Summary
Early life stress (ELS) in the form of child abuse/neglect is associated with an increased risk of developing social dysfunction in adulthood. Little is known, however, about the neural substrates or the neuromodulatory signaling that govern ELS-induced social dysfunction. Here, we show that ELS-induced downregulation of dopamine receptor 3 (Drd3) signaling and its corresponding effects on neural activity in the lateral septum (LS) are both necessary and sufficient to cause social abnormalities in adulthood. Using in vivo Ca2+ imaging, we found that Drd3-expressing-LS (Drd3LS) neurons in animals exposed to ELS show blunted activity in response to social stimuli. In addition, optogenetic activation of Drd3LS neurons rescues ELS-induced social impairments. Furthermore, pharmacological treatment with a Drd3 agonist, which increases Drd3LS neuronal activity, normalizes the social dysfunctions of ELS mice. Thus, we identify Drd3 in the LS as a critical mediator and potential therapeutic target for the social abnormalities caused by ELS.
Keywords: early life stress, early social deprivation, lateral septum, Drd3, social dysfunction, PD128907
Highlights
-
•
Early social deprivation (ESD) causes downregulation of Drd3 signaling in the LS
-
•
Blunted LS Drd3 neuronal activity mediate ESD-induced social dysfunctions
-
•
Drd3 signaling has corresponding effects on neuronal activity in the LS
-
•
Activation of Drd3 signaling in the LS normalize social impairments of ESD mice
Exposure to early adverse experiences is associated with an increased risk of developing social dysfunction later in life. Shin et al. show that Drd3 signaling and its corresponding effects on neural activity in the LS are both necessary and sufficient to cause early life stress-induced social abnormalities in adulthood.
Introduction
Children exposed to early life stress (ELS) such as physical abuse and emotional neglect during a critical period in their development are more likely to display social dysfunction later in life (Bandelow et al., 2004, Heim and Nemeroff, 2001, Kessler et al., 1997). Symptoms of disrupted social behaviors include decreased social motivation and a lack of interest in attending to social stimuli or seeking and enjoying reciprocal social interactions (Kohls et al., 2013). Given that high levels of early adversity are also associated with the asocial behaviors of psychiatric patients (Bolger and Patterson, 2001), identifying the neural mechanisms underlying ELS-induced social dysfunction is essential for the development of treatment strategies for mental illnesses associated with social impairments, such as autism spectrum disorder (ASD) (Rai et al., 2012), schizophrenia (Agid et al., 1999), and major depressive disorder (MDD) (Kendler et al., 1999).
Moreover, clinical studies have suggested that MDD patients with a history of ELS exhibit significantly poorer responses and lower remission rates upon receiving conventional pharmacotherapy and psychotherapy (Bruce et al., 2012, Nanni et al., 2012). This suggests that such individuals possess a distinct endophenotype that will require alternative therapeutic strategies (Nemeroff, 2016). In this context, several recent studies have addressed the neurobiological characteristics of individuals exposed to ELS by pointing to putative dysfunctions in reward-related brain activation (Goff et al., 2013). For example, blunted reactivity to rewarding stimuli in the ventral striatum of adult participants with a history of ELS may constitute a neural mechanism by which ELS increases the risk of many psychiatric symptoms in adulthood (Hanson et al., 2016). However, neither the specific neural substrates nor the precise neuromodulatory mechanisms that transduce ELS into altered neuronal activity resulting in social dysfunction in adulthood are well understood. Thus, the identification of the specific neural mechanisms underlying ELS-induced social abnormalities is a critical next step for the development of therapeutics for psychiatric diseases elicited by ELS.
The lateral septum (LS) is thought to be critical for processing emotional information and for modulating behavioral responses to stress (Guzmán et al., 2013, Sheehan et al., 2004, Singewald et al., 2011). Moreover, the LS has been identified as a potent site for electrical self-stimulation in both rodents and humans (Heath, 1963, Olds and Milner, 1954), and it also has been implicated in reward-related processes such as drug addiction and social behaviors (Harasta et al., 2015, Luo et al., 2011, Mesic et al., 2015). Notably, the LS receives dopaminergic inputs from the ventral tegmental area (VTA) (Reddy et al., 2016)—a critical component of the reward circuitry—and contains subpopulations of neurons that express dopamine receptors that have not yet been subjected to functional dissection.
Here, we identify dopamine receptor 3 (Drd3)-expressing-LS (Drd3LS) neurons as a critical component mediating the detrimental effects of ELS on social behavior. Employing an early social deprivation (ESD) stress paradigm, we found that Drd3 signaling in the LS is significantly downregulated in mice exposed to ESD and that this is accompanied by abnormal social behaviors such as reduced social preferences and severe communication deficits. Furthermore, the Drd3LS neurons of ESD mice show significantly decreased reactivity to social stimuli, while their optogenetic activation ameliorates ESD-induced social impairment. Notably, pharmacological treatment with the Drd3 agonist PD128907, which increases Drd3LS neuron activity, normalizes the abnormal social behaviors of ESD mice. Simultaneous knockdown of Drd3 in the LS, however, abolishes this pharmacological rescue. Taken together, our findings identify Drd3LS neuronal signaling as a critical mediator of the ELS-induced social impairments in adulthood. Drd3 in the LS may therefore constitute an important therapeutic target for the treatment of the severe social impairments commonly observed in numerous neuropsychiatric disorders.
Results
Adult ESD Mice Display Social Dysfunction and Reduced c-fos Induction in the LS in Response to Social Stimuli
To investigate how ELS affects social behavior later in life, we adopted the ESD stress paradigm that disrupts early social bonding by exposing pups to daily infant-mother/littermate separation during their first 2 weeks of postnatal life (Figures 1A and 1B; see STAR Methods). Adult mice (8–12 weeks old) exposed to ESD showed no significant differences from controls in locomotion, anxiety-like behaviors, novel object recognition, or olfactory perception (Figures S1A–S1F).
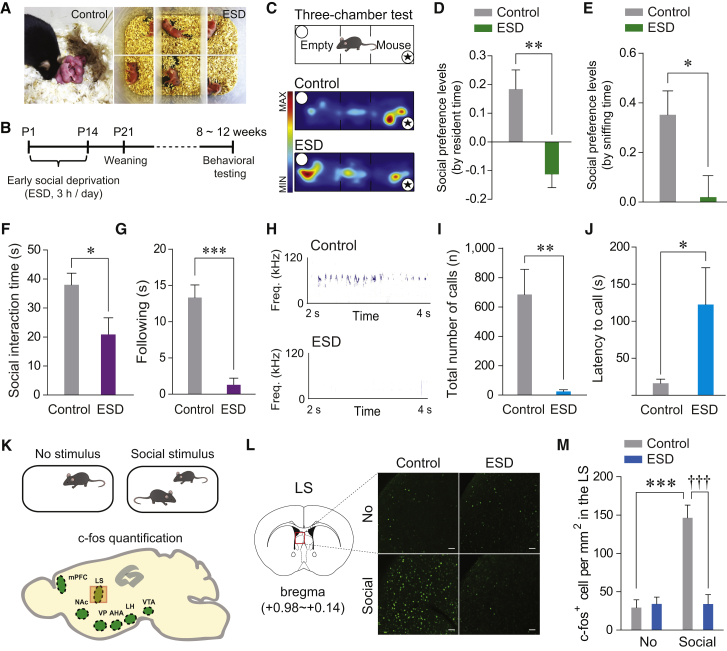
Figure 1.
ESD Mice Exhibit Impaired Social Behaviors and Reduced c-fos Expression in the LS in Response to Social Stimuli
(A) Control pups remained with their dam (left), but ESD pups were removed and separated both from their dam and littermates (right).
(B) The experimental timeline for the ESD stress procedure.
(C) Schematic illustrating the three-chamber social preference test (top) and representative heatmap images during the test session for control (middle) and ESD (bottom) mice. Asterisks indicate the presence of a conspecific stranger.
(D and E) Social preference levels based on resident time (D) and sniffing time (E) in the three-chamber test. ESD mice showed significantly reduced preference for exploring a stranger mouse (n = 7, 11 mice per group).
(F and G) Reciprocal social interaction tests. ESD mice exhibited reduced total duration of direct contacts with a freely moving stranger mouse, as measured by amount of time spent in social interactions (i.e., sniffing, following, mounting, and nose to nose contacts) (F), and in following only (G) (n = 6 mice per group).
(H) Representative images of USVs produced by a control (top) and ESD (bottom) male mouse encountering a female mouse.
(I and J) ESD mice emitted significantly fewer USVs (I) and showed delayed latency to the first USV call (J) compared to controls (n = 12, 7 mice per group).
(K) c-fos expression was examined at baseline (no stimulus) or after social interactions (top). Brain schematic illustrating the target areas analyzed for c-fos quantification (bottom). mPFC, medial prefrontal cortex; NAc, nucleus accumbens; VP, ventral pallidum; AHA, anterior hypothalamus; LH, lateral hypothalamus; VTA, ventral tegmental area.
(L) Representative images of the LS showing c-fos staining in control versus ESD mice following exposure to a social stimulus. Scale bars, 50 μm.
(M) Quantification of c-fos-positive cells in the LS. Social stimuli elicited robust increases in c-fos expression within the LS of control, but not ESD mice (n = 6 mice for each no stimulus group and n = 7 mice for each social stimulus group).
Significance for multiple comparisons: unpaired t test (D–G), Mann-Whitney U test (I and J), and two-way ANOVA, post hoc, Bonferroni (M), ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; †††p < 0.001. Data are expressed as mean ± SEM.
See also Figures S1 and S2.
We first compared ESD and control mice using the three-chamber social preference test. This test quantifies a mouse’s preference for investigating a novel mouse versus an object; it has been used to measure social defects in mouse models of psychiatric disorders that present with social dysfunction (Silverman et al., 2010). In test sessions, ESD mice showed significantly reduced social preference for a stranger mouse over an object compared to control mice, whereas, in habituation sessions, both control and ESD mice showed no preference for the two sides of the three-chambered arena (Figures 1C–1E, S1G, and S1H). We also found that ESD mice exhibited a profound defect in direct social contacts in a reciprocal social interaction test (Figures 1F and 1G). To further explore the social impairments of ESD mice, we measured their ultrasonic vocalizations (USVs) (Jamain et al., 2008). Upon encountering a female, male ESD mice produced significantly fewer calls and show higher latency to their first call than control mice, indicating that ESD mice have defective social communication (Figures 1H–1J).
To identify specific brain regions that contribute to ESD-induced social dysfunction, we quantified the expression of the neuronal activation marker c-fos across several brain regions in response to social stimuli (Figure 1K). Using immunohistochemistry, we found that, after exposing control mice to social stimuli, they showed significant increases in c-fos expression in the LS, but ESD mice did not (Figures 1L and 1M). Notably, control mice showed the largest c-fos induction in the rostral LS rather than the caudal LS (Figures S2A–S2C). We also observed reliable differences in c-fos induction in the VTA of control mice but no distinguishable changes in the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), ventral pallidum (VP), anterior hypothalamus (AHA), or lateral hypothalamus (LH) (Figures S2D–S2I).
Together, these results indicate that ESD stress can induce social dysfunctions in adulthood and that activity in the rostral part of the LS may play a critical role in regulating these processes.
Drd3 Signaling Is Downregulated in the LS Following ESD Stress Exposure
Previous reports indicated that the LS contains heterogeneous cell types, with each expressing different receptors for neuromodulators that regulate social behaviors (Mesic et al., 2015, Risold and Swanson, 1997). It remains unclear, however, how exposure to early adversity affects neuromodulatory signaling through specific receptors in the LS to produce psychiatric symptoms such as asocial behaviors. We therefore examined what molecular signaling in the LS may be altered by ESD stress and whether such changes mediate a disruption of normal social behaviors in adulthood. Using quantitative real-time PCR, we found that ESD mice showed lower Drd3 mRNA expression in the LS compared to control mice. We did not observe any changes for receptors of other neuromodulators including serotonin, oxytocin, glutamate, or glucagon (Figure 2A).
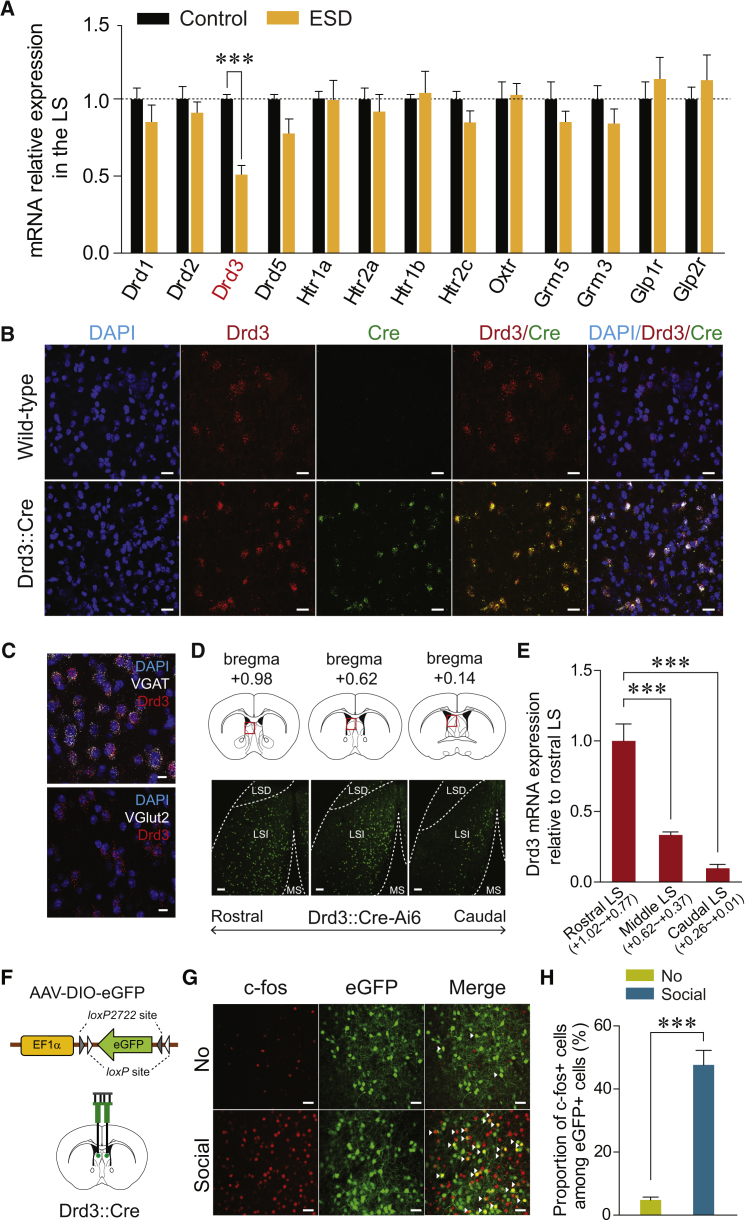
Figure 2.
Reduced Drd3 mRNA in the LS of ESD Mice and Characterization of Drd3LS Neurons
(A) Quantitative real-time PCR analysis of mRNA expression of several neuromodulator receptors in the LS. ESD mice showed significantly reduced Drd3 mRNA expression in the LS, but no changes in the other dopamine receptor subtypes (Drd1, Drd2, or Drd5), the serotonin receptors (Htr1a, Htr2a, Htr1b, Htr2c), an oxytocin receptor (Oxtr), metabotropic glutamate receptors (Grm3, Grm5), or glucagon receptors (Glp1r, Glp2r) (n = 5, 9 mice per group).
(B) Representative images of fluorescent in situ hybridizations of Drd3 mRNA (red) and Cre mRNA (green) expression in the LS of wild-type (top) or Drd3::Cre mice (bottom). Scale bars, 20 μm.
(C) Representative images of fluorescent in situ hybridizations of VGAT or VGlut2 mRNA (white) and Drd3 mRNA (red) in the LS of wild-type mice. Drd3 is expressed primarily in GABAergic neurons of the LS. Scale bars, 10 μm.
(D) Drd3 expression in the LS of Drd3::Cre-Ai6 mice. Coronal diagrams depicting the region analyzed (squared in red; top) and confocal images showing the Drd3 expression pattern in the LS along the rostral-caudal axis (bottom). Scale bars, 100 μm. LSD, lateral septal dorsal part; LSI, lateral septal intermediate part; MS, medial septum.
(E) The rostral, middle, and caudal LS was dissected at the indicated thickness, and Drd3 mRNA expression was measured by quantitative real-time PCR. Drd3 is expressed broadly across the LS, with the highest levels appearing in the rostral LS (n = 3 mice per group).
(F) Schematic for the bilateral injection of AAV expressing a Cre-dependent eGFP into the LS of control Drd3::Cre mice.
(G) Representative images showing c-fos immunoreactivity (red) and eGFP fluorescence (green) in the LS of control Drd3::Cre mice injected with AAV-DIO-eGFP following exposure to a social stimulus. White arrowheads indicate the co-localization of c-fos immunostaining with Drd3 expression. Scale bars, 40 μm.
(H) Quantification of the proportion of c-fos-positive cells among Drd3LS neurons of control Drd3::Cre mice. Social stimuli elicited a robust increase of c-fos expression in Drd3LS neurons (n = 4 mice per group).
Significance for multiple comparisons: unpaired t test (A and H), one-way ANOVA, post hoc, Fisher least significant difference (LSD) (E), ∗∗p < 0.01 ∗∗∗p < 0.001. Data are presented as mean ± SEM.
See also Figure S3.
Drd3, a member of the D2-like receptor family, has been implicated in the development of social deficits associated with ASD (Staal et al., 2015), but its precise roles in mediating social abnormalities following ELS and in regulating neuronal activity in the LS are unknown. Thus, to investigate this, we used Drd3::Cre bacterial artificial chromosome (BAC) transgenic mice, which enable genetic manipulation of Drd3LS neurons. Using dual fluorescent in situ hybridization (FISH), we confirmed that Drd3::Cre mice express Cre recombinase specifically in Drd3LS neurons (Figure 2B). Consistent with previous studies (Zhao et al., 2013), we found that Drd3LS neurons are predominantly GABAergic (Figure 2C). Intriguingly, Drd3::Cre mice crossed with the Ai6 reporter mice line revealed the most prominent Drd3 expression in the rostral part of LS. We then further confirmed this in wild-type mice using quantitative real-time PCR (Figures 2D and 2E). This suggests that Drd3LS neurons may be responsible for the social stimulus-induced c-fos expression that we observed in the rostral LS (Figures S2A–S2C).
To further explore the function of the Drd3LS neurons in social behavior, we asked whether the social stimulus-induced expression of c-fos we observed is occurring in Drd3LS neurons. We injected adeno-associated virus (AAV) expressing the Cre-dependent eGFP (AAV-DIO-eGFP) into the LS of control Drd3::Cre mice to visualize Drd3LS neuronal cell bodies. We then measured the proportion of c-fos-positive cells among eGFP-expressing neurons in the LS. We found that social stimulation enhances c-fos expression in the Drd3LS neuronal population by up to 47.6% (Figures 2F–2H). Conversely, we observed that 50.42% of social stimulus-induced c-fos expression were overlapped with eGFP-expressing cells (data not shown), suggesting that Drd3LS neurons constitute a relevant population preferentially activated by social stimuli (Figures 2F–2H).
To gain a holistic understanding of how Drd3LS neurons contribute to ESD-induced abnormal social behaviors, we next aimed to delineate the circuitry of the Drd3LS neurons. To this end, we injected the LS of wild-type mice with AAV expressing eGFP (AAV-eGFP) or the LS of Drd3::Cre mice with AAV-DIO-eGFP. While LS neurons in general project to the medial preoptic area (MPA), AHA, LH, VTA, and periaqueductal gray (PAG), among other areas, Drd3LS neurons project specifically to the MPA, AHA, and LH (Figures S3A–S3M). Using viral expression strategies that reveal monosynaptic inputs (Figures S3N–S3S) (Callaway and Luo, 2015), we found that Drd3LS neurons receive synaptic inputs from the MPA and AHA, as well as the ventral hippocampus and VTA (Figures S3T–S3Y). These data indicate that Drd3LS neurons are directly connected to several brain areas known to be implicated in the regulation of social behaviors (Figure S3Z) (Gunaydin et al., 2014, Hitti and Siegelbaum, 2014, Paredes, 2003, Wu et al., 2014).
Activity of Drd3LS Neurons Is Blunted in ESD Mice in Response to Social Stimulus
Since Drd3 signaling has been suggested to modulate neuronal activity in several brain areas, it is possible that the endogenous activity of Drd3LS neurons could encode key features of social behaviors (Chen et al., 2006, Gunaydin et al., 2014). We therefore asked how well Drd3LS neuronal activity correlates with the incidence of social interaction behaviors. To do so, we injected the LS of control Drd3::Cre mice with AAV encoding a Ca2+ indicator (GCaMP6f) in a Cre-dependent manner and implanted an imaging cannula with a gradient index (GRIN) lens directly above the virus injection site (Figure 3A). Three weeks post-surgery, we imaged Ca2+ dynamics in Drd3LS neurons with a mini-microscope and then quantified the number of in vivo Ca2+ transients per minute before and after introducing either a conspecific stranger (social stimulus) or a fake mouse (novel object) (Figures 3B, 3C, and S4A). Strikingly, we found significant increases in number of Ca2+ transients per minute in control Drd3::Cre mice during social interactions with the conspecific stranger (Figures S4B–S4D). In contrast, in the presence of a fake mouse, we did not observe any differences in Ca2+ transients of Drd3LS neurons even when control Drd3::Cre mice approached and investigated the fake mouse with physical contacts (Figures S4B–S4D). Notably, we found prominent increases in the average number of Ca2+ transients per minute under social contexts compared to novel object contexts (Figure S4D). This suggests that Drd3LS neuronal activity is more strongly correlated with actual social interactions than general novelty. Furthermore, we found that 64.7% of Ca2+ transients in Drd3LS neurons occurred at the onset of social bouts during male-male interactions (Figures S4E and S4F). Of those interactions, almost half (49.6%) of those Ca2+ transients were correlated with body-sniffing/mounting behaviors, while 33.3%, 17.1% of that occurred during following behaviors or nose to nose contacts, respectively (Figure S4G). We also observed that 56.5% of Ca2+ transients in Drd3LS neurons of control male mice interacting with a female mouse occurred at the onset of USVs (Figures S4H and S4I).
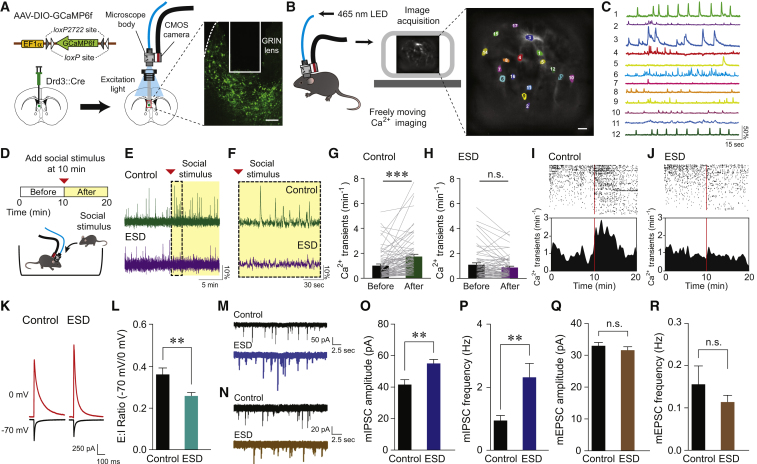
Figure 3.
Drd3LS Neurons of ESD Mice Display Reduced Activity in Response to Social Stimulus and Altered Synaptic Excitatory/Inhibitory Balance
(A) Schematic for the injection of AAV expressing GCaMP6f in a Cre-dependent manner into the LS of Drd3::Cre mice (left). Confocal image showing GRIN lens placement on the GCaMP6f-expressing Drd3LS neurons. Scale bar, 200 μm (right).
(B) Illustration of in vivo Ca2+ imaging setup (left). Sample image of GCaMP6f-expressing Drd3LS neurons and the color-coded regions of interest (ROIs) used for image analysis. Scale bar, 25 μm (right).
(C) Sample Ca2+ activity traces of ΔF/F0 from individual neurons in (B).
(D) Schematic for the experimental setup to record Ca2+ activity in GCaMP6f-expressing Drd3LS neurons.
(E) Representative Ca2+ activity traces from Drd3LS neurons of control (top, green) and ESD (bottom, purple) Drd3::Cre mice, with the yellow-shaded area indicating the presence of a social stimulus.
(F) Magnified view of the dotted box from (E).
(G and H) Average Ca2+ transients per minute in Drd3LS neurons of control (G) and ESD (H) Drd3::Cre mice before and after the presentation of a social stimulus (n = 76 cells from 6 control mice, n = 66 cells from 5 ESD mice).
(I and J) Raster plots (top) and peristimulus time histograms (bottom) showing Drd3LS neuronal activity of control (I) and ESD (J) Drd3::Cre mice (n = 76 cells from 6 control mice, n = 66 cells from 5 ESD mice). The rows and ticks in the raster plots represent individual cells and single Ca2+ transient events, respectively. Vertical red bars mark the time the social stimulus was introduced.
(K and L) Representative traces (K) and quantification (L) of synaptic E:I ratio recorded from Drd3LS neurons of control and ESD Drd3::Cre mice (n = 19 cells from 3 control mice, n = 17 cells from 3 ESD mice).
(M and N) Representative traces of mIPSCs (M) and mEPSCs (N) from Drd3LS neurons of control (top, black) and ESD Drd3::Cre mice (bottom, blue or brown).
(O and P) The Drd3LS neurons of ESD Drd3::Cre mice showed enhanced mIPSC amplitude (O) and frequency (P) (n = 11 cells from 2 control mice, n = 13 cells from 3 ESD mice).
(Q and R) mEPSC amplitude (Q) and frequency (R) did not differ between control and ESD Drd3::Cre mice (n = 10 cells from 2 control mice, n = 14 cells from 3 ESD mice).
Significance for multiple comparisons: Paired t test (G), Mann-Whitney U test (L, O, and P), ∗∗p < 0.01; ∗∗∗p < 0.001; n.s., not significant. Data are presented as mean ± SEM.
See also Figure S4.
In light of our previous finding that ESD mice showed reduced expression of c-fos in the LS in response to social stimuli (Figures 1L and 1M), we hypothesized that the Drd3LS neuronal activity of ESD mice may show blunted responses to social stimuli. To examine this hypothesis, we compared average number of Ca2+ transients per minute in response to social stimuli between control and ESD mice. Consistent with our previous finding, we observed that social stimuli increased Ca2+ transients per minute in Drd3LS neurons of control Drd3::Cre mice. In contrast, the Drd3LS neurons of ESD Drd3::Cre mice exhibited no change in response to social stimuli (Figures 3D–3J and S4J). This suggests that Drd3LS neurons of ESD mice show impaired reactivity to social stimuli.
To uncover the synaptic mechanism by which Drd3LS neurons of ESD mice show blunted activity, we performed a whole-cell patch-clamp recording of Drd3LS neurons. We found a significant reduction in the ratio of evoked excitatory inputs to inhibitory inputs (E/I ratio) in ESD Drd3::Cre mice compared to controls (Figures 3K and 3L). This occurred without any accompanying change in the resting membrane potential, the rheobase, or in the ratio of the amplitudes of AMPA (a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor-mediated excitatory postsynaptic currents (EPSCs) to NMDA (N-methyl-D-aspartate) receptor-mediated EPSCs (AMPAR/NMDAR ratio) (data not shown). Instead, we observed a significant increase in the amplitude and frequency of miniature inhibitory postsynaptic currents (mIPSCs) but not miniature EPSCs (mEPSCs) in the Drd3LS neurons of ESD Drd3::Cre mice (Figures 3M–3R). Together, these data suggest that Drd3LS neurons of ESD mice show blunted responses to social stimuli due to increased inhibitory synaptic inputs.
Bidirectional Modulation of Drd3LS Neuronal Activity Affects ESD-Induced Social Dysfunctions
Next, we asked whether the reduction of Drd3LS neuronal activity recapitulates the asocial behaviors of ESD mice. To determine this, we injected AAV expressing a Cre-dependent Kir2.1 potassium channel (AAV-DIO-GFP-Kir2.1), which reduces cellular activity by chronic hyperpolarization (Rothwell et al., 2014), into the LS of control Drd3::Cre mice (Figure 4A). Strikingly, we found that Kir2.1-induced silencing of Drd3LS neurons itself impairs both social preference and social communication with no accompanying change in locomotion or anxiety, mirroring the deficits of ESD mice (Figures 4B, 4D–4F, S5A, and S5B). However, similar silencing of Drd3-positive neurons in the NAc shell, another area that expresses high levels of Drd3 (Sokoloff et al., 1990), does not affect social preference, suggesting that the LS is a critical neural substrate where Drd3-expressing neurons regulate social behaviors (Figure 4C).
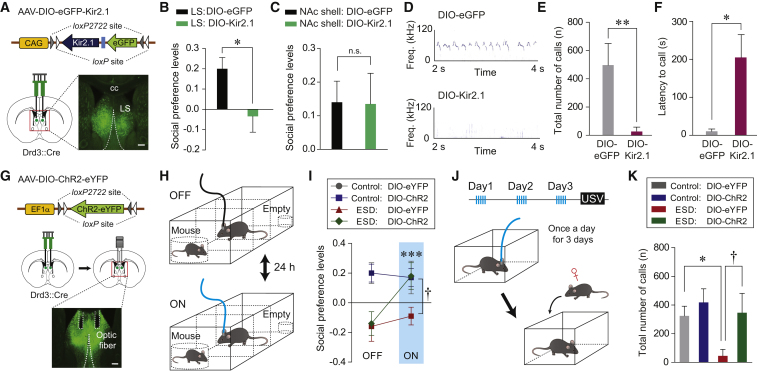
Figure 4.
Modulation of Drd3LS Neuronal Activity Influences Abnormal Social Behavior
(A) Schematic depicting the injection of AAV expressing a Cre-dependent Kir2.1 into the LS of Drd3::Cre mice. Scale bar, 250 μm. cc, corpus callosum.
(B and C) Social preference based on resident time in the three-chamber test. Viral-mediated Kir2.1 expression in Drd3LS neurons reduced social preference (B, n = 8 mice per group), while similar silencing of Drd3NAc shell neurons did not affect social preference (C, n = 4 mice per group).
(D) Representative images of USVs emitted by control Drd3::Cre male mice injected with eGFP-expressing virus (top) or Kir2.1-expressing virus (bottom) in the LS, upon encountering a female mouse.
(E and F) Control Drd3::Cre mice expressing Kir2.1 in Drd3LS neurons produced significantly fewer USVs (E) and showed increased latency to make the first USV call (F) (n = 6 mice per group).
(G) Schematic depicting the injection of AAV-DIO-ChR2-eYFP, followed by implantation of optic fibers above the virus injection site within the LS of Drd3::Cre mice (top and middle). Confocal image showing optic fiber placement in the LS (bottom). Scale bar, 250 μm.
(H) The experimental design of the three-chamber test with optical stimulation. Testing sessions were conducted twice and counterbalanced for order with a 24-hr interval between laser ON and laser OFF condition.
(I) Photostimulation of Drd3LS neurons restored social preference in ESD Drd3::Cre mice (n = 6, 9 mice for each control group and n = 8, 11 mice for each ESD group; ∗∗∗p < 0.001 compared with ESD mice expressing DIO-ChR2 during the OFF state; †p < 0.05 compared with ESD mice expressing DIO-eYFP during the ON state).
(J) The experimental design of the USV tests after repeated delivery of optical stimulation (once a day for 3 days) (top). After the last stimulation, a female mouse was placed for recording USVs produced by male mice (bottom).
(K) Photoactivation of Drd3LS neurons rescued the number of USVs emitted by ESD Drd3::Cre mice to the levels of controls (n = 7, 8 mice for each control group and n = 7, 6 mice for each ESD group).
Significance for multiple comparisons: unpaired t test (B), Mann-Whitney U test (E and F), two-way repeated-measures (RM) ANOVA; post hoc, Fisher LSD (I), and two-way ANOVA; post hoc, Bonferroni (K), ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; †p < 0.05; n.s., not significant. Data are presented as mean ± SEM.
See also Figure S5.
We next examined whether selective activation of Drd3LS neurons can reverse the social dysfunction of ESD mice. To achieve precise excitation of these cells with light, we expressed Channelrhodopsin2 (ChR2) in Drd3LS neurons of control Drd3::Cre or ESD Drd3::Cre mice, followed by the implantation of an optic cannula above the LS (Figure 4G). We subjected each mouse to the three-chamber test or the reciprocal social interaction test on two separate days with and without illumination (Figures 4H, S5C, and S5D). At baseline (light-off), consistent with our previous data, the ESD Drd3::Cre mice showed reduced social preference and fewer direct social contacts compared to control Drd3::Cre mice. In contrast, blue light-elicited activation of the Drd3LS neurons in ChR2-expressing ESD Drd3::Cre mice robustly reversed the impaired social interactions associated with early adversity (Figures 4I and S5E). In addition, ChR2-mediated stimulation of Drd3LS neurons rescued the USV defect of ESD Drd3::Cre mice, suggesting that the activation of Drd3LS neurons has a critical role in the protection against ESD-induced social impairment (Figures 4J, 4K, and S5F). Together, these results indicate that bi-directional modulation of Drd3LS neuronal activity can either aggravate or alleviate the social abnormalities induced by ESD.
Drd3 Agonist Treatment Enhances Drd3LS Neuronal Activity and Reverses ESD-Induced Social Impairments
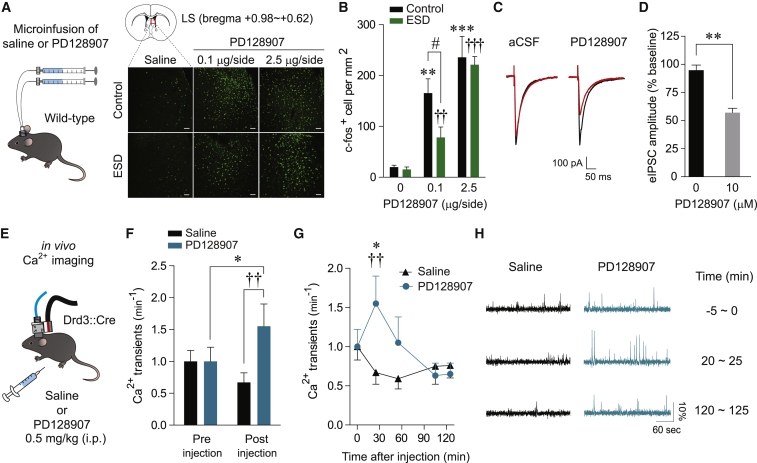
Next, to examine whether Drd3 signaling plays a role in regulating the activity of the LS, we measured the expression of c-fos in the LS of both control and ESD mice 1 hr after intraperitoneal administration of the Drd3 agonist PD128907 (0.1 or 0.5 mg/kg i.p.). We found that PD128907 induced a robust and dose-dependent increase in c-fos immunoreactivity in the LS (Figures S5G and S5H). To further confirm the direct effect of PD128907 on LS neuronal activation, we performed an intracranial microinfusion of either saline or PD128907 (0.1 or 2.5 μg/side) into the LS. We observed that, in both control and ESD mice, local infusion of PD128907 also induced a remarkable increase of c-fos expression in rostral and middle part of the LS (bregma +0.98∼0.62) where most of the cannula tips were found (Figures 5A, 5B, and S5I). In contrast, regardless of drug treatment, we were not able to observe any changes of c-fos induction in caudal part of the LS (bregma +0.35∼+0.14) where we did not place any cannula tips (Figure S5J). Notably, we found that low dose of PD128907 (0.1 μg/side) induced less c-fos expression in the LS of ESD mice than control mice (Figure 5B). This suggests that the submaximal dose of PD128907 elicits a weaker response in the LS of ESD mice compared to controls, which consistent with our previous finding of reduced Drd3 signaling in the LS of ESD mice. Together, these findings demonstrate that pharmacological activation of Drd3 boosts neuronal activity in the LS.
Figure 5.
Administration of the Drd3 Agonist PD128907 Increases Drd3LS Neuronal Activity
(A) Schematic for the microinjection of saline or PD128907 into the LS of control and ESD mice (left). Representative images of c-fos staining in the LS of control and ESD mice following microinfusion of saline or PD128907 (0.1 or 2.5 μg/side). Scale bars, 50 μm (right).
(B) Quantification of c-fos-positive cells in the LS of control or ESD mice 1 hr after saline or PD128907 microinfusion. PD128907 activated the LS neurons both in control and ESD mice (n = 6, 6, and 8 mice for each control group and n = 6, 8, and 6 mice for each ESD group; ∗∗p < 0.01, ∗∗∗p < 0.001 compared with control mice infused with saline; ††p < 0.01, †††p < 0.001 compared with ESD mice infused with saline; #p < 0.05 compared with ESD mice infused with 0.1 μg/side of PD128907).
(C) Representative traces of electrically evoked IPSCs (eIPSCs) recorded from Drd3LS neurons of control Drd3::Cre mice prior to (black) and after (red) bath application of artificial cerebrospinal fluid (aCSF) or PD128907 (10 μM).
(D) The amplitude of eIPSCs showed a significant reduction following a 20-min exposure to 10 μM PD128907 (n = 5, 7 cells per group).
(E) Schematic for recording the activity from GCaMP6f-expressing Drd3LS neurons of control Drd3::Cre mice treated with saline or PD128907 (0.5 mg/kg, i.p.).
(F) Average Ca2+ transients per minute before and 20 min after saline or PD128907 injections (n = 28 cells from 3 mice treated with saline, n = 21 cells from 3 mice treated with PD129907).
(G) Time course of changes in Ca2+ transients per minute was measured during a 5-min window of pre-injection (at −5 min) and post-injection (at 20, 50, 100, 120 min). PD128907 administration induced a significant increase in GCaMP6f activity of Drd3LS neurons, which then gradually returned to baseline levels (n = 28 cells from 3 mice treated with saline, n = 21 cells from 3 mice treated with PD129907; ∗p < 0.05 compared with PD128907-treated mice before injection; ††p < 0.01 compared with saline-treated mice at 20 min after injection).
(H) Representative Ca2+ activity traces from Drd3LS neurons of control Drd3::Cre mice at each 5-min window of pre-injection and post-injection.
Significance for multiple comparisons: two-way ANOVA; post hoc, Bonferroni (B), Mann-Whitney U test (D), and two-way RM ANOVA; post hoc, Fisher LSD (F and G), #p < 0.05; ∗p < 0.05; ∗∗p < 0.01; ††p < 0.01. Data are presented as mean ± SEM.
See also Figure S5.
Consistent with previous reports (Chen et al., 2006, Diaz et al., 2011, Swant et al., 2008), we found that bath application of PD128907 to brain slices of control Drd3::Cre mice significantly reduced the mean amplitude of the evoked IPSCs in Drd3LS neurons (Figures 5C and 5D). We also confirmed via in vivo Ca2+ imaging that PD128907 injection (0.5 mg/kg, i.p.) induced a substantial increase in the average number of Ca2+ transients per minute in Drd3LS neurons of control Drd3::Cre mice, and that this increase then slowly returned to baseline (Figures 5E–5H). These data suggest that the activation of Drd3 signaling via the Drd3 agonist PD128907 enhances the activity of Drd3LS neurons by suppressing their inhibitory synaptic inputs.
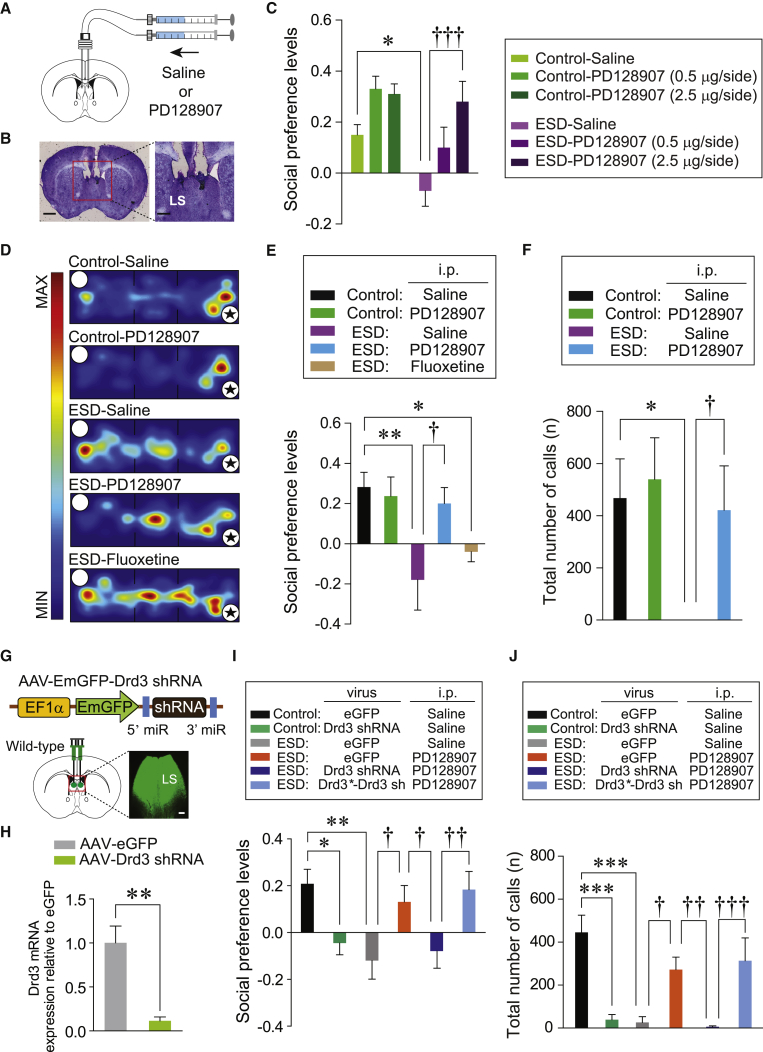
This led us to hypothesize that the activation of Drd3LS neuronal signaling may be able to rescue the social dysfunction of ESD mice by upregulating Drd3LS neuronal activity. To test this, we microinjected either saline or PD128907 directly into the LS of control or ESD mice and found that PD128907 produced a dose-dependent improvement in the social preference of ESD mice (Figures 6A–6C and S6A). Moreover, we further confirmed that systemic injection of PD128907 (0.5 mg/kg, i.p.) normalized ESD-induced abnormal social behaviors both in the three-chamber test and the USV test (Figures 6D–6F, S6B, and S6C). In contrast, chronic injections of fluoxetine (20 mg/kg, i.p.), a commonly prescribed antidepressant to treat psychiatric diseases with accompanying social dysfunctions (Van Ameringen et al., 1993, Hollander et al., 2012), did not ameliorate the impaired social preference of ESD mice (Figures 6D, 6E, and S6B). This suggests that mice exposed to early adversity may have a distinct biological endophenotype that leads to differing responses to conventional treatments.
Figure 6.
Pharmacological Activation of Drd3 Signaling in the LS Prevents Social Impairments of ESD Mice
(A) Schematic for the microinjection of saline or PD128907 into the LS of control and ESD mice.
(B) Image shows the location of the cannula tips in the LS. Scale bars, 1 mm (left) and 250 μm (right).
(C) Microinjection of PD128907 into the LS produced a dose-dependent increase in the social preference of ESD mice in the three-chamber test (n = 5, 5, and 4 mice for each control group; n = 9, 5, and 6 mice for each ESD group).
(D) Representative heatmap images during three-chamber tests for control or ESD mice treated with saline, PD128907 (0.5 mg/kg, i.p.), or fluoxetine (20 mg/kg, i.p.). Asterisks indicate the presence of a conspecific stranger.
(E) Social preference levels based on resident time in the three-chamber test. PD128907 (0.5 mg/kg, i.p.) administration increased social preference in ESD mice to the level of control mice, whereas chronic injection of fluoxetine (20 mg/kg, i.p.) did not (n = 6, 5 mice for each control group, n = 6, 6, and 5 mice for each ESD group).
(F) PD128907 (0.5 mg/kg, i.p.) administration restored the total number of USVs in ESD mice (n = 5, 6 mice for each control group, n = 6, 6 mice for each ESD group).
(G) Schematic depicting the injection of AAV expressing Drd3 shRNA into the LS of wild-type mice. Scale bar, 250 μm.
(H) Quantitative real-time PCR analysis of Drd3 mRNA expression from the LS of mice injected with eGFP- or Drd3 shRNA-expressing AAV (n = 4 mice per group).
(I and J) Knockdown of Drd3 by injection of AAV-Drd3 shRNA into the LS attenuated social preference (I) and total number of USVs (J) in control mice and blocked the PD128907-induced rescue of impaired social preference (I) and communication deficits (J) in ESD mice. However, the expression of shRNA-resistant Drd3 (Drd3∗, the mutant form is indicated by an asterisk) together with the shRNA against Drd3 (AAV-Drd3∗-Drd3 sh) did not block the PD128907-induced rescue of the social dysfunctions in ESD mice. (I, n = 11, 10 mice for each control group, n = 9, 10, 11, and 11 mice for each ESD group). (J, n = 8, 10 mice for each control group, n = 7, 8, 9, and 10 mice for each ESD group).
Significance for multiple comparisons: two-way ANOVA; post hoc, Bonferroni (C and F), one-way ANOVA; post hoc, Fisher LSD (E, I, and J), and unpaired t test (H), ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; †p < 0.05; ††p < 0.01; †††p < 0.001. Data are presented as mean ± SEM.
See also Figures S6 and S7.
To further investigate an extended role for Drd3 in protecting against social defects, we asked whether PD128907 can correct the aberrant social phenotypes of the inbred mouse strain BTBR T+tf/J (BTBR), which is a preclinical model of autism (McFarlane et al., 2008). Remarkably, PD128907 treatment (0.5 mg/kg, i.p.) rescued the impaired social preference of BTBR mice, suggesting that PD128907 may also have an efficacy against for heritable forms of autistic social dysfunction (Figures S6D and S6E). Surprisingly, we also found that BTBR mice showed significantly reduced expression of Drd3 mRNA in the LS compared with age-matched C57/BL6 mice. In addition, this change was not accompanied by alterations in other subtypes of dopamine receptor such as Drd1 and Drd2 (Figures S6F and S6G), indicating a similar molecular defect as the one we observed in ESD mice. This suggests that downregulation of Drd3 signaling in the LS may be the important molecular change leading to impaired social preference in BTBR mice as well as the social dysfunctions of mice exposed to early adversity.
Since systemic treatments generally affect the whole brain, we next asked whether the LS is specifically required for this rescue of ESD-induced social dysfunction by intraperitoneal injection of PD128907. To this end, we took advantage of AAV expressing short hairpin RNA (shRNA) against Drd3 (AAV-Drd3 shRNA), which robustly reduced endogenous Drd3 mRNA levels in the LS (Figures 6G and 6H). After delivering these viruses to the LS of control mice, we found that the resulting knockdown of Drd3 signaling in the LS recapitulated the social impairments (e.g., impaired social preference and communication deficits) of ESD mice (Figures 6I, 6J, S7A, and S7B). A similar knockdown of Drd3 signaling in the LS of ESD mice prevented PD128907 treatment from rescuing their social defects, while ESD mice injected with a control virus (AAV-eGFP) still showed PD128907-induced behavioral normalization (Figures 6I, 6J, S7A, and S7B). To rule out potential off-target effects of shRNA manipulation, we expressed a shRNA-resistant Drd3 (Drd3∗) along with the shRNA against Drd3 (AAV-Drd3∗-Drd3 sh) in the LS. We found that co-expression of Drd3∗ prevented the Drd3 knockdown from inhibiting the PD128907-induced behavioral rescue of the social defects in ESD mice (Figures 6I, 6J, S7A, and S7B). This confirms that effects of shRNA against Drd3 are indeed mediated by knockdown of Drd3 signaling. Finally, to further confirm the specificity of the Drd3 shRNA, we asked whether Drd3LS neuron-specific expression of Drd3∗ can reverse the effect of Drd3 knockdown in the LS of ESD Drd3::Cre mice (Figures S7C and S7D). Consistent with our previous results, the specific knockdown of Drd3 signaling in the LS of ESD Drd3::Cre mice (AAV-DIO-Drd3 shRNA) blocked the PD128907-induced rescue of the social preference defect in ESD mice. However, we did not observe this blockade in ESD Drd3::Cre mice that expressed both Drd3∗ and shRNA against Drd3 (AAV-DIO Drd3∗-Drd3 sh) in the LS (Figure S7E) after PD128907 treatment (0.5 mg/kg, i.p.). This confirms again that shRNA manipulation exerted its effects without any unexpected off-target effects. Together, these data strongly support that the activation of Drd3 signaling in the LS is required for PD128907-induced improvement of the social defects in ESD mice.
Discussion
Exposure to family adversity or social deprivation during childhood is linked to social anxiety, impaired social skills, and poor communication in adulthood. As such, it can be acknowledged to be a predictor of adult antisocial behaviors (Bruce et al., 2012, Dodge et al., 1990, Enoch et al., 2010). Likewise, postnatal stress is also detrimental in rodents. This is particularly true in the critical period of early infancy while the offspring are entirely dependent on their mother for survival. Several “maternal separation” (MS) paradigms in which periodic disruption of social bonding by separating pups from their mother have been devised to study the impact of early life factors on adult life (Huot et al., 2002, Uchida et al., 2010). Here, we adopted a slightly modified version of the maternal separation procedure: the “ESD” paradigm (McCormick et al., 1998, Zimmerberg et al., 2009). Unlike the more common maternal separation paradigms, which keep littermates together, the ESD paradigm subject pups to complete social isolation by physically excluding them from their dams and littermates. Moreover, we employed an unpredictable stress schedule by randomizing the timing of the deprivation period, making it more difficult to anticipate. Given that unpredictable stress has more profound and persistent effects than predictable stress (Enthoven et al., 2008), we believe that our ESD model would better replicate the etiological factors implicated in psychiatric disorders by including this uncertainty. Indeed, we found that our approach with this ESD model allowed us to investigate aberrant social behaviors in adult life, including reduced social preference levels, impaired social interactions, and severe social communication deficits, all of which are core symptoms of psychiatric diseases such as ASD and MDD (Figure 1).
Numerous studies have strongly suggested a functional role for Drd3 in the social disruptions associated with psychiatric disorders such as ASD (de Krom et al., 2009, Staal et al., 2015). For example, polymorphisms in Drd3 are associated with repetitive and stereotyped behaviors in ASD (Staal et al., 2012). Drd3 expression is limited mainly to parts of the limbic system that are involved in motivated behavior (Murray et al., 1994, Sokoloff et al., 1990). In the LS, Drd3 is expressed predominantly in the rostromedial part (Figure 2), a subregion that receives the majority of the LS-projecting dopaminergic terminals from the VTA (Reddy et al., 2016). Interestingly, we found significant increases in c-fos expression in the VTA as well as the LS of control mice in response to social stimuli (Figure S2). This is consistent with previous studies that suggested that the activity dynamics of the VTA projections encode key features of social interactions and that dopaminergic signaling in its downstream structures is involved in social reward and social behaviors (Gunaydin et al., 2014).
We found that Drd3LS neurons have dense reciprocal connections with the MPA and AHA (Figure S3). This is an important observation for three reasons. First, given that the LS receives GABAergic inputs from the hypothalamus (Dietrich and Horvath, 2009, Gallagher et al., 1995), we speculate that the MPA or AHA can be the major sources of these inhibitory inputs onto Drd3LS neurons. It is, therefore, plausible that the MPA or AHA GABAergic inputs to Drd3LS neurons of ESD mice may be increased in number or may display increased release probability, thereby contributing to ESD-induced downregulation of Drd3LS neuronal activity. Second, because both the AHA and MPA are essential nodes for various social behaviors (Ferris et al., 1997, McHenry et al., 2017), engaging LS-MPA and/or LS-AHA circuit activity may override predisposition to social dysfunction induced by early adversity. Moreover, the AHA is a major integrator of neural processes related to aggression and defense during social interactions with same-sex conspecifics (Ferris et al., 1997, Goodson et al., 2012), while the MPA is more likely to be involved in sexual behaviors and encode attractive social cues from opposite-sex conspecifics (McHenry et al., 2017, Paredes et al., 1990). This raises the possibility that the Drd3LS neurons modulate the various aspects of ESD-induced social dysfunction in a projection-specific manner (e.g., reduced social preference during male-male interaction versus communication deficits during male-female interaction). Third, previous studies have shown that AHA-projecting LS neurons regulate the function of the hypothalamus-pituitary-adrenal (HPA) axis as well as corticosterone levels via presumptive di-synaptic disinhibitory connections with the paraventricular nucleus (PVN) (Anthony et al., 2014, Herman et al., 2002). In addition, many clinical and preclinical reports have suggested that early adversity is characterized by lifelong disruptions of the homeostatic mechanisms that regulate the HPA axis (Enthoven et al., 2008, Lyons et al., 1998, Pfeffer et al., 2007). Thus, ESD may exert at least some of its effects by altering the activity of AHA-projecting Drd3LS neurons, which would then contribute to the dysregulation of the HPA axis. Future studies focusing further on Drd3LS neuronal circuitry will expand our understanding of how the cell-type-specific and/or projection-specific neural circuits of the LS interact to precipitate social impairments after early adverse experiences.
Clinical studies showed that the psychiatric symptoms of patients with a history of adverse early life experiences respond more poorly to pharmacotherapy than those of similar patients without such a history (Bruce et al., 2012, Nanni et al., 2012, Nemeroff, 2016). This implies that ELS exerts long-term physiological consequences linked to an individual’s unique endophenotype and that patients with ELS may require alternative treatment options. Interestingly, we observed that, although the commonly prescribed antidepressant fluoxetine had no effect on the social impairments of ESD mice, treatment with the PD128907 rescued their severe social defects (Figure 6). This suggests that manipulation of Drd3 signaling may be an alternative therapeutic strategy for treating the social dysfunction induced or exacerbated by early adversity.
The BTBR mouse is an inbred strain that displays the core symptoms of autism (McFarlane et al., 2008). Although previous studies of BTBR mice have addressed the anatomical features of their brains and changes in neurotransmitter levels (Squillace et al., 2014, Stephenson et al., 2011), little is known about the specific neural substrates or the neuromodulatory signaling whose own modulation affects social dysfunction of BTBR mice. Consistent with previous reports (Stephenson et al., 2011), we found that the LS of BTBR mice showed significant lateral displacement (Figure S6F), implicating that BTBR mice may have altered anatomical LS circuitry. We also found that BTBR mice exhibited downregulation of Drd3 signaling in the LS and that the administration of PD128907 rescues their social impairments (Figure S6). Although further studies are needed, these data support a broad role for Drd3LS neuronal signaling in the pathophysiology of social dysfunctions that are associated with environmental stressors as well as with genetic factors.
It is important to note that the activation of Drd3 signaling in the LS modulates synaptic transmission and neuronal activity. We observed that PD128907 inhibits evoked IPSCs and increases the activity of Drd3LS neurons of control mice (Figure 5). Although the mechanisms that couple Drd3 to intracellular signal transduction systems are not yet well defined in vivo, previous studies have suggested that Drd3 activation increases neuronal activity by inhibiting PKA. This, in turn, would increase the endocytosis of GABAA receptors (Chen et al., 2006, Swant et al., 2008). Furthermore, recent studies have suggested that Drd3’s effects on neuronal activity may depend on the regional localization of Drd3 in glutamatergic versus GABAergic neurons (Li and Kuzhikandathil, 2012). For example, excitatory glutamatergic neurons are likely inhibited by Drd3 activation, whereas GABAergic neurons are activated by Drd3 activation because Drd3 suppresses GABA-receptor-mediated inhibitory synaptic transmission by increasing GABAA receptor endocytosis (Chen et al., 2006, Swant et al., 2008). Given that LS neurons are predominantly GABAergic (Figure 2), we speculate that activation of Drd3 signaling in the LS may modulate GABAA receptor trafficking and enhance the LS neuronal activity by reducing GABAergic transmission.
In summary, we have uncovered a novel mechanism underlying early life stress-induced adult social dysfunction. Our findings reveal a role for the Drd3LS neuronal signaling and its corresponding neuronal activity in mediating ELS-induced social dysfunctions. This is a significant advance in our understanding of the neural mechanisms by which adverse events early in life can alter the activity of specific neural circuits and cause behavioral dysfunction in adulthoods. Our results may provide promising information for the development of novel therapeutic strategies that target adverse childhood experience-related symptomatology associated with numerous neuropsychiatric illnesses.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-c-Fos (9F6) | Cell Signaling Technology | Cat# 2250S; RRID: AB_2247211 |
| Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 | Thermo Fisher Scientific | Cat# A32731; RRID: AB_2633280 |
| Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 555 | Thermo Fisher Scientific | Cat# A32732; RRID: AB_2633281 |
| Bacterial and Virus Strains | ||
| EnvA-pseudotyped glycoprotein (G)-deleted rabies virus expressing eGFP (EnvA-RVΔG-eGFP) | Lim Lab | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| (+)-PD 128907 hydrochloride | Tocris | Cat# 1243 |
| Fluoxetine hydrochloride | Sigma-Aldrich | Cat# F132 |
| NBQX | Tocris | Cat# 0373 |
| Picrotoxin | Sigma | Cat# P1675 |
| Critical Commercial Assays | ||
| Drd3 | Advanced Cell Diagnostics | Cat# 447721 |
| Cre | Advanced Cell Diagnostics | Cat# 312281 |
| Slc32a1 (VGAT) | Advanced Cell Diagnostics | Cat# 319191 |
| Slc17a6 (VGlut2) | Advanced Cell Diagnostics | Cat# 319171 |
| Hybrid-R RNA purification kit | GeneAll Biotechnology | Cat# 305-101 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Drd3::Cre: B6.FVB(Cg)-Tg(Drd3-cre)KI196Gsat/Mmucd | GENSAT | Stock# 036968-UCD; RRID: MMRRC_036968-UCD |
| Mouse: Ai6: B6.Cg-Gt(ROSA)26Sortm6(CAG-ZsGreen1)Hze/J | The Jackson Laboratory | JAX: 007906; RRID: IMSR_JAX:007906 |
| Mouse: C57BL/6J | The Jackson Laboratory | JAX: 000664; RRID: IMSR_JAX:000664 |
| Mouse: BTBR: BTBR T+ Itpr3tf/J | The Jackson Laboratory | JAX: 002282; RRID: IMSR_JAX:002282 |
| Oligonucleotides | ||
| shRNA targeting sequence: Drd3: 5′-TTCTTCTT GACTCACGTTCTT-3′ |
This paper | N/A |
| Primers for mouse Drd3 (F:5′- ATGGCACCTCT GAGCCAGATAAG-3′; R: 5′-TCAGCAGGAT AGAATCTTGAGGAAGG-3′) |
This paper | N/A |
| Drd3 (Mm00432887_m1) | Life Technologies | Cat# 4331182 |
| Drd1 (Mm02620146_s1) | Life Technologies | Cat# 4331182 |
| Drd2 (Mm00438545_m1) | Life Technologies | Cat# 4331182 |
| Drd5 (Mm04210376_s1) | Life Technologies | Cat# 4331182 |
| Htr1a (Mm00434106_s1) | Life Technologies | Cat# 4331182 |
| Htr2a (Mm00555764_m1) | Life Technologies | Cat# 4331182 |
| Htr1b (Mm00439377_s1) | Life Technologies | Cat# 4331182 |
| Htr2c (Mm00434127_m1) | Life Technologies | Cat# 4331182 |
| Oxtr (Mm01329577_g1) | Life Technologies | Cat# 4331182 |
| Grm3 (Mm00725298_m1) | Life Technologies | Cat# 4331182 |
| Grm5 (Mm00690332_m1) | Life Technologies | Cat# 4331182 |
| Glp1r (Mm00445292_m1) | Life Technologies | Cat# 4331182 |
| Glp2r (Mm01329475_m1) | Life Technologies | Cat# 4331182 |
| GAPDH (Mm99999915_g1) | Life Technologies | Cat# 4331182 |
| Recombinant DNA | ||
| AAVDJ-EF1α-eGFP | This paper | N/A |
| AAVDJ-EF1α-DIO-eGFP | This paper | N/A |
| AAVDJ-hSyn-DIO-mRuby2-P2A-TVA-RVG | This paper | N/A |
| AAVDJ-EF1α-DIO-GCaMP6f | This paper | N/A |
| AAVDJ-CAG-DIO-eGFP-P2A-Kir2.1 | This paper | N/A |
| AAVDJ- EF1α-DIO-eYFP | This paper | N/A |
| AAVDJ-EF1α-DIO-hChR2(H134R)-eYFP | Plasmid: Karl Deisseroth Production: Lim Lab | Addgene #55639 |
| AAVDJ- EF1α-EmGFP-Drd3 shRNA | This paper | N/A |
| AAVDJ- EF1α-Drd3∗-P2A-EmGFP-Drd3 shRNA | This paper | N/A |
| AAVDJ- EF1α-DIO-EmGFP-Drd3 shRNA | This paper | N/A |
| AAVDJ- EF1α-DIO-Drd3∗-P2A-EmGFP-Drd3 shRNA | This paper | N/A |
| Software and Algorithms | ||
| QuantStudio Real-Time PCR Software | Applied Biosystems | N/A |
| UltraVox XT (v.3.0) | Noldus | http://www.noldus.com/animal-behavior-research/products/ultravox-xt |
| MATLAB R2015a | MathWorks | N/A |
| Doric Neuroscience Studio software (v.4.0.1) | Doric Lenses | http://doriclenses.com/life-sciences/software/955-doric-neuroscience-studio.html |
| FIJI (ImageJ) | NIH | https://fiji.sc/ |
| Viewer II | BIOBSERVE | http://www.biobserve.com/behavioralresearch/products/viewer |
| SigmaStat (v.3.5) | Aspire Software | N/A |
| Other | ||
| Dual fiber-optic cannula | Doric Lenses | DFC_200/240-0.22_10 mm_GS0.6_FLT |
| Complementary metal-oxide-semiconductor (CMOS) camera | Doric Lenses | SFMB_L_UFGJ_1000_458 |
| Snap-in imaging cannula model L | Doric Lenses | SICL_V_500_5.66_120 |
| Guide cannula | Plastics One | C235GS-5-0.6/SPC |
| Dummy cannula | Plastics One | C235DCS-5/SPC |
| Internal cannula | Plastics One | C235IS-5/SPC |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Byung Kook Lim (bklim@ucsd.edu).
Experimental Model and Subject Details
C57BL/6J and BTBR T+ Itpr3tf/J mice were obtained from the Jackson Laboratory. Drd3::Cre bacterial artificial chromosome (BAC) transgenic mice were from the Gene Expression Nervous System Atlas (GENSAT, Founder line: KI196) and were crossed for several generations to C57BL/6J mice before using. To visualize Drd3-positive neurons, Drd3::Cre mice were crossed with Ai6 mice (ZsGreen1 reporter line; Jackson Laboratory). Adult male mice (8-12 weeks old) were used for behavioral experiments, immunohistochemistry, and slice electrophysiology. Both male and female mice (8-12 weeks old) were used for quantitative real-time PCR, FISH, and anatomical tracing. Mice were housed on a 12-h light/dark cycle with rodent chow and water ad libitum. All behavioral procedures were performed during the light cycle. Prior to testing, mice were acclimated to the test room for at least 1 h. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, San Diego.
Method Details
ESD Procedures
The ESD stress paradigm was adopted from previously published methods with minor modifications (Murgatroyd et al., 2009, Uchida et al., 2010, Zimmerberg et al., 2009). Briefly, pregnant female mice were individually housed when they were 14-16 d pregnant. From postnatal day 1 (P1) to P14, ESD pups were separated from both their dam and littermates for 3 h each day. During this separation, ESD pups were placed individually in a divided small chamber with clean bedding and transferred to an incubator which was maintained at 32 ± 1°C. The timing of the separation period was randomized, but within the light cycle (9:00 a.m. to 4:00 p.m.). At the end of the separation period, ESD pups were reunited with the dam and littermates. Control pups remained undisturbed in the maternal nest. All pups were weaned at P21 and housed in groups of three to five of the same gender until the start of the experiments.
Behavioral Assays
Locomotion
Locomotion was assessed in an open field arena (44 × 44 × 44 cm3). Mice were placed individually to the arena and allowed to explore freely for 15-30 min. Activity was monitored using a webcam mounted above the arena and analyzed by tracking software (BIOBSERVE). For the time course data analysis, the distance traveled was measured in 5-min bins.
Open Field Test
Each individual mouse was placed in the open field arena (44 × 44 × 44 cm3) and allowed to move freely for 15 min. The open field area was subdivided into two zones, a center (20 × 20 cm2) and periphery. The movement of mice was monitored with a webcam and analyzed by tracking software (BIOBSERVE).
Elevated Plus Maze
The elevated plus maze consisted of two open arms, two closed arms, and a center, elevated to a height of 30.5 cm above the ground. Mice were placed in the center and allowed to explore the space for 5 min. The movement of mice was analyzed by tracking software (BIOBSERVE).
Novel Object Recognition Test
Mice were habituated to the open field arena (44 × 44 × 44 cm3) in the absence of objects for 30 min a day before the training session. During the training session, two identical objects were placed in each corner of the arena, and mice were allowed to explore for 10 min. Twenty-four hours after training, mice were placed in the arena where one of the two objects was replaced with a novel object having different color and shape. All movements of mice were monitored with a webcam for 10 min, and the recognition time in each object area (2 cm around the object) was measured by tracking software (BIOBSERVE). Discrimination rate was calculated as [(time spent in a novel object area) / (time spent in a novel object area + time spent in a familiar object area) × 100 (%)] (Lee et al., 2015).
Buried Food Olfactory Test
This experiment was performed as previously described (Chung et al., 2015). Briefly, mice were given strawberry chocolate cookies and water ad libitum for 2 d before testing. Mice were deprived of cookies for 12 h before testing. During testing, a small piece of cookie was buried at the corner of the cage under 2 cm of bedding. The subject mouse was introduced at the opposite end of the cookie burial site. The time for the mouse to find the buried cookie and begin to burrow was measured. Fresh cages and bedding were used for every test.
Three-Chamber Social Preference Test
The test was performed as described previously, with minor modifications (Han et al., 2012). A non-transparent plexiglass box (68 × 22 × 24 cm3) with two partitions that make two side chambers (left and right; 28 × 22 × 24 cm3) separated by a central chamber (12 × 22 × 24 cm3) was used. In the first 10-min session, the test mouse was placed in the middle of the three-chamber apparatus and allowed to habituate to the apparatus where two empty wire cages were located in the left and right chambers. In the second 10-min session, an age and gender-matched stranger mouse was placed in one of the wire cages on the side designated as the ‘social’ compartment, whereas the other side contained an empty wire cage. The designation of the ‘social’ side was randomly assigned in a counterbalanced fashion. The movement of the mouse was monitored by a webcam and was analyzed by tracking software (BIOBSERVE). The amount of time mice spent in each compartment (resident time), and the amount of time mice explored within a 2 cm radius proximal to each wire cage (sniffing time) were measured. Social preference levels were calculated as [(time in the social compartment - time in the empty compartment) / (time in social compartment + time in the empty compartment)] (Bambini-Junior et al., 2014).
Reciprocal Social Interaction Test
As a stranger animal, male juvenile mice (4-5 weeks old) were used instead of adults to avoid mutual aggression (Moretti et al., 2005). Each testing mouse spent 30 min in an open field arena (44 × 44 × 44 cm3) for a habituation. After that, a stranger mouse was introduced to the arena and allowed to explore freely for 5 min. All behaviors were video recorded and analyzed by two experimenters blind to the testing conditions and then the data were averaged. Reciprocal social interactions were defined as activities including close following (< 1 cm), body sniffing, mounting, and nose to nose contact.
USV Test
USVs were examined as described previously (Chung et al., 2015, Fischer and Hammerschmidt, 2011). For habituations, male mice were individually placed inside of a soundproof recording chamber for 30 min. Subsequently, one estrus female was placed in the recording chamber, and the male mouse was allowed to investigate the female for 5 min, during which emitted USVs were recorded using UltraVox XT software (version 3.0; Noldus) (Tsai et al., 2012).
Immunohistochemistry for c-fos
For quantification of c-fos immunoreactivity after social contacts, mice were individually housed on the day before testing day. On the testing day, a stranger male juvenile mouse (social stimulus) was introduced in the cage and allowed to interact with the experimental mouse for 10 min. The experimental mice were transcardially perfused with 4% PFA 30 min after introducing the social stimulus. Brains were extracted and post-fixed overnight in 4% PFA. Coronal sections (50 μm) were immunostained using a rabbit anti-c-fos antibody (1:5000; Cell Signaling Technology) applied overnight in a PBS solution containing 0.3% Triton X-100 (PBS-T), at room temperature. Sections were then washed in PBS-T and incubated in a 1:500 dilution of goat anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibodies, Alexa Fluor Plus 488 or 555 (Thermo Fisher Scientific) in PBS-T for 1 h. The sections were rinsed with PBS-T and mounted using mounting medium. Images were acquired using a confocal microscope (Olympus FluoView FV1200) and quantitatively analyzed with ImageJ.
Brain Dissection and Quantitative Real-Time PCR
Mice were anesthetized with isofluorane and 250 μm slices were prepared in oxygenated aCSF, using a vibratome (Leica VT1200). The LS was microdissected bilaterally, and samples were immediately frozen on dry ice and stored at −80°C prior to RNA isolation. Total RNA was extracted from dissected samples using Hybrid-R RNA purification kit (GeneAll Biotechnology). Purified RNA samples were reverse transcribed by using the SuperScript-III First-strand cDNA synthesis kit (Invitrogen). Quantitative real-time PCR was performed by using TaqMan Gene Expression Assay Kit (Applied Biosystems). All TaqMan probes were purchased from Applied Biosystems and are as follows: Drd3 (Mm00432887_m1), Drd1 (Mm02620146_s1), Drd2 (Mm00438545_m1), Drd5 (Mm04210376_s1), Htr1a (Mm00434106_s1), Htr2a (Mm00555764_m1), Htr1b (Mm00439377_s1), Htr2c (Mm00434127_m1), Oxtr (Mm01329577_g1), Grm3 (Mm00725298_m1), Grm5 (Mm00690332_m1), Glp1r (Mm00445292_m1), Glp2r (Mm01329475_m1) and glyceraldhyde-3-phosphate dehydrogenase (GAPDH; Mm99999915_g1). Target amplification was performed by using ViiA 7 Real-Time PCR System (Applied Biosystems). The relative mRNA expression levels were calculated via a comparative threshold cycle (Ct) method using GAPDH as an internal control: ΔCt = Ct (gene of interest) – Ct (GAPDH). The gene expression fold change was normalized to the control sample and then was calculated as 2−ΔΔCt (Schmittgen and Livak, 2008).
Fluorescent In Situ Hybridization (FISH)
Brains were rapidly extracted and flash frozen with isopentane (Sigma-Aldrich) chilled with dry ice in 70% ethanol. Coronal brain slices (20 μm) containing the LS were sectioned on a cryostat (Leica CM3050S) at −20°C. Brain slices were mounted directly onto slides and stored at −80°C until FISH processing. The FISH was conducted using RNAscope probes (Advanced Cell Diagnostics, ACD) (Tejeda et al., 2017). Slides were fixed in 4% PFA for 15 min at 4°C and subsequently dehydrated for 5-10 min with 50%, 70% and 100% ethanol at room temperature. Sections were then incubated with a Protease pretreat-IV solution for 30 min, and washed with PBS, before being incubated with probes for 2 h at 40°C in the HybEZ oven (ACD). All probes used were commercially available: Mm-Drd3-C1 (NM 007877.1), Cre recombinase-C2 (KC 845567.1), Mm-Slc17a7(VGlut2)-C2 (NM 182993.2) and Mm-Slc32a1(VGAT)-C3 (NM 009508.2). After washing with wash buffer, the signal was amplified by incubating tissue sections in amplification buffers at 40°C. After the final rinse, DAPI solution was applied to the sections. Slides were coverslipped and visualized with a confocal microscope (Olympus FluoView FV1200).
Virus and shRNA Generation
AAV was produced by transfection of 293 cells with three plasmids: an AAV vector expressing target constructs (eGFP, DIO-eGFP, DIO-mRuby2-TVA-RVG, DIO-GCaMP6f, DIO-eGFP-Kir2.1, DIO-ChR2(H134R)-eYFP, DIO-eYFP, EmGFP-Drd3 shRNA, Drd3∗-EmGFP-Drd3 shRNA, DIO-EmGFP-Drd3 shRNA, DIO-Drd3∗-EmGFP-Drd3 shRNA), AAV helper plasmid (pHELPER; Agilent) and AAV rep-cap helper plasmid (pRC-DJ, gift from M. Kay). At 72 h after transfection, the cells were collected and lysed. Viral particles were then purified by an iodixanol step-gradient ultracentrifugation method. The iodixanol was diluted and the AAV was concentrated using a 100-kDa molecular mass cutoff ultrafiltration device. The genomic titer was determined by quantitative real-time PCR. The AAV vectors were diluted in PBS to a working concentration of approximately 1012 viral particles/ml. To generate EnvA-pseudotyped glycoprotein (G)-deleted rabies virus expressing eGFP (EnvA-RVΔG-eGFP), we followed a published protocol (Osakada and Callaway, 2013). Plasmids expressing the rabies viral components, B7GG, BHK-EnvA and HEK-TVA cells were provided at courtesy of Dr. Edward M. Callaway.
To construct shRNA against Drd3 (Drd3 shRNA), oligonucleotides that contained 21 base-pair sense and antisense sequences (5′-TTCTTCTTGACTCACGTTCTT-3′) targeting Drd3 were connected with a hairpin loop followed by a poly(T) termination signal. This shRNA oligonucleotide was ligated into BLOCK-iT POLII miR RNAi expression vectors (Invitrogen) and then transferred to an AAV vector together with EmGFP. For testing of the efficacy of the shRNA, we stereotaxically injected AAVs expressing Drd3 shRNA into the LS. Two weeks after injection, the virus-infected area labeled EmGFP expression was dissected. Drd3 mRNA levels were measured using quantitative real-time PCR and found to be reduced over 80% (Figures 6G and 6H). To validate the specificity of Drd3 shRNA, a control vector expressing both Drd3 shRNA and shRNA-resistant Drd3 (Drd3∗) was cloned (Ma et al., 2007). A mouse cDNA was amplified by PCR using the following primer sets: Drd3-F: 5′-ATGGCACCTCTGAGCCAGATAAG-3′ and Drd3-R: 5′-TCAGCAGGATAGAATCTTGAGGAAGG-3′. The amplified Drd3 cDNA was inserted into AAV vector to produce AAV-hsyn1-Drd3-eGFP. Site-directed mutagenesis was performed using AAV-hsyn1-Drd3-eGFP as a template to make a shRNA-resistant mutant (Drd3∗). Then, this Drd3∗ cDNA was cloned into AAV vectors expressing Drd3 shRNA to rescue the phenotype induced by knockdown of Drd3.
Stereotaxic Surgeries
Drd3::Cre or wild-type mice (6-12 weeks old) were anesthetized with a mixture of ketamine (100 mg/kg) and dexmedetomidine (0.5 mg/kg). The mouse was mounted in a stereotaxic frame (David Kopf Instruments). Body temperature was kept stable by using a heating pad while recovering from anesthesia. Viral injections were targeted using coordinates based on the Paxinos and Franklin mouse brain atlas (Paxinos and Franklin, 2008). For behavioral or electrophysiology experiments, viral preparations (AAV-DIO-eGFP, AAV-DIO-eGFP-Kir2.1, AAV-EmGFP-Drd3 shRNA, AAV-Drd3∗-EmGFP-Drd3 shRNA, AAV-DIO-EmGFP-Drd3 shRNA and AAV-DIO-Drd3∗-EmGFP-Drd3 shRNA) in volumes 250-350 nL were injected bilaterally into the LS (bregma, anteroposterior +0.85 mm; lateral ± 0.35 mm; dorsoventral −3.2 mm) or NAc shell (bregma, anteroposterior +1.6 mm; lateral ± 0.5 mm; dorsoventral −4.1 mm) at a slow rate (100 nl/min) using a syringe pump. Mice were allowed 2-3 weeks to recover from the virus injections before starting of behavioral tests. Injection sites were confirmed in all animals by preparing coronal sections containing the desired plane, and animals with an incorrect injection placement were excluded from analyses. For anatomical output mapping, AAV-eGFP or AAV-DIO-eGFP (200 nl) were unilaterally injected into the LS of wild-type or Drd3::Cre mice, respectively. For rabies-mediated retrograde tracing, we first unilaterally injected 200 nL of AAV-DIO-mRuby2-TVA-RVG into the LS of Drd3::Cre mice. Two weeks later, mice were injected with EnvA-RVΔG-eGFP into the LS. All mice were sacrificed 1-2 weeks after the last injection for circuit mapping analysis. For in vivo Ca2+ imaging experiments, Drd3::Cre animals received the unilateral injection of AAV-DIO-GCaMP6f (250 nl) into the LS. Two weeks later, the snap-in imaging cannula (Model L-V; 500 μm diameter; 5.66 mm length; Doric Lenses) with guiding gradient-index (GRIN) lens was implanted above the previous injection site. The target depth of the lens was adjusted (∼100 μm above injection site) by observing fluorescent signals through a snap-in fluorescence microscope body (Doric Lenses). The implanted imaging cannula and focusing ring were secured to the skull with an initial layer of adhesive cement (C&B metabond; Parkell) followed by a second layer of dental cement (Ortho-Jet; Lang). In vivo Ca2+ imaging tests were performed 3 weeks after the implantation of the image cannula. For optogenetic stimulation of Drd3LS neurons, Drd3::Cre animals were bilaterally injected with 350 nL of AAV-DIO-eYFP or AAV-DIO-ChR2-eYFP into the LS. Bilateral chronic optic fibers (200 μm diameter, 0.22 NA; Doric Lenses) were implanted with the tip ∼200 μm above the virus injection site. Implanted fibers were adhered to the skull with adhesive and dental cement as described above. Lastly, sutures or sterile tissue adhesive (Vetbond; 3M) were used to close the incision. For microinjection of the Drd3 agonist into the LS, a guide cannula (26 gauge, 5 mm long; Plastics One) was chronically implanted in this brain region (bregma, anteroposterior +0.85 mm; lateral ± 0.35 mm; dorsoventral −2.7 mm). Implanted cannulae were secured to the skull as described above, and then obturators were placed in the guide cannulae. Behavioral testing was performed 2 weeks after the implantation. Upon completion of all behavioral experiments, viral injections or fiber/cannula placements were confirmed.
Optogenetic Stimulation
A 3-m-long fiber-optic patch cord (Doric Lenses) was connected to the chronically implanted optic fiber and suspended above the behavioral testing area to allow animals to move freely while receiving laser stimulations. The patch cord was connected to a 473-nm laser (OEM Laser Systems). For Drd3LS neuronal stimulation, bilateral activation using ChR2 was achieved by delivering blue light in a high-frequency train (5 ms pulses of 20 Hz; 10-15 mW at tips). For the three-chamber test, each mouse was subjected to the test twice, separated by 24 h, with one session (10 min) paired with optical stimulation (ON) and one with no stimulation (OFF). Similarly, each mouse underwent two reciprocal social interaction tests separated by 24 h, with one of the sessions (5 min) paired with optical stimulation (ON) and one without (OFF). A novel social stimulus was used for each session and groups were counterbalanced for order of light stimulation (Felix-Ortiz and Tye, 2014). For the USV test, the blue light stimulations were delivered to each mouse in a new cage for 5 min, once a day over 3 consecutive days. One hour after the last stimulation, mice were subjected to the USV test.
In Vivo Ca2+ Imaging in Freely Moving Mice and Data Analysis
Mice were habituated to an open field arena (44 × 44 × 44 cm3) with the head-mounted microscope body attached to the top of the imaging cannula for 30 min. Images were acquired at 8 frames per second with an average exposure time of 125 ms using Doric Neuroscience Studio software (version 4.0.1; Doric Lenses). LED power was maintained at 30% with analog gain 2. To examine Ca2+ dynamics in response to a social stimulus or a novel object, either a conspecific stranger mouse or a fake mouse (mouse toy) was introduced to the open filed arena after 10 min-recording of baseline fluorescence. Animal behaviors were recorded by a web camera concurrently. This experimental setting was also combined with the USV testing, which allowed the analysis for correlation of neural activity with vocal communications in response to a female mouse. To test whether the Ca2+ activity of individual neurons was altered by PD128907 injection, the rate of Ca2+ transients observed per min was measured during 5-min periods after PD128907 injection (at 20, 50, 100, and 120 min post-injection) and compared to the rate during a 5-min pre-injection period. All image analyses were performed with a Doric Neuroscience Studio software (Doric Lenses) and custom MATLAB-based software (Mathworks). To remove movement artifacts, individual image frames were aligned using a single frame as a reference, and then background fluorescence was removed from the aligned images. Regions of interest (ROI) corresponding to cell bodies were determined using an automated cell-finding function and were visually inspected to ensure accuracy. ΔF/F0 was calculated as (F-F0) / F0, where F0 was the lowest 5% of the fluorescence signal for the imaging session being analyzed. To detect individual Ca2+ transients, we first processed the ΔF/F0 time series to remove slow drifts, estimated by a median filter of a 10 s width. Next, median absolute deviation (MAD) of the entire time series was computed. Ca2+ transients were extracted by sequentially detecting each upward transient that exceeded a 6-MAD threshold (equivalent to 4-σ assuming normal distribution) and following the previous event by an interval no shorter than 2 s (Calipari et al., 2016).
Slice Electrophysiology Recordings
Coronal slices (300 μm) containing the LS were prepared using a vibratome (Leica VT1200S), in a solution containing (in mM): 110 choline chloride, 25 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 7 MgCl2, 25 glucose, 0.5 CaCl2, 11.6 ascorbic acid, and 3.1 pyruvic acid, saturated with 95% O2/5% CO2. Slices were then allowed to recover at 30°C for 20 min, and subsequently at room temperature, in a solution containing (in mM): 118 NaCl, 26 NaHCO3, 11 glucose, 15 HEPES, 2.5 KCl, 1.25 NaH2PO4, 2 pyruvic acid, 0.4 ascorbic acid, 2 CaCl2, and 1 MgCl2, saturated with 95% O2/5% CO2. Following > 1.5 h of recovery, slices were transferred to a recording chamber perfused with (in mM): 119 NaCl, 26.2 NaHCO3, 11 glucose, 2.5 KCl, 1 NaH2PO4, 2.5 CaCl2, and 1.3 MgCl2, saturated with 95% O2/5% CO2, and delivered at 2 mL/min at 30 ± 1°C. Whole cell patch clamp recordings were obtained under visual guidance by differential interference microscopy (Olympus BX61WI), using borosilicate glass pipettes (3-4 MΩ) filled with (in mM): 115 CsMeSO3, 1.5 MgCl2, 1 EGTA, 10 HEPES, 4 Mg-ATP, 0.3 Na3-GTP, 10 Na-phosphocreatine, 2 QX-314, and 10 BAPTA-Cs4 for all voltage clamp recordings, except mIPSCs, in which case the internal solution consisted of 125 CsCl, 8 NaCl, 0.6 EGTA, 10 HEPES, 4 Mg-ATP, 0.3 Na3-GTP, 10 Na-phosphocreatine, and 2 QX-314. For current clamp recordings the pipette internal solution consisted of (in mM): 125 K-gluconate, 4 NaCl, 10 HEPES, 0.5 EGTA, 20 KCl, 4 Mg-ATP, 0.3 Na3-GTP, and 10 Na-phosphocreatine. Liquid junction potentials were left uncompensated. Signals were amplified and filtered (2 kHz) using an Axopatch 700B amplifier, sampled at 10 kHz using a Digidata 1550, and recorded with Clampex (Molecular Devices). Excitatory to inhibitory ratios were calculated as the peak synaptic response at Vh = −70 mV divided by the peak synaptic response at Vh = 0 mV. Similarly, AMPA to NMDA ratios were calculated as the peak synaptic response at Vh = −70 mV divided by the peak synaptic response at Vh = +40 mV, with 100 μM picrotoxin added to the external solution. Miniature postsynaptic currents were recorded in the presence of 0.5 μM tetrodotoxin, in addition to either 100 μM picrotoxin for mEPSCs or 10 μM NBQX for mIPSCs. Rheobase was determined as the minimal current step eliciting at least one action potential, using 10 pA increments of 500 ms duration. Analyses were performed offline using Clampfit (Molecular Devices). Only cells with a stable access resistance of < 25 MΩ throughout the recording period were included in the analysis.
Drugs
(+)-PD 128907 hydrochloride (PD128907, Tocris Bioscience) was dissolved in sterile saline. For behavioral experiments, PD128907 (0.5 mg/kg, i.p.) or saline were administered as a series of triple injections 24, 6 and 1 h before the behavioral test. In the experiments for analyzing c-fos levels, mice received PD128907 (0.1 or 0.5 mg/kg, i.p.; 0.1 or 2.5 μg/side) or saline 1 h before transcardial perfusion. For electrophysiology experiments, 10 mM PD128907 was diluted to a final concentration of 10 μM in aCSF. Fluoxetine (Sigma-Aldrich) was dissolved in sterile saline. Mice received 14 daily injections of fluoxetine (20 mg/kg, i.p.).
Intracranial Drug Injection and Histology
On the day of the intracranial injection, an internal cannula (33 gauge, projecting 0.5 mm below the tip of the guide; Plastics One) connected to 1 μL syringes (Hamilton) via polyethylene (PE)-20 tubing was inserted into the guide. PD128907 (0.5 or 2.5 μg/side) or saline was microinjected bilaterally into the LS 1 h before behavioral testing. Upon completion of behavioral experiments, mice were anesthetized and perfused with 4% PFA. Brains were extracted and further post-fixed in 4% PFA. Coronal sections (50 μm) were subsequently stained with 0.2% cresyl violet (Sigma-Aldrich) for verification of cannula tip placements. Only mice with injection cannula tips located bilaterally in the LS were included in the data analyses.
Quantification and Statistical Analysis
All statistical analyses were performed with SigmaStat software (version 3.5; Aspire Software). Differences across more than two groups were analyzed with one-way or two-way analyses of variance (ANOVA) followed by Bonferroni or Fisher LSD post hoc tests for multiple comparisons. For comparisons between two groups, Two-tailed t tests (paired or unpaired) or Mann-Whitney U-test were used as described in the figure legends. The distribution of data was checked for normality and equal variance. p < 0.05 was considered statistically significant. Data are reported as mean ± SEM.
Acknowledgments
We acknowledge Dr. X. Wang for providing MATLAB code for analyzing behavioral data and in vivo Ca2+ imaging data. We also thank M.N. Askar and J. Chang for technical assistance with analyzing behavioral data, Dr. A. Pinto-Duarte for his scientific input, and K. Kim for her help with illustrations. This study was supported by the Klingenstein foundation, Searle scholar program (15-SSP-229; Kinship foundation), Whitehall foundation (2014-08-63), NARSAD young investigator award (24094), and a grant from NIMH (R01MH107742). S. Shin is supported by a Tobacco-related disease research program (TRDRP, 25FT-0007) postdoctoral fellowship. H. Pribiag is supported by a Canadian Institutes of Health Research (CHIR) Fellowship. V. Lilascharoen is supported by the Anandamahidol Foundation Fellowship.
Author Contributions
S.S. and B.K.L. designed the study and interpreted the results. S.S. performed all the experiments and analyzed the data. H.P. performed and analyzed all the electrophysiological experiments. S.S. and V.L. designed and generated the viruses. D.K. helped with the FISH experiments. X.-Y.W. helped with virus production. S.S. and B.K.L wrote the manuscript with contributions from all authors.
Declaration of Interests
The authors declare no competing interests.
Published: December 21, 2017
Footnotes
Supplemental Information includes six figures and can be found with this article online at https://doi.org/10.1016/j.neuron.2017.11.040.
Supplemental Information
References
- Agid O., Shapira B., Zislin J., Ritsner M., Hanin B., Murad H., Troudart T., Bloch M., Heresco-Levy U., Lerer B. Environment and vulnerability to major psychiatric illness: A case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol. Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Anthony T.E., Dee N., Bernard A., Lerchner W., Heintz N., Anderson D.J. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell. 2014;156:522–536. doi: 10.1016/j.cell.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambini-Junior V., Zanatta G., Della Flora Nunes G., Mueller de Melo G., Michels M., Fontes-Dutra M., Nogueira Freire V., Riesgo R., Gottfried C. Resveratrol prevents social deficits in animal model of autism induced by valproic acid. Neurosci. Lett. 2014;583:176–181. doi: 10.1016/j.neulet.2014.09.039. [DOI] [PubMed] [Google Scholar]
- Bandelow B., Charimo Torrente A., Wedekind D., Broocks A., Hajak G., Rüther E. Early traumatic life events, parental rearing styles, family history of mental disorders, and birth risk factors in patients with social anxiety disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2004;254:397–405. doi: 10.1007/s00406-004-0521-2. [DOI] [PubMed] [Google Scholar]
- Bolger K.E., Patterson C.J. Developmental pathways from child maltreatment to peer rejection. Child Dev. 2001;72:549–568. doi: 10.1111/1467-8624.00296. [DOI] [PubMed] [Google Scholar]
- Bruce L.C., Heimberg R.G., Blanco C., Schneier F.R., Liebowitz M.R. Childhood maltreatment and social anxiety disorder: Implications for symptom severity and response to pharmacotherapy. Depress. Anxiety. 2012;29:131–138. doi: 10.1002/da.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari E.S., Bagot R.C., Purushothaman I., Davidson T.J., Yorgason J.T., Peña C.J., Walker D.M., Pirpinias S.T., Guise K.G., Ramakrishnan C. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc. Natl. Acad. Sci. USA. 2016;113:2726–2731. doi: 10.1073/pnas.1521238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E.M., Luo L. Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J. Neurosci. 2015;35:8979–8985. doi: 10.1523/JNEUROSCI.0409-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Kittler J.T., Moss S.J., Yan Z. Dopamine D3 receptors regulate GABAA receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. J. Neurosci. 2006;26:2513–2521. doi: 10.1523/JNEUROSCI.4712-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W., Choi S.Y., Lee E., Park H., Kang J., Park H., Choi Y., Lee D., Park S.-G., Kim R. Social deficits in IRSp53 mutant mice improved by NMDAR and mGluR5 suppression. Nat. Neurosci. 2015;18:435–443. doi: 10.1038/nn.3927. [DOI] [PubMed] [Google Scholar]
- de Krom M., Staal W.G., Ophoff R.A., Hendriks J., Buitelaar J., Franke B., de Jonge M.V., Bolton P., Collier D., Curran S. A common variant in DRD3 receptor is associated with autism spectrum disorder. Biol. Psychiatry. 2009;65:625–630. doi: 10.1016/j.biopsych.2008.09.035. [DOI] [PubMed] [Google Scholar]
- Diaz M.R., Chappell A.M., Christian D.T., Anderson N.J., McCool B.A. Dopamine D3-like receptors modulate anxiety-like behavior and regulate GABAergic transmission in the rat lateral/basolateral amygdala. Neuropsychopharmacology. 2011;36:1090–1103. doi: 10.1038/npp.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich M.O., Horvath T.L. GABA keeps up an appetite for life. Cell. 2009;137:1177–1179. doi: 10.1016/j.cell.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Dodge K.A., Bates J.E., Pettit G.S. Mechanisms in the cycle of violence. Science. 1990;250:1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- Enoch M.A., Steer C.D., Newman T.K., Gibson N., Goldman D. Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Genes Brain Behav. 2010;9:65–74. doi: 10.1111/j.1601-183X.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enthoven L., Oitzl M.S., Koning N., van der Mark M., de Kloet E.R. Hypothalamic-pituitary-adrenal axis activity of newborn mice rapidly desensitizes to repeated maternal absence but becomes highly responsive to novelty. Endocrinology. 2008;149:6366–6377. doi: 10.1210/en.2008-0238. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz A.C., Tye K.M. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 2014;34:586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C.F., Melloni R.H., Jr., Koppel G., Perry K.W., Fuller R.W., Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J. Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J., Hammerschmidt K. Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: Insights into the evolution of vocal communication. Genes Brain Behav. 2011;10:17–27. doi: 10.1111/j.1601-183X.2010.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J.P., Zheng F., Hasuo H., Shinnick-Gallagher P. Activities of neurons within the rat dorsolateral septal nucleus (DLSN) Prog. Neurobiol. 1995;45:373–395. doi: 10.1016/0301-0082(95)98600-a. [DOI] [PubMed] [Google Scholar]
- Goff B., Gee D.G., Telzer E.H., Humphreys K.L., Gabard-Durnam L., Flannery J., Tottenham N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson J.L., Kelly A.M., Kingsbury M.A., Thompson R.R. An aggression-specific cell type in the anterior hypothalamus of finches. Proc. Natl. Acad. Sci. USA. 2012;109:13847–13852. doi: 10.1073/pnas.1207995109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin L.A., Grosenick L., Finkelstein J.C., Kauvar I.V., Fenno L.E., Adhikari A., Lammel S., Mirzabekov J.J., Airan R.D., Zalocusky K.A. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán Y.F., Tronson N.C., Jovasevic V., Sato K., Guedea A.L., Mizukami H., Nishimori K., Radulovic J. Fear-enhancing effects of septal oxytocin receptors. Nat. Neurosci. 2013;16:1185–1187. doi: 10.1038/nn.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Tai C., Westenbroek R.E., Yu F.H., Cheah C.S., Potter G.B., Rubenstein J.L., Scheuer T., de la Iglesia H.O., Catterall W.A. Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Albert D., Iselin A.M.R., Carré J.M., Dodge K.A., Hariri A.R. Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Soc. Cogn. Affect. Neurosci. 2016;11:405–412. doi: 10.1093/scan/nsv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harasta A.E., Power J.M., von Jonquieres G., Karl T., Drucker D.J., Housley G.D., Schneider M., Klugmann M. Septal glucagon-like peptide 1 receptor expression determines suppression of cocaine-induced behavior. Neuropsychopharmacology. 2015;40:1969–1978. doi: 10.1038/npp.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R.G. Electrical self-stimulation of the brain in man. Am. J. Psychiatry. 1963;120:571–577. doi: 10.1176/ajp.120.6.571. [DOI] [PubMed] [Google Scholar]
- Heim C., Nemeroff C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Cullinan W.E., Ziegler D.R., Tasker J.G. Role of the paraventricular nucleus microenvironment in stress integration. Eur. J. Neurosci. 2002;16:381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- Hitti F.L., Siegelbaum S.A. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E., Soorya L., Chaplin W., Anagnostou E., Taylor B.P., Ferretti C.J., Wasserman S., Swanson E., Settipani C. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am. J. Psychiatry. 2012;169:292–299. doi: 10.1176/appi.ajp.2011.10050764. [DOI] [PubMed] [Google Scholar]
- Huot R.L., Plotsky P.M., Lenox R.H., McNamara R.K. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Jamain S., Radyushkin K., Hammerschmidt K., Granon S., Boretius S., Varoqueaux F., Ramanantsoa N., Gallego J., Ronnenberg A., Winter D. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Karkowski L.M., Prescott C.A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Davis C.G., Kendler K.S. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol. Med. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kohls G., Schulte-Rüther M., Nehrkorn B., Müller K., Fink G.R., Kamp-Becker I., Herpertz-Dahlmann B., Schultz R.T., Konrad K. Reward system dysfunction in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 2013;8:565–572. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Chung C., Ha S., Lee D., Kim D.-Y., Kim H., Kim E. Shank3-mutant mice lacking exon 9 show altered excitation/inhibition balance, enhanced rearing, and spatial memory deficit. Front. Cell. Neurosci. 2015;9:94. doi: 10.3389/fncel.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Kuzhikandathil E.V. Molecular characterization of individual D3 dopamine receptor-expressing cells isolated from multiple brain regions of a novel mouse model. Brain Struct. Funct. 2012;217:809–833. doi: 10.1007/s00429-012-0383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo A.H., Tahsili-Fahadan P., Wise R.A., Lupica C.R., Aston-Jones G. Linking context with reward: A functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons D.M., Kim S., Schatzberg A.F., Levine S. Postnatal foraging demands alter adrenocortical activity and psychosocial development. Dev. Psychobiol. 1998;32:285–291. [PubMed] [Google Scholar]
- Ma H.T., On K.F., Tsang Y.H., Poon R.Y.C. An inducible system for expression and validation of the specificity of short hairpin RNA in mammalian cells. Nucleic Acids Res. 2007;35:e22. doi: 10.1093/nar/gkl1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C.M., Kehoe P., Kovacs S. Corticosterone release in response to repeated, short episodes of neonatal isolation: Evidence of sensitization. Int. J. Dev. Neurosci. 1998;16:175–185. doi: 10.1016/s0736-5748(98)00026-4. [DOI] [PubMed] [Google Scholar]
- McFarlane H.G., Kusek G.K., Yang M., Phoenix J.L., Bolivar V.J., Crawley J.N. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McHenry J.A., Otis J.M., Rossi M.A., Robinson J.E., Kosyk O., Miller N.W., McElligott Z.A., Budygin E.A., Rubinow D.R., Stuber G.D. Hormonal gain control of a medial preoptic area social reward circuit. Nat. Neurosci. 2017;20:449–458. doi: 10.1038/nn.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesic I., Guzman Y.F., Guedea A.L., Jovasevic V., Corcoran K.A., Leaderbrand K., Nishimori K., Contractor A., Radulovic J. Double dissociation of the roles of metabotropic glutamate receptor 5 and oxytocin receptor in discrete social behaviors. Neuropsychopharmacology. 2015;40:2337–2346. doi: 10.1038/npp.2015.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P., Bouwknecht J.A., Teague R., Paylor R., Zoghbi H.Y. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum. Mol. Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C., Patchev A.V., Wu Y., Micale V., Bockmühl Y., Fischer D., Holsboer F., Wotjak C.T., Almeida O.F., Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Murray A.M., Ryoo H.L., Gurevich E., Joyce J.N. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc. Natl. Acad. Sci. USA. 1994;91:11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni V., Uher R., Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am. J. Psychiatry. 2012;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- Nemeroff C.B. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron. 2016;89:892–909. doi: 10.1016/j.neuron.2016.01.019. [DOI] [PubMed] [Google Scholar]
- Olds J., Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Osakada F., Callaway E.M. Design and generation of recombinant rabies virus vectors. Nat. Protoc. 2013;8:1583–1601. doi: 10.1038/nprot.2013.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes R.G. Medial preoptic area/anterior hypothalamus and sexual motivation. Scand. J. Psychol. 2003;44:203–212. doi: 10.1111/1467-9450.00337. [DOI] [PubMed] [Google Scholar]
- Paredes R., Haller A.E., Manero M.C., Alvarado R., Ågmo A. Medial preoptic area kindling induces sexual behavior in sexually inactive male rats. Brain Res. 1990;515:20–26. doi: 10.1016/0006-8993(90)90571-r. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. Third Edition. Academic Press; 2008. The Mouse Brain in Stereotaxic Coordinates; pp. 1–350. [Google Scholar]
- Pfeffer C.R., Altemus M., Heo M., Jiang H. Salivary cortisol and psychopathology in children bereaved by the september 11, 2001 terror attacks. Biol. Psychiatry. 2007;61:957–965. doi: 10.1016/j.biopsych.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Rai D., Golding J., Magnusson C., Steer C., Lewis G., Dalman C. Prenatal and early life exposure to stressful life events and risk of autism spectrum disorders: Population-based studies in Sweden and England. PLoS ONE. 2012;7:e38893. doi: 10.1371/journal.pone.0038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy I.A., Pino J.A., Weikop P., Osses N., Sørensen G., Bering T., Valle C., Bluett R.J., Erreger K., Wortwein G. Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Transl. Psychiatry. 2016;6:e809. doi: 10.1038/tp.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold P.Y., Swanson L.W. Chemoarchitecture of the rat lateral septal nucleus. Brain Res. Brain Res. Rev. 1997;24:91–113. doi: 10.1016/s0165-0173(97)00008-8. [DOI] [PubMed] [Google Scholar]
- Rothwell P.E., Fuccillo M.V., Maxeiner S., Hayton S.J., Gokce O., Lim B.K., Fowler S.C., Malenka R.C., Südhof T.C. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell. 2014;158:198–212. doi: 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sheehan T.P., Chambers R.A., Russell D.S. Regulation of affect by the lateral septum: Implications for neuropsychiatry. Brain Res. Brain Res. Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Silverman J.L., Yang M., Lord C., Crawley J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald G.M., Rjabokon A., Singewald N., Ebner K. The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology. 2011;36:793–804. doi: 10.1038/npp.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P., Giros B., Martres M.P., Bouthenet M.L., Schwartz J.C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Squillace M., Dodero L., Federici M., Migliarini S., Errico F., Napolitano F., Krashia P., Di Maio A., Galbusera A., Bifone A. Dysfunctional dopaminergic neurotransmission in asocial BTBR mice. Transl. Psychiatry. 2014;4:e427. doi: 10.1038/tp.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal W.G., de Krom M., de Jonge M.V. Brief report: the dopamine-3-receptor gene (DRD3) is associated with specific repetitive behavior in autism spectrum disorder (ASD) J. Autism Dev. Disord. 2012;42:885–888. doi: 10.1007/s10803-011-1312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal W.G., Langen M., van Dijk S., Mensen V.T., Durston S. DRD3 gene and striatum in autism spectrum disorder. Br. J. Psychiatry. 2015;206:431–432. doi: 10.1192/bjp.bp.114.148973. [DOI] [PubMed] [Google Scholar]
- Stephenson D.T., O’Neill S.M., Narayan S., Tiwari A., Arnold E., Samaroo H.D., Du F., Ring R.H., Campbell B., Pletcher M. Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis. Mol. Autism. 2011;2:7. doi: 10.1186/2040-2392-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swant J., Stramiello M., Wagner J.J. Postsynaptic dopamine D3 receptor modulation of evoked IPSCs via GABA(A) receptor endocytosis in rat hippocampus. Hippocampus. 2008;18:492–502. doi: 10.1002/hipo.20408. [DOI] [PubMed] [Google Scholar]
- Tejeda H.A., Wu J., Kornspun A.R., Lowell B.B., Carlezon W.A., Tejeda H.A., Wu J. Pathway- and cell-specific kappa-opiod receptor modulation of excitation-inhibition balance differentially gates D1 and D2 accumbens neuron activity. Neuron. 2017;93:147–163. doi: 10.1016/j.neuron.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P.T., Hull C., Chu Y., Greene-Colozzi E., Sadowski A.R., Leech J.M., Steinberg J., Crawley J.N., Regehr W.G., Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Hara K., Kobayashi A., Funato H., Hobara T., Otsuki K., Yamagata H., McEwen B.S., Watanabe Y. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J. Neurosci. 2010;30:15007–15018. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ameringen M., Mancini C., Streiner D.L. Fluoxetine efficacy in social phobia. J. Clin. Psychiatry. 1993;54:27–32. [PubMed] [Google Scholar]
- Wu Z., Autry A.E., Bergan J.F., Watabe-Uchida M., Dulac C.G. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–330. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Eisinger B., Gammie S.C. Characterization of GABAergic neurons in the mouse lateral septum: A double fluorescence in situ hybridization and immunohistochemical study using tyramide signal amplification. PLoS ONE. 2013;8:e73750. doi: 10.1371/journal.pone.0073750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B., Foote H.E., Van Kempen T.A. Olfactory association learning and brain-derived neurotrophic factor in an animal model of early deprivation. Dev. Psychobiol. 2009;51:333–344. doi: 10.1002/dev.20373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.