Abstract
Background
Antihyperglycemic effects of Panax ginseng berry have never been explored in humans. The aims of this study were to assess the efficacy and safety of a 12-wk treatment with ginseng berry extract in participants with a fasting glucose level between 100 mg/dL and 140 mg/dL.
Methods
This study was a 12-wk, randomized, double-blind, placebo-controlled clinical trial. A total of 72 participants were randomly allocated to two groups of either ginseng berry extract or placebo, and 63 participants completed the study. The parameters related to glucose metabolism were assessed.
Results
Although the present study failed to show significant antihyperglycemic effects of ginseng berry extract on the parameters related to blood glucose and lipid metabolism in the total study population, it demonstrated that ginseng berry extract could significantly decrease serum concentration of fasting glucose by 3.7% (p = 0.035), postprandial glucose at 60 min during 75 g oral glucose tolerance test by 10.7% (p = 0.006), and the area under the curve for glucose by 7.7% (p = 0.024) in those with fasting glucose level of 110 mg/dL or higher, while the placebo group did not exhibit a statistically significant decrease. Safety profiles were not different between the two groups.
Conclusion
The present study suggests that ginseng berry extract has the potential to improve glucose metabolism in human, especially in those with fasting glucose level of 110 mg/dL or higher. For a more meaningful benefit, further research in people with higher blood glucose levels is required.
Keywords: antihyperglycemic effects, clinical trial, glucose metabolism, oral glucose tolerance test, Panax ginseng berry
1. Introduction
Diabetes mellitus is a serious metabolic disorder characterized by hyperglycemia and various life-threatening complications. Despite enormous preventive efforts, diabetes mellitus is one of the fastest growing chronic disorders across the world. In 2010, an estimated 285 million people worldwide suffered from diabetes mellitus, and the number of people with diabetes mellitus is expected to rise to 439 million or 7.7% of the global adult population aged 20–79 yr by 2030 [1]. According to the Korean National Health and Nutrition Examination Survey conducted in 2013, the prevalence of diabetes mellitus in Korea for adults over age 30 yr was estimated to be 11.0% [2].
Although no cure is yet available for diabetes mellitus, various pharmacologic agents have been developed and are being used to enable blood glucose control. However, the current pharmaceuticals for diabetes mellitus have a number of limitations, such as having adverse effects and high rates of failure in long-term glycemic control. This has led to research for alternative or complementary approaches, such as natural products or botanicals, which have some degree of efficacy and mostly are without the troublesome side effects associated with the conventional pharmacologic treatments. Furthermore, earlier intervention for glycemic control is being emphasized for prevention or delay of diabetes mellitus in the management of patients with prediabetes [3]. In this context, alternative approaches using natural products may provide additional strategies for the early management of diabetes mellitus or prediabetes.
Ginseng is a slow-growing perennial herb that has been used as a traditional medicine for thousands of years in Asia. Ginseng has been reported to have various pharmacological properties, including anticancer, antiaging, anti-inflammatory, and antiallergy effects [4], [5], [6], [7]. Ginseng has also received attention from medical researchers for its antihyperglycemic effects, which have been demonstrated by in vitro [8], [9] and in vivo animal studies [10], [11], [12], [13], [14], [15], [16], [17], [18], [19] and clinical trials [20], [21], [22]. Although both root and berry of ginseng were reported to possess antihyperglycemic effects, a recent in vivo study demonstrated that ginseng berry had more potent antihyperglycemic effects than its root when used at the same dosages [23]. Indeed, the ginseng berry has a distinct ginsenoside profile and contains significantly more ginsenosides than its root [23], [24]. For this reason, the ginseng berry may exert more potent antihyperglycemic effects than its root. However, the antihyperglycemic effects of Panax ginseng berry have never been explored in humans. Therefore, the objective of the present study was to test the efficacy and safety of a 12-wk treatment with ginseng berry in participants with fasting glucose level between 100 mg/dL and 140 mg/dL, using a randomized, double-blind, placebo-controlled study design.
2. Materials and methods
2.1. Preparation of ginseng berry extract and HPLC analysis
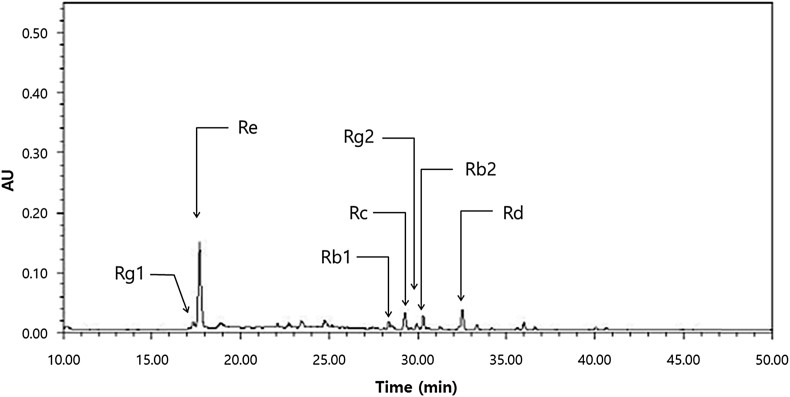
Ginseng berry and placebo capsules were provided by Amorepacific Corporation (Gyeonggi, Korea). Freshly harvested 4-yr-old Korean ginseng berries (P. ginseng Meyer) cultivated in Chungbuk province of South Korea were used. The seeds were separated, and the pulp and juice dried in hot air. The dried ginseng berries were refluxed with 70% ethanol for 10 h. The extract was filtered and evaporated under vacuum at 45°C to obtain standardized Korean ginseng berry extract. The concentration of seven major ginsenosides in ginseng berry extract was analyzed by HPLC (Fig. 1) [25]. Total ginsenoside concentrations (% w/w) were 19.99%. Individual ginsenoside concentrations were 0.77%, 1.90%, 2.11%, 1.65%, 11.06%, 1.66%, and 0.84% for the ginsenosides Rb1, Rb2, Rc, Rd, Re, Rg1, and Rg2, respectively. The content of ginsenoside Re in standardized ginseng berry extract was maintained at 10%. Each 500-mg ginseng berry capsule contained 250 mg of standardized ginseng berry extract. The total treatment dose of ginseng berry extract was 1 g/d. Placebo capsules were identical in appearance and flavor to the ginseng berry capsules. During the trials, four capsules (2 capsules at a single time before breakfast and dinner) of ginseng berry extract or placebo were given daily for 12 wk.
Fig. 1.
HPLC of ginseng berry extract. AU, absorbance units.
2.2. Participants
The study participants were recruited from January 2014 to December 2014 in Dongguk University Ilsan Hospital (Koyang, Gyeonggi, Korea) via hospital and subway advertisement. After the screening test, participants with fasting blood glucose within a range of 100–140 mg/dL, whose age was between 20 yr and 75 yr, were enrolled in this study. Exclusion criteria included: lipid metabolism disorders; acute or chronic inflammatory disease; corticosteroid use within 4 wk of the study; acute cardiovascular disease such as heart failure, myocardial infarction, or stroke; an allergy or hypersensitivity to any of the ingredients in the test products; a history of disease that could interfere with the test products or impede their absorption, such as gastrointestinal diseases or gastrointestinal surgery; participation in other clinical trials within the previous 2 mo; renal disease such as acute/chronic renal failure or nephrotic syndrome; abnormal hepatic liver function; use of antipsychosis drug therapy within 2 mo of the study; a history of alcohol or substance abuse; pregnancy or breast feeding; and laboratory test results, medical, or psychological conditions that the researchers considered unsuitable for this study. In addition, participants taking glucose-lowering medications or insulin injections were excluded from this study. This study was carried out in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Dongguk University Ilsan Hospital (IRB No. 2013-81). A total of 72 participants were enrolled in this study and provided their written informed consent for the study after they were provided with a detailed description of the experimental procedures and informed that they could withdraw from the study at any time.
2.3. Study design
This study was designed as a single-center, randomized, double-blind, placebo-controlled clinical trial that lasted for 12 wk. Following the screening visit, at which inclusion and exclusion criteria were assessed, participants were randomly assigned to receive ginseng berry extract or placebo using a computerized method of random list generation. All participants, investigators, pharmacists and study personnel were blinded to treatment allocation. The enrolled participants were scheduled to visit three times (at 0 wk, 6 wk, and 12 wk) during the trial and their clinical information and trial data were collected during individual interviews conducted by a well-trained interviewer. All participants underwent a thorough medical history review and a physical examination. Height and weight were obtained using standardized techniques and equipment. Height was measured to the nearest 0.1 cm and weight was measured to the nearest 0.1 kg. The body mass index (BMI) was calculated as body weight in kg divided by the square of height in m (kg/m2). Participants were questioned about their daily food intake by the 24-h recall method and physical activity during the past 7 d. Compliance with study restriction and capsule consumption was monitored by means of daily documentation by participants on individualized study calendars and count of returned capsules on the second and third visit. During the intervention period, participants were prohibited from glucocorticoid treatments, glucose-lowering medications or insulin injections, thyroid medications, and any medication or functional food that the researchers considered unsuitable.
2.4. Measurements of efficacy
The primary efficacy measure was changes of fasting and postprandial glucose concentration during the 75 g oral glucose tolerance test (OGTT) before and after intervention. The secondary efficacy measures were changes of fasting and postprandial insulin, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and total cholesterol levels. At 0 wk and 12 wk, a 75 g OGTT was performed after an overnight fast (fasting for at least 8 h). Venous blood samples were then collected at 0 min, 60 min, and 120 min for measurements of serum glucose and insulin levels. Also, fasting blood samples were collected at each visit to measure the lipid profile. Tubes were centrifuged at 2000× g to isolate plasma or serum, and stored at –70°C until analysis. Blood samples were analyzed on a Cobas c702 analyzer (Roche Diagnostics, Basel, Switzerland). Serum glucose concentrations were measured using the hexokinase method with a Cobas c702 analyzer (Roche Diagnostics). Serum insulin concentrations were measured by the electrochemiluminescence immunoassay using a Cobas c602 analyzer (Roche Diagnostics). The homeostasis model assessment of insulin resistance (HOMA-IR) and β cell function (HOMA-β cell) were calculated using the following equations:
| HOMA-IR = [fasting plasma insulin (μIU/mL) × fasting plasma glucose (mM)]/22.5, | (1) |
| HOMA-β cell = [20 × fasting plasma insulin (μIU/mL)]/[fasting plasma glucose (mM) – 3.5]. | (2) |
Hemoglobin A1c (HbA1c) was measured by an G8 glycohemoglobin analyzer (Tosoh Corporation, Tokyo, Japan) using the HPLC method. Fasting serum concentrations of triglyceride and total cholesterol were measured using the enzymatic colorimetric method with a Cobas c702 analyzer (Roche Diagnostics). High-density lipoprotein and low-density lipoprotein cholesterol were measured by the homogeneous enzymatic colorimetric method with a Cobas c702 analyzer (Roche Diagnostics).
2.5. Assessment of safety
Safety was assessed by adverse events reported by participants, physical examination, and laboratory parameters. During the trial, all participants were asked to report potential adverse events. Vital sign of each participant including systolic and diastolic blood pressure and pulse rate was measured at every visit. Systolic and diastolic blood pressure was measured using the left arm of the seated patient with an automatic blood pressure monitor after resting for > 5 min. Pulse rate per min was determined by counting the number of beats on the participant's wrist for 15 s and multiplying this number by 4 to yield beats/min. Laboratory parameters including complete blood count, alkaline phosphatase, aspartate transaminase, alanine aminotransferase, total protein, albumin, blood urea nitrogen, and creatinine were measured at 0 wk and 12 wk as part of a safety assessment.
2.6. Statistical analyses
Differences in mean change from baseline between the treatment groups were examined using the linear mixed model for repeated measures data unless otherwise specified. A paired t test was used to determine significance of change from baseline within each treatment group. The demographic and baseline characteristics of each treatment group were compared by the independent t test for the continuous variables or χ2-test for the categorical data. All p values shown are two-tailed. A p value < 0.05 was considered significant. Calculations and statistical analyses were performed using SPSS (version 18.0.0; SPSS Inc., Chicago, IL, USA). All the data were represented as mean ± standard deviation.
2.7. Analyzing metabolomics profiling from serum and data processing
The metabolomics profiling from serum of study participants were analyzed. UPLC coupled to quadrupole time-of-flight (Q-TOF)–MS analysis was performed using an Acquity UPLC system (Waters, Miliford, MA, USA). The column was an ACQUITY UPLC BEH C18 column (2.1 mm × 100 mm, 1.7μm, Waters) at a flow rate of 0.35 mL/min. The mobile Phase A was water with 0.1% formic acid, and mobile phase B was acetonitrile with 0.1% formic acid. The gradient conditions were 0.5% B rising to 70% in 8 min to a maximum 90% after 8 min and then the equilibrated at 0.5% B for 2 min. The Q-TOF-MS was operated in positive and negative electrospray ionization mode within a mass range of 50–1,000 m/z. The operating parameters were as follows: cone voltage, 30 V; a capillary voltage, 3.0 kV; source temperature, 150°C; desolvation gas temperature, 300°C. The raw data were processed using Progenesis QI data analysis software (Nonlinear Dynamic, Newcastle, UK) for chromatographic alignment, normalization, peak picking, and compound identification. The resulting data sets were imported into SIMCA-P version 12.0.1 (Umetrics, Umeå, Sweden) for multivariate analysis and were mean-centered scaled.
3. Results
3.1. Baseline characteristics of the study participants
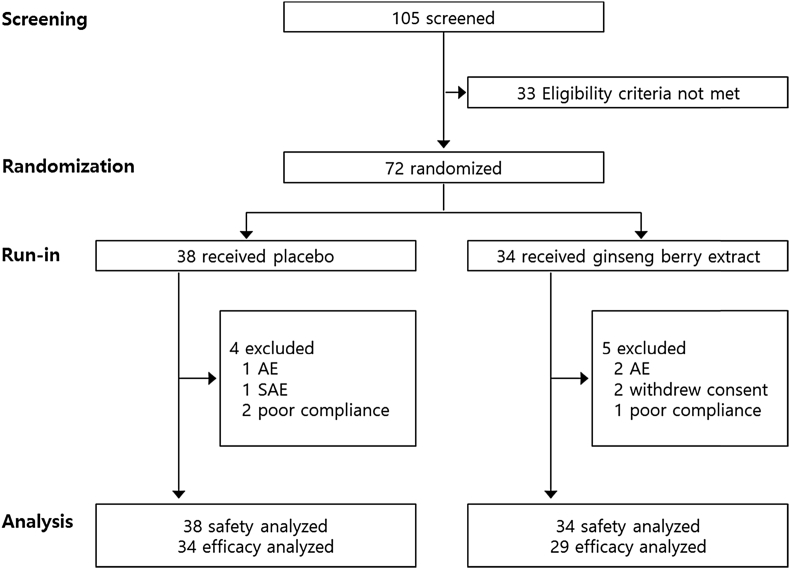
A total of 72 participants were initially randomized in this study. Among these participants, nine participants dropped out. Among the nine dropouts, six participants withdrew consent because of adverse events or personal reasons, and three participants were excluded because of poor compliance. Thus, a total of 63 participants (29 in the ginseng berry group and 34 in the placebo group) who had completed the 12-wk treatment without major protocol violations and had a compliance rate of over 70% were analyzed for efficacy. All participants who entered the study and received at least one dose of the study medication were assessed for safety and demographics (Fig. 2). As shown in Table 1, there was no significant difference between the two groups in baseline demographic characteristics including sex, age, BMI, blood pressure, pulse rates, alcohol consumption, and smoking.
Fig. 2.
Randomization scheme and participant disposition. AE, adverse event; SAE, serious adverse event.
Table 1.
Baseline demographic characteristics of the study participants
| Ginseng berry group (n = 34) | Placebo group (n = 38) | Total (n = 72) |
p1) | ||
|---|---|---|---|---|---|
| Sex (M/F) | 18 / 16 | 24/ 14 | 42 / 30 | 0.3802) | |
| Age (y) | 52.76 ± 10.24 | 51.89 ± 9.46 | 52.31 ± 9.78 | 0.709 | |
| Height (cm) | 164.62 ± 9.23 | 165.79 ± 8.63 | 165.24 ± 8.88 | 0.580 | |
| Weight (kg) | 69.57 ± 12.22 | 70.89 ± 13.04 | 70.27 ± 12.59 | 0.660 | |
| BMI (kg/m2) | 25.52 ± 2.87 | 25.61 ± 3.05 | 25.57 ± 2.95 | 0.908 | |
| SBP (mmHg) | 128.85 ± 13.75 | 128.55 ± 14.56 | 128.69 ± 14.08 | 0.929 | |
| DBP (mmHg) | 82.38 ± 12.32 | 81.16 ± 11.27 | 81.74 ± 11.71 | 0.661 | |
| Pulse (beats/min) | 73.91 ± 12.08 | 73.76 ± 8.64 | 73.83 ± 10.33 | 0.953 | |
| Alcohol | No | 19 (55.88) | 13 (34.21) | 32 (44.44) | 0.0652) |
| Yes | 15 (44.12) | 25 (65.79) | 40 (55.56) | ||
| Amount of alcohol consumption (units3)/wk) | 14.55 ± 19.13 | 12.46 ± 9.64 | 13.26 ± 13.86 | 0.700 | |
| Smoking | No | 27 (79.41) | 27 (71.05) | 54 (75.00) | 0.4142) |
| Yes | 7 (20.59) | 11 (28.95) | 18 (25.00) | ||
| Number of cigarettes/d | 19.29 ± 7.32 | 15.90 ± 11.55 | 17.29 ± 9.90 | 0.506 | |
Values are presented as mean ± standard deviation or n (%)
BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure
Analyzed by independent t test except sex (M/F), alcohol (No/Yes), and smoking (No/Yes)
Analyzed by Chi-square test
1 unit = 10 g of pure alcohol
3.2. Efficacy
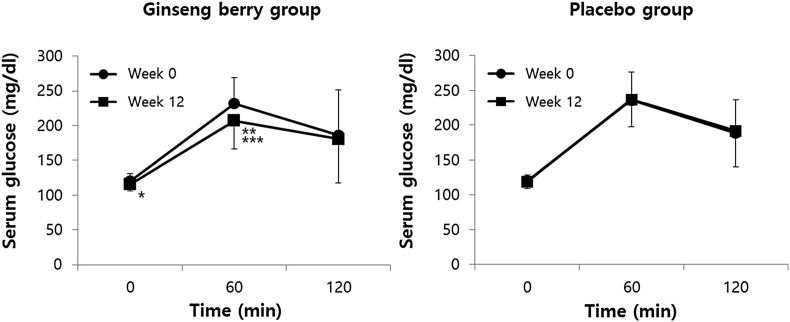
For both groups, within- and between-treatment differences in parameters of glucose or lipid metabolism were assessed (Table 2). There was no difference in serum concentration of glucose and insulin from the 75 g OGTT and HbA1c measures before and after the intervention. No difference for these parameters was found between the two groups. Also, there was no significant difference in the lipid profile for within- and between-treatment differences among participants in this study. In subgroup analyses in those with fasting glucose level of 110 mg/dL or higher (Fig. 3), however, serum concentration of fasting glucose significantly decreased by 3.7% (from a baseline of 120.25 ± 10.32 mg/dL to an endpoint value of 115.81 ± 9.30 mg/dL, p = 0.035) and that of postprandial glucose at 60 min during the 75 g OGTT also significantly decreased by 10.7% (from 231.75 ± 37.58 ng/dL to 206.94 ± 40.28 mg/dL, p = 0.006) in the ginseng berry group after intervention, while the placebo group did not exhibit such a statistically significant decrease. Total glucose area under the curve (AUC) of the ginseng berry group improved by 7.7% (23,090.63 ± 4,273.52 mg*min/dL to 21,307.50 ± 4,298.89 mg*min/dL, p = 0.024). There was a significant difference in between-treatment in 75 g OGTT indices of postprandial glucose level at 60 min (p = 0.017) and total glucose AUC (p = 0.040). However, there was no significant difference for within- and between-treatment in other parameters of glucose metabolism including HbA1c and insulin levels. When we conducted subgroup analyses by sex, we found that ginseng berry extract treatment in men induced a significant decrease of postprandial glucose level at 60 min (from a baseline of 213 ± 52.71 mg/dL to an end point value of 193 ± 44.89 mg/dL) during 75 g OGTT after intervention (p = 0.020), while placebo treatment in men did not show any effect on the same parameter. There was also a significant between-treatment difference in postprandial glucose level at 60 min (p = 0.046). However, there was no within- and between-treatment difference in women.
Table 2.
Changes in serum glucose and insulin during 75 g OGTT, HbA1c, and lipid profile before and after intervention
| Ginseng berry group (n = 29) |
Placebo group (n = 34) |
p2) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 wk | p1) | Baseline | 12 wk | p1) | ||
| Glu 0 min (mg/dL) | 112.31 ± 12.39 | 112.03 ± 10.14 | 0.864 | 112.88 ± 11.06 | 112.79 ± 12.13 | 0.957 | 0.935 |
| Glu 60 min (mg/dL) | 211.07 ± 45.46 | 200.17 ± 44.11 | 0.085 | 215.56 ± 45.91 | 213.71 ± 45.46 | 0.740 | 0.276 |
| Glu 120 min (mg/dL) | 174.03 ± 57.89 | 173.48 ± 57.97 | 0.940 | 171.44 ± 48.23 | 170.76 ± 50.51 | 0.920 | 0.990 |
| AUC-G (mg*min/dL) | 21,254.48 ± 4,490.98 | 20,575.86 ± 4,386.08 | 0.188 | 21,463.24 ± 4,157.40 | 21,329.12 ± 4,271.74 | 0.783 | 0.439 |
| Ins 0 min (μU/mL) | 7.07 ± 4.01 | 7.10 ± 4.38 | 0.962 | 7.06 ± 4.04 | 7.72 ± 5.37 | 0.476 | 0.591 |
| Ins 60 min (μU/mL) | 46.28 ± 21.68 | 47.09 ± 26.11 | 0.864 | 48.66 ± 25.08 | 52.86 ± 28.75 | 0.417 | 0.630 |
| Ins 120 min (μU/mL) | 48.29 ± 23.28 | 49.08 ± 27.44 | 0.877 | 55.28 ± 27.56 | 56.58 ± 30.05 | 0.716 | 0.932 |
| AUC-I (μU*min/mL) | 4,437.76 ± 1,864.94 | 4,510.68 ± 2,330.82 | 0.854 | 4,789.61 ± 2,258.30 | 5,100.60 ± 2,502.90 | 0.424 | 0.668 |
| HbA1c (%) | 5.85 ± 0.38 | 5.89 ± 0.41 | 0.554 | 5.81 ± 0.37 | 5.81 ± 0.47 | 0.897 | 0.675 |
| HOMA-IR | 2.01 ± 1.24 | 1.97 ± 1.24 | 0.857 | 2.00 ± 1.24 | 2.16 ± 1.54 | 0.542 | 0.559 |
| HOMA-β cell | 51.58 ± 27.21 | 52.94 ± 31.57 | 0.779 | 51.65 ± 29.37 | 58.34 ± 40.10 | 0.345 | 0.547 |
| Total cholesterol (mg/dL) | 190.07 ± 31.49 | 191.62 ± 32.57 | 0.770 | 197.88 ± 30.80 | 203.59 ± 30.52 | 0.060 | 0.476 |
| Triglyceride (mg/dL)+ | 157.55 ± 115.56 | 158.29 ± 148.05 | 0.833 | 151.62 ± 76.33 | 150.82 ± 83.46 | 0.945 | 0.814 |
| LDL-cholesterol (mg/dL) | 122.07 ± 33.01 | 117.48 ± 32.64 | 0.355 | 131.15 ± 32.19 | 133.94 ± 29.88 | 0.315 | 0.176 |
| HDL-cholesterol (mg/dL) | 54.28 ± 14.34 | 55.97 ± 18.14 | 0.319 | 53.24 ± 13.68 | 57.71 ± 18.31 | 0.002∗∗ | 0.190 |
Values are presented as mean ± standard deviation
AUC-G, area under the curve for glucose; AUG-I, area under the curve for insulin; Glu, glucose; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment-insulin resistance; HOMA-β cell, homeostasis model assessment-β cell function; Ins, insulin; LDL, low-density lipoprotein; OGTT, oral glucose tolerance test
For within-treatment differences analyzed by paired t test
For between-treatment differences analyzed by linear mixed model for repeated measures data
Fig. 3.
Changes in serum glucose during 75 g oral glucose tolerance test before and after 12-wk intervention in subgroups with fasting glucose level of 110 mg/dL or higher. Values are presented as mean ± standard deviation. *p < 0.05 serum concentration of fasting glucose after 12-wk intervention. **p < 0.01 serum concentration of postprandial glucose at 60 min after 12-wk intervention. ***p < 0.05 serum concentration of postprandial glucose at 60 min in ginseng berry group compared with placebo group.
3.3. Safety
There were no significant differences in number and type of reported adverse events for the two groups (Table 3). Only five adverse events were reported in the ginseng berry group, while 11 adverse events occurred in the placebo group. There was one serious adverse event reported in the placebo group and no cases in the ginseng berry group. In demographic profiles and vital signs, there were no differences in within- and between-treatment groups except for a significant decrease in diastolic blood pressure from a baseline of 79.93 ± 10.97 mmHg to an end point value of 73.93 ± 11.45 mmHg in the ginseng berry group (p = 0.010). When within- and between-treatment differences in safety laboratory parameters were assessed, there was a within-treatment difference in hematocrit (p = 0.047) and lymphocyte count (p = 0.034) in the ginseng berry group and a between-treatment difference in red blood cell count between the two groups (p = 0.042). However, these differences did not have clinical significance.
Table 3.
Adverse events
| Ginseng berry group (n = 34) | Placebo group (n = 38) | |
|---|---|---|
| Overview of AEs | ||
| ≥1 AEs | 5 (14.71) | 11 (28.95) |
| ≥1 serious AEs | 0 | 1 (2.63) |
| AE leading to discontinuation | 2 (5.88) | 2 (5.26) |
| Common AEs1) (≥ 5%) | ||
| Common cold | 1 (2.94) | 2 (5.26) |
| Urticaria | 0 | 2 (5.26) |
| Other AEs | ||
| Gastric soreness | 1 (2.94) | 0 |
| Epigastric pain | 1 (2.94) | 0 |
| Cystitis | 1 (2.94) | 0 |
| Periorbital edema | 0 | 1 (2.94) |
| Herpes zoster | 0 | 1 (2.94) |
| Rheumatoid knee pain | 0 | 1 (2.94) |
| Rheumatoid trigger thumb | 0 | 1 (2.94) |
| Sprain of lumbar spine | 0 | 1 (2.94) |
| Severe cervical dysplasia | 0 | 1 (2.94) |
| Urolithiasis | 1 (2.94%) | 0 |
| Tinea pedis | 0 | 1 (2.94) |
| Serious AEs | ||
| Demyelinating disease | 0 | 1 (2.94) |
Values are presented as n (%)
AEs, adverse events
AEs with frequency ≥ 5% in any group
3.4. Metabolomic profiling of serum of the study participants
For the metabolomic profiling of the participants (who were grouped into 2 as the ginseng berry group and the placebo group), blood samples were collected at the beginning (baseline) and 12 wk after the start of experiment, and the samples were subjected to UPLC/Q-TOF-MS. The metabolomics analysis identified 53 metabolites, listed in Table S1. Also variable importance of projection (VIP) scores of the metabolites corresponding to ginseng berry consumption are presented. The intensity of the identified 53 metabolites in human blood samples is shown in Table S2. There were no significant differences between the ginseng berry group and the placebo group during the 12 wk of treatment. No significant differences in intensity of metabolites were observed in the placebo group prior to the trial and after the 12-wk treatment (p > 0.05). However, as the pre- and post-treatment blood samples in the ginseng berry group were compared, a significant decrease in the levels of the following metabolites was observed: valine (p = 0.027), leucine/isoleucine (p = 0.011), glutamine (p = 0.004), methionine (p = 0.017), xanthine (p = 0.005), and phenylpyruvic acid (p = 0.012). At the same time, there was a significant increase in levels of glycerophosphocholine (p = 0.012), linoleoyl carnitine (p = 0.015), lysophosphatidylethanolamine (18:2; p = 0.011), and lysophosphatidylcholine (20:2; p = 0.053). Pearson's correlation analysis was also conducted to assess the relationship between phenotypic and metabolomic changes. As shown in Fig. S1 and S2, significant metabolomic changes in the ginseng berry group closely correlated with several phenotypic changes. Briefly, changes in fasting blood glucose level positively correlated with levels of xanthine (r = 0.531, p = 0.003), alanine (r = 0.430, p = 0.018), succinic acid (r = 0.668, p < 0.001), phenylalanine (r = 0.450, p = 0.013), creatine (r = 0.385, p = 0.036), and leucine/isoleucine (r = 0.432, p = 0.017; Fig. S1). The postprandial blood glucose at the 60-min level showed a significant positive correlation with levels of alanine (r = 0.559, p = 0.001), succinic acid (r = 0.458, p = 0.012), creatine (r = 0.427, p = 0.019), lysophosphatidylcholine (20:5; r = 0.535, p = 0.002), and lysophosphatidylcholine (22:6; r = 0.471, p = 0.009) and negatively correlated with level of corticosterone (r = 0.499, p = 0.005) (Fig. S2). Based on these results, metabolomic changes for each participant in the ginseng berry group were clearly observed, although the overall changes in the ginseng berry group were not significantly different from those in the placebo group.
4. Discussion
Ginseng consists of several parts including root, leaf, and berry. Generally, ginseng root has been widely used as traditional medicine for thousands of yr in Asia. Compared to the ginseng root, the berry portion of ginseng has not been commonly used for medical purposes. Meanwhile, the major active components of ginseng are reported to be ginsenosides, a class of natural product steroid glycosides and triterpene saponins [26]. Although > 30 ginsenosides have been isolated from various parts of ginseng including root and berry, the most common ginsenosides based on HPLC analysis include Rb1, Rb2, Rc, Rd, Re, Rf, and Rg1 [27]. While the ginseng root has long been the favored source of these active ingredients, recent studies have shown that the ginseng berry contains higher levels of ginsenoside content and with a distinct ginsenoside profile compared with the ginseng root [23], [24]. The ginsenoside Re in particular was four- to six-times higher in the berry than in the root of ginseng [24]. For this reason, each part of the plant may exhibit different pharmacological activities. Since the 1980s, the number of published studies exploring the effects of ginseng on glucose metabolism has remarkably increased. This includes reports demonstrating the therapeutic efficacy and mechanism of antihyperglycemic activity of ginseng root extracts in multiple in vitro [8], [9] and in vivo studies using various diabetic animal models [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. More recent studies have shown that not only root but also berry of ginseng has a potent antihyperglycemic activity [14], [15]. Furthermore, Dey et al [23] compared the antihyperglycemic activity between ginseng root and berry in C57BL/6J ob/ob mice and concluded that ginseng berry is more potent than ginseng root in terms of glucose reduction when used at the same dosage. It was demonstrated that administration of ginsenoside Re, a major constituent of ginseng berry, significantly improved glucose tolerance without affecting body weight in C57BL/6J ob/ob mice [14], [28], which suggests that ginsenoside Re plays a crucial pharmacological role in glucose metabolism.
Several clinical trials investigating the effects of ginseng on diabetes-related parameters in human volunteers have been published, although the results from these studies have varied considerably [20], [21], [22], [29], [30], [31], [32], [33]. Until recently, most clinical studies with ginseng have been conducted with its root. As far as we know, the present study is the first clinical trial to investigate the antihyperglycemic activity of ginseng berry in human volunteers. In this study, although we could not find significant differences within- and between-treatment for glucose and lipid metabolism in the total number of participants, we observed significant improvements in serum concentration of fasting and postprandial glucose at 60 min during 75 g OGTT in those with fasting glucose level of 110 mg/dL or higher, which implies that ginseng berry extract may be more beneficial to people with higher glucose levels.
At present, the mechanisms underlying ginseng's antidiabetic activity are not fully understood. A growing number of evidence from in vitro and in vivo studies suggest that the antihyperglycemic actions of ginseng are complex and attributable to various mechanisms including regulation of insulin secretion or sensitivity, modulation of gastrointestinal absorption, and regulation of inflammatory pathway [34]. A recent animal study showed that treatment with ginseng berry extract significantly increased insulin secretion in β-cell-deficient diabetic mice induced by streptozotocin [35]. In this study, the proliferation of β-cells by ginseng berry extract was also demonstrated in vitro. Another animal study using aged C57BL/6 mice showed that ginseng berry extract feeding significantly decreased HOMA-IR and fasting insulin levels, and ameliorated pancreatic islet hypertrophy, which suggests that the improvement of insulin sensitivity might contribute to a decrease in insulin demand [36]. In the same study, it was also demonstrated that ginseng berry extract could activate insulin receptor substrate-1 and protein kinase B, the important molecules in the insulin signaling pathway. In our study, we showed that ginseng berry treatment induced metabolomic changes, some of which were associated with blood glucose level. This finding suggests that metabolomic changes by ginseng berry treatment might be involved in its antihyperglycemic activity, although we need further investigation to give a detailed account for it.
In the present study, there was no difference of HbA1c levels between control and ginseng berry group at the end of the study. We speculate that the lack of improvement in HbA1c levels by ginseng berry might be due to the participants' well-controlled glucose status when they were enrolled in the study. The baseline mean value of HbA1c was 5.83% at the beginning of the study and most of our participants had a prediabetic condition rather than a diabetes mellitus one. Nevertheless, we found a significant improvement in postprandial glucose levels at 60 min during OGTT in those with fasting glucose levels of 110 mg/dL or higher. Recently, the importance of postprandial hyperglycemia has been highlighted [37], as uncontrolled postprandial hyperglycemia can increase glucose variability. It was suggested that fluctuating glucose produces oxidative stress, thereby inducing endothelial dysfunction and inflammation, both well-known risk factors for cardiovascular disease [38]. The relationship between postprandial hyperglycemia and the risk of cardiovascular event was identified in type 2 diabetes [39]. Furthermore, it was shown that treating postprandial hyperglycemia by acarbose may reduce the incidence of cardiovascular disease in people with impaired glucose tolerance or type 2 diabetes [40], [41].
The limitations of the present study include the following. First, most participants enrolled in the present study were prediabetic. Only a small number of participants were diagnosed with type 2 diabetes. Therefore, caution should be used when we generalize these results to people with type 2 diabetes. In addition, with having only slightly higher than normal levels of fasting glucose in the total population of this study made it difficult to find a significant improvement in parameters related to glucose metabolism. Second, the lack of follow-up data in the dropouts precluded intention-to-treat analyses in order to strengthen the interpretation of the data.
In summary, the present study was the first randomized, double-blind, placebo-controlled clinical trial to examine the efficacy and safety of P. ginseng berry extract on glycemic control in human volunteers. Although the present study failed to show significant antihyperglycemic effects of ginseng berry extract on parameters related to glucose and lipid metabolism in the total study population, it demonstrated that compared with placebo, ginseng berry extract can significantly improve the fasting and postprandial glucose levels over 12 wk in those with fasting glucose levels of 110 mg/dL or higher. This study also showed that there was no safety concern with the long-term consumption of ginseng berry extract. In conclusion, the results of our study suggest that ginseng berry extract has the potential to improve glucose metabolism in humans, particularly those with fasting glucose levels of 110 mg/dL or higher, and without safety concerns. Further research in people with higher glucose levels is required.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Research and Development (R&D) program of MOTIE/KIAT (Establishment of Infra Structure for Anti-aging Industry Support, #N0000697), the Convergence of Conventional Medicine and Traditional Korean Medicine R&D Program funded by the Ministry of Health and Welfare through the Korea Health Industry Development Institute (HI14C0558), and Amorepacific.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jgr.2017.01.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Ha K.H., Kim D.J. Trends in the diabetes epidemics in Korea. Endocrinol Metab. 2015;30:142–146. doi: 10.3803/EnM.2015.30.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association and National Institute of Diabetes Digestive and Kidney Diseases. The prevention or delay of type 2 diabetes. Diabetes Care. 2002;25:742–749. doi: 10.2337/diacare.25.4.742. [DOI] [PubMed] [Google Scholar]
- 4.Bi X., Xia X., Mou T., Jiang B., Fan D., Wang P., Liu Y., Hou Y., Zhao Y. Anti-tumor activity of three ginsenoside derivatives in lung cancer is associated with Wnt/β-catenin signaling inhibition. Eur J Pharmacol. 2014;742:145–152. doi: 10.1016/j.ejphar.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 5.Ramesh T., Kim S.W., Sung J.H., Hwang S.Y., Sohn S.H., Yoo S.K., Kim S.K. Effect of fermented Panax ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Exp Gerontol. 2012;47:77–84. doi: 10.1016/j.exger.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Hong C.E., Lyu S.Y. Anti-inflammatory and anti-oxidative effects of Korean Red Ginseng extract in human keratinocytes. Immune Netw. 2011;11:42–49. doi: 10.4110/in.2011.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung J.W., Kang H.R., Ji G.E., Park M.S., Song W.J., Kim M.H., Kwon J.W., Kim T.W., Park H.W., Cho S.H., Min K.U. Therapeutic effects of fermented red ginseng in allergic rhinitis: a randomized, double-blind, placebo-controlled study. Allergy Asthma Immunol Res. 2011;3:103–110. doi: 10.4168/aair.2011.3.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim K., Kim H.Y. Korean Red Ginseng stimulates insulin release from isolated rat pancreatic islets. J Ethnopharmacol. 2008;120:190–195. doi: 10.1016/j.jep.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Kim J.J., Xiao H., Tan Y., Wang Z.Z., Paul Seale J., Qu X. The effects and mechanism of saponins of Panax notoginseng on glucose metabolism in 3T3-L1 cells. Am J Chin Med. 2009;37:1179–1189. doi: 10.1142/S0192415X09007582. [DOI] [PubMed] [Google Scholar]
- 10.Kimura M., Waki I., Chujo T., Kikuchi T., Hiyama C., Yamazaki K., Tanaka O. Effects of hypoglycemic components in ginseng radix on blood insulin level in alloxan diabetic mice and on insulin release from perfused rat pancreas. J Pharm Dyn. 1981;4:410–417. doi: 10.1248/bpb1978.4.410. [DOI] [PubMed] [Google Scholar]
- 11.Yokozawa T., Kobayashi T., Oura H., Kawashima Y. Studies on the mechanism of the hypoglycemic activity of ginsenoside-Rb2 in straptozotocin-diabetic rats. Chem Pharm Bull. 1985;33:869–872. doi: 10.1248/cpb.33.869. [DOI] [PubMed] [Google Scholar]
- 12.Yun S.N., Moon S.J., Ko S.K., Im B.O., Chung S.H. Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in ICR mice. Arch Pharm Res. 2004;27:790–796. doi: 10.1007/BF02980150. [DOI] [PubMed] [Google Scholar]
- 13.Kimura I., Nakashima N., Sugihara Y., Fu-jun C., Kimura M. The antihyperglycaemic blend effect of traditional Chinese medicine byakko-ka-ninjin-to on alloxan and diabetic KK-CA(y) mice. Phytother Res. 1999;13:484–488. doi: 10.1002/(sici)1099-1573(199909)13:6<484::aid-ptr485>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Attele A.S., Zhou Y.P., Xie J.T., Wu J.A., Zhang L., Dey L., Pugh W., Rue P.A., Polonsky K.S., Yuan C.S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 15.Xie J.T., Zhou Y.P., Dey L., Attele A.S., Wu J.A., Gu M., Polonsky K.S., Yuan C.S. Ginseng berry reduces blood glucose and body weight in db/db mice. Phytomedicine. 2002;9:254–258. doi: 10.1078/0944-7113-00106. [DOI] [PubMed] [Google Scholar]
- 16.Yuan H.D., Kin S.J., Quan H.Y., Huang B., Chung S.H. Ginseng leaf extract prevents high fat diet-induced hyperglycemia and hyperlipidemia through AMPK activation. J Ginseng Res. 2010;34:369–375. [Google Scholar]
- 17.Yuan H.D., Shin E.J., Chung S.H. Anti-diabetic effect and mechanism of Korean red ginseng in C57BL/KsJ db/db mice. J Ginseng Res. 2008;32:187–193. [Google Scholar]
- 18.Lee H.J., Lee Y.H., Park S.K., Kang E.S., Kim H.J., Lee Y.C., Choi C.S., Park S.E., Ahn C.W., Cha B.S. Korean Red Ginseng (Panax ginseng) improves insulin sensitivity and attenuates the development of diabetes in Otsuka Long–Evans Tokushima fatty rats. Metabolism. 2009;58:1170–1177. doi: 10.1016/j.metabol.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z., Wang L.J., Li X., Hu J.N., Chen Y., Ruan C.C., Sun G.Z. Hypoglycemic effects of malonyl-ginsenosides extracted from roots of Panax ginseng on streptozotocin-induced diabetic mice. Phytother Res. 2009;23:1426–1430. doi: 10.1002/ptr.2796. [DOI] [PubMed] [Google Scholar]
- 20.Sotaniemi E.A., Haapakoski E., Rautio A. Ginseng therapy in non-insulin dependent diabetic patients. Diabetes Care. 1995;18:1373–1375. doi: 10.2337/diacare.18.10.1373. [DOI] [PubMed] [Google Scholar]
- 21.Vuksan V., Sievenpiper J.L., Koo V.Y.Y., Francis T., Beljan-Zdravkovic U., Xu Z., Vidgen E. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med. 2000;160:1009–1013. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- 22.Vuksan V., Sung M.K., Sievenpiper J.L., Stavro P.M., Jenkins A.L., Di Buono M., Lee K.S., Leiter L.A., Nam K.Y., Arnason J.T. Korean Red Ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebocontrolled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18:46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Dey L., Xie J.T., Wang A., Wu J., Maleckar S.A., Yuan C.S. Anti-hyperglycemic effects of ginseng: comparison between root and berry. Phytomedicine. 2003;10:600–605. doi: 10.1078/094471103322331908. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y.K., Yoo D.S., Xu H., Park N.I., Kim H.H., Choi J.E., Park S.U. Ginsenoside content of berries and roots of three typical Korean ginseng (Panax ginseng) cultivars. Nat Prod Commun. 2009;4:903–906. [PubMed] [Google Scholar]
- 25.Kim C.K., Cho D.H., Lee K.S., Lee D.K., Park C.W., Kim W.G., Lee S.J., Ha K.S., Taeg O.G., Kwon Y.G. Ginseng berry extract prevents atherogenesis via anti-inflammatory action by upregulating phase II gene expression. Evid Based Complement Alternat Med. 2012;2012:490301. doi: 10.1155/2012/490301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 27.Ji Q.C., Harkey M.R., Henderson G.L., Gershwin M.E., Stern J.S., Hackman R.M. Quantitative determination of ginsenosides by high-performance liquid chromatography-tandem mass spectrometry. Phytochem Anal. 2001;12:320–326. doi: 10.1002/pca.593. [DOI] [PubMed] [Google Scholar]
- 28.Xie J.T., Mehendale S.R., Li X., Quigg R., Wang X., Wang C.Z., Wu J.A., Aung H.H., A Rue P., Bell G.I. Anti-diabetic effect of ginsenoside Re in ob/ob mice. Biochim Biophys Acta. 2005;1740:319–325. doi: 10.1016/j.bbadis.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Ma S.W., Benzie I.F., Chu T.T., Fok B.S., Tomlinson B., Critchley L.A. Effect of Panax ginseng supplementation on biomarkers of glucose tolerance, antioxidant status and oxidative stress in type 2 diabetic subjects: results of a placebo-controlled human intervention trial. Diabetes Obes Metab. 2008;10:1125–1127. doi: 10.1111/j.1463-1326.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 30.Reay J.L., Scholey A.B., Milne A., Fenwick J., Kennedy D.O. Panax ginseng has no effect on indices of glucose regulation following acute or chronic ingestion in healthy volunteers. Br J Nutr. 2009;101:1673–1678. doi: 10.1017/S0007114508123418. [DOI] [PubMed] [Google Scholar]
- 31.Reeds D.N., Patterson B.W., Okunade A., Holloszy J.O., Polonsky K.S., Klein S. Ginseng and ginsenoside Re do not improve β-cell function or insulin sensitivity in overweight and obese subjects with impaired glucose tolerance or diabetes. Diabetes Care. 2011;34:1071–1076. doi: 10.2337/dc10-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh M.R., Park S.H., Kim S.Y., Back H.I., Kim M.G., Jeon J.Y., Ha K.C., Na W.T., Cha Y.S., Park B.H., Park T.S., Chae S.W. Postprandial glucose-lowering effects of fermented red ginseng in subjects with impaired fasting glucose or type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. BMC Complement Altern Med. 2014;14:237. doi: 10.1186/1472-6882-14-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bang H., Kwak J.H., Ahn H.Y., Shin D.Y., Lee J.H. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J Med Food. 2014;17:128–134. doi: 10.1089/jmf.2013.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan H.D., Kim J.T., Kim S.H., Chung S.H. Ginseng and diabetes: the evidences from in vitro, animal and human studies. J Ginseng Res. 2012;36:27–39. doi: 10.5142/jgr.2012.36.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park E.Y., Kim H.J., Kim Y.K., Park S.U., Choi J.E., Cha J.Y., Jun H.S. Increase in insulin secretion induced by Panax ginseng berry extracts contributes to the amelioration of hyperglycemia in streptozotocin-induced diabetic mice. J Ginseng Res. 2012;36:153–160. doi: 10.5142/jgr.2012.36.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo E., Kim S., Lee S.J., Oh B.C., Jun H.S. Ginseng berry extract supplementation improves age-related decline of insulin signaling in mice. Nutrients. 2015;7:3038–3053. doi: 10.3390/nu7043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung H.S. Clinical implications of glucose variability: chronic complications of diabetes. Endocrinol Metab. 2015;30:167–174. doi: 10.3803/EnM.2015.30.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceriello A., Ihnat M. “Glycaemic variability:” a new therapeutic challenge in diabetes and the critical care setting. Diabet Med. 2010;27:862–867. doi: 10.1111/j.1464-5491.2010.02967.x. [DOI] [PubMed] [Google Scholar]
- 39.Cavalot F., Petrelli A., Traversa M., Bonomo K., Fiora E., Conti M., Anfossi G., Costa G., Trovati M. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91:813–819. doi: 10.1210/jc.2005-1005. [DOI] [PubMed] [Google Scholar]
- 40.Chiasson J.L., Josse R.G., Gomis R., Hanefeld M., Karasik A., Laakso M. STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 41.Hanefeld M., Cagatay M., Petrowitsch T., Neuser D., Petzinna D., Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25:10–16. doi: 10.1016/s0195-668x(03)00468-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.