Abstract
Insulin-like growth factor (IGF)-I binds to the ECM protein vitronectin (VN) through IGF binding proteins (IGFBPs) to enhance proliferation and migration of skin keratinocytes and fibroblasts. Although evidence exists for the role of individual components of the complex (IGF-I, IGFBP-3 and VN), the cellular functions stimulated by these proteins together as a complex remains un-investigated in melanoma cells. We report here that the IGF-I:IGFBP-3:VN trimeric complex stimulates a dose-dependent increase in the proliferation and migration of WM35 and Sk-MEL28 melanoma cells. In 3D Matrigel™ and hydrogel cultures, both cell lines formed primary tumor-like spheroids, which increased in size in a dose-dependent manner in response to the trimeric complex. Furthermore, we reveal IGFBP-3:VN protein complexes in malignant melanoma and squamous cell carcinoma patient tissues, where the IGFBP-3:VN complex was seen to be predominantly tumor cell-associated. Peptide antagonists designed to target the binding of IGF-I:IGFBP-3 to VN were demonstrated to inhibit IGF-I:IGFBP-3:VN-stimulated cell migration, invasion and 3D tumor cell growth of melanoma cells. Overall, this study provides new data on IGF:ECM interactions in skin malignancies and demonstrates the potential usefulness of a growth factor:ECM-disrupting strategy for abrogating tumor progression.
Introduction
The high mortality rate of melanoma is associated with the metastasis of malignant melanoma cells to critical organs of the body1. Insulin-like growth factor-I (IGF-I), amongst others, is known to enhance tumor growth and invasion2. IGF-I can act as a paracrine factor that drives malignant cell transformation through the activation of the IGF type-I receptor (IGF-IR)3. All melanocytic cells express the IGF-IR, with increased expression correlated with disease progression4,5. In addition, growth factor interactions with the extracellular matrix (ECM) play important roles in tumor biology, facilitating tumor cell attachment, proliferation and invasion6,7, and resistance against chemotherapeutic drugs8.
Proteins in the IGF system have been shown to interact with ECM proteins such as fibronectin (FN), vitronectin (VN), laminins, as well as integrins, which in turn, modulate the function of IGF-I9,10. Previous studies have demonstrated that IGF-I interacts with VN through IGFBPs to form IGF-I:IGFBP:VN trimeric (TRI) complexes11. Further, IGFBP:VN complexes have been observed in tumor biopsies from breast cancer patients, associating with the invasive front of tumor clusters and around tumor blood vessels12. This is aligned with the concept that VN is a matricellular protein that functions as a scaffold onto which growth factors, such as IGF-I, are captured, exposing cells to concentrated foci of growth factors available for receptor stimulation13. Indeed, complexes of TRI have been shown to promote enhanced cell attachment and migration, as well as protein synthesis, in human keratinocytes14 and breast cancer cell lines11,15,16.
In melanoma VN is known to stimulate cell migration and invasion17,18 through the αvβ3 integrin, the key VN-binding receptor frequently over-expressed in both vertical growth phase (VGP) and malignant melanoma tumors19. However, to our knowledge no published studies address the impact of growth factor interactions with the ECM, in particular IGF-I:VN interactions, in melanoma progression. In addition, given the involvement of TRI complexes in drug resistance15, investigating the therapeutic advantages of disrupting such IGF:VN interactions could be beneficial. To date, therapeutic options to treat aggressive melanoma or melanoma with a propensity to metastasize have not been effective. Nonetheless, immunotherapies have significantly improved overall patient survival20,21. However, efficient targeted therapeutics, which could be used in combination with such immunotherapies, are required to overcome primary resistance. We have designed peptide-antagonists that are capable of disrupting growth factor:ECM interactions, reducing activation of signaling cascades downstream of IGF-I and providing anti-tumor effects in in vitro breast cancer models12. The efficacy of such peptide antagonists on melanoma cell function is explored herein. Our proof-of-concept study demonstrates that targeting IGF:ECM molecular interactions may be a viable option for inhibiting processes facilitating the dissemination of melanoma cells.
Results
Substrate-bound IGF-I:IGFBP-3:VN complex stimulates melanoma cell proliferation and migration
IGF-I:IGFBP-3:VN trimeric (TRI) complex-stimulated melanoma cell proliferation and migration was assessed using the MTS cell proliferation and Transwell migration assays12,15. Cell proliferation was assessed after 72 hours, whereas cell migration was assessed after 15 hours.
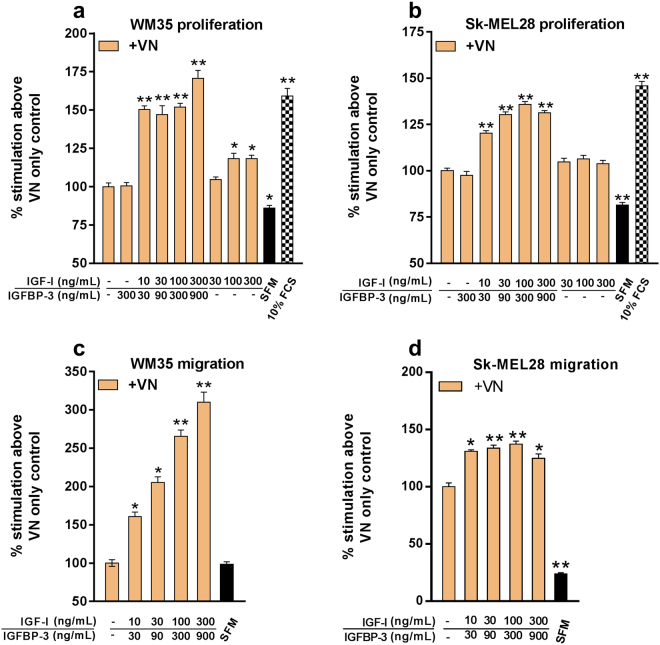
Treatment of both WM35 and Sk-MEL28 cells with substrate-bound TRI significantly enhanced (p < 0.05) cell proliferation in a dose dependent manner compared to VN alone [Fig. 1(a,b)]. The VN and the IGFBP-3 + VN treatment-induced WM35 and Sk-MEL-28 cell proliferation were non-significant to each other. Only marginal increases in proliferation were observed in the VN + IGF-I treatments (without IGFBP-3) in WM35 cells, owing to IGF-I being unable to bind to VN in the absence of IGFBP-3, hence being removed during the washing steps.
Figure 1.
Substrate-bound IGF-I:IGFBP-3:VN complex stimulates melanoma cell proliferation and migration. Proliferation: Serum-starved WM35 (a) and Sk-MEL28 (b) cells were seeded onto 96-well plates pre-coated with VN and combinations of IGF-I and IGFBP-3. Data is expressed as percentage of cells proliferating compared to VN after 72 hours. n = 2 (tested in four wells in each experiment). Migration: Serum-starved WM35 (c) and Sk-MEL28 (d) cells were seeded in the top chamber and allowed to migrate towards VN/IGF-I/IGFBP-3-coated bottom chamber. Data is expressed as percentage of cells migrated compared to VN after 15 hours. Asterisks indicate treatments with significant differences compared to the VN (*p < 0.05, **p < 0.01). SFM = serum-free growth medium. 10% FCS = growth medium containing 10% FCS. Error bars indicate SD.
In terms of cell migration, the TRI complex significantly enhanced (p < 0.05) WM35 cell migration in a dose dependent manner compared to the VN control [Fig. 1(c)]. WM35 cell migration induced by VN was low and similar to that observed in the SFM controls. Additional treatments with increasing doses of IGF-I alone (without VN) did not stimulate migration beyond responses observed with SFM [Fig. S1]).
In Sk-MEL28 cells, however, the basal level of cell migration induced by VN was more pronounced and remained significantly higher (p < 0.01) than the SFM control [Fig. 1(d)]. In spite of the high basal level of migration induced by VN, the addition of IGF-I + IGFBP-3 induced a further increase in Sk-MEL28 cell migration, albeit lesser than that induced in WM35 cells. Taken together, the TRI complex is a potent stimulator of melanoma cell proliferation and migration.
Effect of the TRI complex on melanoma cells cultured in 3D matrices
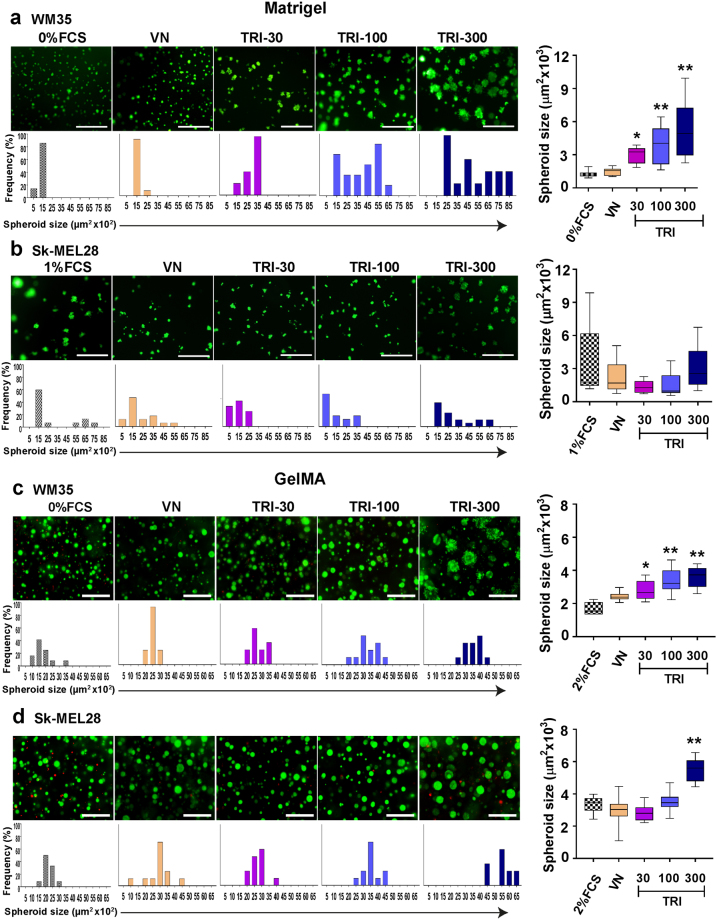
Two different 3D systems mimicking the in vivo tumor microenvironment (MatrigelTM and gelatin methacryloyl; GelMA)22,23 were used to test the effects of the TRI complex on melanoma cells. In both Matrigel™ and GelMA, the non-malignant WM35 cells formed larger spheroids in the presence of the TRI complex, as compared to VN alone [Fig. 2(a,c)]. Size quantification of Sk-MEL28 spheroids in MatrigelTM, revealed non-significant differences between the tr
Figure 2.
IGF-I:IGFBP-3:VN complex stimulates growth of melanoma spheroids in 3D cultures. WM35 and Sk-MEL28 cells were seeded onto 100% Matrigel™ (a,b) or encapsulated in GelMA hydrogel (c,d). On day 10 (Matrigel) or day 21 (GelMA), cells were stained with FDA/PI. TRI 30 = 1 ng/mL VN + 30 ng/mL IGF-I + 90 ng/mL IGFBP-3; TRI 100 = 1 ug/mL VN + 100 ng/mL IGF-I + 300 ng/mL IGFBP-3; TRI 300 = 1 μg/mL VN + 300 ng/mL IGF-I + 900 ng/mL IGFBP-3. Bar graphs represent frequency of spheroid sizes. n = 2 (tested in three wells in each experiment). Asterisks indicate treatments significantly different to VN (*p < 0.05, **p < 0.01). 0% FCS = 3D growth medium without serum. Scale bars indicate 100 μm (matrigel) and 200 μm (GelMA). Error bars indicate SD.
eatments [Fig. 2(b)]. However, Sk-MEL28 cells grown in GelMA demonstrated a significant increase in spheroid growth at the highest dose of the TRI complex [Fig. 2(d)]. Taken together, the MatrigelTM and GelMA results suggest that the TRI complex stimulates WM35 and Sk-MEL28 cell proliferation in a 3D microenvironment.
TRI complex stimulates activation of the IGF-IR and the AKT/ERK cell signaling pathways
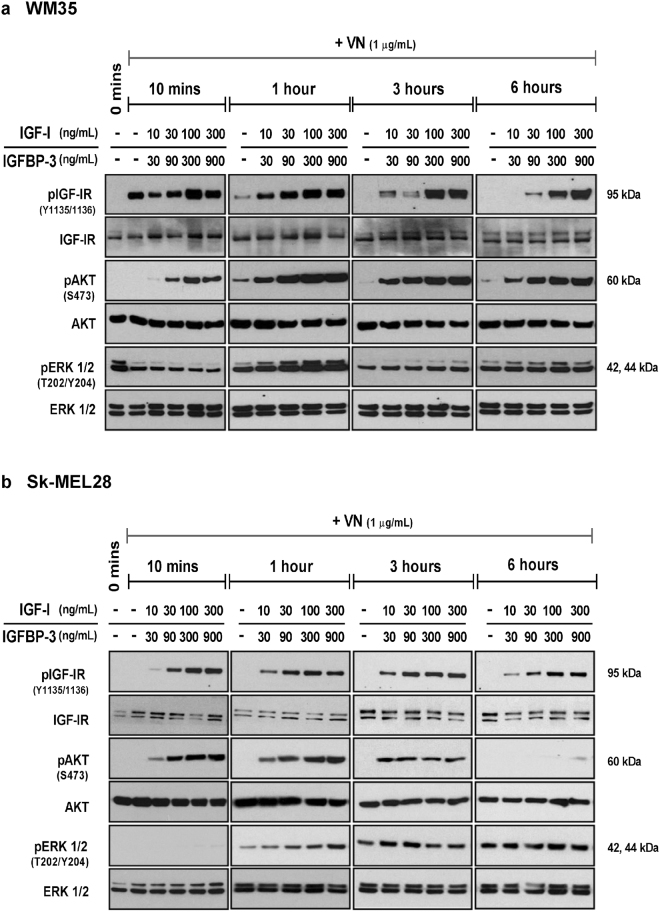
In order to understand the mechanisms underlying the TRI complex-induced proliferation and migration, the activation of IGF-IR and its key downstream signaling intermediates ERK1/2 and AKT were investigated.
In WM35 cells the TRI complex exclusively induced activation of the IGF-IR across all time points tested [Figs 3(a) and S2(a)]. The TRI complex induced a dose-dependent and sustained activation of AKT, whereas minimal activation of AKT was observed with the VN control [Fig. 3(a)]. In WM35 cells, ERK1/2 phosphorylation was detected across all time points tested, even at 0 minutes (no treatment) [Fig. 3(a)]. This indicates that ERK1/2 was activated in WM35 cells, irrespective of the stimulus. Nevertheless, the TRI complex stimulated ERK1/2 activation above VN at the 1-hour time point.
Figure 3.
Activation of the IGF-IR, AKT and ERK1/2 signaling intermediates. Total protein from WM35 (a) and Sk-MEL28 (b) cells exposed to VN, either alone or in combination with increasing concentrations of IGF-I and IGFBP-3, was collected at the indicated time points and assessed by Western blot. Blots were probed for phosphorylated IGF-IR, AKT and ERK1/2 and subsequently stripped and re-probed to determine total levels of IGF-IR, AKT and ERK1/2.
The IGF-IR and AKT activation profile in Sk-MEL28 cells was similar to that observed in WM35 cells [Figs 3(b) and S2(b)]. In contrast to WM35 cells, ERK1/2 in Sk-MEL28 cells remained un-phosphorylated at 0 minutes (no treatment control), as well as the 10 minute time point. The presence of VN within the TRI complex seemed to be a major contributor to the ERK1/2 activation observed [Fig. 3(b)], as phosphorylated ERK1/2 was detected at 1, 3 and 6 hours, not only in response to the TRI complex, but also to the VN only treatment. Nevertheless at 1 hour, TRI-stimulated activation of ERK1/2 was above VN control. We omitted VN + IGF-I treatments (without IGFBP-3) as these were demonstrated in previous publications12,15,16 to not induce activation of IGF-IR, AKT and ERK in the absence of IGFBP-3, due to minimum IGF-I remaining in the wells in the absence of IGFBP-315,16.
IGFBP-3:VN complexes are present in malignant skin tissue sections
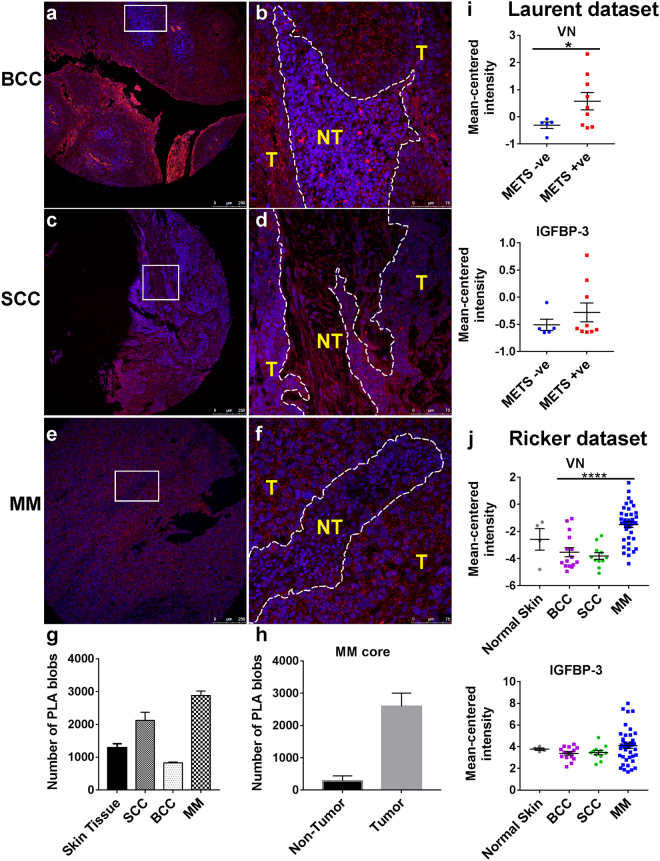
IGF-I, VN and IGFBP-3 have been individually found within the skin epidermis and dermis, however, their presence together in a complex has not been explored in normal and malignant skin tissues. Therefore, IGFBP-3:VN complexes were investigated using the in situ proximity ligation assay (PLA) in normal skin, squamous cell carcinoma (SCC), basal cell carcinoma (BCC) and malignant melanoma (MM) tissue sections. In normal skin tissue IGFBP-3:VN complexes (red PLA “blobs”) were localized in the keratinocyte-rich epidermis [Fig. S3]. The SCC [Fig. 4(c,d)] and MM [Fig. 4(e,f)] sections displayed a higher density of the IGFBP-3:VN PLA “blobs” compared to the BCC sections [Fig. 4(a,b)]. Upon quantification of the PLA signal MM had the highest density of PLA “blobs” and BCC with the least [Fig. 4(g)]. Figure S6 outlines the quantification process. Interestingly, the IGFBP-3:VN PLA “blobs” were predominantly tumor cell associated compared to the non-tumor regions of MM cores [Fig. 4(h)]. These results provide the first identification of IGFBP:VN complexes in skin cancer and melanoma tissues. We further investigated VN and IGFBP-3 expression in publically available clinical datasets24,25. Mean-centered signal intensity for IGFBP-3 and VN were calculated in metastasis positive and negative patients (Laurent dataset), and across SCC, BCC and MM (Riker dataset). While no significant changes in expression of IGFBP-3 were detected, VN expression was higher in metastatic tumors [Fig. 4(i)]. Interestingly, expression of VN was also significantly higher in tumors of MM compared to BCC and SCC [Fig. 4(j)]; supporting our PLA data. It was therefore of interest to explore therapeutic strategies focused on disrupting these IGFBP:VN complexes.
Figure 4.
Detection of IGFBP-3:VN complex in FFPE skin cancer TMAs using in situ PLA. The VN:IGFBP-3 complexes are observed as bright red dots in: basal cell carcinoma (BCC) core (a,b); squamous cell carcinoma (SCC) core (c,d); and malignant melanoma (MM) core (e,f). The IGFBP-3:VN interaction transcript was visualized with a Cy3-labeled detection oligonucleotide (red), and nuclei are in blue. Tumor regions (T) are demarcated from non-tumor (NT) regions by white dotted lines (b,d,f). Scale bars indicate 250 μm and 75 μm. The number of PLA blobs was quantified for each tumor type and normal skin (g) and between non-tumor and tumor areas of the MM core (h). Clinical datasets from oncomine were used to quantify VN and IGFBP-3 expression in metastatic and non-metastatic tumors (i) and between normal and malignant skin sections (j).
Peptide antagonist disrupts TRI complex-stimulated cell migration and tumor spheroid growth
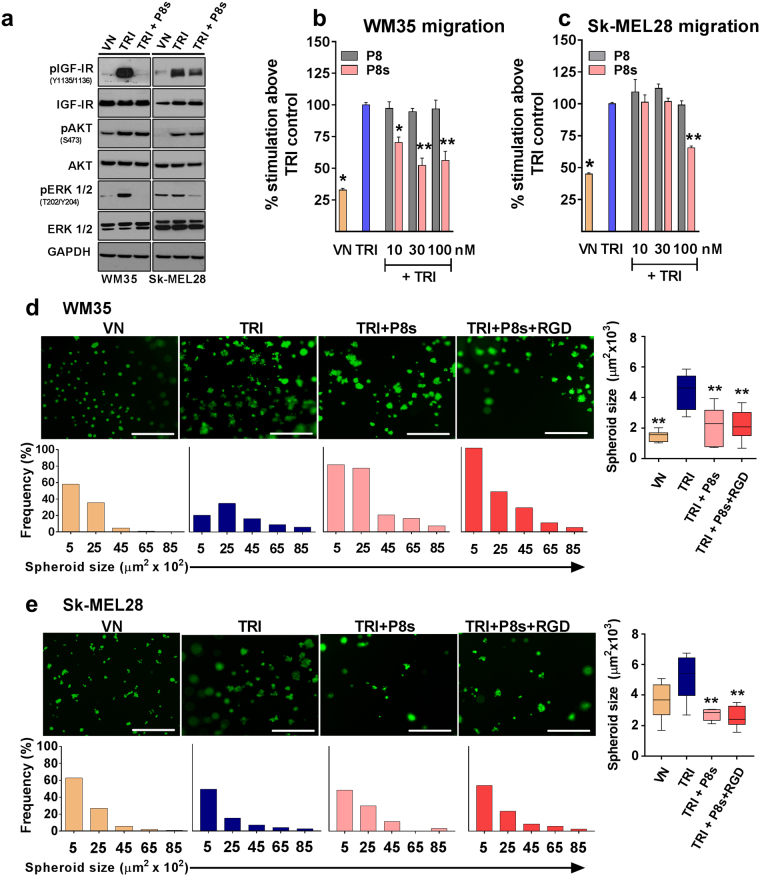
We have previously outlined the design and functional relevance of peptide antagonists (termed P8) targeting the TRI complex. By disrupting the association between IGFBP-3 and VN, the peptide prevents IGF-I from binding to VN and thereby inhibits the downstream effects of IGF-I12. Here we generated a shorter version of P8, termed P8s (sequence: RRVDTV). P8s and P8 had similar ability to disrupt the TRI complex [Fig. S4]. Addition of the P8s peptide substantially reduced the TRI complex-induced activation of the IGF-IR and ERK1/2 phosphorylation [Fig. 5(a)] in both WM35 and Sk-MEL28 cells. However, P8s did not alter TRI-induced activation of AKT in both cell lines. In summary, peptide P8s interferes with TRI complex-induced IGF-IR and ERK1/2 activation in both the WM35 and Sk-MEL28 melanoma cells.
Figure 5.
Effect of the peptide P8s on TRI-stimulated signalling, cell migration and spheroid growth. Western blot: Cell lysates were collected after 1 hour and levels of phosphorylated and total IGF-IR, AKT, and ERK1/2 were determined by Western blot analysis (a). Migration: Serum-starved WM35 (b) and Sk-MEL28 (c) cells were allowed to migrate for 15 hours. Data is expressed as percentage of cells that migrated compared to TRI control. MatrigelTM: WM35 (e) and Sk-MEL28 (f) cells were seeded onto 100% Matrigel™ and subsequently treated with VN or TRI supplemented with P8s. TRI = 1 μg/mL VN + 300 ng/mL IGF-I + 900 ng/mL IGFBP-3. n = 2 (tested in three wells in each experiment). Asterisks indicate treatments with significant differences compared to the TRI control (*p < 0.05, **p < 0.01). Scale bars indicate 100 μm. Error bars indicate SD.
Transwell migration assays were performed in the presence of increasing doses of the P8s peptide. The larger P8 peptide12 was also included for comparative purposes. In both WM35 and Sk-MEL28 cells, the larger P8 peptide had no inhibitory effect on TRI complex-stimulated migration [Fig. 5(b,c)]. In contrast, the smaller P8s peptide significantly reduced (p < 0.05) the TRI complex-induced migration of WM35 and SK-Mel28 cells [Fig. 5(b)].
The ability of the P8s peptide to inhibit tumor-spheroid growth in 3D Matrigel™ culture was further investigated. When WM35 or Sk-MEL28 cells were grown in the presence of the TRI complex + P8s peptide there was a decrease in spheroid size compared to the TRI complex control. ImageJ analysis revealed that cells treated with TRI + P8s and TRI + P8s + RGD had fewer spheroids, and they were of smaller size compared to cells grown in the presence of TRI alone [Fig. 5(d,e)]. These data suggest that P8s can competitively disrupt the TRI complex and inhibit IGF-I-induced cell migration and spheroid formation of melanoma cells. Co-treatment of P8s (IGF-I targeting) and RGD peptide (VN-targeting) did not result in any additive effect in both 2D [Fig. S7] and 3D assays [Fig. 5(d,e)].
In summary our in vitro and ex vivo data provide evidence for the role of IGF-I: matrix protein interactions in cell growth and migration. In addition, peptides that disrupt growth factor:ECM interactions appear promising for targeting growth factor-induced melanoma progression.
Discussion
Experimental evidence strongly suggests growth factor:ECM interactions play critical roles in promoting a variety of skin diseases6,26. In vivo it can regulate growth factor diffusion rates, facilitate the storage of growth factors in a readily available form, and modulate growth factor:receptor interactions27,28. We have previously demonstrated that IGF-I associates with the ECM glycoprotein VN, through IGFBP-2, -3, -4 and -5, to form IGF-I:IGFBP:VN (TRI) complexes11. Such TRI complexes were found to enhance cellular functions in human keratinocytes14,29, and normal as well as malignant breast cells12,15,16. These effects were mediated through VN and IGF-I co-operation where IGFBP-bound IGFs enhance signalling by activating integrin/IGF-IR cross-talk, followed by the recruitment of the PI3-K/AKT and the ERK/MAPK pathway15,16.
Our study investigated the consequence of IGF-I:IGFBP:VN interactions on melanoma cell function and provides insights into a new therapeutic approach that targets the formation of such multiprotein complexes. In vivo, sequestered delivery from the matrix is known to occur for many growth factors, including IGF-I. There is significant evidence that growth factors and ECM proteins interact29, facilitating local storage of growth factors30 in a “bioavailable” form. This localizes ligands in close proximity to their cognate cell surface receptors, thereby prolonging the activation of downstream signaling cascades31, and in turn, enhancing growth factor-induced biological functions. In melanoma all three components of the IGF-I:IGFBP:VN complex are independently shown to be associated with different stages of the disease32–34, wherein IGF-I:IGFBP-3 would more likely be associated with ECM proteins like VN and not present in “solution”. Hence, to mimic this in vivo situation, we adopted a substrate substrate-bound approach, which better demonstrates the influence of growth factors and ECM interactions on tumor cell properties. Using substrate-bound 2D assays, the TRI complex was demonstrated to be a potent stimulator of proliferation and migration in both WM35 and Sk-MEL28 melanoma cells [Fig. 1]. Since the dimeric IGFBP-3:VN complex stimulated similar responses to that found with VN alone, it suggests IGF-I was the predominant effector of TRI complex-induced cell proliferation. Unlike early-stage melanoma, late stage melanoma has been shown to be largely growth factor independent34. Of note, VN ligation to its key integrin receptors, particularly αvβ3, which is highly expressed in Sk-MEL28 cells31, has been shown to activate IGF-IR signaling independent of IGF-I10,35. This may explain why the metastatic Sk-MEL28 cells were only weakly responsive to the TRI complex compared to early-stage WM35 cells.
In vitro 3D models, like Matrigel and GelMA22,36, better recapitulate the in vivo tumor microenvironment, wherein their ECM can facilitate growth factor:ECM protein interactions37,38. Both the Sk-Mel28 and WM35 melanoma cells formed metabolically active spheroids resembling primary tumors [Fig. 2]. Importantly, these long-term assays were conducted using minimal levels of serum (0–2%), providing evidence that the TRI complex is sufficient to support the growth of melanoma cells over prolonged periods. Possibly due to the sustained activation induced by the TRI complex of the PI3K/AKT and ERK1/2 pathways [Figs 3 and S2], known to be activated in response to IGF-I39–41 and deregulated in melanoma42.
While the individual components of the complex have been studied in tissues, the clinical relevance of the TRI complex has not yet been investigated. Indeed, overexpression of IGFBP-3 is found in advanced melanoma tumors32,39. Likewise, serum VN was significantly elevated in patients with melanoma33,43, with tumor growth and metastasis shown to also be dependent on the presence of VN in the ECM44,45. When assessed for co-expression of VN and IGFBP-3, we found IGFBP-3:VN interactions predominantly in malignant melanoma and SCC patient tumors, and mainly tumor-cell associated [Fig. 4]. However, when assessed individually using publically available gene expression data only VN expression correlated with our in situ PLA data [Fig. 4], which suggests that evaluating growth factor:matrix interactions, as opposed to studying them in isolation, may add value to their prognostic and predictive potential. Taken together, growth factor:ECM interactions may play an important role in the progression of cutaneous neoplasia, hence should be considered in the design and the development of growth factor/ECM targeting therapeutics.
Interestingly, the resistance observed to growth factor receptor-targeting therapies may be attributed, at least in part, to growth factor:ECM interactions. For instance, IGF-IR inhibitors such as AVE1642 and R1507 have only been sub-optimal in late-stage clinical trials46–48. In such cases growth factor:ECM interactions may trigger additional compensatory cell signaling pathways, including integrin-mediated signaling that may dampen the therapeutic response8,49. Thus, there is a need for alternative therapeutic strategies that take into account the role of the ECM in facilitating growth factor responses.
We recently reported the development of peptide-based antagonists capable of disrupting the TRI complex and its downstream effects in IGF-IR expressing breast cancer cells12. Interestingly, both WM35 and Sk-Mel28 melanoma cells had a reduced capacity to proliferate and migrate in the presence of the P8s peptide compared to the TRI complex [Figs 5(b,c), S7]. In spite of the IGF-I remaining in the wells during the entire duration of the 3D Matrigel experiment, the peptide was able to dampen IGF-I responses, potentially due to the weak tethering of IGF-I to ECM components (including VN) in the presence of P8s, leading to less IGF-I being available for IGF-IR stimulation. Interestingly, P8 (the longer version of P8s) was less effective compared to P8s [Fig. 5], despite its similar ability to dislodge IGF-I:IGFBP-3 from VN [Fig. S4]. This was also observed in a previous study with another peptide P7, which efficiently disrupted the complex but offered minimal functional effects12. Indeed, the impact of the IGFBP-3-binding peptides on IGF-I-independent effects will need to be investigated to fully understand the off-target effects of these peptides. Nevertheless, it is known that residues within P8s bind to IGFBP-3 at sites distinct to IGF-I-binding, thereby maintaining normal IGFBP:IGF-I–binding interactions12. Currently, P8s is still being optimized for pre-clinical investigations.
Newer approaches to overcome drug resistance of melanoma cells to MAPK/MEK inhibitors are being investigated. A number of co-targeting approaches have been used50,51, however, achieving robust abrogation of this cascade has remained a formidable challenge52. We demonstrate here the use of peptide antagonists for inhibition of IGF-I induced MAPK/ERK signaling in BRAF-mutant Sk-MEL28 and WM35 melanoma cells [Figs 5(a) and S5]. However, AKT phosphorylation remained unaffected upon treatment with the peptide. This suggests that AKT compensation may, in part, undermine the effects of the peptides. This is in agreement with study reporting AKT activation upon MAPK/ERK inhibition, leading to de novo resistance in BRAF-mutant Sk-MEL28 melanoma cells exposed to epidermal growth factor (EGF)53. Recently, Barbara Herkert et al. (2016) reported that combinations of AKT + IGF-IR, and MAPK pathway inhibitors is required to achieve maximal response in BRAF-mutant melanoma patients. Together with the findings demonstrated in this study, we propose that IGFBP:VN targeting peptides are a viable therapeutic option and in combination with AKT/PI3K inhibitors could be employed for the treatment of skin malignancies, namely SCC and melanoma, in particular for patients with BRAF-mutant tumors and/or tumors overexpressing components of the IGF:VN axis.
Materials and Methods
Proteins and reagents
Plasma purified human VN was from Promega (NSW, Australia). Human recombinant IGF-I and IGFBP-3 were from GroPep (SA, Australia). Bovine Serum Albumin (BSA) Fraction-V was from Life Technologies (NSW, Australia). Monoclonal antibodies for phospho-IGF-I Receptor (Tyr1135/1136) (19H7), phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204) (E10), phospho-AKT (Ser473) (D9E) and GAPDH (14C10) were from Cell Signaling Technology (MA, USA). Rabbit anti-IGF-IRβ (C-20) was from Santa Cruz Biotechnology (CA, USA). Mouse anti-VN was from Promega (WI, USA). Rabbit anti-IGFBP-3 antibody was obtained from Robert Baxter (Kolling Institute of Medical Research, Australia). Growth factor-reduced (GFR) phenol red-free Matrigel™ was from BD Biosciences (NJ, USA). The peptides P8 (RVNLRTRRVDTVDPPYPRS-NH2), P8s (RRVDTV-NH2) and RGD (H-CRGDSCG-OH) were synthesized by Mimotopes (VIC, Australia).
Cell culture
WM35, a radial growth phase cell line, was provided Dr. Nikolas Haass and the malignant SK-Mel28 cell line was provided by Dr. Brian Gabrielli (both University of Queensland, Australia). WM35 cells were maintained in MCDB 153 medium (Sigma-Aldrich) supplemented with 4% FCS, 20% Leibovitz’s medium (Life Technologies), bovine insulin (5 µL/mL), CaCl2 (1.68 mM), sodium bicarbonate (7.5% w/v). SK-Mel-28 cells were maintained in RPMI 1640 medium supplemented with 2 mM L-glutamine, 25 mM HEPES, 10% FCS. Both media contained Penicillin (50 U/mL) + Streptomycin (50 µg/mL) and 0.01% Gentamycin (Life Technologies). The identity of both these cell lines was authenticated using short tandem repeat (STR) profiling by CellBank Australia and tested for mycoplasma using the MycoAlertTM mycoplasma detection kit (Lonza).
Pre-binding of proteins
The pre-binding of proteins to culture ware followed previously published procedures15,16. Briefly, culture plates were coated with 1 µg/mL VN in serum-free medium (SFM) and incubated for 3 hours at 37 °C. Unbound VN was aspirated and wells were blocked with 0.5% BSA/SFM. Subsequently, IGF-I and IGFBP-3 suspended in 0.05% BSA/SFM at various concentrations was added to the plates and incubated overnight at 4 °C. For assays using the P8s and P8 peptides, the plates were coated with VN, IGF-I and IGFBP-3 suspended in 0.05% BSA/SFM, with or without P8s/P8 and incubated at 37 °C for 3 hours. In all cases, unbound growth factors were removed with washing prior to seeding the cells. The lower chambers and under-side of 8 µm-pore Transwells were also coated following the same procedure. In all experiments containing peptides, peptide + IGF-I alone controls were not included as these peptides do not bind IGF-I but are designed to bind to VN-binding sites on IGFBP-3.
MTS cell proliferation assay
The cells were serum-starved for 4 hours and seeded at a density of 5 × 103 cells/well onto 96-well plates pre-bound with the respective protein treatment combinations. The cells were incubated for 72 hours and cellular proliferation was measured using the CellTitre 96® AQueous One Solution Cell Proliferation Assay (Promega). The absorbance was recorded at 490 nm (Benchmark Plus, Bio-Rad).
Transwell® migration assays
Cell migration was assessed as previously described13,15,16. The cells were serum-starved for 4 hours and seeded at a density of 6 × 104 cells/well in the upper chambers of pre-bound Transwells and incubated for 15 hours. Cells that had migrated to the lower surface of the membrane were fixed in 3.7% para-formaldehyde and stained with 0.01% crystal violet. The relative number of migrated cells was assessed by extracting the crystal violet stain in 10 mM acetic acid. The optical density of the extracted stain was determined at 595 nm (Benchmark Plus, BioRad.
Western blot analysis for protein expression
Cells were serum-starved for 4 hours and 8 × 105 cells/well were seeded in 6-well plates pre-bound with the respective protein treatments. Cells were lysed using radioimmunoprecipitation assay (RIPA) protein lysis buffer containing phosphatase and protease inhibitors (Pierce, Thermo Fisher Scientific). Twenty microgram total protein was separated on SDS-PAGE gels (Life Technologies) under reducing conditions prior to being transferred onto nitrocellulose membranes (Life Technologies). The membranes were probed with antibodies and visualized using the enhanced chemiluminescence (ECL) system (Amersham Biosciences).
3D Matrigel™ assay
Optical-bottom 96-well plates were coated with 100% growth-factor-reduced-Matrigel™. Using the 3D On-Top method54, 1 × 103 cells/well cells were suspended in 3D growth medium (2.5% Matrigel™ +/− 1% FCS). The cells were incubated for two days to allow spheroid formation, following which 3D growth medium containing VN, IGF-I and IGFBP-3 +/− peptides P8s or RGD were added to the wells and replaced every two days for up to ten days. Live cells were stained using fluorescein diacetate (FDA) dye and imaged using fluorescence microscopy (Nikon Eclipse TS) and analyzed using ImageJ (Supplementary Methods).
3D GelMA assay
GelMA was synthesized following previously published protocols22. To encapsulate cells, GelMA was dissolved in PBS containing 0.05% (w/v) 1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propanone (Irgacure 2959; BASF; Ludwigshafen, Germany) at 37 °C. Sk-MEL28 and WM35 cells were suspended in the hydrogel precursor solution at a density of 2 × 105 cells/mL (6,400 cells per gel), transferred to a custom casting mold to produce hydrogel constructs with the dimension of 4 mm × 4 mm × 2 mm, and then photocrosslinked by exposure to 365 nm light at an approximate intensity of 2.6 mW/cm2 in a CL-1000 crosslinker (UVP; CA, USA) for 15 min. The hydrogel constructs were cultured in stripped green’s medium with 2% FCS containing VN, IGF-I and IGFBP-3 +/− P8s peptide at 37 °C, 5% CO2 for the duration of the experiment.
Proximity ligation assay
Human tissue microarrays (TMA) incorporating 10 cases of skin tumor and 2 non-neoplastic tissues, in duplicates, were purchased from US Biomax Inc. (Cat# SK242). The TMA information is outlined in Figure S8. The TMA slides were deparaffinised and subjected to antigen retrieval for 20 mins at 98 °C in 10 mM Tris-EDTA (pH 9). The IGFBP-3:VN interactions were detected with in situ PLA, performed as published12,55 using anti-VN (1:200) and anti-IGFBP-3 (1:200) antibodies. The tissue sections were incubated with secondary antibodies conjugated with oligonucleotide probes (Olink Bioscience). The IGFBP-3:VN transcript was detected using Cy3-labelled detection probes. The PLA signals were analyzed with confocal microscopy and quantified using ImageJ (Supplementary Methods).
Data availability
The Laurent and Ricker datasets analysed during the current study are available in the Oncomine repository, www.oncomine.com. All other data generated in this study including supplementary information are included in this published article.
Electronic supplementary material
Acknowledgements
The authors wish to thank Leo de Boer (Cell Imaging Facility, Institute of Health and Biomedical Innovation) for help with microscopy. This work was funded by Early Career Researcher Fellowship (Queensland University of Technology) received by A.S.K. and A.S. and the John Williams Cancer Fellowship received by A.S.K., as well as the Tissue Repair and Regeneration Program of the Institute of Health and Biomedical Innovation.
Author Contributions
B.M., A.S., L.M., J.M. and A.K. wrote the main manuscript text. B.M. and A.S. performed the experiments and interpreted the data. A.K. supervised the study. J.M. and C.M. performed and analysed the 3D-GelMA data. L.M. and A.K. performed and generated the P.L.A. data. B.G.H., A.Z., Z.U. and A.K. conceptualized the experiments and helped to interpret the data. All the authors have read and approved the manuscript.
Competing Interests
The Queensland University of Technology has filed a patent related to the peptides outlined in this study (Patent# WO 2014078896 A1) and this has been acquired by Factor Therapeutics Ltd. A.S.K., Z.U. and B.G.H. are named inventors on this patent. Z.U. has purchased stock in Factor Therapeutics. The other authors have nothing to declare.
Footnotes
Berline Murekatete and Ali Shokoohmand contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-19073-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sandru A, Voinea S, Panaitescu E, Blidaru A. Survival rates of patients with metastatic malignant melanoma. Journal of medicine and life. 2014;7:572. [PMC free article] [PubMed] [Google Scholar]
- 2.Lázár-Molnár E, Hegyesi H, Tóth S, Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine. 2000;12:547–554. doi: 10.1006/cyto.1999.0614. [DOI] [PubMed] [Google Scholar]
- 3.Satyamoorthy K, Li G, Vaidya B, Kalabis J, Herlyn M. Insulin-like growth factor-I-induced migration of melanoma cells is mediated by interleukin-8 induction. Cell growth and differentiation. 2002;13:87–93. [PubMed] [Google Scholar]
- 4.Lee JT, Brafford P, Herlyn M. Unraveling the mysteries of IGF-1 signaling in melanoma. Journal of Investigative Dermatology. 2008;128:1358–1360. doi: 10.1038/jid.2008.124. [DOI] [PubMed] [Google Scholar]
- 5.Kanter-Lewensohn L, Dricu A, Girnita L, Wejde J, Larsson O. Expression of Insulin-Like Growth Factor-1 Receptor (IGF-1R) and p27Kip1 in Melanocyte Tumors: A Potential Regulatory Role of IGF-1 Pathway in Distribution of p27Kip1 between Different Cyclins. Growth Factors. 2000;17:193–202. doi: 10.3109/08977190009001068. [DOI] [PubMed] [Google Scholar]
- 6.Kim S-H, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. Journal of Endocrinology. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 7.Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. The FASEB Journal. 1997;11:51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 8.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nature Reviews Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 9.Beattie J, McIntosh L, Van der Walle CF. Cross‐talk between the insulin‐like growth factor (IGF) axis and membrane integrins to regulate cell physiology. Journal of cellular physiology. 2010;224:605–611. doi: 10.1002/jcp.22183. [DOI] [PubMed] [Google Scholar]
- 10.Clemmons DR, Maile LA. Minireview: Integral Membrane Proteins that Function Coordinately with the Insulin-Like Growth Factor I Receptor to Regulate Intracellular Signaling. Endocrinology. 2003;144:1664–1670. doi: 10.1210/en.2002-221102. [DOI] [PubMed] [Google Scholar]
- 11.Kricker JA, Towne CL, Firth SM, Herington AC, Upton Z. Structural and functional evidence for the interaction of insulin-like growth factors (IGFs) and IGF binding proteins with vitronectin. Endocrinology. 2003;144:2807–2815. doi: 10.1210/en.2002-221086. [DOI] [PubMed] [Google Scholar]
- 12.Kashyap AS, et al. Antagonists of IGF: vitronectin interactions inhibit IGF-I–induced breast cancer cell functions. Molecular cancer therapeutics. 2016;15:1602–1613. doi: 10.1158/1535-7163.MCT-15-0907. [DOI] [PubMed] [Google Scholar]
- 13.Leavesley DI, et al. Vitronectin—Master controller or micromanager? IUBMB life. 2013;65:807–818. doi: 10.1002/iub.1203. [DOI] [PubMed] [Google Scholar]
- 14.Hyde C, Hollier B, Anderson A, Harkin D, Upton Z. Insulin-like growth factors (IGF) and IGF-binding proteins bound to vitronectin enhance keratinocyte protein synthesis and migration. Journal of Investigative Dermatology. 2004;122:1198–1206. doi: 10.1111/j.0022-202X.2004.22527.x. [DOI] [PubMed] [Google Scholar]
- 15.Kashyap AS, et al. Insulin-like growth factor-I: vitronectin complex-induced changes in gene expression effect breast cell survival and migration. Endocrinology. 2011;152:1388–1401. doi: 10.1210/en.2010-0897. [DOI] [PubMed] [Google Scholar]
- 16.Hollier BG, Kricker JA, Van Lonkhuyzen DR, Leavesley DI, Upton Z. Substrate-bound insulin-like growth factor (IGF)-I-IGF binding protein-vitronectin-stimulated breast cell migration is enhanced by coactivation of the phosphatidylinositide 3-kinase/AKT pathway by αv-integrins and the IGF-I receptor. Endocrinology. 2008;149:1075–1090. doi: 10.1210/en.2007-0740. [DOI] [PubMed] [Google Scholar]
- 17.Aznavoorian S, Stracke ML, Parsons J, McClanahan J, Liotta LA. Integrin Mediates Chemotactic and Haptotactic Motility in Human Melanoma Cells through Different Signaling Pathways. Journal of Biological Chemistry. 1996;271:3247–3254. doi: 10.1074/jbc.271.6.3247. [DOI] [PubMed] [Google Scholar]
- 18.Stahl A, Mueller BM. Melanoma cell migration on vitronectin: regulation by components of the plasminogen activation system. International journal of cancer. 1997;71:116–122. doi: 10.1002/(SICI)1097-0215(19970328)71:1<116::AID-IJC19>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Kuphal S, Bauer R, Bosserhoff A-K. Integrin signaling in malignant melanoma. Cancer and Metastasis Reviews. 2005;24:195–222. doi: 10.1007/s10555-005-1572-1. [DOI] [PubMed] [Google Scholar]
- 20.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nature reviews. Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smalley KS, Eroglu Z, Sondak VK. Combination Therapies for Melanoma: A New Standard of Care? American journal of clinical dermatology. 2016;17:99–105. doi: 10.1007/s40257-016-0174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loessner D, et al. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nature protocols. 2016;11:727–746. doi: 10.1038/nprot.2016.037. [DOI] [PubMed] [Google Scholar]
- 23.Beaumont, K. A., Mohana-Kumaran, N. & Haass, N. K. In Healthcare. 27–46 (Multidisciplinary Digital Publishing Institute).
- 24.Riker AI, et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Medical Genomics. 2008;1:13–13. doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurent C, et al. High PTP4A3 phosphatase expression correlates with metastatic risk in uveal melanoma patients. Cancer Res. 2011;71:666–674. doi: 10.1158/0008-5472.CAN-10-0605. [DOI] [PubMed] [Google Scholar]
- 26.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound repair and regeneration. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 27.Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Current opinion in cell biology. 2012;24:645–651. doi: 10.1016/j.ceb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollier B, Harkin DG, Leavesley D, Upton Z. Responses of keratinocytes to substrate-bound vitronectin: growth factor complexes. Experimental cell research. 2005;305:221–232. doi: 10.1016/j.yexcr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Beattie J, McIntosh L, van der Walle CF. Cross-talk between the insulin-like growth factor (IGF) axis and membrane integrins to regulate cell physiology. J Cell Physiol. 2010;224:605–611. doi: 10.1002/jcp.22183. [DOI] [PubMed] [Google Scholar]
- 31.Klein A, Capitanio JS, Maria DA, Ruiz IRG. Gene expression in SK-Mel-28 human melanoma cells treated with the snake venom jararhagin. Toxicon. 2011;57:1–8. doi: 10.1016/j.toxicon.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Xi Y, et al. Association of insulin-like growth factor binding protein-3 expression with melanoma progression. Molecular cancer therapeutics. 2006;5:3078–3084. doi: 10.1158/1535-7163.MCT-06-0424. [DOI] [PubMed] [Google Scholar]
- 33.Ortega‐Martínez I, et al. Vitronectin and dermcidin serum levels predict the metastatic progression of AJCC I–II early‐stage melanoma. International journal of cancer. 2016;139:1598–1607. doi: 10.1002/ijc.30202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satyamoorthy K, Li G, Vaidya B, Patel D, Herlyn M. Insulin-like growth factor-1 induces survival and growth of biologically early melanoma cells through both the mitogen-activated protein kinase and β-catenin pathways. Cancer research. 2001;61:7318–7324. [PubMed] [Google Scholar]
- 35.Clemmons DR, Maile LA, Ling Y, Yarber J, Busby WH. Role of the integrin αVβ3 in mediating increased smooth muscle cell responsiveness to IGF-I in response to hyperglycemic stress. Growth Hormone & IGF Research. 2007;17:265–270. doi: 10.1016/j.ghir.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nature methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Øy GF, et al. Biological effects induced by insulin‐like growth factor binding protein 3 (IGFBP‐3) in malignant melanoma. International journal of cancer. 2010;126:350–361. doi: 10.1002/ijc.24727. [DOI] [PubMed] [Google Scholar]
- 40.Davies MA. The role of the PI3K-AKT pathway in melanoma. The Cancer Journal. 2012;18:142–147. doi: 10.1097/PPO.0b013e31824d448c. [DOI] [PubMed] [Google Scholar]
- 41.Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annual Review of Pathology: Mechanisms of Disease. 2014;9:239–271. doi: 10.1146/annurev-pathol-012513-104658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier F, et al. Combined targeting of MAPK and AKT signalling pathways is a promising strategy for melanoma treatment. British Journal of Dermatology. 2007;156:1204–1213. doi: 10.1111/j.1365-2133.2007.07821.x. [DOI] [PubMed] [Google Scholar]
- 43.Tas F, Karabulut S, Bilgin E, Tastekin D, Duranyildiz D. Clinical significance of serum fibronectin and vitronectin levels in melanoma patients. Melanoma research. 2014;24:475–479. doi: 10.1097/CMR.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 44.Janik ME, Przybylo M, Pochec E, Pokrywka M, Litynska A. Effect of α3β1 and αvβ3 integrin glycosylation on interaction of melanoma cells with vitronectin. Acta Biochimica Polonica. 2010;57:55. [PubMed] [Google Scholar]
- 45.Mesnard, L. et al. In Journal of Investigative Dermatology. S140–S140.
- 46.Gombos A, Metzger-Filho O, Dal Lago L, Awada-Hussein A. Clinical development of insulin-like growth factor receptor—1 (IGF-1R) inhibitors: At the crossroad? Investigational new drugs. 2012;30:2433–2442. doi: 10.1007/s10637-012-9811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pappo AS, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. Journal of Clinical Oncology. 2011;29:4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macaulay, V. et al. Phase I study of humanized monoclonal antibody AVE1642 directed against the type 1 insulin-like growth factor receptor (IGF-1R), administered in combination with anticancer therapies to patients with advanced solid tumors. Annals of oncology, mds511 (2012). [DOI] [PMC free article] [PubMed]
- 49.Smalley, K. S. & McArthur, G. A. In Seminars in oncology. 204–214 (Elsevier). [DOI] [PMC free article] [PubMed]
- 50.Cao, J. et al. Targeting of the MAPK and AKT pathways in conjunctival melanoma shows potential synergy. Oncotarget5 (2016). [DOI] [PMC free article] [PubMed]
- 51.Herkert B, et al. Maximizing the efficacy of MAPK-targeted treatment in PTENLOF/BRAFMUT melanoma through PI3K and IGF1R inhibition. Cancer research. 2016;76:390–402. doi: 10.1158/0008-5472.CAN-14-3358. [DOI] [PubMed] [Google Scholar]
- 52.Solit DB, Rosen N. Towards a unified model of RAF inhibitor resistance. Cancer discovery. 2014;4:27–30. doi: 10.1158/2159-8290.CD-13-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Won J-K, et al. The crossregulation between ERK and PI3K signaling pathways determines the tumoricidal efficacy of MEK inhibitor. Journal of molecular cell biology. 2012;4:153–163. doi: 10.1093/jmcb/mjs021. [DOI] [PubMed] [Google Scholar]
- 54.Weigelt, B. & Bissell, M. J. In Seminars in cancer biology. 311–321 (Elsevier). [DOI] [PMC free article] [PubMed]
- 55.Söderberg O, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nature methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Laurent and Ricker datasets analysed during the current study are available in the Oncomine repository, www.oncomine.com. All other data generated in this study including supplementary information are included in this published article.