Abstract
Background
The Panax ginseng plant is used as an herbal medicine. Phytosterols of P. ginseng have inhibitory effects on inflammation-related factors in HepG2 cells.
Methods
Phytosterols (e.g., stigmasterol and β-sitosterol) in the roots of P. ginseng grown under various conditions were analyzed using high-performance liquid chromatography. The P. ginseng roots analyzed in this study were collected from three cultivation areas in Korea (i.e., Geumsan, Yeongju, and Jinan) and differed by cultivation year (i.e., 4 years, 5 years, and 6 years) and production process (i.e., straight ginseng, red ginseng, and white ginseng).
Results
The concentrations of stigmasterol and β-sitosterol in P. ginseng roots were 2.22–23.04 mg/g and 7.35–59.09 mg/g, respectively. The highest concentrations of stigmasterol and β-sitosterol were in the roots of 6-year-old P. ginseng cultivated in Jinan (82.14 mg/g and 53.23 mg/g, respectively).
Conclusion
Six-year-old white ginseng and white ginseng cultivated in Jinan containing stigmasterol and β-sitosterol are potentially a new source of income in agriculture.
Keywords: Panax ginseng, quantitative analysis, root, sterol
1. Introduction
The Panax ginseng plant is a very widely used herbal medicine globally. It was first mentioned in East Asian folklore more than 4,000 years ago [1], [2]. Dried P. ginseng has diverse pharmacological benefits on the central nervous system and cardiovascular system and has immune-modulating functions. Panax ginseng enhances lymphocyte proliferation, stimulates macrophages in cytokine production, and improves the phagocytic activity of polymorphonuclear leukocytes, and is beneficial in the treatment of diabetes, inflammation in aging, oxidative damage, and cancer [1], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12].
The increasing demand for alternative health remedies has expanded the global nutraceutical market. Plant sterols (i.e., phytosterols) represent an important group of compounds in unsaponifiable plant oils that confer biological activities to the oils [13]. Phytosterols reduce serum low-density lipoprotein cholesterol levels by decreasing intestinal cholesterol absorption [14]. Therefore, phytosterol-enriched food products have been engineered and marketed to lower serum cholesterol and reduce cardiovascular risks [15], [16]. In addition to their cholesterol-lowering effect, phytosterols possess anti-inflammatory, antifungal, antiulcerative, antibacterial, and antitumor activities [17], [18], [19], [20]. Phytosterols are also valuable in the treatment of benign prostatic hyperplasia and colon cancer [21], [22].
To date, much research has focused on the analysis of saponins in P. ginseng, but only few studies have focused on phytosterols in P. ginseng. In this paper, we aimed to analyze the phytosterol content in the roots of P. ginseng grown under different conditions using high-performance liquid chromatography (HPLC).
2. Materials and methods
2.1. Plant materials
The dried and powdered roots of P. ginseng from different cultivation areas in Korea (i.e., Geumsan, Yeongju, and Jinan), cultivation years (i.e., 4 years, 5 years, and 6 years), and production processes (i.e., straight ginseng, red ginseng, and white ginseng) were obtained from Korea Food Research Institute in Sungnam, Korea. In brief, the cultivation conditions of ginseng in the experiment consisted of the expected annual average temperature of 4–10°C and average precipitation of 800–900 mm. Ginseng requires 80% shade to thrive and prefers a deep rich loamy soil. The roots can be harvested once in the fall after the leaves have died. White ginseng was produced by sun-drying fresh ginseng.
2.2. Instruments and reagents
For thin layer chromatography (TLC) analysis, a TLC precoated silica gel 60 F254 (No. 5715; Merck Co., Darmstadt, Germany) was used. We used silica gel (No. 7734; Merck Co.) as the stationary phase for repeated column chromatography. A medium-pressure liquid chromatography (MPLC) system (Biotage, Uppsala, Sweden) was used; it was equipped with KP-SIL cartridges (39 mm × 225 mm; Biotage). The HPLC analysis was conducted using the Waters 1525 Binary HPLC Pump (Waters, Miami, FL, USA), which was equipped with a UV/visible spectroscopy detector (model 2489; Waters, Miami, FL, USA). The water and acetonitrile were of HPLC grade, and all other reagents were of analytical grade.
2.3. Extraction, fractionation, and isolation of Compounds 1 and 2
The dried and powdered root of P. ginseng (7.0 kg) was extracted with ethanol (3 × 21 L) and heated to reflux. The extracts were combined and concentrated to a brown residue (139 g). The residue was dissolved in water (7 L) and partitioned successively with n-hexane, trichloromethane (CHCl3), ethyl acetate (EtOAc), and n-butanol (n-BuOH) to yield n-hexane-soluble (50 g), CHCl3-soluble (11 g), EtOAc-soluble (11 g), and n-BuOH-soluble (50 g) fractions. A portion of the CHCl3 fraction was separated by MPLC with a gradient system of n-hexane-EtOAc and EtOAc-methanol (EtOAc-MeOH). The flow rate was maintained at 20.0 mL/min. The peaks in MPLC were detected by UV absorbance at 254 nm. The CHCl3 fraction gained 38 subfractions. Compound 1 was isolated from subfraction 6 (n-hexane:EtOAc was 85:15). Compound 2 was isolated from subfraction 7 (n-hexane:EtOAc was 80:20) [23], [24].
2.4. The preparation of Compounds 1 and 2 and the samples for HPLC
To quantify the amounts of Compounds 1 and 2, we dissolved 3 mg of each compound in 300 μL of CHCl3. The dried and powdered roots of P. ginseng were extracted by refluxing using ethanol (300 mL × 3). The extracts were then combined and evaporated to obtain a brown residue. The brown residue (2 mg) was dissolved 1 mL MeOH. The resultant solutions were filtered through a 0.45-μm filter (Whatman Cat No. 6779-1304, NJ, USA), and used for HPLC analysis.
2.5. The HPLC conditions
The HPLC separation of Compounds 1 and 2 for qualitative and quantitative analyses was performed using a reverse phase system. A reverse phase column (SunFire C-18 stainless steel, 2.1 mm × 50 mm, 5 μm; Waters Corporation, Milford, MA, USA) was used with MeOH and acetonitrile. The gradient solvent system was initially 30:70, and was increased in a linear gradient to 30:70 for 20 minutes, 0:100 for 10 minutes, and finally 30:70 for 15 minutes. UV detection was conducted at 210 nm. The injection amount was 10 μL and the flow rate was 1.0 mL/min. All injections were performed three times.
2.6. Calibration
A stock solution (1 mg/mL) of Compounds 1 and 2 was prepared using CHCl3. The concentration of the analytes was determined, based on the corresponding calibration curves. The peak area (Y), concentration (X; mg/10 μL), and the mean concentration value (of three measurements) ± standard deviation were calculated.
3. Results and discussion
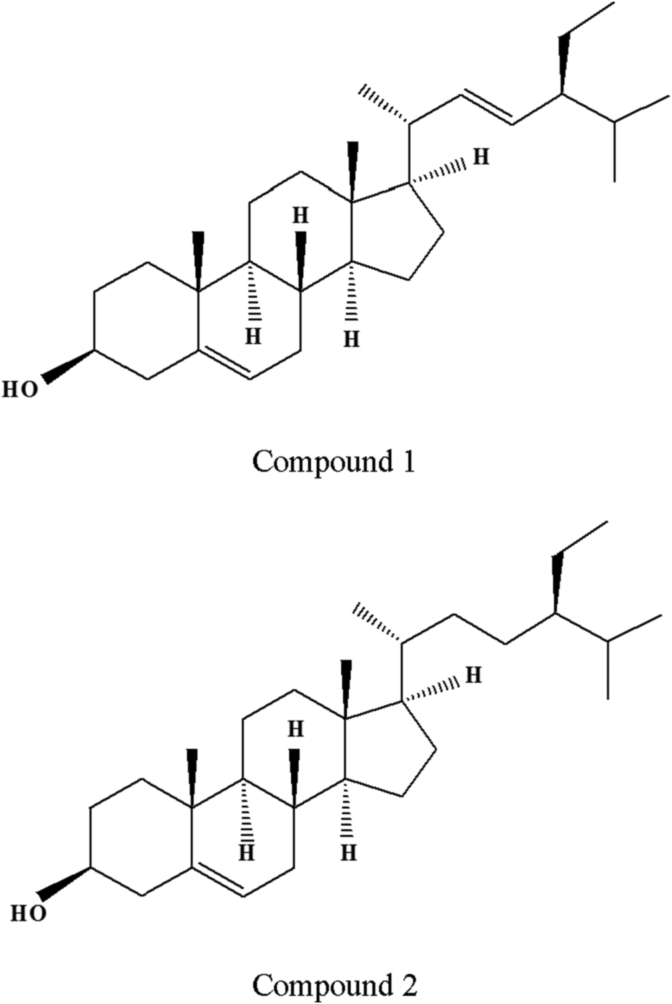
Compounds 1 and 2 were obtained in the form of a white powder. These compounds were phytosterols, based on mass spectrometry and proton (1H) nuclear magnetic resonance (NMR) and carbon-13 (13C)-NMR analyses. The typical 1H-NMR and 13C-NMR spectral patterns of Compounds 1 and 2 indicated the presence of a sterol skeleton. The structures of Compounds 1 and 2 were stigmasterol and β-sitosterol, respectively (Fig. 1), based on the interpretation of spectroscopic data in the literature [25], [26]. Simultaneous determination of stigmasterol (i.e., Compound 1) and β-sitosterol (i.e., Compound 2) in the P. ginseng roots exposed to different conditions was conducted using HPLC. Previous studies [27], [28] have shown that stigmasterol has antioxidant, thyroid-inhibitory, antiperoxidative, and hypoglycemic activities. Several other studies [29], [30] have shown that β-sitosterol has anti-inflammatory, positive immune response, anticancer defense, and apoptosis activities. These phytosterols were isolated from P. ginseng root.
Fig. 1.
The chemical structure of stigmasterol (i.e., Compound 1) and β-sitosterol (i.e., Compound 2). Stigmasterol and β-sitosterol are representative phytosterols in the plants and have the chemical structure of a terpenoid compound.
In a previous paper [31], the phytosterol content in Panax quinquefolium seed oil, based on gas chromatography and gas chromatography/mass spectrometry analysis, were as follows: squalene, oxidosqualene, campesterol, stigmasterol, clerosterol, β-sitosterol, β-amyrin, Δ(5)-avenasterol, Δ(5,24(25))-stigmasterol, lupeol, Δ(7)-sitosterol, Δ(7)-avenasterol, 24-methylenecycloartanol, and citrostadienol. These phytosterols in ginseng seed are more varied than in its root.
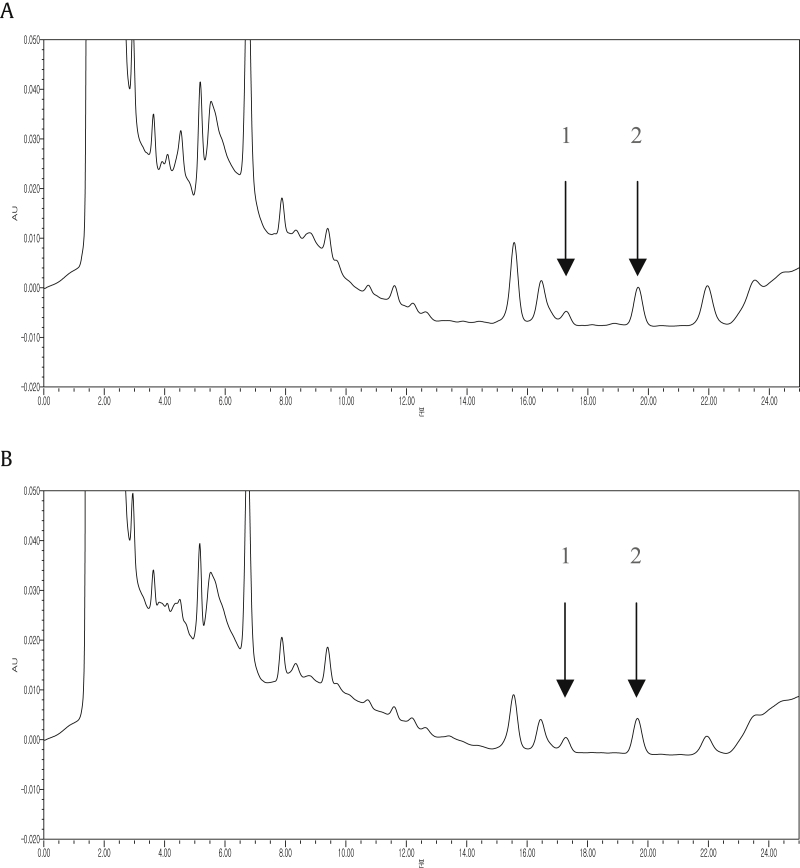
Standard calibration curves for stigmasterol and β-sitosterol were obtained at a detection wavelength of 210 nm (Table 1 and Fig. 2). By using an optimized analytical method, we successfully and simultaneously determined the levels of stigmasterol and β-sitosterol in the P. ginseng roots. The results of these analyses are shown in Table 2, Table 3 and Fig. 3. The total amount of stigmasterol and β-sitosterol in the roots of P. ginseng cultivated in Geumsan was 9.57 mg/g, whereas the amount of these chemicals in P. ginseng cultivated in Yeongju and Jinan was 26.78 mg/g and 53.23 mg/g, respectively. Thus, the total content of stigmasterol and β-sitosterol was the highest in P. ginseng from Jinan (Table 2). We also measured the total content of stigmasterol and β-sitosterol in the roots of P. ginseng exposed to different production processes and harvested at different cultivation years. Thus, the total content of stigmasterol and β-sitosterol was highest in 6-year-old white ginseng (82.14 mg/g; Table 3).
Table 1.
Linearity of the standard curves for stigmasterol (Compound 1) and β-sitosterol (Compound 2)
| Compound | tR | Calibration equation1) | Correlation factor (r2)2) |
|---|---|---|---|
| 1 | 17.1 | Y = 1037.7X + 29.186 | 0.9992 |
| 2 | 19.5 | Y = 1047.1X + 17.006 | 0.9993 |
The variable X is the concentration of the standard (mg/mL) and the variable Y is the peak area
The variable r2 is the correlation coefficient, based on three data points in the calibration curves
Fig. 2.
The phytosterols in the standard solution, as measured by HPLC analysis. The HPLC chromatograms of the standard compounds show the peaks of (A) stigmasterol and (B) β-sitosterol. HPLC, high-performance liquid chromatography.
Table 2.
The content of stigmasterol (Compound 1) and β-sitosterol (Compound 2) in the roots of Panax ginseng cultivated in different areas of Korea
| Sample source | Content (mg/g) |
||
|---|---|---|---|
| Compound 1 | Compound 2 | Total | |
| Geumsan | 2.22 ± 0.10 | 7.35 ± 0.33 | 9.57 ± 0.44 |
| Yeongju | 6.73 ± 0.42 | 20.04 ± 1.55 | 26.78 ± 1.97 |
| Jinan | 13.52 ± 0.68 | 39.70 ± 1.20 | 53.23 ± 1.89 |
Data are presented as the mean (of three measurements) ± the standard deviation in mg/g of the dried samples
Table 3.
The content of stigmasterol (Compound 1) and β-sitosterol (Compound 2) in the roots of Panax ginseng, based on the production process and cultivation year
| Sample and cultivation year | Content (mg/g) |
|||
|---|---|---|---|---|
| Compound 1 | Compound 2 | Total | ||
| Straight ginseng | Year 4 | 9.22 ± 0.49 | 24.51 ± 0.78 | 33.77 ± 1.27 |
| Year 5 | 2.64 ± 0.23 | 9.18 ± 0.43 | 11.82 ± 0.67 | |
| Year 6 | 4.71 ± 1.03 | 10.35 ± 6.51 | 15.07 ± 7.54 | |
| Red ginseng | Year 4 | 4.75 ± 0.09 | 13.49 ± 0.62 | 18.25 ± 0.72 |
| Year 5 | 5.07 ± 0.35 | 18.77 ± 0.43 | 23.84 ± 0.78 | |
| Year 6 | 6.24 ± 0.29 | 21.21 ± 0.57 | 27.46 ± 0.86 | |
| White ginseng | Year 4 | — | 22.63 ± 0.14 | 22.63 ± 0.14 |
| Year 5 | 18.66 ± 1.20 | 53.91 ± 3.43 | 72.58 ± 4.63 | |
| Year 6 | 23.04 ± 1.30 | 59.09 ± 2.82 | 82.14 ± 4.12 | |
The data are presented as the mean (of three measurements) ± the standard deviation in mg/g of the dried samples
Fig. 3.
The HPLC chromatograms of the standard compounds show the peaks of stigmasterol (1) and β-sitosterol (2) in Panax ginseng roots. The roots are (A) from Jinan and (B) from 6-year-old white ginseng. HPLC, high-performance liquid chromatography.
Stigmasterol has previously been isolated from seeds and has inhibitory effects on inflammation-related factors in HepG2 cells, as determined by luciferase reporter gene assays [32]. β-Sitosterol was previously isolated from P. ginseng root [23]. It induces diverse pharmacological effects on serum cholesterol levels and prevents cardiovascular risks by inhibiting cholesterol absorption in the intestines [28].
To our knowledge, a simple, accurate, and rapid HPLC method for simultaneously detecting stigmasterol and β-sitosterol in P. ginseng root was developed for the first time in the present study. Our results showed that 6-year-old white ginseng and ginseng cultivated in Jinan (which contains high levels of stigmasterol and β-sitosterol) are potentially an economically profitable crop because these plants can be used in the production of natural medicinal products, health supplements, and beverages.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgements
This study was conducted with the support of the Korea Food Research Institute (2012; Sungnam, Korea), the Cooperative Research Program for Agriculture Science and Technology Development (project no. PJ011582052016; Eumseong, Korea), and the Rural Development Administration (Eumseong, Korea). The authors specifically thank the staff and crew of the National Center for Inter-University Research Facilities (Seoul National University, Seoul, Korea) for their assistance with the nuclear magnetic resonance and gas chromatography/mass spectrometry experiments.
References
- 1.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 2.Hofseth L.J., Wargovich M.J. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137(1 Suppl.):183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 3.Kim S.K., Park J.H. Trends in ginseng research in 2010. J Ginseng Res. 2011;35:389–398. doi: 10.5142/jgr.2011.35.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun B.S., Gu L.J., Fang Z.M., Wang C.Y., Wang Z., Lee M.R., Li Z., Li J.J., Sung C.K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J Pharm Biomed Anal. 2009;50:15–22. doi: 10.1016/j.jpba.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Chen C.F., Chiou W.F., Zhang J.T. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol Sin. 2008;29:1103–1108. doi: 10.1111/j.1745-7254.2008.00868.x. [DOI] [PubMed] [Google Scholar]
- 6.Yue P.Y., Mak N.K., Cheng Y.K., Leung K.W., Ng T.B., Fan D.T., Yeung H.W., Wong R.N. Pharmacogenomics and the yin/yang actions of ginseng: anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin Med. 2007;2:1–21. doi: 10.1186/1749-8546-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung C.H., Seog H.M., Choi I.W., Cho H.Y. Antioxidant activities of cultivated and wild Korean ginseng leaves. Food Chem. 2005;92:535–540. [Google Scholar]
- 8.Rai D., Bhatia G., Sen T., Palit G. Anti-stress effects of Ginkgo biloba and Panax ginseng: a comparative study. J Pharmacol Sci. 2003;4:458–464. doi: 10.1254/jphs.93.458. [DOI] [PubMed] [Google Scholar]
- 9.Choi S. Epidermis proliferative effect of the Panax ginseng ginsenoside Rb2. Arch Pharm Res. 2002;25:71–76. doi: 10.1007/BF02975265. [DOI] [PubMed] [Google Scholar]
- 10.Surh Y.J., Na H.K., Lee J.Y., Keum Y.S. Molecular mechanisms underlying antitumor promoting activities of heat-processed Panax ginseng C. A. Meyer. J Kor Med Sci. 2001;16:38–41. doi: 10.3346/jkms.2001.16.S.S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang L.K., Whitaker D.C. The impact of herbal medicine on dermatologic surgery. Dermatol Surg. 2001;27:759–763. doi: 10.1046/j.1524-4725.2001.01089.x. [DOI] [PubMed] [Google Scholar]
- 12.Keum Y.S., Park K.K., Lee J.M., Chun K.S., Park J.H., Lee S.K., Kwon H., Surh Y.J. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41–48. doi: 10.1016/s0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- 13.Jones P.J.H., MacDougall D.E., Ntanios F., Vanstone C.A. Dietary phytosterols as cholesterol-lowering agents in humans. Can J Physiol Pharmacol. 1997;75:217–227. doi: 10.1139/cjpp-75-3-217. [DOI] [PubMed] [Google Scholar]
- 14.Miettinen T.A., Puska P., Gylling H., Vanhanen H., Vartiainen E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N Engl J Med. 1995;333:1308–1312. doi: 10.1056/NEJM199511163332002. [DOI] [PubMed] [Google Scholar]
- 15.Ronco A., De Stefani E., Boffetta P., Deneo-Pellegrini H., Mendilaharsu M., Leborgne F. Vegetables, fruits, and related nutrients and risk of breast cancer: a case-control study in Uruguay. Nutr Cancer. 1999;35:111–119. doi: 10.1207/S15327914NC352_3. [DOI] [PubMed] [Google Scholar]
- 16.Berges R.R., Windeler J., Trampisch H.J., Senge T. Randomised, placebo-controlled, double-blind clinical trial of β-sitosterol in patients with benign prostatic hyperplasia. β-Sitosterol study group. Lancet. 1995;345:1529–1532. doi: 10.1016/s0140-6736(95)91085-9. [DOI] [PubMed] [Google Scholar]
- 17.Akihisa T., Yasukawa K., Yamaura M., Ukiya M., Kimura Y., Shimizu N., Arai K. Triterpene alcohol and sterol ferulates from rice bran and their anti-inflammatory effects. J Agric Food Chem. 2000;48:2313–2319. doi: 10.1021/jf000135o. [DOI] [PubMed] [Google Scholar]
- 18.Arisawa M., Kinghorn A.D., Cordell G.A., Phoebe C.H., Farnsworth N.R. Plant anticancer agents. XXXVI. Schottenol glucoside from Baccharis cordifolia and Ipomopsis aggregata. Planta Med. 1985;6:544–545. [PubMed] [Google Scholar]
- 19.Recio M.C., Giner R.M., Máñez S., Ríos J.L. Structural requirements for the anti-inflammatory activity of natural triterpenoids. Planta Med. 1995;61:182–185. doi: 10.1055/s-2006-958045. [DOI] [PubMed] [Google Scholar]
- 20.Ling W.H., Jones P.J. Dietary Phytosterols: a review of metabolism, benefits and side effects. Life Sci. 1995;57:195–206. doi: 10.1016/0024-3205(95)00263-6. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi Y., Sugaya Y., Tokue A. Clinical effects of β-sitosterol (phytosterol) on benign prostatic hyperplasia: preliminary study. Hinyokika Kiyo. 1998;44:865–868. [in Japanese] [PubMed] [Google Scholar]
- 22.Pegel K.H. The importance of sitosterol and sitosterol in human and animal nutrition. S Afr J Sci. 1997;93:263–268. [Google Scholar]
- 23.Lee D.G., Kim K.T., Lee S. Taste profile characterization of white ginseng by electronic tongue analysis. Afr J Biotechnol. 2012;11:9280–9287. [Google Scholar]
- 24.Lee D.G., Lee A.Y., Kim K.T., Cho E.J., Lee S. Novel dammarane-type triterpene saponins from Panax ginseng root. Chem Pharm Bull (Tokyo) 2015;63:927–934. doi: 10.1248/cpb.c15-00302. [DOI] [PubMed] [Google Scholar]
- 25.Chang I.M., Yun H.S., Yamasaki K. Revision of 13C- NMR assignments of β-sitosterol and β-sitostery-3-O-β-d-glucopyranoside isolated from Plantago asiatica seed. Kor J Pharmacogn. 1981;12:12–14. [Google Scholar]
- 26.Chaturvedula V.S.P., Prakash I. Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus. Int Curr Pharm J. 2012;1:239–242. [Google Scholar]
- 27.Panda S., Jafri M., Kar A., Meheta B.K. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia. 2009;80:123–126. doi: 10.1016/j.fitote.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Jamaluddin F., Mohamed S., Lajis M.N. Hypoglycaemic effect of Parkia speciosa seeds due to the synergistic action of β-sitosterol and stigmasterol. Food Chem. 1994;49:339–345. [Google Scholar]
- 29.Bouic P.J. Sterols and sterolins: new drugs for the immune system? Drug Discov Today. 2002;7:775–778. doi: 10.1016/s1359-6446(02)02343-7. [DOI] [PubMed] [Google Scholar]
- 30.Cho I.H. Volatile compounds of ginseng (Panax sp.): a review. J Korean Soc Appl Biol Chem. 2015;58:67–75. [Google Scholar]
- 31.Beveridge T.H., Li T.S., Drover J.C. Phytosterol content in American ginseng seed oil. J Agric Food Chem. 2002;50:744–750. doi: 10.1021/jf010701v. [DOI] [PubMed] [Google Scholar]
- 32.Kim J.A., Son J.H., Song S.B., Yang S.Y., Kim Y.H. Sterols isolated from seeds of Panax ginseng and their anti-inflammatory activities. Pharmacogn Mag. 2013;9:182–185. doi: 10.4103/0973-1296.111288. [DOI] [PMC free article] [PubMed] [Google Scholar]