Abstract
Biogenic volatile organic compounds (BVOCs) affect atmospheric chemistry, climate and regional air quality in terrestrial and marine atmospheres. Although isoprene is a major BVOC produced in vascular plants, and marine phototrophs release dimethyl sulfide (DMS), lakes have been widely ignored for their production. Here we demonstrate that oligotrophic Lake Constance, a model for north temperate deep lakes, emits both volatiles to the atmosphere. Depth profiles indicated that highest concentrations of isoprene and DMS were associated with the chlorophyll maximum, suggesting that their production is closely linked to phototrophic processes. Significant correlations of the concentration patterns with taxon-specific fluorescence data, and measurements from algal cultures confirmed the phototrophic production of isoprene and DMS. Diurnal fluctuations in lake isoprene suggested an unrecognised physiological role in environmental acclimation similar to the antioxidant function of isoprene that has been suggested for marine biota. Flux estimations demonstrated that lakes are a currently undocumented source of DMS and isoprene to the atmosphere. Lakes may be of increasing importance for their contribution of isoprene and DMS to the atmosphere in the arctic zone where lake area coverage is high but terrestrial sources of BVOCs are small.
Introduction
Surface-to-atmosphere emissions of reactive BVOCs control the atmosphere’s oxidation capacity and secondary aerosol formation. These aerosols contribute considerably to the formation of particles affecting biogeochemical cycling, atmospheric processes, climate, and regional air quality in terrestrial1 and marine atmospheres2. Although lakes are recognised as hot-spots for CO2 exchange and the release of methane3, freshwater biomes have received little attention for their total contribution to the atmospheric BVOC burden. Here, we demonstrate a flux of isoprene (2-methyl-1,3-butadiene; C5H8) and DMS ((CH3)2S) out of Lake Constance and suggest that oligotrophic lakes can be a source of these BVOCs to the overlying atmosphere. Our findings are of particular importance for our understanding of BVOC emissions at night and suggest that lakes may sustain a substantial flux to the atmosphere at high latitudes where lake area density is exceptionally high but terrestrial emission very low.
Isoprene comprises about a third of all BVOCs in the terrestrial atmosphere and is recognised for its function in the physiological acclimation in vascular plants4–6. In contrast, this gas is unreported in lakes despite the demonstration that heterotrophic bacteria7, marine cyanobacteria, phytoplankton and seaweeds also produce isoprene8. Two biosynthesis pathways exist for isoprene that result in isopentenyl diphosphate, the universal isoprenoid precursor. They are named after their key intermediate metabolites, mevalonate (MVA) and 2-C-methyl-d-erythritol 4-phosphate (MEP). Under low light heterotrophic growth conditions, several freshwater eukaryotic microalgae and a cyanobacterium differentially expressed one or both pathways9 but the production of isoprene by freshwater biota is undocumented and not represented in Earth system models.
Marine environments are a predominant source of DMS10 and various physiological and ecological functions have been attributed to the production of this BVOC from its cellular precursor dimethylsulfoniopropionate (DMSP) in algae and bacteria11. These include cryoprotection, an overflow mechanism under unbalanced algal growth, as grazing deterrents, an antioxidant system that quenches reactive oxygen species10 or as chemical cues12. Molecular genetic evidence for various DMSP catabolic pathways that produce DMS exists for bacteria, fungi and algae13,14. DMS is also produced by trees and soils15 and in freshwater systems16,17. However, eutrophic lakes are suggested to be a minor source of DMS-sulfur to the atmosphere during periods of stratification since increased concentrations are associated with the anoxic hypolimnion16, likely as a result of microbial biomethylation of hydrogen sulfide17.
Concentrations and production rates of isoprene and DMS have previously been reported for estuarine and marine environments18–23 and such information has facilitated the estimation of the source strength of these climate-active BVOCs to the atmosphere24,25. A transect study from the North to South Atlantic21 indicated that isoprene and DMS do not correlate with concentrations of chlorophyll-a (chl-a) but positively correlate with the concentration of 19′-hexanoyloxyfucoxanthin, an accessory pigment occurring in the primarily marine haptophyte and some dinoflagellate algae, in areas characterised by low nitrogen concentrations. Limited information exists on the production and flux of DMS from lakes and freshwater sediments26,27 but similar data for isoprene is lacking. This shortage of ecosystem observations precludes the accurate estimation of global gas fluxes15.
BVOCs have important roles for the physiology of producers and consumers in aquatic food webs12,28. Isoprene and DMS are produced in response to oxidative stress from, for example, high light and temperature conditions in terrestrial plants (isoprene:29), phytoplankton (isoprene:30; DMS:31) and air exposure in corals (DMS:32). Further evidence suggests that the strong relationship between isoprene and photoprotective carotenoids in marine phytoplankton could relate to a photoprotective function33 and that marine phytoplankton use DMS and/or isoprene to mitigate ROS-induced metabolic damage under sublethal environmental stresses6. Hence, it is possible that the production of these BVOCs also assists with physiological acclimation to environmental conditions in freshwater phytoplankton. To date this has been largely unexplored.
This study investigated the concentrations of isoprene and DMS in Lake Constance (see Supplementary Fig. S1), the third largest body of freshwater in central Europe and a well-studied model for north temperate deep lakes. We quantified DMS and isoprene production in 10 species of freshwater algae from four different taxonomic classes using gas chromatography with flame-ionisation detection. Particular focus was on the vertical distribution of isoprene and DMS in depth profiles, their concentrations in surface samples over a diurnal cycle and the flux of these gases between Lake Constance and its overlying atmosphere.
Results
Depth Profiles
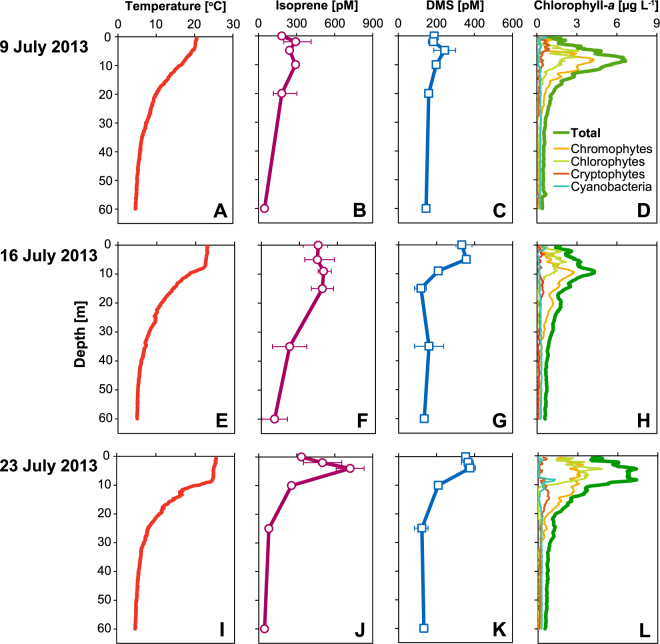
Our weekly depth profiles showed a typical distribution of temperature and phytoplankton pigments in stratified lakes during summer with increasing stratification from 9–23 July 2013. We observed relatively high concentrations of isoprene (183 to 722 pM) and DMS (185 to 377 pM) associated with phototrophic processes in the epilimnion, which progressively deepened from approximately 4.5 to 8.3 m (Fig. 1). Lowest concentrations were generally found at the deepest sampling depth of 60 m (isoprene: 45 pM; DMS: 133 pM). Data from an optical profiler provided information on the vertical distribution of chl-a and fluorescence fingerprints were used to estimate the relative contribution of specific taxonomic groups to total chl-a. Total and taxon-specific chl-a (Fig. 1D,H,L) showed maxima at 8.7 m on 9 July (6.6 µg L−1), 9.0 m on 16 July (4.3 µg L−1) and 4.6 and 8.3 m on 23 July (both 7.4 µg L−1). The majority of biomass from the surface to the chl-a maxima had optical characteristics of chromophytes (including diatoms, dinoflagellates and chrysophytes: 36 to 43% of total chl-a) and chlorophytes (31 to 50%). Linear regression analysis indicated a significant positive correlation between BVOC and total chl-a concentrations (Pearson correlation, P ≤ 0.004, n = 18; for details see Supplementary Table S1). The taxon-specific data on chl-a concentration indicated significant positive correlations between isoprene and DMS with chlorophytes (Pearson correlation, P ≤ 0.003, n = 18), and between isoprene with chromophytes (Pearson correlation, P = 0.004, n = 18). Cryptophyte- and cyanobacteria-derived chl-a abundance was relatively low (2 to 17% of total chl-a) and did not correlate significantly with trace gas concentrations (P > 0.05). The fluorescence data provide a basic indication that the production of both BVOCs is relatively wide-spread across the different algal taxonomic groups.
Figure 1.
Depth profiles of temperature, isoprene, DMS and chl-a on 9, 16 and 23 July 2013. Concentrations of isoprene (B,F,J) and DMS (C,G,K) are shown as the arithmetic mean ± range of data (n = 2–3). Chl-a data (D,H,L) are shown as total and split based on fluorescence characteristics into four major phytoplankton groups (chromophytes, chlorophytes, cryptophytes, and cyanobacteria). Chl-a data were smoothed using a simple moving mean (running average) covering 0.80 ± 0.128 m depth.
Phytoplankton incubations
The importance of phototrophic processes for the production of isoprene and DMS was confirmed by screening unialgal phytoplankton cultures of ten algal species from four algal classes. These measurements represent net rates resulting from the interplay between gross production and gross consumption processes in the alga and associated microbiota. After normalisation of our data to chl-a and carbon concentration in the phytoplankton cultures (Table 1), we found culture-specific production rates (Table 2) that ranged from no production of either isoprene or DMS (Cyclotella meneghiniana, Chlamydomonas reinhardtii, Ulothrix fimbriata) to isoprene only (Cryptomonas sp., Anabaena variabilis, Microcystis aeruginosa, Synechococcus elongatus), DMS only (Chlorella vulgaris, Aphanizomenon flos-aquae) or both isoprene and DMS production (Scenedesmus obliquus). This suggests that cryptophytes and cyanobacteria may have contributed to DMS and isoprene production in the lake despite the low abundance indicated by the optical profiler.
Table 1.
Phytoplankton class, species and strain information, growth form, growth media, chlorophyll-a (chl-a) and particulate organic carbon (POC) concentrations in cultures used for trace gas production measurements. Algal cultures were grown in 4 L volumes at a temperature of 20 °C and a light intensity of ~100 µmol m−2 s−1 from fluorescent tubes. Cyanobacteria were grown in Cyano medium74, Chlorophyceae and Cryptomonas sp. were cultivated in Woods Hole (WC) medium either with or without vitamins75, and diatoms were grown in a modified M III medium with vitamins (M III KS)76. Data show mean ± standard deviation (n = 3).
| Class and Species | Strain IDa | Growth form | Medium | chl-a [mg L−1] | POC [mg L−1] |
|---|---|---|---|---|---|
| Bacillariophyceae | |||||
| Cyclotella meneghiniana | SAG 1020-1a | Unicellular | M III KS + Vit | 1.0 ± 0.21 | 71.4 ± 8.30 |
| Chlorophyceae | |||||
| Chlamydomonas reinhardtii | SAG 11-31 | Unicellular | WC | 4.6 ± 0.62 | 73.2 ± 11.87 |
| Chlorella vulgaris | SAG 211-11b | Unicellular | WC + Vit | 10.1 ± 1.04 | 112.7 ± 3.17 |
| Scenedesmus obliquus | SAG 276-3a | Unicellular | WC | 5.8 ± 1.70 | 99.1 ± 17.48 |
| Ulothrix fimbriata | SAG 36.86 | Filamentous | WC | 4.4 ± 0.42 | 94.9 ± 4.18 |
| Cryptophyceae | |||||
| Cryptomonas sp. | SAG 26.80 | Unicellular | WC + Vit | 5.4 ± 0.43 | 106.1 ± 5.26 |
| Cyanophyceae | |||||
| Anabaena variabilis | LI 81a | Filamentous | Cyano | 6.4 ± 0.67 | 135.0 ± 16.63 |
| Aphanizomenon flos-aquae | LI 83 | Filamentous | Cyano | 1.3 ± 0.02 | 42.1 ± 1.27 |
| Microcystis aeruginosa | LI 78 | Unicelluar | Cyano | 1.4 ± 0.07 | 38.1 ± 0.63 |
| Synechococcus elongatus | SAG 89.79 | Unicellular | Cyano | 4.6 ± 1.10 | 101.2 ± 24.59 |
aSAG = Culture collection of algae, University of Göttingen; LI = Culture collection of the Limnological Institute, University of Konstanz.
Table 2.
Isoprene and DMS production in four classes of freshwater phytoplankton from 10 species after normalization to particulate organic carbon (POC) or chlorophyll-a (chl-a). ‘NS’ indicates that incubations with algae were not significantly different from controls with alga medium (two-tailed t-test, P > 0.05).
| Class and Species | n | Isoprene | DMS | ||
|---|---|---|---|---|---|
| nmol [g org-C]−1 h−1 | nmol [g chl-a]−1 h−1 | nmol [g org-C]−1 h−1 | nmol [g chl-a]−1 h−1 | ||
| Bacillariophyceae | |||||
| Cyclotella meneghiniana | 3 | NS | NS | NS | NS |
| Chlorophyceae | |||||
| Chlamydomonas reinhardtii | 3 | NS | NS | NS | NS |
| Chlorella vulgaris | 3 | NS | NS | 0.3 ± 0.03 | 3.5 ± 0.03 |
| Scenedesmus obliquus | 6 | 3.1 ± 2.31 | 49.2 ± 35.66 | 0.5 ± 0.28 | 9.0 ± 5.90 |
| Ulothrix fimbriata | 3 | NS | NS | NS | NS |
| Cryptophyceae | |||||
| Cryptomonas sp. | 3 | 0. 7 ± 0.53 | 12.6 ± 9.46 | NS | NS |
| Cyanophyceae | |||||
| Anabaena variabilis | 3 | 0.9 ± 0.15 | 18.7 ± 2.99 | NS | NS |
| Aphanizomenon flos-aquae | 3 | NS | NS | 0.7 ± 0.19 | 21.1 ± 5.30 |
| Microcystis aeruginosa | 3 | 6.2 ± 0.93 | 174.3 ± 27.21 | NS | NS |
| Synechococcus elongatus | 6 | 7.3 ± 1.63 | 159.3 ± 35.14 | NS | NS |
Diel study
We also explored the diurnal differences in lake isoprene and DMS since strong diel pattern of isoprene production are observed in seaweed incubations and rock pools34, and terrestrial environments have no or negligible isoprene in the atmosphere during the night4,35. Aqueous isoprene level was significantly lower in the morning than in the afternoon (Fig. 2A) with mean aqueous isoprene concentrations (±standard deviation) of 455 ± 44.7 pM between 06:25 h and 12:22 h, and 548 ± 75.7 pM between 13:34 h and 20:26 h (two-tailed t-test: P = 0.04, n = 5). The atmospheric concentrations of isoprene showed a similar pattern but concentration differences between morning and afternoon were more pronounced with a mean isoprene concentration of 0.6 ± 0.15 ppb between 07:34 h and 13:00 h and 1.9 ± 0.39 ppb between 14:27 h and 20:03 h (two-tailed t-test, P < 0.001, n = 5). No significant difference was observed in aqueous DMS between morning (466 ± 47.3 pM) and afternoon (459 ± 11.6 pM; two-tailed t-test, P > 0.05), with a mean concentration of 462 ± 31.0 pM (atmospheric DMS was below the limit of detection).
Figure 2.
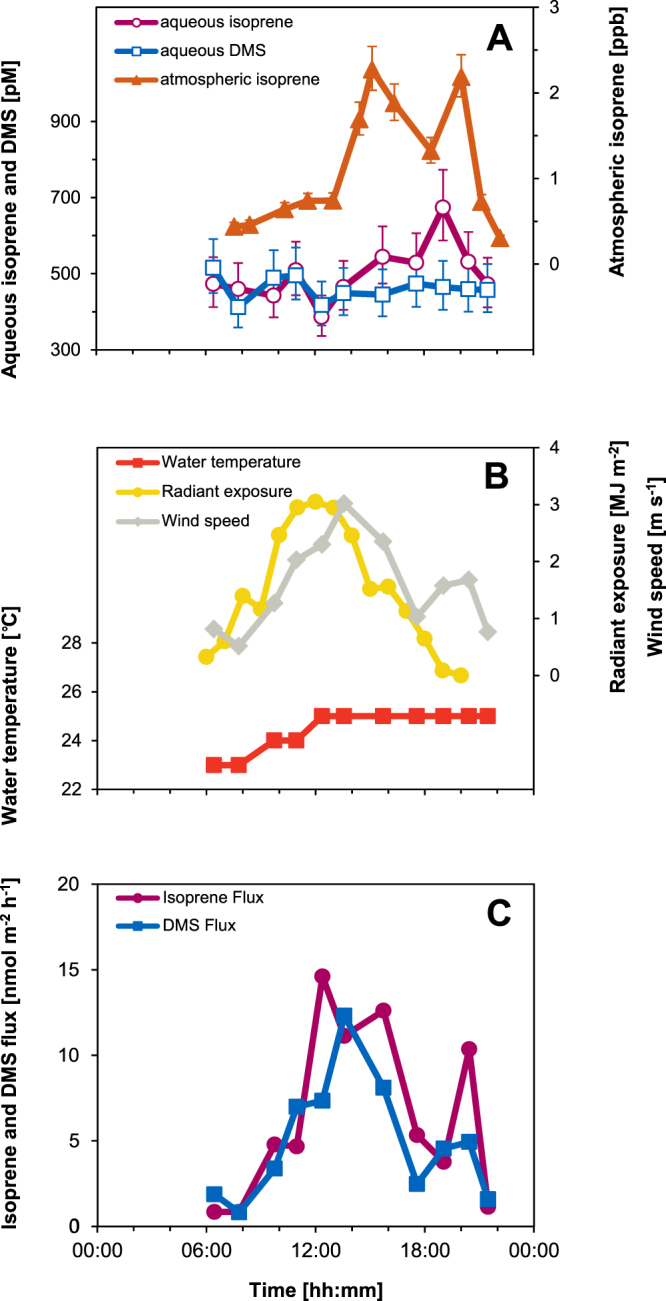
Diurnal study on 23 July 2013 showing concentrations of aqueous isoprene and DMS, and atmospheric isoprene (A), water temperature, radiant exposure and wind speed (B), calculated isoprene and DMS flux (C). Error bars in (A) indicate the coefficient of variation based on repeated calibrations.
Isoprene and DMS fluxes
Using air and water temperatures, and wind speeds (Fig. 2B), the concentration measurements allowed us to calculate the flux of isoprene and DMS across the water-atmosphere interface (Fig. 2C). Wind speeds were low throughout the diurnal study (1.6 ± 0.79 m s−1), constraining the transfer of gases into the atmosphere during our investigation. Isoprene flux was relatively small at the beginning (07:47 h: 0.8 nmol m−2 h−1) and towards the end of our diurnal study (21:29 h: 1.2 nmol m−2 h−1). Highest fluxes were observed between 12:22 h and 15:43 h (11.1 to 14.6 nmol m−2 h−1). Isoprene fluxes were likely similar at Sites 1 and 2 since they showed similar surface concentrations (around mid-day at Site 1: 337 pM on 23 July; Site 2: 387 pM on 25 July) and were driven by the diurnal variation in wind speed that directly affects the gas-transfer velocity used in our calculations. Using chl-a concentrations for the epilimnion on 23 July (48 mg m−2 or mean of 6.2 ± 1.36 µg chl-a L−1 from surface to 8.3 m depth), we can further calculate a biomass-normalised maximum isoprene flux of 304 nmol [g chl-a]−1 h−1. Flux of DMS showed a similar pattern to isoprene and ranged from 0.8 nmol m−2 h−1 at 07:47 h to a maximum of 12.3 nmol m−2 h−1 (256 nmol [g chl-a]−1 h−1) at 13:34 h to 1.6 nmol m−2 h−1 at 21:29 h.
Discussion
We first compared isoprene concentrations and fluxes from the lake with measurements from a temperate mixed-deciduous forest of beech (48%), oak (44%) and birch (8%) at a location 416 km to the north-northeast of Lake Constance in July 2003. This is an example for a high isoprene-producing terrestrial environment in the northern European temperate zone where atmospheric isoprene concentrations ranged from near zero at night to about 3 ppb around noon indicating mean hourly fluxes from the terrestrial vegetation of 1 to 2 µg m−2 s−1 (equivalent to 53 to 106 µmol m−2 h−1)35. Using a conservative estimate of the leaf area index (5.5 m2 m−2 for beech and oak)36, this hourly flux equates to 10 to 19 µmol m−2 h−1 based on the one-sided leaf surface area. Our data on atmospheric isoprene concentrations were similar (0.3 to 2.3 ppb during the diurnal study) but flux from the lake (14.6 nmol m−2 h−1) was substantially lower than the flux from terrestrial vegetation. We also normalised the isoprene flux to depth-integrated chl-a for the lake epilimnion on 23 July, compared this with chl-a normalised terrestrial fluxes and find that these are about 80 to 160-fold higher than fluxes from the lake.
We then compared our measured fluxes with examples from low isoprene-producing terrestrial environments. The arctic tundra is relatively poorly vegetated and only 17–20% of plant species from cold environments produce isoprene37. Fluxes in Lake Constance are similar to typical fluxes from arctic tundra vegetation (9 to 39 nmol m−2 h−1)38,39. This raises the question whether arctic lakes may be a substantial source of isoprene to the local atmosphere, provided their production and resulting flux is similar to that of Lake Constance. Pelagic mean chl-a concentration in arctic lakes ranges from 0.3 to 5.6 µg chl-a L−1 (overall mean of 1.9 µg chl-a L−1)40 but the typically shallow arctic lakes have large parts of the benthic sediments located in the euphotic zone. This provides a surface for growth of attached algae resulting in substantial epilithic chl-a concentrations (258 to 458 mg m−2) that generate 28 to 77% of total primary production in six arctic lakes41. Although pelagic chl-a was higher in Lake Constance (6.2 ± 1.36 µg chl-a L−1), its morphometry suggests that epilithic primary production was small and restricted to the immediate shoreline. Furthermore, the taxonomic composition of arctic and subarctic lakes is similar to oligotrophic temperate lakes with frequent domination by diatoms and chlorophytes, cryptophytes only temporally important in the seasonal succession and a low abundance of cyanobacteria42–45. This generally matches the taxonomic composition of oligotrophic Lake Constance based on the fluorescence characteristics from the optical profiler that showed a high abundance of chromophytes (including diatoms) and chlorophytes, and a lower abundance of cryptophytes and cyanobacteria (Fig. 1). Taken together, this suggests that primary productivity of arctic lakes could likely support at least a similar isoprene flux as that of Lake Constance. Additionally, since lakes are an increasingly dominant feature in the landscape from northern temperate to arctic zones46 and much of the Arctic has an exceptionally high lake area density (limnicity) of 10 to 50%47, the relative importance of lakes in the release of isoprene to the atmosphere may exceed that of terrestrial sources in the arctic where lake area is large and terrestrial inputs are small. This suggests that, relative to terrestrial sources, lakes in cold-temperate and subarctic climates could add substantially to the local atmospheric isoprene burden.
We then assessed our data against measurements from marine environments. Using recent reviews on marine isoprene33,48, we calculated an overall mean marine concentration of 30 pM (mean range 4.7 to 126.7 pM; n = 12–14). Typical marine fluxes range from 2.8 nmol m−2 h−1 in the Southern North Sea19 to 313 nmol m−2 h−1 in the Southern Indian Ocean49. These fluxes are strongly controlled by wind speed owing to the relatively small isoprene concentrations in the marine atmosphere and its relatively high concentrations in seawater34. In comparison to the marine examples, isoprene concentration in Lake Constance was higher and ranged from 183 to 722 pM in the epilimnion. Even at the relatively low wind speed during our study, high concentrations resulted in a substantial flux (maximum of 14.6 nmol m−2 h−1 around noon). Hence, similar to the marine example, Lake Constance was an important source of isoprene to the local atmosphere with an extrapolated emission of 59 moles (4 kg) of isoprene on the day of our diurnal measurements alone. Since aqueous isoprene concentrations can build up during periods of low wind speed when loss due to water-to-air transfer is limited, Lake Constance also provides a reservoir of isoprene. It is further possible that, depending on wind conditions, isoprene flux can be sustained into the night-time as indicated by the increased flux from the lake when light intensity was relatively low and wind speed temporarily increased from 19:00 to 20:30 h (Fig. 2). We then simulated the potential flux of isoprene using night-time concentrations and temperatures from our diurnal study (see Supplementary Fig. S2) and wind-speed data for 28 July 2013 when the calm conditions during our measurements were interrupted by a three-hour moderate breeze (maximum of 6.8 m s−1), and calculated an initial flux of 49.0 nmol m−2 h−1. Hence, the lake likely acts as an important source of night-time isoprene when terrestrial production ceases due to the strong light and temperature dependency of biological isoprene production4. This night-time release is unrecognised but of particular relevance since day-time isoprene is predominantly and rapidly oxidised (lifetime of few hours) by the light-generated hydroxyl radical (•OH), whilst isoprene emitted during the night will be mostly rapidly oxidised by the typically 100-fold more abundant nitrate radical (NO3; formed from anthropogenic NO2 and ozone). This should then impact the type, yield and fate of the isoprene-nitrates formed locally and consequently the NOX recycling, ozone and particle formation that may affect polluted urban atmospheres in the vicinity of lakes50.
DMS is the largest natural source of sulfur in the remote marine atmosphere and, similar to isoprene, may play some role in the formation and growth of atmospheric aerosol51 and impact on the night-time chemistry of the NO3 radical50. The transfer of DMS-sulfur into the atmosphere is estimated at 19.6 Tg per year25 which equates to a flux of 193 nmol m−2 h−1. As expected, the flux of DMS from Lake Constance (maximum of 12.3 nmol m−2 h−1) was lower than the marine flux and similar to the earlier estimates from Lake Kinneret that showed an estimated DMS-flux of 0.1 mmol m−2 month−1 (equivalent to 13.7 nmol m−2 h−1)52 and the mean flux from 10 Canadian lakes (7.1 nmol m−2 h−1) that, extrapolated to the Canadian boreal region, sustains an important 83% of biogenic sulfur in the atmosphere27.
Five of the phytoplankton cultures showed net-production rates for isoprene ranging from 12.6 to 174.3 nmol [g chl-a]−1 h−1 and three produced DMS at 3.5 to 21.1 nmol [g chl-a]−1 h−1. The isoprene production rates in the algal cultures were lower than the calculated lake flux after normalisation to chl-a biomass (304 nmol [g chl-a]−1 h−1). This could indicate that important isoprene-producing taxa were excluded from our screening or that environmental conditions (e.g. light, temperature) can significantly affect isoprene production rates in freshwater algae. This supports the idea that light-stress may drive the production of freshwater isoprene since it is linked to photoprotection in marine algae6,33. Our data agree with net-production rates in 21 marine algal strains from 7 taxonomic groups that varied by two orders of magnitude between strains (30 to 1340 nmol [g chl-a]−1 h−1)8. This suggests that the physiological processes involved in the production of isoprene are fundamentally similar between marine and freshwater environments.
As far as we are aware, surprisingly little information on the rates of DMS production in algal cultures is available in the literature. The high DMS-producing marine haptophyte Emiliania huxleyi (CCMP 373) produces DMS at rates of 10.1 ± 0.60 and 8.2 ± 1.80 nmol DMS L−1 h−1 during the day and night, respectively, at culture cell densities of 200 to 800 × 106 cells L−153. Using a cell density of 500 × 106 cells L−1 and a mean chl-a concentration of 0.22 ng cell−154, this equates to 91.6 ± 5.5 and 74.2 ± 16.4 nmol [g chl-a]−1 h−1. This is about 4 times higher than the DMS-production rate in our culture of the freshwater cyanobacterium Aphanizomenon flos-aquae but 21-times higher than in the chlorophyte Chlorella vulgaris. Marine dinoflagellates are among the highest producers of DMSP and DMS55. For example, the dinoflagellate symbiont Symbiodinium sp. produces DMS at 20 to 107 µmol [g chl-a]−1 h−156, at least three orders of magnitude higher than the freshwater phytoplankton in our study.
It is likely that isoprene and DMS are of ecological importance57–60. Freshwater algae are recognised as a rich source of volatiles that are documented for their effects on drinking water quality61, and used as directional cues to find food in freshwater gastropods62, hence can affect food web structure and function12. It is timely and important to address the ecological and physiological relevance of isoprene and DMS in freshwater environments and assess their roles in the infochemistry and structuring of freshwater food webs.
Methods
Sampling sites
Water samples were collected in July 2013 from two sites in Upper Lake Constance, a large (571 km2), deep (zmax = 252 m), warm-monomictic, oligotrophic lake in south-western Germany at the northern fringe of the Alps (Supplementary Fig. S1). Site 1 was at the long-term sampling site of the Limnological Institute of the University of Konstanz located in Lake Überlingen, a fjordlike appendix of Upper Lake Constance, which was accessed via boat (47°45′43.6″N, 9°07′50.0″E; depth about 140 m). Site 2 was accessed via a mooring and located close to the Limnological Institute, about 30 m offshore (47°41′44.3″N, 9°11′38.1″E) with a water depth of about 3 m.
Depth profiles
Water was collected from 6 depths (surface to 60 m) using a Niskin sampler at Site 1 at approximately 11:00 h on 9, 16 and 23 July 2013. Depths for discrete samples were selected based on in situ chl-a profiles recorded using a multi-channel fluorescence probe (bbe FluoroProbe, bbe Moldaenke, Schwentinental, Germany) and included samples from the surface (0 m) and from a maximum depth of 60 m. This probe has been shown to resolve the distribution of the four different taxonomic groups of chromophytes (including diatoms, dinoflagellates and chrysophytes), chlorophytes, cryptophytes, and cyanobacteria in laboratory cultures63 and lakes64 so that their abundances can be recorded based on fluorescence characteristics. For the quantification of discrete chlorophyll-a (chl-a) and organic carbon, samples were filtered immediately onto glass-fibre filters (Whatman GF/F; 25 mm diameter) and stored in a cool box before freezing filters at −20 °C at the Institute for subsequent analysis. For trace gas analysis, water was filled bubble-free into 250 mL gas-tight Winkler bottles (acid-washed and rinsed with ultrapure water prior to sampling) with a short length of silicone rubber tubing allowing for copious overflow before bottle closure. Samples were taken in analytical replicates (n = 3) and stored in a cool box equipped with several ice-packs before analysis of trace gases (n = 2 to 3) commenced ~1 hour after sampling.
Diurnal study
Water was collected bubble-free using an inverted aspirator approximately every 1.5 h at Site 2 between 06:25 and 21:29 h on 25 July 2013. Water was transferred into gas-tight bottles as described above and analysis of trace gases commenced about 10 min later. Air samples were taken from outside the institute located in a rural setting approximately 80 m from the lake shore with an air intake at 7 m above the lake level by sucking air through a 10 m long 1/8 inch (3.2 mm) OD Teflon tube using a vacuum pump. Air was flushed for 10 min at 80 mL min−1 into the cryo-focussing apparatus to trap trace gases from the atmosphere as described below.
Isoprene and DMS production in phytoplankton cultures
Algal cultures were aerated with compressed and filtered (0.2 µm pore size) air and grown under constant growth conditions using culture media depending on the cultures’ specific requirements (Table 1). The cultures were diluted by replacing 1 L of culture with fresh medium every 2 to 3 days and experiments were conducted 2 d after the last replacement.
On the day of the experiment, duplicate glass bottles were filled with algal medium (controls) or culture (treatment) at time zero (t0) and one bottle was immediately sacrificed for the quantification of isoprene and DMS. The other bottle was incubated under culture growth conditions and gases quantified at t1 after approximately 4 h. This was repeated twice using a staggered protocol resulting in 3 bottles each quantified for gases at t0 and t1. Treatments with significant difference to the controls (two-tailed t-test, P < 0.05) were considered for further analysis by subtracting control production rates and normalisation to culture chl-a and particulate organic carbon (POC) concentrations. It is important to note that previous incubation experiments with filtered seawater suggest that isoprene can also be produced at very low rates by photochemical processes with the bulk of this production controlled by ultraviolet light65. However, these experiments were affected by the presence of bacteria that could potentially lead to isoprene production from dissolved organic carbon. Furthermore, since we used borosilicate bottles and light derived from fluorescent tubes in our experiments photochemical production of isoprene was likely negligible during the incubations but small photochemical isoprene production may have added to the biological production processes at the lake surface.
Quantification of discrete chl-a and POC
Glass-fibre filters (Whatman GF/F; 25 mm diameter) loaded with aliquots of the algal suspensions were used for photometric chl-a determination after wet extraction in ethanol66. Particulate organic carbon (POC) was quantified with an EuroEA3000 elemental analyser (HEKAtech GmbH; Wegberg, Germany; Table 1).
Analysis of isoprene and DMS
Gas chromatography with flame ionisation detection combined with a purpose-built purge-and-trap system for the cryogenic enrichment of BVOCs was used for the analysis of isoprene and DMS following established protocols8,67 while using best practices for sampling and storage68. Calibration stocks for aqueous measurements of isoprene and DMS were volumetrically prepared, and a commercially-sourced isoprene gas standard was used for the calibration of atmospheric isoprene measurements. For method details see Supporting Information.
Quantification of water-to-air flux
Concentrations of isoprene in water (Cw) and air (Ca) together with water temperature, air temperature and wind speeds measured at the Meteorological Station Konstanz (see Supplementary Fig. S1) were used to calculate water-to-air isoprene fluxes: Flux = k(Cw − Ca × Hc), where k is the wind speed-dependent gas transfer velocity (cm hr−1)69, adjusted to the in situ Schmidt number70, and Hc is the Henry’s Law constant for isoprene (1.3 × 10−2 M atm−1)71. DMS flux calculations used the same approach and wind speed-based parametrisation of gas transfer velocity, but assumed Ca = 0 as atmospheric DMS levels were below the level of detection.
To compare the water-to-air flux with terrestrial flux estimates based on area or chl-a, we used a conservative estimate of the leaf area index of 5.5 m2 m−2 for beech and oak36, and literature data for chlorophyll (a + b) concentrations of 400 mg m−2 and chl-a/chl-b ratios in oak of 3.472,73.
Data analysis
Commercial software (GC Solution Lite version 2.41; Shimadzu UK, Milton Keynes, UK) was used for peak integration and data retrieval. We confirmed that test assumptions were met before conducting statistical analyses (two-tailed t-test and regression analysis) in MS Excel version 14.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
We are thankful to the captain and crew of ‘MS Robert Lauterborn’. Technical assistance was provided by Tania Cresswell-Maynard, John Green, Pia Mahler, Petra Merkel and Martin Wolf. Comments by Ian Colbeck, Richard Geider and Terry McGenity have greatly improved earlier versions of the manuscript. Financial support from the Konstanz-Essex Development Fund was provided to M.S. and D.M-C.
Author Contributions
M.S. and D.M.-C. conceived the original project. M.S., B.H., R.S. and D.M.-C. conducted the sampling, performed the incubations and measurements, and processed the data. T.G.B. calculated the gas fluxes and simulated the night-time release of isoprene from the lake. M.S. wrote the manuscript. All authors edited and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18923-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hallquist M, et al. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 2009;9:5155–5236. doi: 10.5194/acp-9-5155-2009. [DOI] [Google Scholar]
- 2.O’Dowd CD, de Leeuw G. Marine aerosol production: a review of the current knowledge. Philos. T. Roy. Soc. A. 2007;365:1753–1774. doi: 10.1098/rsta.2007.2043. [DOI] [PubMed] [Google Scholar]
- 3.Bastviken D, Tranvik LJ, Downing JA, Crill PM, Enrich-Prast A. Freshwater Methane Emissions Offset the Continental Carbon Sink. Science. 2011;331:50–50. doi: 10.1126/science.1196808. [DOI] [PubMed] [Google Scholar]
- 4.Kesselmeier J, Staudt M. Biogenic Volatile Organic Compounds (VOC): An Overview on Emission, Physiology and Ecology. J. Atmos. Chem. 1999;33:23–88. doi: 10.1023/A:1006127516791. [DOI] [Google Scholar]
- 5.Loreto F, Fineschi S. Reconciling functions and evolution of isoprene emission in higher plants. New Phytol. 2014;206:578–582. doi: 10.1111/nph.13242. [DOI] [PubMed] [Google Scholar]
- 6.Dani KGS, Loreto F. Trade-off between dimethyl sulfide and isoprene emissions from marine phytoplankton. Trends Plant Sci. 2017;22:361–372. doi: 10.1016/j.tplants.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Fall R, Copley SD. Bacterial sources and sinks of isoprene, a reactive atmospheric hydrocarbon. Environ. Microbiol. 2000;2:123–130. doi: 10.1046/j.1462-2920.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- 8.Exton DA, Suggett DJ, McGenity TJ, Steinke M. Chlorophyll-normalized isoprene production in laboratory cultures of marine microalgae and implications for global models. Limnol. Oceanogr. 2013;58:1301–1311. doi: 10.4319/lo.2013.58.4.1301. [DOI] [Google Scholar]
- 9.Disch A, Schwender J, Müller C, Lichtenthaler HK, Rohmer M. Distribution of the mevalonate and glyceraldehyde phosphate/pyruvate pathways for isoprenoid biosynthesis in unicellular algae and the cyanobacterium Synechocystis PCC 6714. Biochem. J. 1998;333:381–388. doi: 10.1042/bj3330381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefels J, Steinke M, Turner S, Malin G, Belviso S. Environmental constraints on the production and removal of the climatically active gas dimethylsulphide (DMS) and implications for ecosystem modelling. Biogeochemistry. 2007;83:245–275. doi: 10.1007/s10533-007-9091-5. [DOI] [Google Scholar]
- 11.Curson ARJ, et al. Dimethylsulfoniopropionate biosynthesis in marine bacteria and identification of the key gene in this process. Nat. Microbiol. 2017;2:17009. doi: 10.1038/nmicrobiol.2017.9. [DOI] [PubMed] [Google Scholar]
- 12.Pohnert G, Steinke M, Tollrian R. Chemical cues, defence metabolites and the shaping of pelagic interspecific interactions. Trends Ecol. Evol. 2007;22:198–204. doi: 10.1016/j.tree.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Curson AR, Todd JD, Sullivan MJ, Johnston AW. Catabolism of dimethylsulphoniopropionate: microorganisms, enzymes and genes. Nat. Rev. Microbiol. 2011;9:849–859. doi: 10.1038/nrmicro2653. [DOI] [PubMed] [Google Scholar]
- 14.Alcolombri U, et al. Identification of the algal dimethyl sulfide–releasing enzyme: A missing link in the marine sulfur cycle. Science. 2015;348:1466–1469. doi: 10.1126/science.aab1586. [DOI] [PubMed] [Google Scholar]
- 15.Jardine, K. et al. Dimethyl sulfide in the Amazon rain forest. Global Biogeochem. Cycles, 2014GB004969, 10.1002/2014GB004969 (2015).
- 16.Hu HY, Mylon SE, Benoit G. Volatile organic sulfur compounds in a stratified lake. Chemosphere. 2007;67:911–919. doi: 10.1016/j.chemosphere.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Sela-Adler M, et al. Isotopic evidence for the origin of dimethylsulfide and dimethylsulfoniopropionate-like compounds in a warm, monomictic freshwater lake. Environ. Chem. 2016;13:340–351. doi: 10.1071/EN15042. [DOI] [Google Scholar]
- 18.Exton DA, Suggett DJ, Steinke M, McGenity TJ. Spatial and temporal variability of biogenic isoprene emissions from a temperate estuary. Global Biogeochem. Cycles. 2012;26:GB2012. doi: 10.1029/2011GB004210. [DOI] [Google Scholar]
- 19.Broadgate WJ, Liss PS, Penkett SA. Seasonal emissions of isoprene and other reactive hydrocarbon gases from the ocean. Geophys. Res. Lett. 1997;24:2675–2678. doi: 10.1029/97GL02736. [DOI] [Google Scholar]
- 20.Bell TG, Malin G, McKee CM, Liss PS. A comparison of dimethylsulphide (DMS) data from the Atlantic Meridional Transect (AMT) programme with proposed algorithms for global surface DMS concentrations. Deep-Sea Res. Pt. II. 2006;53:1720–1735. doi: 10.1016/j.dsr2.2006.05.013. [DOI] [Google Scholar]
- 21.Zindler C, Marandino CA, Bange HW, Schütte F, Saltzman ES. Nutrient availability determines dimethyl sulfide and isoprene distribution in the eastern Atlantic Ocean. Geophys. Res. Lett. 2014;41:GL059547. doi: 10.1002/2014GL059547. [DOI] [Google Scholar]
- 22.Bonsang B, Polle C, Lambert G. Evidence for marine production of isoprene. Geophys. Res. Lett. 1992;19:1129–1132. doi: 10.1029/92GL00083. [DOI] [Google Scholar]
- 23.Baker, A. R. et al. Distribution and sea-air fluxes of biogenic trace gases in the eastern Atlantic Ocean. Global Biogeochem. Cycles14, 871-886 (2000).
- 24.Lana A, et al. An updated climatology of surface dimethylsulfide concentrations and emission fluxes in the global ocean. Global Biogeochem. Cycles. 2011;25:GB1004. doi: 10.1029/2010GB003850. [DOI] [Google Scholar]
- 25.Land PE, Shutler JD, Bell TG, Yang M. Exploiting satellite earth observation to quantify current global oceanic DMS flux and its future climate sensitivity. J. Geophys. Res. - Oceans. 2014;119:7725–7740. doi: 10.1002/2014JC010104. [DOI] [Google Scholar]
- 26.Yoch DC, Carraway RH, Friedman R, Kulkarni N. Dimethylsulfide (DMS) production from dimethylsulfoniopropionate by freshwater river sediments: phylogeny of Gram-positive DMS-producing isolates. FEMS Microbiol. Ecol. 2001;37:31–37. doi: 10.1111/j.1574-6941.2001.tb00850.x. [DOI] [Google Scholar]
- 27.Sharma S, Barrie LA, Hastie DR, Kelly C. Dimethyl sulfide emissions to the atmosphere from lakes of the Canadian boreal region. J. Geophys. Res. - Atmos. 1999;104:11585–11592. doi: 10.1029/1999JD900127. [DOI] [Google Scholar]
- 28.Alvarez LA, Exton DA, Timmis KN, Suggett DJ, McGenity TJ. Characterization of marine isoprene-degrading communities. Environ. Microbiol. 2009;11:3280–3291. doi: 10.1111/j.1462-2920.2009.02069.x. [DOI] [PubMed] [Google Scholar]
- 29.Sharkey TD, Wiberley AE, Donohue AR. Isoprene emission from plants: Why and how. Ann. Bot. 2008;101:5–18. doi: 10.1093/aob/mcm240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meskhidze N, Sabolis A, Reed R, Kamykowski D. Quantifying environmental stress-induced emissions of algal isoprene and monoterpenes using laboratory measurements. Biogeosciences. 2015;12:637–651. doi: 10.5194/bg-12-637-2015. [DOI] [Google Scholar]
- 31.Sunda W, Kieber DJ, Kiene RP, Huntsman S. An antioxidant function for DMSP and DMS in marine algae. Nature. 2002;418:317–320. doi: 10.1038/nature00851. [DOI] [PubMed] [Google Scholar]
- 32.Hopkins FE, Bell TG, Yang M, Suggett DJ, Steinke M. Air exposure of coral is a significant source of dimethylsulfide (DMS) to the atmosphere. Sci. Rep. 2016;6:36031. doi: 10.1038/srep36031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hackenberg, S. C. et al. Potential controls of isoprene in the surface ocean. Global Biogeochem. Cycles31, 10.1002/2016GB005531 (2017).
- 34.Broadgate WJ, Malin G, Küpper FC, Thompson A, Liss PS. Isoprene and other non-methane hydrocarbons from seaweeds: a source of reactive hydrocarbons to the atmosphere. Mar. Chem. 2004;88:61–73. doi: 10.1016/j.marchem.2004.03.002. [DOI] [Google Scholar]
- 35.Spirig C, et al. Eddy covariance flux measurements of biogenic VOCs during ECHO 2003 using proton transfer reaction mass spectrometry. Atmos. Chem. Phys. 2005;5:465–481. doi: 10.5194/acp-5-465-2005. [DOI] [Google Scholar]
- 36.Bréda NJJ. Ground‐based measurements of leaf area index: a review of methods, instruments and current controversies. J. Exp. Bot. 2003;54:2403–2417. doi: 10.1093/jxb/erg263. [DOI] [PubMed] [Google Scholar]
- 37.Rinnan R, Steinke M, McGenity T, Loreto F. Plant volatiles in extreme terrestrial and marine environments. Plant, Cell Environ. 2014;37:1776–1789. doi: 10.1111/pce.12320. [DOI] [PubMed] [Google Scholar]
- 38.Lindwall F, Schollert M, Michelsen A, Blok D, Rinnan R. Fourfold higher tundra volatile emissions due to arctic summer warming. J. Geophys. Res. – Biogeo. 2016;121:895–902. doi: 10.1002/2015JG003295. [DOI] [Google Scholar]
- 39.Schollert M, Burchard S, Faubert P, Michelsen A, Rinnan R. Biogenic volatile organic compound emissions in four vegetation types in high arctic Greenland. Polar Biol. 2014;37:237–249. doi: 10.1007/s00300-013-1427-0. [DOI] [Google Scholar]
- 40.Rautio M, et al. Shallow freshwater ecosystems of the circumpolar Arctic. Ecoscience. 2011;18:204–222. doi: 10.2980/18-3-3463. [DOI] [Google Scholar]
- 41.Whalen SC, Chalfant BA, Fischer EN. Epipelic and pelagic primary production in Alaskan Arctic lakes of varying depth. Hydrobiologia. 2008;614:243–257. doi: 10.1007/s10750-008-9510-1. [DOI] [Google Scholar]
- 42.Kalff, J. Arctic lake ecosystems in AntarcticEcology (ed. Holdgate, M. W.) 651–663 (Academic Press, 1970).
- 43.Harris, G. P. Phytoplankton Ecology - Structure, Function and Fluctuation (Chapman and Hall, 1986).
- 44.Forsström L, Sorvari S, Korhola A, Rautio M. Seasonality of phytoplankton in subarctic Lake Saanajärvi in NW Finnish Lapland. Polar Biol. 2005;28:846–861. doi: 10.1007/s00300-005-0008-2. [DOI] [Google Scholar]
- 45.Sheath R, Munawar M. Phytoplankton composition of a small subarctic lake in the Northwest Territories, Canada. Phycologia. 1974;13:149–161. doi: 10.2216/i0031-8884-13-2-149.1. [DOI] [Google Scholar]
- 46.Vincent, W. F., Laurion, I., Pienitz, R. & Walter Anthony, K. M. Climate impacts on Arctic lake ecosystems in Climatic Change and Global Warming of Inland Waters: Impacts and Mitigation for Ecosystems and Societies (eds Goldman, C. R., Kumagai, M. & Robarts, R. D.) 27–42 (John Wiley & Sons, 2013).
- 47.Messager ML, Lehner B, Grill G, Nedeva I, Schmitt O. Estimating the volume and age of water stored in global lakes using a geo-statistical approach. Nat. Commun. 2016;7:13603. doi: 10.1038/ncomms13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw SL, Gantt B, Meskhidze N. Production and emissions of marine isoprene and monoterpenes: A review. Adv. Meteorol. 2010;2010:408696. doi: 10.1155/2010/408696. [DOI] [Google Scholar]
- 49.Kameyama S, et al. High-resolution observations of dissolved isoprene in surface seawater in the Southern Ocean during austral summer 2010–2011. J. Oceanogr. 2014;70:225–239. doi: 10.1007/s10872-014-0226-8. [DOI] [Google Scholar]
- 50.Brown SS, Stutz J. Nighttime radical observations and chemistry. Chem. Soc. Rev. 2012;41:6405–6447. doi: 10.1039/c2cs35181a. [DOI] [PubMed] [Google Scholar]
- 51.Vallina SM, Simó R. Strong relationship between DMS and the solar radiation dose over the global surface ocean. Science. 2007;315:506–508. doi: 10.1126/science.1133680. [DOI] [PubMed] [Google Scholar]
- 52.Ginzburg B, et al. DMS formation by dimethylsulfoniopropionate route in freshwater. Environ. Sci. Technol. 1998;32:2130–2136. doi: 10.1021/es9709076. [DOI] [Google Scholar]
- 53.Green BC, Suggett DJ, Hills A, Steinke M. Optimisation of a fast DMS sensor (FDS) for real time quantification of dimethyl sulfide production by algae. Biogeochem. 2012;110:163–172. doi: 10.1007/s10533-011-9678-8. [DOI] [Google Scholar]
- 54.Wolfe GV, Steinke M. Grazing-activated production of dimethyl sulfide (DMS) by two clones of Emiliania huxleyi. Limnol. Oceanogr. 1996;41:1151–1160. doi: 10.4319/lo.1996.41.6.1151. [DOI] [Google Scholar]
- 55.Caruana AMN, Malin G. The variability in DMSP content and DMSP lyase activity in marine dinoflagellates. Prog. Oceanogr. 2014;120:410–424. doi: 10.1016/j.pocean.2013.10.014. [DOI] [Google Scholar]
- 56.Steinke M, Brading P, Kerrison P, Warner ME, Suggett DJ. Concentrations of dimethylsulfoniopropionate and dimethyl sulfide are strain-specific in symbiotic dinoflagellates (Symbiodinium sp., Dinophyceae) J. Phycol. 2011;47:775–783. doi: 10.1111/j.1529-8817.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- 57.Savoca MS, Nevitt GA. Evidence that dimethyl sulfide facilitates a tritrophic mutualism between marine primary producers and top predators. Proc. Natl Acad. Sci. 2014;111:4157–4161. doi: 10.1073/pnas.1317120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nevitt GA, Veit RR, Kareiva P. Dimethyl sulphide as a foraging cue for Antarctic Procellariiform seabirds. Nature. 1995;376:680–682. doi: 10.1038/376680ao. [DOI] [Google Scholar]
- 59.Steinke M, Stefels J, Stamhuis E. Dimethyl sulfide triggers search behavior in copepods. Limnol. Oceanogr. 2006;51:1925–1930. doi: 10.4319/lo.2006.51.4.1925. [DOI] [Google Scholar]
- 60.Seymour JR, Simó R, Ahmed T, Stocker R. Chemoattraction to Dimethylsulfoniopropionate Throughout the Marine Microbial Food Web. Science. 2010;329:342–345. doi: 10.1126/science.1188418. [DOI] [PubMed] [Google Scholar]
- 61.Watson SB. Cyanobacterial and eukaryotic algal odour compounds: signals or by-products? A review of their biological activity. Phycologia. 2003;42:332–350. doi: 10.2216/i0031-8884-42-4-332.1. [DOI] [Google Scholar]
- 62.Fink P, von Elert E, Juettner F. Volatile foraging kairomones in the littoral zone: Attraction of an herbivorous freshwater gastropod to algal odors. J. Chem. Ecol. 2006;32:1867–1881. doi: 10.1007/s10886-006-9115-y. [DOI] [PubMed] [Google Scholar]
- 63.Kring SA, Figary SE, Boyer GL, Watson SB, Twiss MR. Rapid in situ measures of phytoplankton communities using the bbe FluoroProbe: evaluation of spectral calibration, instrument intercompatibility, and performance range. Can. J. Fish. Aquat. Sci. 2014;71:1087–1095. doi: 10.1139/cjfas-2013-0599. [DOI] [Google Scholar]
- 64.Catherine A, et al. On the use of the FluoroProbe (R), a phytoplankton quantification method based on fluorescence excitation spectra for large-scale surveys of lakes and reservoirs. Water Res. 2012;46:1771–1784. doi: 10.1016/j.watres.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 65.Ratte M, Bujok O, Spitzy A, Rudolph J. Photochemical alkene formation in seawater from dissolved organic carbon: Results from laboratory experiments. J. Geophys. Res. – Atmos. 1998;103:5707–5717. doi: 10.1029/97JD03473. [DOI] [Google Scholar]
- 66.Stich HB, Brinker A. Less is better: Uncorrected versus pheopigment-corrected photometric chlorophyll-a estimation. Arch. Hydrobiol. 2005;162:111–120. doi: 10.1127/0003-9136/2005/0162-0111. [DOI] [Google Scholar]
- 67.Franchini, F. & Steinke, M. Protocols for the quantification of dimethyl sulfide (DMS) and other volatile organic compounds in aquatic environments in Hydrocarbon and Lipid Microbiology Protocols (eds McGenity, T. J., Timmis, K. N. & Nogales, B.) 161–177 (Springer, 2017).
- 68.Stefels, J. Determination of DMS, DMSP, and DMSO in Seawater in Practical Guidelines for the Analysis of Seawater (ed. Wurl, O.) 223–234 (CRC Press, 2009).
- 69.Nightingale PD, et al. In situ evaluation of air-sea gas exchange parameterizations using novel conservative and volatile tracers. Global Biogeochem. Cycles. 2000;14:373–387. doi: 10.1029/1999GB900091. [DOI] [Google Scholar]
- 70.Johnson MT. A numerical scheme to calculate temperature and salinity dependent air-water transfer velocities for any gas. Ocean Sci. 2010;6:913–932. doi: 10.5194/os-6-913-2010. [DOI] [Google Scholar]
- 71.Sander R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015;15:4399–4981. doi: 10.5194/acp-15-4399-2015. [DOI] [Google Scholar]
- 72.Rossini M, Panigada C, Meroni M, Colombo R. Assessment of oak forest condition based on leaf biochemical variables and chlorophyll fluorescence. Tree Physiol. 2006;26:1487–1496. doi: 10.1093/treephys/26.11.1487. [DOI] [PubMed] [Google Scholar]
- 73.Gond V, de Pury DGG, Veroustraete F, Ceulemans R. Seasonal variations in leaf area index, leaf chlorophyll, and water content; scaling-up to estimate fAPAR and carbon balance in a multilayer, multispecies temperate forest. Tree Physiol. 1999;19:673–679. doi: 10.1093/treephys/19.10.673. [DOI] [PubMed] [Google Scholar]
- 74.Jüttner F, Leonhardt J, Möhren S. Environmental factors affecting the formation of mesityloxide, dimethylallylic alcohol and other volatile compounds excreted by Anabaena cylindrica. J. Gen. Microbiol. 1983;129:407–412. [Google Scholar]
- 75.Guillard, R. R. L. Culture of phytoplankton for feeding marine invertebrates in Culture of Marine Invertebrate Animals (eds Smith, W. L. & Chanley, M. H.) 29–60 (Plenum Press, 1975).
- 76.Körner S, Nicklisch A. Allelopathic growth inhibition of selected phytoplankton species by submerged macrophytes. J. Phycol. 2002;38:862–871. doi: 10.1046/j.1529-8817.2002.t01-1-02001.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.