Key Points
Alternative donor HCT can be performed in patients with FA without using radiation.
All 26 patients younger than 10 years of age undergoing HCT for marrow failure using lower-dose busulfan-containing regimen survived.
Abstract
Fanconi anemia (FA) is an inherited bone marrow failure syndrome characterized by chromosomal fragility, progressive marrow failure, and cancer predisposition. Hematopoietic cell transplantation (HCT) is curative for FA-related marrow failure or leukemia, but both radiation exposure during transplant and graft-versus-host disease (GVHD) may increase risk of later malignancies of the head and neck and anogenital area. In this study, we tested a radiation-free conditioning regimen with a T-cell–depleted graft to eliminate radiation exposure and minimize early and late toxicities of transplant. Forty-five patients (median age, 8.2 years; range 4.3-44) with FA underwent HCT between June 2009 and May 2014. The preparative regimen included busulfan, cyclophosphamide, fludarabine, and rabbit anti-thymocyte globulin. Busulfan levels were monitored to avoid excess toxicity. All grafts were CD34-selected/T-cell–depleted using the CliniMacs CD34 columns (Miltenyi). Thirty-four patients (75.6%) with marrow failure and 11 (24.4%) with myelodysplastic syndrome underwent HCT using matched unrelated (n = 25, 55.5%), mismatched unrelated (n = 14, 31.1%), or mismatched related donors (n = 6, 13.4%). One year probabilities of overall and disease-free survival for the entire cohort, including patients with myeloid malignancy and those receiving mismatched related/haploidentical grafts, were 80% (±6%) and 77.7% (±6.2%), respectively (median follow-up 41 months). All young children (<10 years of age) undergoing HCT for marrow failure using low-dose busulfan-containing regimen survived. No patients developed acute grade 3-4 GVHD. Sequential reduction of busulfan dose was successfully achieved per study design. Our results show excellent outcomes in patients with FA undergoing alternative donor HCT without radiation exposure. The study is registered to www.clinicaltrials.gov as #NCT01082133.
Introduction
Fanconi anemia (FA) is an inherited bone marrow failure syndrome characterized by congenital anomalies, progressive marrow failure, and predisposition to myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), and solid tumors, specifically squamous cell carcinoma of head and neck and genital region.1 Mutations in at least 20 genes in pathways involved in DNA damage sensing and repair have been described in association with a Fanconi phenotype.2-5 Impaired DNA repair leads to clinically significant chemotherapy and radiation sensitivity, making delivery of the preparative regimen for allogeneic hematopoietic cell transplantation (HCT), the only curative option for the hematologic complications of patients with FA, challenging.6-8
Initial efforts at HCT for FA in the late 1970s and early 1980s demonstrated excessive toxicity and high mortality rates.9 Subsequently, further understanding of the biology of the disease and incremental improvements in preparative regimens for HCT for FA have improved overall outcomes significantly. Specifically the addition of antithymocite globulin (ATG) and T-cell depletion of donor grafts have decreased rates of graft-versus-host disease (GVHD) and the addition of the immunosuppressive agent fludarabine (FLU) has decreased graft rejection, a major obstacle to successful HCT for FA.10-12 Five-year survival after a matched sibling transplant is now ∼90%. Similarly, more recent results for unrelated donor transplant show continued improvements in the 5-year survival rates.11,13-15 Improved outcomes led to the elimination of total body irradiation (TBI) from the preparative regimen for FA HCT using fully matched sibling donors in the past decade.16-19 With improved HCT outcomes, more patients with FA have survived to adulthood,20-22 and long-term side effects of HCT, including the increased risk of secondary cancers have become critical considerations for long-term care.
Patients with FA have significantly increased cancer susceptibility because of defective DNA repair, which is a major phenotype of the disease. The risk of head and neck squamous cell carcinoma is >500-fold higher among individuals with FA compared with the general population.1,23 Head and neck squamous cell carcinoma occurs at strikingly early ages and, in general, treatment is difficult and survival poor.24 Epidemiologic data suggest that both radiation exposure and the occurrence of chronic GVHD may increase the risk of subsequent solid tumors.25 T-cell depletion of the graft has successfully reduced the occurrence of chronic GVHD. Elimination of radiation exposure is therefore a logical next step in reduction of late complications of HCT for FA. In this paper, we report results from the first prospective, multi-institutional US study of alternative donor HCT for FA without use of radiation.
Patients and methods
Study design
We report a prospective, single-arm, phase 2 multicenter study. The 5 participating US centers were Cincinnati Children’s Hospital Medical Center, Boston Children’s Hospital, Fred Hutchinson Cancer Research Center/Seattle Children Hospital, Children’s Hospital of Wisconsin, and Memorial Sloan-Kettering Cancer Center (MSKCC), enrolling 29, 3, 2, 1, and 10 patients respectively. A description of the study protocol is available online (clinicaltrials.gov #NCT01082133). The individual Institutional Review Board of each of the participating centers approved the study. A Food and Drug Administration Investigational New Device was filed and approved at each of the participating centers for use of CD34 selection and T-cell depletion of the graft using the CliniMacs CD34 columns (Miltenyi). MSKCC, the trial-leading institution, served as the central data reporting center. FA patients of all ages were eligible. Patients or parents/legal guardians of all minor patients gave written informed consent for participating in the study, and patients provided assent where age appropriate.
Patients
Patients with FA and progressive marrow failure (pancytopenia or single-lineage cytopenia that were red cell– or platelet transfusion–dependent or unresponsive to granulocyte colony-stimulating factor), or MDS or AML for whom an allogeneic HCT was indicated but who lacked an HLA-genotypically matched related donor, were eligible. MDS was defined using World Health Organization (WHO) System.26 Patients with refractory cytopenia with excess blasts –1 (RAEB1) and higher (WHO classification) were considered to have high-grade MDS. Others were classified as low-grade MDS.
The exclusion criteria on this protocol include patients with active central nervous system leukemic involvement; active uncontrolled viral, bacterial, or fungal infection at the time of enrollment; and patients who are seropositive for HIV-I/II; HTLV-I/II. All patients were hospitalized in single rooms with high-efficiency particulate air filtration with positive pressure and strict isolation until discharge. Institutional standard of care guidelines were followed for transplant-related supportive care, including provision of antiviral, Pneumocystis jiroveci pneumonia and antifungal prophylaxis. Cytomegalovirus (CMV)-safe and irradiated blood products were used for all patients. Patients were monitored for viral reactivation (for CMV, Epstein-Barr virus [EBV], and adenovirus) weekly or twice per week (depending on institutional standards) until at least day 100 after transplant. CMV reactivation was treated with ganciclovir or foscarnet depending on time of reactivation post-HCT.
Stem cells were collected from either HLA-compatible (8/8 or 7/8 allele-matched) unrelated donors or HLA-mismatched related donors (7/8 to 4/8 allele-matched). Donor peripheral blood stem cells were processed to obtain a CD34+ selected T-cell–depleted graft. Target CD34+ cell dose was >5.0 × 106 cells/kg recipient weight (min 2.5 × 106 cells/kg) and maximum allowed CD3+ cell dose was <5.0 × 104 cells/kg recipient weight.
Preparative regimen
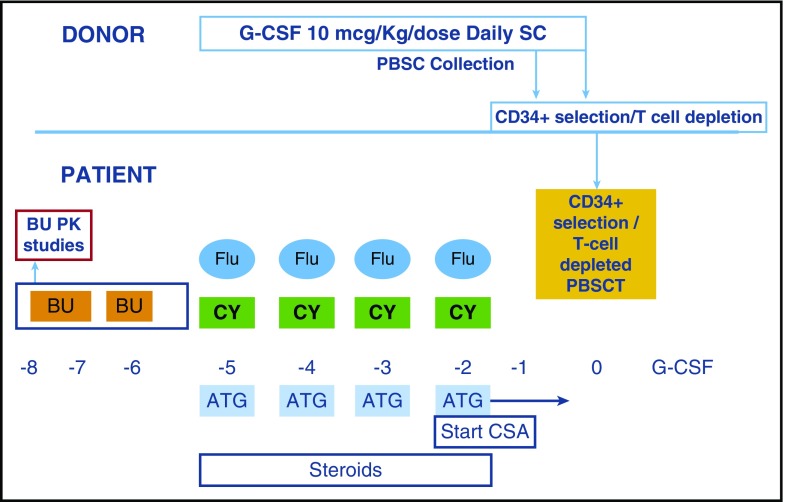
All patients received the preparative regimen shown in Figure 1. Patients received the first dose of busulfan IV on day −8 and blood levels for pharmacokinetics (PK) of busulfan were obtained around this dose. Once the PK results returned on day –7, the next 3 doses of busulfan were administered IV every 12 hours. A steady-state concentration (Css) target of ≤450 ng/mL was established for busulfan to limit toxicity. There was no contingency to increase busulfan dose based on PK results. After one of the early patients developed sinusoidal obstruction syndrome (SOS), the busulfan PK goal was lowered to a Css of ≤350 ng/mL, which was followed throughout the rest of the study. There were no further cases of SOS. A dose de-escalation of busulfan was built into the study. The first cohort of 25 patients received an initial busulfan dose at 0.8 or 1 mg/kg per dose (dose/kg of 0.8 or 1 mg selected based on patient age and weight as previously reported27-29), whereas for the next 20 patients, the initial busulfan dose was reduced to 0.6 or 0.8 mg/kg per dose. In both groups, the subsequent 3 doses of busulfan were reduced (if needed), based on PK results. On days –5 through −2, FLU 35 mg/m2 per dose IV, cyclophosphamide (CY) 10 mg/kg per dose IV and thymoglobulin (rabbit ATG) 2.5 mg/kg per dose IV were administered. A CD34+ selected T-cell–depleted peripheral blood stem cell graft was infused on day 0. Immunosuppression for GVHD prophylaxis consisted of cyclosporine started on day –2 and continued through day +90, then weaned over 10 weeks. Filgrastim was administered from day +1 and continued until absolute neutrophil count of >2×109/L for 3 days.
Figure 1.
Conditioning regimen. BU, busulfan; CSA, cyclosporine; G-CSF, granulocyte colony stimulating factor; PBSCT, peripheral blood stem cell transplant.
Outcomes
Regimen-related toxicities were graded according to the WHO Toxicity Grading Scale (v3.0 and v4.0). Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count ≥0.5 × 109/L and platelet recovery as ≥20 × 109/L without transfusion for 1 week. Engraftment studies were performed weekly until day 100 on peripheral blood using XY fluorescence in situ hybridization in the case of opposite-sex donors or short tandem repeat analysis in the case of same sex donors. Patients were considered to have full chimerism if ≥95% of cells were of donor origin in peripheral blood without evidence of relapse. Overall survival (OS) was defined as time from date of transplant to event (death from any cause) or last follow-up. Disease-free survival was defined as time from date of transplant to relapse, graft rejection or graft failure, or death. Relapse of MDS was defined as disease recurrence supported by cytogenetic and/or molecular analyses. Acute GVHD was graded according to the modified Glucksberg system.30 Chronic GVHD was graded according to the National Institutes of Health Consensus Conference.31
Statistical analyses
Categorical and continuous data are summarized by median (range) and frequency, respectively. Patient characteristics and complications were compared among groups using Fisher’s exact test for categorical variables or Wilcoxon’s rank-sum test for continuous variables. Disease-free survival was defined as time from date of transplant to relapse, graft rejection or graft failure, or death. Survival and cumulative incidence curves were plotted using the Kaplan-Meier method.32 Survival was compared for the entire study duration by group using log-rank tests. Confidence intervals and P values for the difference in 1-year survival by group were computed using Wald’s method with Greenwoods variance approximation. Acute GVHD and recovery of CD4 were compared by group using Gray’s competing risk analysis.33 In each case, time of death and relapse were treated as competing risks. All tests were 2-sided and statistical significance was assessed at the 0.05 level. All calculations were carried out in R version 3.2.4 (Vienna, Austria).
Results
Forty-five patients were prospectively enrolled between June 2009 and May 2014. Median age was 8.2 years (range 4.3-44). Patient demographics, disease characteristics, and donor sources are described in Table 1. Indications for transplant included severe marrow failure (N = 34) or MDS (N = 11). Seven patients had low-grade and 4 patients had high-grade MDS. Three of 26 patients <10 years of age (11.5%) were transplanted for MDS compared with 5 of 14 (35.7%) in patients 10 to 18 years group and 3 of 5 (60%) in the >18 years group. None of the patients had AML, and none of the patients with MDS (including high-grade MDS) received chemotherapy before transplant. One or more clonal abnormalities present in the patients with MDS included trisomy 1q25 (n = 3), trisomy or 4 copies of 3q27 (n = 4), del7q31 (n = 3), monosomy 7 (n = 1), and deletion 20q (n = 1).
Table 1.
Patient demographics, disease characteristics, and donor sources
| Characteristics | Standard-dose busulfan group (n = 25) | Lower-dose busulfan group (n = 20) | P |
|---|---|---|---|
| Median age (y) | 8.2 (4.3-31.4) | 9.8 (4.3-44) | .86 |
| <10 | 15 | 11 | .77 |
| ≥10 | 10 | 9 | |
| Gender | |||
| Males | 12 | 8 | .76 |
| Females | 13 | 12 | |
| Indication for HCT | .82 | ||
| Severe single-lineage cytopenia | 3 | 2 | |
| Severe aplastic anemia | 17 | 12 | |
| Myelodysplastic syndrome | 5 | 6 | |
| Low-grade | 3 | 4 | |
| High-grade | 2 | 2 | |
| Unrelated donors | 19 | 20 | .027 |
| 8/8 allele-matched | 16/19 | 10/20 | .041 |
| 7/8 allele-matched | 3/19 | 10/20 | |
| Mismatched related donors (7/8, 6/8, 5/8, and 4/8 allele-matched) | 6 | 0 | |
| CD34+ cells/kg (median) | 10.5 × 106 (1.1-42.7) | 14.6 × 106 (3-63.3) | .044 |
| CD3 cells/kg (median) | 0.4 (0-5) | 0.95 (0.1-5) | .68 |
| Median follow-up (mo) | 54.9 (39.9-76.8) | 26.6 (17.36-39.62) | < .0001 |
Thirty-two (71%) patients had received prior red cell and 39 (86%) had prior platelet transfusions. Eighteen patients (40%) had received androgens before transplant. Twenty-six patients were seropositive for CMV before transplant. Pre-HCT comorbidities identified in children with FA (<18 years) in this cohort included maple syrup urine disease, history of patent ductus arteriosus, urinary tract infections, borderline renal dysfunction, hypertension and history of transfusions, and use of androgens in the past.
Five adult patients were transplanted at a median age of 30.1 years (range 22.4-44). Transplant indication was progressive marrow failure in 2 and MDS in 3 of these patients. Adult patients had increased comorbidities, which included but were not limited to asthma, history of transfusions and use of androgens in the past, West Nile virus infection, glucose intolerance, hyperlipidemia, dysplastic nevus syndrome, CMV infection, basal cell carcinoma, bronchitis, squamous cell carcinoma of the uterus requiring hysterectomy, pneumonia, and, in one patient, history of transfusions for 10 years.
All patients received an adequate stem cell dose while limiting CD3+ cell dose per protocol (Table 1). There were no significant demographic differences between the 2 busulfan dosing groups.
Engraftment
Forty-four of 45 patients achieved neutrophil engraftment. Median time to ANC ≥0.5 × 109/L in those that achieved engraftment was 9 days (range 7-15) from stem cell infusion. One adult patient with MDS had progressive disease and did not achieve primary neutrophil recovery. Median time to platelet recovery was 16 days (range 11-230). Time to neutrophil or platelet recovery was not different in the 2 busulfan dose groups (data not shown). Immunologic graft rejection followed by secondary graft failure (day +36) occurred in a single patient in the first cohort. This patient subsequently achieved neutrophil engraftment after a second transplant using a different donor and a preparative regimen containing fludarabine and ATG. Mixed chimerism was seen in 6 patients (3 in each busulfan dosing group) and was successfully reversed with increased immune suppression (n = 5) or donor lymphocyte infusion (n = 1).
Busulfan pharmacokinetics and regimen-related toxicity
Busulfan PK after the first dose led to reduction in subsequent doses to achieve target Css in 10 patients in the standard-dose cohort, with a mean dose reduction of 17.4%. In contrast, only 4 children in the lower-dose cohort had a dose reduction (P = .02). In the lower-dose busulfan group, mean busulfan dose reduction of 19.3% was required to keep the Css <350 ng/mL.
Regimen-related toxicities
Regimen-related toxicities were graded according to the WHO Toxicity Grading Scale (v3.0 and v4.0). All grade 3-5 severe adverse events (unexpected, definite, probable, and possibly related) were reported to the data coordinating center. Regimen related toxicities are shown in Table 2. Mucositis, hyperbilirubinemia, and hypertension were less common in the lower busulfan group. Viral reactivation represented the major infectious complication and included adenovirus, BK, cytomegalovirus, and Epstein-Barr virus reactivation/infections, along with others (Table 2).
Table 2.
Regimen-related toxicities
| Complications | Standard dose busulfan group (n = 25) | Lower dose busulfan group (n = 20) | P |
|---|---|---|---|
| Oral mucositis (≥grade 3) | 21 | 11 | .048 |
| Hyperbilirubinemia (≥grade 3) | 9 | 3 | .019 |
| Hypertension (≥grade 3) | 8 | 3 | .025 |
| Sinusoidal obstruction syndrome (SOS) | 1 | 0 | 1 |
| Infections (number of patients) | 17 | 15 | .10 |
| Bacterial | 4 | 4 | |
| Viral | 12 | 7 | |
| CMV (viremia/disease) | 5 (5/0) | 2 (2/0) | |
| EBV (viremia/disease) | 1 (1/0) | 1 (0/1) | |
| Adenovirus (viremia/disease) | 2 (0/2) | 1 (0/1) | |
| BK (viremia/disease) | 3 (1/2) | 0 | |
| HHV-6 (viremia/disease) | 0 | 1 (0/1) | |
| HSV (viremia/disease) | 0 | 1 (0/1) | |
| Influenza virus | 0 | 1 (0/1) | |
| RSV | 1 (0/1) | ||
| Fungal | 2 | 3 |
EBV, Epstein-Barr virus; HHV 6, human herpesvirus 6; HSV, herpes simplex virus; RSV, respiratory syncytial virus.
GVHD
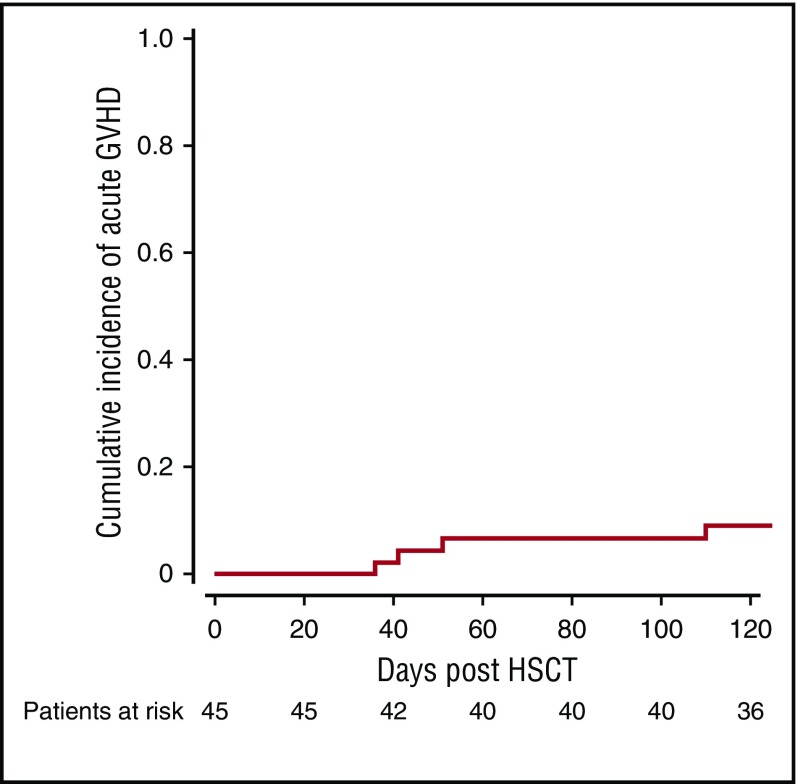
The cumulative incidence of acute GVHD was 6.7% at 100 days (Figure 2). None of the patients developed grade 3-4 acute GVHD. Three patients developed limited chronic GVHD and none developed extensive chronic GVHD. None of the adult patients developed GVHD. All patients with GVHD had a complete response to treatment and no deaths were attributed to GVHD.
Figure 2.
Cumulative incidence of any acute GVHD.
Immune recovery
Median time to CD4 count recovery to >200 × 109/L was 6.6 months (range 2.6-13) (n = 31 with available data). Median time to normal T-cell function (phytohemagglutinin proliferation) was 7.5 months (range 3.7-25.5) (n = 25). No differences in immune recovery were seen in the 2 busulfan dosing cohorts (data not shown). Cumulative incidence of recovery to CD4 >200 × 109/L was earlier in those who received CD34 cell dose >13 × 106/kg (median CD34 cell dose) compared with those whose cell dose was lower than median CD34 cell dose (P = .02).
Survival
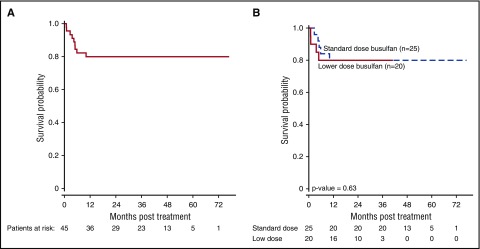
Median follow-up for the entire cohort was 41 months (range 17.4-76.8). Both 1- and 3-year OS was 80.0% (±6%) (Figure 3A). There was no significant difference in OS between the standard-dose and the low-dose busulfan cohort (Figure 3B). Disease-free survival at 1 and 3 years was 77.8% (± 6.2%), with no late events.
Figure 3.
OS for the entire group (n = 45) (A), and according to 2 busulfan dosing groups (B).
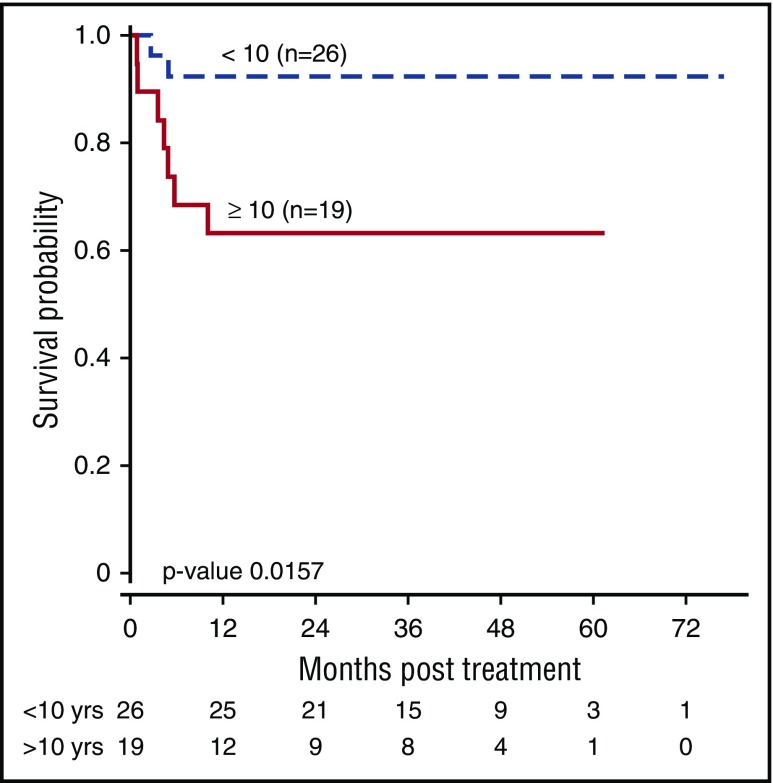
Survival was superior in younger patients (OS 92.3% in <10 years vs 63.2% ≥10 years; P = .02) (Figure 4A). Patients transplanted for marrow failure fared better than those transplanted for MDS, although this difference was not statistically significant, likely because of the small numbers (OS 85.3% vs 63.6%; P = .11). Patients younger than 10 years of age who were transplanted for severe marrow failure and with lower-dose busulfan all survived (Figure 4B). One of 5 adult patients survived for a median of 4.81 months (range 0.89-34.08).
Figure 4.
OS by age.
Patients who had received androgens before HCT (n = 18) had an 88.9% survival rate at 1 year compared with those without androgen exposure (n = 27) at 66.7% (P = .078; 1-year confidence interval [-0.02 – 0.47]). Prior red blood cell and platelet transfusions did not affect outcome (P = .47 and .10, respectively).
Causes of death
Thirty-five of 45 patients are alive and well. Causes of death included progressive disease: MDS (n = 1), relapsed MDS (n = 1), infections (n = 5), multiorgan failure (n = 2), and severe pulmonary hypertension (n = 1). Infections causing death included community-acquired Clostridium botulinum (n = 1), fungal infection (n = 1), viral + fungal infection (n = 2), and viral infection (n = 1). Viral infections responsible for death (with or without additional fungal infection) included adenoviral infection (n = 2) and human herpesvirus 6 (HHV-6) (n = 1). Two deaths in the adult cohort (N = 5) were not primarily related to HCT. One patient died of a sudden cardiac arrest of unknown etiology in the operating room after a cystoscopy (performed for hemorrhagic cystitis) and 1 patient died of botulism. Two additional adult deaths were from progressive MDS and fungal infection. One adult patient is alive and well.
Subsequent malignancies
One patient, transplanted at the age of 32 years, developed a gingival squamous cell carcinoma 3 years posttransplant. This patient did not develop GVHD during HCT or have any additional comorbidities before transplant. No other patient has developed malignancy at a second site, but follow-up remains short.
Discussion
This is the first prospective multi-institutional US study showing that radiation can be safely omitted from the preparative regimen of patients with FA undergoing alternative-donor transplantation. Our results show excellent overall and disease-free survival (comparable with previous radiation containing regimens) with a chemotherapy-only preparative regimen. Outcomes for the younger patients without MDS are comparable with outcomes of matched-sibling donor HCT for FA.16,18,19,22
Increasingly better survival after HCT for FA increases the importance of minimizing late consequences of transplant caused by the risk of subsequent malignancies. Radiation exposure and GVHD are the 2 main HCT-related factors that increase the risk of subsequent malignancies. Ionizing radiation is a known carcinogen, inducing double-strand breaks in DNA and other cell damage, which in aggregate can lead to chromosomal translocations and genomic instability. In childhood cancer studies, there are multiple reports on effect of radiation and/or chemotherapy on risk of subsequent secondary malignant solid tumors.34-37 Irradiation containing conditioning regimens have been identified as a risk factor for posttransplant malignancy in patients with severe aplastic anemia and FA20,38; and similar efforts of removing radiation from the conditioning regimen have been successful in patients with severe39 aplastic anemia. A study by Rosenberg et al showed a trend toward increasing malignancy in patients with FA receiving a TBI-based regimen.40 In addition, evidence suggests that chronic GVHD induces a chronic inflammatory state, leading to microsatellite instability and frequent genomic alterations in epithelial tissues, predisposing to development of cancers.41,42 Several reports have shown increased risk of subsequent malignancy associated with chronic GVHD in FA.14,21,43 T-cell depletion of the donor graft is clearly effective in largely eliminating severe acute and chronic GVHD, as seen in this study and in other reports.13,15 In our study, one patient, transplanted at the age of 32 years, developed a gingival squamous cell carcinoma 3 years posttransplant. Older age of 32 years at the time of transplant and shorter time (3 years) from transplant to development of squamous cell carcinoma bring up an important question of attribution: whether the development of squamous cell carcinoma is caused by increased susceptibility related to baseline diagnosis of FA or a secondary malignancy caused by transplant. No other patient has developed malignancy at a second site. However, the follow-up period for our cohort is not sufficient to fully evaluate rates of subsequent malignancy, but we are hopeful that avoidance of major carcinogenic exposure will reduce risk.
A key goal of our study was to minimize early as well as late toxicities of transplant. The first 25 patients enrolled in this study tolerated transplant well, so by study design, the dose was reduced for the next 20 patients. Engraftment was not compromised by this dose reduction, and toxicity, in particular, less mucositis, and hyperbilirubinemia were significantly reduced. Viral infections are expected complications with use of ATG and T-depleted graft. Approximately 40% of our patients had ≥1 viremia, which are comparable with previous experience with T-depleted grafts.13,15 Standard supportive care, including routine monitoring for viral reactivation and preemptive treatment with antiviral agents and/or viral-specific cytotoxic T cells will help reduce this complication in the future. A key determinant of successful control of viral reactivation is early immune reconstitution. In our patients, immune reconstitution was adequate and timely, perhaps helped by the small size of many of the patients who allowed administration of large stem cell doses. We understand that observation regarding the role of higher CD34 cell dose in faster recovery may need to be confirmed in a larger dataset. In addition, early results from use of interleukin-7,44 T-cell receptor α/β, T-cell depletion, and peritransplant leuprolide45 to improve immune reconstitution look promising and may be explored further in future studies.
Reports from Brazil and Germany of smaller cohorts also demonstrated that good outcomes can be achieved in FA without exposure to radiation.46,47 Our study used a higher dose of busulfan compared with the German study, but used pharmacokinetic monitoring and intravenous administration, perhaps explaining the lower rate of mucositis reported here. Over the years, transplant cytoreductive regimens for patients with FA have used TBI (or thoraco-abdominal irradiation), procarbazine, cyclophosphamide, and fludarabine. Successful T-cell–depleted protocols have in general used TBI, fludarabine, and cyclophosphamide. During the study design, busulfan, thiotepa, and melphalan were among the potential candidate agents considered to replace TBI. No data were available on use of thiotepa and melphalan in patients with FA. In contrast, there were a couple of small reports available on the use of busulfan for patients with FA.48,49 An additional reason for choosing Busulfan was based on our ability to perform and understand PK studies in this high-risk population, and administer PK-directed dosing of busulfan, therefore avoiding excessive toxicity without increase in graft rejection. Cut offs for busulfan PK were based on a limited prior experience at MSKCC with this regimen. A majority of patients enrolled in this study were children, and for them outcomes were excellent. It is clear, however, that outcomes in the 5 adult patients enrolled were unsatisfactory, with only 1 of 5 surviving. It should be noted that 1 adult died of botulism, and one during a procedure—both deaths perhaps unrelated to transplant. Older children and adult patients (10-18 and >18 years) in this cohort were transplanted more for MDS than marrow failure (as expected), which is a known negative prognostic factor, along with having increased comorbidities that negatively affect transplant outcomes. Our next study focuses on improving outcomes in this challenging group.
In summary, our data indicate that HCT for FA can be performed without radiation and with T-cell–depleted peripheral blood grafts, with acceptable toxicity including mild or no GVHD. Longer follow-up is needed to determine whether these strategies reduce risk of subsequent malignancy.
Acknowledgments
The authors thank their patients and their families for their participation in the study; and the nurses, nurse coordinators, nurse practitioners, physician assistants, social workers, and physicians who cared for these patients and their families. They acknowledge the Clinical research staff for assistance in managing the trial at all of the participating centers. Special thanks go to E. Klein and A. Judge from MSKCC and J. Wilhelm from CCHMC for additional assistance with data coordination.
Supported in part by a grant from the Fanconi Anemia Research Fund Inc.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.A.M., S.M.D., and F.B. designed the study, performed research, and wrote the manuscript; T.L. supervised the cell processing and required regulatory aspects; A.L. performed statistical analyses; E.G. provided vital conceptual insights for study design, and interpretation of data; K.M., N.A.K., S.E.P., A.S., R.J.O., D.A.W., L.L., D.M., and K.S.B. assisted with study subject accrual and data collection; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Parinda A. Mehta, Division of Bone Marrow Transplantation and Immune Deficiency, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: parinda.mehta@cchmc.org.
References
- 1.Kutler DI, Singh B, Satagopan J, et al. . A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood. 2003;101(4):1249-1256. [DOI] [PubMed] [Google Scholar]
- 2.Bogliolo M, Surrallés J. Fanconi anemia: a model disease for studies on human genetics and advanced therapeutics. Curr Opin Genet Dev. 2015;33:32-40. [DOI] [PubMed] [Google Scholar]
- 3.Wang AT, Kim T, Wagner JE, et al. . A dominant mutation in human RAD51 reveals its function in DNA interstrand crosslink repair independent of homologous recombination. Mol Cell. 2015;59(3):478-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ameziane N, May P, Haitjema A, et al. . A novel Fanconi anaemia subtype associated with a dominant-negative mutation in RAD51. Nat Commun. 2015;6:8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluteau D, Masliah-Planchon J, Clairmont C, et al. . Biallelic inactivation of REV7 is associated with Fanconi anemia. J Clin Invest. 2016;126(9):3580-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gluckman E, Devergie A, Schaison G, et al. . Bone marrow transplantation in Fanconi anaemia. Br J Haematol. 1980;45(4):557-564. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman E, Devergie A, Dutreix J. Radiosensitivity in Fanconi anaemia: application to the conditioning regimen for bone marrow transplantation. Br J Haematol. 1983;54(3):431-440. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman E. Bone marrow transplantation in Fanconi’s anemia. Stem Cells. 1993;11(Suppl 2):180-183. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman E, Auerbach AD, Horowitz MM, et al. . Bone marrow transplantation for Fanconi anemia. Blood. 1995;86(7):2856-2862. [PubMed] [Google Scholar]
- 10.Boulad F, Gillio A, Small TN, et al. . Stem cell transplantation for the treatment of Fanconi anaemia using a fludarabine-based cytoreductive regimen and T-cell-depleted related HLA-mismatched peripheral blood stem cell grafts. Br J Haematol. 2000;111(4):1153-1157. [DOI] [PubMed] [Google Scholar]
- 11.Wagner JE, Eapen M, MacMillan ML, et al. . Unrelated donor bone marrow transplantation for the treatment of Fanconi anemia. Blood. 2007;109(5):2256-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locatelli F, Zecca M, Pession A, et al. ; Italian pediatric group. The outcome of children with Fanconi anemia given hematopoietic stem cell transplantation and the influence of fludarabine in the conditioning regimen: a report from the Italian pediatric group. Haematologica. 2007;92(10):1381-1388. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhury S, Auerbach AD, Kernan NA, et al. . Fludarabine-based cytoreductive regimen and T-cell-depleted grafts from alternative donors for the treatment of high-risk patients with Fanconi anaemia. Br J Haematol. 2008;140(6):644-655. [DOI] [PubMed] [Google Scholar]
- 14.Peffault de Latour R, Porcher R, Dalle JH, et al. ; FA Committee of the Severe Aplastic Anemia Working Party; Pediatric Working Party of the European Group for Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122(26):4279-4286. [DOI] [PubMed] [Google Scholar]
- 15.MacMillan ML, DeFor TE, Young JA, et al. . Alternative donor hematopoietic cell transplantation for Fanconi anemia. Blood. 2015;125(24):3798-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farzin A, Davies SM, Smith FO, et al. . Matched sibling donor haematopoietic stem cell transplantation in Fanconi anaemia: an update of the Cincinnati Children’s experience. Br J Haematol. 2007;136(4):633-640. [DOI] [PubMed] [Google Scholar]
- 17.Ayas M, Al-Jefri A, Al-Mahr M, et al. . Stem cell transplantation for patients with Fanconi anemia with low-dose cyclophosphamide and antithymocyte globulins without the use of radiation therapy. Bone Marrow Transplant. 2005;35(5):463-466. [DOI] [PubMed] [Google Scholar]
- 18.Bonfim CM, de Medeiros CR, Bitencourt MA, et al. . HLA-matched related donor hematopoietic cell transplantation in 43 patients with Fanconi anemia conditioned with 60 mg/kg of cyclophosphamide. Biol Blood Marrow Transplant. 2007;13(12):1455-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasquini R, Carreras J, Pasquini MC, et al. . HLA-matched sibling hematopoietic stem cell transplantation for fanconi anemia: comparison of irradiation and nonirradiation containing conditioning regimens. Biol Blood Marrow Transplant. 2008;14(10):1141-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anur P, Friedman DN, Sklar C, et al. . Late effects in patients with Fanconi anemia following allogeneic hematopoietic stem cell transplantation from alternative donors. Bone Marrow Transplant. 2016;51(7):938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonfim C, Ribeiro L, Nichele S, et al. . Long-term survival, organ function, and malignancy after hematopoietic stem cell transplantation for Fanconi anemia. Biol Blood Marrow Transplant. 2016;22(7):1257-1263. [DOI] [PubMed] [Google Scholar]
- 22.Svahn J, Bagnasco F, Cappelli E, et al. . Somatic, hematologic phenotype, long-term outcome, and effect of hematopoietic stem cell transplantation. An analysis of 97 Fanconi anemia patients from the Italian national database on behalf of the Marrow Failure Study Group of the AIEOP (Italian Association of Pediatric Hematology-Oncology). Am J Hematol. 2016;91(7):666-671. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93(4):511-517. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg PS, Socié G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105(1):67-73. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo JD, Curtis RE, Socié G, et al. . Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swerdlow SH. WHO classification of tumours of haematopoietic and lymphoid tissues, 4th ed Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 27.Booth BP, Rahman A, Dagher R, et al. . Population pharmacokinetic-based dosing of intravenous busulfan in pediatric patients. J Clin Pharmacol. 2007;47(1):101-111. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen L, Fuller D, Lennon S, Leger F, Puozzo C. I.V. busulfan in pediatrics: a novel dosing to improve safety/efficacy for hematopoietic progenitor cell transplantation recipients. Bone Marrow Transplant. 2004;33(10):979-987. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen L. Integration of modelling and simulation into the development of intravenous busulfan in paediatrics: an industrial experience. Fundam Clin Pharmacol. 2008;22(6):599-604. [DOI] [PubMed] [Google Scholar]
- 30.Glucksberg H, Storb R, Fefer A, et al. . Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295-304. [DOI] [PubMed] [Google Scholar]
- 31.Filipovich AH, Weisdorf D, Pavletic S, et al. . National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945-956. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 33.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141-1154. [Google Scholar]
- 34.Lowsky R, Lipton J, Fyles G, et al. . Secondary malignancies after bone marrow transplantation in adults. J Clin Oncol. 1994;12(10):2187-2192. [DOI] [PubMed] [Google Scholar]
- 35.Bhatia S, Ramsay NK, Steinbuch M, et al. . Malignant neoplasms following bone marrow transplantation. Blood. 1996;87(9):3633-3639. [PubMed] [Google Scholar]
- 36.Bhatia S, Louie AD, Bhatia R, et al. . Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19(2):464-471. [DOI] [PubMed] [Google Scholar]
- 37.Vaxman I, Ram R, Gafter-Gvili A, et al. . Secondary malignancies following high dose therapy and autologous hematopoietic cell transplantation-systematic review and meta-analysis. Bone Marrow Transplant. 2015;50(5):706-714. [DOI] [PubMed] [Google Scholar]
- 38.Deeg HJ, Socié G, Schoch G, et al. . Malignancies after marrow transplantation for aplastic anemia and fanconi anemia: a joint Seattle and Paris analysis of results in 700 patients. Blood. 1996;87(1):386-392. [PubMed] [Google Scholar]
- 39.Bacigalupo A, Socie’ G, Lanino E, et al. ; Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA Working Party. Haematologica. 2010;95(6):976-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg PS, Alter BP, Socié G, Gluckman E. Secular trends in outcomes for Fanconi anemia patients who receive transplants: implications for future studies. Biol Blood Marrow Transplant. 2005;11(9):672-679. [DOI] [PubMed] [Google Scholar]
- 41.Themeli M, Petrikkos L, Waterhouse M, et al. . Alloreactive microenvironment after human hematopoietic cell transplantation induces genomic alterations in epithelium through an ROS-mediated mechanism: in vivo and in vitro study and implications to secondary neoplasia. Leukemia. 2010;24(3):536-543. [DOI] [PubMed] [Google Scholar]
- 42.Khan FM, Sy S, Louie P, et al. . Genomic instability after allogeneic hematopoietic cell transplantation is frequent in oral mucosa, particularly in patients with a history of chronic graft-versus-host disease, and rare in nasal mucosa. Blood. 2010;116(10):1803-1806. [DOI] [PubMed] [Google Scholar]
- 43.Guardiola P, Socié G, Li X, et al. . Acute graft-versus-host disease in patients with Fanconi anemia or acquired aplastic anemia undergoing bone marrow transplantation from HLA-identical sibling donors: risk factors and influence on outcome. Blood. 2004;103(1):73-77. [DOI] [PubMed] [Google Scholar]
- 44.Perales MA, Goldberg JD, Yuan J, et al. . Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120(24):4882-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldberg GL, King CG, Nejat RA, et al. . Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J Immunol. 2009;182(9):5846-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonfim CRL, Nichele S, Loth G, et al. . Excellent outcome for Fanconi anemia patients undergoing hematopoietic stem cell transplantation (HSCT) without radiation: a single center experience on 103 patients. Biol Blood Marrow Transplant. 2015;21:S94a. [Google Scholar]
- 47.Chao MM, Kuehl JS, Strauss G, et al. . Outcomes of mismatched and unrelated donor hematopoietic stem cell transplantation in Fanconi anemia conditioned with chemotherapy only. Ann Hematol. 2015;94(8):1311-1318. [DOI] [PubMed] [Google Scholar]
- 48.Torjemane L, Ladeb S, Ben Othman T, Abdelkefi A, Lakhal A, Ben Abdeladhim A. Bone marrow transplantation from matched related donors for patients with Fanconi anemia using low-dose busulfan and cyclophosphamide as conditioning. Pediatr Blood Cancer. 2006;46(4):496-500. [DOI] [PubMed] [Google Scholar]
- 49.Maschan AA, Trakhtman PE, Balashov DN, et al. . Fludarabine, low-dose busulfan and antithymocyte globulin as conditioning for Fanconi anemia patients receiving bone marrow transplantation from HLA-compatible related donors. Bone Marrow Transplant. 2004;34(4):305-307. [DOI] [PubMed] [Google Scholar]