Abstract
In the present study, biodiesel-derived waste glycerol (WG) was used for the isolation and production of gellan, an exopolysaccharide, on media containing WG as the main carbon source. Two bacterial isolates showed gellan producing potential which were identified as Sphingomonas pseudosanguinis (Accession No. GI:724472387) and Sphingomonas yabuuchiae (GI:724472388) by 16S rRNA gene sequencing. To maximize gellan production by S. pseudosanguinis and S. yabuuchiae, media optimization was performed at different pHs and glycerol concentrations. Morphological observations through microscopic images showed the production of gellan from these isolates. Simple linear regression showed better utilization of WG by S. pseudosanguinis than S. yabuuchiae at pH 6 and pH 7. Though, both the strains showed reverse trend at pH 8. Both the strains were able to produce high amounts of gellan gum (51.6 and 52.6 g/l, respectively) using WG (80 g/l) as the sole carbon source, in a minimal medium. This is the first report on the efficient degradation of WG and low-cost production of gellan. Owing to these characteristics, S. pseudosanguinis and S. yabuuchiae demonstrate great potential for use in the commercial production of gellan and in the bioremediation of WG.
Keywords: Waste glycerol, Biodegradation, 16S rRNA, Exopolysaccharides, Gellan gum
Introduction
Microbes release different types of exopolysaccharides which have diverse applications in various sectors such as food, cosmetic, pharmaceutical, and biotechnological industries (Nampoothiri et al. 2003; Bajaj et al. 2007; Zhang et al. 2015). Microbial derived polysaccharides such as gums have numerous applications and are of industrial relevance compared to those produced from plants or algae (Bajaj et al. 2007). For example, sphingan-producing bacteria belong to diverse genera which includes Azotobacter, Alcaligenes, Pseudomonas, Xanthomonas, Xanthobacter and Sphingomonas (Pollock 1993; Nampoothiri et al. 2003). Sphingomonas genus is known to produce gellan extracellularly in the growth medium (Banerjee et al. 2013; Murillo-Martínez and Tecante 2014). Gellan gum is one such exopolysaccharide with good gelling properties suitable for microbiological applications. There are three types of gellan produced by bacteria, viz., native, deacetylated, and clarified. Native gellan has a backbone of repeating units of β-1,4-d-glucuronic acid, β-1,3-d-glucose, α-1,4-l-rhamnose, and two acyl groups (acetate and glycerate), which are bound to glucose residues adjacent to glucuronic acid. Deacetylated gellan is generated by removing acetyl groups from native gellan while clarified gellan gum is deacetylated gellan, without any protein residue (Bajaj et al. 2007). Based on the acylation degree, gellan can be termed as low acyl gellan (LAG; partially deacylated) and high acyl gellan (HAG; highly acylated) (Prajapati et al. 2013). HAG keeps the same substituent groups present in the native form and when acyl groups are removed by alkaline treatment, LAG is obtained. Gellan gum is a high molecular mass polysaccharide, recovered by precipitation with ethanol or isopropyl alcohol after heating the culture broth to near boiling point followed by the removal of cells. Lyophilization of gellan has been suggested as an alternative strategy for getting dry gellan powder (Bajaj et al. 2007).
Gellan gum has found various applications such as use as an agar substitute with good thermostability in microbiology media, thickening agent in food, drug delivery in pharmaceuticals and as an additive in the cosmetic industry (Fialho et al. 2008; Osmalek et al. 2014). Apart from the various industrial applications, gellan producing organisms (e.g., Sphingomonas sp.) play a positive role in the bioremediation of toxic wastes in the environment. Successful biotransformation of phenanthrene by Sphingomonas sp. has been reported by Tao et al. (2007) and Zhao et al. (2008). The potential of S. capsulate to degrade naphthalene, toluene, and other aromatic compounds has been demonstrated by Fredrickson et al. (1995). Furthermore, many studies have demonstrated gellan production by S. paucimobilis using glucose as the main carbon source (Banik et al. 2007; Arockiasamy and Banik 2008).
To meet the growing energy demand of world population, researchers shift their focus on more and more production of biodiesel which is generated from non-edible oil seeds and algae as its having benefits such as carbon neutral fuel, and less competition with food crops (Vasudevan et al. 2005; Kumar and Sharma 2008, 2011; Kumar et al. 2009, 2010a, b; Chaturvedi et al. 2013; Chen et al. 2013; Gupta et al. 2013; Raghunandan et al. 2014; Singh et al. 2014). Biodiesel production will generate approximately 10% (w/w) glycerol as byproduct and utilization of the seed cakes and glycerol is one of the key choices to reduce the biodiesel production cost (Arora et al. 2017; Kothari et al. 2017). A rapid increase in crude glycerol (CG) production during the transesterification process in biodiesel industry has led to a drastic reduction in its price to such an extent that its disposal has become a burden to the environment (Maru et al. 2012; Kalia et al. 2016). Accumulation of WG is also a rampant problem faced by many other industries such as soap and petro-chemical. The WG, however, could be utilized as a viable alternative source of carbon in microbial fermentations, as opposed to glucose, resulting in value addition. Therefore, the current impetus globally is to produce value-added products from WG to make biodiesel production attractive and economical, with a positive impact on the environment. This paper focuses on the biological degradation of crude glycerol and gellan production potential of Sphingomonas spp. isolated from WG.
Methodology
Screening, isolation and maintenance of glycerol degrading microorganisms
For the isolation of glycerol-utilizing microorganisms, waste glycerol was randomly collected from the top and bottom of the storage vat of a biodiesel processing plant in Durban, South Africa, and a 9-day enrichment technique (Govender and Pillay 2011) was followed, with minor modifications. M9 broth (pH 7) served as the growth medium which comprised (g/l) NH4Cl (1); KH2PO4 (3); NaCl (5); Na2HPO4 (6) and autoclaved at 121 °C for 15 min. CaCl2 (0.1 mM) and MgSO4 (1 mM) were filter sterilized and added to the broth separately (Nimje et al. 2011). All the chemicals and reagents used in the study were procured from Merck (USA) or Sigma-Aldrich (Germany).
For the enrichment experiments, 250-ml Erlenmeyer flasks containing 100 ml M9 broth was supplemented with analytical grade glycerol (10, 15, 20, and 25 g/l; Merck) as the sole carbon source and incubated at 30 °C and 200 rpm for 3 days. Waste glycerol (100 g/l) from biodiesel industry served as the inoculum. This was followed by a second enrichment step, where a 10% culture from the first enrichment served as inoculum. Subsequent to enrichment procedures, serial dilutions (1:10) were made from each flask and 100 µl inoculum was plated onto M9 plates containing 1% pure glycerol. Plates were then incubated at different temperatures (30, 34 and 37 °C) for 48–72 h and single colonies were picked and transferred onto fresh M9 plates. The isolates obtained were designated as K1–K10.
Morphological characterization of the bacterial isolates
The glycerol degrading bacterial isolates were morphologically characterized using light microscopy, SEM, ESEM and TEM. Electron microscopy was performed at the Microscopy and Microanalysis Unit at the University of Kwazulu Natal, Pietermaritzburg Campus, South Africa. For light microscopy, a 10 µl sample broth was placed on a slide, stained with nigrosine, smeared across the slide and the cells were fixed. The morphological features of the cells were observed by an Olympus (Provis) AX 70 Light Microscope equipped with a Nikon digital camera. Samples were processed using standard protocols for SEM (Hitachi S-570) and ESEM. For TEM, samples were processed using standard methods and viewed on a TEM (JEOL JEM-1400 equipped with Orius SC 600A camera).
16S rRNA identification of the isolates
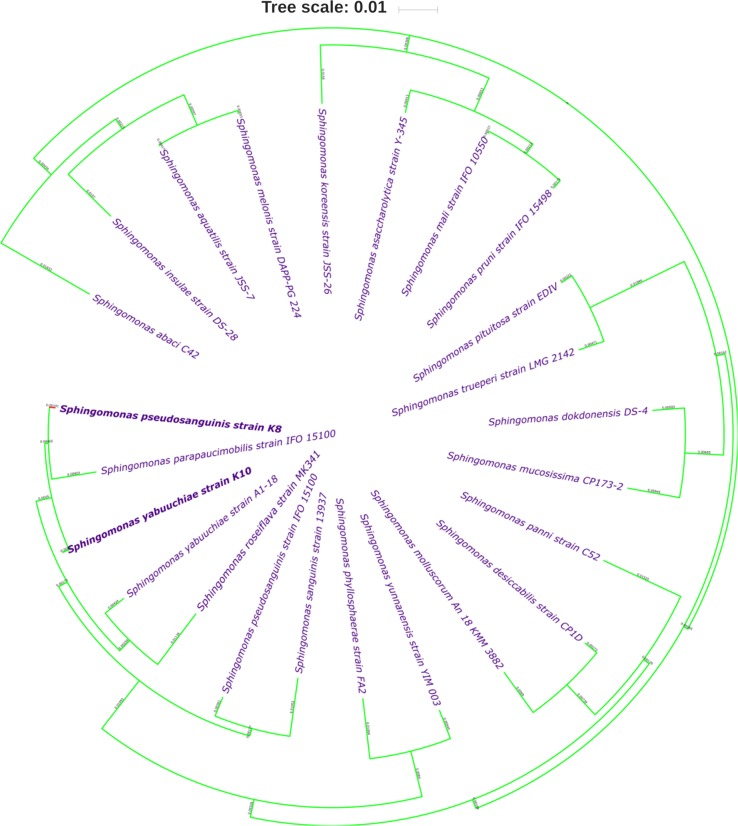
From the promising glycerol degrading bacterial isolates, genomic DNA was extracted and purified using ZR Bacterial DNA MiniPrep Kit (Zymo Research, USA).The extracted DNA was amplified using the 16S rRNA gene based primers (27F-5ʹ-AGAGTTTGATCCTGGCTCAG-3ʹ and 1492R-5ʹ-ACGGCTACCTTGTTACGACTT-3ʹ) in a thermocycler (Bio-Rad, USA). Partial sequencing of the amplicons was performed at Inqaba Biotec, South Africa, followed by BLAST analysis. A phylogenetic tree was constructed using online tool iTOL (Letunic and Bork 2016) after establishing relationship among the similar sequences analysis generated from Mega 5.05 software. Distances and clustering were performed Kimura’s two-parameter model and neighbor-joining method (Kimura 1980; Kumar et al. 2004).
Optimization of glycerol degradation and gellan production in shake flasks
To check the glycerol degradation potential of isolated bacteria, shake-flask fermentations were carried out using modified M9 medium (100 ml) containing different concentrations of WG (60–90 g/l). To ascertain the optimum pH for glycerol degradation, pH of the medium was adjusted using 1 N HCl or 1 M NaOH and inoculated with a log phase bacterial culture (10%, v/v) and incubated at 34 °C and 200 rpm for 7 days. Samples (5 ml) were collected every 24 h to analyze biomass, glycerol degradation and gellan formation.
Scale-up of gellan production in a 5-l fermenter
Gellan production was scaled up in a 5-l vertical glass fermenter with 3-l working volume (Minifors, Infors HT, Switzerland) using the modified M9 medium (pH 7) containing 80 g/l CGW. A 10% (v/v) culture of S. yabuuchiae was used as the inoculum. The agitation (600 rpm), aeration rate (0.5 vvm, gas volume per liquid reactor volume per minute) and temperature (30 °C) were maintained for 7 days. Samples (10 ml) were collected at 24 h intervals for further analysis.
Analytical methods
High-performance liquid chromatography (HPLC)
HPLC (Merck-Hitachi Lachrom) was used to detect and quantify CGW degradation in the fermentation medium using a Hi-Plex H fast acid column (100 × 7.7 mm, Polymer labs, USA) and a refractive index detector. Culture broth was centrifuged (Eppendorf 5415R) at 5000 rpm for 10 min at 4 °C and the supernatant was filtered into HPLC vials using a 0.22 µm Millipore filter and injected. H2SO4 (5 mM) served as the mobile phase at a flow rate of 0.7 ml/min and the column temp was set at 65 °C. HPLC grade glycerol (Merck) was used as standard.
Quantification of deacetylated gellan and dry cell weight
Gellan and bacterial dry cell weight was quantified using a method devised by Nampoothiri et al. (2003). Fermentation broth was boiled initially for 15 min and cooled down to room temperature followed by adjusting the pH to 10.0. The broth was then incubated at 80 °C for 10 min, followed by lowering the pH to 7.0 using 1.0 M HCl. The cell-free supernatant was added with three volumes of ice-cold isopropyl alcohol (− 70 °C) to precipitate the deacetylated gellan followed by centrifugation (10,000 rpm) at 4 °C for 25 min. This precipitate containing deacetylated gellan was then oven dried (80 °C for 24 h) until constant weight was achieved. For dry cell weight determination, the cells were harvested and dried at 80 °C for 12 h until constant weight was obtained.
Statistical analysis
The data were subjected to statistical analysis using Origin 9. Simple linear regression and polynomial were performed to establish a relation between WG degradation and gellan formation. Multiple correlations were performed using Pearson correlations at 95% confidence level to establish the relation between glycerol degradation and gellan production at each level of WG and pH used.
Results and discussion
Screening and isolation of bacterial isolates for WG degradation
Ten potential glycerol degraders were isolated from waste glycerol following enrichment (Table 1). Of the 10 bacterial isolates screened for glycerol degradation, only two (K8 and K10) were selected for further analysis on the basis of their ability to produce gellan from WG.
Table 1.
Screening of bacterial isolates for percentage waste glycerol (50 g/l) degradation at 34 °C, 200 rpm for 72 h
| Strains | 6 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| K1 | 0 | 6 | 14 | 18 |
| K2 | 0 | 22.2 | 22.8 | 24.6 |
| K3 | 4 | 8 | 11.6 | 14 |
| K4 | 2.2 | 10 | 27.8 | 45 |
| K5 | 1.8 | 2 | 3.4 | 4.2 |
| K6 | 5.4 | 13.8 | 26.4 | 30.6 |
| K7 | 0 | 9.6 | 13.2 | 16 |
| K8 | 4.2 | 14.4 | 33.2 | 42.2 |
| K9 | 0 | 8.4 | 19.6 | 41.4 |
| K10 | 0.8 | 8.6 | 38 | 47.4 |
The promising bacterial isolates, K8 and K10 were identified by partial 16 S rRNA sequencing and BLAST analysis displayed 99% similarity to Sphingomonas pseudosanguinis (K8) and Sphingomonas yabuuchiae (K10). The nucleotide sequences were submitted to the GenBank under the following accession numbers; KM453692(GI:724472387) and KM453693(GI:724472388). iTOL online tool has been used to prepare the phylogenetic tree by selecting the neighbor-joining method to explore the strain’s similarity to other species of Sphingomonas (Fig. 1). It was observed that S. pseudosanguinis and S. yabuuchiae were showing close similarity to each other, and also to other strains of the same genus. There are reports on the isolation of Sphingomonas sp. from other industrial wastes. Govender and Pillay (2011) has isolated Sphingomonas paucimobilis UT26 from 1,2-dichloroethane (DCA) containing industrial wastes and Tao et al. (2007) has isolated Sphingomonas GY2B from polycyclic aromatic hydrocarbons (PAHs) contaminated soil.
Fig. 1.
Phylogenetic tree derived from 16SrRNA gene sequence analysis, showing the position of strain K8 and K10 among related members of the Sphingomonas. The tree was generated by the neighbor-joining method
Morphological characterization of S. yabuuchiae
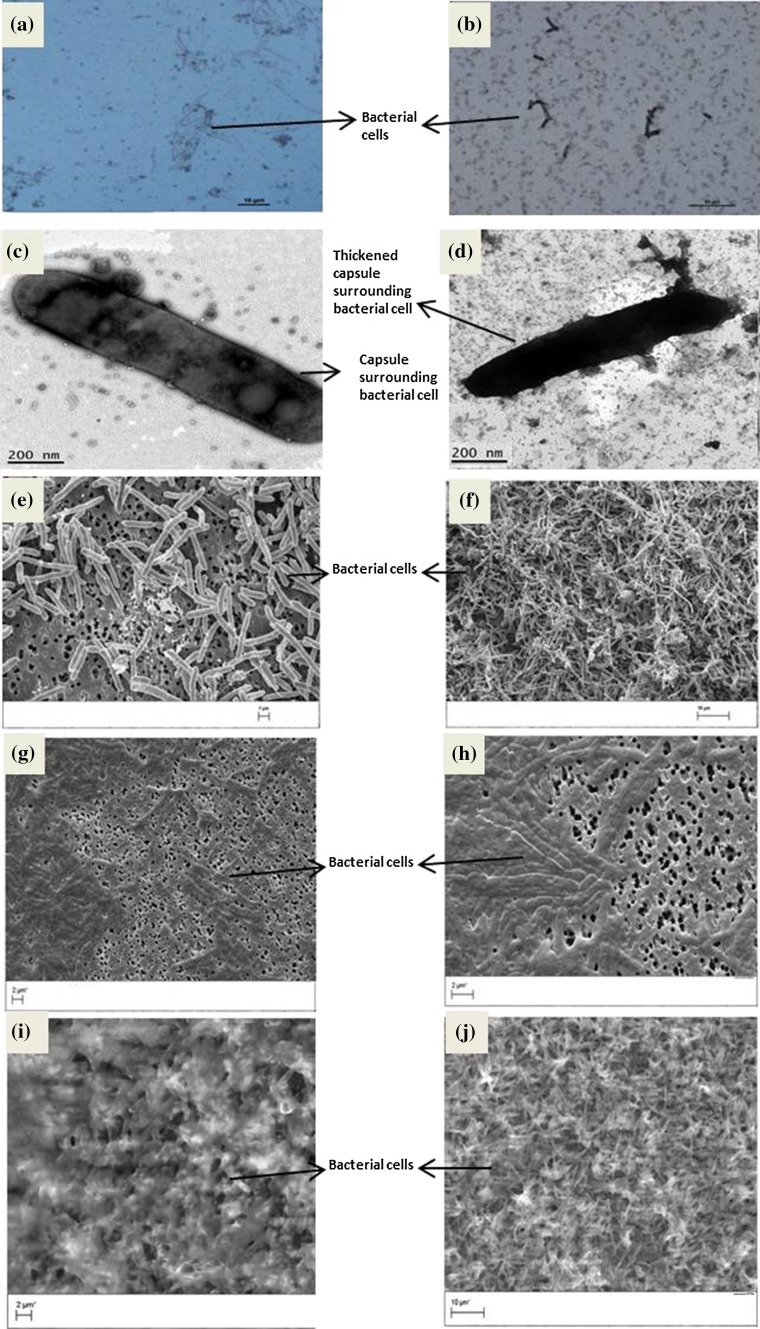
Morphological characters of S. yabuuchiae were observed using various microscopy techniques under different fermentation conditions (Fig. 2). Broth cultures of S. yabuuchiae grown on WG (80 g/l) at pH 7 was analyzed for cell morphology and gellan production using light microscopy (LM), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and environmental scanning electron microscopy(ESEM) for 7 days. Light microscopy coupled with negative staining was used to visualize the bacterial capsule formation (Fig. 2a, b). From microscopy images, it was clear that the bacterial capsule darkened over a period of 6 days, which could be attributed to gellan production. TEM images showed that the cells were more translucent on day 1 in comparison to day 6, where the cells were completely covered by biofilm (Fig. 2c, d). From SEM images, the cell length was estimated to be between 1 to 3 µm and with time, the cells formed long chains (Fig. 2e, f). ESEM images of the air-dried samples have revealed that the broth became more viscous as fermentation progressed (Fig. 2g, h). ESEM analysis could only be performed up to day 3 due to the culture being too viscous for the electrons to pass through. From day 1 to 3, a clear increase in viscosity was observed along with increasing cell numbers (Fig. 2i, j).
Fig. 2.
Microscopic observation of S. yabuuchiae, at day 1 and day 6 at different bar scales. a, b Light microscopy; c, d transmission electron microscopy; e, f Scanning electron microscopy; g, h environmental scanning electron microscopy (ESEM), of air-drying method, and i, j ESEM of fresh samples
Effect of pH on WG degradation
To evaluate the effect of pH on WG degradation, S. pseudosanguinis and S. yabuuchiae were grown on media containing different concentrations of WG (70, 80 and 90 g/l) at different pHs (6, 7 and 8) (Fig. 3). Both the strains could completely degrade WG of concentrations up to 80 g/l on day seven at all pHs tested. However, at a higher concentration of 90 g/l WG, complete degradation was not achieved with both the strains. Linear and polynomial equations were applied on the same data (Table 2) and observed that the polynomial model showed the best fit over simple linear regression in all WG degradation experiments. The only difference was in the choice of the model. The curve fitting showed that degradation was non-linear in nature and followed the quadratic relation with different constants for different concentrations. It is also apparent from the regression coefficient that has the larger value for quadratic fitting. Simple linear regression showed better utilization of WG by S. pseudosanguinis than S. yabuuchiae at pH 6 and pH 7. Though both the strains showed reverse trend at pH 8, overall, both the strains have the ability to metabolize WG. However, prolonged fermentation time was required for both S. yabuuchiae and S. pseudosanguinis for the complete degradation of WG at higher concentrations (90 g/l). This might be due to the accumulation of non-reacted components in the broth from WG, which could be responsible for the inhibition or lowering of the enzyme activities resulting in longer degradation time (Freitas et al. 2011).
Fig. 3.
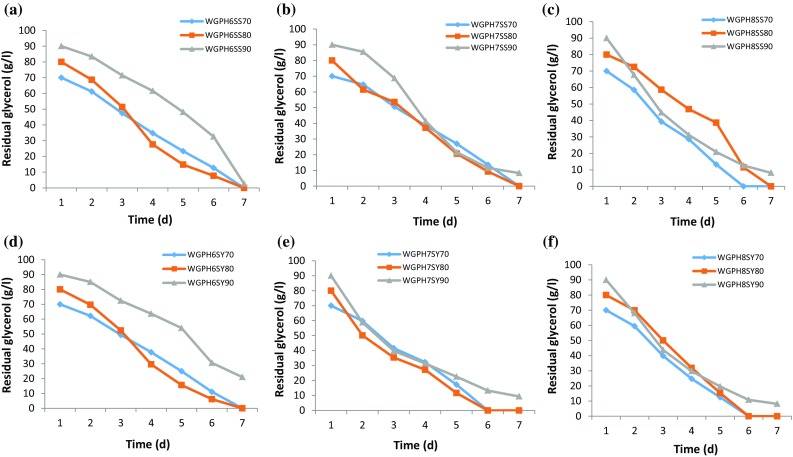
Degradation of waste glycerol (WG) by S. pseudosanguinis (a pH 6, b pH 7 and c pH 8) and S. yabuuchiae (d pH 6, e pH 7 and f pH 8), during shake-flask fermentation at 34 °C and 200 rpm over 7 days using WG conc. of: 70 (◆), 80 (■) and 90 (▲) g/l
Table 2.
Linear and polynomial function on waste glycerol (WG) degradation by Sphingomonas spp. at different pHs and different WG concentrations (70–90 g/l)
| Strains | pHs | WG conc. (g/l) | Linear | Polynomial |
|---|---|---|---|---|
| S. pseudosanguinis | 6 | 70 |
y = − 11.824x + 82.931 R2 = 0.9984 |
y = − 0.0176x2 − 11.683x + 82.72 R2 = 0.9984 |
| 80 |
y = − 14.235x + 92.673 R2 = 0.9685 |
y = 1.0844x2 − 22.91x + 105.69 R2 = 0.9853 |
||
| 90 |
y = − 13.819x + 110.97 R2 = 0.9496 |
y = − 1.689x2 − 0.3069x + 90.704 R2 = 0.9922 |
||
| 7 | 70 |
y = − 11.993x + 85.753 R2 = 0.9929 |
y = − 0.4414x2 − 8.4614x + 80.456 R2 = 0.9969 |
|
| 80 |
y = − 13.469x + 91.317 R2 = 0.9919 |
y = 0.3419x2 − 16.204x + 95.42 R2 = 0.9938 |
||
| 90 |
y = − 15.716x + 109.58 R2 = 0.9562 |
y = 0.6605x2 − 21x + 117.5 R2 = 0.9612 |
||
| 8 | 70 |
y = − 12.612x + 80.417 R2 = 0.9716 |
y = 0.925x2 − 20.012x + 91.517 R2 = 0.9873 |
|
| 80 |
y = − 13.641x + 98.597 R2 = 0.9737 |
y = − 0.9464x2 − 6.0693x + 87.24 R2 = 0.9878 |
||
| 90 |
y = − 13.549x + 93.547 R2 = 0.9367 |
y = 2.0117x2 − 29.642x + 117.69 R2 = 0.9987 |
||
| S. yabuuchiae | 6 | 70 |
y = − 12.019x + 84.569 R2 = 0.9966 |
y = − 0.289x2 − 9.7069x + 81.1 R2 = 0.9983 |
| 80 |
y = − 14.422x + 93.897 R2 = 0.9737 |
y = 0.9213x2 − 21.792x + 104.95 R2 = 0.9856 |
||
| 90 |
y = − 11.938x + 107.27 R2 = 0.9701 |
y = − 0.9371x2 − 4.4407x + 96.02 R2 = 0.988 |
||
| 7 | 70 |
y = − 12.64x + 82.119 R2 = 0.9784 |
y = 0.5294x2 − 16.876x + 88.471 R2 = 0.9835 |
|
| 80 |
y = − 12.996x + 81.173 R2 = 0.9324 |
y = 1.7881x2 − 27.301x + 102.63 R2 = 0.9854 |
||
| 90 |
y = − 12.506x + 87.867 R2 = 0.902 |
y = 2.1962x2 − 30.076x + 114.22 R2 = 0.9855 |
||
| 8 | 70 |
y = − 10.862x + 74.825 R2 = 0.9159 |
y = 1.4898x2 − 24.271x + 97.173 R2 = 0.9848 |
|
| 80 |
y = − 12.74x + 88.335 R2 = 0.9271 |
y = 1.4849x2 − 26.103x + 110.61 R2 = 0.9775 |
||
| 90 |
y = − 12.278x + 89.139 R2 = 0.9166 |
y = 1.7991x2 − 28.47x + 116.13 R2 = 0.9953 |
Several researchers have reported the ability of Sphingomonas sp. to degrade various industrial wastes. For example, Sphingomonas sp. ZP1, isolated from industrial wastes have the ability to degrade various toxic chemicals such as naphthalene, phenanthrene, toluene, methanol, ethanol, salicylic acid and Tween 80 (Zhao et al. 2008). Another report indicates that Sphingomonas strain SS3 isolated from industrial waste contaminated soil in Germany, utilizes diphenyl ether and its 4-fluoro, 4-chloro, and (to a lesser extent) 4-bromo derivatives as a sole carbon and energy source (Schmidt et al. 1992).
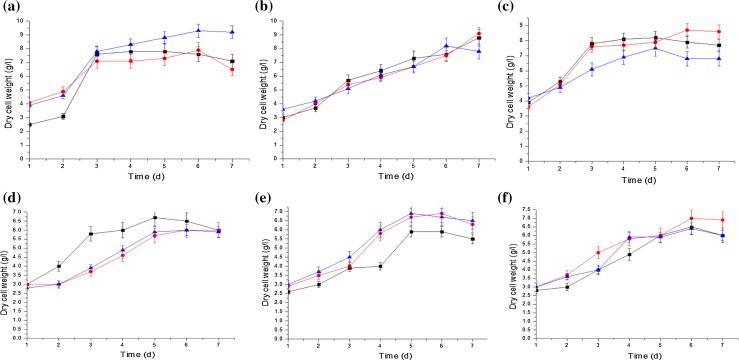
Dry cell weight determination of S. pseudosanguinis was performed and results are presented in Fig. 4a–c. While growing S. pseudosanguinis on WG (90 g/l), a maximum dry cell weight of 9.3 g/l was observed on day 7 at pH 6 (Fig. 4a) followed by other pHs tested under similar conditions (Fig. 4b, c). Of the two strains analyzed, dry cell weight accumulation was recorded less in S. yabuuchiae in comparison to S. pseudosanguinis (Fig. 4d, e).
Fig. 4.
Assessment of dry cell weight by S. pseudosanguinis (a pH 6, b pH 7 and c pH 8) and S. yabuuchiae (d pH 6, e pH 7 and f pH 8) during shake-flask fermentation at 34 °C and 200 rpm over 7 days using waste glycerol (WG) conc. of: 70 (◆), 80 (■) and 90 (▲) g/l
Effect of medium pH on gellan production
Gellan production by S. pseudosanguinis and S. yabuuchiae was studied over 7 days by growing the cultures at different WG concentrations (70–90 g/l) and pH levels (6, 7 and 8) (Fig. 5a–f). At pH 6, S. pseudosanguinis recorded the maximum gellan production of 48.2 g/l followed by 44.2 g/l at a WG concentration of 80 and 90 g/l, respectively (Fig. 5a) after 6 days; while at pH 7, gellan production was increased to 51.6 g/l followed by 48.5 g/l at a WG concentration of 80 and 90 g/l, respectively. However, gellan production was decreased to 46 g/l when pH of the media containing 80 g/l WG was increased to 8. Comparison of gellan production by the two strains has showed that S. yabuuchiae produced a significantly higher amount of gellan (52.6 g/l) at 80 g/l WG on day 6 at pH 7. Even at a higher pH of 8, the gellan production was relatively good (47.4 g/l) at 80 g/l WG under similar conditions (Fig. 5d–f).
Fig. 5.
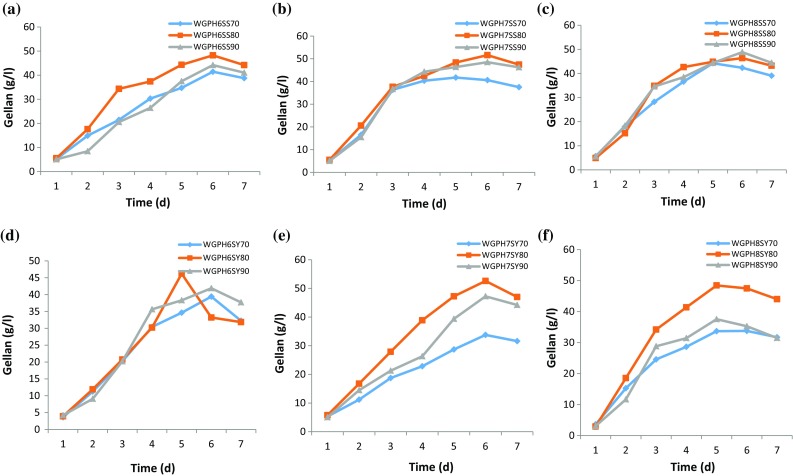
Gellan production by S. pseudosanguinis (a pH 6, b pH 7 and c pH 8) and S. yabuuchiae (pH 6 (d), pH 7 (e) and pH 8 (f)) during shake-flask fermentation at 34 °C and 200 rpm over 7 days using waste glycerol (WG) conc. of 70 (◆), 80 (■) and 90 (▲) g/l
Multiple correlations (Pearson) matrix of glycerol degradation and gellan production by S. pseudosanguinis and S. yabuuchiae at different pHs (6–8) and WG concentrations (70–90 g/l) are presented in Tables 3 and 4. From the data, it can be deduced that Pearson’s r value for WG degradation and gellan production close to + 1 represents perfect direct linear relationships and − 1 represents a perfect decreasing (inverse) linear relationship (anti-correlation). The values between − 1 and 1 in all other cases, indicates the degree of linear dependence between the variables. As it approaches zero, the relationship is relatively less (closer to uncorrelated). The closer the coefficient is to either − 1 or 1, the stronger the correlation between the variables. Comparison of data showed both the strains reveal that degradation of WG and production of gellan was negatively correlated. It has also demonstrated that WG degradation has a positive correlation on the production of gellan by S. yabuuchiae. The gellan production levels by S. pseudosanguinis and S. yabuuchiae in our study are comparatively higher than the previously reported values (35.7 g/l) from glucose (Bajaj et al. 2007). Furthermore, our production technology is more cost effective, as WG was the main carbon source used, in comparison to sugars used in other studies. Crude glycerol being an industrial waste, poses a major problem of disposal and utilizing them for gellan production would assist in reducing their disposal costs and preserving the environment. Published reports have shown that glucose, lactose or sucrose are the most common carbon sources used for gellan production. To the best of our knowledge, this is the first report on the successful production of gellan from WG.
Table 3.
Multiple correlation (Pearson) matrix of waste glycerol (WG) degradation and gellan production by S. pseudosanguinis at different pHs (6–8) and WG concentrations (70–90 g/l)
| WGPH6–70 | GPH6–70 | WGPH6–80 | GPH6–80 | WGPH6–90 | GPH6–90 | WGPH7–70 | GPH7–70 | WGPH7–80 | GPH7–80 | WGPH7–90 | GPH7–90 | WGPH8–70 | GPH8–70 | WGPH8–80 | GPH8–80 | WGPH8–90 | GPH8–90 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WGPH6–70 | 1.000 | |||||||||||||||||
| GPH6–70 | − 0.963 | 1.000 | ||||||||||||||||
| WGPH6–80 | 0.987 | − 0.985 | 1.000 | |||||||||||||||
| GPH6–80 | − 0.913 | 0.973 | − 0.946 | 1.000 | ||||||||||||||
| WGPH6–90 | 0.975 | − 0.883 | 0.928 | − 0.810 | 1.000 | |||||||||||||
| GPH6–90 | − 0.971 | 0.982 | − 0.984 | 0.955 | − 0.903 | 1.000 | ||||||||||||
| WGPH7–70 | 0.998 | − 0.948 | 0.977 | − 0.889 | 0.984 | − 0.963 | 1.000 | |||||||||||
| GPH7–70 | − 0.809 | 0.901 | − 0.867 | 0.969 | − 0.679 | 0.864 | − 0.775 | 1.000 | ||||||||||
| WGPH7–80 | 0.993 | − 0.976 | 0.987 | − 0.923 | 0.957 | − 0.974 | 0.987 | − 0.816 | 1.000 | |||||||||
| GPH7–80 | − 0.898 | 0.968 | − 0.937 | 0.998 | − 0.789 | 0.940 | − 0.871 | 0.978 | − 0.911 | 1.000 | ||||||||
| WGPH7–90 | 0.981 | − 0.973 | 0.993 | − 0.921 | 0.926 | − 0.988 | 0.976 | − 0.824 | 0.982 | − 0.906 | 1.000 | |||||||
| GPH7–90 | − 0.889 | 0.954 | − 0.934 | 0.989 | − 0.778 | 0.929 | − 0.863 | 0.987 | − 0.891 | 0.992 | − 0.902 | 1.000 | ||||||
| WGPH8–70 | 0.985 | − 0.989 | 0.990 | − 0.959 | 0.928 | − 0.995 | 0.978 | − 0.869 | 0.988 | − 0.946 | 0.986 | − 0.934 | 1.000 | |||||
| GPH8–70 | − 0.893 | 0.967 | − 0.945 | 0.985 | − 0.775 | 0.941 | − 0.864 | 0.963 | − 0.913 | 0.988 | − 0.921 | 0.981 | − 0.941 | 1.000 | ||||
| WGPH8–80 | 0.986 | − 0.932 | 0.953 | − 0.863 | 0.983 | − 0.945 | 0.992 | − 0.734 | 0.975 | − 0.843 | 0.954 | − 0.829 | 0.963 | − 0.823 | 1.000 | |||
| GPH8–80 | − 0.880 | 0.951 | − 0.929 | 0.988 | − 0.764 | 0.925 | − 0.853 | 0.989 | − 0.885 | 0.992 | − 0.898 | 1.000 | − 0.928 | 0.983 | − 0.817 | 1.000 | ||
| WGPH8–90 | 0.967 | − 0.990 | 0.983 | − 0.984 | 0.894 | − 0.972 | 0.950 | − 0.928 | 0.972 | − 0.979 | 0.961 | − 0.971 | 0.985 | − 0.971 | 0.927 | − 0.967 | 1.000 | |
| GPH8–90 | − 0.910 | 0.974 | − 0.944 | 1.000 | − 0.806 | 0.952 | − 0.886 | 0.970 | − 0.921 | 0.999 | − 0.918 | 0.990 | − 0.957 | 0.985 | − 0.860 | 0.990 | − 0.983 | 1.000 |
WG waste glycerol, G gellan production
Table 4.
Multiple correlation (pearson) matrix of waste glycerol (WG) degradation and gellan production by S. yabuuchiae at different pHs (6–8) and WG concentrations (70–90 g/l)
| WGPH6–70 | GPH6–70 | WGPH6–80 | GPH6–80 | WGPH6–90 | GPH6–90 | WGPH7–70 | GPH7–70 | WGPH7–80 | GPH7–80 | WGPH7–90 | GPH7–90 | WGPH8–70 | GPH8–70 | WGPH8–80 | GPH8–80 | WGPH8–90 | GPH8–90 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WGPH6–70 | 1.000 | |||||||||||||||||
| GPH6–70 | − 0.904 | 1.000 | ||||||||||||||||
| WGPH6–80 | 0.984 | − 0.959 | 1.000 | |||||||||||||||
| GPH6–80 | − 0.804 | 0.929 | − 0.885 | 1.000 | ||||||||||||||
| WGPH6–90 | 0.992 | − 0.862 | 0.957 | − 0.726 | 1.000 | |||||||||||||
| GPH6–90 | − 0.914 | 0.992 | − 0.970 | 0.928 | − 0.871 | 1.000 | ||||||||||||
| WGPH7–70 | 0.989 | − 0.947 | 0.989 | − 0.844 | 0.976 | − 0.945 | 1.000 | |||||||||||
| GPH7–70 | − 0.964 | 0.979 | − 0.986 | 0.894 | − 0.938 | 0.970 | − 0.991 | 1.000 | ||||||||||
| WGPH7–80 | 0.953 | − 0.960 | 0.968 | − 0.880 | 0.924 | − 0.944 | 0.975 | − 0.988 | 1.000 | |||||||||
| GPH7–80 | − 0.936 | 0.995 | − 0.977 | 0.929 | − 0.897 | 0.987 | − 0.970 | 0.993 | − 0.980 | 1.000 | ||||||||
| WGPH7–90 | 0.933 | − 0.954 | 0.954 | − 0.877 | 0.898 | − 0.941 | 0.956 | − 0.975 | 0.994 | − 0.971 | 1.000 | |||||||
| GPH7–90 | − 0.973 | 0.954 | − 0.980 | 0.873 | − 0.953 | 0.942 | − 0.992 | 0.991 | − 0.980 | 0.976 | − 0.954 | 1.000 | ||||||
| WGPH8–70 | 0.979 | − 0.971 | 0.996 | − 0.884 | 0.955 | − 0.973 | 0.994 | − 0.996 | 0.979 | − 0.986 | 0.966 | − 0.986 | 1.000 | |||||
| GPH8–70 | − 0.873 | 0.973 | − 0.927 | 0.935 | − 0.820 | 0.956 | − 0.918 | 0.960 | − 0.969 | 0.976 | − 0.980 | 0.927 | − 0.943 | 1.000 | ||||
| WGPH8–80 | 0.985 | − 0.962 | 0.997 | − 0.874 | 0.964 | − 0.966 | 0.996 | − 0.992 | 0.972 | − 0.980 | 0.954 | − 0.990 | 0.998 | − 0.927 | 1.000 | |||
| GPH8–80 | − 0.863 | 0.975 | − 0.924 | 0.944 | − 0.806 | 0.962 | − 0.909 | 0.955 | − 0.958 | 0.974 | − 0.971 | 0.917 | − 0.939 | 0.999 | − 0.922 | 1.000 | ||
| WGPH8–90 | 0.952 | − 0.975 | 0.980 | − 0.902 | 0.917 | − 0.972 | 0.974 | − 0.990 | 0.990 | − 0.988 | 0.992 | − 0.968 | 0.988 | − 0.979 | 0.979 | − 0.975 | 1.000 | |
| GPH8–90 | − 0.818 | 0.955 | − 0.887 | 0.939 | − 0.757 | 0.942 | − 0.874 | 0.926 | − 0.921 | 0.949 | − 0.939 | 0.878 | − 0.909 | 0.984 | − 0.888 | 0.990 | − 0.948 | 1.000 |
WG waste glycerol, G gellan production
The use of industrial waste as viable carbon sources for the production of different exopolysaccharides for commercial applications has gained importance recently. Some of the most attractive feedstocks that have been used, for example, cheese whey in the production of gellan from S. paucimobilis (Fialho et al. 1999), sugar cane molasses in the production of cellulose by Acetobacter xylinum ATCC 10245, glycerol by-product to produce an exopolysaccharide from Pseudomonas oleovorans (Freitas et al. 2009) and GalactoPol and FucoPol from biodiesel waste crude glycerol (Alves et al. 2011; Freitas et al. 2011).
Scale-up of gellan production by S. yabuuchiae
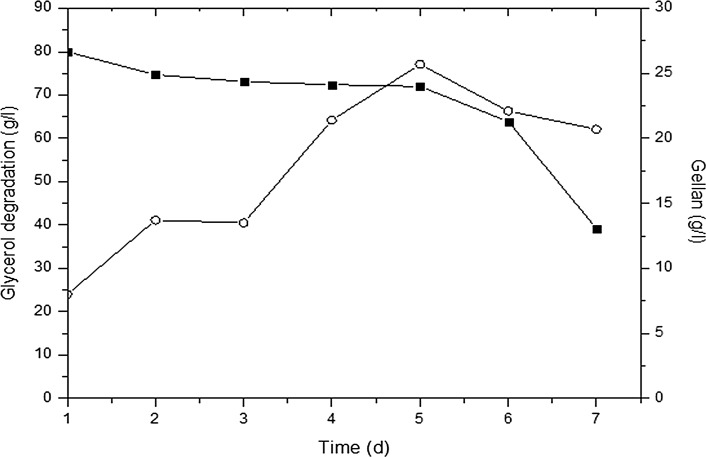
Based on the promising results for gellan production from shake-flask experiments, S. yabuuchiae was selected for further scale-up in a 5-l vertical glass fermenter. The optimum conditions from shake flask experiments (80 g/l glycerol and pH 7) were retained for scale-up. However, in contrast to shake flask experiments, glycerol utilization and gellan production was low in the fermenter and was reduced to 50% after 7 days (Fig. 6). Previous studies have shown similar trends and was noted that at high volumes, the carbon sources did not exhaust in the same pace as in shake-flask experiments (Wang et al. 2006; Freitas et al. 2010). Previously, Wang et al. (2006) reported glycerol degradation (49.17%) in a 30-l batch fermenter with gellan production of 14.75 g/l. In another study, Zhang et al. 2015 reported on the enhancement of gellan production by 47.26% using a statistically optimized medium using S. paucimobilis QHZJUJW. However, the production of gellan gum by different species varied differently. Sphingomonas paucimobilis ATCC 31461 produced 43.6 g/l gellan from starch (Bajaj et al. 2006), 35.7 and 13.8 g/l gellan from glucose and molasses (Bajaj et al. 2006), 15.9 g/l gellan from glucose (Abd-El-Aal and Attallah 2007), 20 g/l gellan from lactose (Nampoothiri et al. 2003), 19.91 g/l gellan from sucrose (Zhang et al. 2015), and 7.9 g/l gellan from cheese whey (Fialho et al. 1999).
Fig. 6.
Waste glycerol (WG) degradation by S. yabuuchiae (80 g/l) (filled square) and production of gellan (g/l) (●) at 34 °C and 200 rpm
Conclusions
The biopolymer market is very competitive which stresses the constant need for procuring low-cost carbon sources to reduce production costs without compromising on productivity. Waste glycerol derived from biodiesel industries would offer a good source of carbon for gellan production. In this study, out of the 10 bacterial isolates tested, S. yabuuchiae and S. pseudosanguinis displayed high WG degradation potential up to 80 g/l. Furthermore, impact of this study is much higher as gellan was produced from WG rather than monomeric sugars. Of further significance, the gellan conversion rate by these strains from WG is much higher than the previously reported values. Pearson’s correlations study proved that S. yabuuchiae performed better than S. pseudosanguinis for WG degradation and gellan production. This study has also demonstrated the use of low-cost carbon sources for the production of industrially attractive biochemical, gellan, in a sustainable manner.
Acknowledgements
KR and SS would like to acknowledge National Research Foundation (NRF), South Africa, and the Durban University of Technology, South Africa, for financial support for this research.
Author contributions statement
KR and AK contributed equally and have made substantial contribution to this manuscript. SKP reviewed the manuscript. SS and KP critically edited and formatted the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Kerisha Raghunandan and Ashwani Kumar contributed equally.
References
- Alves VD, Ferreira AR, Costa N, Freitas F, Reis MA, Coelhoso IM. Characterization of biodegradable films from the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohydr Polym. 2011;83:1582–1590. doi: 10.1016/j.carbpol.2010.10.010. [DOI] [Google Scholar]
- Arockiasamy S, Banik RM. Optimization of gellan gum production by Sphingomonas paucimobilis ATCC 31461 with nonionic surfactants using central composite design. J Biosci Bioeng. 2008;105:204–210. doi: 10.1263/jbb.105.204. [DOI] [PubMed] [Google Scholar]
- Arora K, Sharma S, Krishna SBN, Adam JK, Kumar A (2017) Non-edible oil cakes as a novel substrate for DPA production and augmenting biocontrol activity of Paecilomyces variotii. Front Microbiol 8:1–12. 10.3389/fmicb.2017.00753 [DOI] [PMC free article] [PubMed]
- Abd-El-Aal S, Attallah A (2007) Induction of highly gellan gum productive Sphingomonas paucimobilis. Research J Agri Biol Sci 3(6):554–557
- Bajaj IB, Saudagar PS, Singhal RS, Pandey A. Statistical approach to optimization of fermentative production of gellan gum from Sphingomonas paucimobilis ATCC 31461. J Biosci Bioeng. 2006;102:150–156. doi: 10.1263/jbb.102.150. [DOI] [PubMed] [Google Scholar]
- Bajaj IB, Survase SA, Saudagar PS, Singhal RS. Gellan gum: fermentative production, downstream processing and applications. Food Technol Biotechnol. 2007;45:341–354. [Google Scholar]
- Banerjee S, Ravi R, Bhattacharya S. Textural characterisation of gellan and agar based fabricated gels with carrot juice. LWT Food Sci Technol. 2013;53:255–261. doi: 10.1016/j.lwt.2013.02.011. [DOI] [Google Scholar]
- Banik RM, Santhiagu A, Upadhyay SN. Optimization of nutrients for gellan gum production by Sphingomonas paucimobilis ATCC-31461 in molasses based medium using response surface methodology. Bioresour Technol. 2007;98:792–797. doi: 10.1016/j.biortech.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Chaturvedi S, Kumar A, Singh B, Nain L, Joshi M, Satya S. Bioaugmented composting of Jatropha de-oiled cake and vegetable waste under aerobic and partial anaerobic conditions. J Basic Microbiol. 2013;53:327–335. doi: 10.1002/jobm.201100634. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhou L, Tian K, Kumar K, Singh S, Prior BA, Wang Z. Metabolic engineering of Escherichia coli: a sustainable industrial platform for bio-based chemical production. Biotechnol Adv. 2013;31:1200–1223. doi: 10.1016/j.biotechadv.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Fialho AM, Martins LO, Donval ML, Leitão JH, Ridout MJ, Jay AJ, Morris VJ, Sá-Correia I. Structures and properties of gellan polymers produced by Sphingomonas paucimobilis ATCC 31461 from lactose compared with those produced from glucose and from cheese whey. Appl Environ Microbiol. 1999;65:2485–2491. doi: 10.1128/aem.65.6.2485-2491.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialho AM, Moreira LM, Granja AT, Popescu AO, Hoffmann K, Sá-Correia I. Occurrence, production, and applications of gellan: current state and perspectives. Appl Microbiol Biotechnol. 2008;79:889–900. doi: 10.1007/s00253-008-1496-0. [DOI] [PubMed] [Google Scholar]
- Fredrickson JK, Balkwill DL, Drake GR, Romine MF, Ringelberg DB, White DC. Aromatic-degrading Sphingomonas isolates from the deep subsurface. Appl Environ Microbiol. 1995;61:1917–1922. doi: 10.1128/aem.61.5.1917-1922.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas F, Alves VD, Pais J, Costa N, Oliveira C, Mafra L, Hilliou L, Oliveira R, Reis MA. Characterization of an extracellular polysaccharide produced by a Pseudomonas strain grown on glycerol. Bioresour Technol. 2009;100:859–865. doi: 10.1016/j.biortech.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Freitas F, Alves VD, Pais J, Carvalheira M, Costa N, Oliveira R, Reis MA. Production of a new exopolysaccharide (EPS) by Pseudomonas oleovorans NRRL B-14682 grown on glycerol. Process Biochem. 2010;45:297–305. doi: 10.1016/j.procbio.2009.09.020. [DOI] [Google Scholar]
- Freitas F, Alves VD, Reis MAM. Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol. 2011;29:388–398. doi: 10.1016/j.tibtech.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Govender A, Pillay B (2011) Biochemical activities of 1, 2-dichloroethane (DCA) degrading bacteria. African J Biotechnol 10:11574–11581. 10.5897/ajb10.1685
- Gupta A, Kumar A, Sharma S, Vijay VK. Comparative evaluation of raw and detoxified mahua seed cake for biogas production. Appl Energy. 2013;102:1514–1521. doi: 10.1016/j.apenergy.2012.09.017. [DOI] [Google Scholar]
- Kalia VC, Prakash J, Koul S. Biorefinery for Glycerol Rich Biodiesel Industry Waste. Indian J. Microbiol. 2016;56:113–125. doi: 10.1007/s12088-016-0583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kothari R, Pandey A, Ahmad S, Kumar A. Microalgal cultivation for value-added products: a critical enviro-economical assessment. 2017 doi: 10.1007/s13205-017-0812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Sharma S. An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): a review. Ind Crops Prod. 2008;28:1–10. doi: 10.1016/j.indcrop.2008.01.001. [DOI] [Google Scholar]
- Kumar A, Sharma S. Potential non-edible oil resources as biodiesel feedstock: an Indian perspective. Renew Sustain Energy Rev. 2011;15:1791–1800. doi: 10.1016/j.rser.2010.11.020. [DOI] [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sharma S, Mishra S. Effect of akalinity on growth performance of Jatropha Curcas Inoculated With. Methods. 2009;1:177–184. [Google Scholar]
- Kumar A, Kumar K, Kaushik N, et al. Renewable energy in India: current status and future potentials. Renew Sustain Energy Rev. 2010;14:2434–2442. doi: 10.1016/j.rser.2010.04.003. [DOI] [Google Scholar]
- Kumar A, Sharma S, Mishra S. Influence of arbuscular mycorrhizal (AM) fungi and salinity on seedling growth, solute accumulation, and mycorrhizal dependency of Jatropha curcas L. J Plant Growth Regul. 2010;29:297–306. doi: 10.1007/s00344-009-9136-1. [DOI] [Google Scholar]
- Letunic I, Bork P (2016) Interactive tree of life (iTOL) v3 : an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acid Research 44:242–245. 10.1093/nar/gkw290 [DOI] [PMC free article] [PubMed]
- Maru BT, Bielen AAM, Kengen SWM, Constantí M, & Medina F (2012) Biohydrogen production from glycerol using Thermotoga spp. Energy Procedia 29:300–307
- Murillo-Martínez MM, Tecante A. Preparation of the sodium salt of high acyl gellan and characterization of its structure, thermal and rheological behaviors. Carbohydr Polym. 2014;108:313–320. doi: 10.1016/j.carbpol.2014.02.056. [DOI] [PubMed] [Google Scholar]
- Nampoothiri KM, Singhania RR, Sabarinath C, Pandey A. Fermentative production of gellan using Sphingomonas paucimobilis. Process Biochem. 2003;38:1513–1519. doi: 10.1016/S0032-9592(02)00321-7. [DOI] [Google Scholar]
- Nimje VR, Chen CY, Chen CC, Chen HR, Tseng MJ, Jean JS, Chang YF (2011) Glycerol degradation in single-chamber microbial fuel cells. Bioresource Technol 102(3):2629–2634 [DOI] [PubMed]
- Osmałek T, Froelich A, Tasarek S. Application of gellan gum in pharmacy and medicine. Int J Pharm. 2014;466:328–340. doi: 10.1016/j.ijpharm.2014.03.038. [DOI] [PubMed] [Google Scholar]
- Pollock TJ. Gellan-related polysaccharides and the genus Sphingomonas. J Gen Microbiol. 1993;139:1939–1945. doi: 10.1099/00221287-139-8-1939. [DOI] [Google Scholar]
- Prajapati VD, Jani GK, Zala BS, Khutliwala TA. An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohydr Polym. 2013;93:670–678. doi: 10.1016/j.carbpol.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Raghunandan K, McHunu S, Kumar A, et al. Biodegradation of glycerol using bacterial isolates from soil under aerobic conditions. J Environ Sci Health A Toxicol Hazard Subset Environ Eng. 2014;49:85–92. doi: 10.1080/10934529.2013.824733. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Wittich RM, Erdmann D, et al. Biodegradation of diphenyl ether and its monohalogenated derivatives by Sphingomonas sp. strain SS3. Appl Environ Microbiol. 1992;58:2744–2750. doi: 10.1128/aem.58.9.2744-2750.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NB, Kumar A, Rai S. Potential production of bioenergy from biomass in an Indian perspective. Renew Sustain Energy Rev. 2014;39:65–78. doi: 10.1016/j.rser.2014.07.110. [DOI] [Google Scholar]
- Tao XQ, Lu GN, Dang Z, et al. A phenanthrene-degrading strain Sphingomonas sp. GY2B isolated from contaminated soils. Process Biochem. 2007;42:401–408. doi: 10.1016/j.procbio.2006.09.018. [DOI] [Google Scholar]
- Vasudevan P, Sharma S, Kumar A. Liquid fuel from biomass: an overview. J Sci Ind Res (India) 2005;64:822–831. [Google Scholar]
- Wang X, Xu P, Yuan Y, Liu Y, Zhang D, Yang Z, Yang C, Ma C. Modeling for gellan gum production by Sphingomonas paucimobilis ATCC 31461 in a simplified medium. Appl Environ Microbiol. 2006;72:3367–3374. doi: 10.1128/AEM.72.5.3367-3374.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Dong YC, Fan LL, et al. Optimization of culture medium compositions for gellan gum production by a halobacterium Sphingomonas paucimobilis. Carbohydr Polym. 2015;115:694–700. doi: 10.1016/j.carbpol.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Zhao HP, Wang L, Ren JR, Li Z, Li M, Gao HW. Isolation and characterization of phenanthrene-degrading strains Sphingomonas sp. ZP1 and Tistrella sp. ZP5. J Hazard Mater. 2008;152:1293–1300. doi: 10.1016/j.jhazmat.2007.08.008. [DOI] [PubMed] [Google Scholar]