Abstract
Panax ginseng has been used since ancient times based on the traditional Asian medicine theory and clinical experiences, and currently, is one of the most popular herbs in the world. To date, most of the studies concerning P. ginseng have focused on specific mechanisms of action of individual constituents. However, in spite of many studies on the molecular mechanisms of P. ginseng, it still remains unclear how multiple active ingredients of P. ginseng interact with multiple targets simultaneously, giving the multidimensional effects on various conditions and diseases. In order to decipher the systems-level mechanism of multiple ingredients of P. ginseng, a novel approach is needed beyond conventional reductive analysis. We aim to review the systems-level mechanism of P. ginseng by adopting novel analytical framework–network pharmacology. Here, we constructed a compound-target network of P. ginseng using experimentally validated and machine learning-based prediction results. The targets of the network were analyzed in terms of related biological process, pathways, and diseases. The majority of targets were found to be related with primary metabolic process, signal transduction, nitrogen compound metabolic process, blood circulation, immune system process, cell-cell signaling, biosynthetic process, and neurological system process. In pathway enrichment analysis of targets, mainly the terms related with neural activity showed significant enrichment and formed a cluster. Finally, relative degrees analysis for the target-disease association of P. ginseng revealed several categories of related diseases, including respiratory, psychiatric, and cardiovascular diseases.
Keywords: network pharmacology, Panax ginseng, polypharmacology, traditional Asian medicine
1. Introduction
Panax ginseng is one of the most widely used herbs in the world. It has been frequently used in East Asia since ancient times based on the traditional Asian medicine theory and clinical experiences. For instance, among herbal prescriptions in Shang-Han Lun (Treatise on Cold Damage Diseases) and Donguibogam (Korean Clinical Pharmacopoeia), which are representative publications of traditional Asian medicine, 21 of 113 and 653 of 3,944 formulas (18.6% and 16.6%, respectively) contain P. ginseng [1]. Textbook of formula study that refers to herbal formula also has 78 prescriptions that contain P. ginseng out of the 438 total prescriptions (17.8%) [2]. In prescriptions, P. ginseng is mainly used as a tonic to boost the function of feeble bodies, and therefore applicable to a wide range of diseases [3]. In recent years, many clinical trials have been conducted to reveal the efficacy of P. ginseng for various diseases and symptoms. The results suggest that P. ginseng has effects on pathological conditions, such as ischemic heart disease, common cold, obstructive pulmonary disease, and erectile dysfunction [4], [5], [6], [7].
Numerous studies have investigated the pharmacological mechanisms of P. ginseng. Most of the studies have focused on the actions of ginsenosides, the major active component of P. ginseng. It is commonly believed that most pharmacological effects of P. ginseng are attributed to ginsenosides, including the stimulatory and inhibitory effects on the nervous system, antineoplastic effects, immunomodulatory effects, and nitric oxide release [8], [9], [10], [11]. However, P. ginseng reportedly contains various potentially bioactive ingredients such as phytosterols, sesquiterpenes, flavonoids, polyacetylenes, alkaloids, and phenolic compounds in addition to ginsenosides [12], [13], [14], [15], and these ingredients may also work together with ginsenosides to contribute to the various effects of P. ginseng. Indeed, there have been reports that ginsenosides do not act alone; rather they function in concert with minor ingredients to perform their beneficial effects [16], [17], [18].
Despite the previous efforts to understand the molecular mechanisms, it is still unclear how the combinations of multiple ingredients work together to produce clinical effects of P. ginseng. The conventional pharmacological approaches are unable to capture the systems-level mechanism of herbs; therefore, novel methods are needed. In recent years, emergence of network pharmacology is shedding light on understanding the mechanism of the herbal medicine at the systems level. Network pharmacology integrates computational and experimental methods, focusing on the “multi-component, multi-target effects” [19].
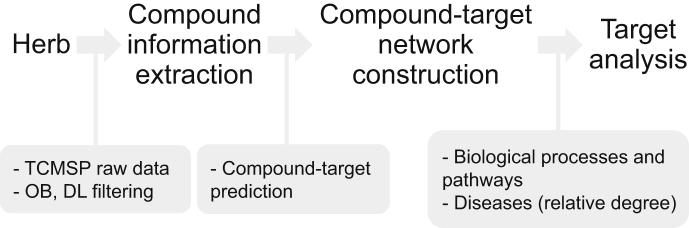
The aim of this article was to review the systems-level mechanism of P. ginseng by adopting network pharmacological analysis, providing new insights into the effects and mechanisms of P. ginseng. First, we briefly reviewed the chemical constituents of P. ginseng including the minor components in addition to the ginsenosides. Next, we constructed a compound-target network using the information from the Traditional Chinese Medicine Systems Pharmacology Database {TCMSP [Institute of Integrated Bioinformedicine and Translational Science (IBTS), Hong Kong], http://tcmspnw.com} [20]. In order to review the related processes and pathways of the compound-network of P. ginseng, the PANTHER [Protein ANalysis THrough Evolutionary Relationships (Paul Thomas, in Keck School of Medicine of USC, Los Angeles, USA), http://pantherdb.org] classification system [21], [22] and Enrichr method were employed, respectively. Finally, the relative degree matrix was constructed from the network of P. ginseng to investigate the related diseases (Fig. 1).
Fig. 1.
Framework of network pharmacological analysis. DL, drug-likeness; OB, oral bioavailability, TCMSP, Traditional Chinese Medicine Systems Pharmacology.
2. The chemical constituents of P. ginseng
2.1. Ginsenosides
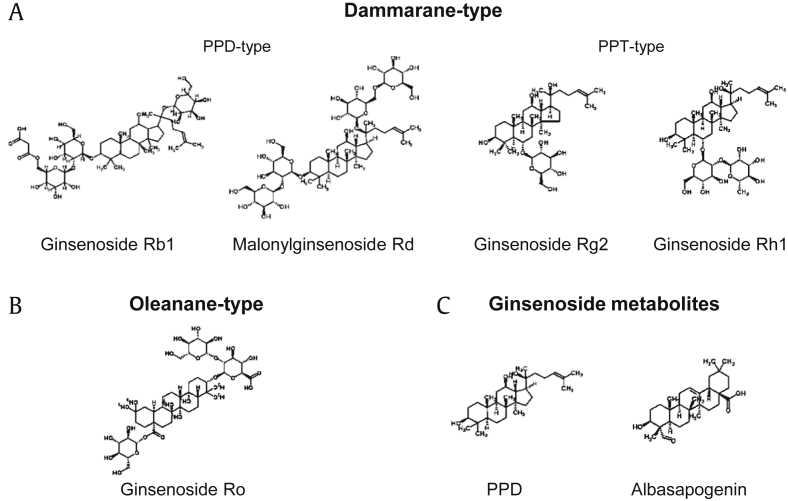
Ginsenosides were isolated in the 1960s for the first time [23], and many types of ginsenosides have been identified. Ginsenosides are triperpene saponins that originated from 2, 3-oxidosqualene. They can be divided into two groups by their skeletal structures: dammarane-type ginsenosides and oleanane-type ginsenosides.
2.1.1. Dammarane-type ginsenosides
Dammarane-type ginsenosides are biosynthesized from protopanaxadiol (PPD) or protopanaxatriol (PPT), both of which are formed when dammarenediol-II is hydroxylated. They can be classified into two groups, PPD-type and PPT-type. PPD-type has the attachment of saccharides to C-3 and/or C-20 and includes ginsenosides Ra1, Rb1, Rc, Rd, etc. PPT-type has the attachment of saccharides to C-6 and/or C-20 and includes ginsenosides Re, Rg2, Rh1, etc. [24] (Fig. 2A).
Fig. 2.
Structures of selected ginsenosides. Dammarane-type is classified into two further types, PPD-type and PPT-type. PPD, protopanaxadiol; PPT, protopanaxatriol.
2.1.2. Oleanane-type ginsenosides
Oleanane-type ginsenosides are biosynthesized from β-amyrin, which are also formed from dammarenediol-II. They have a pentacyclic structure, whereas dammarane-type ginsenosides have a tetracyclic structure. Ginsenoside Ro (Fig. 2B) is a compound that is commonly detected in P. ginseng, and other oleanane-type ginsenosides are rare [25].
2.1.3. Ginsenoside metabolites
The majority of ginsenosides are deglycosylated in the gastrointestinal tract by colonic bacteria. Most of them are finally metabolized to PPD, PPT, compound K, or other compounds [26], [27] (Fig. 2C).
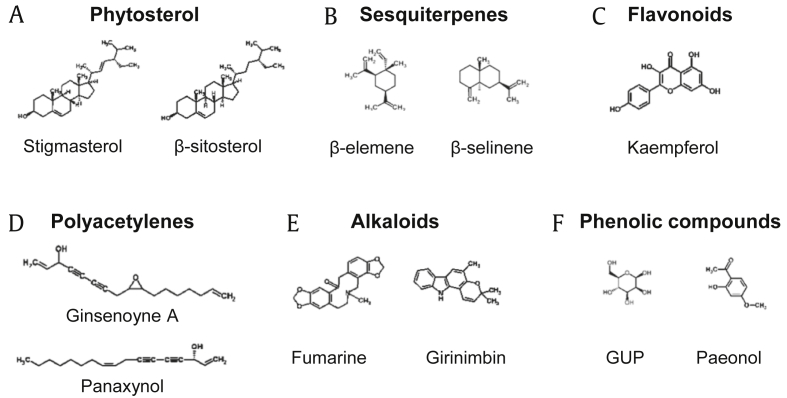
2.2. Phytosterols
Phytosterols are a type of alcohol that has the steroid skeleton and are naturally present in plants. Phytosterols are generally considered to lower the cholesterol level [28]. The representative phytosterol, stigmasterol, and β-sitosterol (Fig. 3A) are commonly detected in P. ginseng.
Fig. 3.
Structures of selected non-ginsenoside constituents. GUP, β-D-mannopyranose.
2.3. Sesquiterpenes
Sesquiterpenes are volatile C15-terpenoids originating from three isoprene units. Many sesquiterpenes including β-elemene and β-selinene (Fig. 3B) have been isolated and identified as compounds of P. ginseng [13], [29].
2.4. Flavonoids
Flavonoids are a group of polyphenolic compounds that consist of two phenyl rings and a heterocyclic ring and are universally present in plants. It is believed that flavonoids have health-promoting properties due to antioxidant activities [30]. Kaempferol (Fig. 3C) is the representative flavonoid in P. ginseng.
2.5. Polyacetylenes
Plenty of polyacetylenes have been identified since the first polyacetylene panaxynol was extracted from P. ginseng [31]. These include panaxynol, ginsenoyne A, etc. (Fig. 3D). Several studies revealed that polyacetylenes in P. ginseng show cytotoxic activity at high concentrations and possess antitumor properties [32].
2.6. Alkaloids
Alkaloids are one of the non-saponin constituents in P. ginseng, including fumarine and girinimbin (Fig. 3E). P. ginseng alkaloids are minor components; they were isolated later than other compounds [15] and relatively less investigated.
2.7. Phenolic compounds
There are > 10 identified phenolic compounds in P. ginseng, such as elemicin and dauricine (Fig. 3F). Numerous studies have reported various biological properties of phenolic compounds, including antitumor, antioxidant, and anti-inflammatory activities [33], [34].
3. Network construction
Compound-target networks are bipartite networks in which the nodes represent compounds and targets, and the edges (links, connections) are defined as compound-target interactions (1 or 0). In order to construct networks, oral bioavailability (OB) and drug-likeness (DL) index information were extracted from TCMSP for each compound of P. ginseng (a total of 190 compounds including 18 microbiota-derived metabolites). OB and DL are calculated by machine learning methods or Tanimoto coefficient, using diverse drugs and drug-like molecule datasets [35]. They are commonly used for filtering out compounds that are unlikely to be drugs and the thresholds are set to ≥ 30 (OB) and ≥ 0.18 (DL) as default suggestive values of TCMSP.
In this review, a wide range of thresholds of OB and DL (10 bins between minimum and maximum values of OB and DL) were applied for compound filtering instead of a single value of threshold since it is not clear to what extent the compounds will be utilized as active compounds. Compound-target interaction information was also extracted from TCMSP for all pairs of candidate compounds and target proteins in the database. It includes experimentally validated interactions, but most of the interactions were predicted ones, based on the machine learning methods (Support Vector Machine and Random Forest) with validated drug-target interaction datasets. The performance of this predictive method for compound-target interactions are proven to be reliable [36].
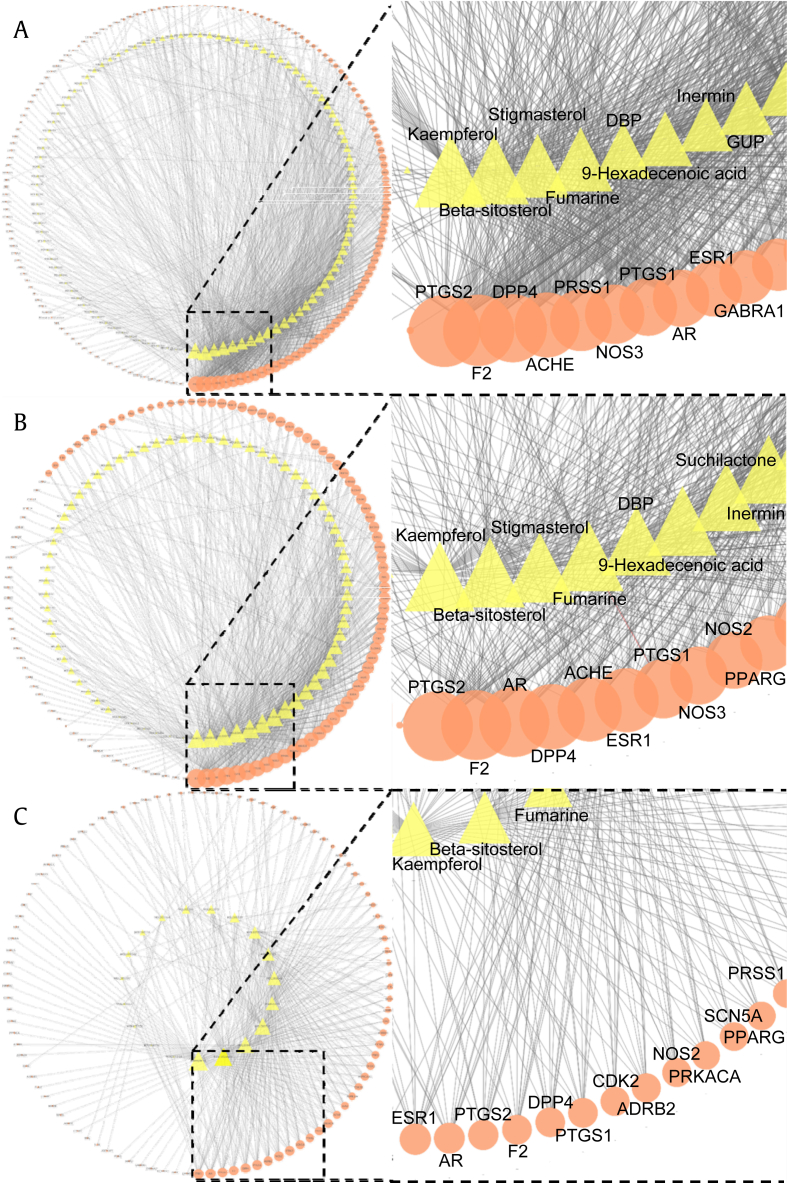
For the purpose of visualization, three representative networks among threshold networks were presented using Cytoscape (Cytoscape Consortium, San Diego, California, USA) [37] (Fig. 4, Table 1).
Fig. 4.
The representative compound-target networks of Panax ginseng with different thresholds for OB and DL. (A) The networks are presented by applying no thresholds for OB and DL (105 compounds and 161 corresponding targets); (B) 13.56 and 0.09 for OB and DL, respectively (71 compounds and 116 corresponding targets); and (C) 30.27 and 0.19 for OB and DL, respectively (20 compounds and 100 corresponding targets). The yellow triangle represents compounds and the orange circle represents targets. The compounds and targets of each network were sorted in degree descending order from the bottom of the figure in a circular layout. The size of compound and target node is proportional to the degree in the network. Top 10 targets of each network are enlarged. ACHE, acetylcholinesterase; ADRB2, beta-2 adrenergic receptor; AR, androgen receptor; CDK2, cell division protein kinase 2; DBP, dibutyl phthalate; DL, drug-likeness; DPP4, dipeptidyl peptidase IV; ESR1, estrogen receptor; F2, thrombin; GABRA1, gamma-aminobutyric acid receptor subunit alpha-1; GUP, β-D-mannopyranose; NOS2, nitric oxide synthase (inducible); NOS3, nitric-oxide synthase (endothelial); OB, oral bioavailability; PTGS2, prostaglandin G/H synthase 2; PKACA, mRNA of PKA catalytic subunit C-alpha; PPARG, peroxisome proliferator activated receptor gamma; PRSS1, trypsin-1; PTGS1, prostaglandin growth hormone synthase 1; SCN5A, sodium channel protein type 5 subunit alpha.
Table 1.
Top 10 targets in each network with different thresholds for OB and DL
| Gene symbol | Target name | Degree (rank) according to each OB, DL threshold |
Total degree | ||
|---|---|---|---|---|---|
| 0.00, 0.00 | 13.56, 0.09 | 30.27, 0.19 | |||
| PTGS2 | Prostaglandin G/H synthase 2 | 54 (1) | 35 (1) | 11 (3) | 100 |
| F2 | Thrombin | 53 (2) | 35 (1) | 10 (4) | 98 |
| DPP4 | Dipeptidyl peptidase IV | 49 (3) | 28 (4) | 10 (4) | 87 |
| AR | Androgen receptor | 35 (8) | 33 (3) | 12 (2) | 80 |
| ACHE | Acetylcholinesterase | 45 (4) | 27 (5) | 7 (14) | 72 |
| ESR1 | Estrogen receptor | 34 (9) | 27 (5) | 13 (1) | 74 |
| PTGS1 | Prostaglandin G/H synthase 1 | 36 (7) | 23 (7) | 10 (4) | 69 |
| PRSS1 | Trypsin-1 | 41 (5) | 16 (14) | 8 (10) | 49 |
| NOS3 | Nitric-oxide synthase, endothelial | 38 (6) | 22 (8) | 4 (32) | 60 |
| NOS2 | Nitric oxide synthase, inducible | 27 (13) | 21 (9) | 9 (7) | 30 |
| GABRA1 | Gamma-aminobutyric acid receptor subunit alpha-1 | 31 (10) | 16 (14) | 6 (22) | 31 |
| PPARG | Peroxisome proliferator activated receptor gamma | 22 (14) | 20 (10) | 8 (10) | 28 |
| CDK2 | Cell division protein kinase 2 | 15 (23) | 14 (16) | 9 (7) | 9 |
| ADRB2 | Beta-2 adrenergic receptor | 16 (21) | 11 (20) | 9 (7) | 9 |
| PRKACA | mRNA of PKA Catalytic Subunit C-alpha | 11 (27) | 11 (20) | 8 (10) | 8 |
| SCN5A | Sodium channel protein type 5 subunit alpha | 10 (31) | 8 (32) | 8 (10) | 8 |
Targets are sorted according to their total degrees (sums of degrees in 3 networks). DL, drug-likeness; OB, oral bioavailability.
4. Pathway analysis
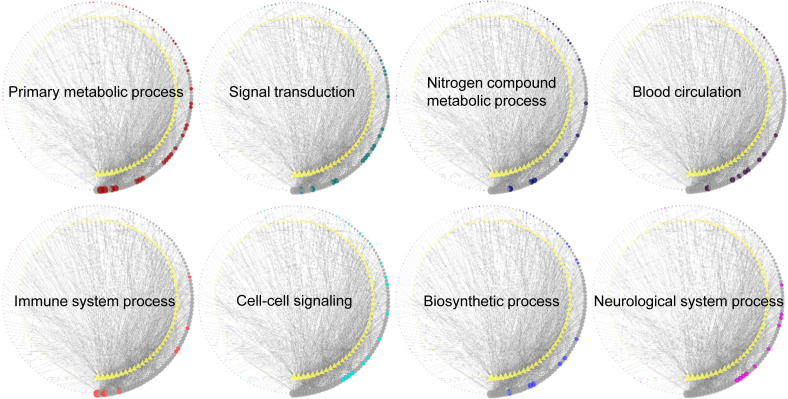
To capture the related biological functions of P. ginseng at coarse-grained level, every target of the compound-target network was assigned to biological processes using the PANTHER classification system [21]. This system provides tools for large-scale gene function analysis by combining information of gene functions, ontology, and pathways. In this review, an ontology system named “PANTHER GO-Slim Biological Process” was used. For the interpretability, 74 biological processes were manually extracted from the first to third level nodes of the taxonomy of PANTHER GO-Slim Biological Process. The majority of targets were included in eight categories as follows: primary metabolic process (55 targets, total degrees = 471), signal transduction (36 targets, total degrees = 313), nitrogen compound metabolic process (27 targets, total degrees = 195), blood circulation (15 targets, total degrees = 191), immune system process (14 targets, total degrees = 186), cell-cell signaling (20 targets, total degrees = 180), biosynthetic process (22 targets, total degrees = 180), and neurological system process (15 targets, total degrees = 155) (Fig. 5).
Fig. 5.
Dominant biological processes of targets of Panax ginseng. Each target is classified using “PANTHER GO-slim Biological Process” in the PANTHER classification System. The top eight biological processes are presented according to the total degrees. The compounds and targets of each network are sorted in degree descending order from the bottom of the figure, in a circular layout. The size of compound and target node is proportional to the degree in the network. This analysis is conducted with unfiltered compound-target network.
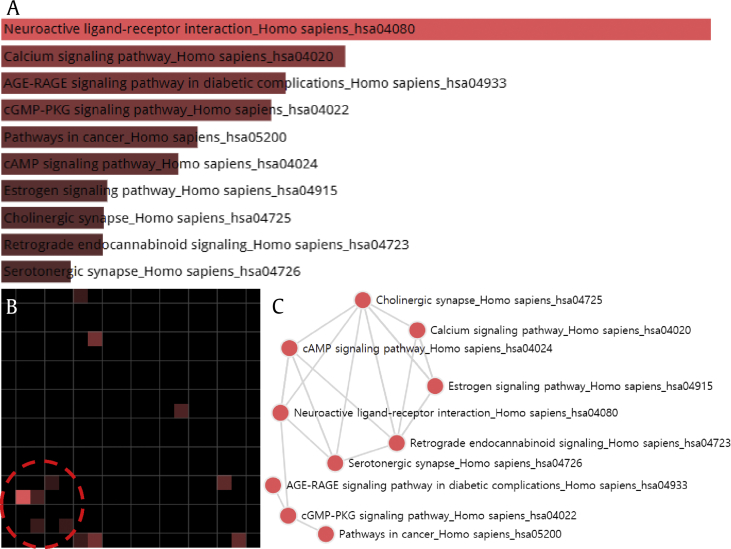
In order to determine the significantly enriched pathways in the targets of the network, pathway enrichment analysis was performed with Enrichr (Avi Ma'ayan in Ma'ayan Laboratory, Center for Bioinformatics, Icahn School of Medicine at Mount Sinai, New York, USA) [38] using the KEGG pathway database (http://www.genome.ad.jp/kegg/pathway.html) [39]. As a result, the top 10 terms were ranked in the descending order as follows: neuroactive ligand–receptor interaction, calcium signaling pathway, advanced glycation end-products (AGEs) and receptor for advanced glycation end-product signaling pathway in diabetic complications, cGMP-PKG signaling pathway, pathways in cancer, cAMP signaling pathway, estrogen signaling pathway, cholinergic synapse, retrograde endocannabinoid signaling, and serotonergic synapse. These enriched terms were visualized as a bar graph, grid, and network using the KEGG 2016 library (Fig. 6). Specifically, it was found that the terms related with neural activities such as neuroactive ligand–receptor interaction, serotonergic synapse, and cholinergic synapse form a cluster, suggesting multidimensional therapeutic effects of P. ginseng on the nervous system.
Fig. 6.
Grid and network analysis of enriched terms using the KEGG 2016 library. The gene symbols of 161 targets are used as an input and the result is displayed in three different manners: a bar graph, a grid, and a network. (A) The top 10 terms are ranked as bar graph in descending order. The length of the bar represents the significance of the specific gene-sets represented by the terms. The brighter the color, the more significant that term is. (B) Each grid square represents a term and is arranged based on term-term similarity, which represents one term's gene-set content similarity with another term. It shows the top 10 terms sorted by enrichment score. The brighter the square, the more significant that term is. A circle is used to highlight the most dominant cluster of enriched terms: neuroactive ligand–receptor interaction, cAMP signaling pathway, serotonergic synapse, cholinergic synapse, and retrograde endocannabinoid signaling (displayed clockwise from the brightest grid). (C). In the network, each node represents the enriched term, and the edge between two nodes means that the two terms have some gene content similarity.
5. Disease analysis
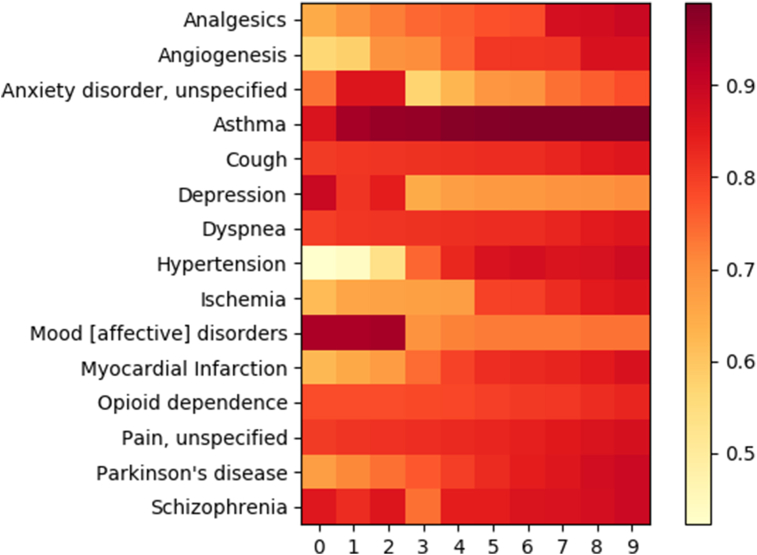
Finally, potential target diseases of P. ginseng were analyzed based on the target-disease information from TCMSP, where the information is extracted from PharmGKB (http://www.pharmgkb.org) and Therapeutic Targets Database (http://bidd.nus.edu.sg/BIDD-Databases/TTD/TTD.asp). First, degrees were calculated for all diseases in TCMSP by counting the number of associations with targets in the constructed compound-target networks of P. ginseng. Since the results differ with the changes in the threshold level, the degrees of diseases were calculated across a wide range of thresholds of OB and DL, resulting in the degree matrix of diseases. However, it turned out that the degrees of diseases tend to be biased to specific diseases (e.g., unspecific cancer) because previous studies about target genes are not evenly distributed for diseases. To avoid this bias, relative degrees were calculated by dividing degrees by the maximum degree of the corresponding disease. The relative degree matrix of major diseases targeted by P. ginseng shows a comparative advantage of this herb over others on various diseases (Fig. 7). Comparative advantage means that P. ginseng has more protein targets than other herbs for the same disease, thereby making the probability of targeting corresponding disease higher.
Fig. 7.
Relative degree matrix of major diseases. Each row and column represents the disease name and threshold level of oral bioavailability and drug-likeness, respectively. Only the top 30% of diseases by total degrees across thresholds are presented. Color bar indicates relative degrees.
As a result, major diseases targeted by P. ginseng were analyzed as follows: angiogenesis, anxiety disorder, asthma, cough, depression, dyspnea, hypertension, ischemia, mood disorder, myocardial infarction, opioid dependence, pain, Parkinson's disease, and schizophrenia (Fig. 7). These diseases could be categorized into respiratory, psychiatric, cardiovascular, and miscellaneous diseases (Table 2).
Table 2.
Category of diseases and corresponding disease names
| Category of diseases | Disease name |
|---|---|
| Respiratory diseases | asthma, cough, dyspnea |
| Psychiatric diseases | anxiety disorder, depression, mood disorder, opioid dependence, schizophrenia |
| Cardiovascular diseases | angiogenesis, hypertension, ischemia, myocardial infarction |
| Miscellaneous diseases | pain, Parkinson's disease |
Interestingly, the results of systems-level target diseases analysis were found to be consistent with the published studies on diseases with the extract of P. ginseng.
5.1. Respiratory effects
P. ginseng is known to produce numerous actions on the respiratory system, especially on asthma related with anti-allergic properties [40]. For example, Babayigit et al. [41] investigated the anti-asthmatic activity of P. ginseng in a murine model of chronic asthma sensitized by ovalbumin. When compared with the placebo group, all the chronic histopathologic changes of airways (thicknesses of basement membrane, epithelium, and subepithelial smooth muscle, goblet cell number, and mast cell number) in P. ginseng group were significantly ameliorated. In accordance with this, it was reported that P. ginseng suppressed airway hyperresponsiveness, ovalbumin-specific IgE levels, and inflammatory cytokine production [42]. Kim and Yang [43] demonstrated that P. ginseng reduced airway inflammation in allergic asthma mice model and investigated the underlying mechanism. The P. ginseng-treated group restored not only the expression of inflammatory cells, such as EMBP, Muc5ac, CD40, and CD40L, but also the mRNA and protein levels of the cytokines [interleukin (IL)-1β, IL-4, IL-5, and tumor necrosis factor-α].
5.2. Psychiatric effects
Several studies have described the beneficial effects of P. ginseng on various psychiatric diseases such as depression and schizophrenia. It has been reported that P. ginseng has curative effects on depression by a plethora of studies. For instance, a report states that the wild P. ginseng extract suppressed the expression of corticotrophin-releasing factor and neuropeptide Y, significantly reducing depression-like behavior in morphine withdrawal rat model [44]. Furthermore, numerous studies revealed the clinical effects of herbal formulas containing P. ginseng on depression, e.g., Kai-Xin-San [45], [46], [47], [48], [49], Sanyuansan [50], Xiaochaihutang [51], and Sho-ju-sen [52]. In parallel with this, there have been several clinical trials on depression. Lee and Ji [53] showed that fermented red P. ginseng had beneficial effects on depression by altering lipids. In addition, Jeong et al. [54] reported that Korean Red P. ginseng at a dose of 3 g/d significantly decreased residual symptoms of major depression in an 8-wk study with 35 female outpatients remitted from major depression.
Several recent studies have suggested that P. ginseng has beneficial effects on schizophrenia. For instance, Tran et al. [44] reported that wild P. ginseng was found to ameliorate phencyclidine-induced schizophrenia-like behavior in mice by positive modulation of glutathione. Kim et al. [55] investigated the influence of P. ginseng on offspring of pregnant rats exposed to prenatal stress. The influence of P. ginseng was examined in the behavioral activity and protein expression analysis. The results demonstrated that the downregulation of some genes after exposure to prenatal stress had influences on behavioral changes, and these phenomena were recovered following the treatment with P. ginseng (300 mg/kg) during pregnancy.
5.3. Cardiovascular effects
P. ginseng also produces numerous effects on the cardiovascular system [6]. There have been studies suggesting the efficacy of P. ginseng on hypertension [56], [57], [58], [59], [60]. It is known that P. ginseng regulates blood pressure to normal and thereby helps to elevate low blood pressure and to lower high blood pressure [61]. It was reported that the effect of regulating high blood pressure is mediated by promoting vascular endothelial cell-derived nitric oxide secretion [62], [63], [64].
Recent studies found a close relationship between angiogenesis and P. ginseng [65], [66]. P. ginseng and its ginsenosides reportedly modulate multiple steps of angiogenesis, such as inhibiting endothelial cell proliferation, formation of capillary tube, and vascular endothelial growth factor (VEGF)-induced chemoinvasion [67], [68]. According to Choi et al. [69], Korean Red Ginseng extracts efficiently decrease several angiogenic factors such as IL-8, hypoxia inducible factor-1a, VEGF, IL-6, and matrix metalloproteinases, implying the underlying mechanism of anti-angiogenesis.
Many studies suggest that P. ginseng has protective effects on ischemia and reperfusion (I/R), especially on the myocardial I/R [70], [71]. Recently, Aravinthan et al. [72] reported that ginseng total saponin ameliorated myocardial injury by improving hemodynamics, such as aortic flow, coronary flow, and cardiac output. Thus, ginseng total saponin significantly suppressed the biochemical parameters, oxidative stress markers, and inflammatory indicators. In consistent with this, Luo et al. [73] suggested that the long-term consumption of P. ginseng lowers the susceptibility of acute myocardial I/R injury in intermediate-aged rats. P. ginseng-treated heart reportedly showed reduced infarct size, improved cardiac performance, and increased survival signals.
5.4. Parkinson's disease
Several studies have recently reported that P. ginseng has a wide range of actions in the central nervous system, with promising effects on Parkinson's disease. Van et al. [74] demonstrated neuroprotective effects of the P. ginseng extract. It significantly reduced dopaminergic cell loss, preventing the development of locomotor deficits in chronic Parkinson's disease model animals. Hu et al. [75] demonstrated that the water extract of P. ginseng has significant protective effects against parkinsonism-inducing cytotoxic agents, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and its active metabolite 1-methyl-4-phenylpyridinium, in mice. It increased the Bax/Bcl-2 ratio, decreased cell death, promoted the release of cytochrome C, and suppressed the overproduction of reactive oxygen species.
5.5. Pain
There have been reports on pain-relieving effects of P. ginseng [76], [77], [78]. Nah et al. [76] reported that ginsenosides could regulate the pain-related behavior of mice with capsaicin-induced pain in a dose-dependent manner. Lee et al. [79] demonstrated analgesic and anti-inflammatory effects of the fraction of P. ginseng in inflammatory pain mice models. Wang et al. [80] showed that glycoproteins extracted from P. ginseng exhibited a dose-dependent analgesic effect in mice by conducting acetic acid-induced writhing and hot-plate. Recently, a study also showed analgesic effect of P. ginseng in neuropathic pain animal models [81].
6. Concluding remarks and future directions
P. ginseng contains various ingredients in addition to ginsenosides, and these components might interact with multiple targets and pathways simultaneously in a complex manner. It is difficult to understand the complex mechanisms of the action of P. ginseng at systems-level using the conventional reductive analysis. Here, we attempted to review the systems-level mechanism of P. ginseng by applying a novel analytical framework, network pharmacology. The constructed compound-target network of P. ginseng based on validated datasets and predictive models provided potential target proteins of P. ginseng. The multiple targets of the network were analyzed in terms of related biological process, pathways, and diseases, revealing the systems-level mechanism of P. ginseng. The majority of targets were related with primary metabolic process, signal transduction, nitrogen compound metabolic process, blood circulation, immune system process, cell-cell signaling, biosynthetic process, and neurological system process. In more detailed pathway enrichment analysis of targets using KEGG pathway database, mainly the terms related with neural activity showed significant enrichment and formed a cluster. Furthermore, relative degrees analysis for target-disease association of P. ginseng revealed several categories of related diseases including respiratory, psychiatric, and cardiovascular diseases. However, it is worthy to note that the network pharmacological approach adopted in this review largely depends on the in silico predictions although the training dataset for the prediction models are experimentally validated. Currently, many different methods exist for each network pharmacological analysis step, such as the prediction of OB, DL, and drug-target interactions [35], and it is not clear which methods are ideal for understanding the systems-level, multidimensional mechanisms of herbs with multiple ingredients. In spite of the limitations of in silico analysis, our results were consistent with the previous researches on diseases with the extract of P. ginseng. The network pharmacological approach will help to comprehensively understand the systems-level mechanism of P. ginseng by combining experimental validation and optimizing the analytical methodologies in future studies.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP; Ministry of Science, ICT & Futue Planning) (No. 2017-0129). This work was also supported by the Korea Food Institute (E0164500-01), Korea.
Contributor Information
Ki Sung Kang, Email: kkang@gachon.ac.
Chang-Eop Kim, Email: eopchang@gachon.ac.kr.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Park H.J., Kim D.H., Park S.J., Kim J.M., Ryu J.H. Ginseng in traditional herbal prescriptions. J Ginseng Res. 2012;36:225–241. doi: 10.5142/jgr.2012.36.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuk Y.B., Kim S.C., Park S.D., Park S.K., Seo B.Y., Seo Y.B., Shin S.S., Lee S.I., Lee J.C., CH L. 1999. Formula study. Younglimsa. [in Korean] [Google Scholar]
- 3.Xiang Y.Z., Shang H.C., Gao X.M., Zhang B.L. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother Res. 2008;22:851–858. doi: 10.1002/ptr.2384. [DOI] [PubMed] [Google Scholar]
- 4.Choi J., Kim T.H., Choi T.Y., Lee M.S. Ginseng for health care: a systematic review of randomized controlled trials in Korean literature. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H.W., Lim H.J., Jun J.H., Choi J., Lee M.S. Ginseng for the treatment of hypertension: a systematic review and meta-analysis of double-blind, randomized, placebo-controlled trials. Curr Vasc Pharmacol. 2017;15:549. doi: 10.2174/1570161115666170713092701. [DOI] [PubMed] [Google Scholar]
- 6.Lee C.H., Kim J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J.H. Cardiovascular diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J Ginseng Res. 2012;36:16–26. doi: 10.5142/jgr.2012.36.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan C.S., Wang C.Z., Wicks S.M., Qi L.W. Chemical and pharmacological studies of saponins with a focus on American ginseng. J Ginseng Res. 2010;34:160–167. doi: 10.5142/jgr.2010.34.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhee Y.H., Ahn J.H., Choe J., Kang K.W., Joe C. Inhibition of mutagenesis and transformation by root extracts of Panax ginseng in vitro. Planta Med. 1991;57:125–128. doi: 10.1055/s-2006-960047. [DOI] [PubMed] [Google Scholar]
- 10.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen X., Lee T.J. Ginsenosides-induced nitric oxide-mediated relaxation of the rabbit corpus cavernosum. Br J Pharmacol. 1995;115:15–18. doi: 10.1111/j.1476-5381.1995.tb16313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee M.H., Jeong J.H., Seo J.W., Shin C.G., Kim Y.S., In J.G., Yang D.C., Yi J.S., Choi Y.E. Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol. 2004;45:976–984. doi: 10.1093/pcp/pch126. [DOI] [PubMed] [Google Scholar]
- 13.Richter R., Basar S., Koch A., Konig W.A. Three sesquiterpene hydrocarbons from the roots of Panax ginseng C.A. Meyer (Araliaceae) Phytochemistry. 2005;66:2708–2713. doi: 10.1016/j.phytochem.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.S. Investigation of phenolic, flavonoid, and vitamin contents in different parts of Korean Ginseng (Panax ginseng C.A. Meyer) Prev Nutr Food Sci. 2016;21:263–270. doi: 10.3746/pnf.2016.21.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han B.H., Park M.H., Han Y.N., Woo L.K. Alkaloidal components of Panax ginseng. Archives of Pharmacal Research. 1986;9:21–23. [Google Scholar]
- 16.Huong N.T., Matsumoto K., Kasai R., Yamasaki K., Watanabe H. In vitro antioxidant activity of Vietnamese ginseng saponin and its components. Biol Pharm Bull. 1998;21:978–981. doi: 10.1248/bpb.21.978. [DOI] [PubMed] [Google Scholar]
- 17.Chang T.K., Chen J., Benetton S.A. In vitro effect of standardized ginseng extracts and individual ginsenosides on the catalytic activity of human CYP1A1, CYP1A2, and CYP1B1. Drug Metab Dispos. 2002;30:378–384. doi: 10.1124/dmd.30.4.378. [DOI] [PubMed] [Google Scholar]
- 18.Rimar S., Lee-Mengel M., Gillis C.N. Pulmonary protective and vasodilator effects of a standardized Panax ginseng preparation following artificial gastric digestion. Pulm Pharmacol. 1996;9:205–209. doi: 10.1006/pulp.1996.0025. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins A.L. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 20.Ru J., Li P., Wang J., Zhou W., Li B., Huang C., Li P., Guo Z., Tao W., Yang Y. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mi H., Huang X., Muruganujan A., Tang H., Mills C., Kang D., Thomas P.D. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 2017;45:D183–D189. doi: 10.1093/nar/gkw1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mi H., Muruganujan A., Casagrande J.T., Thomas P.D. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elyakov G., Strigina L., Uvarova N., Vaskovsky V., Dzizenko A., Kochetkov N. Glycosides from ginseng roots. Tetrahedron Letters. 1964;5:3591–3597. [Google Scholar]
- 24.Tansakul P., Shibuya M., Kushiro T., Ebizuka Y. Dammarenediol-II synthase, the first dedicated enzyme for ginsenoside biosynthesis, in Panax ginseng. FEBS Lett. 2006;580:5143–5149. doi: 10.1016/j.febslet.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 25.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasegawa H., Suzuki R., Nagaoka T., Tezuka Y., Kadota S., Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biol Pharm Bull. 2002;25:861–866. doi: 10.1248/bpb.25.861. [DOI] [PubMed] [Google Scholar]
- 27.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 28.Ling W.H., Jones P.J. Dietary phytosterols: a review of metabolism, benefits and side effects. Life Sci. 1995;57:195–206. doi: 10.1016/0024-3205(95)00263-6. [DOI] [PubMed] [Google Scholar]
- 29.Iwabuchi H., Kato N., Yoshikura M. Studies on the sesquiterpenoids of Panax ginseng C. A. Meyer. IV. Chem Pharm Bull (Tokyo) 1990;38:1405–1407. doi: 10.1248/cpb.38.1405. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. Sci World J. 2013;2013 doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi M., Yoshikura M. Studies on the components of Panax ginseng C.A. Meyer. V. On the structure of a new acetylene derivative “Panaxynol” (3). Synthesis of 1,9-(cis)-heptadecadiene-4,6-diyn-3-ol. Yakugaku Zasshi. 1966;86:1053–1056. doi: 10.1248/yakushi1947.86.11_1053. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 32.Matsunaga H., Katano M., Yamamoto H., Fujito H., Mori M., Takata K. Cytotoxic activity of polyacetylene compounds in Panax ginseng C. A. Meyer. Chem Pharm Bull (Tokyo) 1990;38:3480–3482. doi: 10.1248/cpb.38.3480. [DOI] [PubMed] [Google Scholar]
- 33.Kong Y.-H., Lee Y.-C., Choi S.-Y. Neuroprotective and anti-inflammatory effects of phenolic compounds in Panax ginseng CA Meyer. J Ginseng Res. 2009;33:111–114. [Google Scholar]
- 34.Lee L.S., Cho C.W., Hong H.D., Lee Y.C., Choi U.K., Kim Y.C. Hypolipidemic and antioxidant properties of phenolic compound-rich extracts from white ginseng (Panax ginseng) in cholesterol-fed rabbits. Molecules. 2013;18:12548–12560. doi: 10.3390/molecules181012548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou W., Wang J., Wu Z., Huang C., Lu A., Wang Y. Systems pharmacology exploration of botanic drug pairs reveals the mechanism for treating different diseases. Sci Rep. 2016;6 doi: 10.1038/srep36985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu H., Chen J., Xu X., Li Y., Zhao H., Fang Y., Li X., Zhou W., Wang W., Wang Y. A systematic prediction of multiple drug-target interactions from chemical, genomic, and pharmacological data. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen E.Y., Tan C.M., Kou Y., Duan Q., Wang Z., Meirelles G.V., Clark N.R., Ma'ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanehisa M., Goto S., Kawashima S., Nakaya A. The KEGG databases at GenomeNet. Nucleic Acids Res. 2002;30:42–46. doi: 10.1093/nar/30.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung J.H., Kang I.G., Kim D.Y., Hwang Y.J., Kim S.T. The effect of Korean red ginseng on allergic inflammation in a murine model of allergic rhinitis. J Ginseng Res. 2013;37:167–175. doi: 10.5142/jgr.2013.37.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babayigit A., Olmez D., Karaman O., Bagriyanik H.A., Yilmaz O., Kivcak B., Erbil G., Uzuner N. Ginseng ameliorates chronic histopathologic changes in a murine model of asthma. Allergy Asthma Proc. 2008;29:493–498. doi: 10.2500/aap.2008.29.3137. [DOI] [PubMed] [Google Scholar]
- 42.Lim C.Y., Moon J.M., Kim B.Y., Lim S.H., Lee G.S., Yu H.S., Cho S.I. Comparative study of Korean White Ginseng and Korean Red Ginseng on efficacies of OVA-induced asthma model in mice. J Ginseng Res. 2015;39:38–45. doi: 10.1016/j.jgr.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim D.Y., Yang W.M. Panax ginseng ameliorates airway inflammation in an ovalbumin-sensitized mouse allergic asthma model. J Ethnopharmacol. 2011;136:230–235. doi: 10.1016/j.jep.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 44.Tran T.V., Shin E.J., Ko S.K., Nam Y., Chung Y.H., Jeong J.H., Jang C.G., Nah S.Y., Yamada K., Nabeshima T. Mountain-cultivated ginseng attenuates phencyclidine-induced abnormal behaviors in mice by positive modulation of glutathione in the prefrontal cortex of mice. J Med Food. 2016;19:961–969. doi: 10.1089/jmf.2016.3751. [DOI] [PubMed] [Google Scholar]
- 45.Dang H., Sun L., Liu X., Peng B., Wang Q., Jia W., Chen Y., Pan A., Xiao P. Preventive action of Kai Xin San aqueous extract on depressive-like symptoms and cognition deficit induced by chronic mild stress. Exp Biol Med (Maywood) 2009;234:785–793. doi: 10.3181/0812-RM-354. [DOI] [PubMed] [Google Scholar]
- 46.Zhu K.Y., Mao Q.Q., Ip S.P., Choi R.C., Dong T.T., Lau D.T., Tsim K.W. A standardized Chinese herbal decoction, Kai-Xin-San, restores decreased levels of neurotransmitters and neurotrophic factors in the brain of chronic stress-induced depressive rats. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/149256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan L., Hu Q., Mak M.S., Lou J., Xu S.L., Bi C.W., Zhu Y., Wang H., Dong T.T., Tsim K.W. A Chinese herbal decoction, reformulated from Kai-Xin-San, relieves the depression-like symptoms in stressed rats and induces neurogenesis in cultured neurons. Sci Rep. 2016;6 doi: 10.1038/srep30014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Y., Duan X., Cheng X., Cheng X., Li X., Zhang L., Liu P., Su S., Duan J.A., Dong T.T. Kai-Xin-San, a standardized traditional Chinese medicine formula, up-regulates the expressions of synaptic proteins on hippocampus of chronic mild stress induced depressive rats and primary cultured rat hippocampal neuron. J Ethnopharmacol. 2016;193:423–432. doi: 10.1016/j.jep.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Y., Chao C., Duan X., Cheng X., Liu P., Su S., Duan J., Dong T.T., Tsim K.W. Kai-Xin-San series formulae alleviate depressive-like behaviors on chronic mild stressed mice via regulating neurotrophic factor system on hippocampus. Sci Rep. 2017;7 doi: 10.1038/s41598-017-01561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan S., You Z.L., Zhao Q.Y., Peng C., He G., Gou X.J., Lin B. Antidepressant-like effects of Sanyuansan in the mouse forced swim test, tail suspension test, and chronic mild stress model. Kaohsiung J Med Sci. 2015;31:605–612. doi: 10.1016/j.kjms.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Zhang K., Wang F., Yang J.Y., Wang L.J., Pang H.H., Su G.Y., Ma J., Song S.J., Xiong Z.L., Wu C.F. Analysis of main constituents and mechanisms underlying antidepressant-like effects of Xiaochaihutang in mice. J Ethnopharmacol. 2015;175:48–57. doi: 10.1016/j.jep.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 52.Kuribara H., Tomioka H., Takahashi R., Onozato K., Murohashi N., Numajiri T., Iwata H., Koya S. An antidepressant effect of Sho-ju-sen, a Japanese herbal medicine, assessed by learned helplessness model in mice. Phytother Res. 2004;18:173–176. doi: 10.1002/ptr.1412. [DOI] [PubMed] [Google Scholar]
- 53.Lee K.J., Ji G.E. The effect of fermented red ginseng on depression is mediated by lipids. Nutr Neurosci. 2014;17:7–15. doi: 10.1179/1476830513Y.0000000059. [DOI] [PubMed] [Google Scholar]
- 54.Jeong H.G., Ko Y.H., Oh S.Y., Han C., Kim T., Joe S.H. Effect of Korean Red Ginseng as an adjuvant treatment for women with residual symptoms of major depression. Asia Pac Psychiatry. 2015;7:330–336. doi: 10.1111/appy.12169. [DOI] [PubMed] [Google Scholar]
- 55.Kim Y.O., Lee H.Y., Won H., Nah S.S., Lee H.Y., Kim H.K., Kwon J.T., Kim H.J. Influence of Panax ginseng on the offspring of adult rats exposed to prenatal stress. Int J Mol Med. 2015;35:103–109. doi: 10.3892/ijmm.2014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong S.Y., Kim J.Y., Ahn H.Y., Shin J.H., Kwon O. Panax ginseng extract rich in ginsenoside protopanaxatriol attenuates blood pressure elevation in spontaneously hypertensive rats by affecting the Akt-dependent phosphorylation of endothelial nitric oxide synthase. J Agric Food Chem. 2012;60:3086–3091. doi: 10.1021/jf204447y. [DOI] [PubMed] [Google Scholar]
- 57.Lei Y., Tao L.L., Wang G.L. Effect of extracts from Panax ginseng, Panax notoginseng, and Ligusticum chuanxiong on vascular smooth muscle cells of aging and hypertension rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:1374–1379. [in Chinese] [PubMed] [Google Scholar]
- 58.Zhou W., Chai H., Lin P.H., Lumsden A.B., Yao Q., Chen C.J. Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med Sci Monit. 2004;10:RA187–R192. [PubMed] [Google Scholar]
- 59.Rhee M.Y., Kim Y.S., Bae J.H., Nah D.Y., Kim Y.K., Lee M.M., Kim H.Y. Effect of Korean red ginseng on arterial stiffness in subjects with hypertension. J Altern Complement Med. 2011;17:45–49. doi: 10.1089/acm.2010.0065. [DOI] [PubMed] [Google Scholar]
- 60.Jeon B.H., Kim C.S., Kim H.S., Park J.B., Nam K.Y., Chang S.J. Effect of Korean red ginseng on blood pressure and nitric oxide production. Acta Pharmacol Sin. 2000;21:1095–1100. [PubMed] [Google Scholar]
- 61.Jeon B.H., Kim C.S., Park K.S., Lee J.W., Park J.B., Kim K.J., Kim S.H., Chang S.J., Nam K.Y. Effect of Korea red ginseng on the blood pressure in conscious hypertensive rats. Gen Pharmacol. 2000;35:135–141. doi: 10.1016/s0306-3623(01)00096-9. [DOI] [PubMed] [Google Scholar]
- 62.Kang S.Y., Schini-Kerth V.B., Kim N.D. Ginsenosides of the protopanaxatriol group cause endothelium-dependent relaxation in the rat aorta. Life Sci. 1995;56:1577–1586. doi: 10.1016/0024-3205(95)00124-o. [DOI] [PubMed] [Google Scholar]
- 63.Kim N.D., Kang S.Y., Schini V.B. Ginsenosides evoke endothelium-dependent vascular relaxation in rat aorta. Gen Pharmacol. 1994;25:1071–1077. doi: 10.1016/0306-3623(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 64.Shin W., Yoon J., Oh G.T., Ryoo S. Korean red ginseng inhibits arginase and contributes to endothelium dependent vasorelaxation through endothelial nitric oxide synthase coupling. J Ginseng Res. 2013;37:64–73. doi: 10.5142/jgr.2013.37.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee J.J., Kwon H.K., Jung I.H., Cho Y.B., Kim K.J., Kim J.L. Anti-cancer activities of ginseng extract fermented with Phellinus linteus. Mycobiology. 2009;37:21–27. doi: 10.4489/MYCO.2009.37.1.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hwang S.H., Lee B.H., Choi S.H., Kim H.J., Won K.J., Lee H.M., Rhim H., Kim H.C., Nah S.Y. Effects of gintonin on the proliferation, migration, and tube formation of human umbilical-vein endothelial cells: involvement of lysophosphatidic-acid receptors and vascular-endothelial-growth-factor signaling. J Ginseng Res. 2016;40:325–333. doi: 10.1016/j.jgr.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yue P.Y., Wong D.Y., Wu P.K., Leung P.Y., Mak N.K., Yeung H.W., Liu L., Cai Z., Jiang Z.H., Fan T.P. The angiosuppressive effects of 20(R)- ginsenoside Rg3. Biochem Pharmacol. 2006;72:437–445. doi: 10.1016/j.bcp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 68.Kim J.W., Jung S.Y., Kwon Y.H., Lee J.H., Lee Y.M., Lee B.Y., Kwon S.M. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biol Ther. 2012;13:504–515. doi: 10.4161/cbt.19599. [DOI] [PubMed] [Google Scholar]
- 69.Choi K.S., Song H., Kim E.H., Choi J.H., Hong H., Han Y.M., Hahm K.B. Inhibition of hydrogen sulfide-induced angiogenesis and inflammation in vascular endothelial cells: potential mechanisms of gastric cancer prevention by Korean Red Ginseng. J Ginseng Res. 2012;36:135–145. doi: 10.5142/jgr.2012.36.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou H., Hou S.Z., Luo P., Zeng B., Wang J.R., Wong Y.F., Jiang Z.H., Liu L. Ginseng protects rodent hearts from acute myocardial ischemia-reperfusion injury through GR/ER-activated RISK pathway in an endothelial NOS-dependent mechanism. J Ethnopharmacol. 2011;135:287–298. doi: 10.1016/j.jep.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 71.Kim T.H., Lee S.M. The effects of ginseng total saponin, panaxadiol and panaxatriol on ischemia/reperfusion injury in isolated rat heart. Food Chem Toxicol. 2010;48:1516–1520. doi: 10.1016/j.fct.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 72.Aravinthan A., Kim J.H., Antonisamy P., Kang C.W., Choi J., Kim N.S., Kim J.H. Ginseng total saponin attenuates myocardial injury via anti-oxidative and anti-inflammatory properties. J Ginseng Res. 2015;39:206–212. doi: 10.1016/j.jgr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luo P., Dong G., Liu L., Zhou H. The long-term consumption of ginseng extract reduces the susceptibility of intermediate-aged hearts to acute ischemia reperfusion injury. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Kampen J.M., Baranowski D.B., Shaw C.A., Kay D.G. Panax ginseng is neuroprotective in a novel progressive model of Parkinson's disease. Exp Gerontol. 2014;50:95–105. doi: 10.1016/j.exger.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 75.Hu S., Han R., Mak S., Han Y. Protection against 1-methyl-4-phenylpyridinium ion (MPP+)-induced apoptosis by water extract of ginseng (Panax ginseng C.A. Meyer) in SH-SY5Y cells. J Ethnopharmacol. 2011;135:34–42. doi: 10.1016/j.jep.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 76.Nah J.J., Hahn J.H., Chung S., Choi S., Kim Y.I., Nah S.Y. Effect of ginsenosides, active components of ginseng, on capsaicin-induced pain-related behavior. Neuropharmacology. 2000;39:2180–2184. doi: 10.1016/s0028-3908(00)00048-4. [DOI] [PubMed] [Google Scholar]
- 77.Mogil J.S., Shin Y.H., McCleskey E.W., Kim S.C., Nah S.Y. Ginsenoside Rf, a trace component of ginseng root, produces antinociception in mice. Brain Res. 1998;792:218–228. doi: 10.1016/s0006-8993(98)00133-4. [DOI] [PubMed] [Google Scholar]
- 78.Yin H., Park S.A., Park S.J., Han S.K. Korean Red Ginseng extract activates non-NMDA glutamate and GABAA receptors on the substantia gelatinosa neurons of the trigeminal subnucleus caudalis in mice. J Ginseng Res. 2011;35:219–225. doi: 10.5142/jgr.2011.35.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee J.H., Lee J.H., Lee Y.M., Kim P.N., Jeong C.S. Potential analgesic and anti-inflammatory activities of Panax ginseng head butanolic fraction in animals. Food Chem Toxicol. 2008;46:3749–3752. doi: 10.1016/j.fct.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y., Chen Y., Xu H., Luo H., Jiang R. Analgesic effects of glycoproteins from Panax ginseng root in mice. J Ethnopharmacol. 2013;148:946–950. doi: 10.1016/j.jep.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 81.Suzuki T., Yamamoto A., Ohsawa M., Motoo Y., Mizukami H., Makino T. Effect of ninjin'yoeito and ginseng extracts on oxaliplatin-induced neuropathies in mice. J Nat Med. 2017;71:757–764. doi: 10.1007/s11418-017-1113-6. [DOI] [PubMed] [Google Scholar]