Abstract
The combined use of opioid and benzodiazepine medications increases the risk of hazardous effects, such as respiratory depression. Although recent increases in outpatient use of opioid prescriptions have been documented, there are limited data regarding rates and correlates of combined opioid and benzodiazepines among adults in outpatient settings. Our objective was to examine annual trends in outpatient visits including opioids, benzodiazepines, and their combination among adults as well as clinical and demographic correlates. We used data from the 1993–2014 National Ambulatory Medical Care Survey (NAMCS) among non-elderly (i.e., ages 18–64 years) adults to examine the probability of a visit including an opioid, benzodiazepine, or their combination, in addition to clinical and demographic correlates. From 1993 to 2014, benzodiazepines-with-opioids visits increased from 9.8 to 62.5 (OR = 9.23, 95% CI = 5.45–15.65) per 10,000 visits. Highest-represented groups among benzodiazepines-with-opioids visits were older (50–64 years) (49.1%), white (88.8%), commercially insured (58.0%) patients during their first visit (87.6%) to a primary-care physician (41.9%). We identified a significant increase in the outpatient co-prescription of opioids and benzodiazepines, notably among adults aged 50–64 years during primary-care visits. Educational and policy changes to provide alternatives to benzodiazepine-with-opioid co-prescription and limiting opioid prescription to pain specialists may reduce rates of this potentially hazardous combination.
Abbreviations: CDC, Centers for Disease Control and Prevention; CI, confidence interval; NCHS, National Center for Health Statistics; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; NAMCS, National Ambulatory Medical Care Survey; OR, odds ratio; RFV, reason-for-visit
Keywords: Opioid, Benzodiazepine, Pain, Prescriptions, Medication safety
Highlights
-
•
Current guidelines for prescribing opioids recommend against co-administration with benzodiazepines
-
•
Concurrent use of opioids and benzodiazepines increases the risk of overdose, respiratory depression, and death.
-
•
We examined 22 years of outpatient prescribing patterns of opioids and benzodiazepines using a survey of US physicians.
-
•
Over this period, visits with both opioids and benzodiazepines increased from roughly 9.8 to 62.5 per 10,000 visits.
-
•
These visits were more likely among older (50–64 years), white, privately insured patients with a low-back pain diagnosis.
1. Introduction
In the period between 1993 and 2014, the number of opioid analgesic prescriptions dispensed from retail pharmacies in the United States (US) increased from roughly 113 million to 264 million (Pezalla et al., 2017), with a corresponding increase in opioid-related diversion, abuse, and deaths between 2002 and 2010 (Dart et al., 2015). Similarly, between 1996 and 2013, the percentage of US adults who filled a prescription for a benzodiazepine increased from 4.1% to 5.6%, during which time the rate of deaths attributed to benzodiazepine overdoses increased from 0.58 to 3.07 per 100,000 adults (Bachhuber et al., 2016).
Although rates of outpatient prescription of opioids (Dart et al., 2015) and benzodiazepines (Bachhuber et al., 2016) may be stabilizing or decreasing in the years since 2010, the overall rates remain greatly elevated compared to previous decades. In addition, among adults who are prescribed opioids for daily use, nearly 40% have a concurrent prescription for a benzodiazepine. This is especially concerning given the increased risk of respiratory depression, overdose, and death associated with the co-administration of these two classes of medications (Karaca-Mandic et al., 2017, Saunders et al., 2012, Jann et al., 2014). For instance, in a prospective, outpatient-based study of adult patients prescribed high-dose opioids, those concurrently prescribed benzodiazepines were nearly 10 times more likely to die from overdose (Dasgupta et al., 2016). Similarly, among adults in the Veterans Health Administration who received prescriptions for opioids, nearly half of drug overdose deaths occurred among those concurrently prescribed benzodiazepines (Park et al., 2015). Privately insured, nonelderly adults who received prescriptions for both opioids and benzodiazepines, compared to opioids alone, were more likely to visit the emergency department or have an inpatient admission for opioid overdose (Sun et al., 2017). Furthermore, in the emergency department setting between 2004 and 2011, the percentage of opioid overdose deaths among adults that also involved benzodiazepine use increased steadily from 18% to 31% (Jones and McAninch, 2015).
In light of these and similar data, the Centers for Disease Control and Prevention (CDC) released revised guidelines (Dowell et al., 2016) for prescribing opioid analgesics for chronic pain in 2016, which recommend that “clinicians should avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible.” However, despite the well-documented risk of death from concurrent administration of opioids and benzodiazepines, less is known about the trends in general, outpatient prescribing patterns of these two classes of medications. Recent analyses of US prescription claims has revealed increased rates of co-prescription of benzodiazepines and opioids among non-elderly adults (Hwang et al., 2016) and adults receiving opioids for musculoskeletal pain (Larochelle et al., 2015). However, these studies have generally limited analyses to the years subsequent to 2001; this excludes the 1990s, which was also marked by a steady increase in rates of opioid prescriptions. In addition, previous studies have either used administrative or claims-level data (Sun et al., 2017, Hwang et al., 2016), which may be associated with validity issues (Strom, 2001), or focused on specific populations of adults (Larochelle et al., 2015).
Therefore, to address this gap in the literature, we sought to examine trends in the outpatient administration of opioids, benzodiazepines, and their combination among nonelderly adults from 1993 to 2014, clinical and demographic correlates of prescription patterns, and likelihood of physicians co-prescribing opioids and benzodiazepines. To achieve these aims, we used prospectively collected, nationally representative data from the National Ambulatory Medical Care Survey (NAMCS). We anticipate that results from these analyses will inform clinical and policy decisions by specifically identifying adults and clinicians who are at highest risk for benzodiazepine and opioid coadministration.
2. Material and methods
2.1. Sample
We used data from the 1993–2014 NAMCS, an annual, cross-sectional, nationally representative probability sample survey of non-federally employed office-based physicians, administrated by National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (National Center for Health Statistics, 2017). We limited our analysis to visits by patients aged 18–64 years (n = 402,027). Physicians specializing in anesthesiology, pathology, and radiology are excluded from the survey, as well as home-based visits or those within institutional settings (e.g., nursing homes). Survey response rates varied from 38.7% in 2014 to 73.1% in 1993 (mean = 61.6%). Within each patient visit, either the physician or a staff member recorded information about patient characteristics and medications that were “ordered, supplied, administered, and continued.” Detailed information regarding NAMCS administration and coding can be accessed elsewhere (Centers for Disease Control and Prevention, 2017). Because this research involves use of de-identified data, it was exempt from review by the Institutional Review Board of the University of California, San Francisco.
2.2. Medications
During the years 1993 to 2014, NAMCS forms allowed extraction of five to 30 current medications; to maintain consistency across years, we limited our analyses to the first five medications listed. Starting in 2006, NAMCS medications were coded using Lexicon Plus®, a proprietary database of Cerner Multum, Inc.; medication data from survey years prior to 2006 were recoded into Lexicon Plus® therapeutic classes using syntax developed by the NCHS. Visits were considered benzodiazepine visits if they were included in the Therapeutic Category Level 3, “069 – benzodiazepines” (i.e., alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, estazolam, flurazepam, halazepam, lorazepam, midazolam, nitrazepam, oxazepam, prazepam, quazepam, temazepam, or triazolam). Visits were considered opioid visits if they were included in the Therapeutic Category Level 3, “060 – analgesics” (i.e., alphaprodine, codeine, dezocine, diphenoxylate, fentanyl, glutethimide, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, nalbuphine, opium, oxycodone, oxymorphone, pentazocine, propoxyphene, remifentanil, sufentanil, or tapentadolor).
2.3. Demographic characteristics
We classified visits by patient sex, age at time of visit (18–34, 35–49, or 50–64 years), and race (white, black, or other). Because ethnicity was not recorded consistently among the included survey years, this variable was excluded from our analyses.
2.4. Primary source of payment
Following guidelines established by the NCHS, we grouped visits into mutually exclusive payment categories in descending order: (Pezalla et al., 2017) private-pay or commercial, (Dart et al., 2015) Medicare, (Bachhuber et al., 2016) Medicaid and other government insurance (including the Children's Health Insurance Program), or (Karaca-Mandic et al., 2017) other (self-pay, no charge, or “other”).
2.5. Reasons for visit and diagnoses
Survey forms included up to three patient-generated reasons-for-visit (RFV) as well as up to three visit diagnoses (using the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]). Pain was classified based on physical location into six categories using RFVs: head or neck, chest, abdomen or pelvis, back or hip, extremities, or unspecified. We also identified visits in which any pain was listed as the primary (i.e., first listed) RFV. Visits were also classified by relevant diagnoses based on ICD-9-CM codes: low-back pain (722.10, 722.52, 724.2–724.6, 738.4, 756.11, 839.2, 846.0, 847.2), cancer (140–239, 338.3), anxiety disorders (293.84, 300.0, 300.2–300.3, 308.3, 309.21, 309.81, 313.0), substance use disorders (291–292, 303–305), depressive disorders (296.2, 296.3, 300.4, 311), and insomnia (307.4, 327.00, 327.01, 327.02, 327.09, 780.50, 780.51, 780.52, 780.55, 780.56, 780.59).
2.6. Other visit-level characteristics
We grouped visit status as a first or returning visit by whether the treating physician or anyone in the practice had seen the patient before. In addition, we recoded the specialty of the treating physician as primary care (internal medicine, geriatric medicine, adolescent medicine, pediatrics, family practice, and general practice), psychiatry, or another medical specialty.
2.7. Statistical analysis
We used logistic regression to assess year-by-year time trends in the probability of that visits included any 1 of the 3 medication groups, controlling for patient age and race. We assigned a study-year period variable ([survey year-1993]/22) to examine the strength of association of medication-group prescription from 1993 through 2014. The resultant odds ratios for each medication group estimates the change in odds of a visit containing the medication group relative to a visit in which neither benzodiazepines nor opioids were prescribed over the entire 1993–2014 study period. We compared differences in proportion by visit characteristics using χ2 tests. Among visits with physician-level weights (2005–2014, n = 6338), we similarly used logistic regression to examine time trends of the 3 medication groups controlling for the visits per physician. Analyses were adjusted for visit weights, clustering, and strata using complex survey-design elements; incorporating these elements yields data that are reflective of annual visits to US office-based physicians. We performed analyses using SAS, version 9.4 (SAS Institute), with 2-sided tests (α = 0.05).
3. Results
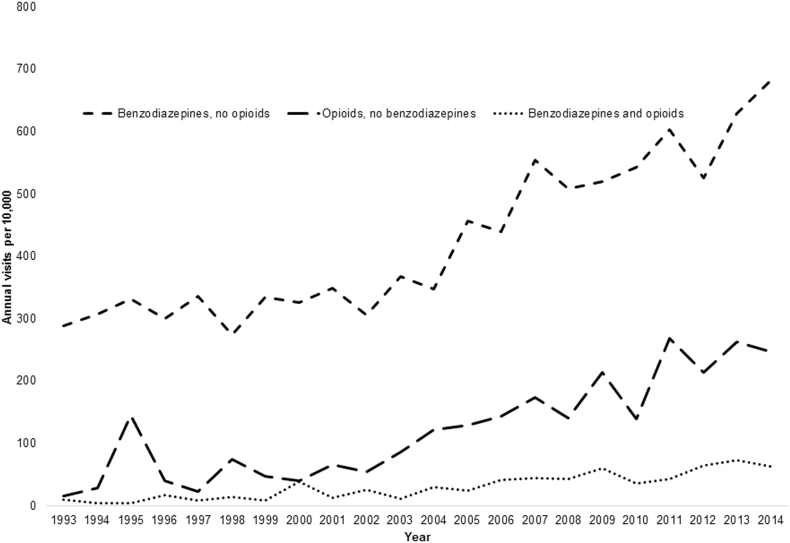
Fig. 1 illustrates the year-by-year frequency with which benzodiazepines-with-opioids visits occurred over the study period for the entire sample. Between 1993 and 2014, annual benzodiazepines-with-opioids visits increased from 9.8 to 62.5 (OR = 9.23, 95% CI = 5.45–15.65) per 10,000 visits, benzodiazepines-only visits from 288.3 to 682.4 per 10,000 visits (OR = 8.18, 95% CI = 4.12, 16.25), and opioids-only visits from 15.4 to 247.2 per 10,000 visits (OR = 5.63, 95% CI = 3.17–10.02). Between 2005 and 2014, an increased proportion of physicians provided benzodiazepines-with-opioids visits (0% in 2005 to 1.41% in 2014; OR = 47.56, 95% CI = 6.95, 325.46), but not benzodiazepines-only (1.61% in 2005 to 10.49% in 2014; OR = 1.09, 95% CI = 0.66, 1.79) or opioids-only visits (0.75% in 2005 to 4.03% in 2014; OR = 2.16, 95% CI = 0.82, 5.72).
Fig. 1.
Trends in annual ambulatory prescribing of Schedule IV benzodiazepines, Schedule II opioids, or both, United States.
Number of annual visits among each medication group, 1993–2014; all analyses were conducted using survey-design elements for visit or physician weight, clustering, and stratification to yield national inferences.
In contrast to Fig. 1, Table 1 uses combined data from all 22 survey years to examine visit characteristics associated with each of the 3 medication groups. Nearly 42% of benzodiazepines-with-opioids visits were among primary care physicians, compared with 2.9% among psychiatrists and 55.2% among other medical specialties. Furthermore, benzodiazepines-with-opioids visits were concentrated among older (50–64 years, 49.1%), white (88.8%), commercially insured (58.0%) adults with a low-back pain diagnosis (19.7%) during their initial visit to the index physician (87.6%). Notably, the distribution of payer types differed significantly between opioids-only and (p < 0.001), with more commercially insured benzodiazepines-only visits compared with opioid-only visits. Compared to benzodiazepines-with-opioids visits, benzodiazepines-only visits (p < 0.0001), but not opioids-only visits, were significantly more likely to be by men than women. In regards to the type of pain, benzodiazepines-with-opioids visits were more likely than opioids-only visits for back/hip pain (p < 0.001); however, benzodiazepines-with-opioids visits and opioids-only visits occurred at similar rates for pain of the head/neck, chest, abdominal/pelvic, extremities, and for unspecified locations. Furthermore, a diagnosis of a substance use disorder occurred among a significantly higher percentage of benzodiazepines-with-opioids visits than among opioids-only visits (p < 0.001), but not among opioids-only visits. Among benzodiazepines-with-opioids visits, low-back pain represented the largest diagnostic category (19.7%).
Table 1.
Volume and distribution of annual ambulatory medical visits including Schedule IV benzodiazepines, Schedule II opioids, or both by background patient demographic and clinical characteristics, United States, 1993–2014.
| Visits, % |
Comparisons a | |||
|---|---|---|---|---|
| 1. Both opioids and benzodiazepines (N = 1350) |
2. Opioids, no benzodiazepines (N = 5351) |
3. Benzodiazepines, no opioids (N = 19,413) |
||
| Sexb | ||||
| Male | 45.1 | 46.3 | 34.3 | 1—3⁎⁎⁎, 2—3⁎⁎⁎ |
| Female | 54.9 | 53.7 | 65.7 | |
| Ageb | ||||
| 18–34 | 15.6 | 16.2 | 17.8 | 1—3⁎, 2—1⁎ |
| 35–49 | 35.3 | 41.5 | 39.4 | |
| 50–64 | 49.1 | 42.4 | 42.8 | |
| Raceb | ||||
| White | 88.8 | 85.3 | 89.5 | 1—2⁎, 2—3⁎⁎⁎ |
| Black | 7.4 | 11.5 | 7.9 | |
| Other | 3.8 | 3.1 | 2.6 | |
| Primary payerb | ||||
| Commercial | 58.0 | 54.9 | 61.6 | 2—3⁎⁎⁎ |
| Medicare | 14.2 | 15.0 | 13.0 | |
| Medicaid | 11.7 | 14.2 | 10.2 | |
| Self-pay or other | 16.1 | 15.9 | 15.6 | |
| Visit statusb | ||||
| First visit | 87.6 | 88.6 | 91.0 | 2—3⁎⁎ |
| Return visit | 12.4 | 11.4 | 9.0 | |
| Pain as reason for first visitc | 27.7 | 37.3 | 13.7 | 3 < 2⁎⁎⁎, 2 > 1⁎⁎, 3 < 1⁎⁎⁎ |
| Type of painc | ||||
| Head/neck | 7.7 | 9.6 | 5.0 | 3 < 2⁎⁎⁎, 3 < 1⁎ |
| Chest | 1.5 | 1.5 | 1.7 | NS |
| Abdominal/pelvic | 3.8 | 3.5 | 2.5 | 3 < 2⁎⁎, 3 < 1⁎ |
| Back/hip | 13.9 | 21.8 | 6.1 | 3 < 2⁎⁎⁎, 2 > 1⁎⁎⁎, 3 < 1⁎⁎⁎ |
| Extremities | 10.3 | 13.4 | 5.1 | 3 < 2⁎⁎⁎, 3 < 1⁎⁎⁎ |
| Unspecified | 8.8 | 8.4 | 2.4 | 3 < 2⁎⁎⁎, 3 < 1⁎⁎⁎ |
| Physician specialtyb | ||||
| Primary care | 41.9 | 47.8 | 49.0 | 2—3⁎⁎⁎, 1—3⁎⁎⁎ |
| Psychiatry | 2.9 | 4.6 | 27.0 | |
| Other | 55.2 | 47.6 | 24.0 | |
| Selected medical or mental health problemc | ||||
| Low-back pain | 19.7 | 15.9 | 5.7 | 3 < 2⁎⁎⁎, 3 < 1⁎⁎⁎ |
| Cancer | 5.5 | 8.2 | 2.8 | 3 < 2⁎⁎⁎, 2 > 1⁎, 3 < 1⁎⁎⁎ |
| Anxiety | 1.2 | 7.4 | 25.8 | 3 > 2⁎⁎⁎, 2 > 1⁎⁎⁎, 3 > 1⁎⁎⁎ |
| Substance use | 6.4 | 2.1 | 3.7 | 2 < 1⁎ |
| Depression | 3.5 | 6.2 | 21.2 | 3 > 2⁎⁎⁎, 2 > 1⁎, 3 > 1⁎⁎⁎ |
| Insomnia | 1.0 | 1.5 | 3.2 | 3 > 2⁎⁎⁎, 3 > 1⁎ |
Analyses were conducted using survey-design elements for visit weight, clustering, and stratification to yield national inferences.
Difference-in-ratio (chi-squared) tests among the 3 medication groups; directionality for significant between-group differences is indicated only for 1 × 3 tables. NS, not significant.
Percentages represent the fraction of the listed categories (e.g., 34.3% of total visits in which benzodiazepines, but no opioids, were prescribed were among males and the remainder, 65.7%, were among females). Values within columns may not sum to 100% because of rounding.
Percentages represent the fraction of visits within each item (e.g., 5.7% of total visits in which benzodiazepines, but no opioids, were prescribed included a diagnosis of lower-back pain and the remainder, 94.3%, did not).
P < 0.05.
P < 0.01.
P < 0.001.
4. Discussion
Between 1993 and 2014, the proportion of outpatient medical visits that included co-prescription of benzodiazepines with opioids more than doubled. The increase more closely followed the prescription of benzodiazepines without opioids than with prescription of opioids alone. Notably, a substantial percentage of these visits were in primary-care settings and occurred during the initial visit, suggesting a need to reduce the inappropriate use of opioids in non-specialist settings. Our finding that low-back pain represented the single highest diagnostic correlate of co-prescription of benzodiazepines and opioids is especially concerning given limited evidence supporting the use of opioids for low-back pain (Abdel Shaheed et al., 2016). Furthermore, the fact that substance use disorders were more frequently observed among benzodiazepines-with-opioids compared with opioids-only visits is alarming given the abuse additive abuse potential of these 2 sedative medications compared with either alone.
These results, in particular increased rates of visits with both benzodiazepines and opioids, are particularly relevant in the context of increased rates of drug overdose deaths in the past 2 decades. For instance, Rudd et al., using data from the National Vital Statistics System multiple cause-of-death mortality files, demonstrated that the number of drug overdose deaths among adults in the US tripled between 2000 and 2014 (Rudd et al., 2016a); in the period from 2014 to 2015 alone, the number of drug-overdose deaths associated with synthetic opioids other than methadone increased by 72.2% from 1.8 to 3.1 per 100,000 population (Rudd et al., 2016b). Concurrent benzodiazepine use has also contributed to the increased rate of opioid-related overdose deaths. For instance, Kandel et al., also using National Vital Statistics System data, demonstrated that the proportion prescription opioid-related deaths attributable to combined use with benzodiazepines increased 1.7 times from 16.8% to 27.9% between 2002–2003 and 2014–2015 (Kandel et al., 2017). Similarly, nationally representative data from US emergency departments reveals that drug overdose deaths involving both opioids and benzodiazepines increased nearly three-fold in the period from 2004 to 2011 from 0.6 to 1.7 per 100,000 population; furthermore, benzodiazepine involvement in opioid-related overdose deaths increased from 18% to 31% in the same 7-year period (Jones and McAninch, 2015). Taken together, these results further support the importance of quantifying co-prescription of opioids and benzodiazepines, as this practice may be contributing to combined opioid-benzodiazepine overdose deaths in the US.
These results should also be considered in the context of a related trend; namely, in the period between 1999 and 2013, the annual all-cause mortality of middle-aged (ages 45–54 years), white, non-Hispanic US adults increased by 33.9 per 100,000 population (Case and Deaton, 2015). Primary causes of these deaths included poisonings and suicides; furthermore, increased mortality in this subpopulation paralleled increases in morbidity, including worsened self-reported mental and physical health, daily functioning, and increased alcohol use. This trend contrasts with decreased morbidity over the same period among most other demographic groups. Increased mortality due to these “diseases of despair” (Kochanek et al., 2016) among middle-aged, white adults is especially pertinent in light of our findings, given the potential use of combined opioids and benzodiazepines in intentional overdose deaths, as well as our finding that white adults were more likely than other racial groups to receive combined opioids and benzodiazepines over the study period.
Taken together, the results of the current study suggest that limiting the prescription of benzodiazepines may slow the increased co-prescription of benzodiazepines with opioids and seeking to confine co-prescription to specialists trained in pain management. This recommendation is hindered, however, by a shortage of pain specialists in the US; in its 2011 report on pain management, the Institute of Medicine estimated that there was only one pain specialist per 28,500 individuals with pain (Institute of Medicine of the National Academies, 2011). When a pain specialist is not available, alternatives to benzodiazepines should be considered for patients with pain and anxiety including selective-serotonin reuptake inhibitors and cognitive-behavioral therapy, as well as alternative interventions such as acupuncture and physical therapy (Rosenberg et al., 2008). Likewise, non-opioid analgesic treatments should be considered, including behavioral approaches (e.g., cognitive-behavioral therapy), acupuncture, physical therapy, and non-opioid medications such as nonsteroidal antiinflammatory drugs (Dowell et al., 2016).
There are several limitations of this study. First, the variable non-response rate among survey years introduces a potential source of bias. Second, we are not able to determine whether the visit physician prescribed the listed medications for those listed as “continued;” for instance, an opioid may have been prescribed by other physician prior to the recorded encounter. Third, NACMS does not contain information regarding medication adherence, strength, duration of treatment, or whether the medication is taken on a scheduled or as-needed basis. Fourth, because only up to three RVFs and visit diagnoses were allowed, the RVF or diagnosis associated with a given medication may have been missing from a visit. Fifth, because NAMCS is limited to office-based visits, it does not capture visits to emergency departments, hospital outpatient clinics, cancer centers, and other outpatient medical settings where opioids and benzodiazepines are prescribed. Similarly, sixth, for the same reason, NAMCS does not capture non-medical use of opioids and benzodiazepines (e.g., use of others' prescribed medications, or receipt from non-medical providers in the community).
As a final consideration, many patients with opioid use disorder receive pharmacologic treatment with either methadone (a full opioid agonist) or buprenorphine (a partial opioid agonist). Increasingly, patients are alternatively offered buprenorphine-naloxone (to reduce abuse potential) or naltrexone (an opioid antagonist). These patients may concurrently use benzodiazepines, which may be as hazardous as the combination of non-methadone opioids and benzodiazepines. Concurrent use of benzodiazepines and methadone has been implicated in methadone-related deaths (Chan et al., 2006, Mikolaenko et al., 2002), likely because benzodiazepines compete for opioid receptors and may inhibit hepatic enzymes responsible for degrading methadone. Furthermore, among patients engaged in methadone maintenance therapy, concurrent benzodiazepine use has been associated with higher rates of opioid-positive urine samples during treatment (Brands et al., 2008). However, although there may be less risk of fatal overdose when benzodiazepines are taken in combination with buprenorphine (instead of methadone) (Nielsen et al., 2007), concurrent benzodiazepine and buprenorphine users are still at higher risk of more frequent emergency department visits for accidental injury (Schuman-Olivier et al., 2013) and higher rates of depression and anxiety (Lavie et al., 2009).
Given our focus on combined benzodiazepine and prescription opioids in this study, as well as the consideration that buprenorphine may not be accurately represented in the NAMCS data because they are primarily prescribed in chemical-dependency treatment facilities, we did not examine visits with methadone or buprenorphine specifically. However, given the widespread use of these medications in pain management and opioid use disorder treatment, trends in use of these medications in combination with benzodiazepines should be pursued in future studies, especially using data that incorporate chemical-dependency treatment centers.
4.1. Implications
Among the 47 studies included in a recent systematic review of causes of increased opioid-related mortality in the United States and Canada between 1990 and 2013, 14 included polydrug toxicity as a major factor (King et al., 2014). In addition, public-health research on the causes of the opioid epidemic has shifted from focus on misuse and diversion of prescription analgesics to iatrogenic addiction (Beauchamp et al., 2014), attributable to the increased prescription of opioids for various indications. In this context, the observed increased rate of visits containing both opioids and benzodiazepines calls for public health action.
Educational initiatives may help to stem the increase in opioid and benzodiazepine co-prescriptions. For instance, the Surgeon General, as part of the “Turn the Tide” campaign, distributed a two-page pocket guide (The Office of the Surgeon General, n.d.) for reducing use of opioids for chronic pain and summarizes the CDC guidelines (Dowell et al., 2016) in an easy-to-access format. Though this guide suggests prescribers monitor for concomitant benzodiazepine use, more directed efforts may be made to explicitly list the clinical hazards of opioid and benzodiazepine co-prescription, and provide safe alternatives in specific clinical scenarios. Other strategies to address this public health issue include simultaneous policy initiatives, such as increased support for and mandated use of prescription-drug monitoring programs (Gugelmann and Perrone, 2011), regulating so-called opioid pill-mill pain clinics, and establishing dosage thresholds (Garcia, 2013).
Furthermore, clinicians should remain vigilant for sleep disordered breathing associated with opioids and benzodiazepines, especially when used together. Among patients with chronic opioid therapy (i.e., > 90 days), as many as 85% may have central, obstructive or mixed-type sleep apnea, as verified by polysomnography (Mogri et al., 2009). In addition, higher chronic benzodiazepine doses are associated with greater incidence of central sleep apnea among patients with chronic opioid therapy (Hassamal et al., 2016). Therefore, physicians treating patients with chronic opioid therapy and concomitant benzodiazepines should consider referral for home- or laboratory-based polysomnography, especially in the presence of other risk factors for obstructive sleep apnea (e.g., elevated body-mass index, thick neck circumference, smoking, male sex) and central sleep apnea (e.g., history of stroke, cardiovascular disease) (Cheatle and Webster, 2015). Other risk-mitigation strategies include reduction of opioid dose, substitution of opioids with non-opioid analgesics, avoidance of polypharmacy with other sedative medications (e.g., benzodiazepines, hypnotics), reduction or elimination of concurrent alcohol use, and referral to a sleep-medicine specialist (Cheatle and Webster, 2015).
Funding sources
This work was funded, in part, by a grant to Dr. Hirschtritt from the National Institute of Mental Health (R25 MH060482). The funding source had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript.
Conflicts of interest
None.
Contributor Information
Matthew E. Hirschtritt, Email: matthew.hirschtritt@ucsf.edu.
Kevin L. Delucchi, Email: kevin.delucchi@ucsf.edu.
Mark Olfson, Email: olfsonm@nyspi.columbia.edu.
References
- Abdel Shaheed C., Maher C.G., Williams K.A., Day R., McLachlan A.J. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Intern. Med. 2016;176(7):958–968. doi: 10.1001/jamainternmed.2016.1251. [DOI] [PubMed] [Google Scholar]
- Bachhuber M.A., Hennessy S., Cunningham C.O., Starrels J.L. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am. J. Public Health. 2016;106(4):686–688. doi: 10.2105/AJPH.2016.303061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp G.A., Winstanley E.L., Ryan S.A., Lyons M.S. Moving beyond misuse and diversion: the urgent need to consider the role of iatrogenic addiction in the current opioid epidemic. Am. J. Public Health. 2014;104(11):2023–2029. doi: 10.2105/AJPH.2014.302147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands B., Blake J., Marsh D.C., Sproule B., Jeyapalan R., Li S. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J. Addict. Dis. 2008;27(3):37–48. doi: 10.1080/10550880802122620. [DOI] [PubMed] [Google Scholar]
- Case A., Deaton A. Rising morbidity and mortality in midlife among white Non-Hispanic Americans in the 21st century. Proc. Natl. Acad. Sci. U. S. A. 2015;112(49):15078–15083. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Ambulatory Health Care Data. 2017. https://www.cdc.gov/nchs/ahcd/index.htm.Accessed: (September 5)
- Chan G.M., Stajic M., Marker E.K., Hoffman R.S., Nelson L.S. Testing positive for methadone and either a tricyclic antidepressant or a benzodiazepine is associated with an accidental overdose death: analysis of medical examiner data. Acad. Emerg. Med. 2006;13(5):543–547. doi: 10.1197/j.aem.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Cheatle M.D., Webster L.R. Opioid therapy and sleep disorders: risks and mitigation strategies. Pain Med. 2015;16(Suppl. 1):S22–6. doi: 10.1111/pme.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart R.C., Surratt H.L., Cicero T.J. Trends in opioid analgesic abuse and mortality in the United States. N. Engl. J. Med. 2015;372(3):241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- Dasgupta N., Funk M.J., Proescholdbell S., Hirsch A., Ribisl K.M., Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med. 2016;17(1):85–98. doi: 10.1111/pme.12907. [DOI] [PubMed] [Google Scholar]
- Dowell D., Haegerich T.M., Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A.M. State laws regulating prescribing of controlled substances: balancing the public health problems of chronic pain and prescription painkiller abuse and overdose. J. Law Med. Ethics. 2013;41(Suppl. 1):42–45. doi: 10.1111/jlme.12037. [DOI] [PubMed] [Google Scholar]
- Gugelmann H.M., Perrone J. Can prescription drug monitoring programs help limit opioid abuse? JAMA. 2011;306(20):2258–2259. doi: 10.1001/jama.2011.1712. [DOI] [PubMed] [Google Scholar]
- Hassamal S., Miotto K., Wang T., Saxon A.J. A narrative review: the effects of opioids on sleep disordered breathing in chronic pain patients and methadone maintained patients. Am. J. Addict. 2016;25(6):452–465. doi: 10.1111/ajad.12424. [DOI] [PubMed] [Google Scholar]
- Hwang C.S., Kang E.M., Kornegay C.J., Staffa J.A., Jones C.M., McAninch J.K. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002–2014. Am. J. Prev. Med. 2016;51(2):151–160. doi: 10.1016/j.amepre.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies . 2011. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. [PubMed] [Google Scholar]
- Jann M., Kennedy W.K., Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J. Pharm. Pract. 2014;27(1):5–16. doi: 10.1177/0897190013515001. [DOI] [PubMed] [Google Scholar]
- Jones C.M., McAninch J.K. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am. J. Prev. Med. 2015;49(4):493–501. doi: 10.1016/j.amepre.2015.03.040. [DOI] [PubMed] [Google Scholar]
- Kandel D.B., MC Hu, Griesler P., Wall M. Increases from 2002 to 2015 in prescription opioid overdose deaths in combination with other substances. Drug Alcohol Depend. 2017;178:501–511. doi: 10.1016/j.drugalcdep.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca-Mandic P., Meara E., Morden N.E. The growing problem of co-treatment with opioids and benzodiazepines. BMJ. 2017;356:j1224. doi: 10.1136/bmj.j1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N.B., Fraser V., Boikos C., Richardson R., Harper S. Determinants of increased opioid-related mortality in the United States and Canada, 1990–2013: a systematic review. Am. J. Public Health. 2014;104(8):e32–42. doi: 10.2105/AJPH.2014.301966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek K.D., Arias E., Bastian B.A. 2016. The Effect of Changes in Selected Age-specific Causes of Death on Non-hispanic White Life Expectancy Between 2000 and 2014. Data Brief no. 250. [PubMed] [Google Scholar]
- Larochelle M.R., Zhang F., Ross-Degnan D., Wharam J.F. Trends in opioid prescribing and co-prescribing of sedative hypnotics for acute and chronic musculoskeletal pain: 2001–2010. Pharmacoepidemiol. Drug Saf. 2015;24(8):885–892. doi: 10.1002/pds.3776. [DOI] [PubMed] [Google Scholar]
- Lavie E., Fatseas M., Denis C., Auriacombe M. Benzodiazepine use among opiate-dependent subjects in buprenorphine maintenance treatment: correlates of use, abuse and dependence. Drug Alcohol Depend. 2009;99(1–3):338–344. doi: 10.1016/j.drugalcdep.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Mikolaenko I., Robinson C.A., Jr., Davis G.G. A review of methadone deaths in Jefferson County, Alabama. Am J Forensic Med Pathol. 2002;23(3):299–304. doi: 10.1097/00000433-200209000-00021. [DOI] [PubMed] [Google Scholar]
- Mogri M., Desai H., Webster L., Grant B.J., Mador M.J. Hypoxemia in patients on chronic opiate therapy with and without sleep apnea. Sleep Breath. 2009;13(1):49–57. doi: 10.1007/s11325-008-0208-4. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics, Centers for Disease Control and Prevention. National Ambulatory Medical Care Survey: 2014 NAMCS Micro-data File Documentation. ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NAMCS/doc2014.pdf. Updated 2014, Accessed May 1, 2017.
- Nielsen S., Dietze P., Lee N., Dunlop A., Taylor D. Concurrent buprenorphine and benzodiazepines use and self-reported opioid toxicity in opioid substitution treatment. Addiction. 2007;102(4):616–622. doi: 10.1111/j.1360-0443.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- Park T.W., Saitz R., Ganoczy D., Ilgen M.A., Bohnert A.S. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezalla E.J., Rosen D., Erensen J.G., Haddox J.D., Mayne T.J. Secular trends in opioid prescribing in the USA. J. Pain Res. 2017;10:383–387. doi: 10.2147/JPR.S129553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E.I., Genao I., Chen I. Complementary and alternative medicine use by primary care patients with chronic pain. Pain Med. 2008;9(8):1065–1072. doi: 10.1111/j.1526-4637.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- Rudd R.A., Aleshire N., Zibbell J.E., Gladden R.M. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb. Mortal. Wkly Rep. 2016;64(50–51):1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- Rudd R.A., Seth P., David F., Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010–2015. MMWR. 2016;65(5051):1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- Saunders K.W., Von Korff M., Campbell C.I. Concurrent use of alcohol and sedatives among persons prescribed chronic opioid therapy: prevalence and risk factors. J. Pain. 2012;13(3):266–275. doi: 10.1016/j.jpain.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman-Olivier Z., Hoeppner B.B., Weiss R.D., Borodovsky J., Shaffer H.J., Albanese M.J. Benzodiazepine use during buprenorphine treatment for opioid dependence: clinical and safety outcomes. Drug Alcohol Depend. 2013;132(3):580–586. doi: 10.1016/j.drugalcdep.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom B.L. Data validity issues in using claims data. Pharmacoepidemiol. Drug Saf. 2001;10(5):389–392. doi: 10.1002/pds.610. [DOI] [PubMed] [Google Scholar]
- Sun E.C., Dixit A., Humphreys K., Darnall B.D., Baker L.C., Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760. doi: 10.1136/bmj.j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Office of the Surgeon General Prescribing Opioids for Chronic Pain. http://Turnthetiderx.org/wp-content/uploads/2016/06/TurnTheTide_PocketGuide_Final.pdf available at: