Abstract
Introduction
In LUX-Lung 3 and LUX-Lung 6, afatinib significantly improved progression-free survival (PFS) versus chemotherapy in patients with tumors harboring common epidermal growth factor receptor (EGFR) mutations (Del19/L858R) and significantly improved overall survival (OS) in patients with tumors harboring Del19 mutations. Patient-reported outcomes stratified by EGFR mutation type are reported.
Patients and Methods
Lung cancer symptoms and health-related quality of life (QoL) were assessed every 21 days until progression using the EORTC Quality of Life Core Questionnaire C30 and its lung cancer-specific module, LC13. Analyses of cough, dyspnea, and pain were prespecified and included analysis of percentage of patients who improved on therapy, time to deterioration of symptoms, and change over time. Global health status (GHS)/QoL was also assessed. Analyses were conducted for all patients with tumors harboring Del19 or L858R mutations and were exploratory.
Results
Compared with chemotherapy, afatinib more commonly improved symptoms of, delayed time to deterioration for, and was associated with better mean scores over time for cough and dyspnea in patients with Del19 or L858R mutations. All three prespecified analyses of pain showed a trend favoring afatinib over chemotherapy. In both Del19 and L858R mutations, afatinib was also associated with improvements in GHS/QoL. Longitudinal analyses demonstrated statistically significant improvements in GHS/QoL for afatinib over chemotherapy for patients with tumors harboring Del19 mutations or L858R mutations.
Conclusions
These exploratory analyses suggest first-line afatinib improved lung cancer-related symptoms and GHS/QoL compared with chemotherapy in patients with non-small-cell lung cancer with tumors harboring common EGFR mutations, with benefits in both Del19 and L858R patients. When considered with OS (Del19 patients only) and PFS benefits, these findings substantiate the value of using afatinib over chemotherapy in these patient groups.
Electronic supplementary material
The online version of this article (10.1007/s40271-017-0287-z) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| The benefits of afatinib compared with chemotherapy, with regard to symptom control of cough and dyspnea, are observed regardless of common epidermal growth factor receptor (EGFR) (Del19 or L858R) mutation type. |
| Improvements reported in lung cancer-related symptoms in patients with advanced non-small-cell lung cancer harboring the Del19 and L858R mutations add further support to use of afatinib as a first-choice treatment in these patient populations. |
Introduction
The first-generation reversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors erlotinib and gefitinib, and the irreversible ErbB family blocker, afatinib, were all approved as first-line therapy in patients with non-small-cell lung cancer (NSCLC) harboring EGFR mutations based on results from phase III trials showing improved progression-free survival (PFS) versus standard platinum-based chemotherapy [1–3]. Findings from these and other studies showed that PFS benefit was less pronounced in patients with EGFR exon 21 L858R point mutations than in patients with exon 19 deletion (Del19) mutations [2, 4–8]. Data also suggest that Del19 and L858R mutations might have distinct biological properties that might affect response to treatment [9, 10].
Differences in overall survival (OS) have not been reported between erlotinib or gefitinib and chemotherapy, irrespective of mutation type [8, 11–16]. However, recently published data have shown differences in OS outcomes between patients with tumors harboring EGFR Del19 mutations and L858R mutations when treated with afatinib compared with chemotherapy [17]. OS was substantially and significantly longer for patients with tumors harboring Del19 mutations treated with afatinib than for those treated with chemotherapy in two independent trials; however, OS was similar in both treatment arms for the L858R mutation subgroup [17]. There is some uncertainty as to how this data should be used to guide treatment selection in patients with NSCLC and if type of common mutation, Del19 or L858R mutation, should influence treatment choice.
Patient-reported outcomes (PROs) including quality of life (QoL) are important and clinically relevant endpoints that can be used to substantiate the clinical benefits of prolonged PFS and guide treatment choice. Although data suggests that erlotinib and gefitinib improve QoL compared with chemotherapy in the total population of patients with EGFR-mutation-positive NSCLC [18–20], data by mutation type has not been reported for either agent. Here, we report the analysis of the PROs, by EGFR mutation type, from two large phase III studies in patients with EGFR-mutation-positive advanced NSCLC that compared afatinib with standard of care chemotherapy (LUX-Lung 3 [3] and LUX-Lung 6 [5]). These analyses were exploratory in nature and designed to investigate whether both types of common EGFR mutation experience similar improvements in PROs and discuss the implications of these findings for daily clinical practice.
Methods
Study Population and Design
The study design, inclusion and exclusion criteria, and methods of LUX-Lung 3 and LUX-Lung 6, have been reported in full elsewhere [3, 5]. Both studies were conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines. In brief, both trials randomized eligible patients with stage IIIB/IV lung adenocarcinoma and confirmed EGFR mutations (Therascreen EGFR 29; Qiagen, Manchester, UK) in a 2:1 fashion to receive once-daily oral afatinib 40 mg or up to six cycles of chemotherapy until disease progression, death, or withdrawal due to adverse events (AEs). Chemotherapy in LUX-Lung 3 was intravenous cisplatin 75 mg/m2 and pemetrexed 500 mg/m2 every 21 days, whereas chemotherapy in LUX-Lung 6 was intravenous gemcitabine 1000 mg/m2 on Day 1 and Day 8 plus cisplatin 75 mg/m2 on Day 1 every 21 days. LUX-Lung 3 was a global study and LUX-Lung 6 was conducted in China, South Korea, and Thailand. Treatment randomization was stratified by EGFR mutation type (Del19 vs L858R vs other uncommon mutations) in both studies and by ethnic origin (Asian vs non-Asian) in LUX-Lung 3.
In both studies, PFS, defined as time from randomization to progression, determined by independent review, was the primary endpoint. OS was a key secondary endpoint in both studies and PROs were an additional secondary endpoint.
Patient-Reported Outcomes (PROs)
Assessment
Both the LUX-Lung 3 and LUX-Lung 6 studies included assessment of lung cancer symptoms and global health status (GHS)/QoL [21, 22]. Symptoms and GHS/QoL were assessed using the validated self-administered 30-item European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire (QLQ-C30) [23, 24], which includes both multi-item and single-item measures covering symptoms as well as adverse events associated with treatment. The 13-question EORTC lung cancer-specific module QLQ-LC13 [25, 26] was also used as it was specifically designed for use in patients with lung cancer undergoing treatment and has been validated for use in this setting. PROs were assessed at randomization and every 3 weeks until disease progression. Further details of these assessments have been reported in detail previously [21, 22].
Prespecified PRO measures of interest included cough (assessed by QLQ-LC13 question 1), dyspnea (assessed by a prespecified composite of QLQ-LC13 questions 3–5), and pain (assessed by a prespecified composite of QLQ-C30 questions 9 and 19). These symptoms were selected as they are established to be key lung cancer symptoms. The five functional scale scores (physical, role, functional, cognitive, and social functioning) were also of interest. Concomitant medications prescribed for cough, dyspnea, and pain were documented to enable analysis of their potential impact on reported symptoms.
Statistical Analysis
Analyses were exploratory in nature and neither study was powered to detect differences in PROs. Data from these studies are reported together due to the similarity in the trial designs; data were not pooled as chemotherapy comparator arms were different. Three analyses were prespecified for each symptom of interest and included: (i) comparison between the treatment groups of the percentage of patients who improved (defined as a ≥ 10-point decrease from baseline at any time during the trial) compared with those without improvement (stable or worsened) using a logistic regression model stratified by race in LUX-Lung 3, without adjustment for baseline scores; (ii) time to deterioration in symptoms analysis (measured in months from randomization to the first instance of a 10-point worsening in symptom from baseline)—treatment groups were compared using a Cox proportional hazards regression model stratified by race in LUX-Lung 3; and (iii) mean difference in symptom scores over time (longitudinal analysis) with the assumption that data are missing at random. For the longitudinal analyses all data up to the median follow-up time (calculated across all patients) were included, this was a constant value used across all analyses. Criteria for clinically meaningful symptom improvement, as well as details of statistical analysis of these outcomes, have been reported previously [21, 22]. Functional scale scores were analyzed using longitudinal analysis only. Updated PRO analyses were completed at the time of primary OS analysis (January 2014). Analyses were conducted on the prespecified lung cancer symptoms of interest as well as AE-related symptoms of interest as they are commonly associated with treatment (nausea, vomiting, diarrhea, and sore mouth).
Each analysis was conducted for the population of patients with tumors harboring each of the common EGFR mutation types (Del19 or L858R), as well as in the total intention-to-treat population. All analyses were exploratory and p-values are provided for information only; there were no adjustments for multiple testing.
Results
Patient Population
Full details of the disposition and the baseline characteristics of patients in LUX-Lung 3 and LUX-Lung 6 have been reported previously [3, 5]. Briefly, the majority of patients were female (afatinib 64% vs chemotherapy 68%), never smokers (71% vs 76%), had stage IV disease (92% vs 90%), and had an Eastern Cooperative Oncology Group performance status of 1 (70% vs 65%). In LUX-Lung 3, 72% of patients were Asian; all patients were Asian in LUX-Lung 6. In LUX-Lung 3, 88% (n = 203: Del19, n = 112; L858R, n = 91) of afatinib-treated patients and 90% (n = 104: Del19, n = 57; L858R, n = 47) of chemotherapy-treated patients had common mutations, whereas in LUX-Lung 6, 89% (n = 216: Del19, n = 124; L858R, n = 92) of afatinib-treated patients and 89% (n = 108: Del 19, n = 62; L858R, n = 46) of chemotherapy-treated patients had common mutations (Fig. 1). Baseline characteristics in patients with Del19 or L858R mutations were similar to those of the overall population in both studies. At the time of analysis reported here, 21 patients in LUX-Lung 3 and 23 patients in LUX-Lung 6 were still receiving afatinib treatment; no patients were receiving chemotherapy.
Fig. 1.
Study profile. † Cisplatin-pemetrexed in LUX-Lung 3; cisplatin-gemcitabine in LUX-Lung 6. EGFR, epidermal growth factor receptor
Mean (standard deviation) baseline symptom scores for cough, dyspnea, pain, and GHS/QoL indicated a low overall symptom burden for patients in both studies and in both treatment arms, although symptom burden was greatest for cough (Table 1). Symptom burden was well balanced between treatment arms and mutation types. Compliance rates for EORTC QLQ-C30 questionnaire completion were high in both trials across treatment arms and mutation types and were above 90% at all study visits. Average completion rates over the course of LUX-Lung 3 were 97.1% in Del19 and 96.5% in L858R patients treated with afatinib and 96.9% in Del19 and 96.1% in L858R patients treated with chemotherapy. Average completion rates in LUX-Lung 6 were 97.7% in Del19 and 97.0% in L858R patients treated with afatinib and 93.9% in Del19 and 93.1% in L858R patients treated with chemotherapy.
Table 1.
Mean (SD) baseline symptom scoresa for all prespecified patient-reported outcomes symptoms of interest (cough, dyspnea, and pain) and GHS/QoL by mutation type (Del19 and L858R)
| Mean (SD) | Del19 | L858R | ||
|---|---|---|---|---|
| Afatinib | Chemotherapy | Afatinib | Chemotherapy | |
| LUX-Lung 3 | n = 110 | n = 56 | n = 90 | n = 45 |
| Cough | 36.7 (27.2) | 33.9 (23.6) | 32.6 (24.6) | 31.8 (27.8) |
| Dyspnea | 22.1 (18.3) | 24.8 (23.8) | 21.9 (19.1) | 23 (23.7) |
| Pain | 22.6 (23.0) | 23.5 (26.2) | 28.8 (24.6) | 23.1 (25.0) |
| GHS/QoL | 66.4 (19.3) | 60 (23.4) | 66.0 (20.8) | 60.4 (20.2) |
| Functional scales | ||||
| Physical | 81.2 (19.0) | 77.4 (22.2) | 80.52 (19.13) | 77.27 (20.70) |
| Role | 76.5 (27.0) | 72.6 (29.9) | 78.28 (26.52) | 73.11 (25.72) |
| Emotional | 80.0 (16.8) | 73.2 (23.0) | 76.97 (18.08) | 72.54 (22.70) |
| Cognitive | 87.6 (15.6) | 87.5 (17.5) | 84.83 (17.52) | 81.82 (19.95) |
| Social | 79.8 (22.3) | 74.1 (25.2) | 78.84 (22.01) | 76.14 (27.23) |
| LUX-Lung 6 | n = 124 | n = 59 | n = 90 | n = 43 |
|---|---|---|---|---|
| Cough | 36.6 (24.5) | 27.5 (23.7) | 37.9 (23.4) | 32.5 (28.4) |
| Dyspnea | 24 (19.4) | 24.2 (19.0) | 25.6 (19.1) | 24.7 (22.8) |
| Pain | 21.2 (20.0) | 24.6 (20.9) | 27.5 (23.1) | 21.1 (25.3) |
| GHS/QoL | 64.5 (20.7) | 65.4 (15.9) | 60.1 (21.2) | 66.9 (22.6) |
| Functional scales | ||||
| Physical | 80.2 (20.1) | 80.5 (18.4) | 77.62 (15.8) | 80.5 (18.5) |
| Role | 79.6 (25.4) | 79.2 (23.2) | 74.90 (23.1) | 82.5 (23.0) |
| Emotional | 84.8 (16.3) | 80.7 (17.4) | 81.13 (18.2) | 79.7 (22.5) |
| Cognitive | 89.3 (13.4) | 86.8 (15.7) | 83.14 (19.6) | 86.6 (16.3) |
| Social | 74.4 (23.5) | 73.1 (21.8) | 73.18 (24.0) | 73.2 (25.0) |
GHS global health status, QoL quality of life, SD standard deviation
aAll scores range from 0 to 100. For the GHS/QoL scale, a value of 100 was equivalent to the best possible score and 0 to the worst possible score. For cough, dyspnea, and pain, 100 was equivalent to the highest burden of symptoms and 0 to the lowest burden. Total patient numbers represent the number of patients with at least one baseline and one on treatment assessment and, as such, differ slightly from the number of patients randomized to treatment
PROs in Patients by Mutation Type
Patients with Lung Cancer Symptom Improvement
The percentages of patients that experienced clinically meaningful improvements in symptom scores by mutation type are shown in Fig. 2. In patients with tumors harboring Del19 mutations, a higher proportion of afatinib-treated patients experienced clinically meaningful improvements in dyspnea symptom scores compared with chemotherapy-treated patients in both studies (LUX-Lung 3: 69% vs 48%; LUX-Lung 6: 70% vs 43%). In the Del19 population, the proportion of patients with improvements in pain was higher for afatinib in LUX-Lung 3 (60% vs 43%) and the proportion of patients with improvements in cough was higher for afatinib in LUX-Lung 6 (75% vs 55%).
Fig. 2.
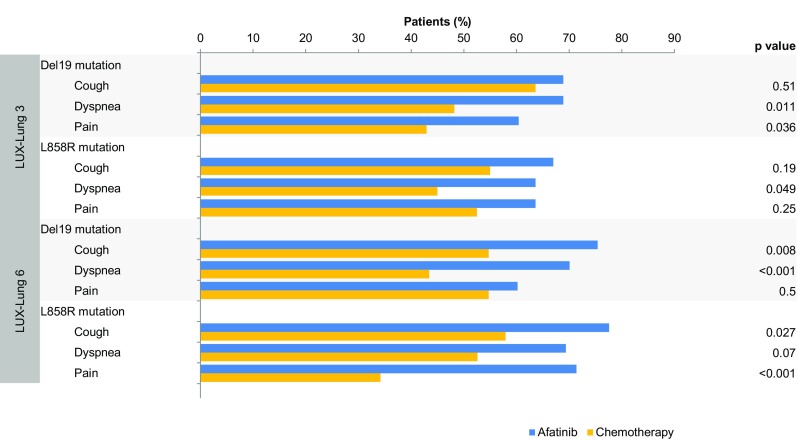
Percentages of patients with improvement in all prespecified PROs symptoms of interest: cough, dyspnea, and pain by mutation type (Del19 and L858R). p-values from logistic regression analysis of ‘improved/not improved’. PRO, patient-reported outcome
For patients with tumors harboring L858R mutations in LUX-Lung 3, a higher proportion of afatinib-treated patients experienced clinically meaningful improvements in dyspnea (64% vs 45%) symptom scores, and a higher proportion of afatinib-treated patients in LUX-Lung 6 experienced clinically meaningful improvements in cough (78% vs 58%) and pain (71% vs 34%) symptom scores compared with chemotherapy-treated patients.
Time to Deterioration of Lung Cancer Symptoms
Afatinib significantly delayed the time to deterioration of cough and dyspnea, compared with chemotherapy, in patients with tumors harboring Del19 mutations in both studies; (Fig. 3 and Supplemental Fig. 1 [see electronic supplementary material]).
Fig. 3.
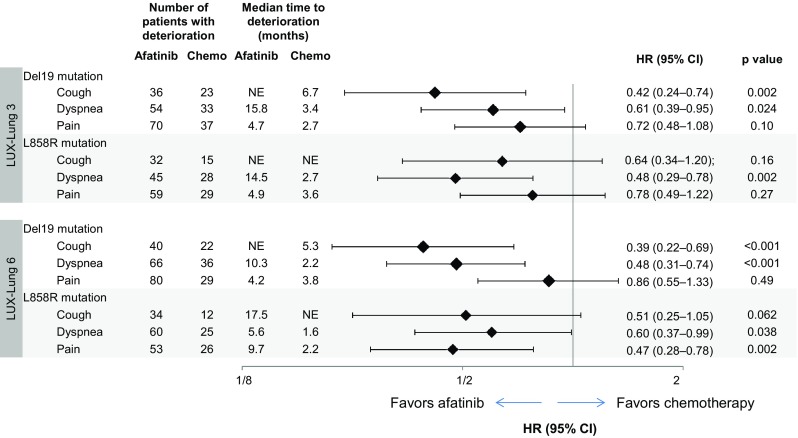
Time to deterioration of all prespecified PROs symptoms of interest: cough, dyspnea, and pain by mutation type (Del19 and L858R). HRs from Cox proportional hazard model stratified by race in LUX-Lung 3. p-values calculated from log-rank test. The median time to deterioration was not evaluable in some groups because there were not sufficient events at the time of analysis for the median value to be reached. CI, confidence interval; HR, hazard ratio; NE, not evaluable; PRO, patient-reported outcome
In the group of patients with tumors harboring L858R mutations, afatinib significantly delayed the time to deterioration of dyspnea in both studies compared with chemotherapy, as well as time to deterioration of pain in LUX-Lung 6. A trend towards delayed time to deterioration of pain in LUX-Lung 3, and time to deterioration in cough, was also observed in patients with tumors harboring L858R mutations receiving afatinib compared with chemotherapy.
Longitudinal Analysis of Lung Cancer Symptoms
In patients with tumors harboring Del19 mutations, differences in mean symptom scores over time significantly favored afatinib over chemotherapy for cough and dyspnea in LUX-Lung 3, and cough, dyspnea, and pain in LUX-Lung 6 (Fig. 4). For patients with tumors harboring L858R mutations, differences in mean symptom scores over time significantly favored afatinib over chemotherapy for cough and dyspnea in LUX-Lung 3, and cough, dyspnea and pain in LUX-Lung 6.
Fig. 4.

Longitudinal analysis of all prespecified PROs symptoms of interest: cough, dyspnea, and pain by mutation type (Del19 and L858R). Scores range from 0 to 100 (100 is equivalent to the highest burden of symptoms and 0 to the lowest burden); mean treatment difference shown as afatinib minus chemotherapy and, as such, a negative score favors afatinib treatment. CI, confidence interval; PRO, patient-reported outcome
Adverse-Event-Related Symptoms
Consistent findings were reported in the time to deterioration analysis in patients with either L858R or Del19 mutations (shorter time to deterioration of nausea and vomiting with chemotherapy and shorter time to deterioration of diarrhea and sore mouth with afatinib). Longitudinal analyses in patients with tumors harboring either L858R or Del19 mutations were also consistent (worse scores for nausea and vomiting with chemotherapy and worse scores for diarrhea and sore mouth with afatinib). Time to deterioration and longitudinal analyses of fatigue were significantly different, favoring afatinib, in patients with Del19 mutations in both studies and in patients with L858R mutations in LUX-Lung 6, although were not significantly different between treatment groups in patients with L858R mutations in LUX-Lung 3.
There were no significant differences in the prescription of concomitant medications for cough, dyspnea, and pain between treatment arms in LUX-Lung 3. In LUX-Lung 6, a lower level of concomitant medication use was observed overall, with greater use of cough (13.6% vs 4.9%) and pain (46.3% vs 28.7%) medication in the afatinib treatment arm compared with chemotherapy.
Global Health Status
In longitudinal analysis of LUX-Lung 3 data, patients with tumors harboring Del19 mutations on afatinib had significantly better mean scores over time for GHS/QoL (Fig. 5). In LUX-Lung 3, no significant difference between treatment arms was observed in the proportion of patients with improvement or time to deterioration analyses of GHS/QoL with tumors harboring Del19 mutations.
Fig. 5.
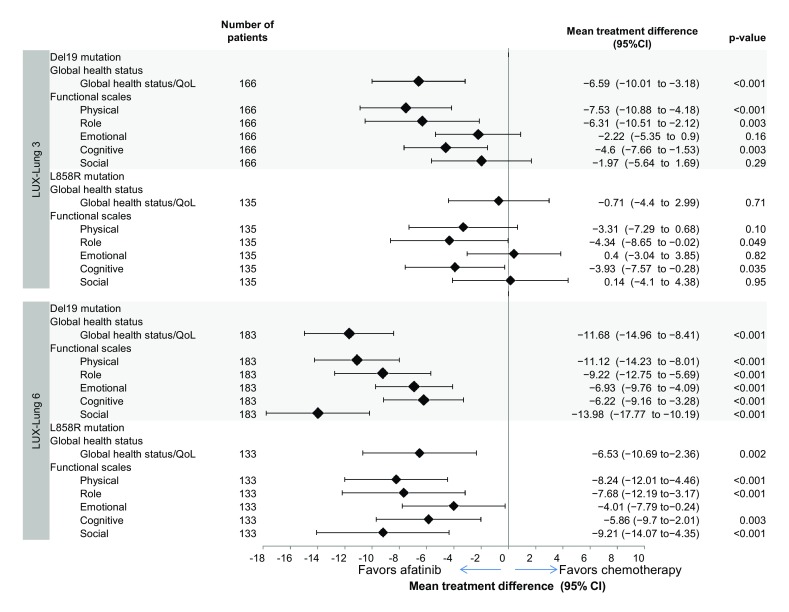
Longitudinal analysis of GHS/QoL and functional scale domains by mutation type (Del19 and L858R). For the GHS/QoL scale, a value of 100 was equivalent to the best possible score and 0 to the worst possible score; mean treatment difference shown as chemotherapy minus afatinib and, as such, a negative score favors afatinib treatment. CI, confidence interval; GHS, global health status; QoL, quality of life
In patients with tumors harboring Del19 mutations in LUX-Lung 6, GHS/QoL improvements in afatinib-treated patients were also observed in all three prespecified methods of analysis; patients on afatinib had significantly better mean scores over time [mean treatment difference: − 11.68 (95% CI: − 14.96 to − 8.41); p < 0.001; Fig. 5]; afatinib significantly delayed time to deterioration for GHS/QoL [HR: 0.53 (95% CI: 0.35–0.82); p = 0.003]; and a significantly greater number of patients had an improvement in GHS/QoL (63% vs 34%; p < 0.001). In patients with tumors harboring L858R mutations in LUX-Lung 6, mean scores over time significantly favored afatinib compared with chemotherapy [mean treatment difference: − 6.53 (95% CI: −10.69 to −2.36); p = 0.002; Fig. 5] and a significantly higher number of patients treated with afatinib had an improvement in GHS/QoL (61.2% vs 34.2%; p = 0.007).
Functional Scales
In the longitudinal analysis of data from LUX-Lung 3, patients with tumors harboring Del19 mutations who received afatinib had significantly better mean scores over time for physical, role, and cognitive functioning than patients treated with chemotherapy (Fig. 5). In LUX-Lung 3, patients on afatinib with tumors harboring L858R mutations had significantly better mean scores over time for role and cognitive functioning. In LUX-Lung 6, patients with tumors harboring common mutations had significantly better mean scores over time for all functional scales; results were observed regardless of whether patients had tumors harboring Del19 mutations or L858R mutations (Fig. 5).
PROs in the Intention-to-treat Population
For the intention-to-treat population, the percentage of patients experiencing clinically meaningful improvements in symptom scores is shown in Supplemental Fig. 2A, time to deterioration of symptom scores is shown in Supplemental Fig. 2B and differences in mean symptom scores over time are shown in Supplemental Fig. 2C. Longitudinal analysis of GHS/QoL and functional scale scores are shown in Supplemental Fig. 2D (see electronic supplementary material). All findings were comparable to the data reported by mutation type.
Discussion
The new analyses described here suggest that the benefits of afatinib compared with chemotherapy, with regard to symptom control of cough and dyspnea, are observed regardless of common EGFR (Del19 or L858R) mutation type. In patients with Del19 or L858R, differences favoring afatinib over chemotherapy for cough and dyspnea were observed in all three prespecified analyses, with differences substantially favoring afatinib over chemotherapy in a number of comparisons. All three prespecified analyses of pain generally showed a trend favoring afatinib over chemotherapy, although the differences between treatments reached significance in only a few of the analyses and were sometimes inconclusive. These new findings support previous analyses that have shown that first-line treatment with afatinib was associated with better control of cough and dyspnea compared with chemotherapy and better control of pain in patients with EGFR-mutation-positive NSCLC [3, 5].
What are the implications of these findings to clinical practice? Use of afatinib as first-line therapy significantly prolongs OS and PFS compared with chemotherapy in patients with advanced NSCLC harboring the Del19 mutation [3, 5]. As such, afatinib should be considered a first-choice, first-line agent in patients with tumors harboring Del19 mutations, with the additional improvements reported in lung cancer-related symptoms and overall GHS adding further support to this recommendation.
Treatment guidelines recommend both erlotinib and gefitinib over chemotherapy as first-line treatment in patients with EGFR-mutation-positive NSCLC [27, 28]. These recommendations are based on data showing that both treatments significantly improve PFS [1, 2, 4, 7, 11, 29, 30] and QoL symptoms [18–20] compared with chemotherapy, as benefits in OS have not been reported in either the overall study population [8, 11–15] or by mutation type; specifically, hazard ratios for patients with L858R mutations, where reported, are greater than (favoring chemotherapy) [14] or close to 1 [12, 13]. In agreement with these recommendations, demonstrated improvements in PFS, combined with improvements in symptom control, substantiate the value of also recommending afatinib over chemotherapy in patients with L858R mutations despite the lack of OS benefit [17]. Data from the LUX-Lung 7 global, randomized, phase IIb trial comparing first-line afatinib with gefitinib showed that afatinib significantly improved PFS versus gefitinib in patients with common EGFR mutations, with efficacy improvements being observed in both L858R and Del19 mutations [31], and a trend towards improved OS with afatinib versus gefitinib in both mutation types [32] adding further support to the use of afatinib in both mutation types.
The longitudinal analysis of these parameters provided statistically significant improvements for afatinib over chemotherapy in both studies for patients with tumors harboring Del19 mutations, as well as for patients with tumors harboring L858R mutations, in LUX-Lung 6. This suggests that the GHS/QoL of patients with NSCLC with common EGFR mutations (both for Del19 and L858R) receiving afatinib is potentially better than with chemotherapy. These new data also indicate that the symptoms of diarrhea and sore mouth, as well as other AEs more commonly observed with afatinib compared with chemotherapy, did not adversely affect patients’ overall QoL. Afatinib treatment also showed consistent significant improvements in scores of all functional scales compared with chemotherapy in LUX-Lung 6, regardless of common mutation type, whereas significant differences in functional scale scores between treatment arms were not as uniformly seen in the LUX-Lung 3 trial. This likely reflects the differential impact of the chemotherapy arms used in each trial on patient outcome rather than the responsiveness of the mutation type to improvements in aspects of patient function with afatinib; the cisplatin/pemetrexed chemotherapy comparator used in LUX-Lung 3 is generally considered to have a better tolerability profile than cisplatin/gemcitabine used in LUX-Lung 6 and, as such, is likely to have less of an impact on functioning compared with the LUX-Lung 6 chemotherapy comparator.
The EORTC QLQ-C30 and QLQ-LC13 instruments used in our analyses have been well validated for the assessment of PROs and, although LUX-Lung 3 and LUX-Lung 6 both used comprehensive and prespecified methods for the assessment of PROs, the analyses reported here were conducted post hoc and should be considered to be exploratory. It should be noted that the studies on which these analyses were based were not powered to detect significant differences in PRO outcomes and the number of analyses conducted does increase the chance of false-positive results being observed (a type I error). The strengths and limitations of each analysis method should also be considered: analysis of the percentage of patients with symptom improvement is of clinical interest yet the threshold that constitutes a clinically relevant change is often debated and the presence of asymptomatic patients at baseline can impact on the results. The event time for symptom improvement or deterioration could occur at any stage during the assessment period; time to deterioration analysis is easy to interpret, yet has limitations associated with censoring due to progression, and longitudinal analysis offers comprehensive use of available data, yet is a complex method of analysis that requires certain assumptions regarding missing data. However, collectively, the three methods of analysis broaden the perspective of the results, thereby enhancing their interpretation. The overall inferential strategy was to provide a comprehensive analysis relying on the consistency (and potential inconsistency) of the results to reflect the strength of the evidence. As such, the general consistency of the results favoring afatinib treatment compared with chemotherapy across studies, mutation groups (Del19 or L858R), and analysis methods suggests that the differences observed represent a true treatment effect rather than a chance occurrence. An additional consideration in interpreting the findings reported here is that baseline pain scores were low; as such, only a limited number of patients had a chance to show improvements in this item. Despite this, a trend towards better control of pain was observed in both studies.
Conclusion
Compared with chemotherapy, first-line treatment with afatinib generally improves lung cancer-related symptoms in patients with EGFR-mutation-positive NSCLC, with comparable benefits being observed regardless of common mutation type. Afatinib also results in improvements in overall QoL and functional improvements compared with chemotherapy, providing further support for the use of afatinib in the first-line treatment of this patient group.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The LUX-Lung 3 and LUX-Lung 6 studies were funded by Boehringer Ingelheim. Editorial support was provided by Suzanne Patel, Origin Health Ltd, funded by Boehringer Ingelheim Pharmaceuticals, Inc. Boehringer Ingelheim Pharmaceuticals, Inc. was given the opportunity to review the manuscript for medical and scientific accuracy, as well as for intellectual property considerations.
Author contributions
JC-HY, Y-LW, KO’B, VH, TM, DM, and LVS were involved in conception and design of the studies. JC-HY, Y-LW, MSc, NY, CZ, C-PH, KO’B, JF, SL, YH, SLG, VH, TM, and LVS were involved in patient enrolment, recruitment, and treatment. JC-HY, Y-LW, MSc, NY, CZ, C-PH, SLG, VH, JB, SO, and TM, were involved in data collection. JC-HY, Y-LW, MSc, NY, CZ, KO’B, JF, SL, SLG, VH, TM, DM, AM, JL, and LVS were involved in data analysis and interpretation. JC-HY, Y-LW, and LVS were involved in study oversight and supervision. All authors were involved in the drafting and reviewing of the manuscript, and approved the final manuscript for submission.
Compliance with Ethical Standards
Funding
This study was supported by Boehringer Ingelheim. We also gratefully acknowledge the patients, their families, and their caregivers for participation in this study.
Conflict of interest
Dr. Wu reports grants and personal fees from Boehringer Ingelheim during the conduct of the study, personal fees from AstraZeneca, Lilly, Pierre Fabre, Pfizer, Sanofi, and Merck, and grants and personal fees from Roche, outside the submitted work. Dr. Hirsh reports personal fees from Boehringer Ingelheim during the conduct of the study; and personal fees from AstraZeneca, Roche, Pfizer, Amgen, and Merck outside the submitted work. Dr. Sequist reports grants and other from Boehringer Ingelheim during the conduct of the study; and personal fees from AstraZeneca, ARIAD, and Genentech, and other from Merrimack, Clovis Oncology, Novartis, and Taiho Pharmaceutical, outside the submitted work. Dr. Hu has nothing to disclose. Dr. Feng has nothing to disclose. Dr. Lu reports personal fees from Boehringer Ingelheim during the conduct of the study; and personal fees from Bristol-Myers Squibb, non-financial support from Roche, and grants from Lilly outside the submitted work. Dr. Huang has nothing to disclose. Dr. Schuler reports grants, personal fees and non-financial support from Boehringer Ingelheim during the conduct of the study; and personal fees from AstraZeneca, Celgene, Lilly, MSD, Roche, and Alexion, grants and personal fees from Novartis, Boehringer Ingelheim, and Bristol-Myers Squibb, other from Universität Duisburg-Essen, Universitätsklinikum Essen, and Ruhrlandklinik, and non-financial support from Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG) outside the submitted work. Dr. Mok reports grants, personal fees and non-financial support from Boehringer Ingelheim during the conduct of the study; and grants, personal fees and non-financial support from AstraZeneca, Lilly, Amgen, Roche/Genentech, and Pfizer, personal fees and non-financial support from Novartis, grants and personal fees from Bristol-Myers Squibb, Clovis Oncology, Janssen Pharmaceuticals, AVEO & Biodesix Inc., ACEA Biosciences, Vertex, Biomarin, and Oncogenex, personal fees from Prime Oncology, Merck Sharp & Dohme, and GlaxoSmithKline, grants and non-financial support from Merck Serono, and grants from SFJ Pharmaceuticals Group and geneDecode outside the submitted work. Dr. Yamamoto reports grants and personal fees from Boehringer Ingelheim during the conduct of the study. Dr. O’Byrne reports grants, personal fees and non-financial support from Boehringer Ingelheim during the conduct of the study; and personal fees and non-financial support from MSD, AstraZeneca, BMS, Roche, Lilly, Boehringer Ingelheim, and Pfizer, and personal fees from Novartis, outside the submitted work. Dr. Geater reports non-financial support from Boehringer Ingelheim, AstraZeneca, Sanofi Aventis, and Novartis, and grants from Boehringer Ingelheim, AstraZeneca, and Novartis during the conduct of the study. Dr. Zhou reports personal fees from Boehringer Ingelheim during the conduct of the study; and personal fees from AstraZeneca, Pfizer, F. Hoffmann La Roche, and Lilly outside the submitted work. Dan Massey is an employee of Boehringer Ingelheim. Dr. Märten is an employee of Boehringer Ingelheim. Juliane Lungershausen is an employee of Boehringer Ingelheim. Dr. Yang reports grants and personal fees from Boehringer Ingelheim during the conduct of the study; and personal fees from AstraZeneca, Pfizer, Roche, Lilly, Chugai Pharma, MSD, Merck Serono, Celgene, Astellas Pharma, Bayer, Ono Pharmaceutical, Daiichi Sankyo, Clovis Oncology, Yuhan Pharmaceuticals, and Novartis outside the submitted work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s40271-017-0287-z) contains supplementary material, which is available to authorized users.
References
- 1.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 4.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 5.Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 6.Yang JC, Shih JY, Su WC, Hsia TC, Tsai CM, Ou SH, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol. 2012;13(5):539–548. doi: 10.1016/S1470-2045(12)70086-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 8.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29(21):2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 9.Okabe T, Okamoto I, Tamura K, Terashima M, Yoshida T, Satoh T, et al. Differential constitutive activation of the epidermal growth factor receptor in non-small cell lung cancer cells bearing EGFR gene mutation and amplification. Cancer Res. 2007;67(5):2046–2053. doi: 10.1158/0008-5472.CAN-06-3339. [DOI] [PubMed] [Google Scholar]
- 10.Zhu JQ, Zhong WZ, Zhang GC, Li R, Zhang XC, Guo AL, et al. Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett. 2008;265(2):307–317. doi: 10.1016/j.canlet.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 11.Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent IRESSA versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 12.Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24(1):54–59. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- 13.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802) Ann Oncol. 2015;26(9):1877–1883. doi: 10.1093/annonc/mdv276. [DOI] [PubMed] [Google Scholar]
- 14.Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883–1889. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka H, Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I et al. Final overall survival results of WJTOG 3405, a randomized phase 3 trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer (NSCLC) harboring mutations of the epidermal growth factor receptor (EGFR). ASCO Meeting Abstracts. 2014;32(15_suppl):8117.
- 16.Lee C, Davies LC, Wu Y-L, Mitsudomi T, Inoue A, Rosell R et al. The impact on overall survival (OS) of first-line gefitinib (G) and erlotinib (E) and of clinical factors in advanced non-small cell lung cancer (NSCLC) with activating epidermal growth factor receptor mutations (EGFR mut) based on meta-analysis of 1,231 patients (pts) enrolled in 6 major randomized trials. ASCO Meeting Abstracts. 2015;33(15_suppl):8072.
- 17.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Feng J, Zhou C, Wu YL, Liu XQ, Wang C, et al. Quality of life (QoL) analyses from OPTIMAL (CTONG-0802), a phase III, randomised, open-label study of first-line erlotinib versus chemotherapy in patients with advanced EGFR mutation-positive non-small-cell lung cancer (NSCLC) Ann Oncol. 2013;24(6):1615–1622. doi: 10.1093/annonc/mdt012. [DOI] [PubMed] [Google Scholar]
- 19.Oizumi S, Kobayashi K, Inoue A, Maemondo M, Sugawara S, Yoshizawa H, et al. Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: quality of life analysis of North East Japan Study Group 002 Trial. Oncologist. 2012;17(6):863–870. doi: 10.1634/theoncologist.2011-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YL, Fukuoka M, Mok TS, Saijo N, Thongprasert S, Yang JC, et al. Tumor response and health-related quality of life in clinically selected patients from Asia with advanced non-small-cell lung cancer treated with first-line gefitinib: post hoc analyses from the IPASS study. Lung Cancer. 2013;81(2):280–287. doi: 10.1016/j.lungcan.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Yang JC, Hirsh V, Schuler M, Yamamoto N, O’Byrne KJ, Mok TS, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3342–3350. doi: 10.1200/JCO.2012.46.1764. [DOI] [PubMed] [Google Scholar]
- 22.Geater SL, Xu CR, Zhou C, Hu CP, Feng J, Lu S, et al. Symptom and quality of life improvement in LUX-Lung 6: an open-label phase III study of afatinib versus cisplatin/gemcitabine in Asian patients with EGFR mutation-positive advanced non-small-cell lung cancer. J Thorac Oncol. 2015;10(6):883–889. doi: 10.1097/JTO.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 23.Fayers P, Aaronson N, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 scoring manual. 3. Brussels: EORTC; 2001. [Google Scholar]
- 24.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 25.Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer. 1994;30A(5):635–642. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 26.Earle CC, Weeks JC. The science of quality-of-life measurement in lung cancer. In: Lipscomb J, Gotay CC, Snyder C, editors. Outcomes assessment in cancer: measures, methods, and applications. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- 27.National Comprehensive Cancer Network. NCCN Guidelines: Non-Small Cell Lung Cancer. V 1. 2015. Available at: http://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf.
- 28.Reck M, Popat S, Reinmuth N, De Ruysscher D, Kerr KM, Peters S et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii27-39. 10.1093/annonc/mdu199. [DOI] [PubMed]
- 29.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 30.Wu YL, Liam CK, Zhou C, et al. First-line erlotinib versus cisplatin/gemcitabine (GP) in patients with advanced EGFR mutation-positive non-small-cell lung cancer (NSCLC): interim analyses from the phase 3, open-label, ENSURE study. J Thorac Oncol. 2013;8(Suppl. 2):S603. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 31.Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589. doi: 10.1016/S1470-2045(16)30033-X. [DOI] [PubMed] [Google Scholar]
- 32.Paz-Ares L, Tan EH, O’Byrne K, Zhang L, Hirsh V, Boyer M, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28(2):270–277. doi: 10.1093/annonc/mdw611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



