Abstract
In the current study, extracellular biosynthesis of silver nanoparticles (AgNPs) was carried out using aqueous extracts of green Calligonum comosum stem, besides Fusarium sp. Synthesized AgNPs were characterized using ultraviolet (UV)–Vis spectrophotometer, transmission electron microscopy (TEM) and zeta potential. Moreover, biosynthesized AgNPs were estimated for the scavenging ability on DPPH radical as well as tested for their antibacterial activity using well diffusion method against Gram-positive bacteria Staphylococcus aureus. On the other hand, DNA content from untreated and AgNPs treated bacterial cells was evaluated by (UV)–Vis spectrophotometer and agarose gel electrophoresis. Results revealed the formation of AgNPs, which was first detected by color change of the reaction mixture. The characteristic surface plasmon resonance absorption was detected at 450 and 410 nm for the plant and myco-synthesized AgNPs. Furthermore, TEM micrograph and zeta sizer showed formation of spherical particles with an average size of about 105.8 and 228.4 nm for plant and myco-synthesized AgNPs, respectively. Plant-synthesized AgNPs exhibited higher scavenging of DPPH radicals than that of the myco-synthesized one. For bactericidal action, plant-synthesized AgNPs showed higher inhibition zone compared with myco-synthesized one, which was negatively correlated with the nanoparticle size. Furthermore, low DNA concentration was detected for AgNPs treated bacteria, which might be a consequence of inactivation for DNA replication. Further experimental work is required to find out if there is any correlation between nanoparticles size and efficacy against bacteria.
Keywords: AgNPs, Fungi, Plant extract, DPPH, DNA, Nanotechnology
Introduction
Nanotechnology is an emerging field of science, which involves synthesis and development of various nano-materials. At present, different types of metal nanomaterial are being produced using copper, zinc, titanium, magnesium, gold, alginate and silver (Naveen et al. 2010). Applications of nanoparticles (NPs) in Nano-medicine have been well documented. Therapeutic applications to target definite sites, such as lung tissue, vaccinations and cancer therapy have been recently employed (Thorley and Tetley 2013). Currently, the increased use of NPs in medicine is closely associated with the potential of NPs to circumvent microbial resistance. Furthermore, to overcome the microbial resistance, NPs were used in a form of metallic NPs, chitosan NPs and nitric oxide-releasing NPs (Pelgrift and Friedman 2013). Amon the metallic NPs, AgNPs possess excellent antibacterial, antifungal and anti-viral potentials (dos Santos et al. 2014). Chemical and physical methods for AgNPs formation were used, but recently plant and microbial extracts were used as bio-mediators for AgNPs formation (Singh and Raja 2011; Mohammed 2015). The antimicrobial potential of AgNPs is assumed because nanoparticles (NPs) are defined as having about 100 nm in size they tend to react differently than larger particles of the same composition, allowing them to be utilized in unique applications (Raudabaugh et al. 2013). AgNPs can cause cell lysis, inhibit cell transduction, changes in the cell membrane permeability and destruction of microbial genome and DNA fragmentation (Prabhu and Poulose 2012; Ouda 2014). Danilcauk et al. (2006) and Kim et al. (2007) suggested that, silver nanoparticles mode of action when contacted with microbes could be related to the formation of free radicals which have the ability to damage cell membrane. Recently, utilization of bacteria, fungi, yeasts and plant extracts have been used in synthesis of nanoparticles. Aspergillus fumigatus, Penicillium citrinum, Bryophilous rhizoctonia were used recently for this technology (Ratnasri and Hemalatha 2014; Honary et al. 2013; Raudabaugh et al. 2013). It is well documented that certain bacteria, yeasts and fungi play an important role in remediation of toxic metals through reduction of the metal ions (Vahabi et al. 2011). Furthermore, microorganisms were used as bio-mediators for synthesis of metallic nanoparticles such as cadmium sulphide, gold and silver (Sastry et al. 2003). Plant phytochemicals such as terpenoids, flavones, carboxylic acids in addition to plant-derived polysaccharides play important roles as reducing and stabilizing agents in conversion of silver ions to silver nanoparticles (Jha et al. 2009). Furthermore, the microorganisms extracellular enzymes present in the cell wall might be the main reason for the reduction of metal ions to metal nanoparticles (Mukherjee et al. 2001; Sahayaraj and Rajesh 2011). On the other hand, the cell wall of the microorganisms play a major role in the intracellular synthesis of nanoparticles since the cell wall being negatively charged interacts electrostatically with the positively charged metal ions (Deepak et al. 2011). In the current study, Fusarium sp. extract and Calligonum comosum extracts were used for the formation of AgNPs. C. comosum L’Her., “Arta”, is a plant with a wide distribution in Saudi Arabia. Anthraquinones and flavonoids are the common chemical constituents in Arta (Ghazanfar 1994; Kamil et al. 2000). Using biomaterial for AgNPs formation is an eco-friendly approach since the processing of biomass are simple and it is easy to handle plant and microbial cultures (Ingle et al. 2008). The main objective of this study is to use C. comosum green stem besides Fusarium sp. extracts to mediate reduction in the silver ions present in the aqueous solution of silver nitrate into AgNPs. Furthermore, the bactericidal impact of biosynthesized AgNPs on Staphylococcus aureus was evaluated. To realize that goal inhibition zone was assessed.
Materials and methods
Chemicals
Potato dextrose agar (PDA), potato dextrose broth (PDB) and silver nitrate (AgNO3).
Sample collection
Fungi were isolated and screened from some tomato fruits and C. comosum (Arta) green stem was collected from Riyadh, SA.
Isolation of fungal cultures
Conducted to isolate fungi by taking apiece by a sterile scalpel and placed in Petri dishes, then incubated for 5 days at a temperature of 25 °C.
Colony characterization
Colony morphology of the fungal isolates was recorded with respect to color, shape, size and nature of colony.
Plant extract as biomediator in AgNPs formation
The aqueous extract of C. comosum prepared by mixing 10 g of the dry sample with 100 ml of highly purified water. The mixture was heated for 10 min at 80 °C to denature the enzymes of the extracts. The solution was filtered through a Whatman filter papers (pore size 100, 70, 55 mm) conseqatively. For synthesis of the AgNPs, 12 ml of each aqueous extract of C. comosum as reducing agents was mixed with 88 ml of a 1 mM AgNO3 aqueous solution in an Erlenmeyer flask and allowed to react at room temperature in dark condition. The AgNPs colloidal solution was stored at 4 °C until further analysis (Mohammed 2015).
Fungal extract as biomediator in AgNPs formation
The Fusarium sp. was selected for production of silver nanoparticles. The Fusarium sp. was inoculated in Potato Dextrose Broth (PDB) broth at 25 °C. The biomass was harvested after 72 h of growth. The biomass was washed with sterilized distilled water to remove any medium component. 5 g of biomass (fresh weight) was mixed with 100 ml of sterile distilled water in a 500 ml flask and agitated for 72 h at 25 °C. After the incubation, the cell filtrate was obtained by passing it through Whatman filter paper. For the synthesis of silver nanoparticles, 55 ml of 1 mM AgNO3 solution was mixed with 10 ml fungal filtrate.
Characterization of AgNPs
The reduction of silver ions to AgNPs in the solution was monitored by measuring the ultraviolet–visible spectrum of the solution using a UV–Vis 2450 double-beam spectrophotometer (Shimadzu, Tokyo, Japan).
Transmission electron microscopy (TEM)
The analysis by TEM technique was used to visualize the size, shape and morphology of the AgNPs. A drop of AgNPs solution was placed on carbon-coated copper grid. The images were obtained at a voltage of 80 kV.
Dynamic light scattering (DLS) and zeta potential
The particle size distribution and zeta potential analysis of bio-prepared AgNPs were evaluated via dynamic light scattering (DLS) and zeta potential analysis using Zetasizer, Nano series, HT laser, ZEN 3600 (Malvern Instruments Ltd., Malvern, UK). The measurements were taken in the range between 0.1 and 10,000 nm.
2,2-Diphenyl-1-picryl-hydrazylhydrate (DPPH) free radical assay
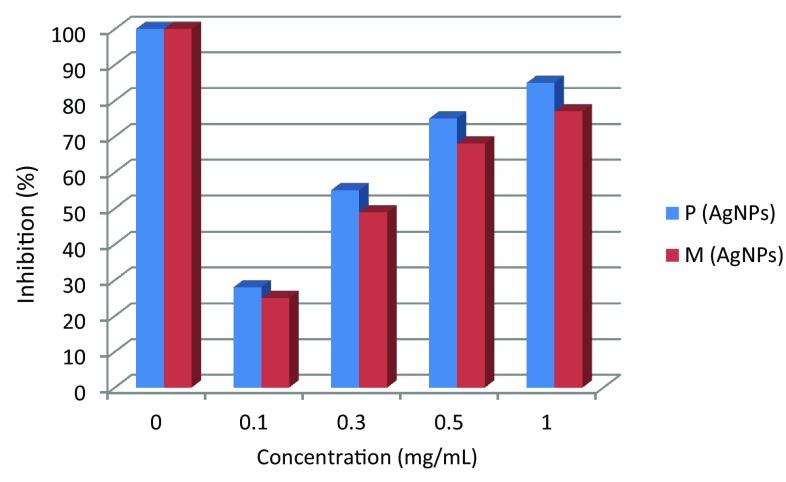
Biosynthesized AgNPs were estimated for the scavenging ability on DPPH radical (Blois 1958). Concentrations of 0.01–1 mg/l of AgNPs solution were added to 3 ml methanolic DPPH solution (0.1 mM) each. Such a combination was incubated at room temperature for 30 min under shaking and the absorbance was recorded at 517 nm. Glutathione (GSH) was used as control. Data expressed as percentage of inhibition according to the following equation (Azizi et al. 2017):
Fourier transform infrared spectroscopy (FTIR)
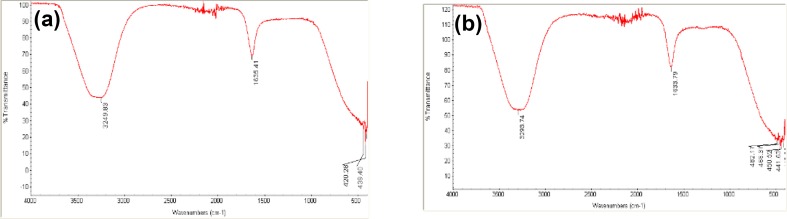
Fourier transform infrared spectroscopy (FTIR) was used to measure infrared absorption and emission spectra for the expected organic compounds found in the biogenic AgNPs that act as reducing and stabilizing agents. A range of 500–5000 cm−1 FTIR (Shimadzu FTIR Prestiage 21) was used and the biomolecules were recorded using diffuse reflectance mode.
Evaluation of antibacterial activity of AgNPs against Staphylococcus aureus
The antibacterial activity of the synthesized AgNPs was measured using the well diffusion methods. Gram-positive bacteria S. aureus was swabbed uniformly onto individual nutrient agar plates using sterile swabs. Subsequently, two adequately spaced wells of 4 mm diameter reach were made per plate at the culture agar surface using sterile metal cup borer. In each well, 0.4 ml of each biosynthesized AgNPs extract and AgNO3 were put under aseptic conditions, kept at room temperature for 1 h to allow the extracts to diffuse into agar medium and incubated accordingly. Sterile distilled water was used as the reference negative control. All the plates were incubated at 25 °C for 24–36 h. The plates were examined for determination of inhibition zone, which appear as a clear space around the wells. The diameter of inhibition zones was measured using a ruler. Experiments were performed in four replicates and mean values were calculated.
DNA extraction of S. aureus
Cells were lysed in 250 μl cell lysis buffer containing 50 mM Tris–HCl, pH 8.0, 10 mM ethylene diamine tetra acetic acid, 0.1 M NaCl, and 0.5% sodium dodecyl sulfate. The lysate was incubated in 0.5 mg/ml RNase A at 37 °C for 1 h, and then in 0.2 mg/ml proteinase K at 50 °C overnight. Phenol extraction of this mixture was carried out, DNA in the aqueous phase was precipitated by 25 μl (1/10 volume) of 7.5 M ammonium acetate and 250 μl (1/1 volume) isopropanol. DNA electrophoresis was performed in 1% agarose gel containing 1 μg/ml ethidium bromide at a constant voltage of 70 V for 2 h. Following electrophoresis, gel was stained with Coomassie Brilliant Blue dye and observed in a gel-imaging system (Chromous Biotech, Bangalore, India).
Results and discussion
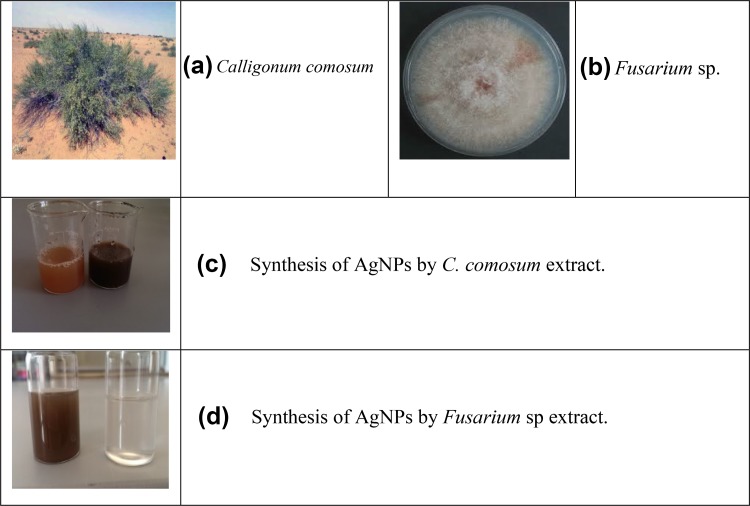
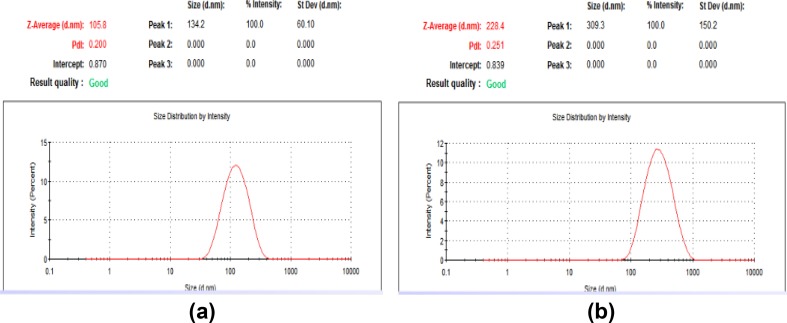
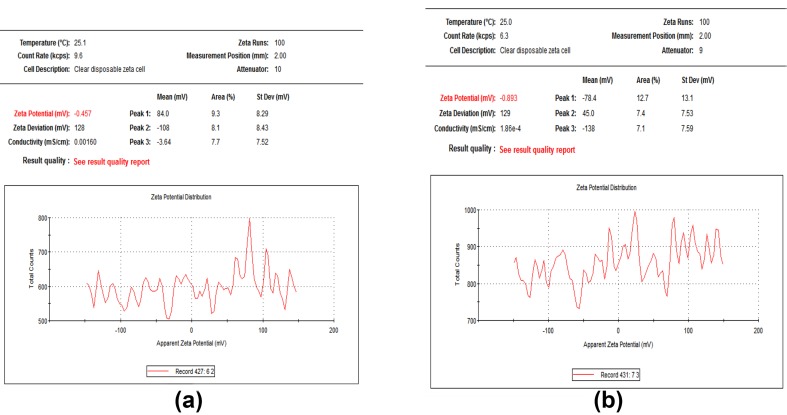
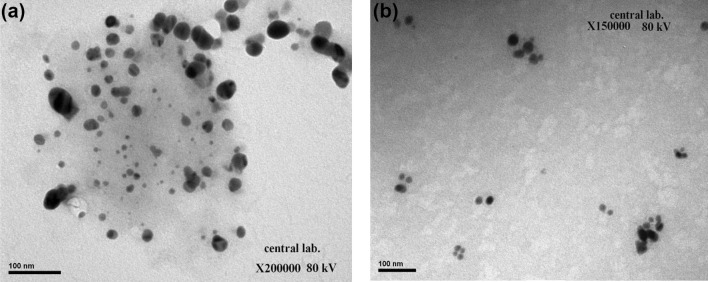
Recently, the development of a new antimicrobial compounds has proved to be efficient in suppressing microorganisms and thus has become widely employed in medical field as a reliable alternative for antibiotics to which certain microbes have developed a relative resistance. The present study aimed to evaluate the capacity of C. comosum green stem and Fusarium sp. as reducing and stabilizing bio-agents for silver nanoparticles formation. A further goal is to confirm and characterize the biosynthesized silver nanoparticles and to study their ability with respect to their antibacterial effect against S. aureus. To realize the first goal, an amount of 12 ml of plant extract, a concentration of 1 mg/ml and with 10 ml fungal filtrate showed an ability to convert the color of reaction mixture from yellow and white to a dark brown color (Fig. 1c, d). This color change is an indication for the conversion of silver ions to silver nanoparticles. The color changes are acquired due to the excitation of surface plasmon resonance (SPR) in the synthesized NPs (Paulkumar et al. 2014). Plant extract showed faster ability in conversion of silver ions to silver nanoparticles (1 h) than fungi (15 days). Furthermore, dynamic light scattering and zeta potential graph of AgNPs biosynthesized by C. comosum and Fusarium sp. showed an average size of 105.8 and 228.4 nm (Fig. 2a, b, respectively). A range between 50 and 300 nm particle size of the synthesized silver nanoparticles using red apple fruit extract was obtained by Umoren et al. (2014). A recent study (Banerjee et al. 2014) documented that different bio-reductant agents provide different AgNPs size when using banana, neem and tulsi leaf extracts. Furthermore, the morphological analysis by atomic force microscopy (AFM) revealed structural arrangements of AgNPs (300 ± 57 nm) when Fusarium oxysporum was used for the biosynthesis (Ishida et al. 2014). Zeta potential graph showed the potential stability of the particles in the solution (Fig. 3a, b). Negative charge of the nanoparticles was well confirmed in this study for the plant-synthesized and myco-synthesized AgNPs. Similar trend of observations was also recently reported by Singh et al. (2014), that silver nanoparticles synthesized from P. amarus and T. cordifolia have showed negative charges. Furthermore, Fig. 4a, b demonstrate TEM images, confirmed the development of silver nanostructures using C. comosum and Fusarium sp., respectively, indicating that the bio-prepared AgNPs are spherical in shape with a smooth surface morphology. Same observations were also recorded when AgNPs prepared by endophytic fungus Penicillium sp. and Oak fruit hull (Singh et al. 2014; Heydari and Rashidipour 2015). On the other hand, color change, spectrophotometer, zeta potential and TEM results revealed that, stable and uniform size of AgNPs could be easily produced by C. comosum and Fusarium sp. aqueous extracts. Such methods might be a feasible, low-cost and environmentally friendly technique. Moreover, it was well documented that C. comosum produce a unique secondary metabolites such as anthraquinones, flavonoids and dehydrodicatechin which had cytotoxic effect and antioxidant activity (Ghazanfar 1994; Kamil et al. 2000). These compounds might be the main reason for the reduction process of silver ions to silver nanoparticles since the plant secondary metabolites can act for reduction/oxidation reactions in the biosynthesis of silver nanoparticles (Prabhu and Poulose 2012). Regarding myco-synthesized AgNPs, it was observed that the filtrate broth obtained from Fusarium sp. contained dehydrogenase enzyme (Duran et al. 2005). The fungal extracellular enzymes and electron shuttle quinones showed excellent redox properties, they can act as electron shuttle in silver ions reduction (Duran et al. 2005; Sahayaraj and Rajesh 2011). FTIR was used for the possible biomolecules that responsible for formation and stabilization of AgNPs. Different peaks were detected, 1635.41 and 3249.83 cm−1 for plant synthesized and 1633.79 and 3293.74 cm−1 for myco-synthesized AgNPs, indicating different expected molecules present (Fig. 5). Amide I vibration for protein might be detected at absorbance between 1600 and 1700 cm−1 due to C=O stretching vibration (Yang et al. 2015). Furthermore, the peaks found in the current study for the plant and myco-synthesized AgNPs were near to 1633 cm−1 that was also reported for native protein indicating that protein cooperated with AgNPs did not changed when binding with AgNPs (Macdonald and Smith 1996). Furthermore, absorption bands in the FTIR spectra at 3293.74 and 3249.83 cm−1 were due to O–H stretching vibration of alcohol and phenol (Jemal et al. 2017). Results from FTIR analyses of plant and myco-synthesized AgNPs indicated presence of some biomolecules such as phenol and amino acid that could be responsible for bio-reduction of Ag+ to AgNPs in aqueous solutions. Regarding the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, The free radical scavenging activity of AgNPs were tested by DPPH scavenging as shown in Fig. 6. DPPH shows absorption peak at 517 nm. The mixtures were changed in color from dark purple to light yellow, which considered as reduction of DPPH and conversion to 1,1-diphenyl-2-picryl hydrazine with decolorization. The radical scavenging quantity was considered as the decrease in absorption rate. The DPPH activity of the AgNPs was found to be increased as the increment in the AgNPs concentration. Among, biogenic nanoparticles the plant-synthesized AgNPs exhibited higher scavenging of DPPH radicals with increasing concentration than that of the myco-synthesized ones it could be related to the more negative charge of plant-synthesized compared to myco-synthesized, same trend of observation was recorded by Azizi et al. (2017). Maximum DPPH scavenging activity was 81% at 1 mg/ml for the plant-synthesized AgNPs. Current results proved that, biogenic AgNPs could serve as free radical inhibitors. Additionally, silver nanoparticles synthesized by plant extract and fungi showed an antibacterial activity (Table 1). Bio-prepared AgNPs had higher ability to suppress the bacterial growth compared with silver nitrate; this might be due to the larger surface areas of the nanoparticles, which increased the interaction zone with biological targets. The biosynthesis of AgNPs from plant origin is an eco-friendly method that is costless with no toxic chemicals used and more efficient than using fungal extract. Plant-synthesized AgNPs showed higher inhibition zone (21.9 ± 3.7 mm) compared with myco-synthesized one (15.1 ± 4.0). Seemingly, the antibacterial activity was nanoparticle size-dependent. Plant-synthesized AgNPs with particle size of 105 nm had greater effect on the treated bacteria resulting in higher inhibition zone when compared with myco-synthesized AgNPs with particle size of 228 nm. Wu et al. (2014), Tamayo et al. (2014), and Franci et al. (2015) have recently documented that AgNPs activity is strongly dependent on the size since the smaller nanoparticles seem to have a superior ability to penetrate into bacteria cell. Banerjee et al. (2014) found that, banana leaf extract showed small AgNPs size which showed higher ability to suppress microbial growth compared with neem and tulsi leaf extracts. Furthermore, sizes and shapes of metal nanoparticles are influenced by a number of factors including pH, reductant concentration, incubation time, temperature as well as method of preparation (Umoren et al. 2014).
Fig. 1.
Photograph of C. comosum plant (a), culture of Fusarium sp. (b), synthesis of AgNPs by C. comosum aqueous extract (c), showing the color of reaction mixture at zero time (left) and after 24 h (right), synthesis of AgNPs by Fusarium sp. biomass filtrate (d), showing the color of AgNO3 aqueous solution (left) and the reaction mixture after 15 days (right)
Fig. 2.
Zetasizer for plant-synthesized (a) and myco-synthesized (b) AgNPs
Fig. 3.
Zeta potential for plant-synthesized (a) and myco-synthesized (b) AgNP
Fig. 4.
Transmission electron microscopy (TEM) images indicating shape, morphology and size for plant-synthesized (a) and myco-synthesized (b) AgNPs
Fig. 5.
FTIR for plant-synthesized (a) and myco-synthesized (b) AgNP
Fig. 6.
Scavenging capacity of different concentration of biogenic AgNPs on 1,1-diphenyl-2-picrylhydrazyl (DPPH), P plant-synthesized and M myco-synthesized
Table 1.
Inhibition zone diameter (mm) of S. aureus treated with plant-synthesized and myco-synthesized silver nanoparticles
| Treated bacteria | Treatments | ||
|---|---|---|---|
| AgNPs (Silver nanoparticles mediated by bioreductant agents) | AgNO3 | ||
| S. aureus | Green stem | Fusarium oxysporum | |
| 21.9 ± 3.7 a | 15.1 ± 4.0 ab | 12.5 ± 0.7 b | |
Data are mean ± SD
Different letters stand for significant differences among treatments
Silver nanoparticles effect on the DNA of Staphylococcus aureus
DNA from normal bacteria cells and from bacteria cells treated by AgNPs was evaluated by agarose gel electrophoresis and (UV)–Vis spectrophotometer in an attempt to understand the mode of action regarding the antibacterial activity of silver nanoparticles against microbial DNA. Low DNA concentration was observed in the treated bacteria with plant-synthesized and myco-synthesized AgNPs compared with untreated controls. Silver ions (Ag+) released from silver nanoparticles can interact with phosphorus moieties in DNA, resulting in inactivation of DNA replication, or can react with sulfur-containing proteins, leading to the inhibition of enzyme functions (Rai and Bai 2011). Furthermore, deformation of DNA from supercoiled to open circular DNA was observed when plasmid DNA was treated with silver nanoparticles which indicated that the nanoparticles have broken the DNA (Vahdati and Sadeghi 2013).
Conclusions
The technique employed in the current investigation for the synthesis of silver nanoparticles is fairly well suited with green chemistry principles as the plant extract and fungi serve as reductants and stabilizing agents. The stability of the nanoparticles was confirmed by zeta potential estimation using DLS. AgNPs exerted strong antibacterial effects on S. aureus tested in-vitro, probably via destruction of membrane integrity or inhibition of DNA replication. It might be concluded that the lower sized nanoparticles diffuse more easily than the larger ones, which partially explains the higher toxicity on S. aureus strain used in this study. Further experimental work is required to test the correlation of the composed particle size and the efficacy against bacteria. The plant and fungal extracts used here might also be considered as a natural environment, which likely interpret the observed differences in AgNPs diameters.
Aknowledgements
Authors are grateful to staff members in the Biology Section, Faculty of Science, PNU for their continuous support. Thankfullness is also extended to King Faisal specialist hospital and research center for providing the necessary facilities during the experimental period of this research work.
Compliance with ethical standards
Conflict of interest
The author has no competing interest.
References
- Azizi S, Rosfarizan M, Mahnaz MS. Green microwave-assisted combustion synthesis of zinc oxide nanoparticles with Citrullus colocynthis (L.) Schrad: characterization and biomedical applications. Molecules. 2017;2:301. doi: 10.3390/molecules22020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P, Satapathy M, Mukhopahayay A, Das P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour Bioprocess. 2014;1:3. doi: 10.1186/s40643-014-0003-y. [DOI] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Danilcauk M, Lund A, Saldo J, Yamada H, Michalik J. Conduction electron spin resonance of small silver particles. Spectrochim Acta A. 2006;63(75):189–191. doi: 10.1016/j.saa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Deepak V, Kalimuthu K, Sureshbabu RKP, Gurunathan S. Deepak et al book chapter. In: Rai MK, Duran N, editors. Metal nanoparticles in microbiology. New York: Springer; 2011. pp. 17–35. [Google Scholar]
- dos Santos CA, Seckler MM, Ingle AP, Gupta I, Galdiero S, Galdiero M, Gade A, Rai M. Silver nanoparticles: therapeutical uses, toxicity and safety issues. J Pharm Sci. 2014;103:1931–1944. doi: 10.1002/jps.24001. [DOI] [PubMed] [Google Scholar]
- Duran N, Marcato PD, Alves OL, De Souza GIH, Esposito E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nano Biotechnol. 2005;3:8–14. doi: 10.1186/1477-3155-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M. Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20:8856–8874. doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar SA, editor. Handbook of Arabian medicinal plants. Boca Raton: CRC Press; 1994. p. 173. [Google Scholar]
- Heydari R, Rashidipour M. Green synthesis of silver nanoparticles using extract of Oak Fruit Hull (Jaft): synthesis and in vitro cytotoxic effect on MCF-7 cells. Int J Breast Cancer. 2015;2015:846743. doi: 10.1155/2015/846743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honary S, Barabadi H, Gharaei-Fathabad E, Naghibi F. Green synthesis of silver nanoparticles induced by the fungus Penicillium citrinum. Trop J Pharm Res. 2013;12(1):7–11. [Google Scholar]
- Ingle A, Gade A, Pierrat S, Sonnichsen C, Rai MK. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr Nanosci. 2008;4:141–144. doi: 10.2174/157341308784340804. [DOI] [Google Scholar]
- Ishida K, Cipriano TF, Rocha GM, Weissmüller G, Gomes F, Miranda K, Rozental S. Silver nanoparticle production by the fungus Fusarium oxysporum: nanoparticle characterisation and analysis of antifungal activity against pathogenic yeasts. Mem Inst Oswaldo Cruz. 2014;109(2):220–228. doi: 10.1590/0074-0276130269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal K, Sandeep BV, Pola S. Synthesis, characterization, and evaluation of the antibacterial activity of Allophylus serratus leaf and leaf derived callus extracts mediated silver nanoparticles. J Nanomater. 2017 [Google Scholar]
- Jha AK, Prasad K, Kulkami AR. Plant system: nature’s nanofactory. Colloids Surf B. 2009;73:219–223. doi: 10.1016/j.colsurfb.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Kamil M, Jayaraj AF, Ahmad F, Gunasekhar C, Samuel S, Habiballah M, Chan K. Pharmacognostic and phytochemical standardisation of Calligonum comosum. J Pharm Pharm. 2000;52(Suppl):262. [Google Scholar]
- Kim J, Kuk E, Yu K, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang C-Y, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Macdonald IDG, Smith WE. Orientation of cytochrome C adsorbed on a citrate-reduced silver colloid surface. Langmuir. 1996;12:706–713. doi: 10.1021/la950256w. [DOI] [Google Scholar]
- Mohammed AE. Green synthesis and antimicrobial activity of Eucalyptus camaldulensis mediated silver nanoparticles. Asian Pac J Trop Biomed. 2015;5:930–934. doi: 10.1016/S2221-1691(15)30373-7. [DOI] [Google Scholar]
- Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar RS, Khan IM, Parishcha R, Ajaykumar VP, Alam M, Kumar R, Sastry M. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelia matrix. A novel biological approach to nanoparticle synthesis. Nano Lett. 2001;1(10):515–519. doi: 10.1021/nl0155274. [DOI] [Google Scholar]
- Naveen HKS, Gaurav K, Karthik L, Rao BKV. Extracellular biosynthesis of silver nanoparticles using the filamentous fungus Penicillium sp. Arch App Sci Res. 2010;2(6):161–167. [Google Scholar]
- Ouda SM. Some nanoparticles effects on Proteus sp. and Klebsiella sp. isolated from water. Am J Infect Dis Microbiol. 2014;2:4–10. [Google Scholar]
- Paulkumar K, Gnanajobitha G, Vanaja M, Rajeshkumar S, Malarkodi C, Pandian K, Annadurai G. Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. Sci World J. 2014 doi: 10.1155/2014/829894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65:1803–1815. doi: 10.1016/j.addr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2:1–10. doi: 10.1186/2228-5326-2-32. [DOI] [Google Scholar]
- Rai RV, Bai JA. Science against microbial pathogens: communicating current research and technological advances. In: Méndez-Vilas A, editor. Nanoparticles and their potential application as antimicrobials. Mysore: University of Mysore, Manasagangotri; 2011. [Google Scholar]
- Ratnasri PV, Hemalatha KPJ. Biological synthesis of silver nanoparticles from Aspergillus fumigatus. Am J Drug Deliv. 2014;2:6. [Google Scholar]
- Raudabaugh DB, Tzolov MB, Calabrese JP, Overton BE. Synthesis of silver nanoparticles by a Bryophilous rhizoctonia species. Nanomater Nanotechnol. 2013;3:2. doi: 10.5772/56207. [DOI] [Google Scholar]
- Sahayaraj K, Rajesh S. Science against microbial pathogens: communicating current research and technological advances. In: Méndez-Vilas A, editor. Bionanoparticles: synthesis and antimicrobial applications. Badajoz: Formatex Research Center; 2011. [Google Scholar]
- Sastry M, Ahmad A, Khan MI, Kumar R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci. 2003;85(2):162–170. [Google Scholar]
- Singh P, Raja RB. Biological synthesis and characterization of silver nanoparticles using the fungus Trichoderma harzianum. Asian J Exp Biol Sci. 2011;2(4):600. [Google Scholar]
- Singh Kh, Panghal M, Kadyan S, Yadav JP. Evaluation of antimicrobial activity of synthesized silver nanoparticles using Phyllanthus amarus and Tinospora cordifolia medicinal plants. J Nanomed Nanotechnol. 2014;5:6. [Google Scholar]
- Tamayo LA, Zapata PA, Vejar ND, Azocar MI, Gulppi MA, Zhou X, Thompson GE, Rabagliati FM, Paez MA. Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater Sci Eng C. 2014;40:24–31. doi: 10.1016/j.msec.2014.03.037. [DOI] [PubMed] [Google Scholar]
- Thorley AJ, Tetley TD. New perspectives in nano-medicine. Pharmacol Ther. 2013;140:176–185. doi: 10.1016/j.pharmthera.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Umoren SA, Obot IB, Gasem ZM. Green synthesis and characterization of silver nanoparticles using red apple (Malus domestica) fruit extract at room temperature. J Mater Environ Sci. 2014;5(3):907–914. [Google Scholar]
- Vahabi KH, Mansoori GA, Sedighe K. Biosynthesis of silver nanoparticles by fungus Trichoderma reesei. Int Sci J. 2011;1(1):65–79. [Google Scholar]
- Vahdati AR, Sadeghi B (2013) A study on the assessment of DNA strand-breaking activity by silver and silica nanoparticles. J Nanostruck Chem 3:7. http://www.jnanochem.com/content/3/1/7
- Wu D, Fan W, Kishen A, Gutmann JL, Fan B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J Endod. 2014;40:285–290. doi: 10.1016/j.joen.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang SH, Kong J, Dong A, Yu Sh. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat Protoc. 2015;10(3):382–396. doi: 10.1038/nprot.2015.024. [DOI] [PubMed] [Google Scholar]