Abstract
Background
Depression is one of the most commonly diagnosed neuropsychiatric diseases, but the underlying mechanism and medicine are not well-known. Although Panax ginseng has been reported to exert protective effects in various neurological studies, little information is available regarding its antidepressant effects.
Methods
Here, we examined the antidepressant effect and underlying mechanism of P. ginseng extract (PGE) in a chronic restraint stress (CRS)-induced depression model in mice.
Results
Oral administration of PGE for 14 d decreased immobility (depression-like behaviors) time in forced swim and tail suspended tests after CRS induction, which corresponded with attenuation of the levels of serum adrenocorticotropic hormone and corticosterone, as well as attenuated c-Fos expression in the amygdala. PGE enhanced messenger RNA expression level of brain-derived neurotrophic factor but ameliorated microglial activation and neuroinflammation (the level of messenger RNA and protein expression of cyclooxygenase-2 and inducible nitric oxide synthase) in the amygdala of mice after CRS induction. Interestingly, 14-d treatment with celecoxib, a selective cyclooxygenase-2 inhibitor, and Nω-nitro-L-arginine methyl ester hydrochloride, a selective inducible nitric oxide synthase inhibitor, attenuated depression-like behaviors after CRS induction. Additionally, PGE inhibited the upregulation of the nuclear factor erythroid 2 related factor 2 and heme oxygenase-1 pathways.
Conclusion
Taken together, our findings suggest that PGE exerts antidepressant-like effect of CRS-induced depression by antineuroinflammatory and antioxidant (nuclear factor erythroid 2 related factor 2/heme oxygenase-1 activation) activities by inhibiting the hypothalamo-pituitary-adrenal axis mechanism. Further studies are needed to evaluate the potential of components of P. ginseng as an alternative treatment of depression, including clinical trial evaluation.
Keywords: antineuroinflammation, chronic restraint stress, depression, nuclear factor erythroid 2 related factor 2, Panax ginseng
1. Introduction
Depression is one of the most prevalent psychiatric disorders that can affect a person's thoughts, behavior, feelings, and sense of well-being [1], [2]. Patients with depression and experimental animal models display structural alterations (dendritic remodeling in neurons) in some brain areas including the prefrontal cortex, hippocampus, and amygdala [1], [2], which may be related to aberrations in neurotrophic factors/neurotransmitter and receptor signaling pathways, disturbances in the hypothalamo-pituitary-adrenocortical (HPA) axis, inflammation, immune dysfunctions, and oxidative stress [1], [2], [3]. Currently, accumulating evidence increases the possibilities of significant roles of amygdala in depression. For example, both acute and chronic experimental stressors cause patterns of dendritic remodeling in neurons and neuronal hypertrophy of the amygdala [4], [5]. However, physiological significance of the amygdala in depression is not clearly understood. Now, patients with depression are normally treated with antidepressants (neurotransmitter-related medicines), such as serotonin-norepinephrine reuptake inhibitors and selective serotonin reuptake inhibitors (SSRIs) [6]. However, the classical antidepressants are only beneficial in ∼60% of patients [7] and often produce significant adverse effects, such as agitation, headache, dizziness, anxiety, constipation, nausea, and lethargy [1]. Efficient and safe drugs to treat depression are required.
Oxidative stress plays a main negative contribution to the neuroprogression that is observed in major depressive diseases [1], [2], [3]. However, nuclear factor erythroid 2 related factor 2 (Nrf2), a major regulator of the antioxidant response, plays a beneficial key role in inflammation which is involved in depression [8]. The deletion of Nrf2 exerts antidepressant effects and pretreatment with the Nrf2 activator sulforaphane prevents the depression-like phenotype induced by repeated social defeat stress [9]. Reactive oxygen species, oxidative stress biomarkers, serve as a crucial secondary messenger in signal transduction and significantly affect inflammatory pathways by activating mitogen-activated protein kinases (MAPKs) and nuclear factor kappa B (NF-κB) signaling pathways in depression [1], [2], [3]. Therefore, significant regulators of Nrf2, MAPKs, and NF-κB pathways might be a therapeutic tool to treat major depressive disorder.
Panax ginseng has been prescribed as a traditional herbal medicine for over 2,000 yr in Eastern Asia including Korea, China, and Japan [10]. Its antidepressant, antianxiety, and cognition-enhancing effects have been recorded by Shi-Zhen Li in Ben Cao Gang Mu, the most comprehensive premodern herbal text that was compiled during the Ming Dynasty in China. Traditional P. ginseng containing formulations, such as Sho-ju-sen, Kai Xin San aqueous extract, and Gincosan, mitigate the symptoms of depression in humans and rodent models [11], [12], [13]. Ginsenoside Rb1 and its metabolite compound K ameliorated depression-like behaviors during a menopausal depressive-like state in female mice through the 5-hydroxytryptamine 2A-receptor [14]. Ginsenoside Rb1 ameliorated chronic stress induced depression-like behaviors by increase of brain-derived neurotrophic factor (BDNF) expression in the amygdala of rats [15] and ameliorated neuroinflammation-induced depression-like behavior in rodents by blunting the upregulation in circulating interleukin (IL)-6 levels [16]. Ginsenoside Rb3 exerted antidepressant-like effects in several animal models by regulating BDNF and the monoamine neurotransmitters 5-hydroxytryptamine, dopamine, and norepinephrine [17]. 20(S)-Protopanaxadiol, an intestinal metabolite of ginseng, has antidepressant-like activity through the regulation of neuropeptide Y expression and hypothalamic corticotrophin-releasing factor, BDNF expression, neurofilament-L, and glucocorticoid receptor [18]. Based on these collective reports, P. ginseng extract (PGE) may exert stronger antidepressive effects than a single component, because PGE contains various active components, such as ginsenosides, gintonin, and polysaccharides [19]. Additionally, it can exert antidepressive effects by antioxidant and antiinflammatory activities, because PGE regulates the Nrf2, MAPKs, and NF-κB pathways in Alzheimer's and Parkinson's diseases [19]. However, the role and molecular mechanisms of antioxidant and antiinflammatory activities of P. ginseng on depression-like behaviors remain poorly understood. Here we demonstrated the antidepressant effects of PGE mediated by the upregulation of the Nrf2-heme oxygenase-1 (HO-1) pathway and downregulation of the neuroinflammatory system (MAPKs and NF-κB pathways) in the amygdala, using a rodent model of chronic restraint stress (CRS)-induced depression.
2. Materials and materials
2.1. Animals and ethical approval
Adult male C57BL/6 mice (Narabiotec Co., Ltd., Seoul, Korea) 8–10 wk of age, and 22–25 g in body weight, were housed at a constant temperature of 23 ± 2°C with a 12-h light-dark cycle (light on from 08:00 am to 08:00 pm), and food and water ad libitum. All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee at Kyung Hee University, Seoul, Korea. In this process, proper randomization of laboratory animals and handling of data were performed in a blinded manner in accordance with recent recommendations from an National Institutes of Health (NIH) workshop on preclinical models of neurological diseases [20].
2.2. Experimental design and CRS procedure
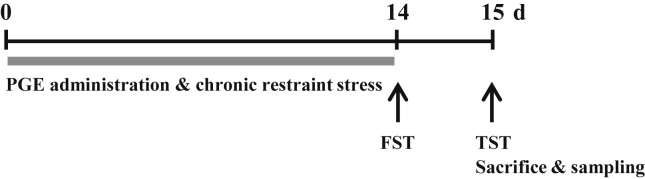
For the behavioral test, the experimental group was subdivided into the following groups (n = 4–6/group): sham group (nonstress + saline, p.o.), CRS group (CRS + saline, p.o.), and CRS + PGE group (CRS + 75 mg/kg, 150 mg/kg, or 300 mg/kg body weight of PGE, p.o.). For determination of serum corticosterone level and histological and molecular biological analyses, the experimental group was subdivided into the following groups (n = 4–6/group): sham group (nonstress + saline, p.o.), CRS group (CRS + saline, p.o.), CRS + PGE group (CRS + 150 mg/kg of PGE, p.o.), and PGE alone group (nonstress + 150 mg/kg of PGE, p.o.). Body weight of each mouse was measured 1 h prior to stress induction. Mice were subjected to CRS similar to a published procedure [21], but with some modifications (Fig. 1). In brief, mice in the sham group were left in their home cages in a noise-free environment, with food and water during the restraint stress period. Mice in the CRS and CRS + PGE groups were exposed to restraint stress for 2 h (from 10:00 am to 12:00 pm) each day for 14 d. For restraint stress, each mouse was placed in a transparent 50 mL polyethylene conical centrifuge tube of 10 cm in length and 2.5 cm in diameter that had many air holes to allow ventilation. After restraint stress induction, the mice were returned to their home cages and allowed free access to food and water for the duration of the experiment.
Fig. 1.
Schema for chronic restraint stress (CRS) and Panax ginseng extract (PGE) treatment, behavioral experiments, and tissue preparation. FST, forced swimming test; TST, tail suspension test.
2.3. Preparation of PGE
Dried roots of P. ginseng Meyer were purchased from a local farm in Yeonpung-myeon, Goesan-gun, Chungcheongbuk-do, Korea. Specimens were taxonomically identified by a Korean medicinal doctor (S.W.L.) at the National Institute of Horticultural and Herbal Science, Rural Development Administration, Eumseong, Korea. A voucher specimen (HPR-207) was deposited at the herbarium of Herbal Crop Research Institute (Eumsung, Korea). Water-based PGE was prepared because most traditional Oriental herbal materials are decocted with boiling water and since ginsenosides are more soluble in water than in organic solvents. The dried and crushed P. ginseng (200 g) was cut into small pieces and incubated in 3.0 L distilled water using a reflux extraction system. The aqueous extract was filtered, concentrated, lyophilized, and stored at −80°C until use. The final yield of dried P. ginseng was 18.3% (wt/wt).
2.4. Administration of PGE, celecoxib, and Nω-nitro-L-arginine methyl ester hydrochloride
PGE was dissolved in normal saline and orally administrated at a dose of 75 mg/kg, 150 mg/kg, or 300 mg/kg in a constant volume (100 μL) once daily from 30 min prior to stress exposure. Saline and PGE (150 mg/kg) were administrated at the same volume to the sham and PGE alone group, respectively. The selective cyclooxygenase-2 (COX-2) inhibitor celecoxib (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) and the selective inducible nitric oxide synthase (iNOS) inhibitor Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME; Sigma-Aldrich, St. Louis, MO, USA) were dissolved in 1% Tween 80/phosphate buffered saline and 0.9% saline, respectively, and intraperitonelly administrated once daily at a dose of 10 mg/kg and 20 mg/kg in a constant volume (100 μL) 30 min prior to stress exposure. The same volumes of phosphate buffered saline, celecoxib, and L-NAME were administered to the sham, celecoxib, and L-NAME alone groups, respectively.
2.5. Forced swimming test
Some 24 h after the last restraint stress induction, a forced swimming test (FST) was done as previously described [21] with some modifications. Briefly, mice (n = 4–6/group) were placed in a Plexiglas cylinder (15 cm in diameter × 25 cm in height) containing water at 23–24°C to a depth of 14 cm. Mice were individually placed in the cylinder for 6 min. Immobility time was quantified during the last 4 min. Each mouse was judged to be immobile when it ceased struggling and remained floating motionless in the water, making only those movements necessary to keep its head above water. At the end of a test, the wet animal was placed in a holding cage with normal bedding that was covered by an absorbent paper towel.
2.6. Tail suspension test
Some 24 h after the last restraint stress induction, a tail suspension test (TST) was performed according to previously described, with some modifications [21]. Briefly, mice (n = 4–6/group) were suspended individually 50 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail for 6 min. Cumulative immobility time was quantified during the last 4 min. Immobility was defined as the absence of limb or body movements, except for those caused by respiration when the mice hung passively and were completely motionless. During the test, mice were separated from each other to prevent possible visual and acoustical associations.
2.7. Enzyme-linked immunosorbent assay
Whole blood from each group (n = 4–6/group) was collected from the beating heart under ethyl ether anesthesia 24 h after the last restraint stress induction. Blood samples were kept at room temperature for 1 h and centrifuged at 700 g for 10 min. The serum (supernatant fraction) was transferred into a new tube for subsequent assays. The serum level of corticosterone and adrenocorticotropic hormone (ACTH) was measured with commercial enzyme-linked immunosorbent assay kits according to the manufacturer's instructions (Enzo Life Sciences, Farmingdale, NY, USA). To exclude the potential impact of diurnal rhythm on mouse hormone levels, blood samples were collected in the same time window of 12:00 am–01:00 pm.
2.8. Immunohistochemistry and Western blot analysis
Some 24 h after the last restraint stress induction, mice (n = 4–6/group) were perfused with 4% paraformaldehyde and brains were coronally cut into 30 μm thick sections with a crystat (Leica Instruments, Nussloch, Germany). Immunohistochemical analysis was accomplished as described previously [22], [23]. In brief, free-floating sections were reacted with either rabbit anti-c-Fos (1:2000; Oncogene Research Products, San Diego, CA, USA) or rabbit anti-ionized calcium-binding adapter molecule 1 (Iba-1; 1:2,000; WAKO, Osaka, Japan) as primary antibodies, followed by biotinylated rabbit IgG antibody (1:200; Vector Laboratories, Burlingame, CA, USA), and visualized using an ABC Elite kit (Vector Laboratories).
2.9. Western blot analysis
The amygdalas from each group (n = 4–6/group) were immediately removed 24 h after the last restraint stress induction. Western blot analysis was performed as previously described [24]. Briefly, each amygdala was homogenized in lysis buffer, separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the resolved proteins were transferred to polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, USA). Blots were incubated with mouse anti-c-Fos (1:2000; EMD Millipore Corp., Billerica, MA, USA), mouse anti-COX-2 (1:2000; BD Biosciences, San Jose, CA, USA), rabbit anti-iNOS (1:1000; Sigma-Aldrich), rabbit anti-Nrf2 (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), or mouse anti-HO-1 (1:1000; Enzo Life Sciences) followed by horseradish peroxidase-conjugated secondary antibody, and specific signals were visualized using chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Western blot images were quantified using Image J analysis software (JAVA image processing program, NIH, Bethesda, MD, USA).
2.10. Reverse transcription-polymerase chain reaction analysis
Some 24 h after the last restraint stress induction, the amygdala from each group (n = 4–6/group) were harvested and total RNA was extracted using TRIsure reagent according to the manufacturer's instructions (Bioline, London, UK) as previously described [24]. The primer sets used were 5′-GAT GCC GCA AAC ATG TCT ATG A -3′ and 5′-TAA TAC TGT CAC ACA CGC TCA GCT C-3′ for BDNF, 5′-TGC TTA CCT GGG TTA TGC TTC TG-3′ and 5′-CCG AGG TGC TCC TAA AAC CA-3′ for CD11b, 5′-CGAGTCCCTAGAGCGGCAAATG-3′ and 5′-CGGATCTGGAGGTTGGAGAAAGTC-3′ for glial fibrillary acidic protein (GFAP), 5′-TTG TGG CTG TGG AGA AGC TGT-3′ and 5′-AAC GTC ACA CAC CAG CAG GTT-3′ for IL-1ß, 5′-CAG TAT CAG AAC CGC ATT GCC-3′ and 5′-GAG CAA GTC CGT GTT CAA GGA-3′ for COX-2, 5′-GGC AAA CCC AAG GTC TAG GTT-3′ and 5′-TCG CTC AAG TTC AGC TTG GT-3′ for iNOS, and 5′-AGG TCA TCC CAG AGC TGA ACG-3′ and 5′-CAC CCT GTT GCT GTA GCC GTA T-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Expression levels of each gene were normalized to that of GAPDH.
2.11. Statistical analyses
Statistical analyses were performed by using the SPSS 21.0 package (SPSS Inc., Chicago, IL, USA) for Windows. Two-sample comparisons were carried out using the Student t test and multiple comparisons were made using one-way analysis of variance with Tukey post hoc test. All data are presented as mean ± standard error and statistical difference was accepted at the 5% level unless otherwise indicated.
3. Results
3.1. Antidepressant effect of PGE in a mouse CRS model
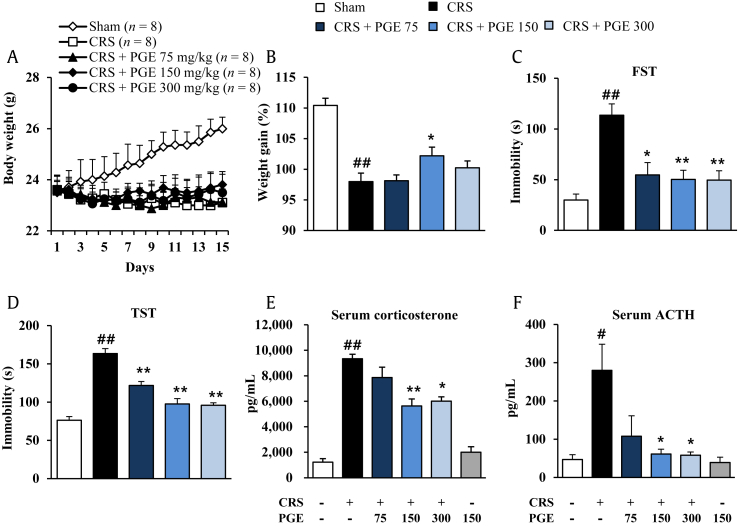
Since chronic exposure to stress alters body weight gain [25], we evaluated whether PGE had beneficial effects in the normal gain of body weight in mice during CRS induction. As shown in Fig. 2A and B, CRS significantly decreased normal increase of body weight for 14 d compared with the sham group, while administration of PGE (150 mg/kg) mitigated the significant CRS-mediated reduction of body weight. PGE at doses of 75 mg/kg and 300 mg/kg did not significantly influence reduction of the body weight by CRS. FST and TST have long been used to screen antidepressants, because these models cause a state of lower “mood” in animals that may be a component of clinical depression in humans [26]. Here, the antidepressant effects of PGE administration were evaluated in the FST and TST in mice. In the FST, CRS produced significantly increased immobility time (113.6 ± 11.2 seconds) compared with the sham group (29.9 ± 5.8 seconds), while administration of PGE significantly decreased immobility time (54.7 ± 12.1, 50.3 ± 9.1, and 49.6 ± 9.2 seconds, in 75 mg/kg, 15 mg/kg, and 300 mg/kg PGE, respectively) compared with the stress group (Fig. 2C). In the TST, the CRS significantly enhanced immobility (163.4 ± 6.6 seconds), compared with the sham group (76.2 ± 4.9 seconds), whereas administration of PGE significantly reduced immobility (121.8 ± 5.2, 97.5 ± 7.1, and 95.9 ± 3.3 seconds, in 75 mg/kg, 15 mg/kg, and 300 mg/kg PGE, respectively) compared with the sham group (76.2 ± 4.9 seconds) (Fig. 2D). Since the serum ACTH (of HPA axis) and corticosterone (stress hormone) level is increased by CRS [27], we evaluated the effects of PGE in levels of serum ACTH and corticosteroid. The levels of serum ACTH and corticosterone were significantly increased by CRS (280.2 ± 68.2 pg/mL and 9333.6 ± 351.6 pg/mL, respectively), compared with the sham group (46.9 ± 12.8 pg/mL and 1,221.4 ± 275.8 pg/mL, respectively), whereas the increase was significantly inhibited by PGE administration (107.8 ± 53.6, 61.5 ± 12.6, and 58.2 ± 8.4 pg/mL, respectively, and 7,865.2 ± 805.1, 5,638.2 ± 549.1, and 6,016.7 ± 331.9 pg/mL, in 75 mg/kg, 15 mg/kg, and 300 mg/kg PGE, respectively), compared to the CRS group (Fig. 2E and F). PGE alone did not affect the level of serum ACTH and corticosteroid. According to the results from depression-like behavior and serum ACTH and corticosterone analysis, administration of PGE at a dose of 150 mg/kg body weight produced the best antidepressant effect in the CRS model. Thus, this dose was used for the subsequent histopathological and cellular analyses.
Fig. 2.
Effects of Panax ginseng extract (PGE) on the body weight gain, depressive-like behaviors, and serum levels of corticosterone and adrenocorticotropic hormone (ACTH). (A) Time course and (B) sum of body weight gain during chronic restraint stress (CRS) and PGE treatment. Immobility time in the (C) forced swimming test (FST) and (D) tail suspension test (TST). Serum levels of (E) ACTH and (F) corticosterone by enzyme-linked immunosorbent assay analysis. The results are expressed as the mean ± SE. ANOVA test; #p < 0.05, ##p < 0.01 CRS vs. sham or PGE alone group; *p < 0.05, **p < 0.01 CRS + PGE vs. CRS group. ANOVA, analysis of variance.
3.2. Effect of PGE on BDNF level in the amygdala
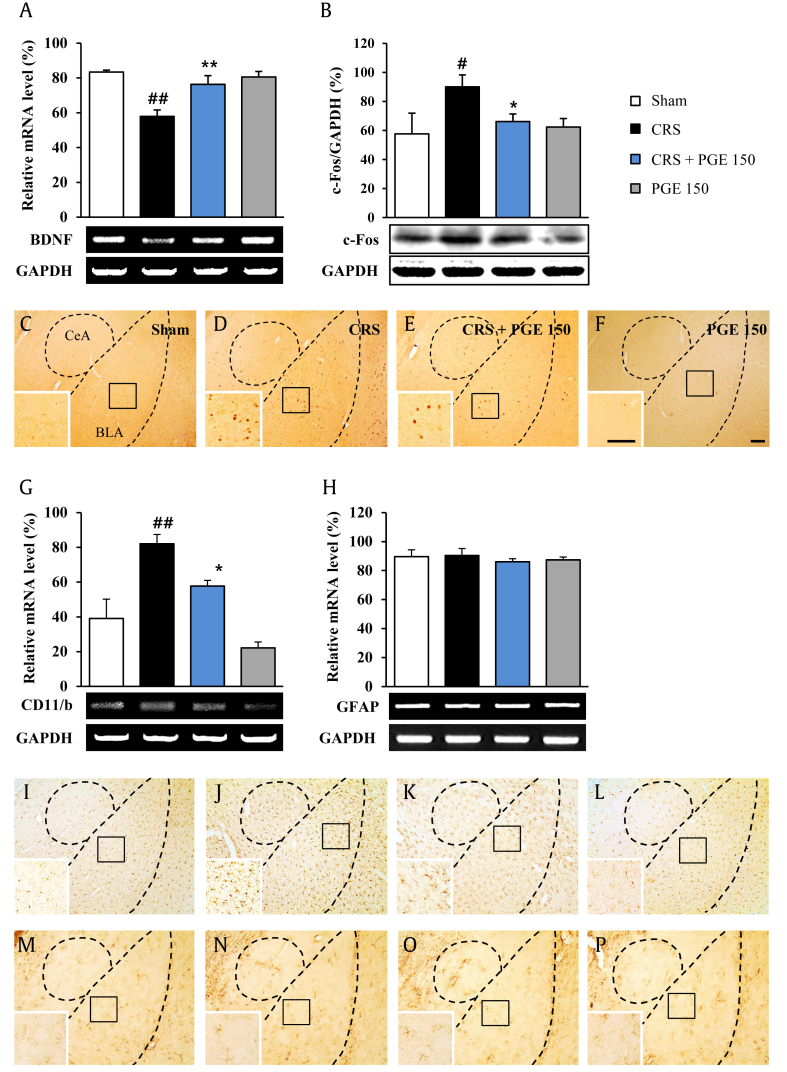
Since chronic corticosterone reduces expression of BDNF messenger RNA (mRNA) and protein in rats [28], the effect PGE on BDNF expression was evaluated by reverse transcription-polymerase chain reaction. Exposure to CRS significantly decreased amygdaloid BDNF mRNA level as compared to the sham group. However, daily administration of 150 mg/kg PGE significantly inhibited the decrease in the mRNA level of BDNF in the amygdala of CRS-induced mice as compared to the sham group. PGE itself was not significantly different from the sham group (Fig. 3A).
Fig. 3.
Effects of Panax ginseng extract (PGE) on the brain-derived neurotrophic factor (BDNF), c-Fos, and microglia in the amygdala. (A) Messenger RNA (mRNA) expression of BDNF by RT-PCR analysis. (B) Expression of c-Fos by Western blot is shown. (C-F) Immunohistochemical data are shown. mRNA expression of (G) CD11b and (H) GFAP by RT-PCR and the expression of (I-L) Iba-1 and(M-P) GFAP by immunohistochemical analysis. Insets are enlargements of rectangle. Scale bars = 100 μm. The results are expressed as the mean ± SE. ANOVA test; #p < 0.05, ##p < 0.01 chronic restraint stress (CRS) vs. sham or PGE alone group; *p < 0.05, **p < 0.01 CRS + PGE vs. CRS group. ANOVA, analysis of variance; BLA, basolateral amygdala; CeA, central amygdala; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT-PCR, reverse transcription-polymerase chain reaction.
3.3. Effect of PGE on neuronal insult in amygdala
Chronic BDNF downregulation may induce neuronal insult [29]. To investigate whether PGE can affect neural responses in mice exposed to CRS, expression of c-Fos (an indicator of neuron activation/damage) was measured in the amygdala using Western blot (Fig. 3B) and immunohistochemistry (Fig. 3C–F). Increased expression of c-Fos was observed in the amygdala following CRS. Interestingly, administration of 150 mg/kg PGE significantly inhibited the CRS-mediated increase in c-Fos protein expression compared with the CRS group by Western blot analysis (Fig. 3B), which corresponded to the change in c-Fos activation revealed by immunohistochemistry (Fig. 3C–F).
3.4. Effect of PGE on activation of microglia and inflammatory mediators in amygdala
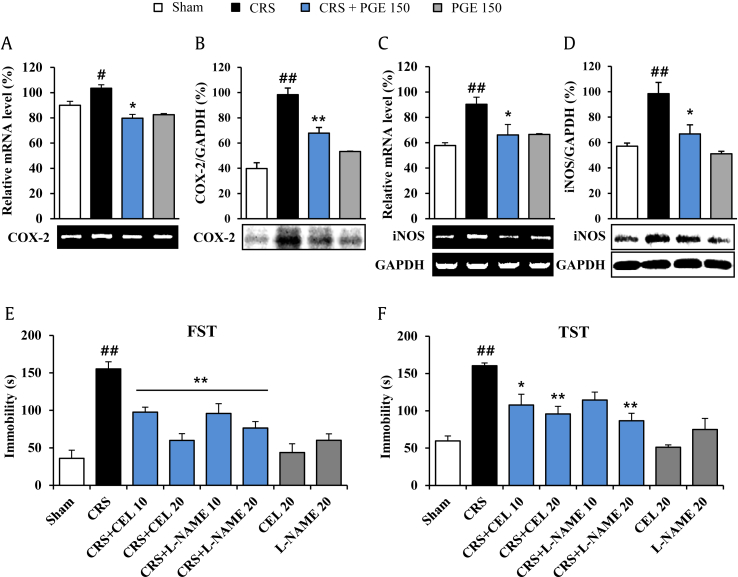
The traditional culprits of depression (glucocorticoids, biogenic amines, BDNF) affect glia (microglia and astrocyte) functioning, whereas antidepressant treatments (SSRIs, electroshocks, deep brain stimulation) recover glia function [30]. The effect on the change in glia in amygdala was investigated. The mRNA expression of CD11b (a microglia marker) was significantly upregulated in amygdala from the CRS group, compared to the sham group. However, the increase in mRNA expression of CD11b was blocked by PGE administration, compared to the CRS group (Fig. 3G). In agreement with this result, Iba-1 (a microglia marker) immunopositive microglia was slightly activated in the amygdala, while the activation was inhibited by administration with PGE (Fig. 3I–L). However, significant changes in mRNA expression and immunohistochemical expression of glial fibrillary acidic protein were not detected by CRS and PGE treatment, compared to the sham group (Fig. 3H, M–P). Normally, activation of microglia can release inflammatory mediators [30]. Thus, the effect of PGE on several representative inflammatory mediators was evaluated. The mRNA and protein levels of COX-2 and iNOS were increased in the CRS group compared with the sham group. These increased expressions were significantly inhibited by PGE treatment (Fig. 4A–D). The results suggested that inhibition of COX-2 and iNOS expressions may have beneficial effects in CRS-induced depression. Therefore, we investigated whether treatment with celecoxib or L-NAME for 14 d could attenuate depression-like behaviors after CRS (Fig. 4E and F). In the FST, administration of celecoxib and L-NAME significantly decreased immobility time (97.7 ± 6.6 s and 59.8 ± 9.1 s for 10 mg/kg and 20 mg/kg celecoxib, respectively, and 95.8 ± 13.1 s and 76.3 ± 8.6 s for 10 mg/kg and 20 mg/kg L-NAME, respectively) compared with the stress group (155.3 ± 9.4 s) (Fig. 4E). In the TST, administration of celecoxib and L-NAME significantly reduced immobility (107.8 ± 14.4 s and 95.8 ± 10.2 s for 10 mg/kg and 20 mg/kg celecoxib, respectively, and 86.7 ± 9.9 s for 20 mg/kg L-NAME) compared with the stress group (160.5 ± 3.6 s) (Fig. 4F). Celecoxib or L-NAME alone did not affect the level of normal behavior.
Fig. 4.
Effects of Panax ginseng extract (PGE) on proinflammatory mediators in the amygdala. The bands of RT-PCR (A and C) and Western blot analysis (B and D) are a representative of results obtained from three separate experiments, respectively. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Quantification of the messenger RNA (mRNA) or protein levels of cyclooxygenase-2 (COX-2) (A and B), inducible nitric oxide synthase (iNOS) (C and D), and GAPDH is determined by Image J analysis software. The GAPDH bands in (C) and (D) were shared with (A) and (B). The results are expressed as the mean ± SE. ANOVA test; #p < 0.05, ##p < 0.01 chronic restraint stress (CRS) vs. sham or PGE alone group; *p < 0.05, **p < 0.01 CRS + PGE vs. CRS group. ANOVA, analysis of variance; CEL, celecoxib; FST, forced swimming test; L-NAME, Nω-nitro-L-arginine methyl ester hydrochloride; RT-PCR, reverse transcription-polymerase chain reaction; TST, tail suspension test.
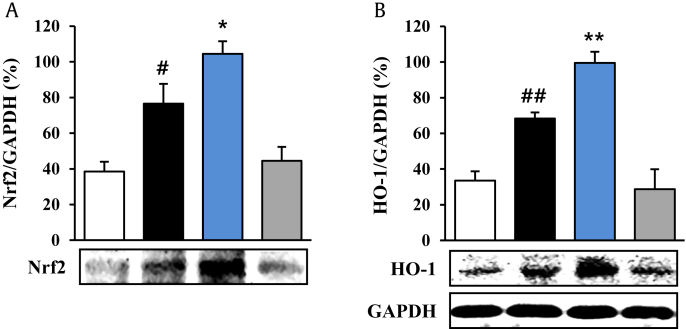
3.5. Effect of PGE on Nrf2 pathway in the amygdala
Since the Nrf2 pathway may regulate BDNF expression involved in pathophysiology and treatment of major depressive disorders [31], we further investigated whether PGE might exert antidepressant effects by regulating the antioxidant system. As shown in Fig. 5, the protein expression of Nrf2 was markedly enhanced 1.85-fold (76.6 ± 11.1%) in the amygdala compared to the sham group (38.6 ± 5.4%), whereas the increased expression was further increased (1.45-fold; 104.5 ± 7.0%) by PGE (150 mg/kg) administration, compared to the CRS group (Fig. 5A). The protein expression of HO-1, as one of the representative products of the Nrf2 pathway, was increased 2.0-fold (68.4 ± 3.3%) in the amygdala of the CRS group, compared to the sham group (33.5 ± 5.2%), whereas the elevated protein expression was further increased (1.45-fold; 99.4 ± 6.3%) in the PGE-administrated group, compared to the CRS group (Fig. 5B). PGE itself was not significantly different from the sham group (Fig. 5A and B). Collectively, the results indicate that administration with PGE activates the Nrf2 pathway, leading to upregulation of HO-1 in the amygdala, which contributes to its antioxidant activity in mice.
Fig. 5.
Effects of Panax ginseng extract (PGE) on the nuclear factor erythroid 2 related factor 2 (Nrf2) pathway in the amygdala. Western blot bands shown are a representative of results obtained from three separate experiments. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control. Quantification of the protein levels of Nrf2 and heme oxygenase-1 (HO-1) and GAPDH is determined by Image J analysis software. The GAPDH band in (B) was shared with (A). The results are expressed as the mean ± standard error. ANOVA test; #p < 0.05, ##p < 0.01 chronic restraint stress (CRS) vs. sham or PGE alone group; *p < 0.05, **p < 0.01 CRS + PGE vs. CRS group. ANOVA, analysis of variance.
4. Discussion
There is a growing need to develop effective and safe antidepressant medications. This study explored the utility of PGE as a potential antidepressant. Administration of PGE for 14 d ameliorated the depressive-like state (immobility time) in FST and TST, and inhibited the increase of serum corticosterone and ACTH level after CRS induction. These specific effects were associated with enhancement in Nrf2 pathway and antiinflammatory activity in the amygdala. Our findings suggest that PGE might be a potential antidepressant through further study.
4.1. PGE has antidepressant effects in behavioral tests
Chronic stress is one of critical risk factors for mental illnesses such as major depressive diseases and anxiety [32]. The chronic stress-treated animals can evoke behavioral and psychiatric changes similar to clinical depression, such as deficit in locomotor activity, increased immobility, decreased sucrose consumption, and a decrease in responsiveness to rewarding stimuli [32]. Antidepressant medicines can recover these symptoms [12], [14], [17], [18], [33], [34]. In the present study, PGE decreased immobility time during FST and TST in the CRS-induced depression model. These findings were also in agreement with previous studies using resveratrol, ginseng total saponin, and ginsenoside Rb3 [13], [17], [34]. The results suggest that PGE may exert antidepressant efficacy for CRS-induced depression.
4.2. Antidepressant properties of PGE are involved in the regulation of HPA axis activity
The most important neuroendocrine dysfunction in depression is HPA axis hyperactivity [35], [36]. Activation of the HPA axis stimulates the secretion of corticotropin releasing hormone and arginine vasopressin from the hypothalamic paraventricular nucleus, which in turn stimulates the secretion of ACTH from the pituitary, finally leading to the secretion of glucocorticoids (cortisol in primates and corticosterone in rodents) from the adrenal glands. Therefore, ACTH and glucocorticoids are used clinically as diagnostic agents in HPA axis function [35], [36]. Although serum concentrations of ACTH and glucocorticoids are increased during chronic stress, their increases are blocked by antidepressants and antidepressant-like materials [35], [36]. Here, we observed that PGE inhibits the increase in serum concentrations of ACTH and glucocorticoids in the CRS-induced depression model. The positive effect could be supported by the fact that ginseng total saponin and ginsenoside Rc inhibit the immobilization stress-induced increase in plasma corticosterone levels by blocking ACTH action in the adrenal gland [37]. The results indicate that antidepressant efficacy of PGE may be related with regulation of the HPA axis mechanism.
4.3. Antidepressant properties of PGE are involved in inhibition of microglial activation and expression of inflammatory mediators
Activation of microglia, caused by either short-term stress or by chronic unpredictable stress, can lead to depression and associated impairments in neuroplasticity and neurogenesis [38]. Long-term treatment with antidepressants like fluoxetine and imipramine prevents microglial activation in vivo, as well as the depressive-like behavioral alterations induced by chronic unpredictable stress [39], [40]. Microglial activation might be critical in depressive disorders. In the present study, the microglia displayed slightly activated morphology in the amygdala after CRS induction, while treatment with PGE for 14 d ameliorated the microglial activation corresponding to the decrease in immobilization time in FST and TST (Fig. 2). The results indicate that PGE may exert an antidepressive effect that is associated with the inhibition of microglial activation. Normally, activated microglia may release neurotoxic (cytokines/chemokines, COX-2, and iNOS) or neurotrophic mediators (BDNF and nerve growth factor), which can be toxic or detrimental in neurological lesions [39], [40]. Thus, controlling microglial activation and inflammation may be an attractive therapeutic strategy for the treatment of neuropsychological diseases including depression [39], [40]. Nonsteroidal antiinflammatory drugs showed antidepressive effects, as add-ons to conventional antidepressants. A meta-analysis of 14 randomized clinical trials (10 with nonsteroidal antiinflammatory drugs, 1 with an IL-12/IL-23 blocker, and 2 with TNF-ɑ/inhibitors) revealed that antiinflammatory drugs produced a significant reduction in depressive symptoms [41]. Selective COX-2 inhibitors, such as celecoxib (selective COX-2 inhibitor), display antidepressive efficacy, while nonselective COX-2 inhibitors like aspirin do not [42]. In agreement with these reports, here, PGE inhibited decrease in mRNA expression of BDNF and increase in protein activation of COX-2 and iNOS in the amygdala after CRS induction. Interestingly, treatment with celecoxib and L-NAME attenuated depression-like behaviors during FST and TST after CRS induction. The results corresponded with the inhibition of microglial activation and reduction of depression-like behaviors. Taken together, these results suggest that PGE may decrease depressive symptoms induced by CRS by inhibiting microglial activation and inflammation in the amygdala.
4.4. Antidepressant properties of PGE are involved in inhibition of oxidative stress
Oxidative stress may be one of the major pathogenesis of depressive diseases. Major redox-sensitive transcription factor Nrf2 is one of potential target for the treatment of major depressive disorders [3]. Various antioxidants, such as catechin, curcumin, and sulforaphane, have a potential as future antidepressants [22], [43]. Nrf2 activators have antidepressant-like effects in animal models of anxiety/depression [44]. Prozac (fluoxetine), an antidepressant belonging to the SSRI class of drugs, can reverse the expression of genes involved in oxidative stress in neurons and astrocytes in the dentate gyrus of the hippocampus from a rodent model of anxiety/depression by the chronic mild stress paradigm [45]. Nrf2 knockout mice recover depressive-like behaviors (by an increase in the immobility time in the TST and by a decrease in the grooming time in the splash test) associated with the reduction of the levels of dopamine and serotonin in the prefrontal cortex [46]. Furthermore, induction of Nrf2 by sulforaphane, in an inflammatory model of depression elicited by lipopolysaccharide, produced antidepressant-like effects [46]. These reports indicate that the antioxidant may involve neuroprotection against the neurotoxic effects by depressions. Our findings indicate that, in a rodent model of CRS-induced depression, the expression level of Nrf2 protein was significantly increased in the amygdala and the enhanced levels were more increased by PGE administration, correspondence with the change of protein expression of HO-1 (a representative cytoprotective protein). Interestingly, a subchronic antiinflammatory treatment (rofecoxib daily for 7 d) reversed the depressive-like behavior in Nrf-2 knock-out mice [46]. In addition, ginsenoside Rg1, ginsenoside Rh3, and ginsenoside R1 (notoginsenoside isolated from Panax notoginseng) increased Nrf2 nuclear translocation potential in cultured neurons [47], [48]. Notoginsenoside R1 or protopanaxtriol extracted from P. ginseng induced the expression of Phase II antioxidant enzymes, such as HO-1, NADPH quinone oxidase 1, and gamma-glutamylcysteine synthetase after 3-nitropropionic acid induced damage or in PC12 cells [48], [49]. Taken together, our findings suggest that PGE reverses the CRS-induced depression-like phenotype in mice through a mechanism involving activation of Nrf2 signaling in the amygdala. Interestingly, in our study, PE did not induce significant enhancement of Nrf1 and HO-1 in sham mice. The result can be explained as follows. Although under physiological conditions, low levels of nuclear Nrf2 are sufficient for the maintenance of cellular homeostasis, and constitutive hyperactivity of Nrf2 is associated with tumorigenesis [50]. Sulforaphane as an inducer of Nrf2 [22] and PGE in the present study did not increase Nrf2 activation in normal mice. Therefore, we suggest that the basal level of Nrf2 may be regulated to maintain cellular homeostasis. Further studies are needed to explore this suggestion.
4.5. Antidepressant properties of PGE involve enhanced BDNF expression
BDNF is a neurotrophic factor involved in the pathophysiology and treatment of major depressive disorders and is a critical regulator between inflammation/oxidative stress and depression [51]. Serum BDNF activity is decreased in the depressive status and increased under the actions of antidepressant drugs [52]. Interestingly, BDNF expression level was decreased in the cortex and hippocampus of Nrf2 knockout mice [46] and chronic fluoxetine treatment increased BDNF protein levels in the cortex and hippocampus of corticosterone-treated Nrf2 knockout mice. Ginsenoside Rg1 or ginseng total saponins upregulated the BDNF signaling pathway in the hippocampus during the chronic mild stress or corticosterone-induced depression [15], [53]. However, little is known about the influence of PGE on BDNF in the amygdala. Here, we demonstrate that PGE reversed decreased BDNF expression in the amygdala after CRS induction. Interestingly, a link between Nrf2/HO-1 and BDNF was confirmed in a recent study showing that the antiapoptotic effects of HO-1 in the ischemic animal brain are mediated through upregulation of the BDNF-TrkB-PI3K/Akt signaling pathway [54]. Taken together, our findings suggest that PGE may reverse CRS-induced depression by enhancing BDNF expression through the upregulation of Nrf2 signaling.
5. Conclusions
This study demonstrates the antidepressant-like effects and mechanisms of PGE in a CRS-induced depression model in mice. Administration of PGE for 14 d blocked the increase in serum concentration of ACTH and corticosterone, upregulated Nrf2 and HO-1 activities, and inhibited inflammatory activity (COX-2 and iNOS) in the amygdala after CRS treatment, which resulted in enhanced BDNF activity and finally, normalized depression-like behaviors. These findings suggest that PGE has antidepressant activity in CRS-induced depression by virtue of antioxidant and antiinflammatory activities through positive regulation of the HPA axis mechanism. Further studies are required to determine if further cellular mechanism of PGE on antioxidation and antiinflammation increases the efficacy of antidepressants and whether ginseng's components are a useful tool for a future antidepressant.
Acknowledgments
This study was supported by the Cooperative Research Program for Agriculture Science and Technology Development, Rural Development Administration, Republic of Korea (Project Number PJ011582042015). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
All the authors of this manuscript have no conflicts of interest in this subject.
References
- 1.Lang U.E., Borgwardt S. Molecular mechanisms of depression: perspectives on new treatment strategies. Cell Physiol Biochem. 2013;31:761–777. doi: 10.1159/000350094. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakunina N., Pariante C.M., Zunszain P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology. 2015;144:365–373. doi: 10.1111/imm.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henckens M.J., van der Marel K., van der Toorn A., Pillai A.G., Fernández G., Dijkhuizen R.M., Joëls M. Stress-induced alterations in large-scale functional networks of the rodent brain. Neuroimage. 2015;105:312–322. doi: 10.1016/j.neuroimage.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Mitra R., Sapolsky R.M. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holtzheimer P.E., 3rd, Nemeroff C.B. Advances in the treatment of depression. NeuroRx. 2006;3:42–56. doi: 10.1016/j.nurx.2005.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souery D., Papakostas G.I., Trivedi M.H. Treatment-resistant depression. J Clin Psychiatry. 2006;67(Suppl 6):16–22. [PubMed] [Google Scholar]
- 8.Vilhardt F., Haslund-Vinding J., Jaquet V., McBean G. Microglia antioxidant systems and redox signaling. Br J Pharmacol. 2017;174:1719–1732. doi: 10.1111/bph.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao W., Zhang J.C., Ishima T., Dong C., Yang C., Ren Q., Ma M., Han M., Wu J., Suganuma H. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci Rep. 2016;6:30659. doi: 10.1038/srep30659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2016;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartley D.E., Elsabagh S., File S.E. Gincosan (a combination of Ginkgo biloba and Panax ginseng): the effects on mood and cognition of 6 and 12 weeks' treatment in post-menopausal women. Nutr Neurosci. 2004;7:325–333. doi: 10.1080/10284150400015557. [DOI] [PubMed] [Google Scholar]
- 12.Kuribara H., Tomioka H., Takahashi R., Onozato K., Murohashi N., Numajiri T., Iwata H., Koya S. An antidepressant effect of Sho-ju-sen, a Japanese herbal medicine, assessed by learned helplessness model in mice. Phytother Res. 2004;18:173–176. doi: 10.1002/ptr.1412. [DOI] [PubMed] [Google Scholar]
- 13.Dang H., Sun L., Liu X., Peng B., Wang Q., Jia W., Chen Y., Pan A., Xiao P. Preventive action of Kai Xin San aqueous extract on depressive-like symptoms and cognition deficit induced by chronic mild stress. Exp Biol Med (Maywood) 2009;234:785–793. doi: 10.3181/0812-RM-354. [DOI] [PubMed] [Google Scholar]
- 14.Yamada N., Araki H., Yoshimura H. Identification of antidepressant-like ingredients in ginseng root (Panax ginseng C.A. Meyer) using a menopausal depressive-like state in female mice: participation of 5-HT2A receptors. Psychopharmacology (Berl) 2011;216:589–599. doi: 10.1007/s00213-011-2252-1. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z., Qi Y., Cheng Z., Zhu X., Fan C., Yu S.Y. The effects of ginsenoside Rg1 on chronic stress induced depression-like behaviors, BDNF expression and the phosphorylation of PKA and CREB in rats. Neuroscience. 2016;322:358–369. doi: 10.1016/j.neuroscience.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 16.Zheng X., Liang Y., Kang A., Ma S.J., Xing L., Zhou Y.Y., Dai C., Xie H., Xie L., Wang G.J. Peripheral immunomodulation with ginsenoside Rg1 ameliorates neuroinflammation-induced behavioral deficits in rats. Neuroscience. 2014;256:210–222. doi: 10.1016/j.neuroscience.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Cui J., Jiang L., Xiang H. Ginsenoside Rb3 exerts antidepressant-like effects in several animal models. J Psychopharmacol. 2012;26:697–713. doi: 10.1177/0269881111415735. [DOI] [PubMed] [Google Scholar]
- 18.Xu C., Teng J., Chen W., Ge Q., Yang Z., Yu C., Yang Z., Jia W. 20(S)-protopanaxadiol, an active ginseng metabolite, exhibits strong antidepressant-like effects in animal tests. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1402–1411. doi: 10.1016/j.pnpbp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Cho I.H. Effects of Panax ginseng in neurodegenerative diseases. J Ginseng Res. 2012;36:342–353. doi: 10.5142/jgr.2012.36.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landis S.C., Amara S.G., Asadullah K., Austin C.P., Blumenstein R., Bradley E.W., Crystal R.G., Darnell R.B., Ferrante R.J., Fillit H. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K.S., Han P.L. Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res. 2006;83:497–507. doi: 10.1002/jnr.20754. [DOI] [PubMed] [Google Scholar]
- 22.Jang M., Cho I.H. Sulforaphane ameliorates 3-nitropropionic acid-induced striatal toxicity by activating the Keap1-Nrf2-ARE pathway and inhibiting the MAPKs and NF-kappaB pathways. Mol Neurobiol. 2016;53:2619–2635. doi: 10.1007/s12035-015-9230-2. [DOI] [PubMed] [Google Scholar]
- 23.Lee M.J., Jang M., Choi J., Chang B.S., Kim D.Y., Kim S.H., Kwak Y.S., Oh S., Lee J.H., Chang B.J. Korean Red Ginseng and ginsenoside-Rb1/-Rg1 alleviate experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating regulatory T cells. Mol Neurobiol. 2016;53:1977–2002. doi: 10.1007/s12035-015-9131-4. [DOI] [PubMed] [Google Scholar]
- 24.Choi J.H., Lee M.J., Jang M., Kim E.J., Shim I., Kim H.J., Lee S., Lee S.W., Kim Y.O., Cho I.H. An oriental medicine, Hyungbangpaedok-San attenuates motor paralysis in an experimental model of multiple sclerosis by regulating the T cell response. PLoS One. 2015;10:e0138592. doi: 10.1371/journal.pone.0138592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marin M.T., Cruz F.C., Planeta C.S. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 2007;90:29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Porsolt R.D., Bertin A., Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 27.Dallman M.F., Akana S.F., Strack A.M., Scribner K.S., Pecoraro N., La Fleur S.E., Houshyar H., Gomez F. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann N Y Acad Sci. 2004;1018:141–150. doi: 10.1196/annals.1296.017. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen J.P., Mork A. Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mRNA and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Res. 2006;1110:221–225. doi: 10.1016/j.brainres.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 29.Lipsky R.H., Marini A.M. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci. 2007;1122:130–143. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- 30.Rial D., Lemos C., Pinheiro H., Duarte J.M., Gonçalves F.Q., Real J.I., Prediger R.D., Gonçalves N., Gomes C.A., Canas P.M. Depression as a glial-based synaptic dysfunction. Front Cell Neurosci. 2016;9:521. doi: 10.3389/fncel.2015.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vavakova M., Durackova Z., Trebaticka J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/898393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slavich G.M., Irwin M.R. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anacker C. Fresh approaches to antidepressant drug discovery. Expert Opin Drug Discov. 2014;9:407–421. doi: 10.1517/17460441.2014.892071. [DOI] [PubMed] [Google Scholar]
- 34.liu S., Li T., Liu H., Wang X., Bo S., Xie Y., Bai X., Wu L., Wang Z., Liu D. Resveratrol exerts antidepressant properties in the chronic unpredictable mild stress model through the regulation of oxidative stress and mTOR pathway in the rat hippocampus and prefrontal cortex. Behav Brain Res. 2016;302:191–199. doi: 10.1016/j.bbr.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 35.Pariante C.M., Lightman S.L. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Varghese F.P., Brown E.S. The hypothalamic-pituitary-adrenal axis in major depressive disorder: a brief primer for primary care physicians. Prim Care Companion J Clin Psychiatry. 2001;3:151–155. doi: 10.4088/pcc.v03n0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D.H., Moon Y.S., Jung J.S., Min S.K., Son B.K., Suh H.W., Song D.K. Effects of ginseng saponin administered intraperitoneally on the hypothalamo-pituitary-adrenal axis in mice. Neurosci Lett. 2003;343:62–66. doi: 10.1016/s0304-3940(03)00300-8. [DOI] [PubMed] [Google Scholar]
- 38.Yirmiya R., Rimmerman N., Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–658. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Kreisel T., Frank M.G., Licht T., Reshef R., Ben-Menachem-Zidon O., Baratta M.V., Maier S.F., Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- 40.Mahmoud R., Wainwright S.R., Chaiton J.A., Lieblich S.E., Galea L.A. Ovarian hormones, but not fluoxetine, impart resilience within a chronic unpredictable stress model in middle-aged female rats. Neuropharmacology. 2016;107:278–293. doi: 10.1016/j.neuropharm.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 41.Köhler O., Benros M.E., Nordentoft M., Farkouh M.E., Iyengar R.L., Mors O., Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 42.Eyre H.A., Air T., Proctor S., Rositano S., Baune B.T. A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:11–16. doi: 10.1016/j.pnpbp.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Lee S.Y., Lee S.J., Han C., Patkar A.A., Masand P.S., Pae C.U. Oxidative/nitrosative stress and antidepressants: targets for novel antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:224–235. doi: 10.1016/j.pnpbp.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Maes M., Fišar Z., Medina M., Scapagnini G., Nowak G., Berk M. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates–Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology. 2012;20:127–150. doi: 10.1007/s10787-011-0111-7. [DOI] [PubMed] [Google Scholar]
- 45.Patrício P., Mateus-Pinheiro A., Irmler M., Alves N.D., Machado-Santos A.R., Morais M., Correia J.S., Korostynski M., Piechota M., Stoffel R. Differential and converging molecular mechanisms of antidepressants' action in the hippocampal dentate gyrus. Neuropsychopharmacology. 2015;40:338–349. doi: 10.1038/npp.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martín-de-Saavedra M.D., Budni J., Cunha M.P., Gómez-Rangel V., Lorrio S., Del Barrio L., Lastres-Becker I., Parada E., Tordera R.M., Rodrigues A.L. Nrf2 participates in depressive disorders through an anti-inflammatory mechanism. Psychoneuroendocrinology. 2013;38:2010–2022. doi: 10.1016/j.psyneuen.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 47.Du X., Xu H., Jiang H., Xie J. Akt/Nrf2 activated upregulation of heme oxygenase-1 involves in the role of Rg1 against ferrous iron-induced neurotoxicity in SK-N-SH cells. Neurotox Res. 2013;24:71–79. doi: 10.1007/s12640-012-9362-3. [DOI] [PubMed] [Google Scholar]
- 48.Meng X., Sun G., Ye J., Xu H., Wang H., Sun X. Notoginsenoside R1-mediated neuroprotection involves estrogen receptor-dependent crosstalk between Akt and ERK1/2 pathways: a novel mechanism of Nrf2/ARE signaling activation. Free Radic Res. 2014;48:445–460. doi: 10.3109/10715762.2014.885117. [DOI] [PubMed] [Google Scholar]
- 49.Gao Y., Chu S.F., Li J.P., Zhang Z., Yan J.Q., Wen Z.L., Xia C.Y., Mou Z., Wang Z.Z., He W.B. Protopanaxtriol protects against 3-nitropropionic acid-induced oxidative stress in a rat model of Huntington's disease. Acta Pharmacol Sin. 2015;36:311–322. doi: 10.1038/aps.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maes M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett. 2008;29(3):287–291. [PubMed] [Google Scholar]
- 52.Castren E. Neurotrophins and psychiatric disorders. Handb Exp Pharmacol. 2014;220:461–479. doi: 10.1007/978-3-642-45106-5_17. [DOI] [PubMed] [Google Scholar]
- 53.Dang H., Chen Y., Liu X., Wang Q., Wang L., Jia W., Wang Y. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1417–1424. doi: 10.1016/j.pnpbp.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 54.Qi D., Ouyang C., Wang Y., Zhang S., Ma X., Song Y., Yu H., Tang J., Fu W., Sheng L. HO-1 attenuates hippocampal neurons injury via the activation of BDNF-TrkB-PI3K/Akt signaling pathway in stroke. Brain Res. 2014;1577:69–76. doi: 10.1016/j.brainres.2014.06.031. [DOI] [PubMed] [Google Scholar]