manchikanti KN, Cash KA, Pampati V, Manchikanti L. Facet joint pain in chronic spinal pain: an evaluation of prevalence and false-positive rate of diagnostic blocks. J Spinal Disord Tech. 2007 Oct;20(7):539–545. doi: 10.1097/BSD.0b013e3180577812. [DOI] [PubMed] [Google Scholar]
- 7.Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation: avoidance and treatment of complications of neurostimulation therapies for the treatment of chronic pain. Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014 Aug;17(6):571–597. doi: 10.1111/ner.12206. discussion 597–578. [DOI] [PubMed] [Google Scholar]
- 8.Gharibo C, Laux G, Forzani BR, Sellars C, Kim E, Zou S. State of the field survey: spinal cord stimulator use by academic pain medicine practices. Pain Med. 2014 Feb;15(2):188–195. doi: 10.1111/pme.12264. [DOI] [PubMed] [Google Scholar]
- 9.Shamji MF, Westwick HJ, Heary RF. Complications related to the use of spinal cord stimulation for managing persistent postoperative neuropathic pain after lumbar spinal surgery. Neurosurg Focus. 2015 Oct;39(4):E15. doi: 10.3171/2015.7.FOCUS15260. [DOI] [PubMed] [Google Scholar]
- 10.Reig E, Abejon D. Spinal cord stimulation: a 20-year retrospective analysis in 260 patients. Neuromodulation. 2009 Jul;12(3):232–239. doi: 10.1111/j.1525-1403.2009.00220.x. [DOI] [PubMed] [Google Scholar]
- 11.Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004 Mar;100(3 Suppl Spine):254–267. doi: 10.3171/spi.2004.100.3.0254. [DOI] [PubMed] [Google Scholar]
- 12.Zan E, Kurt KN, Yousem DM, Christo PJ. Spinal cord stimulators: typical positioning and postsurgical complications. AJR Am J Roentgenol. 2011 Feb;196(2):437–445. doi: 10.2214/AJR.10.4789. [DOI] [PubMed] [Google Scholar]
- 13.Turner JA, Loeser JD, Bell KG. Spinal cord stimulation for chronic low back pain: a systematic literature synthesis. Neurosurgery. 1995 Dec;37(6):1088–1095. doi: 10.1227/00006123-199512000-00008. discussion 1095–1086. [DOI] [PubMed] [Google Scholar]
- 14.Bendersky D, Yampolsky C. Is spinal cord stimulation safe? A review of its complications. World Neurosurg. 2014 Dec;82(6):1359–1368. doi: 10.1016/j.wneu.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Kumar K, Buchser E, Linderoth B, Meglio M, Van Buyten JP. Avoiding complications from spinal cord stimulation: practical recommendations from an international panel of experts. Neuromodulation. 2007 Jan;10(1):24–33. doi: 10.1111/j.1525-1403.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- 16.Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain practice : the official journal of World Institute of Pain. 2011 Mar-Apr;11(2):148–153. doi: 10.1111/j.1533-2500.2010.00407.x. [DOI] [PubMed] [Google Scholar]
- 17.Gazelka HM, Freeman ED, Hooten WM, et al. Incidence of Clinically Significant Percutaneous Spinal Cord Stimulator Lead Migration. Neuromodulation. 2015 Feb;18(2):123–125. doi: 10.1111/ner.12184. [DOI] [PubMed] [Google Scholar]
- 18.North RB, Recinos VR, Attenello FJ, Shipley J, Long DM. Prevention of percutaneous spinal cord stimulation electrode migration: a 15-year experience. Neuromodulation. 2014 Oct;17(7):670–676. doi: 10.1111/ner.12151. discussion 676–677. [DOI] [PubMed] [Google Scholar]
- 19.Babu R, Hazzard MA, Huang KT, et al. Outcomes of percutaneous and paddle lead implantation for spinal cord stimulation: a comparative analysis of complications, reoperation rates, and health-care costs. Neuromodulation. 2013 Sep-Oct;16(5):418–426. doi: 10.1111/ner.12065. discussion 426–417. [DOI] [PubMed] [Google Scholar]
- 20.Henderson JM, Schade CM, Sasaki J, Caraway DL, Oakley JC. Prevention of mechanical failures in implanted spinal cord stimulation systems. Neuromodulation. 2006 Jul;9(3):183–191. doi: 10.1111/j.1525-1403.2006.00059.x. [DOI] [PubMed] [Google Scholar]
- 21.Torre-Amione G, Alo K, Estep JD, et al. Spinal cord stimulation is safe and feasible in patients with advanced heart failure: early clinical experience. Eur J Heart Fail. 2014 Jul;16(7):788–795. doi: 10.1002/ejhf.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bir SC, Konar S, Maiti T, Nanda A, Guthikonda B. Neuromodulation in intractable pain management: outcomes and predictors of revisions of spinal cord stimulators. Neurosurg Focus. 2016 May;40(5):E4. doi: 10.3171/2016.3.FOCUS15634. [DOI] [PubMed] [Google Scholar]
- 23.Marola O, Cherala R, Prusik J, et al. BMI as a Predictor of Spinal Cord Stimulation Success in Chronic Pain Patients. Neuromodulation. 2016 Aug 05; doi: 10.1111/ner.12482. [DOI] [PubMed] [Google Scholar]
- 24.Sastry SND, Gillespy A, Berger K. Technical Challenges of Spinal Cord Stimulation Lead Placement in Morbidly Obese Patients. The Open Anesthesiology Journal. 2017;10:8–11. [Google Scholar]
- 25.De la Garza-Ramos R, Bydon M, Abt NB, et al. The impact of obesity on short- and long-term outcomes after lumbar fusion. Spine (Phila Pa 1976) 2015 Jan 1;40(1):56–61. doi: 10.1097/BRS.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 26.Kalanithi PA, Arrigo R, Boakye M. Morbid obesity increases cost and complication rates in spinal arthrodesis. Spine (Phila Pa 1976) 2012 May 15;37(11):982–988. doi: 10.1097/BRS.0b013e31823bbeef. [DOI] [PubMed] [Google Scholar]
- 27.Telfeian AE, Reiter GT, Durham SR, Marcotte P. Spine surgery in morbidly obese patients. J Neurosurg. 2002 Jul;97(1 Suppl):20–24. doi: 10.3171/spi.2002.97.1.0020. [DOI] [PubMed] [Google Scholar]
- 28.Patel N, Bagan B, Vadera S, et al. Obesity and spine surgery: relation to perioperative complications. J Neurosurg Spine. 2007 Apr;6(4):291–297. doi: 10.3171/spi.2007.6.4.1. [DOI] [PubMed] [Google Scholar]
- 29.Puvanesarajah V, Cancienne JM, Pehlivan H, et al. Morbid Obesity and Lumbar Fusion in Patients over 65 Years of Age: Complications, Readmissions, Costs, and Length of Stay. Spine (Phila Pa 1976) 2016 May 18; doi: 10.1097/BRS.0000000000001692. [DOI] [PubMed] [Google Scholar]
- 30.Elsamadicy AA, Adogwa O, Vuong VD, et al. Patient BMI is an Independent Predictor of 30-Day Hospital Readmission after Elective Spine Surgery. World Neurosurg. 2016 Sep 1; doi: 10.1016/j.wneu.2016.08.097. [DOI] [PubMed] [Google Scholar]
- 31.Seicean A, Alan N, Seicean S, et al. Impact of increased body mass index on outcomes of elective spinal surgery. Spine (Phila Pa 1976) 2014 Aug 15;39(18):1520–1530. doi: 10.1097/BRS.0000000000000435. [DOI] [PubMed] [Google Scholar]


