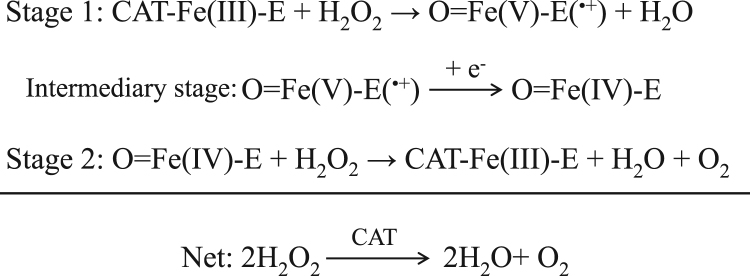Abstract
Maximal velocity (Vmax) is a well established biomarker for the assessment of tissue redox status. There is scarce evidence, though, that it does not probably reflect sufficiently in vivo tissue redox profile. Instead, the Michaelis constant (Km) could more adequately image tissue oxidative stress and, thus, be a more physiologically relevant redox biomarker. Therefore, the aim of the present study was to side-by-side compare Vmax and Km of an antioxidant enzyme after implementing an in vivo set up that induces alterations in tissue redox status. Forty rats were divided into two groups including rats injected with blood plasma originating from rats that had previously swam until exhaustion and rats injected with blood plasma originating from sedentary rats. Tail-vein injections were performed daily for 21 days. Catalase Vmax and Km measured in gastrocnemius muscle were increased after administration of the exercise-conditioned plasma, denoting enhancement of the enzyme activity but impairment of its affinity for the substrate, respectively. These alterations are potential adaptations stimulated by the administered plasma pointing out that blood is an active fluid capable of regulating tissue homeostasis. Our findings suggest that Km adequately reflects in vivo modifications of skeletal muscle catalase and seems to surpass Vmax regarding its physiological relevance and biological interpretation. In conclusion, Km can be regarded as an in vivo-like biomarker that satisfactorily images the intracellular environment, as compared to Vmax that could be aptly parallelized with a biomarker that describes tissue oxidative stress in an in vitro manner.
Keywords: Oxidative stress, Plasma administration, In vivo-like, Catalase, Km, Vmax
1. Introduction
During the last five years, several high prolific studies have challenged the common belief that blood is an inert tissue, which passively accepts molecules (including reactive oxygen and nitrogen species and metabolic products) generated by skeletal muscle and other tissues. Indeed, there is much accumulative in vitro and in vivo evidence suggesting that blood is not just an inactive tissue but, on the contrary, a body fluid that can regulate tissue homeostasis. In this sense, a recent in vivo experimental study from our group, probably the first relevant attempt in the field of redox biology, demonstrated that plasma administration from exercised rats to sedentary rats induced blood and tissue inflammation [1]. Thus, this article saliently highlights the ability of plasma molecules to stimulate tissue redox adaptations. In line with this study, in vitro incubation of cells with sera originating from athletes, who participate in different sports resulted in distinguishable redox responses [2]. Relevant in vivo parabiosis studies have also verified the active role of blood as demonstrated by the reversal of age-related cardiac hypertrophy [3], restoration of synaptic plasticity [4] and vascular, and neuronal rejuvenation in old mice surgically joined to young mice [5]. Thus, these sophisticated experiments document clearly that blood is capable of inducing alterations and adaptations in tissues in diverse biochemical and physiological processes, such as inflammation, aging and oxidative stress.
A common practice in the assessment of tissue oxidative stress is the measurement of the maximal velocity (Vmax) of antioxidant enzymes. Catalase Vmax is, in fact, an established redox biomarker for assessing oxidative stress in blood and tissues [6], [7], [8], [9], [10], [11]. Nevertheless, this tenet has been sporadically challenged. Interestingly, the Michaelis constant (Km) has been suggested to be a more reliable index, compared to Vmax, for monitoring structural and functional modifications of redox related enzymes. Indeed, Ji et al. [12] described that metabolic adaptations to exercise training were clearly denoted by changes in the Km values of lactate dehydrogenase (LDH) in rat muscles. In addition, Somani and Husain [13] reported differential alterations of the Km values in several tissues of trained rats, suggesting that exercise training aids in coping with oxidative stress in old age. Favero et al. [14] demonstrated that the functional alterations of skeletal muscle LDH after endurance training in rats are best reflected by the Km values of the enzyme and not by the modifications of LDH isozyme pattern. The above studies support the hypothesis already proposed by Gollnick and Saltin since 1982 [15], that Km is indicative of the energy metabolism enhancement by training. To our knowledge, these are the only studies suggesting an important potential for Km in the assessment of redox-induced alterations. However, they have offered only circumstantial evidence on the potential superiority of Km over Vmax to adequately reflect responses and adaptations in metabolic pathways in vivo. This is because no study to date has side-by-side compared the Vmax and Km of an antioxidant enzyme using an in vivo redox-altering experimental set up.
In light of the above, the main aim of the present study was to directly compare Vmax and Km of an antioxidant enzyme in an in vivo setting. Furthermore, we intended to investigate whether a usually overlooked in vitro kinetic parameter (i.e., Km) of skeletal muscle catalase could describe in an in vivo-like manner the manifestation of a complicated phenomenon, such as oxidative stress. The redox status-altering stimulus used was administration of plasma from exercised rats to sedentary rats, which is an experimental approach capable of inducing blood-borne effects in blood and tissues. We have hypothesized that Km might be more physiologically relevant and, thus, a more useful redox biomarker compared to Vmax, considering that it represents one fundamental biological property of catalase, namely the affinity for its substrate.
2. Materials and methods
2.1. Animals
Adult male Wistar rats (380 ± 27 g) were housed under a 12 h light: 12 h dark cycle, controlled temperature (21–23 °C) and humidity (50–70%). Commercial rat chow and tap water were provided ad libitum. All procedures were in accordance with the European Union guidelines for the care and use of laboratory animals, as well as the “Principles of laboratory animal care” (NIH publication No. 86-23, revised 1985). The project was reviewed and approved by the institutional review board and the appropriate state authority (#359888/3612).
2.2. Study design
Phase 1: Rats were randomly divided in two groups, as described previously [1]. Briefly, the animals of the exercise group underwent swimming until exhaustion [1], [6], [9], whilst the animals of the sedentary group were placed in the water tanks for a mere ten minutes and remained in their cages without any treatment. The rats were familiarized with water according to the protocol previously presented by our group [1], [6], [9]. Whole blood was collected from the rats of both groups, plasma samples were isolated, homogenized into two containers, separated into aliquots of 0.8 ml each and stored at −80 °C for use in phase 2. Plasma from the rats of the exercise group is hereafter considered as the "exercised plasma" and from the rats of the sedentary group as the "resting plasma".
Phase 2: Experimental rats were randomly divided into two groups (20 animals per group) as follows: the first group involved rats that were injected intravenously with resting plasma and the second group consisted of rats that were injected intravenously with exercised plasma. Tail vein injections at the dose of 2 ml/per kg of body weight were performed daily for 21 consecutive days by using 1 ml insulin syringes (Terrumo, Tokyo, Japan). Twenty-four hours after the last injection, rats of both groups were killed and gastrocnemius muscle was collected and stored at −80 °C for further analysis.
2.3. Blood and tissue collection and preparation
Rats were deeply anesthetized by exposure to ether. The depth of anesthesia was assured by the constriction of the pupils as well as simple sensory tests, such as the absence of eye blinking when the eyelid was touched and the absence of foot withdrawal when it was pinched. Subsequently, the thoracic cavity was opened and the rats were killed by blood collection via cardiac puncture in the right ventricle using a 10 ml syringe during both phases 1 and 2. During phase 2, immediately after blood collection, gastrocnemius muscle was quickly excised, snapped frozen in liquid nitrogen and stored at −80 °C. In preparation for analysis, muscle samples were initially ground using a mortar and a pestle under liquid nitrogen and homogenized as previously demonstrated [1].
2.4. Analysis of kinetic parameters
The Vmax and Km values were calculated using the protocol for catalase activity as it has been previously described [11]. Specifically, 40 μl of muscle homogenate diluted 1/2 in distilled water was added to 2955 μl of phosphate buffer (67 mM, pH = 7.4) and the mixture was incubated for 10 min at room temperature. Next, 5 μl of 30% hydrogen peroxide (H2O2) (to a final concentration of 20 mM) was added and the absorbance was monitored at 240 nm for 2 min. This protocol was performed in six different concentrations of the substrate (H2O2), namely 1 mM, 2 mM, 4 mM, 10 mM, 20 mM and 40 mM for each of the 40 muscle samples. Both the Michaelis-Menten parabolic plot of the catalytic rate, corresponding to the μmol of H2O2 decomposed per minute by catalase (i.e., the reaction velocity, V) for all six substrate concentrations versus the substrate concentration [S] and the reciprocal Lineweaver-Burk plot (i.e., the 1/V vs. 1/[S] plot) were built. The straight line Lineweaver-Burk plot was used to calculate Km (x intercept = −1/Km) and Vmax (y intercept = 1/Vmax) values. Each assay was performed in triplicate. All reagents were purchased from Sigma-Aldrich (St. Louis, Mo.).
2.5. Statistical analysis
The distributions of the dependent variables were examined using the Shapiro-Wilk test and were found not to differ significantly from normality. The values of Vmax and Km were analyzed using Student's t-test for independent samples (Excel 2010, Microsoft Corporation, Redmond, WA, USA). Data are presented as mean ± SEM and the significance level was set at P < 0.05.
3. Results
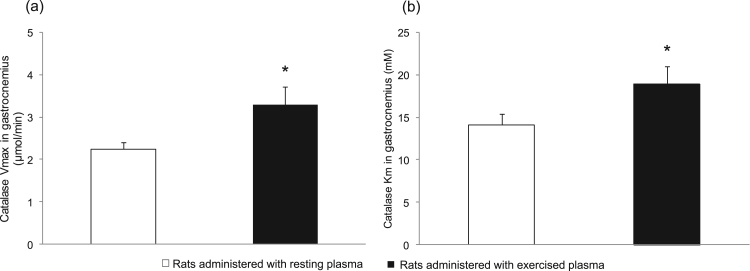
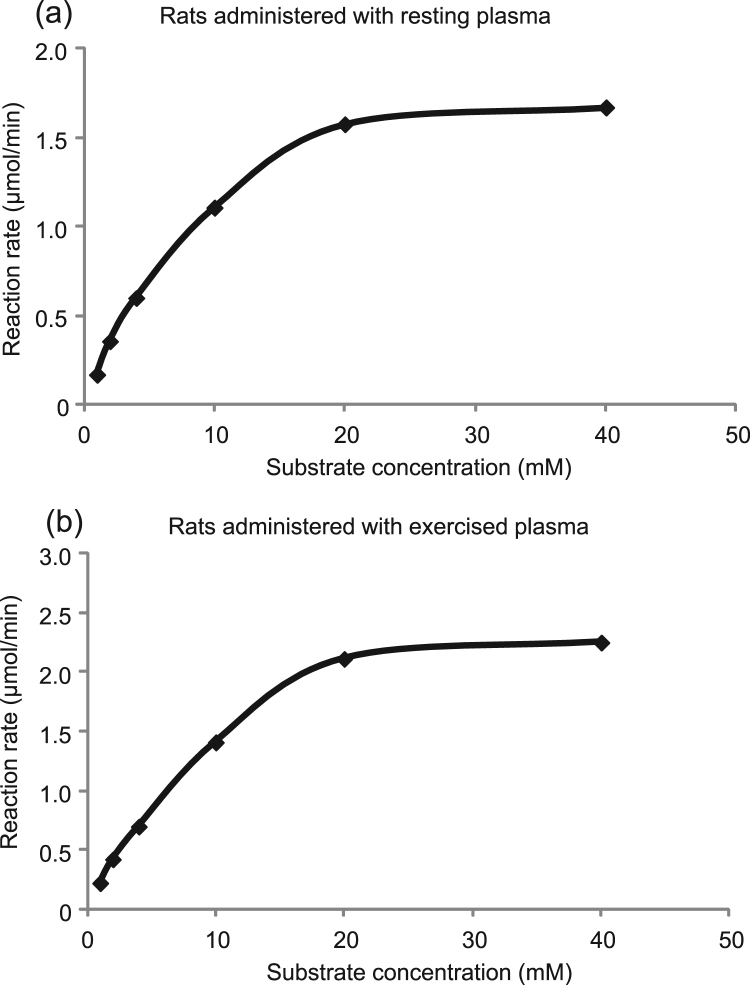
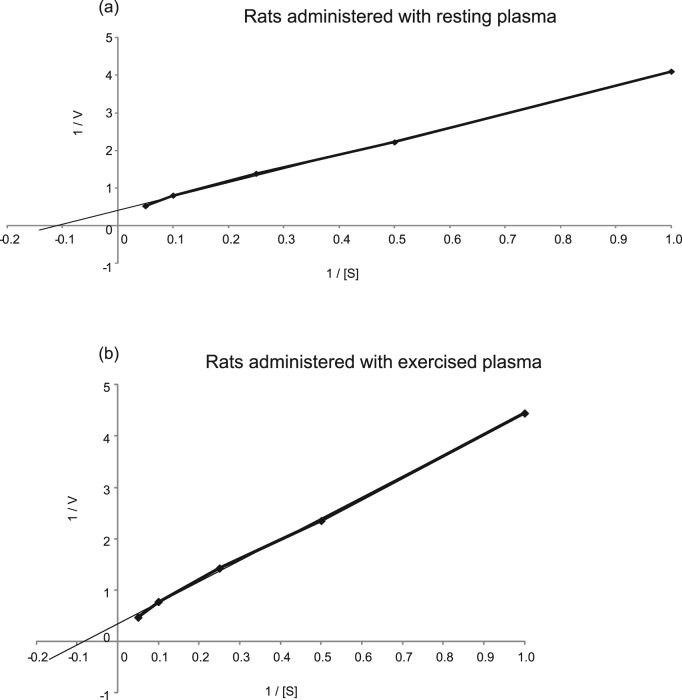
The effect of administration of exercised plasma on catalase Vmax and Km of the gastrocnemius muscle is demonstrated in Fig. 1. Catalase Vmax value was significantly increased (P = 0.02) in the rats administered with exercised plasma (i.e., 3.3 ± 0.4 μmol/min) compared to the rats administered with resting plasma (i.e., 2.2 ± 0.2 μmol/min) (Fig. 1a). Similarly, catalase Km value was also significantly elevated (P = 0.05) in the rats administered with exercised plasma (i.e., 18.9 ± 2.1 mM) compared to the rats administered with resting plasma (i.e., 14.1 ± 1.3 mM) (Fig. 1b). Fig. 2 illustrates two representative plots of the reaction rate (i.e., Michaelis-Menten plots) for the examined groups of rats. Fig. 2a and b depict the catalase reaction rate (i.e., the rate of product generation) in the rats administered with resting plasma and exercised plasma, respectively. The reaction rate of the group administered with exercised plasma is higher as demonstrated by the increase of the Vmax value. Two representative Lineweaver-Burk, double reciprocal plots for the aforementioned groups of rats are shown in Fig. 3. The intersection of the line of the plot with x and y axis is representative of the increase in Km and Vmax values, respectively, in the rats administered with exercised plasma (Fig. 3b) compared to the rats administered with the resting plasma (Fig. 3a).
Fig. 1.
The effect of either resting (open bars) or exercised (closed bars) plasma administration on catalase Vmax (1a) and Km (1b) in gastrocnemius muscle. *Significant difference between the rats administered with resting plasma and the rats administered with exercised plasma.
Fig. 2.
One representative Michaelis-Menten parabolic plot of the reaction rate (V) versus the substrate (H2O2) concentration ([S]) for the rats administered with resting plasma (2a) and the rats administered with exercised plasma (2b).
Fig. 3.
Two representative Lineweaver-Burk plots for reciprocal values of the velocity (1/V) versus the substrate (H2O2) concentration (1/[S]) for the rats administered with resting plasma (3a) and the rats administered with exercised plasma (3b).
4. Discussion
The present study examined whether an overlooked in vitro kinetic parameter, such as Km, is more physiologically relevant than the widely used Vmax in assessing the in vivo behavior of catalase and reflecting tissue oxidative stress, after the implementation of a stimulus disrupting redox equilibrium and inducing adaptations. The levels of catalase Vmax and Km were both increased after administration of exercised plasma in rats, indicating that skeletal muscle adaptations were induced by blood-borne factors. Our findings clearly demonstrate that Km adequately reflects in vivo redox-related modifications of skeletal muscle catalase.
Catalase is a significant enzyme of the antioxidant defence present in almost all aerobes. It catalyzes the dismutation of two H2O2 molecules, that is the reduction of one H2O2 molecule to H2O and the oxidation of the second to O2 [16]. While the exact catalase mechanism is still unresolved, it is currently accepted that it operates in two stages (Fig. 4). During the first stage, H2O2 interacts with asparagine 148 and histidine 75 and the ferric form [Fe(III)] of catalase heam is converted to a nominal valency of Fe(V) oxoporphyrin-cation radical (i.e., compound I). Compound I is subsequently reduced by one electron producing Fe(IV) heam (compound II), which, at the second stage oxidizes a second H2O2 molecule to form O2 [17]. The electron in question may be generated by amino acids present in catalase molecule that consequently are converted to free radicals. Nicotinamide adenine dinucleotide phosphate (NADPH), however, that is bound to all four subunits of catalase usually offers an electron to compound I hindering amino acid oxidation. Furthermore, NADPH recycles catalase regenerating its active ferric form from compound II and, thus, considerably contributes to the regulation of tissue redox equilibrium [16], [18], [19].
Fig. 4.
The reaction mechanism of catalase. CAT: catalase; Fe-E: heam and its iron center attached to the enzyme; O˭Fe(V)-E(•+): compound I [oxoporphyrin-cation radical (haem•+)]; O˭Fe(IV)-E: compound II.
Catalase Km represents the affinity of the enzyme for its substrate (i.e., H2O2). Specifically, the larger the Km value, the weaker the binding of catalase with H2O2. Maximal velocity corresponds to the maximum rate at which catalase works at saturating concentrations of H2O2 and, basically, illustrates the enzyme efficacy. Catalase affinity for H2O2 was compromised in the gastrocnemius muscle of the rats administered with exercised plasma (i.e., Km augmentation) probably due to post-translational modifications of the enzyme caused by irreversible alterations (e.g., carbonyl formation or thiol residue oxidation) [20]. It is plausible to assume that this is a negative adaptation stimulated by the administered exercised plasma pointing out that blood, contrary to the prevailing muscle-centric redox beliefs, is an active body fluid that regulates tissue homeostasis [21]. This finding is in accordance with our previous evidence that exercised plasma induced blood and systemic inflammation [1]. Simultaneously, the enzyme activity was reinforced (i.e., increased Vmax), a fact that could possibly be attributed to the increase in the number of catalase molecules leading to the induction of the enzyme. This could be considered as an adaptive response to inflammation induced oxidative stress, since the antioxidant defence mechanism of skeletal muscle is enhanced to cope with increased H2O2 generation.
Interestingly, apart from the enhancement in the maximal rate of catalase catalysis, exercised plasma administration negatively affected catalase function but this modification could only be reflected by Km and not Vmax. It has been reported that the extracellular and intracellular concentrations of H2O2 in contracting skeletal muscle equal to 15–20 μM and 0.1–0.2 μM, respectively [22]. These values are 1000–10,000 fold lower than the gastrocnemius catalase Km value found in the present study (i.e., approximately 20 mM) in the rats administered with exercised plasma. An enzyme with a high Km value relative to the concentration of its substrate is not normally saturated, thus, its activity will vary because the rate of the product formation depends on the availability of the substrate [23]. Therefore, although the volume of the administered exercised plasma equals to approximately a mere 3% of total blood volume of the rats, there is a possibility that the increased H2O2 levels contained in it have affected catalase kinetics. This is in line with our hypothesis that the exercised plasma is an active fluid capable of altering tissue homeostasis and, specifically, inducing inflammation as we have previously reported [1] and, thus, oxidative stress. However, even though an increase in H2O2 levels may "prime" the enzyme reactivity as indicated by the augmented Vmax levels, the concomitant enhancement of Km levels imply that this is not the case. Specifically, the increased Vmax value indicates a positive effect as the enzyme activity is reinforced, but the augmentation of Km value denotes that the exercise conditioned plasma has negative impact on the enzyme since its affinity for H2O2 is impaired. This is a highly important biological trait of Km that permits a prediction of whether or not the rate of product formation will be affected by the availability and the physiological concentrations of the substrate. Given the fact that exercised plasma administration increased catalase Km value 1000–10,000 times above the normal levels of H2O2 in contracting muscle, it seems that Km is more physiologically relevant (i.e., it reflects the H2O2-related variations of catalase efficacy that are induced by muscle physiology-modifying treatments) and sufficient, than Vmax, in addressing biological questions in oxidative stress context.
One of the major goals of biology is to create comprehensive and quantitative in vitro models that promote the understanding of cellular behavior [24]. A basic question rises here: can in vitro kinetics describe the in vivo function of complex biochemical pathways? [25], [26]. According to the literature, several factors including endogenous metabolite localization and metabolite channelling may modulate enzyme kinetics and improve its translational potential [24], [27], [28]. Noteworthy, with regards to yeast glycolysis, kinetics did describe satisfactorily in vivo function [25]. Presumably, Km reflects more adequately the adaptations induced by administration of exercised plasma, thus could be considered as in vivo-like oxidative stress biomarker. This promotes the comprehension of in vivo redox status in terms of in vitro biochemistry offering the chance to solve in vitro–in vivo discrepancies. Alternatively, in vivo-like kinetics improves the predictive value of kinetic models concerning fundamental biochemical processes [29].
5. Conclusion
This article bears a commentary sentiment intending to investigate whether Vmax is a mechanistically appropriate index adequately reflecting tissue oxidative stress. According to the present as well as previous findings, Vmax could be aptly parallelized with a biomarker that describes a biochemical phenomenon in in vitro terms unlike to Km, which can be regarded as in vivo-like biomarker that more satisfactorily images the intracellular environment. Apparently, Vmax is a well established and reliable redox biomarker. Still, the negative adaptations induced by the exercised plasma administration in sedentary rats could only be reflected by Km, which seems to surpass Vmax as regards its physiological relevance and biological interpretation.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Goutianos G., Veskoukis A.S., Tzioura A., Paschalis V., Margaritelis N.V., Dipla K., Zafeiridis A., Vrabas I.S., Nikolaidis M.G., Kyparos A. Plasma from exercised rats administered to sedentary rats induces systemic and tissue inflammation. Physiol. Rep. 2016;4(24) doi: 10.14814/phy2.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conti V., Corbi G., Russomanno G., Simeon V., Ferrara N., Filippelli W., Limongelli F., Canonico R., Grasso C., Stiuso P., Dicitore A., Filippelli A. Oxidative stress effects on endothelial cells treated with different athletes' sera. Med. Sci. Sports Exerc. 2012;44(1):39–49. doi: 10.1249/MSS.0b013e318227f69c. [DOI] [PubMed] [Google Scholar]
- 3.Loffredo F.S., Steinhauser M.L., Jay S.M., Gannon J., Pancoast J.R., Yalamanchi P., Sinha M., Dall'Osso C., Khong D., Shadrach J.L., Miller C.M., Singer B.S., Stewart A., Psychogios N., Gerszten R.E., Hartigan A.J., Kim M.J., Serwold T., Wagers A.J., Lee R.T. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153(4):828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villeda S.A., Plambeck K.E., Middeldorp J., Castellano J.M., Mosher K.I., Luo J., Smith L.K., Bieri G., Lin K., Berdnik D., Wabl R., Udeochu J., Wheatley E.G., Zou B., Simmons D.A., Xie X.S., Longo F.M., Wyss-Coray T. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014;20(6):659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsimpardi L., Litterman N.K., Schein P.A., Miller C.M., Loffredo F.S., Wojtkiewicz G.R., Chen J.W., Lee R.T., Wagers A.J., Rubin L.L. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344(6184):630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veskoukis A.S., Nikolaidis M.G., Kyparos A., Kokkinos D., Nepka C., Barbanis S., Kouretas D. Effects of xanthine oxidase inhibition on oxidative stress and swimming performance in rats. Appl. Physiol. Nutr. Metab. 2008;33(6):1140–1154. doi: 10.1139/H08-102. [DOI] [PubMed] [Google Scholar]
- 7.Veskoukis A.S., Nikolaidis M.G., Kyparos A., Kouretas D. Blood reflects tissue oxidative stress depending on biomarker and tissue studied. Free Radic. Biol. Med. 2009;47(10):1371–1374. doi: 10.1016/j.freeradbiomed.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Veskoukis A.S., Kyparos A., Stagos D., Kouretas D. Differential effects of xanthine oxidase inhibition and exercise on albumin concentration in rat tissues. Appl. Physiol. Nutr. Metab. 2010;35(3):244–250. doi: 10.1139/H10-013. [DOI] [PubMed] [Google Scholar]
- 9.Veskoukis A.S., Kyparos A., Nikolaidis M.G., Stagos D., Aligiannis N., Halabalaki M., Chronis K., Goutzourelas N., Skaltsounis L., Kouretas D. The antioxidant effects of a polyphenol-rich grape pomace extract in vitro do not correspond in vivo using exercise as an oxidant stimulus. Oxid. Med. Cell Longev. 2012;2012:185867. doi: 10.1155/2012/185867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veskoukis A.S., Goutianos G., Paschalis V., Margaritelis N.V., Tzioura A., Dipla K., Zafeiridis A., Vrabas I.S., Kyparos A., Nikolaidis M.G. The rat closely mimics oxidative stress and inflammation in humans after exercise but not after exercise combined with vitamin C administration. Eur. J. Appl. Physiol. 2016;116(4):791–804. doi: 10.1007/s00421-016-3336-8. [DOI] [PubMed] [Google Scholar]
- 11.Veskoukis A.S., Kyparos A., Paschalis V., Nikolaidis M.G. Spectrophotometric assays for measuring redox biomarkers in blood. Biomarkers. 2016;21(3):208–217. doi: 10.3109/1354750X.2015.1126648. [DOI] [PubMed] [Google Scholar]
- 12.Ji L.L., Stratman F.W., Lardy H.A. Chronic exercise training alters kinetic properties of rat skeletal muscle and myocardial lactate dehydrogenase. FEBS Lett. 1986;208(2):297–300. doi: 10.1016/0014-5793(86)81036-5. [DOI] [PubMed] [Google Scholar]
- 13.Somani S.M., Husain K. Exercise training alters kinetics of antioxidant enzymes in rat tissues. Biochem Mol. Biol. Int. 1996;38(3):587–595. [PubMed] [Google Scholar]
- 14.Favero T.G., Stavrianeas S., Klug G.A. Training-induced alterations in lactate dehydrogenase reaction kinetics in rats: a re-examination. Exp. Physiol. 1999;84(5):989–998. [PubMed] [Google Scholar]
- 15.Gollnick P.D., Saltin B. Significance of skeletal muscle oxidative enzyme enhancement with endurance training. Clin. Physiol. 1982;2(1):1–12. doi: 10.1111/j.1475-097x.1982.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B., Gutteridge J. Oxford University Press; New York: 2015. Free Radicals in Biology and Medicine. [Google Scholar]
- 17.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 18.Nicholls P. Classical catalase: ancient and modern. Arch. Biochem. Biophys. 2012;525(2):95–101. doi: 10.1016/j.abb.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Veskoukis A.S., Margaritelis N.V., Kyparos A., Paschalis V., Nikolaidis M.G. Spectrophotometric assays for measuring redox biomarkers in blood and tissues: the NADPH network. Redox Rep. 2017:1–10. doi: 10.1080/13510002.2017.1392695. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winterbourn C.C., Peskin A.V. Kinetic approaches to measuring peroxiredoxin reactivity. Mol. Cells. 2016;39(1):26–30. doi: 10.14348/molcells.2016.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolaidis M.G., Jamurtas A.Z. Blood as a reactive species generator and redox status regulator during exercise. Arch. Biochem. Biophys. 2009;490(2):77–84. doi: 10.1016/j.abb.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Jackson M.J., Vasilaki A., McArdle A. Cellular mechanisms underlying oxidative stress in human exercise. Free Radic. Biol. Med. 2016;98:13–17. doi: 10.1016/j.freeradbiomed.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Cornish-Bowden A. Portland Press; London: 1995. Fundamentals of Enzyme Kinetics. [Google Scholar]
- 24.Adamczyk M., van Eunen K., Bakker B.M., Westerhoff H.V. Enzyme kinetics for systems biology when, why and how. Methods Enzymol. 2011;500:233–257. doi: 10.1016/B978-0-12-385118-5.00013-X. [DOI] [PubMed] [Google Scholar]
- 25.Teusink B., Passarge J., Reijenga C.A., Esgalhado E., van der Weijden C.C., Schepper M., Walsh M.C., Bakker B.M., van Dam K., Westerhoff H.V., Snoep J.L. Can yeast glycolysis be understood in terms of in vitro kinetics of the constituent enzymes? Testing biochemistry. Eur. J. Biochem. 2000;267(17):5313–5329. doi: 10.1046/j.1432-1327.2000.01527.x. [DOI] [PubMed] [Google Scholar]
- 26.Veskoukis A.S., Tsatsakis A.M., Kouretas D. Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress Chaperon-. 2012;17(1):11–21. doi: 10.1007/s12192-011-0293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Eunen K., Kiewiet J.A., Westerhoff H.V., Bakker B.M. Testing biochemistry revisited: how in vivo metabolism can be understood from in vitro enzyme kinetics. PLoS Comput. Biol. 2012;8(4):e1002483. doi: 10.1371/journal.pcbi.1002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkel B.S. Metabolite channeling and multi-enzyme complexes. Plant-Deriv. Nat. Prod. 2009;2:195–208. [Google Scholar]
- 29.van Eunen K., Bakker B.M. The importance and challenges of in vivo-like enzyme kinetics. Perspect. Sci. 2014;1:126–130. [Google Scholar]