Abstract
The effect of microwave (MW)-assisted acid or alkali pretreatment (300 W, 7 min) followed by saccharification with a triple enzyme cocktail (Cellic, Optimash BG and Stargen) with or without detoxification mix on ethanol production from three cassava residues (stems, leaves and peels) by Saccharomyces cerevisiae was investigated. Significantly higher fermentable sugar yields (54.58, 47.39 and 64.06 g/L from stems, leaves and peels, respectively) were obtained after 120 h saccharification from MW-assisted alkali-pretreated systems supplemented (D+) with detoxification chemicals (Tween 20 + polyethylene glycol 4000 + sodium borohydride) compared to the non-supplemented (D0) or MW-assisted acid-pretreated systems. The percentage utilization of reducing sugars during fermentation (48 h) was also the highest (91.02, 87.16 and 89.71%, respectively, for stems, leaves and peels) for the MW-assisted alkali-pretreated (D+) systems. HPLC sugar profile indicated that glucose was the predominant monosaccharide in the hydrolysates from this system. Highest ethanol yields (YE, g/g), fermentation efficiency (%) and volumetric ethanol productivity (g/L/h) of 0.401, 78.49 and 0.449 (stems), 0.397, 77.71 and 0.341 (leaves) and 0.433, 84.65 and 0.518 (peels) were also obtained for this system. The highest ethanol yields (ml/kg dry biomass) of ca. 263, 200 and 303, respectively, for stems, leaves and peels from the MW-assisted alkali pretreatment (D+) indicated that this was the most effective pretreatment for cassava residues.
Keywords: Cassava residues, Microwave, Acid/alkali pretreatment, SHF, Bioethanol
Introduction
Although fossil fuels have been the primary sources to meet the energy needs of the world, the rapidly depleting scenario due to excessive tapping coupled with the environmental pollution hazards posed by them leading to global warming and climate change issues have urged the search for environmentally benign fuel sources (Sarkar et al. 2012; Wyman 1999). Bioethanol has been recognized as the best transportation fuel owing to its higher oxygen content (35%) capable of reducing vehicular emission of greenhouse gases (Öhgren et al. 2006). The ease of production from starchy or sugar-containing substrates has resulted in the exploitation of maize, sugarcane, wheat, etc. for bioethanol production (Chen et al. 2011). Nevertheless, diversion of food resources for biofuel is implicated to lead to food shortage in future and hence discouraged (Demirbas 2011). As an alternative to this, lignocellulosic biomass (LCB) has received global focus due to the specific advantages such as cheap and abundant availability, renewability and non-competition with food sources (Bussamra et al. 2015; Martin et al. 2002; Mosier et al. 2005). However, sustainable production of bioethanol from LCBs is challenged by several factors such as high recalcitrance necessitating costly and rigorous pretreatment procedures, need for different enzyme cocktails, formation of high level of saccharification/fermentation inhibitors, and cost associated with their removal (Himmel et al. 2007; Mosier et al. 2005; Wyman 1999). The high recalcitrance of LCBs results from the highly ordered structure of lignin-hemicellulose-cellulose matrix where hemicelluloses and cellulose are densely packed and shielded by layers of lignin (Hu and Wen 2008) which thereby resists the attack by enzymes. To enhance the sugar recovery from LCBs, several pretreatment strategies have been attempted by researchers and comprehensive reviews have appeared on this topic (Hendriks and Zeeman 2009; Mosier et al. 2005; Sun and Cheng 2002; Yang and Wyman 2008).
Microwave irradiation has received research focus recently as an effective pretreatment method either alone or in combination with other chemicals, because of the high and uniform heating efficiency, easy operation and short processing time (Ethaib et al. 2015; Tsubaki and Azuma 2011). Microwaves alter the ultrastructure of cellulose through the removal of lignin and hemicellulose and thereby enhancing the accessibility of cellulose to hydrolytic enzymes (Maurya et al. 2013). Microwaves enable homogeneous heating of biomass by causing dipole rotation in which polar molecules align themselves with the rapidly changing electric field and also by ionic conduction leading to instantaneous heating of ionic components in the biomass (Merino-Pérez et al. 2015). As a result, there is effective deconstruction of polysaccharides within a shorter reaction period than in conventional heating (Laghari et al. 2014). Microwave heating creates ‘hot spots’ within the lignocellulose matrix resulting in an explosive action on the recalcitrant structure, enabling its disruption at a faster rate than conventional heating (Saini et al. 2015). Combined MW-chemical pretreatment has been tried by many researchers to enhance sugar release from LCBs during saccharification. While alkali has been combined with microwaves to enable lignin removal, dilute acid-assisted MW irradiation has been reported to remove hemicelluloses (Ethaib et al. 2015). Nomanbhay et al. (2013) found that pretreatment of palm empty fruit bunch with 3% NaOH at a MW power of 180 W for 12 min removed as high as 74% lignin and ca. 25% holocellulose. Binod et al. (2012) treated sugarcane bagasse with 1% NaOH at 600 W MW power for 4 min and reported reducing sugar yields of 66.5 g/100 g. Microwave-assisted alkali pretreatment was reported as highly efficient in deconstructing cellulose and enhancing saccharification of wheat straw (Zhu et al. 2006). MW-assisted dilute acid pretreatment has been studied for many LCBs such as wheat fibres, and sugarcane bagasse (Chen et al. 2011; Palmarola-Adrados et al. 2004).
Although a number of fermentation strategies are adopted to enhance the ethanol yield, the final yield is decided by several factors such as sugar profile of the enzyme hydrolysates, efficiency of the fermenting organisms to ferment the various types of monomeric sugars, level of inhibitors in the hydrolysates and the content of fermentable sugars in the mash (Buruiana et al. 2013). Toxic inhibitors which affect enzyme saccharification and growth of microbes are generated during pretreatment and include sugar degradation products such as furfural and 5-hydroxymethyl furfural (HMF) or lignin degradation products (Modig et al. 2002; Parajó et al. 1998; Taherzadeh and Karimi 2007). Surfactants such as Tween and polyethylene glycol (PEG) have been studied for their effect in reducing the level of inhibitors and enhancing the saccharification rate (Börjesson et al. 2007; Eriksson et al. 2002). Non-ionic surfactants such as Tween 20 (polyethylene glycol sorbitan monolaurate) and Tween 80 (polyethylene glycol sorbitan monooleate) have been reported to prevent the non-productive binding of lignin to cellulases (Börjesson et al. 2007; Eriksson et al. 2002; Tejirian and Xu 2011). Detoxification using sodium borohydride also improved the fermentation of sugarcane bagasse hydrolysate by removing furan aldehydes from the pretreated liquor (Cavka and Jönsson 2013).
Cassava (Manihot esculenta Crantz) is widely cultivated in more than 102 countries for its starchy tubers which meet the hunger needs of approximately 500 million people of the tropics. Globally cassava is grown in an area of 23.867 million hectares, producing 268.28 million tonnes, with a productivity of 11.24 t/ha, while in India it is cultivated in 0.228 million hectares with a total production of 8.14 million tonnes (FAOSTAT 2014). During the cultivation and processing of cassava, several waste residues are produced that find rare alternative uses. The main agricultural residues from cassava are stems and leaves while the secondary processing waste includes cassava peels. The above-ground parts of cassava such as stems and leaves are not economically utilized and only 10–20% of stems are further needed for replanting (Ahamefule 2005). Kosugi et al. (2009) reported that the non-food parts of cassava could play a significant role in the production of energy, because of the huge volume of biomass. Cassava processing generates peels as a waste and it accounts for 10–15% of the fresh weight of roots. These residues are often poisonous due to the high content of cyanoglucosides (Guo et al. 2009) and hence could be exploited for bioethanol production. Kongkiattikajorn (2012) reported that cassava peels contained 35.86% cellulose, 9.27% hemicelluloses, 12.52% lignin and 15.82% starch and hence could be used for ethanol production. Previous studies conducted by Pooja and Padmaja (2015a) showed that cassava peels contained ca. 30% starch, besides 14% cellulose and 23% hemicellulose, while the stems contained 15% starch, 23% cellulose and 28% hemicellulose. Cassava leaves had the least content of starch (2.4%) besides 17% cellulose and 27% hemicelluloses (Table 1). Steam pretreatment of moist samples or microwave-assisted dilute sulphuric acid pretreatment were earlier reported as effective techniques to enhance the fermentable sugar yield from cassava peels, but not optimal for the other two residues during saccharification with Accellerase (Pooja and Padmaja 2015a). Further studies using another cellulolytic complex, Cellic CTec2 showed that very high yield of sugars was possible from peels, while optimum hydrolysis of polysaccharides could not be achieved for the other two biomasses using the single enzyme (Pooja and Padmaja 2015b). MW-assisted alkali pretreatment followed by saccharification with the enzyme complex Cellic in the presence of Tween 20 could convert as high as 83–93% potential sugar yielding carbohydrates to reducing sugars (Pooja and Padmaja 2017). Previous studies showed that a mix of detoxification chemicals such as Tween and Polyethylene glycol was highly effective in eliminating soluble phenolics from the pretreated root crop residues (Mithra and Padmaja 2016).
Table 1.
Compositional data (% dry weight basis)a of the cassava residues under study
| Parameters | Cassava stem | Cassava leaves | Cassava peels |
|---|---|---|---|
| Starch | 15.00 | 2.43 | 29.84 |
| Cellulose | 22.80 | 17.30 | 14.17 |
| Hemicellulose | 28.80 | 27.65 | 23.40 |
| Lignin | 22.10 | 20.10 | 10.88 |
| Crude protein | 3.68 | 19.96 | 5.29 |
| Ash | 1.90 | 2.50 | 3.70 |
| Total sugars | 2.00 | 2.05 | 4.36 |
| Total carbohydrateb | 68.60 | 49.43 | 71.77 |
aMean value from two observations
bTotal carbohydrate indicates the sum of cellulose, hemicelluloses, starch and total sugars in the biomass; Source: Pooja and Padmaja (2015a)
The objective of the present study was to compare the efficacy of ethanol production from MW-assisted dilute acid or alkali-pretreated cassava residues saccharified using a full complement of three enzymes such as Cellic, Optimash BG and Stargen either with or without a detoxification chemical mix comprising Tween 20, Polyethylene glycol (PEG 4000) and sodium borohydride and fermented using Saccharomyces cerevisiae.
Materials and methods
Samples
Stems and leaves were collected from healthy and mature (10 months old) cassava plants (variety: Sree Jaya) grown at the Institute farm. Leaves along with the stalk were separated from the stems and allowed to wilt in the shade for 18 h which helped to reduce the cyanoglucoside content (based on earlier studies (Padmaja 1989) and further dried in the sun for 24 h. Stems were chopped to small pieces (ca. 5.0 cm long) and separately dried in the sun for 36–48 h. Dry stems and leaves were powdered in a hammer mill to particles of size of ca. 850 µm. Peels (skin + rind) were manually separated from the roots and chopped into pieces of ca. 2–3 cm length. These were further dried in the sun for 36–48 h. Dry peels were powdered in a hammer mill to particles of similar size as before and were stored in airtight bottles until use. In the case of all the residues, unscreened powders were used with the objective of maximum utilization of the biomass waste. The composition of the cassava residues as given in Table 1 indicated that stems and peels had high content of starch also, while hemicellulose ranged from 23 to 29% in the residues (Pooja and Padmaja 2015a).
Pretreatment
In the microwave-assisted dilute sulphuric acid pretreatment, the dry biomass powders (20 g each of stems, leaves and peels) were suspended in 0.1 M H2SO4 (200 ml) in Erlenmeyer flasks (250 ml capacity) and kept for proofing at room temperature (30 ± 1 °C) for 10 min. The slurries were then exposed to microwave irradiation power of 300 W and irradiation time of 7 min in a Microwave oven (M/s Samsung, Thailand) as reported (Mithra et al. 2017). MW-assisted alkali pretreatment was carried out by preparing the alkaline biomass slurry (20 g in 200 ml 3% NaOH) for each sample, which was then exposed to MW irradiation at 300 W for 7 min (Pooja and Padmaja 2017).
One set of slurry from each biomass was immediately adjusted to pH 5.0 and treated with a detoxification chemical mix containing Tween 20 (0.50 ml), polyethylene glycol (PEG 4000; 0.50 g) and sodium borohydride (0.30 g), and the other set was adjusted to pH 5.0 and used as such for saccharification without detoxification chemicals.
Enzymes used
Cellic® CTec2, the major enzyme used for the study was gifted by M/s Novozymes, Bagsvaerd, Denmark, and this enzyme cocktail contained cellulase, β-glucosidase as well as xylanase, with reportedly high tolerance to product inhibition (Anon 2014). The optimum temperature and pH of Cellic standardized on cassava residues were 50 °C and 5.5, respectively (Pooja and Padmaja 2015b). Stargen™002 contained Aspergillus kawachii α-amylase (E.C. 3.2.1.1) expressed in Trichoderma reesei and a glucoamylase (E.C. 3.2.1.3) from Trichoderma reesei that work synergistically to hydrolyse granular starch substrate to glucose. It has an activity of 570 glucoamylase units (GAU) per gram, and one GAU is the amount of enzyme that will liberate 1 g of reducing sugars (as glucose) per hour from soluble starch substrate under the conditions of the assay (Anon 2009a). Optimash™BG and Stargen™002 used as supplementary enzymes were gifted by M/s Genencor International Inc. USA (presently Genencor-Danisco, Beloit, WI, USA). Optimash BG is a combination of β-glucanase and xylanase which could hydrolyse cellobiose and hemicellulose, respectively, during saccharification. It is produced by the submerged fermentation using a genetically modified Trichoderma reesei and is reported to have a pH and temperature optima of 4.0–4.5 and 60–70 °C, respectively, although these could vary depending on the type of substrates. It has an activity of 10,300 carboxymethyl cellulase Units/g (CMC U/g) (Anon 2009b). Cellic, Stargen and Optimash BG had crude protein contents of 156, 216.0 and 94.6 g/L, respectively (AOAC 2005).
OptimashBG was incorporated in the enzyme cocktail to supplement the xylanase activity of Cellic, as the residues had high content of hemicellulose and also based on the previous studies where Cellic alone resulted in poor saccharification yield from leaves and stems (Pooja and Padmaja 2015b). Stargen was used in the cocktail (as different from the enzymatic saccharification of typical lignocellulosic biomass) because of the high content of starch in the peels and stem samples.
Enzymatic saccharification of MW-assisted acid or alkali-pretreated biomass
The slurries (200 ml each; 10% w/v) from MW-assisted acid or MW-assisted alkali pretreatments were taken in 250-ml Erlenmeyer flasks. After pH adjustment to 5.0 (as all the three enzymes were added simultaneously), the slurries were equilibrated in a thermostatic water bath at 50 °C for 10 min. An enzyme cocktail consisting of Cellic (1.0 g enzyme protein/200 ml), Optimash BG (1.0 ml equivalent to 94.6 mg enzyme protein) and Stargen (0.20 ml equivalent to ca. 44 mg enzyme protein) were added together to one set of flasks. The flasks were incubated for 120 h at a shaking speed of 100 rpm, with sampling at every 24 h for reducing sugars.
A similar set of flasks were kept for studies on the effect of detoxifying chemicals in enhancing the fermentable sugar yield from the MW-assisted acid and alkali-pretreated biomass. In this set, a chemical mix containing Tween 20 (0.50 ml), polyethylene glycol (PEG 4000; 0.50 g) and sodium borohydride (0.30 g) were added to 200 ml slurry volume and exposed to room temperature for 30 min. These detoxification chemicals and their levels were based on an earlier study from the laboratory (Mithra and Padmaja 2016). After pH adjustment to 5.0, the slurries were equilibrated in a thermostatic water bath at 50 °C for 10 min. The rest of the experiment was as described above for the first set.
Reducing sugar content and characterization of the hydrolysates
The total reducing sugar content of the enzymatic hydrolysates from the three sets of experiments was determined using arsenomolybdate method (Nelson 1944). Enzyme blanks as well as substrate blanks were kept during the assay of RS to nullify the interference from sugars already present in the commercial enzyme samples and original biomass, respectively. The sugar profile of the hydrolysates after 120 h saccharification was determined for the most effective combinations (MW-assisted alkali-pretreated residues saccharified with or without detoxification chemicals) using HPLC. The enzyme-saccharified mash was centrifuged at 8000 rpm to obtain clear hydrolysate. At the time of analysis, the filtrates were again filtered through 0.20-µm Millipore filtres. Monomeric sugars such as glucose, galactose, mannose, arabinose and xylose were identified and quantified using HPLC (M/s Shimadzu, Kyoto, Japan) under an isocratic mode and the conditions were: Column: SUPELCOSIL LC-NH2 (250 × 4.6 mm), mobile phase: acetonitrile:water (75:25), flow rate: 1.0 ml/min; column temperature: ambient (30 ± 1 °C); RID-10 A Refractive index detector; sample injection volume: 20 μl and run time: 30 min.
Ethanol fermentation using S. cerevisiae
Fermentation experiments were conducted using enzymatic hydrolysates in 250-ml Erlenmeyer flasks. The enzyme-saccharified mash from each of the above experiments was adjusted to pH 4.5 using 1 M HCl, temperature brought down to 30 ± 1 °C and squeezed through muslin cloth to remove the unhydrolysed residue. The filtrate was again centrifuged at 8000 rpm for 10 min to obtain clear hydrolysate which was used in fermentation studies.
Activation of yeast
20 g dry granulated Baker’s yeast (S. cerevisiae) was suspended in 100 ml solution containing 10 g sucrose and kept in a water bath at 37 °C for 1 h. Ten millilitres of yeast suspension were used for 200 ml of saccharified mash.
Fermentation
The clear hydrolysate (200 ml each) from the MW-assisted acid/alkali-pretreated and saccharified mash was taken in 250-ml Erlenmeyer flasks and 200 mg urea were added to it as nitrogen source. A mineral mix containing MgSO4·7H2O (100 mg), CaCl2·7H2O (20 mg) and FeCl3·2H2O (20 mg) was added and mixed well. Each flask was inoculated with 10 ml yeast suspension and after thorough mixing the flasks were closed with aluminium foil and allowed to ferment for 48 h at room temperature (30 ± 1 °C). Ethanol content was determined in the fermented liquor after 48 h of fermentation as per the spectrophotometric method of Caputi et al. (1968) using potassium dichromate reagent.
The fermented broth (48 h) was also distilled using a Rotary vacuum evaporator (M/s BUCHI India Pvt. Ltd., India) at 70 °C to confirm the analytical results. The distilled ethanol was mixed with anhydrous sodium sulphate (10 g/100 ml ethanol) to remove the last traces of water. The volume of pure ethanol was quantified and expressed as ethanol recovery in ml/kg dry biomass.
Fermentation experiments were repeated for the MW-assisted acid or alkali-pretreated and saccharified biomass also where systems saccharified with or without the detoxification chemicals were compared for the fermentation performance. Residual reducing sugars in the fermented broth were quantified as earlier. Enzyme blanks as well as substrate blanks were kept to nullify the interference from sugars already present in the commercial enzyme samples and original biomass, respectively.
Calculation for yield parameters
The various parameters related to ethanol fermentation were computed based on the following formulae (Barcelos et al. 2011; Pereira et al. 2015; Yadav et al. 2011).
| 1 |
where S1 is the Initial sugar concentration in the hydrolysate and S2 is the residual sugar concentration in the fermented broth.
| 2 |
| 3 |
| 4 |
| 5 |
where Ef is the ethanol concentration (g/L) in fermented broth and W1 is the weight of dry biomass in one litre slurry.
| 6 |
where 0.82 is the specific gravity of ethanol.
Statistical analysis
Three replicates were kept for each experiment and duplicate analyses were performed on each replicate. The data were subjected to Analysis of Variance (ANOVA) for statistical testing of the mean values and was followed by least significant difference (LSD) for pair-wise comparison of mean values using the statistical package, SAS 9.3 (SAS 2010).
Results and discussion
Changes in RS during saccharification and fermentation
Saccharification
Microwave-assisted alkali (3% NaOH) pretreatment was earlier found to be reasonably effective in enhancing the fermentable sugar yield from cassava residues during saccharification with Cellic alone, which otherwise was only poorly deconstructed after steam pretreatment (Pooja and Padmaja 2017). Hence, it was thought worthwhile to compare the effects of MW-assisted dilute acid or alkali pretreatments on fermentable sugar yield during saccharification with the triple enzyme cocktail comprising Cellic + Optimash BG + Stargen to find out the improvements over Cellic alone-aided saccharification (with or without Tween 20). Also the comparative effect on ethanol yield under SHF mode in systems with or without detoxification chemical mix (Tween 20 + PEG + sodium borohydride) was studied.
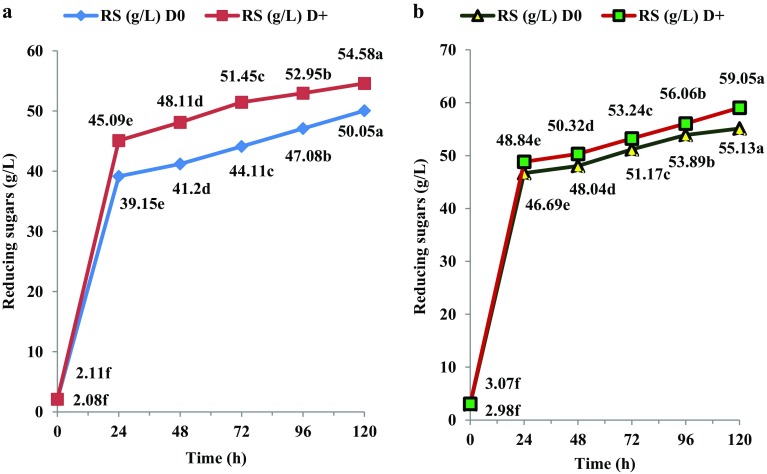
The progressive release of RS during 120 h saccharification of MW-assisted dilute acid-pretreated cassava stems during saccharification with C + S + OBG with (D+) or without (D0) the detoxification chemical mix is given in Fig. 1a. It was seen that there was significantly higher RS release in saccharification system supplemented with detoxification chemical mix (D+) at all periods of saccharification from 24 to 120 h. The final RS yield in (D+) samples of cassava stems was 54.58 g/L vis-à-vis 50.05 g/L in (D0) samples (Fig. 1a). Parallel MW-assisted alkali-pretreated cassava stems subjected to similar saccharification conditions gave higher RS contents in (D0) and (D+) systems, 55.13 and 59.05 g/L, respectively (Fig. 1b).
Fig. 1.
Time course release of reducing sugars from MW-assisted pretreated cassava stems during saccharification; a acid pretreatment; b alkali pretreatment; statistical comparison between different time periods for each sample; lines with different alphabets in each set are significant at p < 0.05
However, when MW-assisted alkali-pretreated cassava stems were saccharified using Cellic alone with or without Tween 20, much lower RS contents were reported (Pooja and Padmaja 2017), which indicated that the triple enzyme cocktail was beneficial in enhancing RS release from MW-assisted alkali-pretreated stems. Optimash BG used in the cocktail had component activities such as β-glucanase and xylanase, while Stargen had high amylolytic activity and these might have facilitated high hydrolysis of both hemicelluloses and starch.
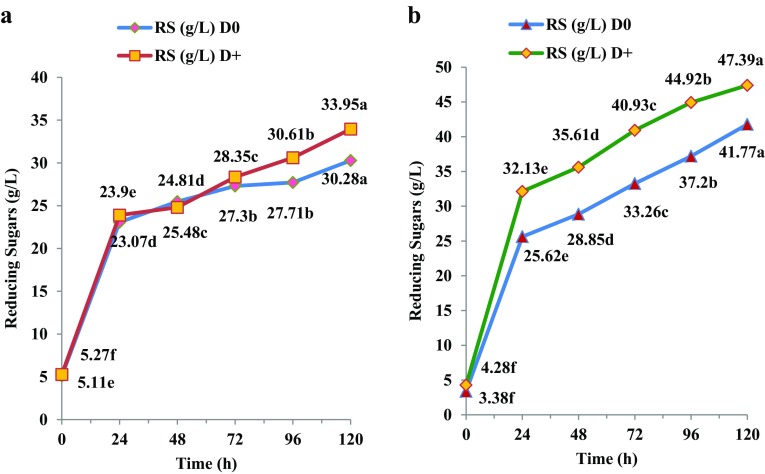
The time course release of RS during saccharification of MW-assisted acid or alkali-pretreated cassava leaves with C + S + OBG under SHF mode for 120 h is presented in Fig. 2a, b. As in the case of cassava stems, the MW-assisted alkali-pretreated leaves performed better during saccharification in systems without (D0) or with (D+) detoxification chemical mix and the final RS values were, respectively, 41.77 and 47.39 g/L (Fig. 2b).
Fig. 2.
Time course release of reducing sugars from MW-assisted pretreated cassava leaves during saccharification; a acid pretreatment; b alkali pretreatment; statistical comparison between different time periods for each sample; lines with different alphabets in each set are significant at p < 0.05
It was reported earlier that in the case of MW-assisted alkali-pretreated cassava leaves saccharified with Cellic alone without or with Tween 20 in the system, RS values of 41.60 and 46.23 g/L were, respectively, obtained (Pooja and Padmaja 2017), which showed that the effect of triple enzyme cocktail or detoxification chemical mix used in the present study was not highly significant for cassava leaves. MW-assisted dilute acid-pretreated cassava leaves on saccharification with triple enzyme (C + S + OBG) without (D0) or with (D+) detoxification chemical mix gave RS values of 30.28 and 33.95 g/L, respectively, after 120 h (Fig. 2a). Previous studies showed that MW-assisted alkali pretreatment followed by saccharification with Cellic alone in presence of Tween 20 enhanced removal of lignin from cassava leaves (Pooja and Padmaja 2017). Increased cellulose accessibility consequent to lignin removal from LCBs has been reported by others also (Singh et al. 2011; Zhu et al. 2006). Saini et al. (2015) found that the ester linkages between lignin and hemicelluloses were broken down during MW-irradiation and this facilitated rapid hydrolysis of polysaccharides.
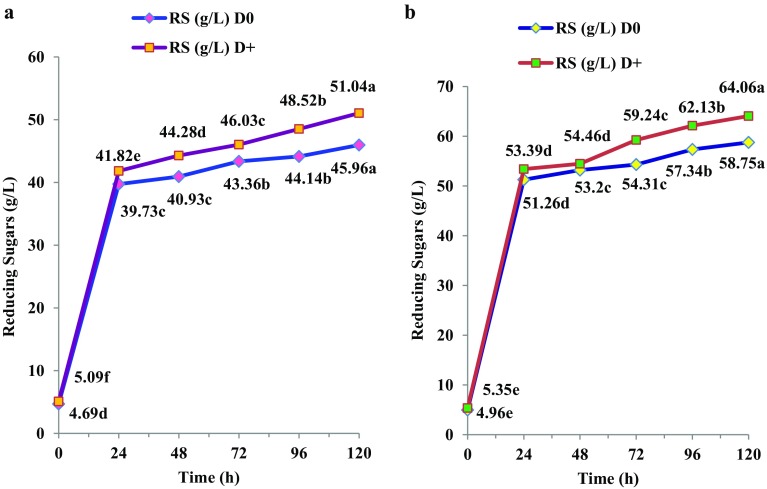
While only 45.96 and 51.04 g/L RS were released from MW-assisted acid-pretreated peels on saccharification without (D0) or with (D+) detoxification chemical mix, respectively (Fig. 3a), the corresponding values for alkali-pretreated peels were 58.75 and 64.06 g/L, respectively (Fig. 3b). In MW-assisted alkali-pretreated cassava peels saccharified with Cellic alone without or with Tween 20, 56.25 and 59.55 g/L RS were released, respectively, as reported earlier (Pooja and Padmaja 2017) compared to 58.75 and 64.06 g/L in the triple enzyme-based saccharification without (D0) or with (D+) detoxification chemicals (Fig. 3b).
Fig. 3.
Time course release of reducing sugars from MW-assisted pretreated cassava peels during saccharification; a acid pretreatment; b alkali pretreatment; statistical comparison between different time periods for each sample; lines with different alphabets in each set are significant at p < 0.05
MW-assisted alkali pretreatment led to the swelling of cassava reisudes (Pooja and Padmaja 2017) and this might have enhanced cellulose accessibility to hydrolytic enzymes. Hu and Wen (2008) also reported 90% conversion of carbohydrates to sugars in MW-assisted alkali (1% NaOH) pretreated switchgrass during saccharification. Zhu et al. (2015) reported twelve times more sugar yield during saccharification of MW-assisted (300 W; 10 min) alkali (0.2 M NaOH) pretreated Miscanthus sp. than conventional alkali or dilute acid pretreatment which corroborated our findings.
The ability of non-ionic surfactants such as polyethylene glycol or Tween 20 or Tween 80 to enhance fermentable sugar yield has been reported by several researchers (Eriksson et al. 2002; Kim et al. 2007; Kurakake et al. 1994). Many mechanisms have been suggested for the enhancing effect of surfactants which include alteration in the lignocellulosic structure (Helle et al. 1993; Kaar and Holtzalpple 1998), stabilization of enzymes and prevention of denaturation (Kaar and Holtzapple 1998), prevention of the non-productive binding of lignin to cellulase (Eriksson et al. 2002), etc. Haven and Jørgensen (2013) observed the binding of as high as 65% of the β- glucosidase in Cellic CTec2 to lignin from pretreated wheat straw and found that PEG or bovine serum albumin could prevent this adsorption. Alkasrawi et al. (2003) found that the free cellulase in the liquid fraction of spruce hydrolysates was much higher when Tween 20 was added than the non-supplemented systems which were supportive of the findings from the present study as well.
The HPLC characterization of monomeric sugars present in the hydrolysates from the MW-assisted alkali-pretreated biomass residues saccharified with (D+) or without (D0) detoxification chemicals is presented in Table 2. Glucose was the predominant monosaccharide in all the hydrolysates, with a significantly higher quantity in the peel hydrolysates. Consistent with the analytical data, lowest levels of glucose were obtained in the leaf hydrolysates. Although the quantity of glucose and other sugars were similar to the steam pretreated and saccharified hydrolysates from peels (unpublished data), the content of sugars in the hydrolysates from the other two residues were significantly higher from MW-assisted alkali-pretreated and saccharified set indicating the advantage of the former pretreatment in the case of these residues.
Table 2.
HPLC sugar profile in the hydrolysates (120 h) from MW-assisted alkali-pretreated cassava residues saccharified using Cellic + Stargen + Optimash BG
| Type of sugars | Reducing sugar content (g/L) | |||||
|---|---|---|---|---|---|---|
| Cassava stems | Cassava leaves | Cassava peels | ||||
| D0 | D+ | D0 | D+ | D0 | D+ | |
| Glucose | 38.46 | 42.35 | 28.86 | 31.11 | 46.55 | 48.26 |
| Xylose | 1.85 | 2.20 | 1.80 | 1.92 | 2.48 | 2.51 |
| Mannose | 0.28 | 0.38 | ND | ND | ND | ND |
| Arabinose | 1.22 | 1.38 | 1.15 | 1.65 | 1.90 | 1.85 |
| Galactose | ND | ND | ND | ND | 0.24 | 0.22 |
| Total | 41.81 | 46.31 | 31.81 | 34.68 | 51.17 | 52.84 |
Mean from two runs; D0 indicates without detoxification chemicals and D+ indicates system supplemented with detoxification chemicals
ND nothing detected
There was also definite improvement in the yield of sugars in systems supplemented with the detoxification chemical mix, which corroborated with the analytical data on total RS release.
Fermentation
Sugar utilization pattern
The initial RS content (before start of fermentation), final RS content in the fermented broth (after 48 h fermentation), RS consumption (g/L) during the period and the percentage utilization of RS are given in Table 3 for the three cassava residues. It could be seen that the initial RS content in saccharified liquor was the highest and the residual RS in the fermented broth was the least in MW-assisted acid-pretreated cassava stems (Table 2). Nevertheless, the initial and residual RS contents were significantly higher in MW-assisted alkali-pretreated cassava peels after saccharification and fermentation, respectively, compared to stems (Table 3).
Table 3.
Initial RS, residual RS, Sugar consumption and Percentage sugar utilization in MW-assisted acid and alkali-pretreated cassava residues subjected to saccharification with or without detoxification chemicals (120 h) followed by fermentation for 48 h
| Treatments* | Initial RS content (g/L) | Residual RS content (g/L) | RS consumption (g/L) | Percentage RS utilization |
|---|---|---|---|---|
| Cassava stems | ||||
| MW-acid (D0) | 50.05d | 5.04b,c | 45.01d | 89.93a |
| MW-acid (D+) | 54.58c | 4.67c | 49.91c | 91.44a |
| MW-alkali (D0) | 55.13c | 5.97b | 49.16c | 89.17a,b |
| MW-alkali (D+) | 59.05b | 5.30b | 53.75b | 91.02a |
| Cassava leaves | ||||
| MW-acid (D0) | 30.28i | 8.69a | 21.59i | 71.37h |
| MW-acid (D+) | 33.95h | 8.19a | 25.76h | 75.88g |
| MW-alkali (D0) | 41.77g | 8.52a | 33.25 g | 79.60f |
| MW-alkali (D+) | 47.39e | 6.11b | 41.29e | 87.13c |
| Cassava peels | ||||
| MW-acid (D0) | 45.96f | 8.50a | 37.46f | 81.51e |
| MW-acid (D+) | 51.04d | 6.01b | 45.03d | 88.22b |
| MW-alkali (D0) | 58.75b | 8.67a | 50.08c | 85.24d |
| MW-alkali (D+) | 64.06a | 6.59b | 57.47a | 89.71a,b |
*D0: without detoxification chemicals; D+: with detoxification chemicals; statistical comparison was made within each column and values with different superscripts are significant at p < 0.05
The RS consumption during the 48 h fermentation period was higher in systems supplemented with the detoxification chemical mix for all the three biomasses indicating a positive effect of surfactants and sodium borohydride in enhancing RS yield. Among the biomasses, the highest RS consumption of 57.47 g/L was observed for MW-assisted alkali-pretreated cassava peels saccharified with detoxification chemicals (Table 3), while least value of 21.59 g/L RS consumption was obtained for MW-assisted acid-pretreated cassava leaves saccharified without detoxification chemicals (Table 3). The percentage utilization of RS was the highest in cassava stems for all the four treatments and it ranged from 89.17 to 91.44%. This was followed by cassava peels and least percentage utilization was observed in cassava leaves (Table 3). The impact of detoxification chemical mix in enhancing RS utilization by yeast was evident in the case of all the three biomasses.
MW-assisted heating is widely recognized as an effective means of deconstructing lignocellulosic biomass, as both thermal and non-thermal effects accelerate the breakdown of cellulose crystallinity (Chen et al. 2011; de la Hoz et al. 2005). Whilst MW-assisted dilute acid pretreatment has been adopted by some researchers (Chen et al. 2012; Palmarola-Adrados et al. 2004; Zhu et al. 2015), others have used MW-assisted alkali pretreatment (Hu and Wen 2008; Singh et al. 2014; Zhu et al. 2006). It was found in the present study that MW-assisted alkali pretreatment was superior to the MW-assisted acid pretreatment in the case of cassava stems, leaves and peels. Singh et al. (2014) studied the efficacy of MW-assisted alkali pretreatment for enhancing the enzymatic digestibility of wheat straw and found that 2% NaOH pretreatment for 3.16 min was optimal.
Hu and Wen (2008) also reported the hydrolysis of 90% of potential carbohydrates when switchgrass was pretreated with 0.1 g NaOH/g biomass at 190 °C for 30 min and hydrolysed with Celluclast and Novozyme 188. Zhu et al. (2015) obtained a hydrolysis of 75.3% when Miscanthus biomass was pretreated with 0.2 M H2SO4 for 20 min at 300 W MW power and saccharified using Celluclast and Novozyme 188, while much higher conversions (81–88% for MW-assisted acid-pretreated peels and 90–91% for stems) were reported in the present study with 0.1 M H2SO4 at 300 W for 7 min followed by enzymatic hydrolysis using the triple enzyme cocktail (Cellic + Optimash BG + Stargen) because of the hydrolysis of starch as well. Nomanbhay et al. (2013) used 3% NaOH at 180 W MW power for 12 min to pretreat empty fruit bunch fibre from oil palm and found that the saccharification yield was increased by 5.8-fold compared to conventional alkali pretreatment and enzymatic hydrolysis, and approximately 17.8 g RS were released during combined pretreatment and digestion. Unlike the empty fruit bunch, biomasses such as peels and stems used in the present study were having more starch and less lignin (Pooja and Padmaja 2015a) and hence higher hydrolysis could be achieved.
Ethanol yield and fermentation efficiency
The ethanol yield-related parameters such as ethanol content (g/L), ethanol yield, YE (g ethanol produced/g RS consumed), volumetric ethanol productivity (g/L/h) and ethanol recovery (ml/kg dry biomass) are presented in Table 4 for the three residues. Among the three residues, the highest ethanol contents (24.86 g/L) were obtained from cassava peels pretreated using MW-assisted alkali treatment and further saccharified with the triple enzyme cocktail and fermented for 48 h followed by cassava stems (21.56 g/L) under the same conditions of pretreatment, saccharification and fermentation (Table 4). SHF mode was adopted in the present study and hence the overall processing time was 168 h (120 h for saccharification + 48 h for fermentation). Significantly, lower ethanol yields were obtained from MW-assisted acid-pretreated biomasses on saccharification and fermentation. In the case of all the biomasses, there was significantly high ethanol yield from saccharification system supplemented with the detoxification chemical mix. The ethanol productivity (YE) was also the highest for cassava peels. Although the ethanol contents (g/L) were the least for the saccharification systems from cassava leaves, the ethanol yield (YE) was not significantly different from cassava stems (Table 4), indicating that the low ethanol contents resulted from the low levels of RS available for fermentation by yeast. This is also evident from the FE (%) values for cassava stems and leaves, which were closely tallying except in the case of MW-assisted acid-pretreated leaves saccharified in the presence of detoxification chemicals (D+). The highest FE for cassava peels was 84.65% followed by stems (78.49%) and for leaves, FE was 77.71% (for MW-assisted alkali-pretreated, saccharified and fermented systems). The volumetric ethanol productivity (VEP) was found to be significantly higher for the MW-assisted acid/alkali-pretreated stems and peels (Table 4). The higher VEP values also contributed to high ethanol yields (ml/kg dry biomass) from these residues compared to leaves (Table 4).
Table 4.
Ethanol yield-related parameters from MW-assisted acid and alkali-pretreated cassava stems subjected to saccharification with or without detoxification chemicals (120 h) followed by fermentation for 48 h
| Treatments* | Ethanol content (g/L) | Ethanol yield (YE) | FE (%) | Volumetric ethanol productivity (g/L/h) | Ethanol recovery (ml/kg dry biomass) | |
|---|---|---|---|---|---|---|
| A* | D* | |||||
| Cassava stems | ||||||
| MW-acid (D0) | 13.66e | 0.304a,b | 59.43g | 0.285b | 166.64h | 160.00 |
| MW-acid (D+) | 16.54d | 0.331a | 64.85f | 0.345b | 201.73e | 190.00 |
| MW-alkali (D0) | 17.84c | 0.363a | 71.01e | 0.372a,b | 217.50d | 210.00 |
| MW-alkali (D+) | 21.56b | 0.401a | 78.49b | 0.449a | 262.90b | 250.00 |
| Cassava leaves | ||||||
| MW-acid (D0) | 6.43h | 0.297b | 58.18g | 0.134c | 78.40 k | 70.00 |
| MW-acid (D+) | 9.60g | 0.373a | 72.99d | 0.200c | 117.05j | 110.00 |
| MW-alkali (D0) | 12.33f | 0.371a | 72.61d | 0.257b,c | 150.41i | 140.00 |
| MW-alkali (D+) | 16.37d | 0.397a | 77.71b | 0.341b | 199.64f | 190.00 |
| Cassava peels | ||||||
| MW-acid (D0) | 14.40e | 0.384a | 75.21c | 0.300b | 175.57 g | 165.00 |
| MW-acid (D+) | 17.93c | 0.398a | 77.90b | 0.373a,b | 218.60d | 205.00 |
| MW-alkali (D0) | 20.15b | 0.402a | 78.74b | 0.420a | 245.76c | 235.00 |
| MW-alkali (D+) | 24.86a | 0.433a | 84.65a | 0.518a | 303.19a | 290.00 |
*D0: without detoxification chemicals; D+: with detoxification chemicals; statistical comparison was made within each column and values with different superscripts are significant at p < 0.05; A*: ethanol recovery based on analytical data; D*: ethanol recovery from distillation (mean from two runs)
Significantly, higher ethanol yields could be obtained for cassava stems and peels from the MW-pretreated samples (Table 4) compared to the steam pretreated samples saccharified and fermented under identical conditions (unpublished data) indicating the superiority of MW-pretreatment for these two biomasses. In the case of cassava peels also, higher ethanol yield (303.19 ml/kg dry peels) could be obtained when MW-assisted alkali-pretreated biomass was saccharified and fermented (Table 4). The ethanol recovery from distillation was closely tallying with the analytical data with only insignificant decrease indicating the precision of the analytical method adopted (Table 4). The small decrease has occurred due to the smearing loss on the sides of the receiver flask which was unrecoverable.
There are reports that pretreatment of lignocellulosic biomass in the presence of non-ionic surfactants could reduce the amount of lignin remaining in the material and thus enhance its enzymatic digestibility (Kim et al. 2007; Kurakake et al. 1994). Non-ionic surfactants such as Tween 20 or Tween 80 and polymers such as polyethylene glycol (PEG) have been reported to enhance the enzymatic digestibility and thus the fermentable sugar yields (Eriksson et al. 2002; Kristensen et al. 2007). A definite improvement in the fermentable sugar yield was obtained in the present study, when the saccharification system of MW-assisted acid or alkali-pretreated biomasses was supplemented with the detoxification chemicals. Qi et al. (2010) found that Tween 20-assisted dilute acid-pretreated wheat straw during saccharification had more of free cellulase and hence resulted in enhanced ethanol content (11.2 g/L) under SSF mode. Singh et al. (2014) reported ethanol yields (YE) of 0.40 g/g glucose from MW-assisted alkali-pretreated rice husk after saccharification and fermentation with S. cerevisiae, although these researchers have not reported on the effect of detoxification on ethanol yield. Godson and Allen (2015) studied acid hydrolysis of cassava peels using 13.1 M H2SO4 and obtained total reducing sugar yield of 85 mg/kg and ethanol yield of 160 ml/kg without any surfactant application and this was much lower than that obtained in the present study. The bioethanol production from pretreated cassava peels by monoculture of Saccharomyces diastaticus or S. cerevisiae and co-culture of S. diastaticus and Candida tropicalis under simultaneous saccharification and fermentation was investigated by Kongkiattikajorn and Sornvoraweat (2011) and found that ethanol production was the highest in co-culture with maximum ethanol yield of 0.44 g/g dry peels. Mechanism for inhibition of growth and ethanol production by yeast has been reviewed by Palmqvist and Hahn-Hägerdal (2000). Surfactants such as Tween 20 or PEG have a definite effect on binding the phenols and thereby reducing their toxic effect on yeast. The increased ethanol yields from surfactant added systems might be due to such channelling out of phenols by the surfactants. Cavka and Jönsson (2013) reported the beneficial role of sodium borohydride in removing inhibitory compounds such as furfural. They found that sodium borohydride-treated slurries from SO2 pretreated spruce gave ethanol yields (YE) of 0.30 g/g glucose compared to only 0.02 g/g from the system without borohydride. It could be noted that Tween 20, PEG and sodium borohydride were used as a detoxification chemical mix in the present study and this might have led to the high ethanol yields from all the three biomasses.
Conclusions
The effect of microwave (MW)-assisted dilute acid or alkali pretreatment of agricultural residues of cassava such as stems, leaves and peels followed by triple enzyme-based saccharification on the ethanol yield during fermentation by Saccharomyces cerevisiae was investigated. Improvement in saccharification yield in system supplemented with detoxification chemical mix comprising Tween 20, polyethylene glycol (PEG 4000) and sodium borohydride was compared with the non-supplemented system and it was found that the former had a significant effect on enhancing the reducing sugar (RS) yield from all the residues. Among the residues, the highest RS yield was obtained from MW-assisted alkali-pretreated peels saccharified in the presence of detoxification chemicals and the RS consumption during fermentation was also the highest for peels. HPLC studies on the MW-assisted alkali-pretreated and saccharified hydrolysates indicated that glucose was the predominant sugar and high levels were present in the stem and leaf hydrolysates which confirmed that this pretreatment was very effective for these residues, which were otherwise recalcitrant after steam pretreatment. Highest ethanol content (24.86 g/L) and fermentation efficiency (84.65%) were also obtained from this system for cassava peels. Ethanol recovery from stems, leaves and peels was 263, 200 and 303 ml/kg dry biomass, respectively, using this pretreatment and saccharification system which indicated that both peels and stems have potential as bioethanol feedstock because of the cheap and unutilized status of these residues.
Acknowledgements
The first author gratefully acknowledges the research fellowship granted for the study by the Kerala State Council for Science, Technology and Environment (KSCSTE), Govt. of Kerala. Authors are thankful to the Director, ICAR-CTCRI for the facilities provided for the study and Dr. J. Sreekumar, Principal Scientist (Agricultural Statistics), ICAR-CTCRI for the help extended in statistical analyses. The support extended for the HPLC analyses by Dr. A. N. Jyothi, Principal Scientist and Mr. V. R. Vishnu, Senior Research Fellow, ICAR-CTCRI is also thankfully acknowledged.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ahamefule FO (2005) Evaluation of pigeon pea-cassava peel based diets for goat production in South-Eastern Nigeria. Ph.D. Thesis, Michael Okpara University of Agriculture, Umudike
- Alkasrawi M, Eriksson T, Borjesson J, Wingren A, Galbe M, Tjerneld F, Zacchi G. The effect of Tween-20 on simultaneous saccharification and fermentation of softwood to ethanol. Enzyme Microb Technol. 2003;33:71–78. doi: 10.1016/S0141-0229(03)00087-5. [DOI] [Google Scholar]
- Anon (2009a) STARGEN™002: Granular starch hydrolyzing enzyme for ethanol production. Product information published by Genencor International, a Division of Danisco, Danisco US Inc. http://www.genencor.com. 22 Dec 2014
- Anon (2009b) OPTIMASH™BG and OPTIMASH™XL: Product information published by Genencor International, a division of Danisco. http://www.genencor.com. Accessed 26 Nov 2015
- Anon (2014) Product information on Cellic. http://www.bioenergy.novozymes.com. Accessed 23 Mar 2014
- AOAC (2005) Official methods of analysis of AOAC international, 18th edn. Horwitz W, Latimer GW (eds.)
- Barcelos CA, Maeda RN, Betancur GJV, Pereira N., Jr Ethanol production from sorghum grains [Sorghum bicolor (L.) Moench]: evaluation of the enzymatic hydrolysis and the hydrolysate fermentability. Braz J Chem Eng. 2011;28(4):597–604. doi: 10.1590/S0104-66322011000400005. [DOI] [Google Scholar]
- Binod P, Satyanagalakshimi K, Sindhu R, Janu U, Sukumaran RK, Pandey A. Short duration microwave assisted pretreatment enhances the enzymatic saccharification and fermentable sugar yield from sugarcane bagasse. Renew Energy. 2012;37:109–116. doi: 10.1016/j.renene.2011.06.007. [DOI] [Google Scholar]
- Börjesson J, Peterson R, Tjerneld F. Enhanced enzymatic conversion of softwood lignocelluloses by poly (ethylene glycol) addition. Enzyme Microb Technol. 2007;40:754–762. doi: 10.1016/j.enzmictec.2006.06.006. [DOI] [Google Scholar]
- Buruiana C-T, Garrote G, Vizireanu C. Bioethanol production from residual lignocellulosic materials: a review—Part 2. The annals of the University Dunarea de Jos of Galati Fascicle VI. Food Technol. 2013;37(1):25–38. [Google Scholar]
- Bussamra BC, Freitas S, da Costa AC. Improvement on sugarcane bagasse hydrolysis using enzymatic mixture designed cocktail. Bioresour Technol. 2015;187:173–181. doi: 10.1016/j.biortech.2015.03.117. [DOI] [PubMed] [Google Scholar]
- Caputi JA, Ueda M, Brown T. Spectrophotometric determination of ethanol in wine. Amer J Enol Vitic. 1968;19(3):160–165. [Google Scholar]
- Cavka A, Jönsson LJ. Detoxification of lignocellulosic hydrolysates using sodium borohydride. Bioresour Technol. 2013;136:368–376. doi: 10.1016/j.biortech.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Chen WH, Tu YJ, Sheen HK. Disruption of sugarcane bagasse lignocellulosic structure by means of dilute sulfuric acid pretreatment with microwave-assisted heating. Appl Energy. 2011;88:2726–2734. doi: 10.1016/j.apenergy.2011.02.027. [DOI] [Google Scholar]
- Chen W, Ye S, Sheen H. Hydrolysis characteristics of sugarcane bagasse pretreated by dilute acid solution in a microwave irradiation environment. Appl Energy. 2012;93:237–244. doi: 10.1016/j.apenergy.2011.12.014. [DOI] [Google Scholar]
- de la Hoz A, Diaz-Ortiz A, Moreno A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem Soc Rev. 2005;34:164–178. doi: 10.1039/B411438H. [DOI] [PubMed] [Google Scholar]
- Demirbas A. Competitive liquid biofuels from biomass. Appl Energy. 2011;88:17–28. doi: 10.1016/j.apenergy.2010.07.016. [DOI] [Google Scholar]
- Eriksson T, Börjesson J, Tjerneld F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb Technol. 2002;31:353–364. doi: 10.1016/S0141-0229(02)00134-5. [DOI] [Google Scholar]
- Ethaib S, Omar R, Kamal SMM, Biak DRA. Microwave-assisted pretreatment of lignocellulosic biomass: a review. J Eng Sci Technol. 2015;21:97–109. [Google Scholar]
- FAOSTAT (2014) Production statistics. http://faostat.fao.org. Accessed 20 May 2016
- Godson AREE, Allen SAA. Bioethanol yield from selected lignocellulosic wastes. Int J Sustain Green Energ. 2015;4:141–149. [Google Scholar]
- Guo AY, Webb BT, Miles MF, Zimmerman MP, Kendler KS, Zhao Z. An ethanol-related gene resource. Nucleic Acids Res. 2009;37(Database issue):D840–D845. doi: 10.1093/nar/gkn816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haven MO, Jørgensen H. Adsorption of β-glucosidases in two commercial preparations onto pretreated biomass and lignin. Biotechnol Biofuels. 2013;6:165. doi: 10.1186/1754-6834-6-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle SS, Duff SJB, Cooper DG. Effect of surfactants on cellulose hydrolysis. Biotechnol Bioeng. 1993;42:611–617. doi: 10.1002/bit.260420509. [DOI] [PubMed] [Google Scholar]
- Hendriks ATWM, Zeeman G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol. 2009;100:10–18. doi: 10.1016/j.biortech.2008.05.027. [DOI] [PubMed] [Google Scholar]
- Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, et al. Biomass recalcitrance, engineering plants and enzymes for biofuels production. Science. 2007;325:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- Hu Z, Wen Z. Enhancing enzymatic digestibility of switchgrass by microwave-assisted alkali pretreatment. Biochem Eng J. 2008;38(3):369–378. doi: 10.1016/j.bej.2007.08.001. [DOI] [Google Scholar]
- Kaar WE, Holtzapple MT. Benefits from Tween during enzymic hydrolysis of corn stover. Biotechnol Bioeng. 1998;59:419–427. doi: 10.1002/(SICI)1097-0290(19980820)59:4<419::AID-BIT4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim SB, Kim CJ. The effects of non-ionic surfactants on the pretreatment and enzymatic hydrolysis of recycled newspaper. Biotechnol Bioprocess Eng. 2007;12:147–151. doi: 10.1007/BF03028641. [DOI] [Google Scholar]
- Kongkiattikajorn J. Ethanol production from dilute acid pretreated cassava peel by fed-batch simultaneous saccharification and fermentation. Inter J Computer Internet Management. 2012;20:22–27. [Google Scholar]
- Kongkiattikajorn J, Sornvoraweat B. Comparative study of bioethanol production from cassava peels by monoculture and co-culture of yeast. Kasetsart J (Nat Sci) 2011;45:268–274. [Google Scholar]
- Kosugi A, Kondo A, Ueda M, Murata Y, Vaithanomsat P, Thanapase W, et al. Production of ethanol from cassava pulp via fermentation with a surface-engineered yeast strain displaying glucoamylase. Renew Energy. 2009;34(5):1354–1358. doi: 10.1016/j.renene.2008.09.002. [DOI] [Google Scholar]
- Kristensen JB, Börjesson J, Bruun MH, Tjerneld F, Jørgensen H. Use of surface active additives in enzymatic hydrolysis of wheat straw lignocellulose. Enzyme Microb Technol. 2007;40:888–895. doi: 10.1016/j.enzmictec.2006.07.014. [DOI] [Google Scholar]
- Kurakake M, Ooshima H, Kato J, Harano Y. Pretreatment of bagasse by non-ionic surfactant for the enzymatic hydrolysis. Bioresour Technol. 1994;49(3):247–251. doi: 10.1016/0960-8524(94)90048-5. [DOI] [Google Scholar]
- Laghari SM, Isa MH, Abdullah AB, Laghari AJ, Saleem H. Microwave individual and combined pre-treatments on lignocellulosic biomasses. IOSR J Eng. 2014;4(2):14–28. doi: 10.9790/3021-04261427. [DOI] [Google Scholar]
- Martin C, Galbe M, Wahlboma CF, Hahn-Hägerdal B, Jönsson LJ. Ethanol production from enzymatic hydrolysates of sugarcane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae. Enzyme Microb Technol. 2002;31:274–282. doi: 10.1016/S0141-0229(02)00112-6. [DOI] [Google Scholar]
- Maurya DP, Vats S, Rai S, Negi S. Optimization of enzymatic saccharification of microwave pretreated sugarcane tops through response surface methoD0logy for biofuel. Ind J Exp Biol. 2013;51(11):992–996. [PubMed] [Google Scholar]
- Merino-Pérez O, Martínez-Palou R, Labidi J, Luque R (2015) Microwave-assisted pretreatment of lignocellulosic biomass to produce biofuels and value-added products. In: Fang Z, Smith Jr, Richard L, Qi X (eds) Production of biofuels and chemicals with microwave. Biofuels Biorefin. 3:197–224. 10.1007/978-94-017-9612-5_10
- Mithra MG, Padmaja G. Phenolic inhibitors of saccharification and fermentation in lignocellulo-starch prehydrolysates and comparative efficacy of detoxification treatments. J Biomass Biofuel. 2016 [Google Scholar]
- Mithra MG, Padmaja G, Sreekumar J. Optimization of microwave-assisted dilute acid pretreatment for enhanced structural breakdown and enzymatic saccharification of lignocellulo-starch biomass. Curr Microwave Chem. 2017;4:1–13. doi: 10.2174/2213335604666170718163955. [DOI] [Google Scholar]
- Modig T, Lidén G, Taherzadeh MJ. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem J. 2002;363:769–776. doi: 10.1042/bj3630769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier NS, Wyman CE, Dale BE, Elander RT, Lee YY, Holtzapple MT, et al. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol. 2005;96:673–686. doi: 10.1016/j.biortech.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Nelson N. A photometric adaptation of the Somogyi method for determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- Nomanbhay M, Saifuddin Hussain R, Palanisamy K. Microwave-assisted alkaline pretreatment and microwave assisted enzymatic saccharification of oil palm empty fruit bunch fiber for enhanced fermentable sugar yield. J Sustain Bioenergy Syst. 2013;3(1):7–17. doi: 10.4236/jsbs.2013.31002. [DOI] [Google Scholar]
- Öhgren KH, Hahn B, Zacchi G. Simultaneous saccharification and co- fermentation of glucose and xylose in steam pretreated corn stover at high fiber content with S. cerevisiae. J Biotechnol. 2006;126:488–496. doi: 10.1016/j.jbiotec.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Padmaja G. Evaluation of techniques to reduce assayable tannin and cyanide in cassava leaves. J Agric Food Chem. 1989;37(3):712–716. doi: 10.1021/jf00087a029. [DOI] [Google Scholar]
- Palmarola-Adrados B, Galbe M, Zacchi G. Combined steam pretreatment and enzymatic hydrolysis of starch-free wheat fibers. Appl Biochem Biotechnol. 2004;115:989–1002. doi: 10.1385/ABAB:115:1-3:0989. [DOI] [PubMed] [Google Scholar]
- Palmqvist E, Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates. I. Inhibition and detoxification. Bioresour Technol. 2000;74:17–24. doi: 10.1016/S0960-8524(99)00160-1. [DOI] [Google Scholar]
- Parajó JC, Domínguez H, Domínguez JM. Biotechnological production of xylitol. Part 3: operation in culture media made from lignocellulose hydrolysates. Bioresour Technol. 1998;66:25–40. doi: 10.1016/S0960-8524(98)00037-6. [DOI] [Google Scholar]
- Pereira SC, Maehara L, Macha DCMM, Farinas CS. 2G ethanol from the whole sugarcane lignocellulosic biomass. Biotechnol Biofuels. 2015;8:44. doi: 10.1186/s13068-015-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooja NS, Padmaja G. Effect of single and sequential cellulolytic enzyme cocktail on the fermentable sugar yield from pretreated agricultural residues of cassava. Am J Biomass Bioener. 2015;4(1):39–53. [Google Scholar]
- Pooja NS, Padmaja G. Enhancing the enzymatic saccharification of agricultural and processing residues of cassava through pretreatment techniques. Waste Biomass Valor. 2015;6:303–315. doi: 10.1007/s12649-015-9345-8. [DOI] [Google Scholar]
- Pooja NS, Padmaja G. Microwave-Assisted alkali delignification coupled with non-Ionic surfactant effect on the fermentable sugar yield from agricultural residues of cassava. Internat J Environ Agric Biotechnol. 2017;2(2):1–13. [Google Scholar]
- Qi B, Chen X, Wan Y. Pretreatment of wheat straw by nonionic surfactant-assisted dilute acid for enhancing enzymatic hydrolysis and ethanol production. Bioresour Technol. 2010;101:4875–4883. doi: 10.1016/j.biortech.2010.01.063. [DOI] [PubMed] [Google Scholar]
- Saini A, Aggarwal NK, Sharma A, Yadav A. Prospects for irradiation in cellulosic ethanol production. Biotech Res Int. 2015 doi: 10.1155/2015/157139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N, Ghosh SK, Bannerjee S, Aikat K. Bioethanol production from agricultural wastes: an overview. Renew Energy. 2012;37:19–27. doi: 10.1016/j.renene.2011.06.045. [DOI] [Google Scholar]
- SAS/STAT Software Version 9.3, SAS Institute Inc., Cary, NC, 2010
- Singh A, Tuteja S, Singh N, Bishnoi NR. Enhanced saccharification of rice straw and hull by microwave-alkali pretreatment and lignocellulolytic enzyme production. Bioresour Technol. 2011;102:1773–1782. doi: 10.1016/j.biortech.2010.08.113. [DOI] [PubMed] [Google Scholar]
- Singh A, Bajar S, Bishnoi NR. Enzymatic hydrolysis of microwave alkali pretreated rice husk for ethanol production by Saccharomyces cerevisiae, Scheffersomyces stipitis and their co-culture. Fuel. 2014;116:699–702. doi: 10.1016/j.fuel.2013.08.072. [DOI] [Google Scholar]
- Sun Y, Cheng JY. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83:1–11. doi: 10.1016/S0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Taherzadeh MJ, Karimi K. Enzyme-based hydrolysis processes for ethanol from lignocellulosic materials: a review. BioResources. 2007;2(4):707–738. [Google Scholar]
- Tejirian A, Xu F. Inhibition of enzymatic cellulolysis by phenolic compounds. Enzyme Microb Technol. 2011;48:239–247. doi: 10.1016/j.enzmictec.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Tsubaki S, Azuma JI (2011) Application of microwave technology for utilization of recalcitrant biomass, In: Stanis Å, Grundas AW (eds) Advances in induction and microwave heating of mineral and organic materials, ISBN, 978-953-307-522-8, InTech. http://www.intechopen.com/books/advances-in-induction-and-microwave-heating-of-mineral-and-organicmaterials/application-of-microwave-technology-for-utilization-of-recalcitrant-biomass
- Wyman CE. Biomass ethanol: technical progress, opportunities and commercial challenges. Annu Rev Energ Env. 1999;24:189–226. doi: 10.1146/annurev.energy.24.1.189. [DOI] [Google Scholar]
- Yadav KS, Naseeruddin S, Sai Prashanthi G, Sateesh L, Rao LV. Bioethanol fermentation of concentrated rice straw hydrolysate using co-culture of Saccharomyces cerevisiae and Pichia stipitis. Bioresour Technol. 2011;102(11):6473–6478. doi: 10.1016/j.biortech.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Yang B, Wyman CE. Pretreatment: the key to unlocking low cost cellulosic ethanol. Biofuels Bioprod Biorefin. 2008;2(1):26–40. doi: 10.1002/bbb.49. [DOI] [Google Scholar]
- Zhu S, Wu Y, Yu Z, Chen Q, Wu G, Yu F, et al. Microwave-assisted alkali pretreatment of wheat straw and its enzymatic hydrolysis. Biosys Eng. 2006;94:437–442. doi: 10.1016/j.biosystemseng.2006.04.002. [DOI] [Google Scholar]
- Zhu Z, Simister R, Bird S, McQueen-Mason SJ, Gomez LD, Macquarrie DJ. Microwave-assisted acid and alkali pretreatment of Miscanthus biomass for biorefineries. AIMS Bioeng. 2015;2:449–468. doi: 10.3934/bioeng.2015.4.449. [DOI] [Google Scholar]