Abstract
Cylindrocarpon destructans/Ilyonectria radicicola is thought to cause both rusty symptom and root-rot disease of American and Korean ginseng. Root-rot disease poses a more serious threat to ginseng roots than rusty symptoms, which we argue result from the plant defense response to pathogen attack. Therefore, strains causing rotten root are characterized as more aggressive than strains causing rusty symptoms. In this review, we state 1- the molecular evidence indicating that the root-rot causing strains are genetically distinct considering them as a separate species of Ilyonectria, namely I. mors-panacis and 2- the physiological and biochemical differences between the weakly and highly aggressive species as well as those between rusty and rotten ginseng plants. Eventually, we postulated that rusty symptom occurs on ginseng roots due to incompatible interactions with the weakly aggressive species of Ilyonectria, by the established iron-phenolic compound complexes while root-rot is developed by I. mors-panacis infection due to the production of high quantities of hydrolytic and oxidative fungal enzymes which destroy the plant defensive barriers, in parallel with the pathogen growth stimulation by utilizing the available iron. Furthermore, we highlight future areas for study that will help elucidate the complete mechanism of root-rot disease development.
Keywords: Cylindrocarpon destructans, I. radicicola-species complex, Panax ginseng, root-rot, rusty root
1. Introduction
The genus Panax is a member of the Araliaceae family. The name Panax, given by Carl A. Meyer, originates from the Greek word meaning “cure-all” and includes members used for many decades as oriental medicinal plants [1], [2]. There are 11 accepted species in the genus Panax (http://www.theplantlist.org/browse/A/Araliaceae/Panax/). Six of these species are well-known: P. quinquefolius and P. trifolius, which are native to North America [3], [4], and P. ginseng, P. notoginseng, P. vietnamensis and P. japonicus which are located in Asia. P. quinquefolius, P. ginseng, and P. notoginseng are considered important cash crops due to their pharmaceutical properties [5], [6]. These pharmaceutical properties are due to the major components of the ginseng root, dammarane-type saponins, which are referred to as ginsenosides [1], [2], [7].
Ginseng plants are slow-growing perennial herbs that require several continuous growing seasons for optimal growth and ginsenoside accumulation. Four- to six-year-old ginseng roots have the best shape, growth, and ginsenoside content [8]. Accordingly, optimal growth is required over a long cultivation period before harvesting.
During the six years of cultivation, ginseng plants can suffer from several diseases that lead to dramatic crop losses and reduction of root quality [9], [10], [11]. Diseases can be divided into two types: foliar-borne diseases and soil-borne diseases. Examples of foliar disease are blight, anthracnose, and mildew. Blight can occur in different forms. The first form appears on the leaves during the initial stage as circular, water-soaked spots. Over time, the spots dry and turn to brown with a targeted-board appearance and clear dark brown margin. This type of blight is caused by Alternaria panax and accordingly is called Alternaria blight [8], [12], [13], [14]. The second form appears mostly at the leaf tip and expands backward through the midrib, resulting in a characteristic V-shape infection. The lesions have a targeted-board appearance like Alternaria infection; however, at the late stage of infection, the lesions cover the leaf surface, the color of the leaves becomes reddish to brown, and the pathogen grows densely and sporulates, giving the infected leaves a characteristic gray color. This blight disease can be differentiated from Alternaria blight by its ability to infect flowers and fruits. This blight is caused by Botrytis cinerea and is accordingly called Botrytis blight [12], [15]. Anthracnose disease is found on leaves exposed to direct sunlight. The symptoms of this disease are similar to those of Alternaria blight; however, anthracnose disease is characterized by a felt-like appearance. The causal agent of anthracnose disease has been reported to be Colletotrichum panacicola [12], [16], [17]. Mildew disease had been considered as a type of blight; however, the name was changed to refer to symptoms completely different from those of Alternaria and Botrytis blights. At the early stage of disease, the blights appear similar to Alternaria and Botrytis blights, but later, the blight turns to dark green with a whitish center. In addition, mildew blights lack the yellowish-brown margins seen in Alternaria blights or a grayish surface as seen in Botrytis blights. Mildew disease is induced by Phytophthora cuctorum [12], [18], [19], [20].
Soil-borne diseases include damping-off, rusty root, and root-rot disease. Damped-off ginseng plants usually have intact and erect stems with drooping leaves. Leaves finally turn yellowish, the stem collapses, and the plant dies. Damping-off disease has been reported to be induced by Pythium spp. [21]. Rusty root disease is usually characterized by small or quite large reddish brown areas at the crown of the tap root that can be easily scraped off to show the inner white healthy tissue, while rotten root disease is characterized by the complete absence or only partial presence of hollow roots with dark brown discoloration. Interestingly, rusty root disease and root-rot disease are both caused by Cylindrocarpon destructans var. destructans [21], [22], [23]. Although foliar diseases cause plant losses, the root mostly remains intact and is not physically harmed. Sometimes, limited growth or lowered production of bioactive metabolites is observed because of defoliation and reduction in leaf area, as in the case of A. panax blight [8], but overall, the reduction in yield is minimal. Root diseases, in contrast, pose the greatest threat to ginseng cultivation and crop yield because the infection directly harms the roots, leading to changes in root shape and reduction in root quality, in addition to physiological changes [24], [25]. Damping-off disease threatens the roots of ginseng crop at the seedling stage; however, careful handling and treatment during ginseng cultivation at this stage can prevent this disease from causing damage, as the causal agent cannot invade older roots [21]. Root-rot and rusty root diseases are the most dangerous diseases for ginseng crops as they cause a great decrease in yield and damage root shape and quality in plants of all ages [25]. As explained earlier, these two diseases have been attributed to infection by Cylindrocarpon destructans var. destructans [22], [25]. Previous studies have reported that this pathogen is genetically diverse and can be divided into several species [26]. In this review, we detail the main morphological differences between these two diseases and environmental factors leading to their development. Furthermore, we detail the re-classification of this pathogen into several species, and we describe, for the first time, how this correlates with the morphological and biochemical characteristics of root-rot and rusty root-causing strains. In addition, we discuss the mechanism of root-rot and rusty root development. Finally, we highlight points that need to be clarified in the future for a comprehensive understanding of how root-rot is initiated and measures that can be taken to combat this disease.
2. Description of ginseng root-rot and rusty root diseases
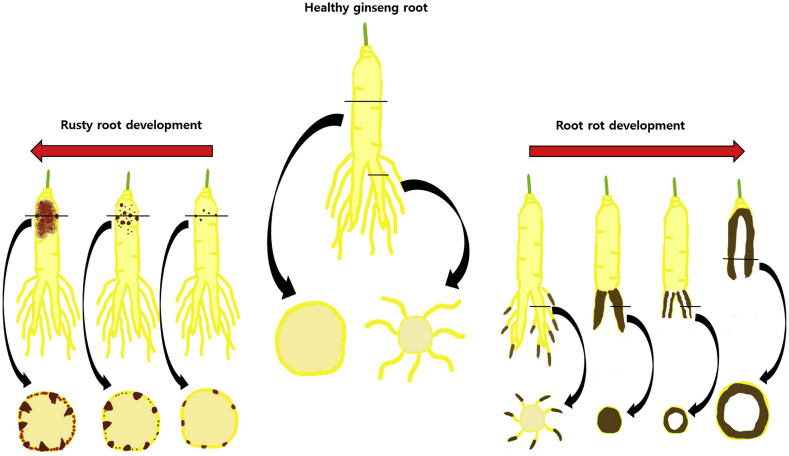
Root-rot is a soil-borne disease that causes huge loss of ginseng crops. The disease is detected at all stages of plant growth. In addition, the causal agent has the ability to overwinter in the soil in a resting stage, so that newly planted crops and existing crops can be re-invaded each season [12], [22], [23], [25]. Therefore, it is considered the most serious disease in most ginseng cultivation areas. Root-rot disease in American ginseng was first described by Zinnsmeister [27]. Similar symptoms were then observed on Korean ginseng, and the same causative pathogen was discovered by Chung [28]. The disease usually appears as a dark brown discolored area on the tip of the tap root and extends over time upward to the crown. Sometimes, rotting can affect any part of the root. Due to invasion, the outer surface of the plants is damaged, while the inner part totally disintegrates, leaving a hollowed-out root in the soil (Fig. 1). At the late stage of root-rot disease, the leaves turn yellow, wilt, and finally the end of the stem get easily separated from the crown. Because of these characteristic symptoms, this kind of rot disease is called disappearing root-rot [12], [25]. The causal agent of such symptoms have been reported to be, as previously named, C. destructans var. destructans [21], [22], [23]. Fusarium species exist in association with root-rot symptoms; however, there is doubt that they are the primary causal agents of rooting disorder [25] as artificial infection of ginseng seedlings by Fusarium species causes root-rot [29], and these species have not been reported to cause rotting symptoms in older roots. Another uncommon rot disease that has been detected on ginseng root differs from Cylindrocarpon root-rot by the absence of brown discoloration and the presence of soft, water-soaked rot in the cortex. This type of rotting is caused by Phytophthora cactorum [12].
Fig. 1.
A schematic showing the morphological development of root-rot and rusty root symptoms.
Rusty root or rusted root is another common disease that affects ginseng roots at all stages of growth. The disease has been reported in North American countries, e.g., Canada, as well as in Asian countries, e.g., South Korea and China [12], [24]. Infected roots have reddish scabs covering the entire root or part of the root. These scabs do not cause plant death; however, they can reduce root quality and price by up to 40%. Symptoms usually start at the crown of the tap root as small or large, raised reddish-brown areas and can sometimes be present on other parts of the root (Fig. 1). The symptoms are usually superficial, and can be easily scraped off to show the inner, white healthy tissues [12], [24], [25]. Rusty root disease had been observed to be associated with C. destructans var. destructans; in particular, weak strains of C. destructans var. destructan are unable to cause intensive root rotting [22], [25].
3. Causal agent
3.1. Taxonomy of the pathogen; from single species to species complex
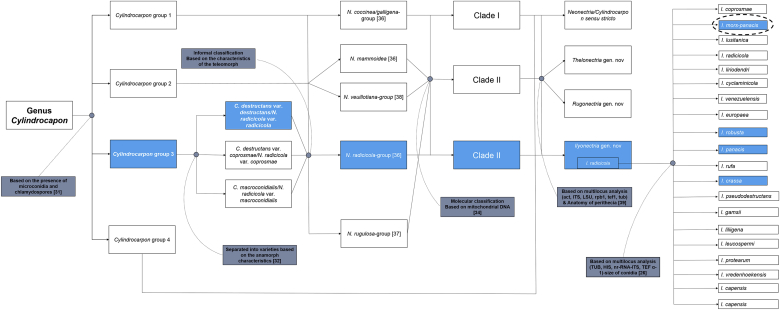
The genus Cylindrocarpon was originally proposed by Wollenweber [30] as the anamorph of the teleomorphic state of the Nectria section Willkommiotes Wollenw and was characterized by the inability to produce chlamydospores. A few years later, the generic name was expanded to include species able to produce chlamydospores, including C. destructans. Then, Booth [31] divided the genus into four different groups based on the presence or absence of microconidia and chlamydospores: C. magnusianum (Sacc.) Wollenw.; C. cylindroides Wollenw., the type species of the genus Cylindrocarpon; the group of Cylindrocarpon species considered the anamorph of Nectria mammoidea; and C. destructans, the anamorph of N. radicicola. C. destructans is characterized by the presence of both microconidia and chlamydospores. Later, morphological variation among C. destructans strains was observed, and their teleomorphs were divided into varieties accordingly. Those infecting ginseng plants were given the name C. destructans var. destructans, and the teleomorph is named N. radicicola var. radicicola [32]. By this time, all Nectria species with the anamorph Cylindrocarpon were included in the genus Neonectria [33], [34], [35]. Based on the morphological characteristics of the teleomorph of the genus Cylindrocarpon, they were divided into five informal groups: (1) Neonectria coccinea/galligena-group (Neonectria sensu stricto) [36]; (2) N. mammoidea-group [36]; (3) N. rugulosa-group [37]; (4) N. radicicola-group [36]; and (5) N. veuillotiana-group [38]. This classification was revised based on analysis of mitochondrial DNA data into three different clades; the C. destructans/N. radicicola group formed clade III [34]. The morphological and molecular variation found among Cylindrocarpon and its teleomorph, Neonectria, suggested the presence of several genera within the genus Cylindrocarpon/Neonectria. Multilocus analysis [α-actin (ACT), internal transcribed spacer (ITS), large subunit (LSU), RNA polymerase II subunit 1 (rpb1), translation elongation factor 1-α (TEF-1-α), β-tubulin (TUB)] revealed the presence of five different groups that overlapped with both Booth [31] and Mantiri [34] classifications. The phylogenetic analysis correlated with observed morphological differences, particularly macroconidial septation and perithecia anatomy. Therefore, each group was considered a distinct genus, and the N. radicicola group was given the generic name Ilyonectria. Accordingly, N. radicicola var. radicicola (the teleomorph of C. destructans var. destructans) was named I. radicicola [39]. The description of I. radicicola in this study is based on the amended description of Booth [31], [40] and Samuels and Brayford [32]: perithecia usually solitary with papillate, conical shape apex, smooth to slightly roughed surface containing 1-septate smoothed ascospores; anamorph produces straight cylindrical macroconidia with rounded ends and conspicuous hilum, ellipsoidal microconidia with or without conspicuous hilum; chlamydospores intercalary, single or in chains, and becoming brown after aging. A few months later, it was proposed that the anamorph of I. radicicola is a species complex; multigene analysis [TUB, histone H3 (HIS), TEF-1-α, and nuclear ribosomal RNA-internal transcribed spacer (nrRNA-ITS)] of I. radicicola anamorphs isolated from many hosts proved that members of that genus are not monophyletic. Rather, they clustered in many groups, each of which was considered a separate species. In this study, both teleomorphic and anamorphic states are referred to by a single generic name, as for the other pleomorphic fungi [41], [42], [43], [44], [45]. Of 68 isolates obtained from many hosts, 21 isolates obtained from ginseng plants were resolved into four different Ilyonectria species: I. mors-panacis, I. robusta, I. panacis, and I. crassa [26]. The taxonomic position of ginseng plant-infecting Cylindrocarpon within each classification study from 1966 to the present is illustrated in Fig. 2. Despite the high morphological similarity among the four resolved Ilyonectria species, some differences have been observed, particularly in the morphology and size of the conidia, in addition to colony diameter at 25°C (Table 1).
Fig. 2.
Schematic overview of the taxonomic position of ginseng plant-infecting Cylindrocarpon species within each classification study from Booth [32] until Cabral [26]. Blue-colored squares refer to the group of Cylindrocarpon/Ilyonectria in which those infecting ginseng plants are included. Dotted circle shows the Ilyonectria species, I. mors-panacis that has been reported only in ginseng plants.
Table 1.
Summary of the morphological differences between Ilyonectria radicicola-species complex infecting American and Korean ginseng [26]
| Type of conidia | characteristic | I. crassa | I. robusta | I. panacis | I. mors-panacis |
|---|---|---|---|---|---|
| Macroconidia | Origin of production | Produced on simple and complex of conidiophores | Produced on simple conidiophore | Produced on simple and complex of conidiophores | Produced only on the simple conidiophores |
| Shape of ends | Both ends are rounded, but sometime narrowing at the tip | both ends are rounded but sometime narrowing at the tip | both ends are rounded | both ends are rounded | |
| Hilum existence | Hilum exist | No hilum exist | Hilum exist | No hilum exist | |
| Mean size of (μm) | |||||
| 1-septate | 26.5 × 5.1 | 23.7 × 6.5 | 24.8 × 4.8 | 29.9 × 6.1 | |
| 2-septate | 29.4 × 5.4 | 27.2 × 7.0 | 28.7 × 5.2 | 34.4 × 6.4 | |
| 3-septate | 35.1 × 5.7 | 33.5 × 7.4 | 33.1 × 5.6 | 41.0 × 7.2 | |
| Microconidia | Hilum existence | Hilum exist | No hilum exist | Hilum exist | No hilum exist |
| Mean size of (μm) | |||||
| 0-septate | 10.3 × 3.5 | 8.7 × 3.8 | 8.9 × 3.8 | 9.6 × 3.8 | |
| 1-septate | 14.7 × 4.0 | 14.1 × 4.9 | 12.5 × 4.0 | 13.3 × 4.6 | |
| Growth at temperature (25°C) (mm) | 19–34 | 35–48 | 15 | 31–40 |
3.2. Correlations between morphological, biochemical, and genetic diversity and pathogenicity
Before the Cabral [26] classification, many differences in morphology, optimal temperature for growth, enzymatic activity, and genetic profiles were detected among strong and weak strains and were assumed to be linked to the observed differences in pathogenicity. Rahman and Punja [25] observed that highly and weakly aggressive strains were morphologically different; the highly aggressive strain had a dark to rust brown-colored appearance after two weeks of growth on PDA media, while the weakly aggressive strain had a beige to light brown-colored appearance. Additionally, the optimal temperature for growth differed between the strains; they observed that the optimal radial growth of highly aggressive and weakly aggressive strains on PDA media plates occurred at 18 and 21°C, respectively. Production of enzymes to degrade plant cell wall materials (e.g., pectinase and cellulase) and oxidative enzymes to detoxify the accumulated polyphenolic compounds by highly and weakly aggressive was also characteristic; Rahman and Punja [25] reported that virulent strains produced pectinase and polyphenol oxidase in greater quantities than avirulent strains. In another study, it was proposed that root-rot-causing strains are able to produce cellulose in high quantities, but the ability of weakly aggressive strains to produce cellulose has not been explored [46]. The morphological and biochemical differences between highly and weakly aggressive strains suggested that the highly aggressive strains are genetically distinct. Combined phylogenetic analysis of both ITS and TUB from weak and aggressive strains revealed that the aggressive strains were phylogenetically distinct. Neither geographical location nor host type factor appear to play a role in the genetic distinctiveness of the aggressive strains. For example, strains isolated from Korean ginseng in Korea and Japan were recorded as aggressive strains on American ginseng. In addition, they were found to be genetically similar to those originally infect American ginseng in Canada. The aggressive strains were therefore considered to be a forma specialis and were given the name C. destructans f. sp. panacis [47]. In the study of Cabral [26], which proved that I. radicicola is a species complex, weak and strong pathogenic strains derived from ginseng root segregated phylogenetically into four different groups, each of which was considered a different species of Ilyonectria. Interestingly, all aggressive strains were clustered together in one group, as occurred previously [47], and were re-named I. mors-panacis, while the remaining strains were divided into three groups (I. robusta, I. panacis, and I. crassa). The genetic properties of the ginseng-derived aggressive species were found to be distinct. On the other hand, ginseng-derived weakly aggressive strains were found to be genetically similar to the strains derived from different hosts. For example, I. robusta derived also from the roots of Loroglossum hircinum, Tilia petiolaris, Quercus robur, Prunus cerasus, Vitis vinifera, and Thymus spp. I. crassa has also been observed on the roots of Narcissus spp. and bulbs of Lilium spp. [26]. The identity of the original host of I. panacis is unclear, as only one ginseng-derived strain has been investigated in detail. These findings suggest that American and Korean ginseng are not susceptible hosts to I. Robusta and I. crassa. In addition, there may be a host-specific interaction between I. mors-panacis and its sole host, ginseng.
4. Environmental factors involved in disease development
Beside the main biotic factor, the pathogen, the density of root-rot disease in ginseng crops is influenced by many abiotic factors that can either favor pathogen growth or increase plant susceptibility to pathogen infection. For example, if wounds made by soil insects or cultivation tools exist on the root surface, they may play a role in root-rot development as weak points through which the pathogen can invade the root aggressively; wounded roots have been found to be more susceptible to infection than unwounded ones [25]. Soil pH also has an effect on disease intensity; generally, the pH of soils with good ginseng root yields is weakly acidic (5.5-6) [48], [49], while more acidic soil (pH < 5) has been observed to be associated with disease abundance. This is consistent with the observation that root-rot-causing I. radicicola-species complex strains develop better under acidic than neutral conditions [25]. Mineral addition to ginseng is also linked to disease abundance; for example, the foliar application of iron [50] and absence of calcium [51] in ginseng soil promote root-rot development. High moisture is generally required for the induction of rot diseases, and the same is true for root-rot of ginseng [25]. Another factor that determines the severity of root-rot disease is the age of the root; although the disease can be found in all ages of ginseng plant, young roots, in particular two-year-old-roots, appear to be the most susceptible [25], possibly because the root cell wall is still immature and not yet well developed. Temperature also plays a critical role in determining the intensity of root-rot disease; rotting symptoms were observed to occur in the temperature range of 13-23°C, while no symptoms were observed at 28°C [52].
Environmental factors that influence rusty root development are similar to those that influence root-rot development., in particular, the presence of metal ions in the ginseng soil; high content of certain cations (e.g., iron, aluminum, and silicon) has been noted in rusty root lesions versus healthy tissues, which conversely have high calcium and potassium levels [24], [53]. It was deduced that such metal variation, in combination with pathogen invasion, may be responsible for the appearance of rust.
5. Mechanism of infection and disease development
As mentioned earlier, rusty root disease causes less damage to ginseng roots than root-rot [25]. Rusty root symptoms have also been reported to be caused by other fungi, e.g., Fusarium spp. [54], Rhexocercosporidium carotae [55], Pseudomonas marginalis, Microbacterium oxydans, Lysobacter gummosus, Rhizobium leguminosarum, Pseudomonas veronii, and Agrobacterium tumefaciens [56]. Therefore, rusty-colored lesions on the root surface may represent a plant’s defensive response to invasion by microorganisms that are unable to cause rotting. As rusty symptoms are economically less important than root-rot disease, it is important to elucidate the interaction between ginseng roots and the two groups of Ilyonectria that cause rusty root and root-rot symptoms to determine how to manage root-rot-causing pathogens and limit disease. In rusty root, the levels of phenolic compounds and iron are much higher in the rusty tissues than adjacent healthy tissues [24], [53]. In addition, production of other defense-related enzymes, e.g., phenylalanine ammonia-lyase, polyphenoloxidase, and peroxidase, are stimulated in rusty tissue. The formation of iron-phenolic compound complexes is thought to be the reason for the rust color formation [24]. Rust-causing Ilyonectria species produce low levels of plant cell wall hydrolytic enzymes and phenolic-detoxifying enzymes [25]. There is little information about physiological and biochemical changes in root-rot infected roots, but the pathogen can produce large amounts of hydrolytic enzymes and is able to detoxify phenolic compounds [25]. In addition, iron has been found to be essential for root-rot development and pathogen growth [50].
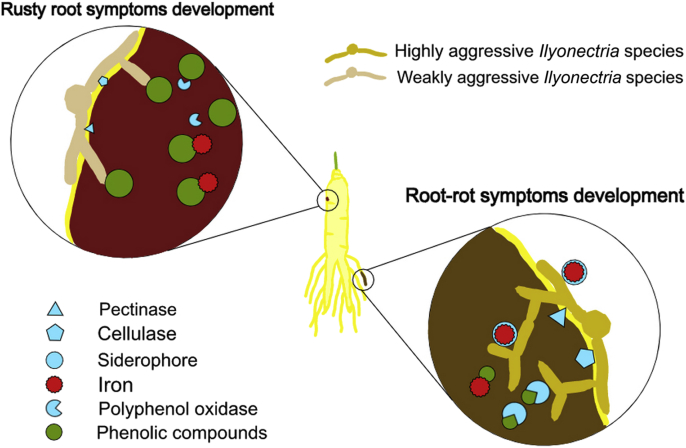
These findings indicate that rusty symptoms are not actually a sign of disease but the result of incompatible interactions between ginseng root and microorganisms, e.g., I. robusta, I. crassa, and I. panacis and others; the ability of these organisms to degrade the plant cell wall is very low; therefore, when they attempt to invade the root surface, they are not very successful, and the process is slow. The plant responds by producing defense-related enzymes, e.g., phenylalanine ammonia-lyase, polyphenoloxidase, peroxidase and secondary metabolites such as phenolic compounds. As these fungi do not have sufficient ability to detoxify these phenolic compounds, invasion is retarded. We argue that root-rot occurs due to compatible interactions between ginseng root and the aggressive Ilyonectria species I. mor-panacis; when the pathogen spore or mycelium attaches to the root surface, it rapidly produces high quantities of hydrolytic enzymes such as cellulase and pectinase, allowing rapid invasion of the epidermal layer and fast extension of the inoculum to the cortical and inner tissues. As the plant starts to respond to the invasion by producing phenolic compounds, the pathogen responds by producing enzymes that can break down phenolic compounds such as polyphenoloxidases. In parallel, the pathogen sequesters iron from the plant to support its growth using siderophores. As the cell wall components degrade, the plant defense response declines, and the growth of the pathogen is supported by the plant and rhizosphere iron; eventually, all barriers to defeat the invading pathogen are overcome, and rotting symptoms are established (Fig. 3).
Fig. 3.
Illustration of ginseng root infection by the highly aggressive Ilyonectria species, I. mors-panacis, and other weakly aggressive Ilyonectria species, leading to the occurrence of rotting and rusty symptoms, respectively. I. mors-panacis produces large amounts of cellulases and pectinases, and the plant rapidly responds to invasion by the production of phenolic compounds. However, the pathogen suppresses the toxicity of the phenolic compounds by producing polyphenol oxidases. In parallel, the pathogen sequesters plant and rhizospheric iron by producing siderophores. The other Ilyonectria species cannot penetrate the host plant as rapidly as I. mors-panacis because they produce lower amounts of hydrolytic and oxidative enzymes. The plant is therefore able to successfully retard their invasion. Iron exists on the root surface and within the plant cells and binds to the accumulated phenolic compounds, forming complexes that are likely responsible for the rusty coloration.
6. Summary and future directions
Cylindrocarpon destructans var. destructans is associated with both rusty and rotting symptoms in ginseng roots. Rusty symptoms do not appear to reflect disease, but to result from defense of ginseng roots to incompatible interactions with soil microorganisms [24]. Therefore, C. destructans var. destructans strains that cause rusting symptoms are unable to cause root-rot disease [22], [25]. This raises several questions. Why are two different kinds of symptoms caused by the same fungus? Are the fungus strains genetically similar or distinct? If distinct, what is the extent of the genetic differences? Are they different genotypes of the same species or different species? Finding the answers to these questions may explain how aggressive strains cause severe rotting and suggest suitable strategies to limit disease development. These questions started to be investigated when it was proposed that strains causing ginseng root-rot have a particular genotype. These strains were accordingly named C. destructans f. sp. panacis [47]. Nevertheless, the teleomorph of genus Cylindrocarpon was segregated into four different groups. Each group was considered to be a different genus, and C. destructans var. destructans teleomorph was re-named Ilyonectria radicicola [39]. Shortly thereafter, it was reported that the anamorphic state of I. radicicola is genetically polyphyletic. In addition, the genetic differences correlated with morphological variation, which provided sufficient evidence to consider each phylogenetic group to be a different species of Ilyonectria. Strains infecting ginseng plants were segregated into four different species: I. crassa, I. robusta, I. panacis, and I. mor-panacis. Interestingly, strains that cause severe root-rot diseases were clustered in one group: the I. mor-panacis group [26]. However, this correlation did not resolve the pathogenicity of other species complexes of Ilyonectria on their hosts. For example I. liriodendri strains infecting grape trees have been reported to be a species complex, but their virulence does not correlate with genetic differences [57]. Other than genetic diversity, several pathological criteria have been used to distinguish the aggressive Ilyonectria species, I. mors-panacis, from others, e.g., the production of high quantities of hydrolytic and oxidative enzymes to rapidly degrade plant cell wall components and detoxify phenolic compounds produced by plant roots upon invasion. However, additional studies need to be performed to fully clarify the mechanism of I. mors-panacis infection. For example, the role of the saponins in disease development should be investigated; generally, saponins have antifungal activity and accordingly protect plants from soil-borne fungi [58], [59], [60], [61], [62]. Many studies have demonstrated the ability of some plant pathogens to tolerate the lethal activity of saponins, possibly through the production of saponin hydrolytic enzymes [60], [63]. No studies have investigated if ginseng saponins play a role in plant protection. Only a few studies have investigated the role of ginsensosides in the pathogenicity of Pythium irregulare, which causes damping-off of ginseng seedlings [62], [64], [65], [66]; these studies concluded that the pathogenicity of this oomycetous pathogen depends on ginsenoside degradation [66]. Therefore, it is unknown how I. mors-panacis interacts with ginsenosides to negate their antifungal properties. Does it degrade the ginsenoside or has another mechanism? Furthermore, the role of reactive oxygen species (ROS) during disease progression of I. mors-panacis needs to be investigated; in general, ROS are produced in plant cells when the plant is exposed to abiotic or biotic stresses [67], [68] and act either as signaling molecules or cause oxidative damage, depending on their concentration [69]. When some pathogenic fungi invade plants, they cause fluctuations in ROS production. For example, the necrotrophic fungus Botrytis cinerea performs nectrotrophic invasion through rapid induction of ROS while being resistant to the oxidative effect of these ROS, leading to the death of plant tissues [70]. In contrast, the semi-biotrophic fungus Ustilago maydis deals with ROS by inhibiting the activity of the peroxidase enzyme, which leads to suppression of ROS production and accordingly a decrease in defense-related signaling [71]. It must therefore be determined how I. mors-pancis copes with ROS. Investigating these points will increase our understanding of the mechanism of root-rot development and provide information that can potentially be exploited to manage and limit disease occurrence.
7. Conclusion
In this review, we conclude the genetic diversity among Ilyonectria radicicola-species complex infecting ginseng plants which state root-rot causing strains as a separate species named I. mors-panacis from others associated with rusty symptoms which named I. crassa, I. robusta, I. panacis. Furthermore, we figure out the physiological and biochemical differences between highly and weakly aggressive species as well as those between the rusty and rotten roots to elucidate the mechanism of the two diseases symptoms developed by weakly and highly aggressive species infection, respectively. Accordingly, we postulated that the rusty symptoms is developed during the incompatible interaction between ginseng roots and weakly aggressive microorganisms, including the species of Ilyonectria other than I. mors-panacis, possibly by the established iron-phenolic compound complexes while rotting symptom is developed during the infection of I. mors-panacis due to the production of high quantities of hydrolytic and oxidative fungal enzymes which destroy the plant defensive barriers, in parallel with the pathogen growth stimulation by utilizing the available iron.
Conflicts of interest
The authors have no conflicts of interest to report.
Acknowledgements
This research was supported by a grant from the Next-Generation BioGreen 21 Program (SSAC, grant#: PJ0120342016), Rural Development Administration, Republic of Korea.
Contributor Information
Yeon-Ju Kim, Email: yeonjukim@khu.ac.kr.
Deok-Chun Yang, Email: deokchunyang@yahoo.co.kr.
References
- 1.Kim Y.J., Jeon J.N., Jang M.G., Oh J.Y., Kwon W.S., Jung S.K., Yang D.C. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J Ginseng Res. 2013;38:66–72. doi: 10.1016/j.jgr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung K.W., Wong A.S. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen J., Zimmer E.A. Phylogeny and biogeography of Panax L. (the ginseng genus, Araliaceae): inferences from ITS sequences of nuclear ribosomal DNA. Mol Phylogenet Evol. 1996;6:167–177. doi: 10.1006/mpev.1996.0069. [DOI] [PubMed] [Google Scholar]
- 4.Choi H.K., Wen L. A phylogenetic analysis of Panax (Araliaceae): Integrating cpDNA restriction site and nuclear rDNA ITS sequence data. Plant Syst Evol. 2000;224:109–120. [Google Scholar]
- 5.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy D.O., Scholey A.B. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav. 2003;75:687–700. doi: 10.1016/s0091-3057(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 7.Lu J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proctor J.T.A., Bailey W.G. Ginseng: Industry, botany and culture. Hortic Rev. 1987;9:187–236. [Google Scholar]
- 9.Ohh S.H., Yu Y.H., Kim K.H., Cho D.H. Ginseng cultivation bulletin. Korea Ginseng and Tobacco Research Inst.; 1992. Studies on control of soil-borne diseases and insects of ginseng and development of antifungal compound; pp. 121–184. [Google Scholar]
- 10.Yu Y.H., Ohh S.H. Research on ginseng diseases in Korea. Korean J Ginseng Sci. 1993;17:61–68. [Google Scholar]
- 11.Ziezold M., Reeleder R.D., Hall R., Proctor J.T.A. Effect of drenching soil with benomyl, propiconazole, and fluazinam on incidence of disappearing root rot of ginseng. J Ginseng Res. 1998;22:237–243. [Google Scholar]
- 12.Howard R.J., Garland J.A., Seaman W.L. Entomological Society of Canada & Canadian Phytopathological Society; 1994. Diseases and pests of vegetable crops in Canada: an illustrated compendium. [Google Scholar]
- 13.Putnam M.L., du Toit L.J. First report of Alternaria blight caused by Alternaria panax on ginseng (Panax quinquefolius) in Oregon and Washington, USA. Plant Pathol. 2003;52:406. [Google Scholar]
- 14.Kim Y.C., Lee J.H., Bae Y.S., Sohn B.K., Park S.K. Development of effective environmentally-friendly approaches to control Alternaria blight and anthracnose diseases of Korean ginseng. Eur J Plant Pathol. 2010;127:443–450. [Google Scholar]
- 15.Cho H.-S., Jeon Y.-H., Do G.-R., Cho D.-H., Yu Y.-H. Mycological characteristics of Botrytis cinerea causing gray mold on ginseng in Korea. J Ginseng Res. 2008;32:26–32. [Google Scholar]
- 16.Takimoto S. Colletotrichum panacicola Uyeda and Takimoto. Chosen Nokwai ho. 1919;14:24–25. (In Japanase) [Google Scholar]
- 17.Chung H.S., Bae H.W. Ginseng anthracnose in Korea: Factors affecting primary inoculum, growth of the pathogen, disease development and control. Korean J Plant Prot. 1979;18:35–41. [Google Scholar]
- 18.Darmono T.W., Owen M.L., Parke J.L. Isolation and pathogenicity of Phytophthora cactorum from forest and ginseng garden soils in Wisconsin. Plant Dis. 1991;75(6):610–612. [Google Scholar]
- 19.Bobev S.G., Baeyen S., Crepel C., Maes M. First report of Phytophthora cactorum on American ginseng (Panax quinquefolius) in Bulgaria. Plant Dis. 2003;87:752. doi: 10.1094/PDIS.2003.87.6.752C. [DOI] [PubMed] [Google Scholar]
- 20.Hill SN, Hausbeck, MK. Virulence and fungicide sensitivity of Phytophthora cactorum isolated from American ginseng gardens in Wisconsin and Michigan 2008;92:1183−1189. [DOI] [PubMed]
- 21.Reeleder R.D., Brammall R.A. Pathogenicity of Pythium species, Cylindrocarpon destructans, and Rhizoctonia solani to ginseng seedlings in Ontario. Can J Plant Pathol. 1994;16:311–316. [Google Scholar]
- 22.Hildebrand A.A. Root rot of ginseng in Ontario caused by members of the genus Ramularia. Can J Res. 1935;12:82–114. [Google Scholar]
- 23.Reeleder R.D., Roy R., Capell B. Seed and root rots of ginseng (Panax quinquefolius L) caused by Cylindrocarpon destructans and Fusarium spp. J Ginseng Res. 2002;26:151–158. [Google Scholar]
- 24.Rahman M, Punja ZK. Biochemistry of ginseng root tissues affected by rusty root symptoms 2005;43:1103–1114. [DOI] [PubMed]
- 25.Rahman M., Punja Z.K. Factors influencing development of root rot on ginseng caused by Cylindrocarpon destructans. Phytopathology. 2005;95:1381–1390. doi: 10.1094/PHYTO-95-1381. [DOI] [PubMed] [Google Scholar]
- 26.Cabral A., Groenewald J.Z., Rego C., Oliveira H., Crous P.W. Cylindrocarpon root rot: multi-gene analysis reveals novel species within the Ilyonectria radicicola-species complex. Mycol Prog. 2012;11:655–688. [Google Scholar]
- 27.Zinnsmeister C.L. Ramularia root-rots of ginseng. Phytopathology. 1918;8:557–571. [Google Scholar]
- 28.Chung H.S. Korean Society of Plant Protection; Seoul: 1979. Ginseng disease. Research reports of the Korean Society of Plant Protection; pp. 107–144. [Google Scholar]
- 29.Punja Z.K., Wan A., Goswami R.S. Root rot and distortion of ginseng seedling roots caused by Fusarium oxysporum. Can J Plant Pathol. 2008;30:565–574. [Google Scholar]
- 30.Wollenweber H.W. Ramularia, Mycosphaerella, Nectria, Calonectria. Phytopathology. 1913;3:197–242. [Google Scholar]
- 31.Booth C. The genus Cylindrocarpon. Myc Papers. 1966;104:1–56. [Google Scholar]
- 32.Samuels G.J., Brayford D. Variation in Nectria radicicola and its anamorph, Cylindrocarpon destructans. Mycol Res. 1990;94:433–442. [Google Scholar]
- 33.Rossman A.Y., Samuels G.J., Rogerson C.T., Lowen R. Genera of the Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes) Stud Mycol. 1999;42:1–248. [Google Scholar]
- 34.Mantiri F.R., Samuels G.J., Rahe J.E., Honda B.M. Phylogenetic relationships in Neonectria species having Cylindrocarpon anamorphs inferred from mitochondrial ribosomal DNA sequences. Can J Bot. 2001;79:334–340. [Google Scholar]
- 35.Brayford D., Honda B.M., Mantiri F.R., Samuels G.J. Neonectria and Cylindrocarpon: the Nectria mammoidea group and species lacking microconidia. Mycologia. 2004;96:572–597. [PubMed] [Google Scholar]
- 36.Booth C. Studies of pyrenomycetes. IV. Nectria (part 1) Myc Papers. 1959;73:1–115. [Google Scholar]
- 37.Samuels G.J., Brayford D. Species of Nectria (sensu lato). with red perithecia and striate ascospores. Sydowia. 1994;46:75–161. [Google Scholar]
- 38.Brayford D., Samuels G.J. Some didymosporous species of Nectria with nonmicroconidial Cylindrocarpon anamorphs. Mycologia. 1993;85:612–637. [Google Scholar]
- 39.Chaverri P., Salgado C., Hirooka Y., Rossman A.Y., Samuels G.J. Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon like anamorphs. Stud Mycol. 2011;68:57–78. doi: 10.3114/sim.2011.68.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Booth C. Nectria radicicola. C.M.I. Descriptions of Pathogenic Fungi and Bacteria 1967;148:1–2.
- 41.Crous P.W., Slippers B., Wingfield M.J., Rheeder J., Marasas W.F.O., Philips A.J.L., Alves A., Burgess T., Barber P., Groenewald J.Z. Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol. 2006;55:235–253. doi: 10.3114/sim.55.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crous P.W., Schoch C.L., Hyde K.D., Wood A.R., Gueidan C., de Hoog G.S., Groenewald J.Z. Phylogenetic lineages in the Capnodiales. Stud Mycol. 2009;64:17–47. doi: 10.3114/sim.2009.64.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gräfenhan T., Schroers H.-J., Nirenberg H.I., Seifert K.A. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutellax. Stud Mycol. 2011;68:79–113. doi: 10.3114/sim.2011.68.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lombard L., Crous P.W., Wingfield B.D., Wingfield M.J. Phylogeny and systematics of the genus Calonectria. Stud Mycol. 2010;66:31–69. doi: 10.3114/sim.2010.66.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroers H.-J., Gräfenhan T., Nirenberg H.I., Seifert K.A. A revision of Cyanonectria and Geejayessia gen. nov., and related species with Fusarium-like anamorphs. Stud Mycol. 2011;68:115–138. doi: 10.3114/sim.2011.68.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee C., Kim K.Y., Lee J.E., Kim S., Ryu D., Choi J.E., An G. Enzymes hydrolyzing structural components and ferrous ion cause rusty-root symptom on ginseng (Panax ginseng) J Microbiol Biotechnol. 2011;21:192–196. doi: 10.4014/jmb.1008.08010. [DOI] [PubMed] [Google Scholar]
- 47.Seifert K.A., McMullen C.R., Yee D., Reeleder R.D., Dobinson K.F. Molecular differentiation and detection of ginseng-adapted isolates of the root rot fungus Cylindrocarpon destructans. Phytopathology. 2003;93:1533–1542. doi: 10.1094/PHYTO.2003.93.12.1533. [DOI] [PubMed] [Google Scholar]
- 48.Lee SS. Korean ginseng (ginseng cultivation), Korean ginseng and T. Research institute 2007;18–40.
- 49.Hankins A. Produced by Communications and Marketing, College of Agriculture and Life Sciences, Virginia Polytechnic Institute and State University; 2009. Producing and Marketing Wild Simulated Ginseng in Forest and Agroforestry Systems. [Google Scholar]
- 50.Rahman M., Punja Z.K. Influence of iron on Cylindrocarpon root rot development on ginseng. Phytopathology. 2006;96:1179–1187. doi: 10.1094/PHYTO-96-1179. [DOI] [PubMed] [Google Scholar]
- 51.Stoltz L.P. In Pro. 4th Natl. Ginseng Conf.; Lexington: Ky: 1982. Mineral nutrition studies of American ginseng. [Google Scholar]
- 52.Kim J.H., Kim S.G., Kim M.S., Jeon Y.H., Cho D.H., Kim Y.H. Different structural modifications associated with development of ginseng root rot caused by Cylindrocarpon destructans. Plant Pathol J. 2009;25:1–5. [Google Scholar]
- 53.Yang D.-C., Kim Y.-H., Yun K.-Y., Lee S.-S., Kwon J.-N., Kang H.-M. Red-colored phenomena of ginseng (Panax ginseng C. A. Meyer): root and soil environment. J Ginseng Sci. 1997;21:91–97. [Google Scholar]
- 54.Punja Z.K., Wan A., Goswami R.S., Verma N., Rahman M., Barasubiye T., Seifert K.A., Lévesque C.A. Diversity of Fusarium species associated with discolored ginseng roots in British Columbia. Can J Plant Pathol. 2007;29:340–353. [Google Scholar]
- 55.Reeleder R.D., Hoke S.M.T., Zhang Y. Rusted root of ginseng (Panax quinquefolius) is caused by a species of Rhexocercosporidium. Phytopathology. 2006;96:1243–1254. doi: 10.1094/PHYTO-96-1243. [DOI] [PubMed] [Google Scholar]
- 56.Choi J.E., Ryuk J.A., Kim J.H., Choi C.H., Chun J.S., Kim Y.J., Lee H.B. Identification of endophytic bacteria isolated from rusty-colored root of Korean ginseng (Panax ginseng) and its induction. Korean J Med Crop Sci. 2005;13:1–5. [Google Scholar]
- 57.Pathrose B., Jones E.E., Jaspers M.V., Ridgway H.J. High genotypic and virulence diversity in Ilyonectria liriodendri isolates associated with black foot disease in New Zealand vineyards. Plant Pathol. 2014;63:613–624. [Google Scholar]
- 58.Crombie W.M.L., Crombie L., Green J.B., Lucas J.A. Pathogenicity of the take all fungus to oats: its relationship to the concentration and detoxification of the four avenacins. Phytochemistry. 1986;25:2075–2083. [Google Scholar]
- 59.Fewell A.M., Roddick J.G. Interactive antifungal activity of the glycoalkaloid solanine and chaconine. Phytochemistry. 1993;33:323–328. [Google Scholar]
- 60.Morrissey J.P., Osbourn A.E. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol Mol Biol Rev. 1999;63:708–724. doi: 10.1128/mmbr.63.3.708-724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papadopoulou K., Melton R.E., Leggett M., Daniels M.J., Osbourn A.E. Compromised disease resistance in saponin-deficient plants. Proc Natl Acad Sci. 1999;96:12923–12928. doi: 10.1073/pnas.96.22.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicol R.W., Traquair J.A., Bernards M.A. Ginsenosides as host resistance factors in American ginseng (Panax quinquefolius) Can J Bot. 2002;80:557–562. [Google Scholar]
- 63.Osbourn A. Saponins and plant defence – a soap story. Trends in Plant Sci. 1996;1:4–9. [Google Scholar]
- 64.Yousef L.F., Bernards M.A. In vitro metabolism of ginsenosides by the ginseng root pathogen Pythium irregulare. Phytochemistry. 2006;67(16):1740–1749. doi: 10.1016/j.phytochem.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 65.Nicol R.W., Yousef L., Traquair J.A., Bernards M.A. Ginsenosides stimulate the growth of soilborne pathogens of American ginseng. Phytochemistry. 2003;64:257–264. doi: 10.1016/s0031-9422(03)00271-1. [DOI] [PubMed] [Google Scholar]
- 66.Ivanov D.A., Bernards M.A. Ginsenosidases and the pathogenicity of Pythium irregulare. Phytochemistry. 2012;78:44–53. doi: 10.1016/j.phytochem.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 67.Shah K., Kumar R.G., Verma S., Dubey R.S. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 2001;161:1135–1144. [Google Scholar]
- 68.Sharma P., Dubey R.S. Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep. 2007;26:2027–2038. doi: 10.1007/s00299-007-0416-6. [DOI] [PubMed] [Google Scholar]
- 69.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;2012:26. [Google Scholar]
- 70.Shlezinger N., Minz A., Gur Y., Hatam I., Dagdas Y.F., Talbot N.J., Sharon A. Anti-apoptotic machinery protects the necrotrophic fungus Botrytis cinerea from host-induced apoptotic-like cell death during plant infection. PLoS Pathog. 2011;7:e1002185. doi: 10.1371/journal.ppat.1002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hemetsberger C., Herrberger C., Zechmann B., Hillmer M., Doehlemann G. The Ustilago maydis effector Pep1 suppresses plant immunity by inhibition of host peroxidase activity. PLoS Pathog. 2012;8:e1002684. doi: 10.1371/journal.ppat.1002684. [DOI] [PMC free article] [PubMed] [Google Scholar]