Abstract
Pain is a significant clinical problem, and there is a need for effective pharmacotherapies with fewer adverse effects than currently available drugs (e.g., mu opioid receptor agonists). Cannabinoid receptor agonists enhance the antinociceptive effects of mu opioid receptor agonists, but it remains unclear which drugs and in what proportion will yield the most effective and safest treatments. The antinociceptive effects of the mu opioid receptor agonists etorphine and morphine alone and in combination with the cannabinoid receptor agonists Δ9-THC and CP55940 were studied in male Sprague-Dawley rats (n=16) using a warm water tail withdrawal procedure. The ratio of opioid to cannabinoid (3:1, 1:1, and 1:3) varied for each mixture. Drugs administered alone or as pairwise mixtures of an opioid and a cannabinoid dose-dependently increased tail withdrawal latency. Mixtures with morphine produced supra-additive (CP55940) and additive (Δ9-THC) effects, whereas mixtures with etorphine and either cannabinoid were sub-additive. The interactions were not different among ratios for a particular mixture. The nature of the interaction between opioids and cannabinoids with regard to antinociceptive effects varies with the particular drugs in the mixture, which can have implications for designing combination therapies for pain.
Keywords: antinociception, thermal nociception, cannabinoid receptor agonist, mu opioid receptor agonist, drug-drug interactions, rats
1. Introduction
Pain is a significant clinical problem (Institute of Medicine 2011) and mu opioid receptor agonists remain the most common treatment for moderate to severe pain despite significant adverse effects and a growing abuse problem (Kolodny et al. 2015; Rudd et al. 2016). One strategy for limiting the adverse effects of opioids is to co-administer a non-opioid drug, thereby reducing the dose of opioid required to treat pain. Recent guidelines for opioid prescribing recommend administering the smallest doses necessary for achieving the desired therapeutic effect (e.g., Dowell et al. 2016).
Studies indicate that combining opioids and cannabinoids might be an effective and safe strategy for treating pain. Cannabinoid receptor agonists such as Δ9-THC enhance the antinociceptive potency of opioids in nonhuman subjects (Welch and Stevens 1992; Li et al. 2008) as well as humans (Roberts et al. 2006) and could be effective adjunct treatments for pain (Nielsen et al. 2017). Moreover, cannabinoids attenuate discriminative stimulus and positive reinforcing effects of opioids (Li et al. 2008; Li et al. 2012; Maguire et al. 2013; Maguire and France 2016a; Maguire and France 2016b), suggesting that cannabinoids do not increase, and might decrease, abuse (e.g., Bachhuber et al. 2014).
Enhancement of the antinociceptive potency of opioids by cannabinoids varies depending on the opioid in the mixture. In mice, Δ9-THC increased the potency of opioids in a tail flick assay, with the magnitude of increase varying from 2 (morphine) to 26 (codeine) fold (Cichewicz et al. 1999). In rhesus monkeys, the dose of CP55940 increasing the potency of fentanyl more than 50 fold increased the potency of morphine 8 fold. CP55940 increased the potency of all opioids more than did Δ9-THC (Maguire and France 2014).
The proportion of drugs in mixtures also impacts interactions (Wessinger 1986; Woolverton 1987; Tallarida 2000). In several studies pretreatments of a cannabinoid were administered prior to varying doses of an opioid (Welch and Stevens 1992; Smith et al. 1998; Cichewicz et al. 1999; Yesilyurt et al. 2003; Pugh et al. 2006; Smith et al. 2007; Li et al. 2008; Williams et al. 2006; Miller et al. 2012; Maguire et al. 2013; Maguire and France 2014) such that the opioid/cannabinoid ratio varied with each dose, making comparisons within and across studies difficult. Studies examining multiple doses in mixtures have generally used only one ratio of opioid to cannabinoid (Cichewicz et al. 2005; Tham et al. 2005; Cox et al. 2007; Wakely and Craft 2011; Kazantzis et al. 2016; Auh et al. 2016), although one study examined two ratios (Cichewicz and McCarthy 2003).
The current study examined antinociceptive effects of opioid/cannabinoid mixtures in rats using a warm water tail withdrawal procedure. Mixtures with morphine or etorphine and Δ9-THC or CP55940 were tested at ratios of 1:3, 1:1, and 3:1, with interactions assessed quantitatively (Tallarida 2000; Grabovsky and Tallarida 2004; Tallarida 2006). A previous study using monkeys (Maguire and France 2014) showed that mixtures of a high efficacy opioid (e.g., etorphine) and a high efficacy cannabinoid (e.g., CP55940) maximally increased antinociceptive potency. This study tested the hypothesis that mixtures of high efficacy drugs (etorphine and CP55940) would increase potency more than mixtures with low efficacy drugs (morphine and Δ9-THC).
2. Materials and methods
2.1 Animals
Sixteen adult male Sprague-Dawley rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN), approximately 4 months old at the beginning of the experiment, were housed individually in 45 x 24 x 20 cm plastic cages containing bedding (Sani-chips, Harlan Teklad, Madison, WI) in a colony room maintained on a 14:10 light/dark cycle with lights on at 0600 h. Test sessions occurred no more than once weekly and began at approximately 1000 h. Rats remained in their home cages except when tail withdrawal latencies were being measured. Tap water and standard rat chow (Rat Sterilizable Diet, Harlan Teklad) were available continuously in the home cage. Animals used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources on Life Sciences, National Research Council, National Academy of Sciences).
2.2 Procedure
The testing procedure was similar to that described previously (Maguire and France 2016c). Water baths (EW-14576-00, Cole-Parmer, Vernon Hills, IL, USA) maintained water at 40, 50, or 55°C. During tests approximately 5 cm of the tail was immersed in one of the water baths, and the time until the tail was removed was recorded with a hand-held stopwatch. If the tail was not withdrawn within 15 s, a latency of 15 s was recorded (maximum effect). Each temperature was tested once per cycle, and the order in which temperatures were tested varied randomly across cycles. Control tail withdrawal latencies were measured first. Immediately following the control measurements, the drug vehicle was administered intraperitoneal, and the rat was returned to the home cage; 30 min later tail withdrawal latencies were measured again to establish the effect of vehicle. The rat received an injection of vehicle or drug and was returned to its home cage, followed 30 min later by another measurement of tail withdrawal latency. Sessions ended when tail withdrawal latency reached 15 s with 50°C water, after 7 cycles (the control test plus up to 6 injections), or after administration of the maximum allowable dose, whichever occurred first.
2.3 Experimental design
Dose-effect curves for all drugs alone were determined within session by administering increasing cumulative doses of morphine (1.78–17.8 mg/kg), etorphine (0.0032–0.01 mg/kg), CP55940 (0.1–1.0 mg/kg), or Δ9-THC (3.2–32.0 mg/kg) in quarter-log unit increments across cycles, intraperitoneal. Rats were divided in to two groups with 8 rats per group. Both groups were tested with morphine and etorphine alone and in combination with one cannabinoid. One group was tested with CP55940, the other with Δ9-THC. Dose-effect curves for each drug alone were doubly determined prior to tests with mixtures, which were determined once per ratio of opioid to cannabinoid. Effects of CP55940 and Δ9-THC alone were doubly determined prior to being tested in combination with each opioid, resulting in a total of four determinations during the course of the study. Tests with mixtures were conducted in the same manner as the drugs alone with increasing cumulative doses of an opioid and a cannabinoid administered in a single injection. The doses of each drug included in the mixture (Table 1) were determined based on the relative potency of the opioid and of the cannabinoid, each administered alone, to increase tail withdrawal latency with 50°C water, estimated by linear regression using group dose-effect curves. The ratio of opioid to cannabinoid in the mixture (3:1, 1:1, or 1:3) remained constant for a particular session and varied across sessions. All rats within a group were tested with the same doses. Although the doses were not tailored for each individual rat, the analysis takes into account individual differences in sensitivity (i.e., potency and maximal effect) to each of the constituent drugs when administered alone (see section 2.4).
Table 1.
Doses (mg/kg) of opioid receptor agonists and cannabinoid receptor agonists in mixtures.
| Drug mixture dose (mg/kg)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Ratio (Opioid:Canna binoid) | Etorphine | CP55940 | Morphine | CP55940 | Etorphine | Δ9- THC | Morphine | Δ9- THC |
| 3:1 | 0.001 | 0.04 | 1.6 | 0.02 | 0.001 | 1.4 | 1.3 | 0.7 |
| 0.003 | 0.07 | 3.1 | 0.04 | 0.003 | 2.7 | 2.6 | 1.3 | |
| 0.005 | 0.14 | 6.3 | 0.08 | 0.005 | 5.4 | 5.2 | 2.6 | |
| 0.010 | 0.29 | 12.5 | 0.16 | 0.010 | 10.9 | 10.5 | 5.2 | |
| 0.020 | 0.58 | 25.1 | 0.33 | 0.020 | 21.7a | 20.9 | 10.4 | |
| 1:1 | 0.001 | 0.07 | 1.0 | 0.04 | 0.001 | 2.7 | 0.9 | 1.3 |
| 0.002 | 0.14 | 2.1 | 0.08 | 0.002 | 5.4 | 1.7 | 2.6 | |
| 0.003 | 0.29 | 4.2 | 0.16 | 0.003 | 10.9 | 3.5 | 5.2 | |
| 0.007 | 0.58 | 8.4 | 0.33 | 0.007 | 21.7a | 7.0 | 10.4 | |
| 0.013 | 1.16 | 16.7 | 0.65 | 0.014 | 43.4a | 14.0 | 20.7a | |
| 1:3 | 0.000 | 0.11 | 0.5 | 0.06 | 0.000 | 4.1 | 0.4 | 1.9 |
| 0.001 | 0.22 | 1.0 | 0.12 | 0.001 | 8.1 | 0.9 | 3.9 | |
| 0.002 | 0.43 | 2.1 | 0.24 | 0.002 | 16.3 | 1.7 | 7.8 | |
| 0.003 | 0.87 | 4.2 | 0.49 | 0.003 | 32.6a | 3.5 | 15.5 | |
| 0.007 | 1.73 | 8.4 | 0.98 | 0.005 | 48.8a,b | 7.0 | 31.0a | |
Doses of Δ9-THC requiring an injection volume between 1.0 and 2.0 mg/kg; all others were 1.0 mg/kg.
Largest dose of Δ9-THC that could be studied.
2.4 Data Analyses
Tests with 40°C water were conducted as a control to confirm that rats would leave the tail in water for the maximum duration, and latencies with 40°C were rarely (< 1% of all tests) less than 15 s; therefore, no further analysis was conducted with these data. Latencies from tests with 50 and 55°C water were expressed as a percentage of maximal possible effect (MPE) according to the following formula: %MPE = [(test latency–control latency) / (20 s–control latency)] x 100. Control latencies were determined each session before the first injection. Effects with 55°C water did not consistently exceed 20% of the MPE; therefore, these data were not analyzed further.
Individual-subject data from tests with 50°C water were fit to a hyperbolic dose-effect function in order to determine the ED50, maximum effect (Emax), and slope for each drug when administered alone and as part of a drug mixture; curves for drugs alone were fit to the average of the two baseline determinations. Increases in opioid potency (ED50) were quantified by dividing the ED50 for the opioid alone by the ED50 for the opioid for each dose ratio, yielding a potency ratio. A potency ratio greater than 1 indicates a leftward shift in the opioid dose-effect curve and increased potency. Effects of dose ratio on the opioid ED50 were statistically analyzed separately for each pair of drugs using a one-way, repeated-measures analysis of variance. Post-hoc analyses were conducted using Dunnett’s test, wherein the opioid ED50 values from each dose ratio were compared with the corresponding control (opioid alone); this test corrects for multiple comparisons (Dunnett, 1955).
In order to characterize the nature of the interaction between drugs, the parameters from the dose-effect curve for the drugs alone were used to convert the cannabinoid dose in a drug mixture to its opioid-equivalent dose (Equation 7, Tallarida 2006). For some individual rats, CP55940 and Δ9-THC produced a larger maximal effect than morphine; thus, there was no morphine equivalent dose. Therefore, cannabinoid doses in the mixture that alone would produce a greater maximal effect than that of morphine were considered equivalent to 32 mg/kg of morphine, one-quarter log unit higher than the largest dose of morphine tested alone. The opioid-equivalent dose of the cannabinoid and the opioid dose in the mixture were then summed, yielding the total additive dose, which was then used to calculate the predicted effect of that mixture for individual rats. Predicted and observed data were plotted as a function of total additive, opioid-equivalent dose. The ED50 (log transformed), Emax, and slope for predicted effects were subtracted from those of the observed effects for individual rats. Differences between predicted and observed parameters were analyzed statistically using a two-tailed, repeated measures t-test with an alpha value of 0.05. The null hypothesis was that the difference between the observed parameter and the predicted parameter was not different from zero.
Dose-effect curves were fit using the solver function of Microsoft Excel 2016 (Redmond, WA, USA) and the method of least squares nonlinear regression (Motulsky and Christopoulos 2003). Analyses of variance were conducted using GraphPad Prism version 7.03 (La Jolla, CA, USA).
2.5 Drugs
CP55940 (2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol [Sigma-Adlrich, St. Louis, MO]) and delta-9-tetrahydrocannabinol (Δ9-THC; National Institute on Drug Abuse [NIDA] Drug Supply Program; Research Technology Branch, Rockville, MD) were dissolved in a 1:1:18 solution of absolute ethanol, Alkamuls EL-620 (Rhodia, Cranbury, NJ, USA), and 0.9% sterile saline. Morphine sulfate and etorphine hydrochloride (NIDA Drug Supply Program) were dissolved in 0.9% saline. Drug mixtures were dissolved in the 1:1:18 solution. Doses of morphine, etorphine, and CP55940 are expressed in terms of the salt; Δ9-THC was stored in absolute ethanol and diluted as necessary. Injections were administered intraperitoneal in a volume of 1.0–2.0 ml/kg (Table 1).
3. Results
The mean (± 1 standard error of the mean) control latencies with 40, 50, and 55°C water were 15 (± 0), 4.6 (± 0.15), and 1.8 (± 0.07) s, respectively, in the group (n=8) tested with CP55940 and 15 (± 0), 4.8 (± 0.15), and 1.9 (± 0.07) s, respectively, in the group (n=8) tested with Δ9-THC. Control latencies did not vary systematically across sessions, and repeated injection of saline or vehicle within a session did not impact tail withdrawal latencies (Supplemental Fig. 1).
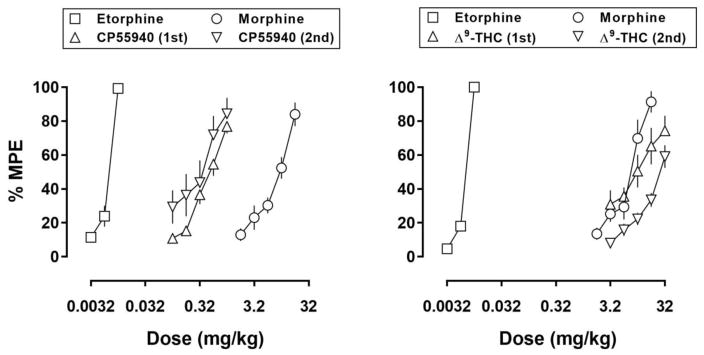
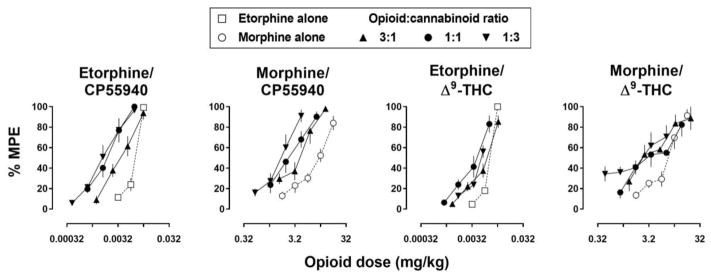
When administered alone, each drug dose-dependently increased tail withdrawal latencies with 50°C water (Fig. 1). Etorphine was the most potent drug tested and produced 100% of the MPE. CP55940, morphine, and Δ9-THC were less potent than etorphine, producing 60–80% of the MPE at the largest doses that were studied. ED50 values for each drug alone with 50°C water are shown in Table 1. All drugs were more effective with 50°C compared with 55°C water, across the range of doses tested (Supplemental Fig. 2). Mixtures also dose-dependently increased tail withdrawal latencies (filled symbols, Fig. 2). In most cases, effects of smaller doses of an opioid were enhanced when administered with a cannabinoid and, generally, doses of an opioid required to produce a particular level of effect decreased as the proportion of cannabinoid in the mixture increased, as indicated by progressively increasing potency ratios (Table 3). Increased potency with the mixtures was also evident with 55°C water, although over the dose ranges studied there was no apparent increase in the maximum effect obtained with 55°C (Supplemental Fig. 3).
Fig. 1.
Antinociceptive effects of cumulative doses of etorphine (squares), morphine (circles), CP55940 (triangles, left panel), and Δ9-THC (triangles, right panel) alone with 50°C water with an inter-injection interval of 30 min. Data in the left panel were collected in one group of 8 rats whereas data in the right panel were collected in another group of 8 rats. Each curve represents the average of two determinations. Abscissae: dose in milligrams per kilogram body weight. Ordinate: percentage of the maximum possible effect (MPE; mean ± 1 standard error of the mean).
Fig. 2.
Antinociceptive effects of cumulative doses of etorphine (open squares) or morphine alone (open symbols) and in combination (filled symbols) with CP55940 or Δ9-THC with 50°C water. The ratio of opioid to cannabinoid in the mixture, 3:1 (upright triangles), 1:1 (circles), and 1:3 (inverted triangles), varied across tests. Data in the two leftmost panels were collected in one group of 8 rats whereas data in the two rightmost panels were collected in another group of 8 rats. Abscissae: dose of the opioid administered alone or as part of a mixture in milligrams per kilogram body weight. Ordinate: percentage of the maximum possible effect (MPE; mean ± 1 standard error of the mean).
Table 3.
Potency ratios [95% confidence intervals] for the antinociceptive effects of the opioid/cannabinoid mixtures (50°C water).
| Test | Potency ratio |
|---|---|
| Etorphine:CP55940 | |
| 3:1 | 1.9 [1.5, 2.3] a |
| 1:1 | 3.4 [2.1, 4.6] a |
| 1:3 | 4.1 [2.5, 5.8] a |
| Morphine:CP55940 | |
| 3:1 | 1.9 [1.2, 2.6] a |
| 1:1 | 2.7 [2.0, 3.5] a |
| 1:3 | 3.7 [2.8, 4.7] a |
| Etorphine: Δ9-THC | |
| 3:1 | 1.5 [1.2, 1.9] a |
| 1:1 | 2.0 [1.5, 2.6] a |
| 1:3 | 2.2 [1.9, 2.6] a |
| Morphine: Δ9-THC | |
| 3:1 | 2.6 [1.0, 4.2] |
| 1:1 | 3.5 [1.7, 5.3] a |
| 1:3 | 9.8 [3.9, 15.7] a |
Shifts were considered significant if the confidence interval did not include 1.
For the mixtures of etorphine and CP55940, there was a significant effect of dose ratio on the potency of etorphine [F3,21=67.9, P<.001], and Dunnett’s post-hoc test indicated that the potency of etorphine for all dose ratios was significantly different from the potency of etorphine alone. For mixtures of morphine and CP55940, there was a significant effect of dose ratio [F3,21=11.0, P<.001]; the potency of morphine for all dose ratios was significantly different from morphine alone. For mixtures of etorphine and Δ9-THC, there was a significant effect of dose ratio [F3,21=17.9, P<.001]; the potency of etorphine for all dose ratios was significantly different from etorphine alone. For mixtures of morphine and Δ9-THC; there was a significant effect of dose ratio [F3,21=9.2, P<.001]; the potency of morphine for ratios of 1:1 and 1:3 but not 3:1 (morphine: Δ9-THC) were significantly different from morphine alone.
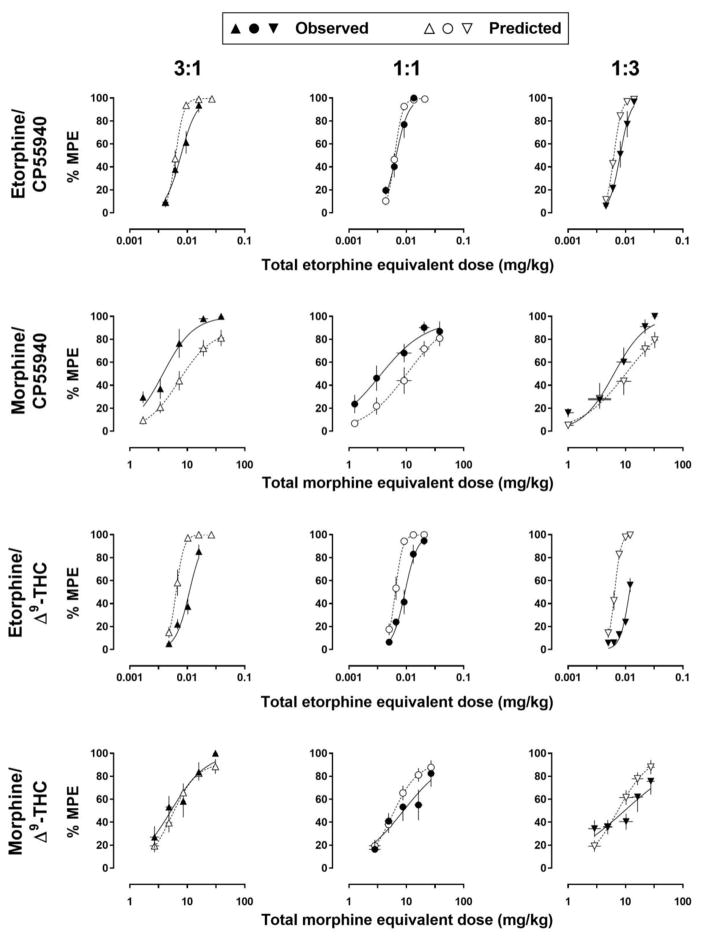
Comparison of observed effects with those predicted for an additive interaction (Fig. 3, top row) indicates that two etorphine/CP55940 mixtures (ratios of 3:1 and 1:3) were sub-additive, as indicated by higher than predicted ED50 values (Table 4). Morphine/CP55940 mixtures (second row) were supra-additive, indicated either by greater than predicted maximal effects (3:1 and 1:3) or lower that predicted ED50 values (1:1; second row, Fig. 3). Etorphine/Δ9-THC mixtures (third row) were sub-additive, indicated by lower than predicted maximal effects (3:1 and 1:3) and/or higher than predicted ED50 values (3:1, 1:1, and 1:3; third row, Fig. 3). Finally, morphine/Δ9-THC mixtures (bottom row) did not differ from additive for any ratio. Slopes for the observed curves did not differ significantly from those of the predicted curves for any mixture (Table 4).
Fig. 3.
Comparison of the predicted effects of the drug mixtures based on the assumption of an additive interaction (open symbols) with the observed effects (filled symbols) for each dose ratio and each dose pair. Abscissae: additive, opioid-equivalent total dose in milligrams per kilogram body weight; horizontal error bars indicate the standard error of the mean for the opioid-equivalent dose (see Data Analyses for details). Ordinate: percentage of the maximum possible effect (MPE; mean ± 1 standard error of the mean).
Table 4.
Mean difference [95% confidence intervals] and t-test statistics for comparison of parameters for predicted and observed dose-effect curves (observed minus predicted).
| Test | Maximum effect | ED50 | Slope | |||
|---|---|---|---|---|---|---|
| Etorphine: | ||||||
| CP55940 | ||||||
| 3:1 | −5.48 [−18.6, 7.7] | t(7)=1.0, P=.36 | 0.10 [0, 0.2] | t(7)=2.6, P=.03a | −1.35 [−2.8, 0.1] | t(7)=2.2, P=.07 |
| 1:1 | 0.86 [−0.8, 2.5] | t(7)=1.3, P=.25 | 0.05 [0, 0.1] | t(7)=1.4, P=.18 | 0.02 [0, 0.1] | t(7)=1.0, P=.35 |
| 1:3 | −2.22 [−7.0, 2.6] | t(7)=1.1, P=.31 | 0.14 [0.1, 0.2] | t(7)=4.6, P<.01a | −0.84 [−2.8, 1.1] | t(7)=1.0, P=.35 |
| Morphine: | ||||||
| CP5594 | ||||||
| 0 | ||||||
| 3:1 | 18.77 [2.0, 35.5] | t(7)=2.7, P=.03a | −0.16 [−0.4, 0.1] | t(7)=1.5, P=.25 | 0.87 [−1.6, 3.4] | t(7)=0.8, P=.44 |
| 1:1 | 13.75 [−2.1, 29.6] | t(7)=2.0, P=.08 | −0.26 [−0.4, − 0.1] | t(7)=3.4, P=.04a | 1.18 [−2.9, 5.3] | t(7)=0.7, P=.52 |
| 1:3 | 20.63 [3.9, 37.3] | t(7)=2.9, P=.02a | 0.03 [−0.2, 0.3] | t(7)=0.3, P=.80 | 1.77 [−2.4, 6] | t(7)=1.0, P=.35 |
| Etorphine: | ||||||
| Δ9-THC | ||||||
| 3:1 | −14.58 [−29, − 0.2] | t(7)=2.4, P<.05a | 0.18 [0.1, 0.3] | t(7)=4.1, P<.01a | −0.29 [−1, 0.4] | t(7)=1.0, P=.33 |
| 1:1 | −5.39 [−13.9, 3.1] | t(7)=1.5, P=.18 | 0.17 [0.1, 0.2] | t(7)=5.6, P<.01a | −0.88 [−2.3, 0.5] | t(7)=1.5, P=.19 |
| 1:3 | −43.46 [−58.0, − 28.9] | t(7)=7.0, P<.01a | 0.16 [0.1, 0.2] | t(7)=6.8, P<.01a | −1.40 [−3.4, 0.6] | t(7)=1.6, P=.14 |
| Morphine: | ||||||
| Δ9-THC | ||||||
| 3:1 | 3.95 [−21.0, 28.9] | t(7)=0.4, P=.72 | 0.01 [−0.1, 0.2] | t(7)=0.2, P=.82 | 0.96 [−1.4, 3.3] | t(7)=1.0, P=.37 |
| 1:1 | −3.24 [−30.3, 23.8] | t(7)=0.3, P=.79 | 0.10 [0, 0.3] | t(7)=1.6, P=.13 | 0.46 [−2.2, 3.2] | t(7)=0.4, P=.70 |
| 1:3 | −11.68 [−40.2, 16.8] | t(7)=1.0, P=.37 | −0.10 [−0.3, 0.1] | t(7)=1.4, P=.17 | −1.81 [−3.9, 0.3] | t(7)=2.0, P=.08 |
Indicates a significant difference between predicted and observed effects (i.e., not an additive interaction) according to a two-tailed, repeated measures t-test with alpha equal to 0.05.
4. Discussion
There is a need for pain treatments that are more effective and have fewer adverse effects than currently available treatments (i.e., mu opioid receptor agonists such as morphine and oxycodone). One strategy for possibly increasing the therapeutic window of opioids is to combine an opioid with another drug, such that smaller doses of the opioid (in combination with another drug) produce the desired therapeutic effect, while reducing or eliminating adverse effects. In preclinical and clinical studies cannabinoid receptor agonists enhance the antinociceptive potency of mu opioid receptor agonists suggesting that opioid/cannabinoid mixtures might be particularly effective and safe for treating pain. Presumably, mixtures that yield the greatest increase in antinociceptive potency would have the lowest risk of adverse effects insofar as the doses of each constituent drug in the mixture are smaller than those that alone produce adverse effects. However, it is unclear which opioid/cannabinoid mixture yields the greatest enhancement of potency. The current study examined the impact of drug and dose ratio on the interaction between opioids (etorphine and morphine) and cannabinoids (CP55940 and Δ9-THC) using a warm water tail withdrawal procedure in rats.
Administered alone, each drug dose-dependently increased the latency for rats to remove the tail from warm water, being more effective, and in some cases more potent, at increasing tail withdrawal latencies from 50° compared with 55°C water. The relative potencies of the two opioids tested (etorphine>morphine) is consistent with previous studies in rats (Paronis and Holtzman 1992; Walker et al. 1998; Smith et al. 1999; Cook et al. 2000). Etorphine had a steeper dose-effect curve, perhaps reflecting higher intrinsic efficacy than morphine at mu opioid receptors (Paronis and Holtzman 1992; Walker et al. 1998). The relative potency of the two cannabinoids tested (CP55940>Δ9-THC) is also consistent with previous studies in rats (Lichtman and Martin 1992 [intravenous route]; Lichtman et al. 1996 [Intracerebroventricular route]; Tseng and Craft 2001 [intraperitoneal route]).
All opioid/cannabinoid mixtures dose-dependently increased tail withdrawal latencies; however, the potency and maximal effects of the mixtures varied across drugs and dose ratios. Mixtures including morphine and/or CP55940 produced greater maximal effects as well as the largest increases in antinociceptive potency. Mixtures containing morphine were consistent with a supra-additive (CP55940) or additive (Δ9-THC) interaction, whereas mixtures containing etorphine and either cannabinoid generally were sub-additive. These results extend previous reports suggesting that drug/drug interactions vary with the particular opioid in the mixture (e.g., Cichewicz et al. 1999). Moreover, these results systematically replicate a recent study in mice showing that mixtures of morphine and the high-efficacy cannabinoid receptor agonist WIN55212 synergistically attenuate mechanical and cold allodynia induced by chronic constriction injury of the sciatic nerve (Kazantzis et al. 2016). Together, these studies support the use of opioid/cannabinoid mixtures for treating acute and persistent pain.
Some results from the current study are different from those reported in a study with rhesus monkeys (Maguire and France 2014); in the current study enhancement of antinociceptive potency by cannabinoids was greater with morphine compared with the higher efficacy opioid etorphine, whereas in monkeys enhancement was greater with etorphine. This difference between studies might reflect a difference between species with regard to the nature of opioid/cannabinoid interactions. However, there are procedural differences between these studies that might also be important. For example, in the current study an opioid and a cannabinoid were administered in a single injection with increasing cumulative doses. In contrast, many studies, including those with rhesus monkeys (Li et al. 2008; Maguire et al. 2013; Maguire and France 2014), administered a single dose of one drug (e.g., as a pretreatment) separately from administration of a second drug, either in single or cumulative doses. Nevertheless, studies in both species indicate that the particular opioid in the mixture plays an important role in the magnitude of enhancement (see also Cichewicz et al. 2005).
Mixtures with CP55940 (combined with morphine or etorphine) appeared to produce greater enhancement in the antinociceptive potency of the opioid than those with Δ9-THC. Although CP55940 and Δ9-THC were studied in different groups of rats, the two groups did not differ in sensitivity to the thermal stimuli (i.e., baseline tail withdrawal latencies were not markedly different; Supplemental Fig. 1) or to drugs that were tested in both groups (etorphine and morphine; Fig. 1; Table 2). Thus, differences in interactions between CP55940 and Δ9-THC were most likely due to the particular cannabinoid studied. That CP55940 enhanced potency more than Δ9-THC is consistent with a study in rhesus monkeys in which the antinociceptive potency of opioids was increased more by CP55940 than by Δ9-THC (Maguire and France 2014).
Table 2.
ED50 values [95% confidence intervals] for the antinociceptive effects of opioid receptor agonists and cannabinoid receptor agonists administered alone and in mixtures (50°C water).
| Test | Opioid ED50 (mg/kg) | Cannabinoid ED50 (mg/kg) |
|---|---|---|
| Etorphinea | 0.0063 [0.0057, 0.0069] | |
| CP55940a | 0.36 [0.29, 0.45] | |
| Etorphine:CP55940 | ||
| 3:1 | 0.0034 [0.0028, 0.0042] | 0.10 [0.08, 0.12] |
| 1:1 | 0.0021 [0.0015, 0.0029] | 0.16 [0.12, 0.22] |
| 1:3 | 0.0018 [0.0012, 0.0027] | 0.44 [0.33, 0.58] |
| Morphinea | 6.99 [5.23, 9.36] | |
| CP55940a | 0.31 [0.18, 0.55] | |
| Morphine:CP55940 | ||
| 3:1 | 3.57 [2.49, 5.13] | 0.05 [0.03, 0.07] |
| 1:1 | 2.23 [1.52, 3.28] | 0.09 [0.06, 0.13] |
| 1:3 | 1.63 [1.23, 2.16] | 0.19 [0.14, 0.25] |
| Etorphinea | 0.0066 [0.0063, 0.0068] | |
| Δ9-THCa | 13.74 [10.15, 18.60] | |
| Etorphine:Δ9-THC | ||
| 3:1 | 0.0045 [0.0036, 0.0057] | 4.73 [3.79, 5.89] |
| 1:1 | 0.0035 [0.0025, 0.0048] | 10.80 [8.10, 14.40] |
| 1:3 | 0.0030 [0.0026, 0.0034] | 29.41 [24.24, 35.69] |
| Morphinea | 6.68 [4.89, 9.15] | |
| Δ9-THCa | 7.69 [5.26, 11.23] | |
| Morphine:Δ9-THC | ||
| 3:1 | 3.40 [2.14, 5.40] | 1.71 [1.07, 2.75] |
| 1:1 | 2.38 [1.64, 3.46] | 3.54 [2.44, 5.14] |
| 1:3 | 0.87 [0.55, 1.39] | 4.01 [2.63, 6.11] |
Dose effect curves for each drug alone were determined twice before tests with mixtures; dose-effect curves for CP55940 and Δ9-THC were determined four times.
Collectively, results from these studies suggest that cannabinoids with high efficacy at cannabinoid receptors (Breivogel et al. 1998) might be most effective at enhancing the potency of opioids (i.e., opioid-sparing effect). Currently, no high efficacy cannabinoid receptor agonists are approved for use in humans and a majority of clinical studies on opioid/cannabinoid mixtures for treating pain use Δ9-THC or one of its analogues, all of which have modest efficacy at cannabinoid receptors. To the extent that mixtures also reduce the dose of the cannabinoid necessary for treating pain, these data support the use of higher efficacy cannabinoids insofar as mixtures would limit adverse effects of both drugs (opioids and cannabinoids). Other things being equal, repeated treatment with a higher efficacy drug typically produces less tolerance as compared to treatment with an equi-effective dose of lower efficacy drugs (e.g., Paronis and Holtzman 1992), due in part to the lower receptor occupancy that is required for higher efficacy drugs to produce a particular effect. In principle, this possibility might also extend to cannabinoids (e.g., Hruba et al. 2012). Thus, repeated treatment with mixtures containing a higher efficacy cannabinoid such as CP55940 might produce less tolerance compared to treatment with an equi-effective mixture containing a lower efficacy cannabinoid such as Δ9-THC. Reduced tolerance would be particularly appealing for treating chronic pain, which often requires repeated drug treatment for extended periods.
Only male rats were tested in the current study, although sex differences that have been reported for the antinociceptive effects of opioids and cannabinoids could translate into differences in the nature the interaction between these drugs. Opioids are generally more potent and/or effective in male rats compared with females (see Craft 2003 for a review); however, the magnitude of the difference can depend on many factors including the intrinsic efficacy of the test drug (e.g., Cook et al. 2000). In contrast, cannabinoids are generally more potent and/or effective in female rats (e.g., Tseng and Craft, 2001; Romero et al., 2002; Craft et al., 2012). Some studies have reported that cannabinoid impacts the behavioral effects of opioids in a sex-dependent manner predominantly late in development (e.g., Ambrosio et al. 1999; Biscaia et al. 2008; Vela et al. 1995); due to a paucity of data, it is unclear whether the nature of the interaction between opioids and cannabinoids are sex-dependent. The approach used in the current study takes into account sensitivity to the drugs administered alone; thus, the same analysis could be extended to examine differences in opioid/cannabinoid interactions based on many biological variables, including sex.
In summary, pain is a significant clinical problem, and there is a need for effective pharmacotherapies with fewer adverse effects than currently available drugs (e.g., mu opioid receptor agonists). Cannabinoid receptor agonists enhance the antinociceptive effects of mu opioid receptor agonists, but it is unclear which drug and in what proportion will yield the safest and most effective treatment. This study examined the antinociceptive effects of the mu opioid receptor agonists etorphine and morphine alone and in combination with the cannabinoid receptor agonists Δ9-THC and CP55940 using a warm water tail withdrawal procedure in rats. Mixtures with morphine were supra-additive (CP55940) or additive (Δ9-THC), whereas mixtures with etorphine were additive or sub-additive; interactions did not vary systematically among different ratios of opioid to cannabinoid in the mixture. These data indicate that the nature of the interaction between opioids and cannabinoids with regard to antinociceptive effects varies with the drugs in the mixture, which can have implications for designing combination therapies for pain.
Supplementary Material
Acknowledgments
Funding: This work was supported, in part, by the United States National Institute on Drug Abuse, National Institutes of Health [K05DA17918] and the Welch Foundation [AQ-0039].
The authors wish to thank Elise Rush and Steven P. Garza for excellent technical assistance in conducting this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrosio E, Martín S, García-Lecumberri C, Crespo JA. The neurobiology of cannabinoid dependence: sex differences and potential interactions between cannabinoid and opioid systems. Life Sci. 1999;65:687–694. doi: 10.1016/S0024-3205(99)00291-X. [DOI] [PubMed] [Google Scholar]
- Auh QS, Chun YH, Melemedjian OK, Zhang Y, Ro JY. Peripheral interactions between cannabinoid and opioid receptor agonists in a model of inflammatory mechanical hyperalgesia. Brain Res Bull. 2016;125:211–217. doi: 10.1016/j.brainresbull.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med. 2014;174:1668–1673. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscaia M, Fernández B, Higuera-Matas A, Miguéns M, Viveros MP, García-Lecumberri C, Ambrosio E. Sex-dependent effects of periadolescent exposure to the cannabinoid agonist CP-55,940 on morphine self-administration behaviour and the endogenous opioid system. Neuropharmacology. 2008;54:863–873. doi: 10.1016/j.neuropharm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Selley DE, Childers SR. Cannabinoid receptor agonist efficacy for stimulating [35S] GTPγS binding to rat cerebellar membranes correlates with agonist-induced decreases in GDP affinity. J Biol Chem. 1998;273:16865–16873. doi: 10.1074/jbc.273.27.16865. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Martin ZL, Smith FL, Welch SP. Enhancement of μ opioid antinociception by oral Δ9-tetrahydrocannabinol: dose-response analysis and receptor identification. J Pharmacol Exp Ther. 1999;289:859–867. [PubMed] [Google Scholar]
- Cichewicz DL, McCarthy EA. Antinociceptive synergy between Δ9-tetrahydrocannabinol and opioids after oral administration. J Pharmacol Exp Ther. 2003;304:1010–1015. doi: 10.1124/jpet.102.045575. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Welch SP, Smith FL. Enhancement of transdermal fentanyl and buprenorphine antinociception by transdermal Δ9-tetrahydrocannabinol. Eur J Pharmacol. 2005;525:74–82. doi: 10.1016/j.ejphar.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the μ opioid receptor. Psychopharmacology (Berl) 2000;150:430–442. doi: 10.1007/s002130000453. [DOI] [PubMed] [Google Scholar]
- Cox ML, Haller VL, Welch SP. Synergy between Δ9-tetrahydrocannabinol and morphine in the arthritic rat. Eur J Pharmacol. 2007;567:125–130. doi: 10.1016/j.ejphar.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in opioid analgesia: “from mouse to man”. Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Craft RM, Wakley AA, Tsutsui KT, Laggart JD. Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by Δ9-tetrahydrocannabinol and CP55, 940 in the rat. J Pharmacol Exp Ther. 2012;340:787–800. doi: 10.1124/jpet.111.188540. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA. 2016;315:1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett C. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- Grabovsky Y, Tallarida RJ. Isobolographic analysis for combinations of a full and partial agonist: curved isoboles. J Pharmacol Exp Ther. 2004;310:981–986. doi: 10.1124/jpet.104.067264. [DOI] [PubMed] [Google Scholar]
- Hruba L, Ginsburg BC, McMahon LR. Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by Δ9-tetrahydrocannabinol treatment in rhesus monkeys. J Pharmacol Exp Ther. 2012;342:843–849. doi: 10.1124/jpet.112.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Kazantzis NP, Casey SL, Seow PW, Mitchell VA, Vaughan CW. Opioid and cannabinoid synergy in a mouse neuropathic pain model. Br J Pharmacol. 2016;173:2521–2531. doi: 10.1111/bph.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, Alexander GC. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–574. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- Li JX, Koek W, France CP. Interactions between Δ9-tetrahydrocannabinol and heroin: self-administration in rhesus monkeys. Behav Pharmacol. 2012;23:754–761. doi: 10.1097/FBP.0b013e32835a3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, McMahon LR, Gerak LR, Becker GL, France CP. Interactions between Δ9-tetrahydrocannabinol and mu opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology (Berl) 2008;199:199–208. doi: 10.1007/s00213-008-1157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Spinal and supraspinal components of cannabinoid- induced antinociception. J Pharmacol Exp Ther. 1991;258:517–523. [PubMed] [Google Scholar]
- Maguire DR, France CP. Impact of efficacy at the μ-opioid receptor on antinociceptive effects of combinations of μ-opioid receptor agonists and cannabinoid receptor agonists. J Pharmacol Exp Ther. 2014;351:383–389. doi: 10.1124/jpet.114.216648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP. Effects of daily delta-9-tetrahydrocannabinol treatment on heroin self-administration in rhesus monkeys. Behav Pharmacol. 2016a;27:249–257. doi: 10.1097/FBP.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP. Interactions between cannabinoid receptor agonists and mu opioid receptor agonists in rhesus monkeys discriminating fentanyl. Eur J Pharmacol. 2016b;784:199–206. doi: 10.1016/j.ejphar.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP. Additive antinociceptive effects of mixtures of the κ-opioid receptor agonist spiradoline and the cannabinoid receptor agonist CP55940 in rats. Beh Pharm. 2016c;27:69–72. doi: 10.1097/FBP.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Yang W, France CP. Interactions between μ-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. J Pharmacol Exp Ther. 2013;345:354–62. doi: 10.1124/jpet.113.204099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LL, Picker MJ, Umberger MD, Schmidt KT, Dykstra LA. Effects of alterations in cannabinoid signaling, alone and in combination with morphine, on pain-elicited and pain-suppressed behavior in mice. J Pharmacol Exp Ther. 2012;342:177–187. doi: 10.1124/jpet.112.191478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H, Christopoulos A. Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. Oxford University Press; 2003. [Google Scholar]
- Nielsen S, Sabioni P, Trigo JM, Ware MA, Betz-Stablein BD, Murnion B, … Le Foll B. Opioid-sparing effect of cannabinoids: a systematic review and meta-analysis. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.51. advance online publication 5 April 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paronis CA, Holtzman SG. Development of tolerance to the analgesic activity of mu agonists after continuous infusion of morphine, meperidine or fentanyl in rats. J Pharmacol Exp Ther. 1992;262:1–9. [PubMed] [Google Scholar]
- Pugh G, Smith PB, Dombrowski DS, Welch SP. The role of endogenous opioids in enhancing the antinociception produced by the combination of Δ 9-tetrahydrocannabinol and morphine in the spinal cord. J Pharmacol Exp Ther. 1996;279:608–16. [PubMed] [Google Scholar]
- Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur J Pharm. 2006;530:54–58. doi: 10.1016/j.ejphar.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Romero EM, Fernández B, Sagredo O, Gomez N, Urigüen L, Guaza C, De Miguel R, Ramos JA, Viveros MP. Antinociceptive, behavioural and neuroendocrine effects of CP 55,940 in young rats. Brain Res Dev Brain Res. 2002;136:85–92. doi: 10.1016/S0165-3806(02)00306-1. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016 Jan 1;64:1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- Smith MA, Barrett AC, Picker MJ. Antinociceptive effects of opioids following acute and chronic administration of butorphanol: influence of stimulus intensity and relative efficacy at the mu receptor. Psychopharmacology (Berl) 1999;143:261–269. doi: 10.1007/s002130050945. [DOI] [PubMed] [Google Scholar]
- Smith PA, Selley DE, Sim-Selley LJ, Welch SP. Low dose combination of morphine and Δ 9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur j Pharmacol. 2007;571:129–137. doi: 10.1016/j.ejphar.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FL, Cichewicz D, Martin ZL, Welch SP. The enhancement of morphine antinociception in mice by Δ9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1998;60:559–566. doi: 10.1016/s0091-3057(98)00012-4. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism and dose-effect data analysis. Boca Raton: Chapman Hall/CRC Press; 2000. [Google Scholar]
- Tallarida RJ. An overview of drug combination analysis with isobolograms. J Pharmacol Exp Ther. 2006;319:1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- Tham SM, Angus JA, Tudor EM, Wright CE. Synergistic and additive interactions of the cannabinoid agonist CP55,940 with mu opioid receptor and alpha2-adrenoceptor agonists in acute pain models in mice. Br J Pharmacol. 2005;144:875–884. doi: 10.1038/sj.bjp.0706045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol. 2001;430:41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- Vela G, Fuentes JA, Bonnin A, Fernández-Ruiz J, Ruiz-Gayo M. Perinatal exposure to delta 9-tetrahydrocannabinol (delta 9-THC) leads to changes in opioid-related behavioral patterns in rats. Brain Res. 1995;680:142–147. doi: 10.1016/0006-8993(95)00255-O. [DOI] [PubMed] [Google Scholar]
- Wakley AA, Craft RM. THC methadone and THC naltrexone interactions on discrimination, antinociception, and locomotion in rats. Behav Pharmacol. 2011;22:489–497. doi: 10.1097/FBP.0b013e328348ed22. [DOI] [PubMed] [Google Scholar]
- Walker EA, Zernig G, Young AM. In vivo apparent affinity and efficacy estimates for μ opiates in a rat tail-withdrawal assay. Psychopharmacology (Berl) 1998;136:15–23. doi: 10.1007/s002130050534. [DOI] [PubMed] [Google Scholar]
- Welch SP, Stevens DL. Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine, in mice. J Pharmacol Exp Ther. 1992;262:10–18. [PubMed] [Google Scholar]
- Wessinger WD. Approaches to the study of drug interactions in behavioral pharmacology. Neurosci Biobehav Rev. 1986;10:103–110. doi: 10.1016/0149-7634(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Williams IJ, Edwards S, Rubo A, Haller VL, Stevens DL, Welch SP. Time course of the enhancement and restoration of the analgesic efficacy of codeine and morphine by Δ9-tetrahydrocannabinol. Eur J Pharmacol. 2006;539:57–63. doi: 10.1016/j.ejphar.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. Analysis of drug interactions in behavioral pharmacology. In: Thompson T, Dews PB, Barrett JE, editors. Neurobehavioral pharmacology. Vol. 6. Lawrence Erlbaum; Hillsdale, NJ, USA: 1987. pp. 275–302. [Google Scholar]
- Yesilyurt O, Dogrul A, Gul H, Seyrek M, Kusmez O, Ozkan Y, Yildiz O. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303–308. doi: 10.1016/s0304-3959(03)00245-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.