Abstract
Background
The aim of the present study was to evaluate the potential protective effects of six ginsenosides (Rb1, Rb2, Rc, Rd, Rg1, and Rg3) isolated from Panax ginseng against tacrolimus (FK506)-induced apoptosis in renal proximal tubular LLC-PK1 cells.
Methods
LLC-PK1 cells were treated with FK506 and ginsenosides, and cell viability was measured. Protein expressions of mitogen-activated protein kinases, caspase-3, and kidney injury molecule-1 (KIM-1) were evaluated by Western blotting analyses. The number of apoptotic cells was measured using an image-based cytometric assay.
Results
Reduction in cell viability by 60μM FK506 was ameliorated significantly by cotreatment with ginsenosides Rg1 and Rb1. The phosphorylation of p38, extracellular signal-regulated kinases, and KIM-1, and cleavage of caspase-3, increased markedly in LLC-PK1 cells treated with FK506 and significantly decreased after cotreatment with ginsenoside Rb1. The number of apoptotic cells decreased by 6.0% after cotreatment with ginsenoside Rb1 (10μM and 50μM).
Conclusion
The antiapoptotic effects of ginsenoside Rb1 on FK506-induced apoptosis were mediated by the inhibition of mitogen-activated protein kinases and caspase activation.
Keywords: caspase-3, FK506-induced nephrotoxicity, ginsenoside Rb1, KIM-1, MAPKs
1. Introduction
Tacrolimus (FK506) is one of the principal immunosuppressive drugs used after solid-organ transplantations to reduce rejection rates [1], [2], because it acts by disrupting the signaling events mediated by the calcium-dependent serine/threonine protein phosphatase, calcineurin, in T lymphocytes [3]. However, in the clinical setting, nephrotoxicity resulting from FK506 may emerge as a significant side effect, which may diminish its overall benefits for long-term graft survival [4]. Transforming growth factor-β and cyclooxygenase-2 are important regulators of inflammatory processes when glomerular and tubular cells are damaged. FK506 seems to induce fewer hemodynamic and fibrogenic effects [5] and changes in glomerular and tubular function [6]. Previous studies suggest that the ability of FK506 to produce reactive oxygen species via the activation of the nicotinamide adenine dinucleotide phosphate oxidase pathway may be responsible for its nephrotoxicity [5]. Apoptosis plays a central role in nephrotoxic agent-induced nephrotoxicity, and a number of other drugs such as cisplatin, cyclosporine A, and amphotericin are also known to induce renal cell apoptosis in vitro or in vivo [7]. However, the molecular pathways for FK506 are poorly understood.
Mitogen-activated protein kinases (MAPKs) are upstream modulators of apoptosis that are induced by nephrotoxic agents [8]. The caspase family plays a central role in apoptosis. In particular, caspase-3 is an important effector enzyme in renal injury associated with apoptosis [9]. Kidney injury molecule-1 (KIM-1) is a receptor involved in the phagocytosis and internalization of apoptotic bodies produced from cells undergoing apoptosis and also a sensitive injury biomarker for detecting the early acute FK506-induced nephrotoxicity in proximal tubules [2], [10]. Studies on natural products that may minimize FK506-induced nephrotoxicity are still marginal. Green tea extract and polyphenols abrogated FK506-induced nephrotoxicity in mice, rats, and LLC-PK1 cells (a porcine proximal tubule cell line). In addition, they significantly suppressed the increased intracellular reactive oxygen species levels as well as caspase-3 activation [11], [12], [13], [14].
Ginsenosides are the biologically active components of ginseng, which are generally obtained from the root of Panax ginseng, and they are divided into 20(S)-protopanaxadiols (ginsenosides Rb1, Rb2, Rc, Rd, and Rg3) and 20(S)-protopanaxatriols (ginsenosides Re and Rg1) groups on the basis of their aglycone moieties [15], [16], [17]. In previous studies, ginsenosides were shown to act as anticancer agents [15], [16], [17], renoprotective agents [16], [18], [19], antioxidant agents [20], [21], [22], and neuroprotective agents [23], [24], [25], [26]. In particular, ginsenosides Rg3, Rg5, and Rk1 generated during the heat treatment of ginseng ameliorate renal damage by regulating inflammation and apoptosis, both in vitro and in vivo [16]. The aim of the present study was to investigate as yet unidentified protective effects of six kinds of ginsenoside (Rb1, Rb2, Rc, Rd, Rg1, and Rg3) against FK506-induced apoptosis in renal proximal tubular LLC-PK1 cells.
2. Materials and methods
2.1. Chemicals
FK506 was purchased from Sigma Aldrich (Saint Louis, MO, USA). Six ginsenosides (Rb1, Rb2, Rc, Rd, Rg1, and Rg3) were purchased from Ambo Institute (Seoul, Korea). An Ez-Cytox cell viability assay kit was purchased from Dail Lab Service Co. (Seoul, Korea). Caspase-3 colorimetric assay kits were purchased from BioVision (Milpitas, CA, USA). A Tali Apoptosis Kit was purchased from Invitrogen (Carlsbad, CA, USA). Dulbecco's modified Eagle's medium and fetal bovine serum were purchased from Invitrogen Co. (Grand Island, NY, USA).
2.2. Cell culture and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell viability assay
LLC-PK1 (pig kidney epithelium, CL-101) cells were used to evaluate the renoprotective activity of ginsenosides against FK506-induced cytotoxicity as reported previously [12]. LLC-PK1 cells were purchased from the American Type Culture Collection (Rockville, MD, USA) and cultured in Dulbecco's modified Eagle's medium (Cellgro, Manassas, VA, USA) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Invitrogen Co., Grand Island, NY, USA), and 4mM l-glutamine in an atmosphere of 5% CO2 at 37°C. Viability of cells was determined by an Ez-Cytox cell viability detection kit (Dail Lab Service Co.). When the cells are approximately 80% confluent, they were seeded in 96-well culture plates at 1 × 104 cells/well and incubated for 24 h for adhesion. Then, cells were treated with control (0.5% dimethyl sulfoxide), or indicated concentrations of six ginsenosides (Rb1, Rb2, Rc, Rd, Rg1, or Rg3). After incubation for 2 h, 60μM of FK506 was added to each well and cells were further incubated for 24 h. After incubation, 10 μL of Ez-Cytox reagent was added to each well and cells were incubated for 2 h. Cell viability was measured by absorbance at 450 nm using a microplate reader (PowerWave XS; Bio-Tek Instruments, Winooski, VT, USA).
2.3. Western blotting analysis
LLC-PK1 cells cultured in six-well plates were treated with ginsenoside Rb1 (10μΜ or 50μΜ) for 24 h; then, the cells were lysed with RIPA buffer (20mM Tris–HCl (pH 7.5), 150mM NaCl, 1mM Na2EDTA, 1mM EGTA [ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid], 1% NP-40, 1% sodium deoxycholate, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, and 1 μg/mL leupeptin (Cell Signaling, Danvers, MA, USA), and supplemented with 1mM phenylmethylsulfonyl fluoride immediately prior to use. Each protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). Equal amounts (20 μg/lane) of protein (whole-cell extracts) were separated by electrophoresis in a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred onto polyvinylidene fluoride transfer membranes. Specific proteins were analyzed with epitope-specific primary antibodies to p38 MAP kinase, phospho-p38, p44/42 MAP kinase (ERK), phospho-p44/42 (p-ERK), cleaved caspase-3, KIM-1, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and horseradish peroxidase-conjugated antirabbit antibodies (Cell Signaling, Boston, MA, USA). Bound antibodies were detected using ECL Advance Western blotting detection reagents (GE Healthcare, Buckinghamshire, UK) and visualized with a FUSION Solo chemiluminescence system (PEQLAB Biotechnologie GmbH, Erlangen, Germany).
2.4. Caspase activity assay
Caspase-3 activities were determined by using caspase-3 colorimetric assay kits according to the protocol of the manufacturer (BioVision, Milpitas, CA, USA). In brief, LLC-PK1 cells were seeded in 100-mm dishes at 1 × 106 and were treated with ginsenoside Rb1 (10μM or 50μM). After incubation for 2 h, 60μM of FK506 was added to each well and they were incubated for 3 h, then cells were lysed in 50 μL of lysis buffer and incubated on ice for 10 min. Then, 50 μL of 10mM dithiothreitol 2× reaction buffer containing 10mM dithiothreitol and 5 μL of the 4mM DEVD-pNA substrate were added to each of the lysed samples and incubated for an additional 1 h at 37°C. Subsequently, caspase activities were measured by absorbance at 400 nm using a microplate reader (PowerWave XS; Bio-Tek Instruments).
2.5. Image-based cytometric assay
LLC-PK1 cells seeded in 6-well plates were treated with ginsenoside Rb (10μM and 50μM). After incubation for 2 h, 60μM of FK506 was added to each well, which were incubated for 3 h, and then cells were stained with annexin V Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) in darkness for 20 min after suspension in annexin binding buffer and stained with propidium in darkness for 5 min after suspension in annexin binding buffer. The number of dead and apoptotic cells was measured using a Tali image-based cytometer (Invitrogen, Carlsbad, CA, USA). This assay identified apoptotic cells as stained with green annexin V Alexa Fluor 488, dead cells as stained with both red propidium iodide and green annexin V Alexa Fluor 488, and nonstained live cells in the population.
2.6. Statistical analysis
Statistical significance was determined through analysis of variance followed by a multiple comparison test with a Bonferroni adjustment. A p value < 0.05 was considered statistically significant. The analysis was performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA).
3. Results and discussion
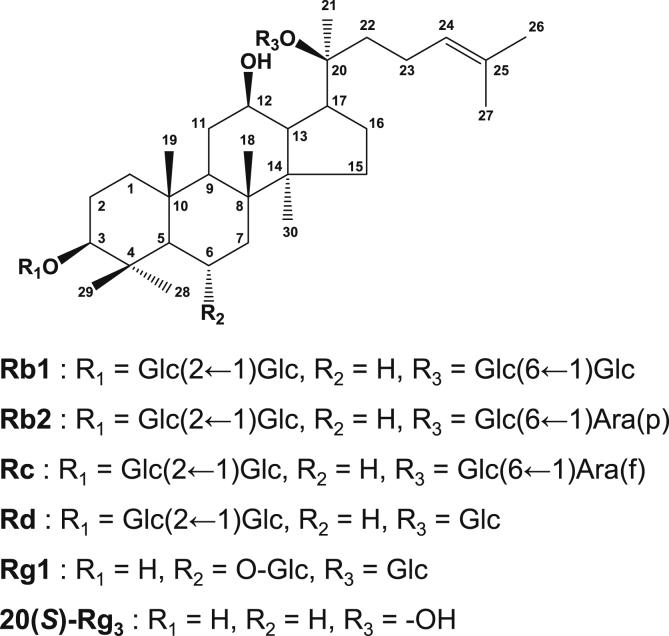
Ginseng and its active components ginsenosides are known to act as renal protective agents [18], [19], [22], [27], [28], [29], [30]. Ginsenosides have a common four-ring hydrophobic structure (Fig. 1). Ginsenoside Rg1 is known to relieve d-galactose-induced renal damage in mice by alleviating oxidative stress [31]. Ginsenoside Rg1 also relieved aldosterone-induced renal damage in rat renal tubular NRK-52E cells by alleviating oxidative stress [32]. Similarly, ginsenoside Rb1 relieved intestinal ischemia–reperfusion-induced renal damage in mice by regulating apoptosis and alleviating renal dysfunction through the Nrf2/ARE signaling pathway [33]. Ginsenosides Rg3, Rg5, and Rk1 are also known to abrogate cisplatin-induced renal damage in porcine renal proximal tubular LLC-PK1 cells by regulating inflammation and apoptosis [30].
Fig. 1.
Structures of ginsenosides Rb1, Rb2, Rc, Rd, Rg1, and Rg3.
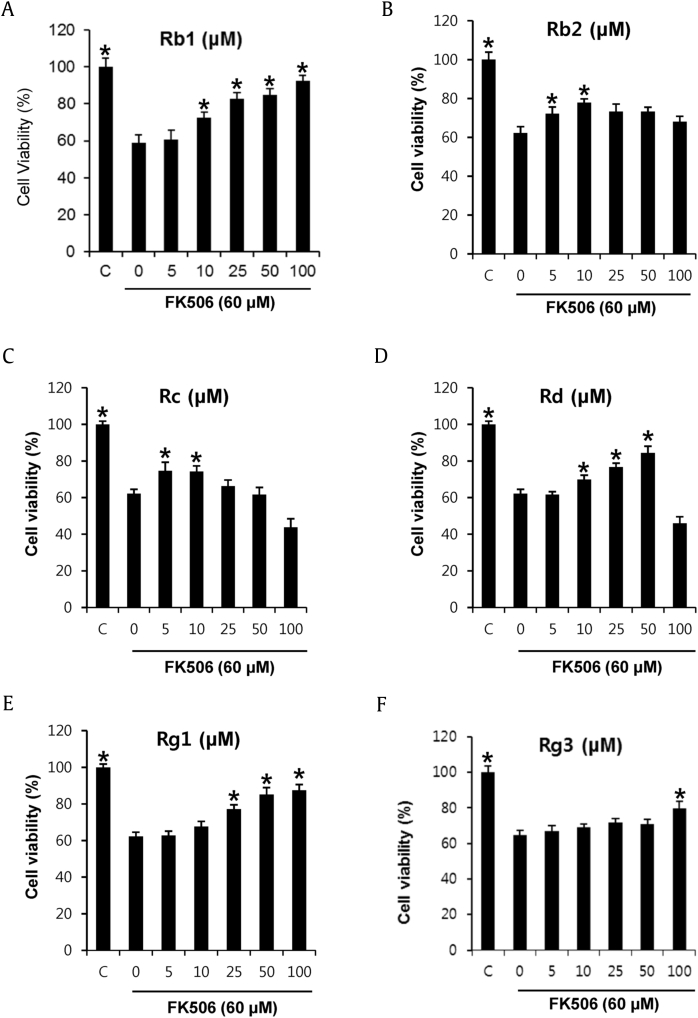
In the present study, the unidentified protective effects of six ginsenosides (Rb1, Rb2, Rc, Rd, Rg1, and Rg3) against FK506-induced apoptosis were evaluated in cell-based assays. We performed comparative experiments of these ginsenosides on FK506-induced renal damage in porcine renal proximal tubular LLC-PK1 cells. After treatment of with 60μM FK506 for 24 h, the LLC-PK1 cell viability was reduced by 61.2%. FK506 is very cytotoxic to renal proximal tubular cells, which are a principal target of FK506 in the kidney [25]. The reduction in cell viability by 60μM of FK506 was recovered by cotreatment with the six ginsenosides (Fig. 2). In particular, the effects of ginsenosides Rg1 and Rb1 were the most prominent. They showed protective effects of more than 80% at concentrations of 25–100μM. This effect was more pronounced from ginsenoside Rb1 than from Rg1 (Fig. 2A). The protective effect of 100μM of ginsenoside Rb1 was similar to that of the control group (0.5% DMSO), and we used ginsenoside Rb1 in the next experiment for identification of molecular pathways related to the protective effect against FK506-induced nephrotoxicity.
Fig. 2.
Comparison of the protective effects of the six ginsenosides (Rb1, Rb2, Rc, Rd, Rg1, and Rg3) on FK506-induced nephrotoxicity in LLC-PK1 cells. *p < 0.05 compared to the FK506-treated value.
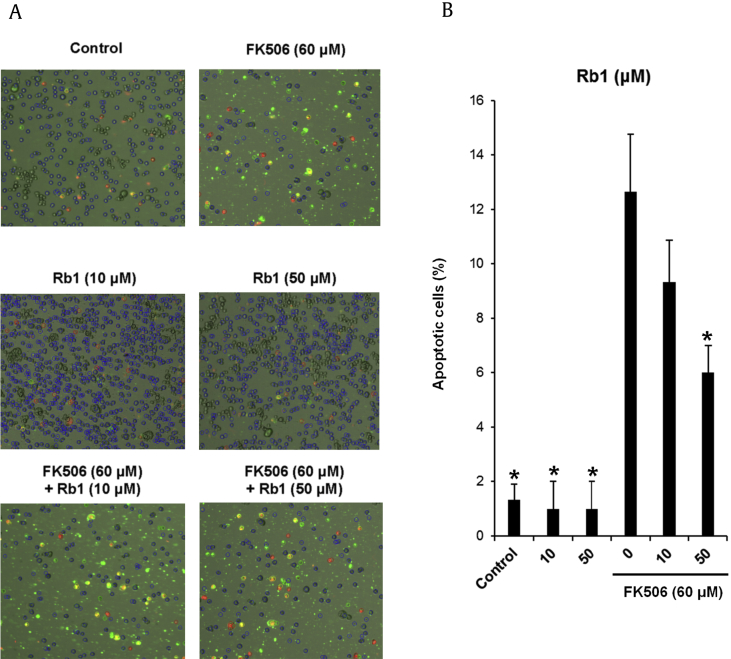
Cell death results from a variety of reasons including apoptosis and necrosis. A number of studies have found that FK506-induced cell damage results in apoptosis [12], [13], [34]. In earlier studies, apoptotic LLC-PK1 cell death, which were stained with annexin V, increased from 2.6% to 14.5% after treatment with 50μM of FK506 [13]. We treated LLC-PK1 cells with ginsenoside Rb1 (10μM and 50μM) to explore whether ginsenoside Rb1 could decrease FK506-induced apoptosis, using annexin V and propidium iodide staining. As shown in Fig. 3A, apoptotic cell death (indicated by annexin V staining) increased from 1.3% to 12.6% after treatment with 60μM of FK506. The percentage of apoptotic cells is shown in the bar graph (Fig. 3B). The number of apoptotic cells decreased by 6.0% after cotreatment with ginsenoside Rb1 (10μM and 50μM).
Fig. 3.
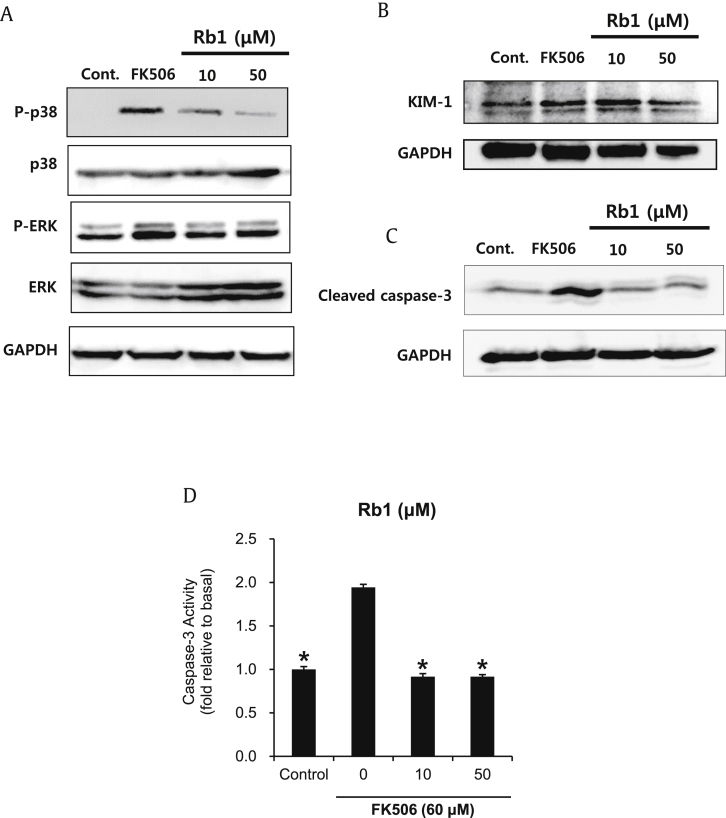
Effect of ginsenoside Rb1 on MAPKs, KIM-1, and caspase-3 protein expressions in LLC-PK1 cells with FK506-induced apoptosis. (A–C) Protein expression levels of p-p38, p38, p-ERK, ERK, KIM-1, cleaved caspase-3, and GAPDH. (D) Activity level of caspase-3. *p < 0.05 compared to the FK506-treated value. ERK, p44/42 MAP kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; KIM-1, kidney injury molecule-1; MAPKs, mitogen-activated protein kinases; p-ERK, phospho-p44/42.
We further evaluated the effect of ginsenoside Rb1 (10μM and 50μM) on MAPK protein expressions in LLC-PK1 cells. In earlier studies, FK506-induced apoptosis resulted from nuclear fragmentation and caspase-3 protease activation [12], [13], [34]. MAPKs are important in apoptosis resulting from caspase-3 protease activation [35], [36]. MAPKs can be activated by growth factors, mitogens, hormones, cytokines, or environmental stress [37]. Although ERK is involved in cell growth and differentiation, according to the cell type and under certain conditions, it is involved in apoptosis through caspase-3 activation and poly ADP ribose polymerase (PARP) cleavage [36], [38]. In addition, p38 MAPK, a stress-activated kinase, is involved in apoptosis through the activation of caspase-3 and phosphorylation of Bcl-2 family proteins [39], [40], [41], [42]. The phosphorylation of p38 MAPK, p44/42 MAPK (ERK), and KIM-1, cleavage of caspase-3 (Figs. 4A–4C), and activity levels of caspase-3 were increased significantly after treatment with 60μM of FK506, whereas pretreatment with ginsenoside Rb1 abrogated the FK506-induced phosphorylation of MAPKs and caspase-3 activity to near basal levels (Fig. 4D). We also confirmed that ginsenoside Rb1 alone did not lead to changes in the protein expressions of p-p38, p-ERK, KIM-1, and cleaved caspase-3 (data not shown).
Fig. 4.
Effect of ginsenoside Rb1 on FK506-induced apoptosis in LLC-PK1 cells. (A) Visualization of apoptosis detection. (B) Percentage of apoptotic cells stained with annexin V. *p < 0.05 compared to the FK506-treated value. ERK, p44/42 MAP kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; KIM-1, kidney injury molecule-1; p-ERK, phospho-p44/42.
In summary, pretreatment with ginsenosides (Rb1, Rb2, Rc, Rd, Rg1, and Rg3) abrogated FK506-induced cytotoxicity in LLC-PK1 cells. Among the six ginsenosides, Rb1 was the most effective against FK506-induced nephrotoxicity in LLC-PK1 cells. Our findings provide evidence that ginsenoside Rb1 produces its antiapoptotic effect by inhibiting MAPK and caspase activation. In addition, the elevated KIM-1 expression resulting from FK506 was decreased by cotreatment with ginsenoside Rb1. These findings may contribute to the utilization of ginsenoside Rb1 as an adjuvant therapy in order to reduce the adverse effects of FK506 in the kidney.
Conflicts of interest
The authors have declared no conflicts of interest.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, & Future Planning (NRF-2016R1C1B1012787). This work was also supported by a grant from the Korea Food Research Institute, Republic of Korea (E0164500-01).
Contributor Information
Ki Hyun Kim, Email: khkim83@skku.edu.
Ki Sung Kang, Email: kkang@gachon.ac.kr.
References
- 1.Wallemacq P.E., Reding R. FK506 (tacrolimus), a novel immunosuppressant in organ transplantation: clinical, biomedical, and analytical aspects. Clin Chem. 1993;39:2219–2228. [PMC free article] [PubMed] [Google Scholar]
- 2.Cosner D., Zeng X., Zhang P.L. Proximal tubular injury in medullary rays is an early sign of acute tacrolimus nephrotoxicity. J Transplant. 2015;2015:142521. doi: 10.1155/2015/142521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumont F.J. FK506, an immunosuppressant targeting calcineurin function. Curr Med Chem. 2000;7:731–748. doi: 10.2174/0929867003374723. [DOI] [PubMed] [Google Scholar]
- 4.Tada H., Nakashima A., Awaya A., Fujisaki A., Inoue K., Kawamura K., Itoh K., Masuda H., Suzuki T. Effects of thymic hormone on reactive oxygen species-scavengers and renal function in tacrolimus-induced nephrotoxicity. Life Sci. 2002;70:1213–1223. doi: 10.1016/s0024-3205(01)01495-3. [DOI] [PubMed] [Google Scholar]
- 5.Al-Harbi N.O., Imam F., Al-Harbi M.M., Iqbal M., Nadeem A., Al-Shahrah O.A., Korashy H.M., Al-Hosaini K.A., Ahmed M., Bahashwar S. Treatment with aliskiren ameliorates tacrolimus-induced nephrotoxicity in rats. J Renin Angiotensin Aldosterone Syst. 2015;16:1329–1336. doi: 10.1177/1470320314530178. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen F.T., Leyssac P.P., Kemp E., Starklint H., Dieperink H. Nephrotoxicity of FK-506 in the rat. Studies on glomerular and tubular function, and on the relationship between efficacy and toxicity. Nephrol Dial Transplant. 1995;10:334–340. [PubMed] [Google Scholar]
- 7.Servais H., Ortiz A., Devuyst O., Denamur S., Tulkens P.M., Mingeot-Leclercq M.P. Renal cell apoptosis induced by nephrotoxic drugs: cellular and molecular mechanisms and potential approaches to modulation. Apoptosis. 2008;13:11–32. doi: 10.1007/s10495-007-0151-z. [DOI] [PubMed] [Google Scholar]
- 8.Havasi A., Borkan S.C. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B., El Nahas A.M., Thomas G.L., Haylor J.L., Watson P.F., Wagner B., Johnson T.S. Caspase-3 and apoptosis in experimental chronic renal scarring. Kidney Int. 2001;60:1765–1776. doi: 10.1046/j.1523-1755.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 10.Humphreys B.D., Xu F., Sabbisetti V., Grgic I., Movahedi Naini S., Wang N., Chen G., Xiao S., Patel D., Henderson J.M. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. 2013;123:4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Back J.H., Ryu H.H., Hong R., Han S.A., Yoon Y.M., Kim D.H., Hong S.J., Kim H.L., Chung J.H., Shin B.C. Antiproteinuric effects of green tea extract on tacrolimus-induced nephrotoxicity in mice. Transplant Proc. 2015;47:2032–2034. doi: 10.1016/j.transproceed.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Hisamura F., Kojima-Yuasa A., Huang X., Kennedy D.O., Matsui-Yuasa I. Synergistic effect of green tea polyphenols on their protection against FK506-induced cytotoxicity in renal cells. Am J Chin Med. 2008;36:615–624. doi: 10.1142/S0192415X08006028. [DOI] [PubMed] [Google Scholar]
- 13.Hisamura F., Kojima-Yuasa A., Kennedy D.O., Matsui-Yuasa I. Protective effect of green tea extract and tea polyphenols against FK506-induced cytotoxicity in renal cells. Basic Clin Pharmacol Toxicol. 2006;98:192–196. doi: 10.1111/j.1742-7843.2006.pto_284.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhong Z., Connor H.D., Li X., Mason R.P., Forman D.T., Lemasters J.J., Thurman R.G. Reduction of ciclosporin and tacrolimus nephrotoxicity by plant polyphenols. J Pharm Pharmacol. 2006;58:1533–1543. doi: 10.1211/jpp.58.11.0015. [DOI] [PubMed] [Google Scholar]
- 15.Park E.H., Kim Y.J., Yamabe N., Park S.H., Kim H.K., Jang H.J., Kim J.H., Cheon G.J., Ham J., Kang K.S. Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J Ginseng Res. 2014;38:22–27. doi: 10.1016/j.jgr.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park J.Y., Choi P., Kim H.K., Kang K.S., Ham J. Increase in apoptotic effect of Panax ginseng by microwave processing in human prostate cancer cells: in vitro and in vivo studies. J Ginseng Res. 2016;40:62–67. doi: 10.1016/j.jgr.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang H.J., Han I.H., Kim Y.J., Yamabe N., Lee D., Hwang G.S., Oh M., Choi K.C., Kim S.N., Ham J., Eom D.W. Anticarcinogenic effects of products of heat-processed ginsenoside Re, a major constituent of ginseng berry, on human gastric cancer cells. J Agric Food Chem. 2014;62:2830–2836. doi: 10.1021/jf5000776. [DOI] [PubMed] [Google Scholar]
- 18.Kang K.S., Kim H.Y., Yamabe N., Park J.H., Yokozawa T. Preventive effect of 20(S)-ginsenoside Rg3 against lipopolysaccharide-induced hepatic and renal injury in rats. Free Radic Res. 2007;41:1181–1188. doi: 10.1080/10715760701581740. [DOI] [PubMed] [Google Scholar]
- 19.Kang K.S., Yamabe N., Kim H.Y., Park J.H., Yokozawa T. Therapeutic potential of 20(S)-ginsenoside Rg(3) against streptozotocin-induced diabetic renal damage in rats. Eur J Pharmacol. 2008;591:266–272. doi: 10.1016/j.ejphar.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 20.Kang K.S., Kim H.Y., Baek S.H., Yoo H.H., Park J.H., Yokozawa T. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30:724–728. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y.J., Kim H.Y., Kang K.S., Lee J.G., Yokozawa T., Park J.H. The chemical and hydroxyl radical scavenging activity changes of ginsenoside-Rb1 by heat processing. Bioorg Med Chem Lett. 2008;18:4515–4520. doi: 10.1016/j.bmcl.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 22.Lee W., Park S.H., Lee S., Chung B.C., Song M.O., Song K.I., Ham J., Kim S.N., Kang K.S. Increase in antioxidant effect of ginsenoside Re–alanine mixture by Maillard reaction. Food Chem. 2012;135:2430–2435. doi: 10.1016/j.foodchem.2012.06.108. [DOI] [PubMed] [Google Scholar]
- 23.Cong L., Chen W. Neuroprotective effect of ginsenoside Rd in spinal cord injury rats. Basic Clin Pharmacol Toxicol. 2016;119:193–201. doi: 10.1111/bcpt.12562. [DOI] [PubMed] [Google Scholar]
- 24.Dong X., Zheng L., Lu S., Yang Y. Neuroprotective effects of pretreatment of ginsenoside Rb1 on severe cerebral ischemia-induced injuries in aged mice: involvement of anti-oxidant signaling. Geriatr Gerontol Int. 2015 doi: 10.1111/ggi.12699. [DOI] [PubMed] [Google Scholar]
- 25.Zhou T., Zu G., Zhang X., Wang X., Li S., Gong X., Liang Z., Zhao J. Neuroprotective effects of ginsenoside Rg1 through the Wnt/beta-catenin signaling pathway in both in vivo and in vitro models of Parkinson's disease. Neuropharmacology. 2016;101:480–489. doi: 10.1016/j.neuropharm.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Li Y.B., Wang Y., Tang J.P., Chen D., Wang S.L. Neuroprotective effects of ginsenoside Rg1-induced neural stem cell transplantation on hypoxic–ischemic encephalopathy. Neural Regen Res. 2015;10:753–759. doi: 10.4103/1673-5374.156971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin H.S., Yu M., Kim M., Choi H.S., Kang D.H. Renoprotective effect of red ginseng in gentamicin-induced acute kidney injury. Lab Invest. 2014;94:1147–1160. doi: 10.1038/labinvest.2014.101. [DOI] [PubMed] [Google Scholar]
- 28.Doh K.C., Lim S.W., Piao S.G., Jin L., Heo S.B., Zheng Y.F., Bae S.K., Hwang G.H., Min K.I., Chung B.H. Ginseng treatment attenuates chronic cyclosporine nephropathy via reducing oxidative stress in an experimental mouse model. Am J Nephrol. 2013;37:421–433. doi: 10.1159/000349921. [DOI] [PubMed] [Google Scholar]
- 29.Lim S.W., Doh K.C., Jin L., Jin J., Piao S.G., Heo S.B., Chung B.H., Yang C.W. Ginseng treatment attenuates autophagic cell death in chronic cyclosporine nephropathy. Nephrology. 2014;19:490–499. doi: 10.1111/nep.12273. [DOI] [PubMed] [Google Scholar]
- 30.Park J.Y., Choi P., Kim T., Ko H., Kim H.K., Kang K.S., Ham J. Protective effects of processed finseng and its active ginsenosides on cisplatin-induced nephrotoxicity: in vitro and in vivo studies. J Agric Food Chem. 2015;63:5964–5969. doi: 10.1021/acs.jafc.5b00782. [DOI] [PubMed] [Google Scholar]
- 31.Fan Y., Xia J., Jia D., Zhang M., Zhang Y., Huang G., Wang Y. Mechanism of ginsenoside Rg1 renal protection in a mouse model of d-galactose-induced subacute damage. Pharm Biol. 2016;54:1815–1821. doi: 10.3109/13880209.2015.1129543. [DOI] [PubMed] [Google Scholar]
- 32.Wang L., Mao N., Tan R.Z., Wang H.L., Wen J., Liu Y.H., Furhad M., Fan J.M. Ginsenoside Rg1 reduces aldosterone-induced autophagy via the AMPK/mTOR pathway in NRK-52E cells. Int J M Med. 2015;36:518–526. doi: 10.3892/ijmm.2015.2242. [DOI] [PubMed] [Google Scholar]
- 33.Sun Q., Meng Q.T., Jiang Y., Liu H.M., Lei S.Q., Su W.T., Duan W.N., Wu Y., Xia Z.Y., Xia Z.Y. Protective effect of ginsenoside Rb1 against intestinal ischemia–reperfusion induced acute renal injury in mice. PloS One. 2013;8:e80859. doi: 10.1371/journal.pone.0080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi S.J., You H.S., Chung S.Y. Tacrolimus-induced apoptotic signal transduction pathway. Transplant Proc. 2008;40:2734–2736. doi: 10.1016/j.transproceed.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 35.Harris R.C. COX-2 and the kidney. J Cardiovasc Pharmacol. 2006;47:S37–S42. doi: 10.1097/00005344-200605001-00007. [DOI] [PubMed] [Google Scholar]
- 36.Cagnol S., Chambard J.C. ERK and cell death: mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 37.Cassidy H., Radford R., Slyne J., O'Connell S., Slattery C., Ryan M.P., McMorrow T. The role of MAPK in drug-induced kidney injury. J Sig Transduct. 2012;2012:463617. doi: 10.1155/2012/463617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Z., Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB life. 2006;58:621–631. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- 39.Deschesnes R.G., Huot J., Valerie K., Landry J. Involvement of p38 in apoptosis-associated membrane blebbing and nuclear condensation. Mol Biol Cell. 2001;12:1569–1582. doi: 10.1091/mbc.12.6.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai B., Chang S.H., Becker E.B., Bonni A., Xia Z. p38 MAP kinase mediates apoptosis through phosphorylation of BimEL at Ser-65. J Biol Chem. 2006;281:25215–25222. doi: 10.1074/jbc.M512627200. [DOI] [PubMed] [Google Scholar]
- 41.Farley N., Pedraza-Alva G., Serrano-Gomez D., Nagaleekar V., Aronshtam A., Krahl T., Thornton T., Rincon M. p38 mitogen-activated protein kinase mediates the Fas-induced mitochondrial death pathway in CD8+ T cells. Mol Cell Biol. 2006;26:2118–2129. doi: 10.1128/MCB.26.6.2118-2129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou W., Zeng J., Zhuo M., Xu W., Sun L., Wang J., Liu X. Involvement of caspase-3 and p38 mitogen-activated protein kinase in cobalt chloride-induced apoptosis in PC12 cells. J Neurosci Res. 2002;67:837–843. doi: 10.1002/jnr.10168. [DOI] [PubMed] [Google Scholar]