Abstract
Background
Ginsenosides have been reported to have many health benefits, including anti-inflammatory effects, and the resolution of inflammation is now considered to be an active process driven by M2-type macrophages. In order to determine whether ginsenosides modulate macrophage phenotypes to reduce inflammation, 11 ginsenosides were studied with respect to macrophage polarization and the resolution of inflammation.
Methods
Mouse peritoneal macrophages were polarized into M1 or M2 phenotypes. Reverse transcription-polymerase chain reaction, Western blotting, and measurement of nitric oxide (NO) and prostaglandin E2 levels were performed in vitro and in a zymosan-induced peritonitis C57BL/6 mouse model.
Results
Ginsenoside Rg3 was identified as a proresolving ginseng compound based on the induction of M2 macrophage polarization. Ginsenoside Rg3 not only induced the expression of arginase-1 (a representative M2 marker gene), but also suppressed M1 marker genes, such as inducible NO synthase, and NO levels. The proresolving activity of ginsenoside Rg3 was also observed in vivo in a zymosan-induced peritonitis model. Ginsenoside Rg3 accelerated the resolution process when administered at peak inflammatory response into the peritoneal cavity.
Conclusion
These results suggest that ginsenoside Rg3 induces the M2 polarization of macrophages and accelerates the resolution of inflammation. This finding opens a new avenue in ginseng pharmacology.
Keywords: ginseng, ginsenoside Rg3, inflammation, resolution
1. Introduction
Pharmacognostic studies on ginseng, the root of Panax ginseng Meyer, have determined that ginsenosides, triterpenoid saponins, are the bioactive ingredients of ginseng [1], [2]. Ginsenosides are believed to mediate most of the pharmacological effects of ginseng, which include anticancer, anti-inflammatory, and antidiabetic activities [1], [3], [4]. Moreover, of the ginsenosides, ginsenoside Rg3 is one of the most effective steroidal saponins in steamed ginseng [5]. Ginsenoside Rg3 exhibits a wide range of therapeutic and pharmacological properties, which include anti-inflammatory, anticancer, antioxidant, and antiobesity effects [4], [6], [7], [8], [9], [10].
The resolution of inflammation is now considered to be an active process orchestrated by proresolving mediators and their receptors [11], [12]. M2 polarized macrophages are supposed to play key roles during the resolution of inflammation [13], [14]. Although anti-inflammatory effects of ginseng and ginsenosides have been studied at the molecular level, the effects of ginsenosides on macrophage polarization and on the resolution of inflammation have not been studied. Therefore, we aimed: (1) to find whether any ginsenoside could induce M2 polarization of macrophages; (2) to confirm induction of M2 polarization and suppression of M1 polarization at molecular and cellular levels in vitro; and (3) to verify M2 polarization effect on inflammation resolution in a mouse peritonitis model in vivo.
First, we investigated the effects of ginsenosides on macrophage polarization, in order to determine which ginsenoside could accelerate the resolution of inflammation [15]. Of the 11 ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh2, and Ro) tested, Rg3 was found to inhibit M1 polarization and to induce M2 polarization in mouse peritoneal macrophages, and to promote the resolution of inflammation in a murine peritonitis model. The first report on ginsenoside Rg3's effects on M2 polarization and resolution of inflammation would be the novelty of the present study.
2. Materials and methods
2.1. Materials
Ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh2, and Ro) were purified in Kang-ju Choi's laboratory, the Korea Ginseng and Tobacco Research Institute (Daejeon, Republic of Korea), and the purities were more than 99.9%. They were dissolved in absolute methanol and stored at −20°C. All other chemicals were purchased from Sigma (St. Louis, MO, USA).
2.2. Animals
Eight- to ten-week-old male C57BL/6 (19–22 g) mice were purchased from Daehan Biolink (DBL; Seoul, Korea), housed in a laboratory animal facility at Pusan National University (Busan, Korea), and provided food and water ad libitum. The animal protocol used in this study was reviewed and approved beforehand by the Pusan National University-Institutional Animal Care Committee with respect to ethicality and scientific care.
2.3. Isolation and culture of mouse peritoneal macrophages
Mouse peritoneal macrophages were isolated from the peritoneal cavity of a 3% thioglycollate-treated C57BL/6 mouse 4 d after treatment and cultured at 37°C in a 5% CO2 humidified incubator. Isolated macrophages were maintained in RPMI1640 containing 10% (v/v) heat-inactivated fetal bovine serum, 100 units/mL penicillin, 50 μg/mL streptomycin, 2 mM glutamine, and 1 mM sodium pyruvate for 18 h and then incubated in 0.5% fetal bovine serum-containing media for 24 h. RNA and protein samples were prepared after 5 h or 24 h of lipopolysaccharide (LPS) treatment (10 ng/mL or 100 ng/mL), respectively. Ginsenosides were added 1 h prior to adding LPS [15].
2.4. Reverse transcriptase-polymerase chain reaction
To determine the expressions of marker proteins of M1 or M2 polarization in macrophages by reverse transcription-polymerase chain reaction (RT-PCR), first-strand cDNA was synthesized with total RNA isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Synthesized cDNA products and primers for each gene were used for PCR, which was conducted using Promega Go-Taq DNA polymerase (Promega, Madison, WI, USA). Specific primers for tumor necrosis factor-alpha (TNF-α) (sense 5′-GAC CCT CAC ACT CAG ATC AT-3′, antisense 5′-TTG AAG AGA ACC TGG GAG TA-3′), transforming growth factor-beta 1 (TGF-β1) (sense 5′-TTGCTTCAGCTCCACAGAGA-3′, antisense 5′-TGGTTGTAGAGGGCAAGGAC-3′), Ym-1 (sense 5′-ACT TTG ATG GCC TCA ACC TG-3′, antisense 5′-AAT GAT TCC TGC TCC TGT GG-3′), and interleukin (IL)-10 (sense 5′-CCAAGCCTTATCGGAAATGA-3′, antisense 5′-TTTTCACAGGGGAGAAATCG-3′) were used to amplify gene fragments. PCR was performed over 30 amplification cycles (denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and elongation at 72°C for 30 s) in an Eppendorf Mastcycler gradient PCR machine (Eppendorf, Hamburg, Germany) [16]. Specific primers for arginase-1 (sense 5′-GTG AAG AAC CCA CGG TCT GT-3′, antisense 5′-CTG GTT GTC GGG GAG TGT T-3′), inducible nitric oxide synthase (iNOS) (sense 5′-ACC TAC CAC ACC CGA GAT GGC CAG-3′, antisense 5′-AGG ATG TCC TGA ACA TAG ACC TTG GG-3′), cyclooxygenase-2 (COX-2) (sense 5′-CCG TGG GGA ATG TAT GAG CA-3′, antisense 5′-CCA GGT CCT CGC TTA TGA TCT G -3′), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (sense 5′-TTCACCACCATGGAGAAGGC-3′, antisense 5′-GGCATGGACTGTGGTCATGA-3′) were used, and annealing was performed at 60°C. For IL-1β (sense 5′-GGAGAAGCTGTGGCAGCTA-3′, antisense 5′-GCTGATGTACCAGTTGGGGA-3′), annealing was undertaken at 57°C. Aliquots (7 μL) were electrophoresed in 1.2% agarose gels and stained with ethidium bromide [15].
2.5. Western blotting
Macrophages were harvested and resuspended in RIPA lysis buffer (GenDEPOT, Baker, TX, USA). Concentrations of proteins were determined using a BCA protein assay (ThermoScientific, Rockford, IL, USA). Proteins (30 μg) were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose. Membranes were blocked in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) and 5% skim milk, incubated with specific primary antibodies recognizing β-actin, COX-2, iNOS, and arginase-1, and then incubated HRP-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA, USA). Signals were developed using an enhanced chemiluminescence system (Pierce Biotechnology Inc., Rockford, IL) [16].
2.6. Nitrites measurement
NO production was estimated by measuring the amount of nitrite (a stable metabolite of NO) in medium using Griess reagent, as previously described [17]. Cells were pretreated with different concentrations of ginsenoside Rg3 for 1 h and subsequently stimulated with LPS (100 ng/mL) for 24 h. Nitrite concentrations in medium were determined using the Griess Reagent System (Promega).
2.7. Prostaglandin E2 production
Peritoneal macrophages were incubated with ginsenoside Rg3 for 1 h and subsequently stimulated with LPS (100 ng/mL) for 24 h. Macrophage culture supernatants were harvested and immediately assayed using a prostaglandin E2 (PGE2) EIA kit (Cayman Chemical, Ann Arbor, MI) [18].
2.8. Induction of peritonitis and peritoneal cell counting
Peritonitis was induced by injecting 30 mg/kg of zymosan (Sigma) intraperitoneally (i.p.), and 12 h later, mice were treated i.p. with vehicle, 1 mg/kg, or 5 mg/kg ginsenoside Rg3. Peritoneal washing was performed 24 h after zymosan treatment using 4 mL of ice-cold RPMI1640. Total cell numbers in peritoneal washings were calculated by counting after trypan blue staining. The cells obtained were washed with 0.1M phosphate buffer (pH 6.8), which was prepared by mixing 153 mL of 0.2M NaH2PO4 (Monobasic; AMRESCO, Solon, OH) and 147 mL of 0.2M Na2HPO4 (Dibasic; AMRESCO) and adding distilled water to a final volume 900 mL. The cells were attached to slides using a Cellspin (Hanil, Anyang, Korea), which was operated at 500 rpm for 5 min. Slides were then dried at room temperature for 30 min and fixed in methanol for 30 s. Cells were then stained with May–Grünwald solution (Sigma) and Giemsa solution (Fluka, Buchs, Switzerland) to identify individual cell types [19].
2.9. Statistics
Results are expressed as the means ± standard errors of the indicated numbers of determinations. The statistical significances of differences were determined by analysis of variance using Tukey's post hoc, and statistical significance was accepted for p values < 0.05. Analyses were performed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
3.1. Effects of 11 ginsenosides on macrophage phenotypes
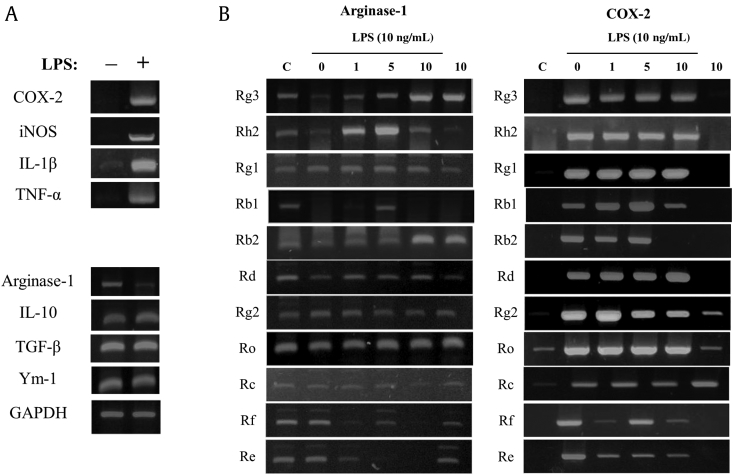
Initially, we tested the effects of the 11 ginsenosides on the M1 and M2 polarizations of peritoneal macrophages. As shown Fig. 1A, isolated peritoneal macrophages expressed M2 marker genes, such as arginase-1, IL-10, TGF-β, and Ym-1, but not M1 marker genes. However, after stimulation with LPS (a cell wall component of Gram-negative bacteria) macrophage phenotype changed, that is, they strongly expressed M1 marker genes, such as COX-2, iNOS, IL-1β, and TNF-α. Furthermore, the representative M2 marker gene, arginase-1, was downregulated after LPS stimulation (Fig. 1A) [15]. These observations show that the isolated peritoneal macrophages were M2 polarized, and that the LPS-stimulated macrophages were M1 polarized [15]. We used this polarization phenomenon to assess the effects of the 11 ginsenosides on macrophage polarization. Of these ginsenosides, Rg3 most prevented LPS-induced loss of arginase-1 expression, which it did in a concentration-dependent manner (Fig. 1B). In addition, it slightly suppressed LPS-induced COX-2 induction. Based on these results, we focused on ginsenoside Rg3 in the following experiments on macrophage polarization and the resolution of inflammation (the structure of ginsenoside Rg3 is provided in Fig. 2A).
Fig. 1.
Macrophage polarization and effects of ginsenosides on the expressions of M1 and M2 marker genes in peritoneal macrophages. (A) Mouse peritoneal macrophages were treated with vehicle or LPS 10 ng/mL for 5 h, and RT-PCR was then performed for proinflammatory and anti-inflammatory genes. The data shown are representative of three independent experiments. (B) Mouse peritoneal macrophages were treated with the indicated concentrations of each ginsenoside for 1 h, and then treated with vehicle or LPS 10 ng/mL for 5 h. RT-PCR for proinflammatory genes and anti-inflammatory genes was performed by targeting arginase-1 and COX-2. COX-2, cyclooxygenase-2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IL-10, interleukin 10; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; RT-PCR, reverse transcription-polymerase chain reaction; TGF, transforming growth factor; TNF, tumor necrosis factor.
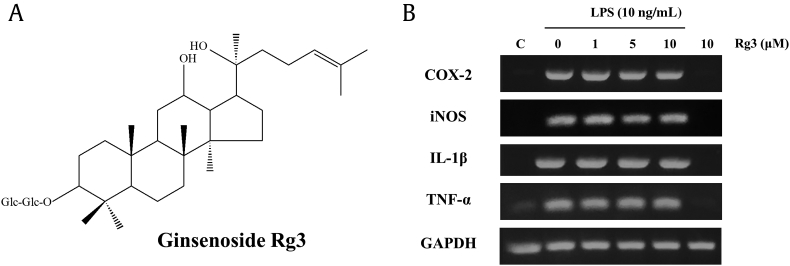
Fig. 2.
Structure of ginsenoside Rg3 and its effect on the expressions of M1 marker genes in macrophages. (A) The structure of ginsenoside Rg3. (B) Mouse peritoneal macrophages were treated with the indicated concentrations of ginsenoside Rg3 for 1 h, and then treated with vehicle or LPS 10 ng/mL for 5 h, and RT-PCR was performed for the proinflammatory genes, COX-2, iNOS, IL-1β, and TNF-α. The data shown are representative of three independent experiments. COX-2, cyclooxygenase-2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IL-1β, interleukin-1 beta; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; RT-PCR, reverse transcription-polymerase chain reaction; TNF, tumor necrosis factor.
3.2. Effects of ginsenoside Rg3 on M1 polarization
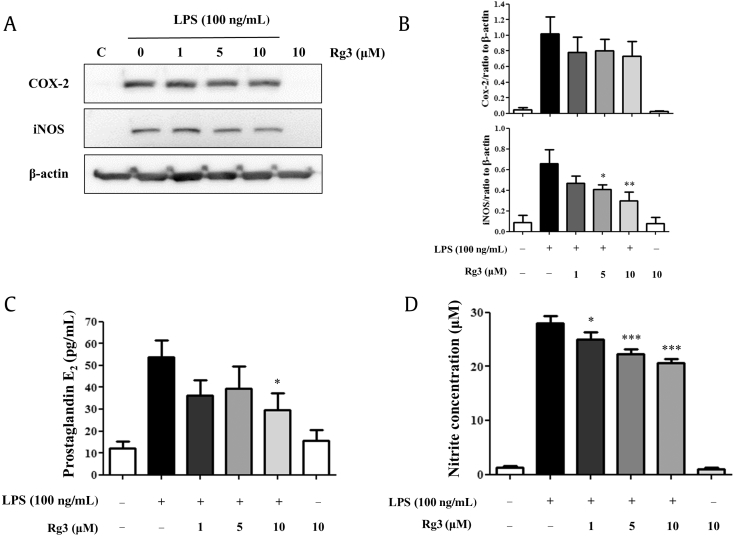
In mouse peritoneal macrophages, stimulation with 10 ng/mL LPS induced the expressions of M1 proinflammatory marker genes, such as COX-2, iNOS, IL-1β, and TNF-α (Fig. 2B). Of the four genes tested, iNOS and COX-2 mRNAs appeared to be reduced concentration-dependently by Rg3, but not significantly so (data not shown). By contrast, the iNOS mRNA expression was much more affected than that of COX-2 mRNA. We also examined the inhibitory effects of Rg3 at the protein level. As shown in Fig. 3A, the expressions of iNOS and COX-2 protein were obviously induced by LPS. Ginsenoside Rg3 strongly and concentration-dependently inhibited iNOS protein induction, but its effect on COX-2 protein induction was not significant (Fig. 3B). At concentrations 5 μM and 10 μM, ginsenoside Rg3 had significant inhibitory effects on iNOS protein induction. In addition, the inhibitory effects of ginsenoside Rg3 on the expressions of iNOS and COX-2 were confirmed by measuring the products of iNOS and COX-2, that is, nitric oxide (NO) and PGE2, respectively. As shown in Fig. 3C, Rg3 significantly and concentration-dependently inhibited LPS-induced PGE2 production (Fig. 3C), and as shown in Fig. 3D, Rg3 markedly inhibited NO production in a concentration-dependent manner from a concentration of 1 μM.
Fig. 3.
Effect of ginsenoside Rg3 on the protein expressions of COX-2 and iNOS and on NO and PGE2 production in macrophages. Mouse peritoneal macrophages were treated with the indicated concentrations of ginsenoside Rg3 for 1 h, and then treated with LPS 100 ng/mL for 24 h. Western blotting was conducted on cell lysates. (A) The data shown are representative of three independent experiments. (B) Relative protein levels of each protein versus β-actin are presented as histograms. (C) LPS-induced production of PGE2. (D) LPS-induced production of nitrite. Ginsenoside Rg3 (C) concentration-dependently inhibited PGE2 production and (D) showed a tendency to inhibit nitrite production. Results are the means ± SEs of three independent experiments. Statistically significant at *p < 0.05, **p < 0.01, and ***p < 0.001 levels versus the LPS-treated macrophages. COX-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; NO, nitric oxide; PGE2, prostaglandin E2; SE, standard error; TNF, tumor necrosis factor.
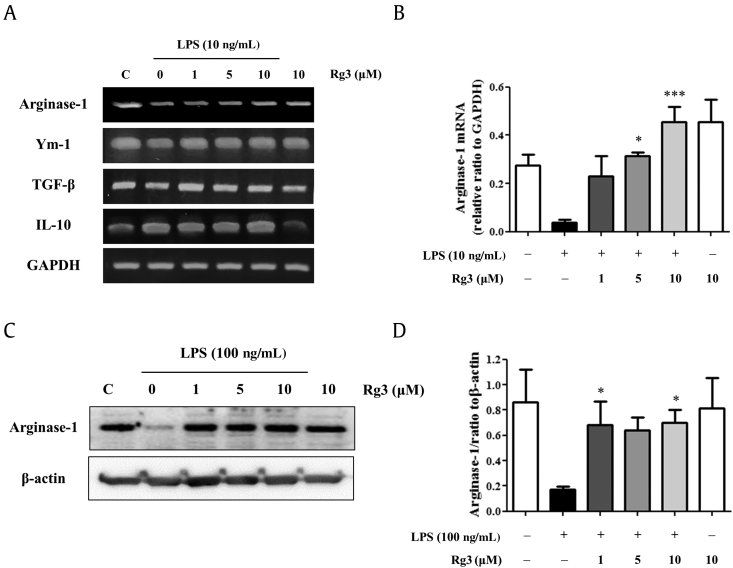
3.3. Effects of ginsenoside Rg3 on M2 polarization
After stimulating macrophages with LPS, the mRNA expression of arginase-1 (a representative M2 marker protein) disappeared, but its expression was retained by pretreating Rg3 (Fig. 4A). By contrast, the mRNA expressions of other M2 markers were not much changed by 10 ng/mL LPS (Fig. 4A and B). The protective effect of ginsenoside Rg3 on arginase-1 expression was also confirmed at the protein level (Fig. 4C and D). Therefore, ginsenoside Rg3 not only inhibited LPS-induced M1 polarization, but also protected the M2 polarization of macrophages in vitro.
Fig. 4.
Effect of ginsenoside Rg3 on the expressions of M2 marker genes in macrophages. Mouse peritoneal macrophages were treated with the indicated concentrations of ginsenoside Rg3 for 1 h, and then treated with vehicle or LPS 10 ng/mL for 24 h. RT-PCR was performed for the M2 marker genes, arginase-1, IL-10, TGF-β, and Ym-1. (A) The results shown are representative of three independent experiments. (B) Relative mRNA levels of each gene versus GAPDH are shown as histograms. Mouse peritoneal macrophages were treated with the indicated concentrations of ginsenoside Rg3 for 1 h, and then treated with LPS 100 ng/mL for 24 h. Western blotting was conducted on cell lysates. (C) The results shown are representative of three independent experiments. (D) Relative protein levels of each protein versus β-actin are presented as histograms. The values shown are means ± SEs (n = 3). Statistically significant at *p < 0.05 and ***p < 0.001 levels versus LPS-treated macrophages. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IL-10, interleukin 10; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; RT-PCR, reverse transcription-polymerase chain reaction; SE, standard errors; TGF, transforming growth factor.
3.4. Ginsenoside Rg3 accelerated the disappearance of polymorphonuclear lymphocytes in peritoneal cavities
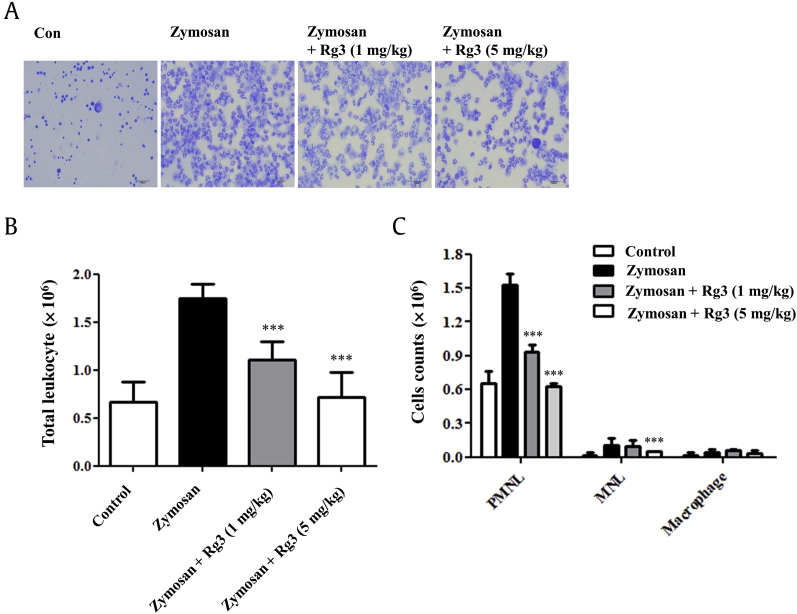
The inhibitory effect of Rg3 on LPS-induced M1 polarization and its protection of M2 polarization of macrophages in vitro were also examined in a zymosan-induced mouse peritonitis model. Zymosan is a glucan found on the surfaces of fungi, such as yeast, and its injection into the peritoneal cavity induces inflammatory response, that is, the migration of immune cells (e.g., neutrophils) into the peritoneal cavity. In a preliminary experiment, we found that peak inflammatory response was observed 12 h after zymosan stimulation, and therefore, Rg3 (1 mg/kg or 5 mg/kg) or vehicle was administered i.p. at this time. Peritoneal cells were stained and counted 24 h after zymosan administration. Cell numbers and cell population distributions were analyzed. As shown in Fig. 5A and B, total cell numbers increased by 161% in the zymosan-induced peritonitis group versus phosphate buffered saline-treated controls (Fig. 5A), and this increase was inhibited by 1 mg/kg or 5 mg/kg of Rg3 by 60% and 93%, respectively (Fig. 5A).
Fig. 5.
Effect of ginsenoside Rg3 on zymosan-induced peritonitis in mice. C57BL/6 mice were treated with zymosan (30 mg/kg, i.p.), and 12 h later treated with vehicle (n = 5) or ginsenoside Rg3 (1 mg/kg or 5 mg/kg; n = 5) by intraperitoneal injection. After a further 12 h, mouse peritoneal cells were collected, May–Grünwald and Giemsa stained, and counted. (A) Representative photos of peritoneal cells. (B) Total cell counts in peritoneal fluids of mice with zymosan-induced peritonitis (zymosan), and ginsenoside Rg3 (1 mg/kg or 5 mg/kg) treated mice with zymosan-induced peritonitis (zymosan + ginsenoside Rg3). (C) Cells counts of polymorph nuclear lymphocytes (PMNLs), mononuclear lymphocytes (MNLs), and macrophages in the peritoneal cavity fluids of zymosan or zymosan+ ginsenoside Rg3 (1 mg/kg or 5 mg/kg) treated mice. The values shown are means ± SEs (n = 5). Statistically significant at ***p < 0.001 level versus mice in the zymosan-treated group. SE, standard error; i.p., intraperitoneally.
Polymorph nuclear lymphocytes (PMNLs; neutrophils, eosinophils, and basophils), mononuclear lymphocytes (MNLs; T cells and B cells), and macrophages can be distinguished by their shapes and by May–Grünwald and Giemsa staining (Fig. 5B). PMNLs were found to be the main population during early peritonitis, and their disappearance was accelerated by Rg3 (1 mg/kg or 5 mg/kg; Fig. 5B). The disappearance of MNLs from peritoneal cavities was also significantly accelerated by Rg3 at 5 mg/kg (Fig. 5B).
4. Discussion
In the present study, ginsenoside Rg3 was shown to suppress the LPS-induced expressions of the M1 marker gene, iNOS, and the production of NO. In addition, LPS-induced COX-2 expression and PGE2 production were also marginally suppressed by ginsenoside Rg3. Previously, the anti-inflammatory effect of ginsenoside Rg3 has been demonstrated by its suppressions of iNOS expression and PGE2 production, that is, ginsenoside Rg3 suppressed LPS-induced iNOS expression and NO, reactive oxygen species (ROS), and PGE2 production in RAW264.7 macrophages in a concentration-dependent manner [4], [20]. Also, topically administered ginsenoside Rg3 pretreatment of the dorsal skins of female ICR mice significantly inhibited 12-O-tetradecanoylphorbol-13-acetate-induced ornithine decarboxylase activity [21]. Furthermore, in Aβ42-treated BV-2 microglial cells, ginsenoside Rg3 reduced inflammatory cytokine expression by inhibiting NF-κB p65 binding to its DNA consensus sequences [22]. In an LPS-induced mouse model of acute lung injury, ginsenoside Rg3 inhibited increases in the levels of proinflammatory cytokines, including those of TNF-α, IL-1β, and IL-6, inhibited increases of neutrophils in bronchoalveolar lavage fluid, and protected lungs from histopathological changes [23]. These effects were supposed to be mediated by the inhibition of NF-κB p65 phosphorylation and downstream COX-2 expression by ginsenoside Rg3 [23]. However, the induction of M2 polarization and acceleration of the resolution process have not been previously proposed as action mechanisms of ginsenoside Rg3.
The present study shows for the first time that ginsenoside Rg3 protects the expression of arginase-1 (the M2 marker gene) in LPS-treated mouse peritoneal macrophages in vitro. In addition, this protective effect on M2 polarization was confirmed in vivo by the accelerated disappearance of neutrophils after peak inflammatory response in the zymosan-induced peritonitis model. Furthermore, because in the in vivo experiment ginsenoside Rg3 was administered after peak inflammatory response had been reached, the observed accelerated disappearance demonstrated a proresolving effect, which differs from its anti-inflammatory effects in zymosan or LPS in vivo models. We do not know how ginsenoside Rg3 accelerates the resolution process, although it is possible that the productions of proresolving mediators, such as lipoxins, resolvins, and protectins, by ginsenoside Rg3 are responsible [11], [12]. Because M2 polarized macrophages have been reported to play key roles in the resolution phase of inflammation [13], [14], ginsenoside Rg3-induced M2 polarization may contribute to inflammation resolution. Further studies are required to determine the nature of the of action mechanisms involved. We hope our findings regarding the effects of ginsenoside Rg3 on macrophage polarization and on the resolution of inflammation can provide a means of devising proresolving drugs based on ginseng pharmacology.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This research was supported by the Korean National Research Foundation funded by the Korean government (MSIP) (Grant no. 2009-0083538) and by Korea–Japan Basic Scientific Cooperation Program for FY 2015 (NRF-2015K2A2A4000081).
References
- 1.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 2.Im D.S., Nah S.Y. Yin and Yang of ginseng pharmacology: ginsenosides vs gintonin. Acta Pharmacol Sin. 2013;34:1367–1373. doi: 10.1038/aps.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nah S.Y., Kim D.H., Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007;13:381–404. doi: 10.1111/j.1527-3458.2007.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin Y.M., Jung H.J., Choi W.Y., Lim C.J. Antioxidative, anti-inflammatory, and matrix metalloproteinase inhibitory activities of 20(S)-ginsenoside Rg3 in cultured mammalian cell lines. Mol Biol Rep. 2013;40:269–279. doi: 10.1007/s11033-012-2058-1. [DOI] [PubMed] [Google Scholar]
- 5.Jovanovski E., Bateman E.A., Bhardwaj J., Fairgrieve C., Mucalo I., Jenkins A.L., Vuksan V. Effect of Rg3-enriched Korean red ginseng (Panax ginseng) on arterial stiffness and blood pressure in healthy individuals: a randomized controlled trial. J Am Soc Hypertens. 2014;8:537–541. doi: 10.1016/j.jash.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Qiu X.M., Bai X., Jiang H.F., He P., Wang J.H. 20-(S)-ginsenoside Rg3 induces apoptotic cell death in human leukemic U937 and HL-60 cells through PI3K/Akt pathways. Anticancer Drugs. 2014;25:1072–1080. doi: 10.1097/CAD.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 7.Park D., Bae D.K., Jeon J.H., Lee J., Oh N., Yang G., Yang Y.H., Kim T.K., Song J., Sun H.L. Immunopotentiation and antitumor effects of a ginsenoside Rg(3)-fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ Toxicol Pharmacol. 2011;31:397–405. doi: 10.1016/j.etap.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Choi S.Y., Cho C.W., Lee Y., Kim S.S., Lee S.H., Kim K.T. Comparison of ginsenoside and phenolic ingredient contents in hydroponically-cultivated ginseng leaves, fruits, and roots. J Ginseng Res. 2012;36:425–429. doi: 10.5142/jgr.2012.36.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang J.T., Lee M.S., Kim H.J., Sung M.J., Kim H.Y., Kim M.S., Kwon D.Y. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-gamma signal pathways. Phytother Res. 2009;23:262–266. doi: 10.1002/ptr.2606. [DOI] [PubMed] [Google Scholar]
- 10.Kim H.S., Lee E.H., Ko S.R., Choi K.J., Park J.H., Im D.S. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Arch Pharm Res. 2004;27:429–435. doi: 10.1007/BF02980085. [DOI] [PubMed] [Google Scholar]
- 11.Serhan C.N., Krishnamoorthy S., Recchiuti A., Chiang N. Novel anti-inflammatory–pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan C.N., Chiang N., Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramon S., Dalli J., Sanger J.M., Winkler J.W., Aursnes M., Tungen J.E., Hansen T.V., Serhan C.N. The protectin PCTR1 is produced by human M2 macrophages and enhances resolution of infectious inflammation. Am J Pathol. 2016;186:962–973. doi: 10.1016/j.ajpath.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ariel A., Serhan C.N. New lives given by cell death: macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front Immunol. 2012;3:4. doi: 10.3389/fimmu.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S.J., Lee K.P., Kang S., Lee J., Sato K., Chung H.Y., Okajima H., Im D.S. Sphingosine 1-phosphate induced anti-atherogenic and atheroprotective M2 macrophage polarization through IL-4. Cell Signal. 2014;26:2249–2258. doi: 10.1016/j.cellsig.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Ryu Y.K., Lee J.W., Moon E.Y. Thymosin beta-4, actin-sequestering protein regulates vascular endothelial growth factor expression via hypoxia-inducible nitric oxide production in HeLa cervical cancer cells. Biomol Ther (Seoul) 2015;23:19–25. doi: 10.4062/biomolther.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J.W., Kim N.H., Kim J.Y., Park J.H., Shin S.Y., Kwon Y.S., Lee H.J., Kim S.S., Chun W. Aromadendrin inhibits lipopolysaccharide-induced nuclear translocation of NF-κB and phosphorylation of JNK in RAW 264.7 macrophage cells. Biomol Ther. 2013;21:216–221. doi: 10.4062/biomolther.2013.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohn Y.A., Hwang S.A., Lee S.Y., Hwang I.Y., Kim S.W., Kim S.Y., Moon A., Lee Y.S., Kim Y.H., Kang K.J. Protective effect of liriodendrin isolated from Kalopanax pictus against gastric injury. Biomol Ther (Seoul) 2015;23:53–59. doi: 10.4062/biomolther.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H.N., Kundu J.K., Cha Y.N., Surh Y.J. Resolvin D1 stimulates efferocytosis through p50/p50-mediated suppression of tumor necrosis factor-alpha expression. J Cell Sci. 2013;126:4037–4047. doi: 10.1242/jcs.131003. [DOI] [PubMed] [Google Scholar]
- 20.Yoon S.J., Park J.Y., Choi S., Lee J.B., Jung H., Kim T.D., Yoon S.R., Choi I., Shim S., Park Y.J. Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochem Biophys Res Commun. 2015;463:1184–1189. doi: 10.1016/j.bbrc.2015.06.080. [DOI] [PubMed] [Google Scholar]
- 21.Keum Y.S., Han S.S., Chun K.S., Park K.K., Park J.H., Lee S.K., Surh Y.J. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB activation and tumor promotion. Mutat Res. 2003;523–4:75–85. doi: 10.1016/s0027-5107(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 22.Joo S.S., Yoo Y.M., Ahn B.W., Nam S.Y., Kim Y.B., Hwang K.W., Lee D.I. Prevention of inflammation-mediated neurotoxicity by Rg3 and its role in microglial activation. Biol Pharm Bull. 2008;31:1392–1396. doi: 10.1248/bpb.31.1392. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Z., Li L. Ginsenoside Rg3 ameliorates lipopolysaccharide-induced acute lung injury in mice through inactivating the nuclear factor-kappaB (NF-kappaB) signaling pathway. Int Immunopharmacol. 2016;34:53–59. doi: 10.1016/j.intimp.2016.02.011. [DOI] [PubMed] [Google Scholar]