Abstract
Background
BIOGF1K, a compound-K-rich fraction, has been shown to display anti-inflammatory activity. Although Panax ginseng is widely used for the prevention of photoaging events induced by UVB irradiation, the effect of BIOGF1K on photoaging has not yet been examined. In this study, we investigated the effects of BIOGF1K on UVB-induced photoaging events.
Methods
We analyzed the ability of BIOGF1K to prevent UVB-induced apoptosis, enhance matrix metalloproteinase (MMP) expression, upregulate anti-inflammatory activity, reduce sirtuin 1 expression, and melanin production using reverse transcription-polymerase chain reaction, melanin content assay, tyrosinase assay, and flow cytometry. We also evaluated the effects of BIOGF1K on the activator protein-1 signaling pathway, which plays an important role in photoaging, by immunoblot analysis and luciferase reporter gene assays.
Results
Treatment of UVB-irradiated NIH3T3 fibroblasts with BIOGF1K prevented UVB-induced cell death, inhibited apoptosis, suppressed morphological changes, reduced melanin secretion, restored the levels of type I procollagen and sirtuin 1, and prevented mRNA upregulation of MMP-1, MMP-2, and cyclo-oxygenase-2; these effects all occurred in a dose-dependent manner. In addition, BIOGF1K markedly reduced activator-protein-1-mediated luciferase activity and decreased the activity of mitogen-activated protein kinases (extracellular response kinase, p38, and C-Jun N-terminal kinase).
Conclusion
Our results strongly suggest that BIOGF1K has anti-photoaging activity and that BIOGF1K could be used in anti-aging cosmeceutical preparations.
Keywords: anti-aging, BIOGF1K, Panax ginseng, photoaging, UVB irradiation
1. Introduction
The skin is an important barrier that protects the body from external damage. A major cause of skin injury is UVB light. UVB emits light with wavelengths ranging from 280 nm to 320 nm. UVB irradiation of the skin results in various photoaging phenomena, such as induction of apoptosis, enhancement of matrix metalloproteinase (MMP) expression, upregulation of inflammation, and reduction of sirtuin (SIRT)1 expression [1], [2], [3], [4]. In turn, activated MMPs contribute to extracellular matrix (ECM) degradation and synthesis inhibition, as well as that of collagen in connective tissues, thereby making a crucial contribution to photoaging [5]. Suppression of SIRT1 expression leads to upregulation of apoptosis and downregulation of cell survival [6], [7]. These UVB-induced photoaging events are regulated by the activator protein (AP)-1 signaling pathway [8], [9], which is in turn activated by mitogen-activated protein kinases (MAPKs), p38, extracellular response kinase (ERK), and c-Jun N-terminal kinase (JNK). Moreover, upon UVB irradiation, melanin is excessively synthesized by tyrosinase and secreted upon stimulation with α-melanocyte stimulating hormone (MSH) from epidermal melanocytes [10]. Melanin synthesis has also been shown to be predominantly regulated by the AP-1 signaling pathway [11].
The root of Korean ginseng (Panax ginseng) has been prescribed as an herbal medicine in East Asia. In addition to its anti-inflammatory and anticancer effects, this root has also been used as a cosmetic biomaterial due to its whitening, moisturizing, antiwrinkle, and antiaging effects [12], [13], [14]. We recently prepared a fraction containing a high concentration of compound K, BIOGF1K, and found that it displayed antioxidative and anti-inflammatory activities [15]. To determine whether this fraction could have additional applications in various fields, we aimed to test whether BIOGF1K protected cells from UVB-induced photoaging events. To this end, we investigated BIOGF1K effects on UVB irradiation-induced apoptosis, morphological changes, melanin production, enzyme downregulation, inflammatory gene expression, and AP-1 signaling.
2. Materials and methods
2.1. Materials
Phorbol-12-myristate-13 acetate (PMA) and (3-4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTT) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The luciferase construct harboring AP-1 and collagen (Col)1A1 promoter binding sites was used as reported earlier [16], [17]. TRIzol reagent was purchased from Molecular Research Center (Montgomery, OH, USA). Fetal bovine serum and Dulbecco's modified Eagle's medium (DMEM) were purchased from Gibco (Grand Island, NY, USA). The cell lines used in the present experiments (NIH3T3, HEK293, and B16F10 cells) were obtained from American Type Culture Collection (Rockville, MD, USA). All other chemicals were obtained from Sigma Chemical Co. Total and phosphospecific antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Plasmid constructs driving the expression of Smad3 (mothers against decapentaplegic homolog 3) were used as reported previously [18].
2.2. Cell culture
Mouse embryonic fibroblast NIH3T3 and mouse melanoma B16F10 cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% antibiotics (penicillin and streptomycin) in a CO2 incubator at 37°C. For experiments, cells were seeded in six-well plates at 106 cells/well with fresh complete culture medium [19].
2.3. Preparation of BIOGF1K
BIOGF1K, a compound-K-rich fraction, was prepared as described previously [20].
2.4. Drug treatment
A stock solution of BIOGF1K was prepared in dimethyl sulfoxide (DMSO) at a concentration of 100 mg/mL. Target concentrations (15 or microgram/ml and 30 μg/mL) were achieved by dilution with culture medium [21].
2.5. Cell viability assay
NIH3T3 cells were seeded onto 96-well plates at 105 cells/well with fresh complete culture medium. To test the cytotoxicity of BIOGF1K alone, cells were treated with 7.5, 15, or 30 μg/mL BIOGF1K. To test the effect of BIOGF1K on UVB-induced toxicity, cells were irradiated with 30 mJ/cm2 UVB and then cultured in complete culture medium with 15 or 30 μg/mL BIOGF1K for a further 24 h. Cell viability was determined with a conventional 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [22].
2.6. UVB irradiation
Cells were irradiated in six-well plates using a UVB lamp (Bio-Link BLX-312; Vilber Lourmat, Collégien, France) with an emission wavelength peak of 312 nm. Before UVB irradiation, culture medium was replaced with 1 mL phosphate-buffered saline (PBS) per well. After removing the plate lid, cells were irradiated at 30 mJ/cm2. After UVB irradiation, PBS was replaced with complete culture medium with the appropriate compound treatments prior to harvesting [3].
2.7. HPLC analysis
The concentrations of compound K in BIOGF1K were quantified by HPLC as described previously [23], [24].
2.8. Fluorescence-activated cell sorting
Apoptosis was analyzed by flow cytometry after different cell treatments. Cells were treated with or without 15 or 30 μg/mL BIOGF1K after UVB irradiation (30 mJ/cm2) or subjected to a control treatment. For staining, cells were washed twice with cold PBS and resuspended in 1× binding buffer at a concentration of 106 cells/mL. Next, 100 μL of suspension (105 cells) was transferred to e tubes, 10 μL propidium iodide, and 5 μL fluorescein isothiocyanate–Annexin V was added, and cells were incubated for 15 min at room temperature in the dark. Finally, 400 μL 1× binding buffer was added and fluorescence was assessed using a Guava easyCyte flow cytometer (Millipore, Billerica, MA, USA) [25].
2.9. Plasmid transfection and luciferase reporter gene assay
For the luciferase reporter gene assay, HEK293 cells (105 cells/well in 24-well plates) were transfected with 0.8 μg/mL plasmids driving the expression of β-galactosidase, AP-1-Luc, Col1A1-Luc, and FLAG-smad3. Cells were transfected using the polyethyleneimine method and then incubated for 24 h. Finally, HEK293 cells ware treated with 15 or 30 μg/mL BIOGF1K and 100 nM PMA for a further 24 h [15].
2.10. Analysis of mRNA levels by reverse transcription-polymerase chain reaction
To quantify cytokine mRNA expression levels, NIH3T3 cells were treated with or without 15 or 30 μg/mL BIOGF1K after UVB irradiation (30 mJ/cm2). Total RNA was then isolated with TRIzol reagent. Reverse transcription-polymerase chain reaction (RT-PCR) was performed as described previously [26]. Primers used in this study are listed in Table 1.
Table 1.
Polymerase chain reaction primers used in this study
| Name | Sequence (5′ to 3′) | |
|---|---|---|
| MMP1 | F | TCTGACGTTGATCCCAGAGAGCAG |
| R | CAGGGTGACACCAGTGACTGCAC | |
| MMP2 | F | CAAGTGGAGAGCAGTTGAGGACATC |
| R | TGAGGACATCTCCCACGTCAA | |
| Type 1 procollagen | F | AGGGCCAAGACGAAGACATC |
| R | AGATCACGTCATCGCACAACA | |
| SIRT1 | F | GCTCTAGCCACCATGGCGGACGA |
| R | CGCGGATCCTGATTTGTTTGATGGATAGTT | |
| COX-2 | F | CACTACATCCTGACCCACTT |
| R | ATGCTCCTGCTTGAGTATGT | |
| GAPDH | F | CACTCACGGCAAATTCAACGGCA |
| R | GACTCCACGACATACTCAGCAC |
COX-2, cyclo-oxygenase-2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MMP, matrix metalloproteinase; SIRT1, sirtuin 1
2.11. Immunoblotting
Total lysates prepared from NIH3T3 cells were subjected to western blot analysis of the total and phospho-forms of JNK, ERK, p38, IκBα, MAPK/ERK kinase (MEK)1/2, MAPK kinase (MKK)3/6, and β-actin. Immunoreactive bands were visualized as described previously [27].
2.12. Tyrosinase assay
Fifty milliliters of 6 mM l-dopamine (dissolved in 50 mM potassium phosphate buffer, pH 6.8), 50 μL DMSO (with or without 30 μg/mL BIOGF1K), and 200 μM kojic acid (dissolved in potassium phosphate buffer) were mixed at room temperature for 15 min. Mushroom tyrosinase (100 U/mL) dissolved in potassium phosphate buffer was then added to the mixture. The absorbance of the mixture at 475 nm was immediately measured using a multidetection microplate reader [28].
2.13. Melanin formation and secretion test
For the melanin formation assay, B16F10 cells (105 cells/well in 12-well plates) were treated with 100 nM α−MSH, 30 μg/mL BIOGF1K, or 1mM arbutin for 48 h. Melanin secretion was assessed by measuring the absorbance of the culture medium at 475 nm using a multidetection microplate reader. For melanin content analysis, cells were lysed with 20 μL cell lysis buffer (50 mM Tris–HCl pH 7.5, 20 mM NaF, 25 mM β-glycerolphosphate pH 7.5, 120 mM NaCl, and 2% NP-40 in distilled water). The lysed pellets were dissolved in 90 μL 1 M NaOH containing 10% DMSO for 30 min at 55°C, after which, the absorbance of the resulting solutions was measured at 405 nm [12].
2.14. Statistical analysis
All data are presented as mean ± standard deviation and each experiment consisted of three or four replications. The Mann–Whitney U test was used to analyze the statistical difference between groups. A p value < 0.05 was regarded as statistically significant. All statistical tests were performed using SPSS version 22.0, 2013 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. BIOGF1K effects on cell viability
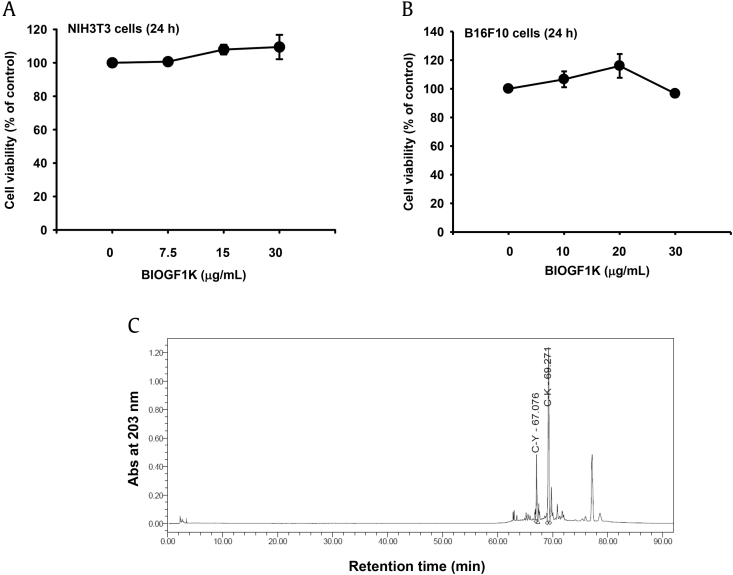
We first investigated the effects of BIOGF1K on the viability of NIH3T3 and B16F10 cells to determine the noncytotoxic BIOFG1K concentrations. BIOGF1K was not cytotoxic at concentrations up to 30 μg/mL, implying that 30 μg/mL of this extract had no cytotoxic effect (Figs. 1A, 1C). In addition, the level of compounds K and Y in BIOGF1K was analyzed by HPLC. Compounds K and Y were observed as major peaks at 67 min and 69 min in the BIOGF1K extract, but another compound also was observed as the third major peak at around 77.5 min (Fig. 1C).
Fig. 1.
Effect of BIOGF1K on the viability of NIH3T3 and B16F10 cells. (A and B) NIH3T3 and B16F10 cells were treated with various concentrations (0–30 μg/mL) of BIOGF1K and then incubated for 24 h. Cell viability was determined using the MTT assay. (C) The phytochemical profiles of compound K and compound Y in BIOGF1K were analyzed by HPLC. MTT, (3-4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide.
3.2. Protective effects of BIOGF1K against UVB irradiation-induced damage to NIH3T3 cells
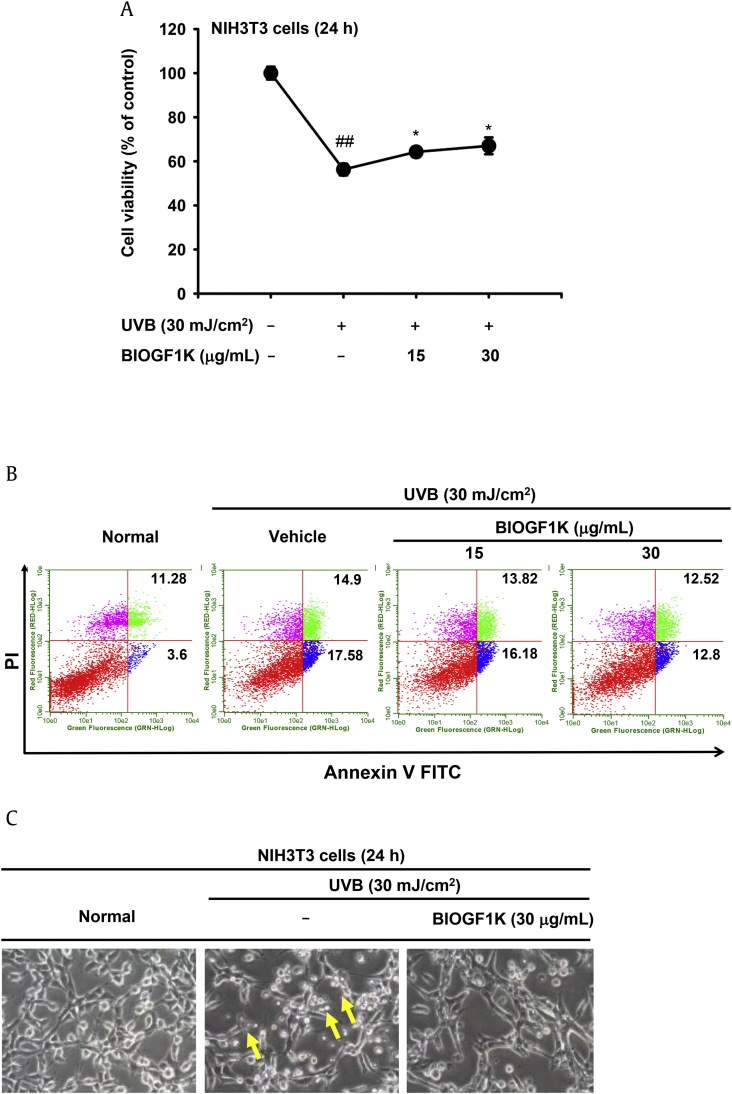
To examine the ability of BIOGF1K to protect against UVB-induced apoptosis in mouse embryonic fibroblasts, we determined whether BIOGF1K decreased UVB-irradiation-induced death of NIH3T3 cells using an MTT assay. Irradiation with UVB (30 mJ/cm2) significantly reduced the viability of NIH3T3 cells by up to 40% (Fig. 2A). However, treatment with BIOGF1K (15 and 30 μg/mL) significantly increased the viability of UVB-exposed fibroblasts; this effect occurred in a dose-dependent manner (Fig. 2A). To determine whether this effect of BIOGF1K was due to suppression of UVB-induced apoptosis, we performed flow cytometric analysis of UVB-treated cells. Apoptosis was increased by 17.58% upon UVB irradiation; this increase was suppressed by BIOGF1K treatment (Fig. 2B). In agreement with this finding, BIOGF1K treatment also reduced morphological changes in UVB-treated NIH3T3 cells (Fig. 2C).
Fig. 2.
Protective effect of BIOGF1K against UVB-irradiation-induced damage in NIH3T3 cells. (A) NIH3T3 cells were irradiated with UVB (30 mJ/cm2) and then treated with BIOGF1K (15 or 30 μg/mL) for 24 h. Cell viability was then determined using the MTT assay. (B) The antiapoptotic effect of BIOGF1K was assessed by analyzing the levels of apoptotic cells after staining with Annexin V–FITC and PI for 15 min. (C) Images of NIH3T3 cells treated with BIOGF1K (30 μg/mL) for 24 h were obtained with a digital camera after irradiation with UVB (30 mJ/cm2). *p < 0.05 compared to the control group. **p < 0.01 compared to the normal group. FITC, fluorescein isothiocyanate; MTT, (3-4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide; PI, propidium iodide.
3.3. Antimelanogenesis effect of BIOGF1K in α-MSH-treated B16F10 cells
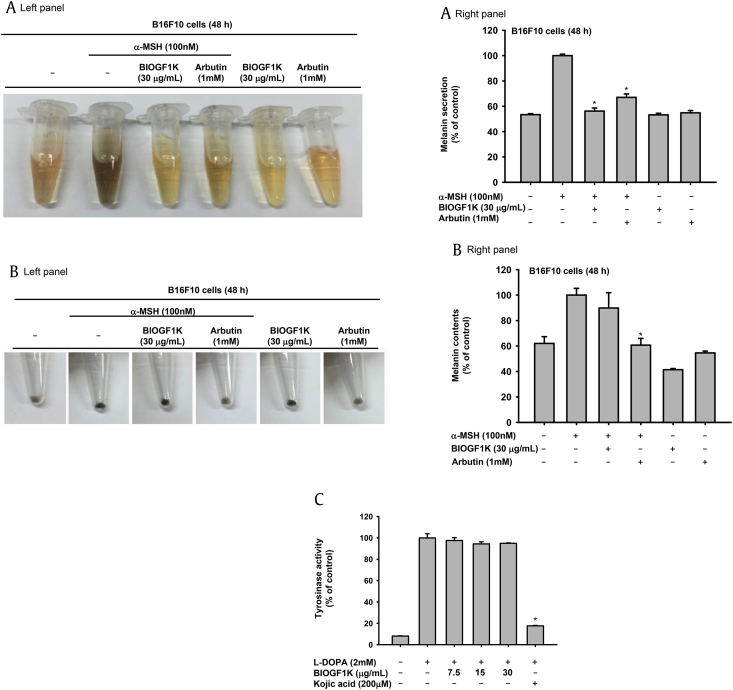
Since UVB irradiation induces melanin production by melanocytes, we also examined whether BIOGF1K treatment suppressed this α-MSH-induced increase in melanogenesis. We used arbutin at 1mM concentration as a positive control drug, because arbutin is well known tyrosine inhibitor suppressing melanin production. The level of secreted melanin and intracellular melanin contents in α-MSH-stimulated B16F10 cells was increased up to twofold (Figs. 3A, 3B). While BIOGF1K (30 μg/mL) suppressed the secretion of melanin, intriguingly, it did not affect melanin production upon UVB irradiation (Figs. 3A, 3B). We used mushroom tyrosinase to investigate whether BIOGF1K-mediated inhibition of melanin secretion was related to the suppression of the enzymes involved in melanin production. BIOGF1K (7.5, 15, and 30 μg/mL) did not inhibit the activity of tyrosinase, while kojic acid (200 μM; positive control) strongly inhibited tyrosinase activity by up to 90% (Fig. 3C).
Fig. 3.
Antimelanogenesis effect of BIOGF1K in α-MSH-treated B16F10 cells. (A, B) B16F10 cells were treated with BIOGF1K (30 μg/mL) and incubated for 24 h. The levels of melanin secretion and production in B16F10 cells treated with α-MSH (100 nM) in the presence or absence of BIOGF1K (30 μg/mL) or arbutin (1 mM) for 48 h were then determined. (C) The effect of BIOGF1K (0–30 μg/mL) or kojic acid (200 μM) on mushroom tyrosinase activity was determined by quantifying the activity of purified tyrosinase. *p < 0.01 compared to the control group. alpha-MSH, α-melanocyte stimulating hormone; l-DOPA, l-dopamine.
3.4. Effect of BIOGF1K on collagen degradation and inflammatory responses in UVB-irradiated NIH3T3 cells
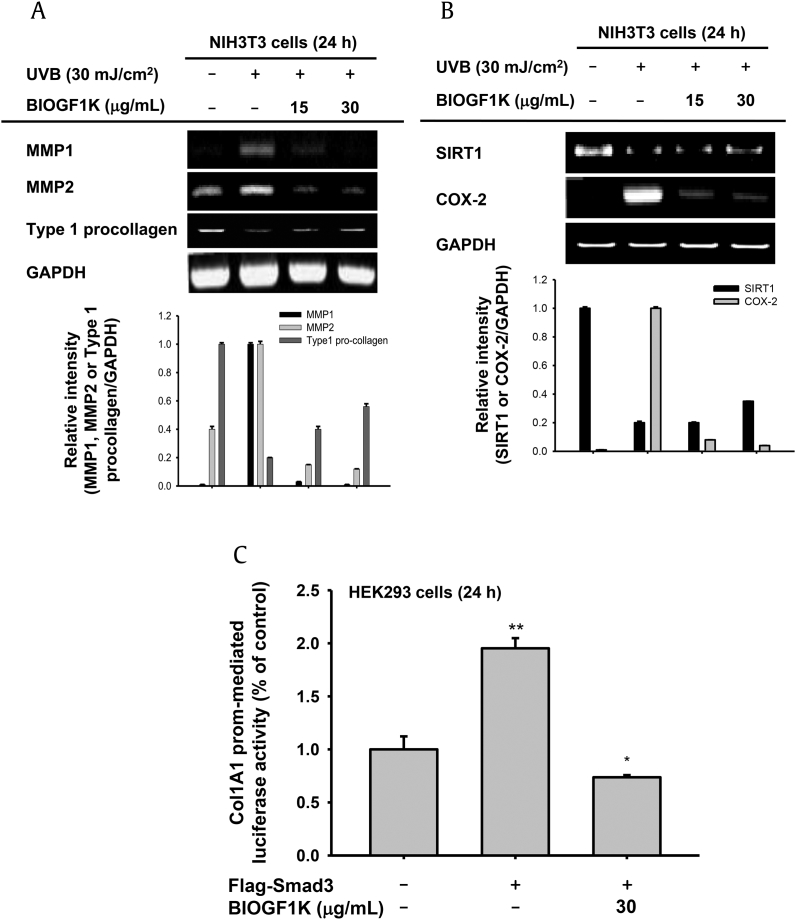
To study the effect of BIOGF1K on collagen degradation in UVB-irradiated NIH3T3 cells, cells were treated with 15 or 30 μg/mL BIOGF1K at 24 h after UVB irradiation and the mRNA levels of MMPs and type 1 procollagen were determined by RT-PCR. UVB irradiation markedly enhanced the mRNA levels of MMP-1, MMP-2, and cyclo-oxygenase (COX)-2 but downregulated the mRNA levels of type 1 procollagen and SIRT1 (Figs. 4A, 4B). Interestingly, treatment of UVB-exposed cells with BIOGF1K (15 or 30 μg/mL) for 24 h downregulated the mRNA levels of MMP-1, MMP-2, and COX-2, and upregulated the mRNA levels of type 1 pro-collagen and SIRT1 to normal levels; these effects occurred in a dose-dependent manner (Figs. 3A, 3B). To assess whether BIOGF1K directly regulated collagen synthesis at the transcriptional level, we performed a luciferase reporter assay in HEK293 cells. This assay utilized a luciferase construct harboring the binding sites of collagen synthesis-regulatory transcriptional factor Col1A1, which is activated by Smad3, an upstream signal molecule of Col1A1, facilitating Col1A1 synthesis. Smad3 increased luciferase activity up to twofold, but BIOGF1K did not block this increase in luciferase activity (Fig. 4C).
Fig. 4.
Effect of BIOGF1K on collagen degradation and the inflammatory response in UV-irradiated NIH3T3 cells. (A, B) NIH3T3 cells were irradiated with UVB (30 mJ/cm2) and then treated with BIOGF1K (15 or 30 μg/mL) for 24 h. The mRNA levels of MMP1, MMP2, COX-2, SIRT1, and type 1 procollagen were then determined by reverse transcription-polymerase chain reaction. (C) The promoter binding activity of the transcription factor Col1A1 was analyzed using a reporter gene assay. HEK293 cells were transfected with plasmids driving the expression of Col1A1-Luc (1 μg/mL), β-galactosidase (as a transfection control), and FLAG-Smad3 (1 μg/mL) and then treated with or without BIOGF1K for 24 h. Luciferase activity was measured using a luminometer. Relative intensity was calculated using total levels by the DNR Bio-imaging system. *p < 0.01 compared to the control group. **p < 0.01 compared to the normal group. Col1A1, collagen 1A1; COX-2, cyclo-oxygenase-2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MMP, matrix metalloproteinase; SIRT1, sirtuin 1.
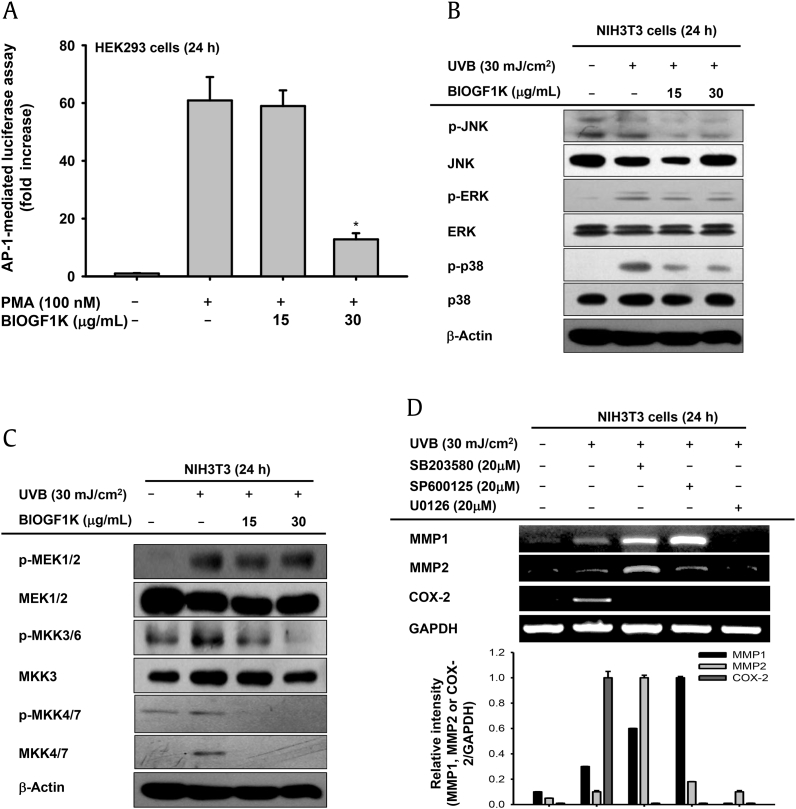
3.5. Effect of BIOGF1K on AP-1 activation and upstream signaling in UVB-irradiated NIH3T3 cells
UVB irradiation has been reported to activate the AP-1 signaling pathway. AP-1 is a critical transcription factor for upregulating production of collagen-degrading enzymes and the proinflammatory factor COX-2 [8], [9], [29]. We therefore tested whether BIOGF1K suppressed UVB-induced AP-1 signaling. BIOGF1K (30 μg/mL) significantly suppressed PMA-induced luciferase activity mediated by AP-1 (Fig. 5A). In addition, UVB-triggered phosphorylation of MAPKs (JNK, ERK, and p38), and MKK3/6 was remarkably suppressed by BIOGF1K (15 and 30 μg/mL; Figs. 5B, 5C). Interestingly, we observed that total form of MKK4/7 was only increased in the UVB-irradiated group (Fig. 5C). Finally, specific MAPK inhibitors (SB203580, a p38 inhibitor; SP600125, a JNK inhibitor; and U0126, an ERK inhibitor) were used to determine which MAPK was responsible for the downregulation of collagen-degrading enzymes and inflammatory genes. U0126 downregulated the expression of all genes tested under UVB irradiation, while SB203580 and SP600125 strongly suppressed the expression of COX-2 mRNA (Fig. 5D).
Fig. 5.
Effect of BIOGF1K on AP-1 activation and upstream signaling in UV-irradiated NIH3T3 cells. (A) The promoter binding activity of the transcription factor AP-1 was analyzed using a reporter gene assay. HEK293 cells were transfected with plasmids driving the expression of AP-1-Luc (1 μg/mL) and β-galactosidase (as a transfection control) in the presence or absence of PMA (100 nM) and BIOGF1K (15 or 30 μg/mL) for 24 h. (B and C) The levels of phospho- and total forms of JNK, ERK, p38, IκBα, MEK 1/2, MKK 3/6, MKK4/7, and β-actin in whole cell lysates of UVB-irradiated NIH3T3 cells treated with BIOGF1K were determined by immunoblotting. (D) The effects of MAPK inhibitors [SB203580 (p38 inhibitor, 20 μM), SP600125 (JNK inhibitor, 20 μM), and U0126 (ERK inhibitor, 20 μM)] on expression of MMP1, MMP2, and COX-2 in UVB-irradiated NIH3T3 cells were determined by reverse transcription-polymerase chain reaction. Relative intensity was calculated using total levels by the DNR Bio-imaging system. *p < 0.01 compared to the control group. AP-1, activator protein-1; COX-2, cyclo-oxygenase-2; ERK, extracellular response kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; JNK, C-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; MKK, MAPK kinase; MMP, matrix metalloproteinase; p, phosphorylated; PMA, Phorbol-12-myristate-13 acetate.
4. Discussion
The objective of this study was to investigate the effects of BIOGF1K on UVB-induced damage associated with aging, including apoptosis, collagen degradation, and melanin formation. The cellular and molecular events involved in UVB-induced mutagenesis, cancer, and aging have been extensively characterized [30], [31], [32], [33], [34]. In particular, UVB affects the DNA repair system, apoptosis, melanin formation, and the cell cycle [35], [36], [37], [38]. In this study, we examined the antiaging, antiapoptosis, and whitening activities of BIOGF1K in UVB-treated NIH3T3 and B16F10 cells in vitro. To assess the potential of BIOGF1K for use in antiaging and whitening remedies, we also investigated the phenomenological effects and molecular mechanisms by which this extract acts.
Apoptosis is a type of programmed cell death regulated by various proteolytic caspases [39]. Previous studies have demonstrated that UVB irradiation induces caspase-3 activation, which in turn is associated with DNA damage, apoptosis, and photoaging [40], [41], [42]. UVB irradiation is also known to induce the production of MMPs, which degrade native fibrillary collagen and inhibit the construction of collagenous extracellular matrix [43]. The extracellular matrix is composed of collagens, which are the major components of bone, cartilage, and dentin. These collagens are related to skin aging, wrinkles, and skin tumors [44], [45], [46]. Collagen metabolism is important to these biological processes. Collagenases are able to degrade triple-helical fibril collagens. MMPs are collagenases capable of degrading fibrillary collagen and are also responsible for the destruction of collagenous extracellular matrix. Thus, UVB is a major cause of collagen degradation and is responsible for skin aging and wrinkling through upregulation of MMPs and inhibition of collagen synthesis.
Interestingly, we found that the compound-K-rich fraction BIOGF1K restored cell viability in the presence of UVB irradiation and inhibited apoptosis in UVB-treated NIH3T3 cells (Fig. 2). Moreover, the BIOGF1K fraction reduced UVB-induced production of MMPs and restored procollagen expression without stimulating collagen synthesis by activating the Smad3-dependent pathway (Fig. 4). These findings imply that BIOGF1K might act specifically against UVB-irradiation-induced photoaging processes. Moreover, α-MSH-induced hyperpigmentation due to melanin secretion, triggered by UVB irradiation, [47] was inhibited by BIOGF1K (Fig. 3A), suggesting that BIOGF1K can act as an antimelanogenic agent that suppresses melanin secretion. Although BIOGF1K was revealed not to have direct suppressive action on melanin formation and tyrosinase activity, present results seem to raise a possibility that BIOGF1K can have another potential merit for the development of cosmetic products in addition to its antiphotoaging properties. In fact, numerous cosmetic products contain fractions previously prepared in Korea from P. ginseng (e.g., BG11001, P. ginseng berry, and AP-SF) [48], [49], [50]. Studies on individual compounds from P. ginseng have indicated that ginsenosides (G)-Rg2, G-F2, G-Re, and G-Rb2 protect skin and possess additional cosmeceutical activities by suppressing melanin biosynthesis, enhancing cyclic growth of hair follicles, improving skin barrier function, and suppressing skin inflammation [51], [52], [53]. Therefore, ginsenosides in BIOGF1K could contribute to its antiphotoaging activity, thereby increasing the value of this fraction as a cosmeceutical biomaterial. So far, it is unclear why BIOGF1K is capable of blocking the process of melanin secretion. Since numerous studies have been explored on how melanosomes are secreted [54], [55], [56], therefore, we will further examine whether this preparation can modulate the functional role of melanosome secretion regulatory proteins.
Many studies have demonstrated that UVB-induced apoptosis and collagen metabolism are predominantly regulated by the AP-1 signaling pathway [8], [9]. In addition, AP-1 upstream signaling events mediated by MAPKs and their associated kinases are activated by UVB irradiation [57]. We also confirmed that UVB irradiation of NIH3T3 cells increased phosphorylated JNK, ERK, and p38 levels, in addition to the phosphorylated levels of their upstream kinases MEK1/2, MKK3/6, and MKK4/7 (Figs. 5B, 5C). As we expected, it was found that BIOGF1K is able to suppress the increased levels of phospho-MKK3/6 and phospho-MKK4/7 during UVB irradiation conditions, although total MKK4/7 was appeared to be also inducible under UVB irradiation conditions (Fig. 5C). Intriguingly, BIOGF1K strongly suppressed the phosphorylation of these enzymes, implying that these enzymes could be targeted by this fraction. In particular, the finding that U0126 (an inhibitor of ERK) strongly suppressed the expression of COX-2, MMP1, and MMP2 (Fig. 5D) indicated that BIOGF1K-mediated ERK inhibition could be a critical factor in the suppression of UVB-induced photoaging events mediated by AP-1. Nonetheless, it is not yet precisely clear how BIOGF1K suppressed the enzymatic activity of MAPKs. Therefore, future studies will focus on elucidating the exact molecular mechanisms of BIOGF1K action.
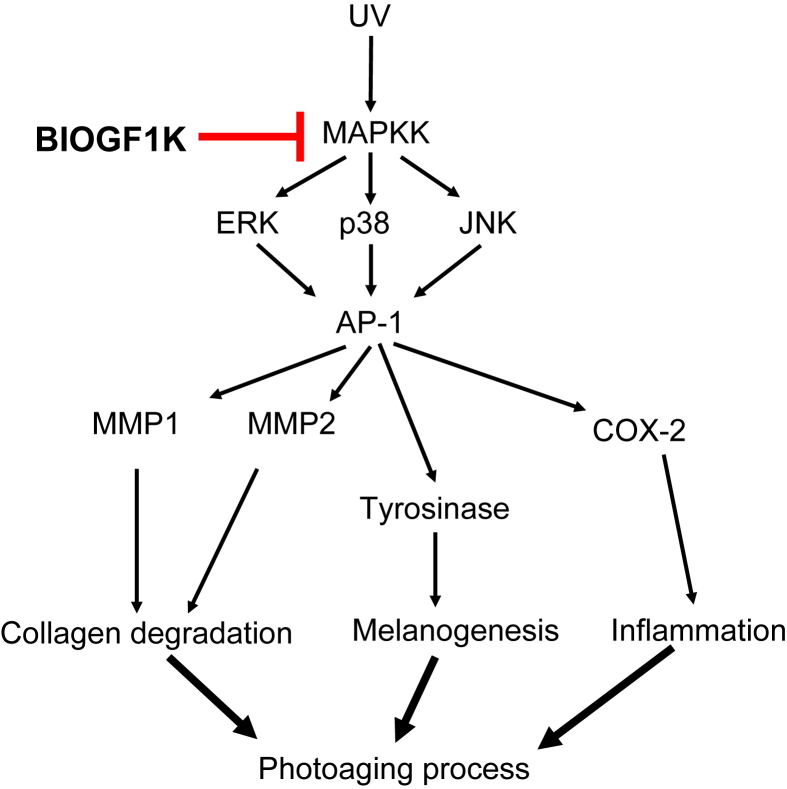
In summary, we demonstrated that BIOGF1K prevented UVB-induced cell death, inhibited apoptosis, suppressed morphological changes, downregulated melanin secretion, restored the levels of type I procollagen and SIRT1, and reduced mRNA levels of MMP-1, MMP-2, and COX-2 in UVB-irradiated NIH3T3 fibroblasts and α-MSH-treated B16F10 cells. Moreover, immunoblot analysis and luciferase reporter gene assays strongly indicated that BIOGF1K suppressed AP-1 activity and the activities of its upstream regulatory enzymes, ERK, p38, and JNK (Fig. 6). Therefore, our results strongly suggest that BIOGF1K can prevent photoaging and that this fraction has potential applications in cosmeceutical preparations.
Fig. 6.
Putative inhibitory pathway of BIOGF1K-mediated antiphotoaging events. AP-1, activator protein-1; COX-2, cyclo-oxygenase-2; ERK, extracellular response kinase; JNK, c-Jun N-terminal kinase; MAPKK, mitogen-activated protein kinase kinase; MMP, matrix metalloproteinase.
Conflicts of interest
The authors report no conflicts of interest.
Contributor Information
Junseong Park, Email: superbody@amorepacific.com.
Jae Youl Cho, Email: jaecho@skku.edu.
References
- 1.Asadamongkol B., Zhang J.H. The development of hyperbaric oxygen therapy for skin rejuvenation and treatment of photoaging. Med Gas Res. 2014;4:1. doi: 10.1186/2045-9912-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch R., Philips N., Suárez-Pérez J.A., Juarranz A., Devmurari A., Chalensouk-Khaosaat J., González S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants. 2015;4:248–268. doi: 10.3390/antiox4020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung K.W., Choi Y.J., Park M.H., Jang E.J., Kim D.H., Park B.H., Yu B.P., Chung H.Y. Molecular insights into SIRT1 protection against UVB-induced skin fibroblast senescence by suppression of oxidative stress and p53 acetylation. J Gerontol A Biol Sci Med Sci. 2014;70:959–968. doi: 10.1093/gerona/glu137. [DOI] [PubMed] [Google Scholar]
- 4.Yan Y., Li L., Xu H., Peng S., Qu T., Wang B. Receptor interacting protein 1 involved in ultraviolet B induced NIH3T3 cell apoptosis through expression of matrix metalloproteinases and reactive oxygen species production. Chin Med J. 2013;126:4327–4333. [PubMed] [Google Scholar]
- 5.Brenneisen P., Sies H., Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases. Ann N Y Acad Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 6.Ming M., Soltani K., Shea C.R., Li X., He Y.Y. Dual role of SIRT1 in UVB-induced skin tumorigenesis. Oncogene. 2015;34:357–363. doi: 10.1038/onc.2013.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao C., Lu S., Kivlin R., Wallin B., Card E., Bagdasarian A., Tamakloe T., Wj Wang, Song X., Wm Chu. SIRT1 confers protection against UVB-and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J Cell Mol Med. 2009;13:3632–3643. doi: 10.1111/j.1582-4934.2008.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J., Guo J.H., Tu X.L., Zhang C., Zhao M., Zhang Q.W., Gao F.H. Tiron Inhibits UVB-induced AP-1 binding sites transcriptional activation on MMP-1 and MMP-3 promoters by MAPK signaling pathway in human dermal fibroblasts. PloS One. 2016;11:e0159998. doi: 10.1371/journal.pone.0159998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H.J., Hwang E., Park B., Zhang M., Zw Sun, Lee D.G., Park S.Y., Yi T.H. Methanol extract of bitter melon alleviates UVB-induced MMPs expression via MAP kinase and AP-1 signaling in human dermal fibroblasts in vitro. Phytother Res. 2016;30:1519–1526. doi: 10.1002/ptr.5656. [DOI] [PubMed] [Google Scholar]
- 10.D'Mello S.A., Finlay G.J., Baguley B.C., Askarian-Amiri M.E. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:1144. doi: 10.3390/ijms17071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englaro W., Rezzonico R., Durand-Clément M., Lallemand D., Ortonne J.P., Ballotti R. Mitogen-activated protein kinase pathway and AP-1 are activated during cAMP-induced melanogenesis in B-16 melanoma cells. J Biol Chem. 1995;270:24315–24320. doi: 10.1074/jbc.270.41.24315. [DOI] [PubMed] [Google Scholar]
- 12.Kim J.H., Baek E.J., Lee E.J., Yeom M.H., Park J.S., Lee K.W., Kang N.J. Ginsenoside F1 attenuates hyperpigmentation in B16F10 melanoma cells by inducing dendrite retraction and activating Rho signalling. Exp Dermatol. 2015;24:150–152. doi: 10.1111/exd.12586. [DOI] [PubMed] [Google Scholar]
- 13.Choi Kt. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng CA Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee H.J., Lee J.Y., Song K.C., Kim J.H., Park J.H., Chun K.H., Hwang G.S. Protective effect of processed Panax ginseng, sun ginseng on UVB-irradiated human skin keratinocyte and human dermal fibroblast. J Ginseng Res. 2012;36:68–77. doi: 10.5142/jgr.2012.36.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hossen M.J., Hong Y.D., Baek K.S., Yoo S., Hong Y.H., Kim J.H., Lee J.O., Kim D., Park J., Cho J.Y. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J Ginseng Res. 2017;41:43–51. doi: 10.1016/j.jgr.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hossen M.J., Baek K.S., Kim E., Yang W.S., Jeong D., Kim J.H., Kweon D.H., Yoon D.H., Kim T.W., Kim J.H. In vivo and in vitro anti-inflammatory activities of Persicaria chinensis methanolic extract targeting Src/Syk/NF-κB. J Ethnopharmacol. 2015;159:9–16. doi: 10.1016/j.jep.2014.10.064. [DOI] [PubMed] [Google Scholar]
- 17.Tavazoie S.F., Alarcón C., Oskarsson T., Padua D., Wang Q., Bos P.D., Gerald W.L., Massagué J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding N., Ruth T.Y., Subramaniam N., Sherman M.H., Wilson C., Rao R., Leblanc M., Coulter S., He M., Scott C. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan S.Y., Ang C.Y., Luo Z., Li P., Nguyen K.T., Zhao Y. An iGlu receptor antagonist and its simultaneous use with an anticancer drug for cancer therapy. Chemistry. 2015;21:6123–6131. doi: 10.1002/chem.201406527. [DOI] [PubMed] [Google Scholar]
- 20.Baek K.S., Yi Y.S., Son Y.J., Yoo S., Sung N.Y., Kim Y., Hong S., Aravinthan A., Kim J.H., Cho J.Y. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J Ginseng Res. 2016;40:437–444. doi: 10.1016/j.jgr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y., Yu T., Jang H.J., Byeon S.E., Song S.Y., Lee B.H., Rhee M.H., Kim T.W., Lee J., Hong S. In vitro and in vivo anti-inflammatory activities of Polygonum hydropiper methanol extract. J Ethnopharmacol. 2012;139:616–625. doi: 10.1016/j.jep.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Kim M.Y., Cho J.Y. 20S-dihydroprotopanaxadiol, a ginsenoside derivative, boosts innate immune responses of monocytes and macrophages. J Ginseng Res. 2013;37:293–299. doi: 10.5142/jgr.2013.37.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starkenmann C., Luca L., Niclass Y., Praz E., Roguet D. Comparison of volatile constituents of Persicaria odorata(Lour.) Sojak (Polygonum odoratum Lour.) and Persicaria hydropiper L. Spach (Polygonum hydropiper L.) J Agric Food Chem. 2006;54:3067–3071. doi: 10.1021/jf0531611. [DOI] [PubMed] [Google Scholar]
- 24.Almela L., Sanchez-Munoz B., Fernandez-Lopez J.A., Roca M.J., Rabe V. Liquid chromatograpic-mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J Chromatogr A. 2006;1120:221–229. doi: 10.1016/j.chroma.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Y., Ren X., Zhang Y., Patel R., Sharma A., Wu H., Robertson G.P., Yan L., Rubin E., Yang J.M. eEF-2 kinase dictates cross-talk between autophagy and apoptosis induced by Akt inhibition, thereby modulating cytotoxicity of novel Akt inhibitor MK-2206. Cancer Res. 2011;71:2654–2663. doi: 10.1158/0008-5472.CAN-10-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H.J., Hyun E.A., Yoon W.J., Kim B.H., Rhee M.H., Kang H.K., Cho J.Y., Yoo E.S. In vitro anti-inflammatory and anti-oxidative effects of Cinnamomum camphora extracts. J Ethnopharmacol. 2006;103:208–216. doi: 10.1016/j.jep.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Cho M.K., Jang Y.P., Kim Y.C., Kim S.G. Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits MAP kinases and AP-1 activation via potent MKK inhibition: the role in TNF-α inhibition. Int Immunopharmacol. 2004;4:1419–1429. doi: 10.1016/j.intimp.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Kubo I., Nitoda T., Ki Nihei. Effects of quercetin on mushroom tyrosinase and B16-F10 melanoma cells. Molecules. 2007;12:1045–1056. doi: 10.3390/12051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanos T., Marinissen M.J., Leskow F.C., Hochbaum D., Martinetto H., Gutkind J.S., Coso O.A. Phosphorylation of c-Fos by members of the p38 MAPK family role in the AP-1 response to UV light. J Biol Chem. 2005;280:18842–18852. doi: 10.1074/jbc.M500620200. [DOI] [PubMed] [Google Scholar]
- 30.Ikehata H., Ono T. The mechanisms of UV mutagenesis. J Radiat Res. 2011;52:115–125. doi: 10.1269/jrr.10175. [DOI] [PubMed] [Google Scholar]
- 31.Brash D.E., Rudolph J.A., Simon J.A., Lin A., McKenna G.J., Baden H.P., Halperin A.J., Ponten J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong B.K., Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 33.Rittié L., Fisher G.J. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705–720. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 34.Brown W.T. Human mutations affecting aging – a review. Mech Ageing Dev. 1979;9:325–336. doi: 10.1016/0047-6374(79)90109-x. [DOI] [PubMed] [Google Scholar]
- 35.Sinha R.P., Häder D.P. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. 2002;1:705–720. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- 36.Guo L., Huang Z.X., Chen X.W., Deng Q.K., Yan W., Zhou M.J., Ou C.S., Ding Z.H. Differential expression profiles of microRNAs in NIH3T3 cells in response to UVB irradiation. Photochem Photobiol. 2009;85:765–773. doi: 10.1111/j.1751-1097.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- 37.Jablonski N.G., Chaplin G. Chaplin, Human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107:8962–8968. doi: 10.1073/pnas.0914628107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang D., Wu D., Hirao A., Lahti J.M., Liu L., Mazza B., Kidd V.J., Mak T.W., Ingram A.J. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem. 2002;277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- 39.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumann L. How to prevent photoaging? J Invest Dermatol. 2005;125:xii–xiii. doi: 10.1111/j.0022-202X.2005.23810.x. [DOI] [PubMed] [Google Scholar]
- 41.Xiao G.H., Jeffers M., Bellacosa A., Mitsuuchi Y., Woude G.F.V., Testa J.R. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A. 2001;98:247–252. doi: 10.1073/pnas.011532898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumura M., Tanaka N., Kuroki T., Ichihashi M., Ohba M. The η isoform of protein kinase C inhibits UV-induced activation of caspase-3 in normal human keratinocytes. Biochem Biophys Res Commun. 2003;303:350–356. doi: 10.1016/s0006-291x(03)00345-0. [DOI] [PubMed] [Google Scholar]
- 43.Cho S., Kim H.H., Lee M.J., Lee S., Park C.-S., Nam S.-J., Han J.-J., Kim J.-W., Chung J.H. Phosphatidylserine prevents UV-induced decrease of type I procollagen and increase of MMP-1 in dermal fibroblasts and human skin in vivo. J Lipid Res. 2008;49:1235–1245. doi: 10.1194/jlr.M700581-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Kang T.H., Park H.M., Kim Y.B., Kim H., Kim N., Do J.H., Kang C., Cho Y., Kim S.Y. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J Ethnopharmacol. 2009;123:446–451. doi: 10.1016/j.jep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 45.Brennan M., Bhatti H., Nerusu K.C., Bhagavathula N., Kang S., Fisher G.J., Varani J., Voorhees J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol. 2003;78:43–48. doi: 10.1562/0031-8655(2003)078<0043:mmitmc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Jung S.K., Lee K.W., Kim H.Y., Oh M.H., Byun S., Lim S.H., Heo Y.S., Kang N.J., Bode A.M., Dong Z. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem Pharmacol. 2010;79:1455–1461. doi: 10.1016/j.bcp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu L., Lu Y., Yu W.G., Zhao X., Lu Y.H. Anti-photoageing and anti-melanogenesis activities of chrysin. Pharm Biol. 2016:1–9. doi: 10.1080/13880209.2016.1179334. [DOI] [PubMed] [Google Scholar]
- 48.Park S.Y., Shin Y.K., Kim H.T., Kim Y.M., Lee D.G., Hwang E., Cho B.G., Yin C.S., Kim K.Y., Yi T.H. A single-center, randomized, double-blind, placebo-controlled study on the efficacy and safety of “enzyme-treated red ginseng powder complex (BG11001)” for antiwrinkle and proelasticity in individuals with healthy skin. J Ginseng Res. 2016;40:260–268. doi: 10.1016/j.jgr.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee D.Y., Jeong Y.T., Jeong S.C., Lee M.K., Min J.W., Lee J.W., Kim G.S., Lee S.E., Ahn Y.S., Kang H.C., Kim J.H. Melanin biosynthesis inhibition effects of Ginsenoside Rb2 isolated from Panax ginseng Berry. J Microbiol Biotechnol. 2015;25:2011–2015. doi: 10.4014/jmb.1505.05069. [DOI] [PubMed] [Google Scholar]
- 50.Baek K.S., Hong Y.D., Kim Y., Sung N.Y., Yang S., Lee K.M., Park J.Y., Park J.S., Rho H.S., Shin S.S., Cho J.Y. Anti-inflammatory activity of AP-SF, a ginsenoside-enriched fraction, from Korean ginseng. J Ginseng Res. 2015;39:155–161. doi: 10.1016/j.jgr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z., Ryu S.W., Lee J., Choi K., Kim S., Choi C. Protopanaxatirol type ginsenoside Re promotes cyclic growth of hair follicles via inhibiting transforming growth factor beta signaling cascades. Biochem Biophys Res Commun. 2016;470:924–929. doi: 10.1016/j.bbrc.2016.01.148. [DOI] [PubMed] [Google Scholar]
- 52.Park S.H., Seo W., Eun H.S., Kim S.Y., Jo E., Kim M.H., Choi W.M., Lee J.H., Shim Y.R., Cui C.H., Kim S.C., Hwang C.Y., Jeong W.I. Protective effects of ginsenoside F2 on 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation in mice. Biochem Biophys Res Commun. 2016;478:1713–1719. doi: 10.1016/j.bbrc.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Chung I., Lee J., Park Y.S., Lim Y., Chang D.H., Park J., Hwang J.S. Inhibitory mechanism of Korean Red Ginseng on GM-CSF expression in UVB-irradiated keratinocytes. J Ginseng Res. 2015;39:322–330. doi: 10.1016/j.jgr.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee E.J., Kim J.Y., Oh S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci Rep. 2016;6:27848. doi: 10.1038/srep27848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park P.J., Lee T.R., Cho E.G. Substance P stimulates endothelin 1 secretion via endothelin-converting enzyme 1 and promotes melanogenesis in human melanocytes. J Invest Dermatol. 2015;135:551–559. doi: 10.1038/jid.2014.423. [DOI] [PubMed] [Google Scholar]
- 56.Won Y.K., Lin C.B., Seiberg M., Chen N., Hu Y., Rossetti D., Saliou C., Loy C.J. Galvanic zinc–copper microparticles inhibit melanogenesis via multiple pigmentary pathways. Arch Dermatol Res. 2014;306:27–35. doi: 10.1007/s00403-013-1369-y. [DOI] [PubMed] [Google Scholar]
- 57.Bode A.M., Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci STKE. 2003;2003:RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]