Abstract
Background
Black ginseng (BG) has greatly enhanced pharmacological activities relative to white or red ginseng. However, the effect and molecular mechanism of BG on muscle growth has not yet been examined. In this study, we investigated whether BG could regulate myoblast differentiation and myotube hypertrophy.
Methods
BG-treated C2C12 myoblasts were differentiated, followed by immunoblotting for myogenic regulators, immunostaining for a muscle marker, myosin heavy chain or immunoprecipitation analysis for myogenic transcription factors.
Results
BG treatment of C2C12 cells resulted in the activation of Akt, thereby enhancing heterodimerization of MyoD and E proteins, which in turn promoted muscle-specific gene expression and myoblast differentiation. BG-treated myoblasts formed larger multinucleated myotubes with increased diameter and thickness, accompanied by enhanced Akt/mTOR/p70S6K activation. Furthermore, the BG treatment of human rhabdomyosarcoma cells restored myogenic differentiation.
Conclusion
BG enhances myoblast differentiation and myotube hypertrophy by activating Akt/mTOR/p70S6k axis. Thus, our study demonstrates that BG has promising potential to treat or prevent muscle loss related to aging or other pathological conditions, such as diabetes.
Keywords: Akt signaling, black ginseng, myoblast differentiation, myogenic conversion, myotube hypertrophy
1. Introduction
Black ginseng (BG) was prepared through the iterative process of steaming and drying nine times [1]. During the steaming process, ginsenosides of white or red ginseng are converted into less polar ginsenosides, and 19 ginsenosides were newly discovered in BG [1]. It has been reported that BG has greatly elevated the pharmacological activities compared with white or red ginseng [2]. Other reports have suggested the beneficial effect of BG on cancer, inflammatory, and oxidant effects [3], [4], [5]. The extract of BG has an anti-hyperglycemia effect via modulation of glucose metabolism in the liver and muscle [6]. However, the detailed mechanism of BG on myogenesis and muscular hypertrophy has not been examined.
Muscle differentiation is a tightly regulated process in which proliferative myoblasts exit the cell cycle and fuse together to generate mature multinucleated myofibers. Myogenic regulatory factors such as MyoD and Mef2 coordinate the process of myogenic specification and differentiation [7]. These factors influence myogenesis during skeletal muscle regeneration after injury in which quiescent muscle stem cells re-enter the process of cell cycle, proliferate, and fuse to repair the injured skeletal muscle [8]. Recent research is inclined toward discovery of natural compounds that have the potential to improve myogenesis and muscle regeneration. Our study demonstrates the beneficial effect of BG on myoblast differentiation and myotube hypertrophy. Thus, BG has great potential as a therapeutic agent for treating muscular atrophy related to aging or other chronic diseases, including diabetes and cancer.
2. Materials and methods
2.1. Cell cultures
C2C12 cells from mouse myoblasts were cultured as previously described [9]. C2C12 cells were maintained in Dulbecco's Modified Eagle's medium (DMEM; Gibco-BRL, Grand Island, NY, USA) with 15% fetal bovine serum (FBS; Gibco-BRL, Grand Island, NY, USA). For induction of myogenic differentiation, C2C12 cells were exchanged into DMEM with 2% horse serum (differentiation medium; Gibco-BRL) after reaching near confluence. The efficiency of the myoblast differentiation was quantified as previously reported [10]. For induction of hypertrophic growth, C2C12 cells were differentiated for 2 d and then treated with BG for an additional 2 d in differentiation medium [11]. For this study that involved the pharmacological inhibitor, C2C12 cells were pre-incubated with 2.5μM LY294003 (CalBiochem, La Jolla, CA, USA) for 30 min and then treated with BG for 2 d. Additional cell lines used in this study included human rhabdomyosarcoma (RMS) and 293T cultured in DMEM containing 10% FBS.
2.2. Immunoblotting and immunoprecipitation analysis
Immunoblotting analysis was performed as described previously [12], [13]. Briefly, cells were extracted in cell extraction buffer (pH 7.2, 1mM EDTA, 10mM Tris-HCl, 150mM NaCl, 1% Triton X-100) containing complete proteinase inhibitor cocktail (Roche Diagnostics, Basel, Switzerland). Cell lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis. The primary antibodies used were MHC (Developmental Studies Hybridoma Bank, Iowa, IA, USA), Akt, mammalian target of rapamycin (mTOR), 4E-BP1, p70S6K, the phosphorylated form of Akt (p-Akt), mTOR (p-mTOR), 4E-BP1 (p-4E-BP1), p70S6K (p-p70S6K) (Cell Signaling Technology, Beverly, MA, USA), pan-Cadherin (Sigma-Aldrich, St. Louis, MO, USA), MyoD, myogenin, E2A, and α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
For immunoprecipitation (IP) assay, cultures were treated with BG and lysed in IP lysis buffer, followed by IP with anti-E2A antibodies overnight at 4°C. The immunoprecipitants were mixed with protein G agarose beads (Roche Diagnostics, Basel, Switzerland) and analyzed by immunoblotting with antibodies against MyoD and E2A.
2.3. Immunocytochemistry
MHC immunostaining was performed as mentioned previously [10], [12]. Briefly, cultures were fixed, permeabilized, blocked, and incubated with MHC antibodies, followed by an Alexa Fluor 568-conjugated secondary antibody (Molecular Probes, Eugene, OR, USA). Images were obtained with a Zeiss LSM-510 Meta confocal microscope and processed using the ZEN-2 software (Carl Zeiss AG, Oberkochen, Germany).
2.4. HPLC analysis
The profile of ginsenosides in BG was determined by HPLC–evaporative light scattering detector method as previously mentioned [4], [14]. For detailed methods, see supplementary methods.
2.5. Cell viability assay
MTT assay was used to examine cell viability. For detailed methods, see supplementary methods.
2.6. Statistics
The experiments were performed three times independently. The participants' t test was used to assess the significance of the difference between two mean values. The p values < 0.01 and < 0.05 were considered to be statistically significant.
3. Results and discussion
3.1. BG enhances myogenic differentiation
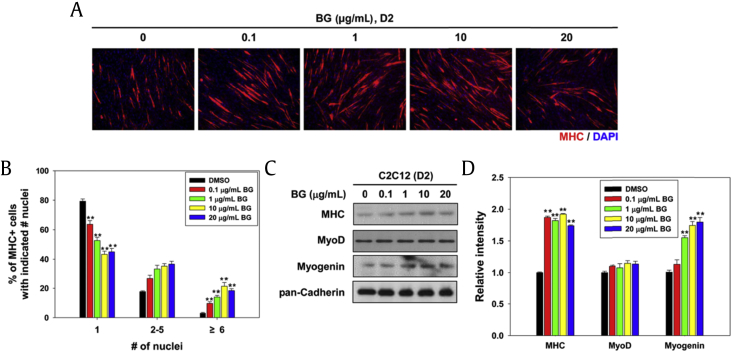
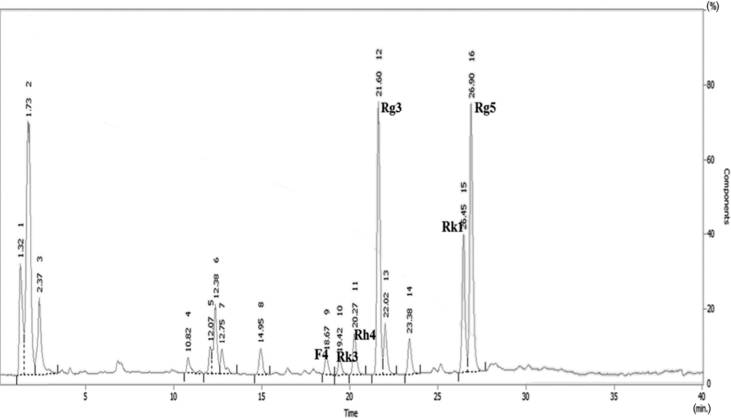
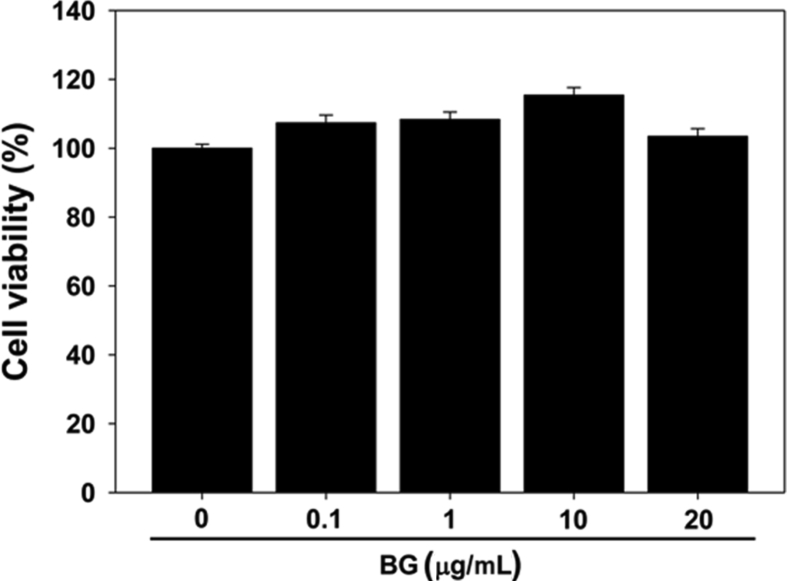
The ginsenoside profile of BG was found to contain Rg3, Rg5, Rk1, and Rh4 using HPLC analysis (Fig. S1). BG is enriched with the bioactive chemical constituents by the process of heating and drying, and the chemical composition of BG is different from that of white and red ginseng [4], [14]. In order to characterize the effect of BG on myogenic differentiation, C2C12 cells were differentiated in differentiation medium with varying concentrations of BG for 2 d, and the myotube formation was assessed by immunostaining. BG treatment enhanced the formation of MHC-positive multinucleated myotube in a dose-dependent manner (Fig. 1A). For quantification of the myotube formation, MHC-positive myotubes were counted and plotted as percentile (Fig. 1B). Treatment with BG significantly increased the ratio of larger myotubes with six or more nuclei per myotube, while mononucleate myocytes decreased in a concentration-dependent manner (Fig. 1B). Furthermore, Figs. 1C and 1D revealed that the expression of MHC and myogenin was gradually increased in BG-treated C2C12 cells compared with that in dimethyl sulfoxide (DMSO)-treated cells. BG had no cytotoxic effect on the growth of C2C12 cells, as assessed by MTT assay (Fig. S2). These results indicate that BG enhances myoblast differentiation at a morphological as well as at a biochemical level.
Fig. 1.
BG promotes myogenesis in C2C12 myoblasts. (A) BG-treated C2C12 cells were differentiated in differentiation medium for 2 d and analyzed by MHC immunostaining. DAPI was used to visualize nuclei. (B) The MHC-positive myocytes shown in panel A were quantified as a number of nuclei per myotube. Values are represented as means ± SD of three independent experiments. **p < 0.01. (C) MHC, MyoD, and myogenin were analyzed by immunoblotting using prepared cell lysates from panel A. (D) The signal intensity of indicated muscle-specific proteins was quantified in three independent experiments and normalized to pan-Cadherin. Values are represented as means ± SD. **p < 0.01. BG, black ginseng; DAPI, 4′,6-diamidino-2-phenylindole stain; MHC, major histocompatibility complex; SD, standard deviation.
3.2. BG activates Akt signaling and promotes heterodimerization of MyoD and E proteins
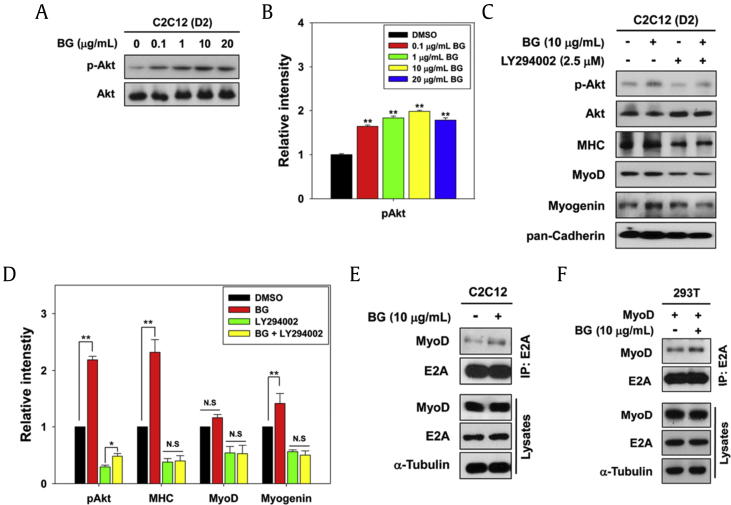
Akt signaling plays a major role in myogenic differentiation and acts as a key promyogenic kinase [12], [13], [15]. To examine the mechanism of BG in the promotion of myoblast differentiation, C2C12 cells were treated with either control DMSO or BG in the differentiation medium for 2 d and the activation status of Akt was examined with an antibody recognizing the active phosphorylated form of Akt (p-Akt). BG treatment elevated the level of p-Akt dose-dependently without altering the total Akt level (Figs. 2A and 2B). To investigate whether the BG effect on myoblast differentiation requires Akt activation, C2C12 cells were pre-incubated with LY294002 for 30 min and then treated with 10 μg/mL of BG for 2 d in differentiation medium, followed by Western blot analysis. Inhibition of Akt decreased the expression of MHC, MyoD, and myogenin compared with the control cells (Figs. 2C and 2D). Furthermore, BG treatment partially restored the phosphorylation level of Akt in LY294002-treated cells; however, it failed to rescue the expression of muscle-specific proteins.
Fig. 2.
BG activates Akt signaling, thereby promoting the heterodimerization of MyoD and E proteins. (A) BG-treated C2C12 cells were differentiated for 2 d. The phosphorylated and total forms of Akt of cell lysates were analyzed by immunoblotting. (B) Quantification of three experiments was independently performed as shown in panel A. The signal intensity of phosphorylated Akt was calculated and normalized to total Akt. Values are presented as means ± SD. **p < 0.01. (C) C2C12 cells were pre-incubated with 2.5μM LY294002 for 30 min and treated with 10 μg/mL BG. These cells were differentiated in differentiation medium for 2 d. Immunoblotting analysis was performed using cell lysates. (D) Quantification of three experiments was independently performed as shown in panel C. The relative intensity of phosphorylated Akt and muscle-specific proteins was calculated and normalized to total Akt and pan-Cadherin, respectively. Values are represented as means ± SD. *p < 0.05, **p < 0.01. (E) C2C12 and (F) MyoD-transfected 293T cells were treated with BG for 2 d and followed by immunoprecipitation with anti-E2A antibodies and immunoblotting using anti-MyoD antibodies. Cell lysates are used as an input control for each protein. BG, black ginseng; DAPI, 4′,6-diamidino-2-phenylindole stain; DMSO, dimethyl sulfoxide; MHC, major histocompatibility complex; N.S., not significant; p-Akt, phosphorylated Akt; SD, standard deviation.
One key step for myoblast differentiation is through MyoD activation which in turn induces myogenin and MHC expression, and MyoD activity is regulated at multiple levels, including the heterodimer formation with E protein [16], [17], [18]. Therefore, we examined whether BG modulates the heterodimerization of MyoD with E protein. C2C12 cells were treated with either DMSO or 10 μg/mL of BG for 2 d, and lysates were analyzed by co-IP with an antibody against E2A, by immunoblotting with MyoD antibodies. BG treatment enhanced the amount of MyoD in the precipitates with E2A antibodies as compared with the control treatment (Fig. 2E). However, MyoD and E2A protein levels were constant in total lysates, regardless of the BG treatment (Fig. 2E). To further confirm, 293T cells expressing MyoD were treated with 10 μg/mL of BG for 2 d, followed by co-IP analysis. Consistent with the endogenous interaction, exogenous MyoD proteins strongly interacted with E2A proteins in the BG-treated cells (Fig. 2F). In order to regulate the target genes expression, the recruitment of MyoD-associated proteins such as BAF60C to the promoter region of muscle-specific genes is required [19]. Promyogenic signalings, p38MAPK, and Akt are critical for sustained recruitment of MyoD protein complex [20], [21]. One of the key steps for early myogenic differentiation is the heterodimer formation of MyoD with E2A gene products, leading to the recruitment of MyoD to the E box of target genes and eventually induction of gene transcription [20]. Our data suggest that BG augments MyoD-dependent myogenic transcription through Akt activation.
3.3. BG enhances myotube hypertrophy through activation of Akt and its downstream signalings
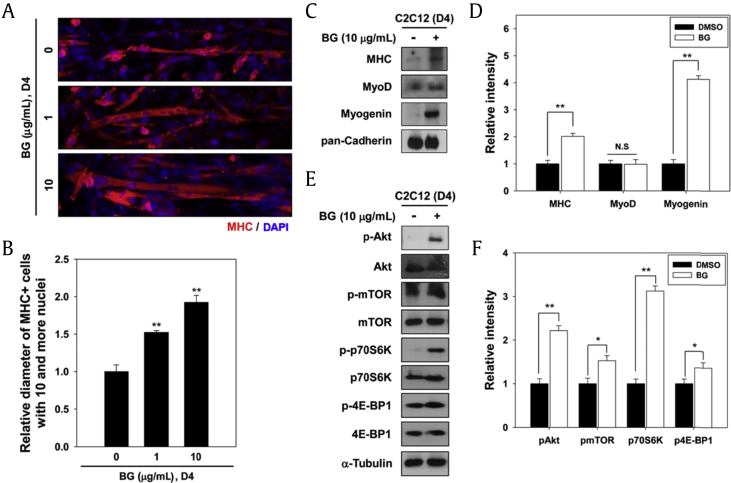
Skeletal muscle atrophy and hypertrophy contribute to modulation of the diameter of pre-existing muscular fibers [11]. Akt signaling promotes protein synthesis pathway leading to muscle hypertrophy mediated by its downstream signaling pathways, such as mTOR/p70S6K [22], [23]. To determine the effect of BG on muscle growth, C2C12 cells were differentiated for 2 d and treated with BG or control DMSO for additional 2 d, followed by immunostaining to assess the myotube diameter. BG treatment enhanced myotube growth (Fig. 3A), and myotubes were thicker with diameter about 1.52- or 1.92-fold when treated with BG (1μg/mL or 10 μg/mL, respectively) compared with control myotubes (Fig. 3B). To further explore the effect of BG on hypertrophic signaling pathway, C2C12 cells were cultured under the same experimental conditions as mentioned above, and subjected to immunoblotting analysis. Similarly to early differentiation, myotubes treated with BG displayed higher expression levels of MHC, MyoD, and myogenin than the DMSO-treated myotubes (Figs. 3C and 3D). Additionally, BG treatment elevated the activation of Akt and its downstream signaling events, as evident by greatly enhanced levels of the active phosphorylated forms of mTOR and p70S6K as well as 4E-BP1 phosphorylation compared with vehicle-treated cells (Figs. 3E and 3F). Total protein levels of mTOR, p70S6K, and 4E-BP1 remained constant regardless of the BG treatment. The protein synthesis pathway controlled by Akt/mTOR/p70S6K pathway plays a critical role in muscle growth, and defects in this signaling pathway have been observed in various conditions associated with muscle atrophy [22], [24]. Akt/mTOR signaling pathway has been involved in muscle regeneration and hypertrophy by counteracting atrophy-related signaling [21]. The activation of Akt and its downstream signaling pathway not only prevent muscle loss but also induce muscle growth [11], [22]. Thus, Akt/mTOR signaling has been investigated for its potential clinical benefit to prevent age-related muscular atrophy [11]. Our current data demonstrate that BG elicits the activation of Akt/mTOR/p70S6K signaling to induce myotube hypertrophy, indicating that BG has a great potential as a therapeutic agent to treat atrophy.
Fig. 3.
BG promotes Akt-dependent myotube hypertrophy. (A) C2C12 myoblasts were differentiated in differentiation medium for 2 d and then treated with 10 μg/mL of BG for additional 2 d. The myotube formation was analyzed by MHC immunostaining. DAPI was used to visualize nuclei. (B) Average myotube diameter shown in panel A was measured using the ZEN-2 software. Data are presented as means determination of six fields ± 1 SD. **p < 0.01. (C) Muscle-specific proteins from panel A were analyzed by immunoblotting. (D) Quantification of three experiments was independently performed as shown in panel C. The relative intensity of muscle-specific proteins such as MHC, MyoD and Myogenin was calculated and normalized to pan-Cadherin. Values are represented as means ± SD. **p < 0.01. (E) Total and phosphorylated forms of Akt, mTOR, p70S6K, and 4E-BP1 from panel A were analyzed by immunoblotting. α-Tubulin was used as loading control. (F) Quantification of three experiments was independently performed as shown in panel E. The relative intensity of phosphorylated Akt, mTOR, p79S6K, and 4E-BP1 was calculated and normalized to total Akt, mTOR, p79S6K, and 4E-BP1, respectively. Values are represented as means ± SD. *p < 0.05, **p < 0.01. BG, black ginseng; DAPI, 4′,6-diamidino-2-phenylindole stain; DMSO, dimethyl sulfoxide; IP, immunoprecipitation; MHC, major histocompatibility complex; mTOR, mammalian target of rapamycin; N.S., not significant; p-Akt, phosphorylated Akt; p-mTOR, phosphorylated mTOR; SD, standard deviation.
3.4. RMS cells are converted into myoblasts by treatment of BG
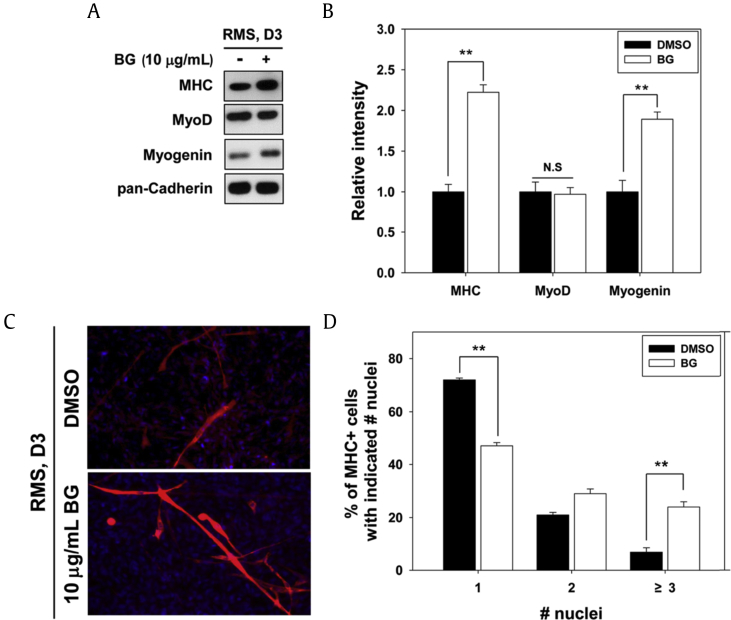
RMS is a malignant cancer that arises from childhood soft tissue sarcoma and has the characteristics of fetal myoblasts with impaired myogenic differentiation [25]. We tested the effect of BG on the impaired differentiation capacity of human RMS cells. RMS cells at near confluence were treated with 10 μg/mL BG for 3 d and subjected to immunoblotting for the expression of muscle-specific proteins. BG treatment dramatically increased the expression of muscle-specific proteins, such as MHC and myogenin, compared with the DMSO treatment, whereas the expression of MyoD remained unchanged by BG treatment (Figs. 4A and 4B). Furthermore, BG treatment also improved MHC-positive myotube formation and enhanced the ratio of larger myotubes relative to DMSO-treated RMS cells (Fig. 4C). BG treatment increased in the ratio of myotubes containing three or more nuclei, whereas it decreased in the portion of mononucleate myocytes (Fig. 4D). These data suggest that BG restores myogenic differentiation of human RMS cells.
Fig. 4.
BG converts RMS cells into myoblasts. (A) BG-treated RMS cells were differentiated for 3 d. Using prepared cell lysates, MHC, MyoD, and myogenin were analyzed by immunoblotting. (B) The signal intensity of indicated muscle-specific proteins was quantified in three independent experiments and normalized to pan-Cadherin. Values are presented as means ± SD. **p < 0.01. (C) C2C12 cells from panel A were analyzed by MHC immunostaining to reveal myotube formation. DAPI was used to visualize nuclei. (D) The MHC-positive myocytes shown in panel C were quantified as the number of nuclei per myotube. Values are presented as means ± SD of three independent experiments. **p < 0.01. BG, black ginseng; DAPI, 4′,6-diamidino-2-phenylindole stain; DMSO, dimethyl sulfoxide; MHC, major histocompatibility complex; N.S., not significant; RMS, rhabdomyosarcoma; SD, standard deviation.
4. Conclusion
Our study provides a mechanistic framework for understanding how BG enhances myoblast differentiation and muscular hypertrophy by activating Akt/mTOR/p70S6K signaling. Furthermore, BG ameliorates the differentiation capacity of RMS cells, suggesting that BG is a promising candidate to treat or prevent muscle atrophy associated with aging or other chronic diseases.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2011-0030074, 2015R1A2A1A15056117 and 2016R1A2B4014868).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jgr.2017.08.009.
Contributor Information
Sang-Jin Lee, Email: lee.sangjin74@sookmyung.ac.kr.
Gyu-Un Bae, Email: gbae@sookmyung.ac.kr.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig. S1.
Ginsenoside profile of BG containing Rg3, Rg5, Rk1, and Rh4 using HPLC analysis. BG, black ginseng.
Fig. S2.
Effects of BG on the growth of C2C12 cells as assessed by MTT assay. BG, black ginseng.2
References
- 1.Sun B.S., Gu L.J., Fang Z.M., Wang C.Y., Wang Z., Lee M.R., Li Z., Li J.J., Sung C.K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J Pharm Biomed Anal. 2009;50:15–22. doi: 10.1016/j.jpba.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Jin Y., Piao J., Lee S.M. Evaluating the validity of the Braden scale using longitudinal electronic medical records. Res Nurs Health. 2015;38:152–161. doi: 10.1002/nur.21642. [DOI] [PubMed] [Google Scholar]
- 3.Liu L., Zhu X.M., Wang Q.J., Zhang D.L., Fang Z.M., Wang C.Y., Wang Z., Sun B.S., Wu H., Sung C.K. Enzymatic preparation of 20(S, R)-protopanaxadiol by transformation of 20(S, R)-Rg3 from black ginseng. Phytochemistry. 2010;71:1514–1520. doi: 10.1016/j.phytochem.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Jung K., An J.M., Eom D.W., Kang K.S., Kim S.N. Preventive effect of fermented black ginseng against cisplatin-induced nephrotoxicity in rats. J Ginseng Res. 2017;41:188–194. doi: 10.1016/j.jgr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu J.N., Liu Z., Wang Z., Li X.D., Zhang L.X., Li W., Wang Y.P. Ameliorative effects and possible molecular mechanism of action of black ginseng (Panax ginseng) on acetaminophen-mediated liver injury. Molecules. 2017;22 doi: 10.3390/molecules22040664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo Y.S., Shon M.Y., Kong R., Kang O.H., Zhou T., Kim D.Y., Kwon D.Y. Black ginseng extract exerts anti-hyperglycemic effect via modulation of glucose metabolism in liver and muscle. J Ethnopharmacol. 2016;190:231–240. doi: 10.1016/j.jep.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 7.Pownall M.E., Gustafsson M.K., Emerson C.P., Jr. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol. 2002;18:747–783. doi: 10.1146/annurev.cellbio.18.012502.105758. [DOI] [PubMed] [Google Scholar]
- 8.Charge S.B., Rudnicki M.A. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 9.Lee S.J., Yoo M., Go G.Y., Kim D.H., Choi H., Leem Y.E., Kim Y.K., Seo D.W., Ryu J.H., Kang J.S. Bakuchiol augments MyoD activation leading to enhanced myoblast differentiation. Chem Biol Interact. 2016;248:60–67. doi: 10.1016/j.cbi.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Bae G.U., Kim B.G., Lee H.J., Oh J.E., Lee S.J., Zhang W., Krauss R.S., Kang J.S. Cdo binds Abl to promote p38alpha/beta mitogen-activated protein kinase activity and myogenic differentiation. Mol Cell Biol. 2009;29:4130–4143. doi: 10.1128/MCB.00199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rommel C., Bodine S.C., Clarke B.A., Rossman R., Nunez L., Stitt T.N., Yancopoulos G.D., Glass D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 12.Bae G.U., Lee J.R., Kim B.G., Han J.W., Leem Y.E., Lee H.J., Ho S.M., Hahn M.J., Kang J.S. Cdo interacts with APPL1 and activates Akt in myoblast differentiation. Mol Biol Cell. 2010;21:2399–2411. doi: 10.1091/mbc.E09-12-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S.J., Hwang J., Jeong H.J., Yoo M., Go G.Y., Lee J.R., Leem Y.E., Park J.W., Seo D.W., Kim Y.K. PKN2 and Cdo interact to activate AKT and promote myoblast differentiation. Cell Death Dis. 2016;7:e2431. doi: 10.1038/cddis.2016.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bak M.J., Jeong W.S., Kim K.B. Detoxifying effect of fermented black ginseng on H2O2-induced oxidative stress in HepG2 cells. Int J Mol Med. 2014;34:1516–1522. doi: 10.3892/ijmm.2014.1972. [DOI] [PubMed] [Google Scholar]
- 15.Serra C., Palacios D., Mozzetta C., Forcales S.V., Morantte I., Ripani M., Jones D.R., Du K., Jhala U.S., Simone C. Functional interdependence at the chromatin level between the MKK6/p38 and IGF1/PI3K/AKT pathways during muscle differentiation. Mol Cell. 2007;28:200–213. doi: 10.1016/j.molcel.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milasincic D.J., Dhawan J., Farmer S.R. Anchorage-dependent control of muscle-specific gene expression in C2C12 mouse myoblasts. In Vitro Cell Dev Biol Anim. 1996;32:90–99. doi: 10.1007/BF02723040. [DOI] [PubMed] [Google Scholar]
- 17.Neuhold L.A., Wold B. HLH forced dimers: tethering MyoD to E47 generates a dominant positive myogenic factor insulated from negative regulation by Id. Cell. 1993;74:1033–1042. doi: 10.1016/0092-8674(93)90725-6. [DOI] [PubMed] [Google Scholar]
- 18.Lluis F., Ballestar E., Suelves M., Esteller M., Munoz-Canoves P. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 2005;24:974–984. doi: 10.1038/sj.emboj.7600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forcales S.V., Albini S., Giordani L., Malecova B., Cignolo L., Chernov A., Coutinho P., Saccone V., Consalvi S., Williams R. Signal-dependent incorporation of MyoD-BAF60c into Brg1-based SWI/SNF chromatin-remodelling complex. EMBO J. 2012;31:301–316. doi: 10.1038/emboj.2011.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tapscott S.J. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- 21.Wilson E.M., Rotwein P. Selective control of skeletal muscle differentiation by Akt1. J Biol Chem. 2007;282:5106–5110. doi: 10.1074/jbc.C600315200. [DOI] [PubMed] [Google Scholar]
- 22.Stitt T.N., Drujan D., Clarke B.A., Panaro F., Timofeyva Y., Kline W.O., Gonzalez M., Yancopoulos G.D., Glass D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 23.Sandri M., Barberi L., Bijlsma A.Y., Blaauw B., Dyar K.A., Milan G., Mammucari C., Meskers C.G., Pallafacchina G., Paoli A. Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology. 2013;14:303–323. doi: 10.1007/s10522-013-9432-9. [DOI] [PubMed] [Google Scholar]
- 24.Hauerslev S., Vissing J., Krag T.O. Muscle atrophy reversed by growth factor activation of satellite cells in a mouse muscle atrophy model. PLoS One. 2014;9:e100594. doi: 10.1371/journal.pone.0100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coda D.M., Lingua M.F., Morena D., Foglizzo V., Bersani F., Ala U., Ponzetto C., Taulli R. SMYD1 and G6PD modulation are critical events for miR-206-mediated differentiation of rhabdomyosarcoma. Cell Cycle. 2015;14:1389–1402. doi: 10.1080/15384101.2015.1005993. [DOI] [PMC free article] [PubMed] [Google Scholar]