Abstract
Objectives
The aim was to develop a clinical outcome assessment (COA) for itching in children with cholestatic pruritus.
Methods
This prospective study aimed to enroll patients aged 4–30 years with Alagille syndrome (ALGS) or progressive familial intrahepatic cholestasis type 1 and caregivers of patients aged 5 months to 14 years. Eligible patients experienced itching during ≥3 of the 7 days before enrollment and had not undergone liver transplant or surgical interruption of the enterohepatic circulation. Open-ended qualitative interviews confirmed that itching was a primary concern for patients and caregivers. Diaries were modified and then evaluated by participants during cognitive debriefing. Interview results were reviewed by clinical, COA and statistical experts. Diary questions were revised following an interim analysis before finalizing the Itch Reported Outcome (ItchRO).
Results
Thirty-six interviews were analyzed, representing 25 families of patients with ALGS. Itching was reported spontaneously (without prompting by the interviewer) by ten of 12 patients with ALGS and 19 of 20 caregivers. Consequences of itching included skin damage (78%), mood changes (59%), and difficulties staying asleep (59%) or falling asleep (53%). Two versions of the ItchRO were developed: ItchRO(Patient) for self-completion by patients and ItchRO(Observer) for caregivers. The ItchRO diaries comprise a single scorable item to assess itch and are to be completed twice daily (morning and evening).
Conclusions
Itching was the most bothersome ALGS symptom reported by study participants. We have developed the ItchRO(Patient) and ItchRO(Observer) to assess itching in children with ALGS and other cholestatic liver diseases. These diaries are being validated for use in clinical trials.
Electronic supplementary material
The online version of this article (doi:10.1007/s40271-017-0266-4) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| Assessment of pruritus associated with Alagille syndrome (ALGS) and other pediatric cholestatic conditions is challenging because the patients most severely affected by itching are often very young and there are currently no widely accepted instruments for evaluating this often debilitating symptom in children. |
| Accordingly, this qualitative, prospective study evaluated the symptoms, signs and impacts of itching in pediatric patients with ALGS, with the aim of developing a clinical outcome tool to assess itching. |
| Based on this research, two versions of the ItchRO were developed for validation in pediatric cholestatic conditions: a patient version designed for completion by individuals aged 9 years and older, and an observer version to enable caregiver assessment of itching behaviors in children with ALGS who are too young to report their own outcomes. |
Background
Alagille syndrome (ALGS) is a rare, autosomal dominant, multisystem disorder with a reported incidence of 1:30,000 live births, based on molecular diagnostics [1]. ALGS arises from defects in the Notch signaling pathway, most commonly mutations or deletions in JAG1; mutations in NOTCH2 account for a minority of cases [2–4]. Clinical features of ALGS include chronic cholestasis associated with paucity of intrahepatic bile ducts; congenital heart disease; dysmorphic facial features; and skeletal, ocular, renal and vascular abnormalities [5–7]. ALGS can significantly impair the quality of life of patients and their caregivers [8–10].
ALGS-associated cholestasis presents in infancy and typically manifests with pruritus in children older than approximately 6 months [5–7]. Severe itching can be unbearable, causing scratching, scarring of the skin and chronic sleep disturbance [8, 9]. In a survey of children with ALGS, 59% of patients experienced itch, with 25% reporting injury of the skin, bleeding or scarring [8]. Indeed, itch is considered the most troublesome symptom of ALGS. It can have a significant impact on a child’s school and social activities, and medically refractory pruritus is an indication for biliary diversion surgery or liver transplant [6, 8].
Itch involves physiologic, psychosocial and behavioral factors that are difficult to quantify [11]. Assessments of itching, therefore, rely on patient- or observer-reported outcome measures. Self-reported measures of pruritus include mono-dimensional intensity scales (e.g., visual analog scale, numerical or verbal rating scales) [12], the Itch Man Scale [13] and the 5-D Itch Scale [14]. However, no widely accepted, standardized and validated instruments for assessment of childhood pruritus exist that comply with the US Food and Drug Administration (FDA) guidelines for patient-reported outcomes [15]. Assessment of pruritus associated with ALGS and other pediatric cholestatic conditions is challenging because the patients most severely affected by itching are often very young. Although a patient-reported outcome is preferred, an observer-reported outcome that could serve as a proxy measure would enable evaluations in children who cannot verbalize their symptoms.
This qualitative, prospective study evaluated the symptoms, signs and impacts of itching in pediatric patients with ALGS, with the aim of developing a clinical outcome assessment to assess itching. Recruitment difficulties necessitated the enrollment of adult patients. This study also targeted patients with progressive familial intrahepatic cholestasis type 1 (PFIC1), an inherited cholestatic liver disease that is also associated with early-onset pruritus [16, 17]. However, as only one individual with PFIC1 was recruited, this report focuses on the ALGS population; results for the patient with PFIC1 are presented as a case study.
Methods
This non-interventional study was conducted between July 2012 and December 2012.
It initially enrolled patients aged 4–18 years with ALGS or PFIC1, and caregivers of patients aged 5 months to 14 years with ALGS or PFIC1. A protocol amendment after study initiation permitted enrollment of adults aged 18–30 years with ALGS or PFIC1. Individuals were recruited through Alagille Syndrome Alliance-sponsored community events throughout the USA, and online forums and support groups for ALGS or PFIC. Recruitment targets were 0–2 years (three to five caregivers), 3–5 years (three to five caregivers), 6–11 years (four to eight patients and their caregivers), 12–17 years (four to eight patients and their caregivers) and 18–30 years (four to eight patients)1. Patients with ALGS or PFIC1 who had experienced itching during at least 3 of the 7 days before the eligibility assessment were eligible to participate. Patients who had undergone liver transplant or surgical interruption of the enterohepatic circulation were excluded.
Study Design
Development of the Itch Reported Outcome (ItchRO) occurred in multiple stages, during which diaries underwent two complete phases of evaluation and revision. The process began with a review of previous studies assessing itch, consultation with clinical experts in ALGS or PFIC and preliminary diary and study protocol development. This was followed by an initial round of interviews and subsequent revision of diaries and study documents based on patient and caregiver feedback. This draft was then evaluated during the second round of interviews, and a revised version was developed to include all patient and caregiver adaptations (Fig. 1). The preliminary patient- and observer-reported diaries were based on a physician-reported outcome measure that was developed to assess signs and behaviors of scratching [18, 19]. Exploratory items assessing the intensity, duration and impacts of itching were added after reviewing existing measures of itching, including the Itch Man Scale [13] and the 5-D Itch Scale [14], and the Children’s Sleep Habits Questionnaire [20].
Fig. 1.
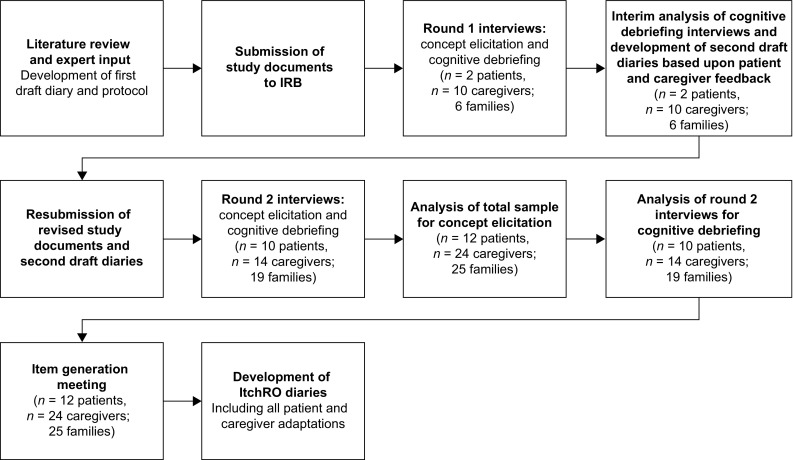
Development of the ItchRO. IRB independent review board, ItchRO Itch Reported Outcome
Patients and their caregivers participated in a 60-min interview that was conducted either face-to-face or using Skype™ (Skype Communications SARL, Luxembourg City, Luxembourg). The minimum age for participation in the interviews was 5 years. Participants were interviewed separately; if required, caregivers could remain in the room during patient interviews. Interviewers had expertise in qualitative interviewing in the context of patient-reported outcomes and followed independent review board-approved study procedures and documentation, including a semi-structured interview guide. They also completed National Institutes of Health Human Participant Protection training [21], as well as data protection and interview training. Audio recordings of interviews were transcribed by an independent transcription agency (Fantastic Transcripts, Boston, MA, USA). Interviewees were reimbursed for their time.
During concept elicitation, interviewers asked open-ended questions to identify clinical features relevant to patients with ALGS or PFIC1 and their caregivers. If required, interviewers could ask follow-up questions. In particular, interviewers determined whether patients experienced itching if this concept was not reported spontaneously (without the topic of itching being raised by the interviewer). During cognitive debriefing, patients and caregivers completed preliminary diaries and commented on their relevance, clarity and comprehensiveness. Child diaries were completed by patients aged 5 years and older (if capable). Caregiver diaries were completed by caregivers of all patients younger than 18 years. During the first round of interviews (n = 2 patients, n = 10 caregivers; n = 6 families), patients completed cognitive debriefing of two draft diaries (morning and evening reports) and caregivers evaluated one diary (daily report). An interim analysis was completed following these interviews and participant feedback incorporated into the diaries. Revised diaries were used in the remaining interviews (n = 10 patients, n = 14 caregivers; n = 19 families). Patients aged 5–11 years and caregivers together completed cognitive debriefing of the caregiver-administered versions of the child morning and evening diaries; caregivers read each item aloud for the patient to respond. Based on FDA guidance [15], patients and caregivers were asked to specify their preferred wording or concepts for diary items and preferred format for the response options (boxes, circles or squares of varying sizes).
Data Analysis
Interview responses were analyzed based on grounded theory methods [22] using qualitative analysis software (ATLAS.ti version 7.0 software; ATLAS.ti Scientific Software Development GmbH, Berlin, Germany) [23]. Each interview was considered a unit of analysis. A concept code book was developed, using a standard iterative process [24], which provided code descriptions and indicated whether concepts were provided spontaneously or following questioning by the interviewer. Similarities and differences in the meaning of concept codes across patients were identified using a constant comparison method [22]. Responses were tabulated based on the questions in the semi-structured interview guides, and the number of times that a unique concept was reported by a participant was recorded in a frequency grid. Information obtained during concept elicitation was summarized in a saturation grid. The number of responses elicited from the first 25% of patient/caregiver interviews was compared to the number elicited from the next 25%, the number elicited from the first 50% was compared to the next 25%, and the number elicited from the first 75% was compared to the last 25%. Itching concepts were also assessed by age group and reporter (patient or caregiver).
Interview results were reviewed during an item generation meeting, and draft diary items, response options and instructions were revised as required. Items were selected based on the frequency and clinical relevance of the concepts expressed spontaneously by patients and caregivers. The ItchRO[Patient (Pt)] and ItchRO[Observer (Obs)] morning and evening diaries were constructed using the words and phrases used by participants. A Flesch–Kincaid grade level readability score and a reading ease score were generated for the ItchRO(Pt) to ensure that it was appropriate for the age group specified.
Results
Study Population
The original analysis set represented patients with ALGS (n = 12 patients, n = 24 caregivers; n = 25 families) or PFIC1 (n = 1 patient). Four of these patients had biliary diversions and one patient had received a liver transplant. These patients would have been excluded owing to protocol violations, but were included in the analysis because their pruritus was refractory to the surgical interventions. Four additional interviews involving three caregivers and one patient (representing three families) were completed, but were excluded owing to protocol violations: patient had never experienced itching (n = 1), patient no longer experienced itching (n = 1), caregiver of deceased patient (n = 1) and caregiver of patient who had received a liver transplant and did not experience itching post-transplant (n = 1). Here, we report the data for the ALGS analysis population (Fig. 2). A case report is presented for the patient with PFIC1.
Fig. 2.

Alagille syndrome analysis population
Demographics, Symptoms and Clinical Features: ALGS Population
Most caregivers were the parents of patients [17 of 22 (77%) mothers, three of 22 (14%) fathers and two of 22 (9%) grandmothers]. Mean age was 37.9 years (range 21.9–65.8 years), and most caregivers had completed a university degree or higher (12 of 22, 55%) and were in full-time employment (14 of 22, 64%). Two caregivers did not provide demographic data.
The mean age of patients was 8.3 years (range 0.44–34.9 years); three patients were adults (Table 1). All four patients with severe itching (16%) were in the youngest age groups (0–5 years). Across all age groups, equal numbers of patients had very mild, mild or moderate itching (seven of 25, 28% for each severity category). The majority of patients (20 of 25, 80%) were currently receiving treatment for ALGS, mostly for itching (18 of 25, 72%). The most common treatments used by patients for itching were rifampin (12 of 25, 48%), hydroxyzine (Atarax) (ten of 25, 40%) and ursodeoxycholic acid (six of 25, 24%). Four patients (16%) were not receiving treatment for itching, and three patients (12%) did not provide treatment information.
Table 1.
ALGS population: patient demographic and clinical characteristics reported by caregivers (for patients under 18 years of age) or patients
| Demographic and clinical characteristics | N = 25 |
|---|---|
| Sex, n (%) | |
| Male | 10 (40.0) |
| Female | 15 (60.0) |
| Age, years | |
| Mean | 8.3 |
| Median | 5.8 |
| Range | 0.44–34.9 |
| Race/ethnicitya, n (%) | |
| Asian | 1 (4.0) |
| Black/African–American | 5 (20.0) |
| Hispanic/Spanish American/Latino (of any race) | 4 (16.0) |
| White/Caucasian | 14 (56.0) |
| Severity of reported itching, n (%) | |
| Very mild | 7 (28.0) |
| Mild | 7 (28.0) |
| Moderate | 7 (28.0) |
| Severe | 4 (16.0) |
| Patient receiving treatment for any aspect of ALGS, n (%) | |
| Yes | 20 (80.0) |
| No | 2 (8.0) |
| Not answered | 3 (12.0) |
| Patient has undergone surgery for any aspect of ALGSb, n (%) | |
| Yes | 9 (36.0) |
| No | 14 (56.0) |
| Not answered | 2 (8.0) |
| Type of surgery for ALGS, n (%) | |
| Biliary diversionc | 3 (12.0) |
| Open biopsy and catheterization | 1 (4.0) |
| Tetralogy of Fallot repair | 1 (4.0) |
| Liver biopsy | 1 (4.0) |
| Liver transplant | 1 (4.0) |
| Bone graft/hardware | 1 (4.0) |
| Not answeredd | 17 (68.0) |
| Patient receiving treatment for itching, n (%) | |
| Yes | 18 (72.0) |
| No | 4 (16.0) |
| Not answered | 3 (12.0) |
| Current itching treatmentse, n (%) | |
| Rifampin | 12 (48.0) |
| Hydroxyzine (Atarax) | 10 (40.0) |
| Ursodeoxycholic acid | 6 (24.0) |
| Naltrexone | 3 (12.0) |
| Clonidine | 1 (4.0) |
| Unspecified medications | 3 (12.0) |
ALGS Alagille syndrome
aRace information was not provided for one participant
bWhile any surgical interruption of the enterohepatic circulation and liver transplant were exclusion criteria for this study, all patients who had undergone these surgery types continued to experience itching following surgery and were, therefore, included in the study
cIncluding partial external biliary diversion, ileal exclusion, biliary diversion and partial internal biliary diversion
dIncludes one patient for whom surgery type was not specified
eMore than one treatment was reported for some participants
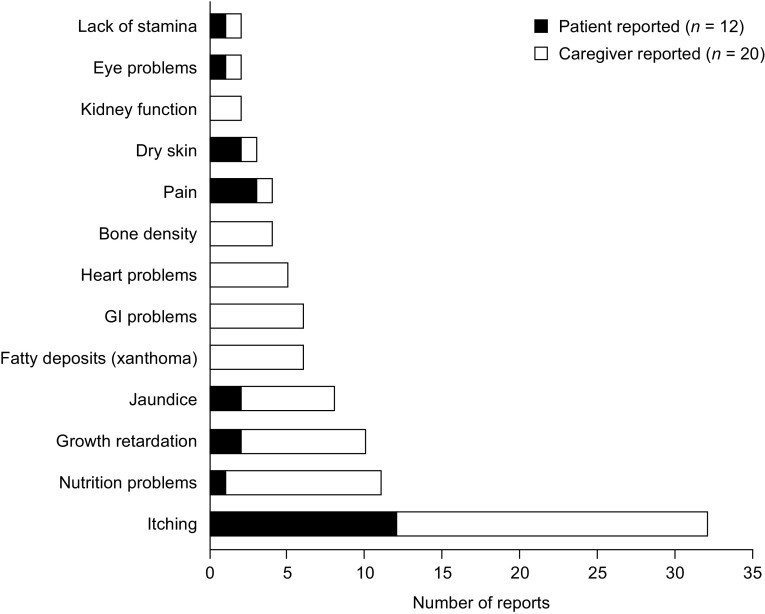
Figure 3 outlines the clinical features of ALGS reported by patients (n = 12) and their caregivers (n = 20) during concept elicitation. The total number for the caregiver population was n = 20 rather than n = 24 because four families each included two caregivers; concepts reported by both caregivers of a patient were counted once. Itching was spontaneously reported by the majority of patients (10 of 12, 83%) and their caregivers (19 of 20, 95%); a further two patients (17%) and one caregiver (5%) discussed itching when questioned about this concept. Example descriptors of itching are provided in the electronic supplementary material (Online Resources 1 and 2). Other medical problems reported spontaneously by patients and caregivers included nutrition problems (11 of 32, 34%), growth retardation (10 of 32, 31%), jaundice (8 of 32, 25%), pain (four of 32, 13%), dry skin (three of 32, 9%), eye problems (2 of 32, 6%) and lack of stamina (2 of 32, 6%). Patients aged 3–5 years and 6–8 years exclusively discussed itching; other clinical features were reported only by patients aged 9 years or older. Most of the language used by patients to describe itching did not vary across age groups; however, only patients aged 9 years and older described the severity or duration of their itching. Patients described itch severity in terms of its frequency, duration and intensity and damage to the skin. Itching often occurred during periods of inactivity (e.g., when lying in bed, watching television or when bored). Caregiver responses in the ItchRO(Obs) diary were to be based on observed behaviors of the child or verbal reports from the patient. Caregivers (n = 20) identified five observations that indicated that the patient was experiencing itching: patient scratching (n = 14, 70%), mood changes (n = 8, 40%), patient-reported itching (n = 7, 35%), evidence of skin damage (n = 6, 30%) and sleep disturbance (n = 5, 25%). Three caregivers (15%) received reports from other caregivers (e.g., nanny or teacher).
Fig. 3.
Clinical features of ALGS reported by caregivers and patents (N = 32)a. Counts are not mutually exclusive; clinical features include concepts reported spontaneously by patients and caregivers, as well as those reported following further questioning by interviewer. ALGS Alagille syndrome, GI gastrointestinal. a N = 32 because four families each included two caregivers who reported the same concept; in these cases, the concept was counted once to avoid inflation of the results
The impacts of itching most frequently reported by patients (n = 12) and caregivers (n = 20) were skin damage (n = 25, 78%), mood changes (n = 19, 59%) and difficulties staying asleep (n = 19, 59%) or falling asleep (n = 17, 53%) (Table 2). Impacts of itching reported only by patients were feeling embarrassed (n = 1 child and n = 1 adult), fingernail damage (n = 1 child), needing to take medicine (n = 1 child), social impacts associated with dating (n = 1 adult), missing school (n = 1 child) and two work-related problems (productivity and finding a job; n = 1 adult).
Table 2.
Impacts of cholestatic pruritus in patients with ALGS
| Impact, n (%) | Patient (N = 12) | Caregiver (N = 20)a | Total (N = 32) |
|---|---|---|---|
| Impacts on skin | |||
| Skin damage | 9 (75.0) | 16 (80.0) | 25 (78.1) |
| Skin damage (spontaneous) | 7 (58.3) | 15 (75.0) | 22 (68.8) |
| Skin damage (probed) | 2 (16.7) | 1 (5.0) | 3 (9.4) |
| Blood from scratching | 3 (25.0) | 8 (40.0) | 11 (34.4) |
| Blood from scratching (spontaneous) | 1 (8.3) | 8 (40.0) | 9 (28.1) |
| Blood from scratching (probed) | 2 (16.7) | 0 | 2 (6.3) |
| Impacts on sleep | |||
| Difficulty staying asleep | 3 (25.0) | 16 (80.0) | 19 (59.4) |
| Difficulty staying asleep (spontaneous) | 3 (25.0) | 15 (75.0) | 18 (56.3) |
| Difficulty staying asleep (probed) | 0 | 1 (5.0) | 1 (3.1) |
| Difficulty falling asleep | 6 (50.0) | 11 (55.0) | 17 (53.1) |
| Difficulty falling asleep (spontaneous) | 4 (33.3) | 10 (50.0) | 14 (43.8) |
| Difficulty falling asleep (probed) | 2 (16.7) | 1 (5.0) | 3 (9.4) |
| Impacts on emotions | |||
| Mood changes | 6 (50.0) | 13 (65.0) | 19 (59.4) |
| Low self-esteem | 0 | 2 (10.0) | 2 (6.3) |
| Impacts on daily life | |||
| Inability to concentrate | 4 (33.3) | 8 (40.0) | 12 (37.5) |
| Inability to concentrate (spontaneous) | 4 (33.3) | 7 (35.0) | 11 (34.4) |
| Inability to concentrate (probed) | 0 | 1 (5.0) | 1 (3.1) |
| Feeling tired in school | 1 (8.3) | 1 (5.0) | 2 (6.3) |
| Difficulty participating in sports/playing | 2 (16.7) | 5 (25.0) | 7 (21.9) |
| Social impact | |||
| Unwanted attention from others | 5 (41.7) | 6 (30.0) | 11 (34.4) |
ALGS Alagille syndrome
aFour families each included two caregivers who reported the same concept; in this case, the concept was counted once to avoid inflation of the results
Most symptoms and impacts of itching (12 of 15) were spontaneously reported for at least one age group in the pediatric patient population (Table 3). Patients aged 3–5 years and 6–8 years reported fewer concepts than those in the older age groups. The 5-year-old patient did not mention any concepts and the 6-year-old patient only mentioned itching. For the caregiver population, most itching-related symptoms and impacts (13 of 20) were reported for patients in more than one age group. Some impacts of itching emerged in specific age groups: pulling out hair (0–2 years), eating difficulties (0–2 years), inability to care for self (3–5 years), attachment to caregiver (3–5 years), feeling tired in school (9–11 years), lowered confidence (12–17 years) and self-pity (12–17 years) (Table 4).
Table 3.
ALGS study population: patient-reported symptoms and impacts of itching by age group (N = 12 patients)
| Concept | Age, years | Total (N = 12) | ||||
|---|---|---|---|---|---|---|
| 3–5 (n = 1) | 6–8 (n = 1) | 9–11 (n = 3) | 12–17 (n = 4) | ≥18 (n = 3) | ||
| Symptom | ||||||
| Itching | 0 | 1 | 2 | 4 | 3 | 10 |
| Patient-reported impacts: physical | ||||||
| Skin damage | 0 | 0 | 2 | 3 | 2 | 7 |
| Blood from scratching | 0 | 0 | 0 | 1 | 0 | 1 |
| Fingernail damage | 0 | 0 | 0 | 1 | 0 | 1 |
| Patient-reported impacts: sleep | ||||||
| Difficulty falling asleep | 0 | 0 | 0 | 3 | 1 | 4 |
| Difficulty staying asleep | 0 | 0 | 2 | 1 | 0 | 3 |
| Patient-reported impacts: activities of daily living | ||||||
| Ability to concentrate | 0 | 0 | 1 | 2 | 1 | 4 |
| Sports/playing | 0 | 0 | 1 | 1 | 0 | 2 |
| Patient-reported impact: school | ||||||
| Feeling tired in school | 0 | 0 | 0 | 1 | 0 | 1 |
| Patient-reported impacts: social | ||||||
| Unwanted attention from others | 0 | 0 | 2 | 1 | 2 | 5 |
| Dating | 0 | 0 | 0 | 0 | 1 | 1 |
| Patient-reported impacts: work | ||||||
| Finding a job | 0 | 0 | 0 | 0 | 1 | 1 |
| Productivity | 0 | 0 | 0 | 0 | 1 | 1 |
| Patient-reported impacts: emotional | ||||||
| Mood changes | 0 | 0 | 1 | 3 | 2 | 6 |
| Embarrassment | 0 | 0 | 0 | 1 | 1 | 2 |
ALGS Alagille syndrome
Table 4.
ALGS study population: caregiver-reported symptoms and impacts of itching by age group (N = 24 caregivers; N = 20 families)
| Concept | Age of child, years | Total (N = 20)a | ||||
|---|---|---|---|---|---|---|
| 0–2 (n = 6) | 3–5 (n = 6) | 6–8 (n = 2) | 9–11 (n = 4) | 12–17 (n = 2) | ||
| Symptom | ||||||
| Itching | 5 | 6 | 3 | 3 | 2 | 19 |
| Impact on child: physical | ||||||
| Skin damage | 5 | 3 | 1 | 4 | 2 | 15 |
| Blood from scratching | 3 | 1 | 1 | 3 | 0 | 8 |
| Physical discomfort | 1 | 1 | 1 | 0 | 0 | 3 |
| Sitting still | 0 | 0 | 1 | 2 | 0 | 3 |
| Pulling out hair | 2 | 0 | 0 | 0 | 0 | 2 |
| Impact on child: sleep | ||||||
| Difficulty staying asleep | 5 | 5 | 1 | 2 | 2 | 15 |
| Difficulty falling asleep | 3 | 2 | 2 | 2 | 1 | 10 |
| Impact on child: activities of daily living | ||||||
| Difficulty participating in sports/playing | 2 | 2 | 0 | 0 | 1 | 5 |
| Inability to concentrate | 1 | 2 | 2 | 1 | 1 | 7 |
| Difficulty with eating | 2 | 0 | 0 | 0 | 0 | 2 |
| Inability to care for self | 0 | 1 | 0 | 0 | 0 | 1 |
| Impact on child: school | ||||||
| Feeling tired in school | 0 | 0 | 0 | 1 | 0 | 1 |
| Impact on child: social | ||||||
| Unwanted attention from others | 0 | 2 | 1 | 1 | 2 | 6 |
| Difficulty/avoiding going out | 1 | 1 | 1 | 0 | 0 | 3 |
| Impact on child: emotional | ||||||
| Mood changes | 5 | 4 | 1 | 2 | 1 | 13 |
| Low self-esteem | 0 | 1 | 0 | 0 | 1 | 2 |
| Attachment to caregiver | 0 | 1 | 0 | 0 | 0 | 1 |
| Lowered confidence | 0 | 0 | 0 | 0 | 1 | 1 |
| Self-pity | 0 | 0 | 0 | 0 | 1 | 1 |
Only spontaneous patient reports are included in the assessment of itching concepts
ALGS Alagille syndrome
aFour families each included two caregivers who reported the same concept; in this case, the concept was counted once to avoid inflation of the results
Saturation of Concepts: ALGS Population
No new clinical features of ALGS were reported spontaneously by patients or caregivers in the last 25% of interviews completed compared with the first 75% of interviews. All patient-reported impacts of itching were saturated in the pediatric population. Two work-related impacts (finding a job and productivity) did not achieve saturation because one adult patient mentioned these concepts in the last round of interviews. All observations used by caregivers to determine itching severity achieved saturation. Most caregiver-reported itching impacts (18 of 19, 95%) were saturated; a caregiver mentioned one emotional impact (attachment to caregiver) in the last round of interviews.
Cognitive Debriefing: ALGS Population
Cognitive debriefing was completed in 11 patients and 24 caregivers; one 5-year-old patient could not complete this part of the interview owing to reading difficulties. Input from patients and caregivers, including their interpretations of items and responses and their suggestions for rewording items, was recorded in an item-tracking matrix. The 5-year-old and 6-year-old patients could not complete the diaries, either on their own or with the help of their caregiver. No 7-year-old or 8-year-old patients were recruited. Patients aged 9 years and older completed the measures and provided feedback on the diaries. Caregivers easily understood the items, although caregivers of children over the age of 9 years found it more difficult to provide reliable reports because they were not spending as much time with their children. During the second round of interviews, five sets of patients and their caregivers together carried out cognitive debriefing of the caregiver-administered versions of the child morning and evening diaries; three sets representing 9-, 10- and 11-year-old patients did not find it helpful for the caregiver to read the questions.
Patients aged 9 years or older reported that they felt more able to give accurate responses when given a recall period of about 12 h compared with over a period of 24 h. Patients reported that they would prefer to complete items about the previous night in a morning diary and respond to items about the day during the evening. Accordingly, the ItchRO(Pt) and ItchRO(Obs) diaries were developed to be completed twice daily. Some caregivers said that completion of a morning diary would be difficult owing to school routines and others said that completion of an evening diary would be difficult owing to the stress of getting an itchy child to sleep.
Patient with PFIC1: Case Study
All signs and symptoms reported by the patient with PFIC1 (20-year-old male) were also reported by the patients with ALGS; these included itching, jaundice, growth retardation and loss of stamina. Most impacts of itching reported by this patient also aligned with those reported by the ALGS population: mood changes, feeling tired at college, skin damage, blood from scratching, difficulty falling asleep, unwanted attention from others, feeling embarrassed and an impact on self-esteem. A financial and emotional impact on family and the need to cease employment were reported only by the patient with PFIC1.
ItchRO Item Generation
The item-tracking matrix was reviewed to derive the instructions, items and response options for the final ItchRO diaries. Item selection and wording was based on findings from the concept elicitation and cognitive debriefing sections of the interviews, respectively. Items that were conceptually clear and relevant to patients were included, while those that were interpreted inconsistently or as irrelevant were excluded (e.g., duration and location of itch). The ItchRO(Pt) received a Flesch–Kincaid reading ease score of 92.0 and a grade level score of 2.0 (7–8 years of age).
The ItchRO includes a single item to score itching severity on a scale of zero [ItchRO(Pt): I didn’t feel itchy; ItchRO(Obs): not itchy at all] to four [ItchRO(Pt): I felt very, very itchy; ItchRO(Obs): extremely itchy] (Figs. 4, 5). The daily score will be the highest (worst) score from the morning and evening reports. It is anticipated that clinical studies will assess changes in ItchRO scores over time (pre- and post-treatment). The ItchRO(Pt) also contains two exploratory items to address the impacts of itching on sleep and the extent of skin damage caused by rubbing or scratching. An exploratory item was also included in the ItchRO(Obs) to capture how caregivers become aware of their child’s itching.
Fig. 4.

ItchRO(Pt) morning and evening diaries. ItchRO(Pt) Itch Reported Outcome (patient)
Fig. 5.

ItchRO(Obs) morning and evening diaries. ItchRO(Obs) Itch Reported Outcome (observer)
Discussion
This qualitative study explored the clinical features of pediatric patients with ALGS, with a focus on one of the most debilitating symptoms: pruritus. Consistent with previous clinical experience, the medical problems most frequently reported by patients with ALGS and their caregivers included itching, nutrition problems, growth retardation and jaundice [5, 6]. The most frequently mentioned impacts of itching associated with ALGS included skin damage due to scratching, as well as mood and sleep disturbances.
We have developed the ItchRO(Pt) and ItchRO(Obs) to assess itching in patients with ALGS and other cholestatic liver diseases. Given the rarity of ALGS, clinical trials evaluating the effectiveness of anti-pruritic treatments are unlikely to enroll large patient populations. Furthermore, this and previous studies indicate that the itching associated with ALGS is most prevalent and severe in young children [5–7]. The ItchRO(Pt) and ItchRO(Obs) have been designed for completion by patients 9 years and older and caregivers of younger patients, respectively. The availability of these two versions of the ItchRO addresses the need to maximize the number of patients for whom itching can be objectively assessed in clinical trials, regardless of their age. Care was taken to maintain conceptual equivalence across both diaries. For example, in response to patient feedback, morning and evening versions of both diaries were developed to ensure adequate coverage of each 24-h period. While adults do not usually have difficulty with a 24-h recall period, caregivers were asked to complete the diary twice daily to maintain symmetry with the ItchRO(Pt). Morning and evening diary reports will help to capture the symptoms and impacts of itching during these different periods of the day.
A review of the literature and existing measures of itching, as well as input from clinical experts, patients with ALGS and their caregivers, and the FDA have ensured that the ItchRO contains the most clinically relevant items to assess itching. Open-ended questioning during the study interviews minimized the potential for biased reporting of clinical features of ALGS. Use of follow-up questions was taken into account during analysis of the data, and concepts elicited through interviewer probing were noted. Each ItchRO item measures a unidimensional concept, and employs the language used by patients and caregivers during concept elicitation to describe itching. Saturation was achieved for the majority of itching-related symptoms and impacts, indicating that the study sample size was sufficient to generate all relevant concepts.
Recruitment was based on narrow age groupings to identify developmental differences in the responses given and to determine the age at which patients could understand the questions. A cut-off age of 9 years was considered appropriate for self-completion of the ItchRO(Pt). For patients under the age of 9 years, caregiver assessments were often more accurate than those of the children themselves. For example, some young children scratched or rubbed themselves repeatedly during the interview, but, when asked, reported that they were not itchy. Children may become accustomed to the sensation of itchiness from a young age. Other reports have indicated that children’s self-reports are more reliable than caregiver reports from the age of 9 years, while caregivers’ reports are more reliable for children under 9 years of age [25]. Some concepts, such as pain, may be reliably reported by children as young as 5 years of age [26]; however, our results suggest that the concept of itching may be difficult for young children to understand.
This study had some limitations and highlighted challenges associated with measuring itching in a pediatric population with a rare disease. Assessment of the relative frequency of the clinical features of ALGS was biased by the requirement for patients to have experienced itching immediately before entering the study. Owing to recruitment difficulties, adults with ALGS have provided input into the development of this pediatric instrument. Although the FDA discourages the use of proxy-reported outcome measures, it recognizes that some patients may be unable to respond for themselves. Caregivers cannot directly experience the symptoms or other clinical features of ALGS, but they can assess itching through observable features such as scratching, mood changes, skin damage and sleep disturbance. Their input was particularly useful for the youngest patients. We were unable to enroll any children aged 7–8 years owing to the challenges inherent to recruiting individuals with a rare disease. It is conceivable that patients in this age range may be more able to self-report than the younger participants in the study, raising the possibility that the cut-off age of 9 years could be lowered. Furthermore, caregivers may spend less time with patients of this age, compared with those aged 5 years or younger, a factor which has been shown to influence the reliability of the caregiver to report on behalf of the patient. However, there is currently insufficient evidence to suggest that individuals below 9 years of age could reliably report using ItchRO(Pt) at this stage. This possibility will be explored further during feasibility and psychometric testing of the instrument. Finally, it should be noted that comparison of the patient with PFIC1 with those with ALGS provides inadequate evidence to support the use of the ItchRO in a PFIC1 population or to confirm that the two diseases present in the same way. However, the agreement between the symptoms and impacts reported by the patient with PFIC1 and those reported by the ALGS population offers promise that similarities exist. Qualitative analysis of additional patients with PFIC1 is required to improve understanding of the relationship between the two diseases.
Since completion of this study, minor modifications have been made to the ItchRO in response to feedback from the FDA. Electronic ItchRO diaries have been generated, as well as a version of the instrument for adults. The present study did not assess the ability of the ItchRO to detect changes in the severity of itching over time. However, the ItchRO has been used to assess responses to treatment with maralixibat (formerly LUM001) in patients with ALGS [27]. The psychometric performance of the ItchRO(Pt) and ItchRO(Obs) electronic diaries has also been evaluated in a 2-week, non-interventional study in patients with ALGS or PFIC [28] (results to be reported separately).
Overall, this qualitative study demonstrated that itching is one of the most impactful symptoms for patients with ALGS. Given the lack of pediatric itch assessments that meet current FDA patient-reported outcome guidelines, we have created a new instrument to assess itch in patients with ALGS. Although patient-reported outcome measures may be the preferred method for assessment of clinical symptoms of pruritus, the ItchRO(Obs) enables assessments in patients with ALGS who are too young to report their own outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the Alagille Syndrome Alliance and PFIC.org for their help in patient recruitment, and all of the patients and caregivers who participated in this research.
Author Contributions
BMK, LA-W, CK, BH, MG, NJ, SM, AD and BLS were involved in the design of the study. LA-W, CK, BH, MG, NJ and SM conducted the research. BMK, LA-W, CK, BH, MG, NJ, SM, AD and BLS were involved in data acquisition or analysis. All authors were involved in the drafting of, and critical revisions to, the manuscript, and all authors approved the final version of the article, including the authorship list. Guarantor of the article: BMK.
Compliance with Ethical Standards
Funding
This study was sponsored by Lumena Pharmaceuticals, one of the Shire group of companies. B.L. Shneider and B.M. Kamath were supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases-sponsored ChiLDReN Network. Under the direction of the authors, and funded by Shire International GmbH, Dr. E. Gandhi of Oxford PharmaGenesis, Oxford, UK, provided writing assistance for this publication. Editorial assistance with formatting, proofreading, copy editing and fact checking was also provided by Oxford PharmaGenesis with funding from Shire International GmbH. M. Harrington and M. Vera-Llonch from Shire Development LLC and J. Wiehn from Shire International GmbH also reviewed and edited the manuscript for scientific accuracy.
Conflict of interest
BM. Kamath has no conflict of interest to declare. L. Abetz-Webb, M. Gauthier and N. Johnson worked for the consulting company (Adelphi Values) that conducted the sponsored research; support for travel to interview patients was also provided to L. Abetz-Webb by the study sponsor (Lumena/Shire). Upon leaving Adelphi Values, L. Abetz-Webb received consulting fees to aid in the design of a psychometric validation study for the measure (ItchRO) developed in this study. C. Kennedy and A. Dorenbaum were employees of Lumena Pharmaceuticals. B. Hepburn and S. Medendorp provided paid consultancy services to Lumena Pharmaceuticals on this project. L. Todorova is an employee of Shire and owns stock or stock options in the company. B. L. Shneider has received research support from Hyperion Therapeutics (now Horizon Pharma) and is a consultant for and stock owner in Bristol-Myers Squibb.
Ethical approval
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines, as well as with other applicable local laws, rules and regulations. An independent review board (Copernicus Group Independent Review Board, Durham, NC, USA) reviewed all study documentation and provided ethical approval to conduct the study. Informed consent was obtained from the parents or legal guardians of all patients under 18 years of age who participated in the study interviews. For children under 5 years of age, the caregiver (a parent or grandparent) completed the informed consent form (ICF) and only the caregiver participated in the interviews. For children aged 5–17 years, both the child and their caregiver(s) participated in the interviews; the caregiver completed the ICF and the child provided either verbal assent (children aged 5–9 years) or written assent (children aged 10–17 years) to participate.
Footnotes
One adult aged 34 years was enrolled and was included in the 18–30 year recruitment target
Binita M. Kamath and Benjamin L. Shneider: Part of the Childhood Liver Disease Research Network (ChiLDReN) and in receipt of National Institutes of Health grant support (NIH NIDDK DK103149).
Electronic supplementary material
The online version of this article (doi:10.1007/s40271-017-0266-4) contains supplementary material, which is available to authorized users.
References
- 1.Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet. 2003;40(12):891–895. doi: 10.1136/jmg.40.12.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16(3):243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 3.McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79(1):169–173. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamath BM, Bauer RC, Loomes KM, Chao G, Gerfen J, Hutchinson A, et al. NOTCH2 mutations in Alagille syndrome. J Med Genet. 2012;49(2):138–144. doi: 10.1136/jmedgenet-2011-100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alagille D, Estrada A, Hadchouel M, Gautier M, Odievre M, Dommergues JP. Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J Pediatr. 1987;110(2):195–200. doi: 10.1016/S0022-3476(87)80153-1. [DOI] [PubMed] [Google Scholar]
- 6.Emerick KM, Rand EB, Goldmuntz E, Krantz ID, Spinner NB, Piccoli DA. Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology. 1999;29(3):822–829. doi: 10.1002/hep.510290331. [DOI] [PubMed] [Google Scholar]
- 7.Turnpenny PD, Ellard S. Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2012;20(3):251–257. doi: 10.1038/ejhg.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elisofon SA, Emerick KM, Sinacore JM, Alonso EM. Health status of patients with Alagille syndrome. J Pediatr Gastroenterol Nutr. 2010;51(6):759–765. doi: 10.1097/MPG.0b013e3181ef3771. [DOI] [PubMed] [Google Scholar]
- 9.Kamath BM, Chen Z, Romero R, Fredericks EM, Alonso EM, Arnon R, et al. Quality of life and its determinants in a multicenter cohort of children with Alagille syndrome. J Pediatr. 2015;167(2):390–396.e3. doi: 10.1016/j.jpeds.2015.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mells GF, Pells G, Newton JL, Bathgate AJ, Burroughs AK, Heneghan MA, et al. Impact of primary biliary cirrhosis on perceived quality of life: the UK-PBC national study. Hepatology. 2013;58(1):273–283. doi: 10.1002/hep.26365. [DOI] [PubMed] [Google Scholar]
- 11.Weisshaar E, Gieler U, Kupfer J, Furue M, Saeki H, Yosipovitch G, et al. Questionnaires to assess chronic itch: a consensus paper of the special interest group of the International Forum on the Study of Itch. Acta Derm Venereol. 2012;92(5):493–496. doi: 10.2340/00015555-1402. [DOI] [PubMed] [Google Scholar]
- 12.Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92(5):502–507. doi: 10.2340/00015555-1246. [DOI] [PubMed] [Google Scholar]
- 13.Morris V, Murphy LM, Rosenberg M, Rosenberg L, Holzer CE, 3rd, Meyer WJ., 3rd Itch assessment scale for the pediatric burn survivor. J Burn Care Res. 2012;33(3):419–424. doi: 10.1097/BCR.0b013e3182372bfa. [DOI] [PubMed] [Google Scholar]
- 14.Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010;162(3):587–593. doi: 10.1111/j.1365-2133.2009.09586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stander S, Augustin M, Reich A, Blome C, Ebata T, Phan NQ, et al. Pruritus assessment in clinical trials: consensus recommendations from the International Forum for the Study of Itch (IFSI) Special Interest Group Scoring Itch in Clinical Trials. Acta Derm Venereol. 2013;93(5):509–514. doi: 10.2340/00015555-1620. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. 2014;4(1):25–36. doi: 10.1016/j.jceh.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. Progressive familial intrahepatic cholestasis. Orphanet J Rare Dis. 2009 doi: 10.1186/1750-1172-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitington PF, Whitington GL. Partial external diversion of bile for the treatment of intractable pruritus associated with intrahepatic cholestasis. Gastroenterology. 1988;95(1):130–136. doi: 10.1016/0016-5085(88)90301-0. [DOI] [PubMed] [Google Scholar]
- 19.Evaluating the Genetic causes and Progression of Cholestatic Liver Diseases (LOGIC). National Institute of Diabetes and Digestive and Kidney Diseases: 2007. https://clinicaltrials.gov/ct2/show/NCT00571272. Accessed 7 June 2017.
- 20.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. doi: 10.1093/sleep/23.8.1d. [DOI] [PubMed] [Google Scholar]
- 21.NIH Office of Extramural Research. Protecting human research participants. https://phrp.nihtraining.com/users/login.php. Accessed 10 Feb 2016 (password-protected resource).
- 22.Glaser BG, Strauss A. The discovery of grounded theory: strategies for qualitative research. Chicago: Aldine Transaction; 1967. [Google Scholar]
- 23.ATLAS.ti 7 User Guide and Reference. Berlin: 2013. http://atlasti.com/wp-content/uploads/2014/05/atlasti_v7_manual_201312.pdf?q=/uploads/media/atlasti_v7_manual_201312.pdf. Accessed 17 Dec 2015.
- 24.Bowen GA. Grounded theory and sensitizing concepts. Int J Qual Methods. 2006;5(3):12–23. doi: 10.1177/160940690600500304. [DOI] [Google Scholar]
- 25.Edelbrock C, Costello AJ, Dulcan MK, Kalas R, Conover NC. Age differences in the reliability of the psychiatric interview of the child. Child Dev. 1985;56(1):265–275. doi: 10.2307/1130193. [DOI] [PubMed] [Google Scholar]
- 26.Varni JW, Limbers CA, Burwinkle TM. How young can children reliably and validly self-report their health-related quality of life? An analysis of 8,591 children across age subgroups with the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:1. doi: 10.1186/1477-7525-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker A, Kelly D, McClean P, Karthikeyan P, McKiernan P, Dorenbaum A et al. A phase 2, randomized, placebo-controlled study (IMAGO) of LUM001, a novel inhibitor of the apical sodium-dependent bile acid transporter (ASBT), in paediatric patients with Alagille syndrome (ALGS). In: Late-breaker oral presentation at The International Liver Congress™ 2015, 50th Annual Meeting of the European Association for the Study of the Liver (EASL); 22–26 April; Vienna, Austria.
- 28.Validation of the Itch Reported Outcome (ItchRO) diaries in pediatric cholestatic liver disease. https://clinicaltrials.gov/ct2/show/NCT02131623?term=NCT02131623&rank=1. Accessed 07 June 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.