Abstract
The hypoxic response is a stress response triggered by low oxygen tension. Hypoxia-inducible factors (HIFs) play a prominent role in the pathobiology of hypoxia-associated conditions, including pulmonary hypertension (PH) and polycythemia. The c-Jun N-terminal protein kinase (JNK), a stress-activated protein kinase that consists of two ubiquitously expressed isoforms, JNK1 and JNK2, and a tissue-specific isoform, JNK3, has been shown to be activated by hypoxia. However, the physiological role of JNK1 and JNK2 in the hypoxic response remains elusive. Here, using genetic knockout cells and/or mice, we show that JNK2, but not JNK1, up-regulates the expression of HIF-1α and HIF-2α and contributes to hypoxia-induced PH and polycythemia. Knockout or silencing of JNK2, but not JNK1, prevented the accumulation of HIF-1α in hypoxia-treated cells. Loss of JNK2 resulted in a decrease in HIF-1α and HIF-2α mRNA levels under resting conditions and in response to hypoxia. Consequently, hypoxia-treated Jnk2−/− mice had reduced erythropoiesis and were less prone to polycythemia because of decreased expression of the HIF target gene erythropoietin (Epo). Jnk2−/− mice were also protected from hypoxia-induced PH, as indicated by lower right ventricular systolic pressure, a process that depends on HIF. Taken together, our results suggest that JNK2 is a positive regulator of HIFs and therefore may contribute to HIF-dependent pathologies.
Keywords: polycythemia, c-Jun N-terminal kinase (JNK), hypoxia-inducible factor, pulmonary hypertension, hypoxia, erythropoiesis
Introduction
The last 2 decades have brought insight into the mechanisms of hypoxia-associated diseases, including pulmonary hypertension (PH)2 and polycythemia (i.e. excessive peripheral blood erythrocyte count), primarily through the discovery and understanding of the master regulators of hypoxic adaptation, hypoxia-inducible factor (HIF)-1α and -2α (1, 2). PH is a commonly recognized complication of respiratory diseases that are associated with hypoxia, including chronic obstructive pulmonary disease (COPD), and has been consistently linked to an increase in morbidity and mortality (3–9). Polycythemia is estimated to occur in 6% of people with stable COPD (10) and, by increasing blood viscosity, is associated with PH and reduced cerebral blood flow (11, 12).
HIFs play a central role in the hypoxic response. In an environment of normal oxygen tension, the tumor suppressor protein von Hippel–Lindau (VHL) binds the biologically active α-subunits of HIF-1 and HIF-2 (13). VHL is part of a multiprotein ubiquitin E3 ligase complex (VHLE3), which then mediates degradation of these subunits via the ubiquitin–proteasome system. The binding of VHL to HIF, however, is dependent on the hydroxylation of HIF at specific proline residues (Pro-402 and Pro-564) within the oxygen-dependent degradation domain by prolyl-4-hydroxylase domain proteins, which act in an oxygen-dependent manner (14). Therefore, in low-oxygen tension states (i.e. hypoxia), HIF-1 and HIF-2 are not bound by VHL and accumulate to induce the transcription of their target genes, such as vascular endothelial growth factor (Vegf) and erythropoietin (Epo).
A large body of evidence demonstrates that HIFs are critical for the development of hypoxia-induced PH. It has been reported that PH due to chronic hypoxia can be mitigated in either Hif-1α or Hif-2α heterozygous-null mice (15, 16). Smooth muscle cell–specific HIF-1α knock-out mice similarly showed less pulmonary vascular remodeling and PH after chronic hypoxia (17).
A similar role for HIFs in the development of polycythemia is exemplified by people with the condition known as Chuvash polycythemia, an autosomal recessive disorder of the VHL gene mutation resulting in congenital up-regulation of HIF-1α and severe polycythemia (18). A mouse model of Chuvash polycythemia was made by generating mice homozygous for the Chuvash VHL mutation, which causes up-regulation of HIF-2α and results in polycythemia and spleen enlargement due to splenic erythropoiesis (19).
JNK (also known as SAPK (stress-activated protein kinase)) is a member of the MAPK (mitogen-activated protein kinase) superfamily (20, 21). JNK is activated by a wide variety of extracellular stimuli, from pro-inflammatory cytokines to environmental stresses, including hypoxia (22). Upon activation, JNK regulates the activity of several transcription factors, including c-Jun, ATF-2, Elk-1, p53, and c-Myc, as well as other factors, such as members of the Bcl-2 family (23), and is involved in the regulation of many cellular activities from proliferation to cell death. Two of the three JNK isoforms, JNK1 and JNK2, are ubiquitously expressed among all tissues and are highly homologous to each other (24). They had been considered redundant isoforms; however, our group and others have previously demonstrated that JNK1 is the main JNK isoform that is activated by various extracellular stimuli, whereas the kinase activity of JNK2 is negligible (25). Rather, JNK2 targets its substrates for proteasomal degradation in unstimulated cells independent of its kinase function (26–30). The pathophysiological role of JNK1 and JNK2 under hypoxic conditions remains elusive. Here, we show that JNK2, but not JNK1, positively regulates HIFs in a murine model of ambient hypoxia and thereby contributes to hypoxia-induced PH and polycythemia.
Results
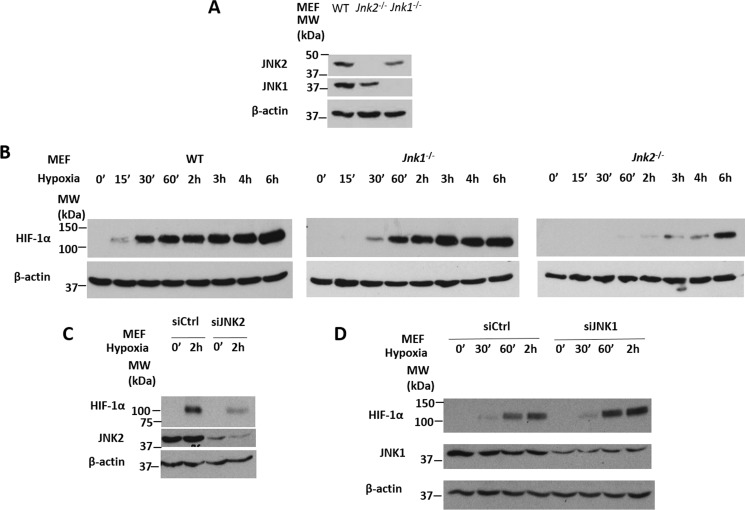
JNK2, but not JNK1, positively regulates HIF-1α in vitro
It is known that both HIF-1α and HIF-2α proteins are constitutively degraded by the ubiquitin-proteasome system in oxygen-replete states but stabilized under conditions of low oxygen tension (31). To investigate whether JNK regulates HIF under resting and hypoxic conditions, we treated wild-type, Jnk1−/−, or Jnk2−/− mouse embryonic fibroblasts (MEFs) (Fig. 1A, Fig S1, A and B) with normobaric hypoxia (1.5% oxygen, 5% carbon dioxide) for different times. In wild-type cells, HIF-1α proteins started to accumulate after hypoxia treatment for 15 min and peaked at 6 h (Fig. 1B, Fig. S1C). HIF-1α protein accumulation in hypoxia-treated Jnk1−/− cells was similar or slightly increased compared with that in wild-type cells (Fig. 1B, Fig. S1C). Interestingly, HIF-1α protein accumulation was delayed and decreased in hypoxia-treated Jnk2−/− cells compared with wild-type cells (Fig. 1B, Fig. S1C). Our data suggest that JNK2, but not JNK1, positively regulates HIF-1α, which is in line with recent studies by our group and others that JNK1 and JNK2 have shared but also distinct functions (25, 32). Similarly, silencing of JNK2 by siRNA in wild-type cells also resulted in decreased accumulation of HIF-1α in response to hypoxia (Fig. 1C), whereas silencing of JNK1 by siRNA in wild-type cells showed a slight increase in HIF-1α accumulation in response to hypoxia (Fig. 1D). Taken together, these data suggest that JNK2 specifically up-regulates HIF-1α in vitro.
Figure 1.
JNK2, but not JNK1, positively regulates HIF-1α in vitro. A, JNK1 and JNK2 immunoblots from WT, Jnk2−/−, or Jnk1−/− MEFs. B, HIF-1α immunoblots from WT, Jnk1−/−, or Jnk2−/− MEFs treated with hypoxia for 0 min, 15 min, 30 min, 60 min, 2 h, 3 h, 4 h, or 6 h. C, HIF-1α and JNK2 immunoblots from WT MEFs transfected with control siRNA or JNK2-specific siRNA and then treated with hypoxia for 0 min or 2 h. D, HIF-1α and JNK1 immunoblots from WT MEFs transfected with control siRNA or JNK1-specific siRNA and then treated with hypoxia for 0 min, 30 min, 60 min, or 2 h. All immunoblots are representative of at least three independent experiments that yielded similar results.
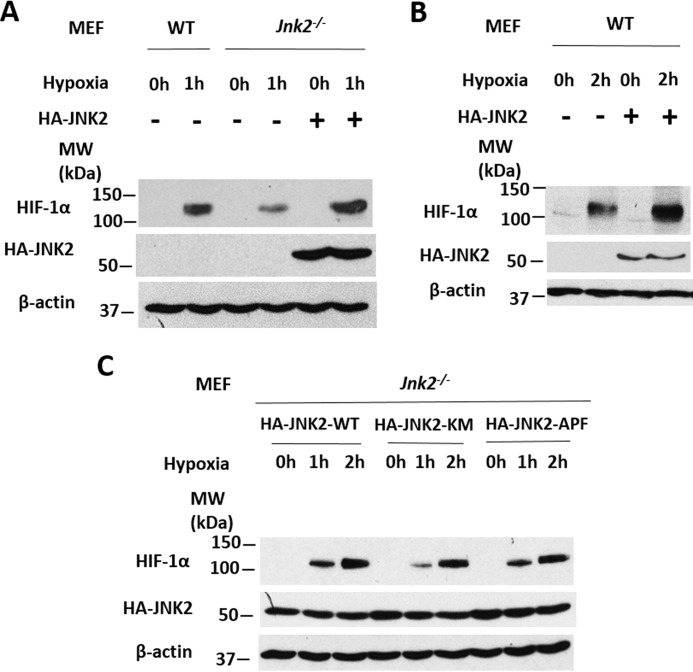
JNK2, independently of its kinase function, is both necessary and sufficient for HIF-1α expression
To further investigate the role of JNK2 in HIF-1α up-regulation, we transfected Jnk2−/− cells with either empty vector or expression vector encoding HA-JNK2 and then treated the cells with hypoxia. Complementation of JNK2 into Jnk2−/− cells restored HIF-1α levels under hypoxic conditions (Fig. 2A). In addition, overexpression of JNK2 in wild-type cells further increased HIF-1α protein levels under hypoxic conditions (Fig. 2B). Reports by our group and others have shown that JNK2 can regulate gene/protein expression in a kinase-independent manner (33). To elucidate whether the kinase activity of JNK2 is required for its regulation of HIF-1α, we transfected Jnk2−/− cells with plasmids expressing wild-type JNK2 or either of the two kinase activity–deficient mutants of JNK2 (JNK2-KM, in which the catalytically active lysine 149 was mutated to methionine, or JNK2-APF, in which the consensus phosphorylation motifs threonine 183 and tyrosine 185 that are required for JNK2 kinase activation were replaced by non-phosphorylatable residues alanine and phenylalanine, respectively). Under hypoxic conditions, we observed similar levels of HIF-1α protein compared with wild-type JNK2–expressing cells (Fig. 2C). Taken together, these data provide evidence that JNK2 is both necessary and sufficient for the expression of HIF-1α protein during hypoxia and that this regulation is independent of the kinase activity of JNK2.
Figure 2.
JNK2, independent of its kinase function, is both necessary and sufficient for HIF-1α expression. A, HIF-1α and HA-JNK2 immunoblot from WT or Jnk2−/− MEFs treated with hypoxia for 0 or 1 h after transfection with HA-JNK2–expressing plasmid or an empty vector. B, HIF-1α and HA-JNK2 immunoblot from WT MEFs treated with hypoxia for 0 or 2 h after transfection with HA-JNK2 or empty vector. C, HIF-1α and HA-JNK2 immunoblot from Jnk2−/− MEFs treated with hypoxia for 0, 1, or 2 h after transfection with HA-JNK2 wild type–expressing plasmid or either kinase-dead mutant, HA-JNK2-KM or HA-JNK2-APF. All immunoblots are representative of at least three independent experiments that yielded similar results.
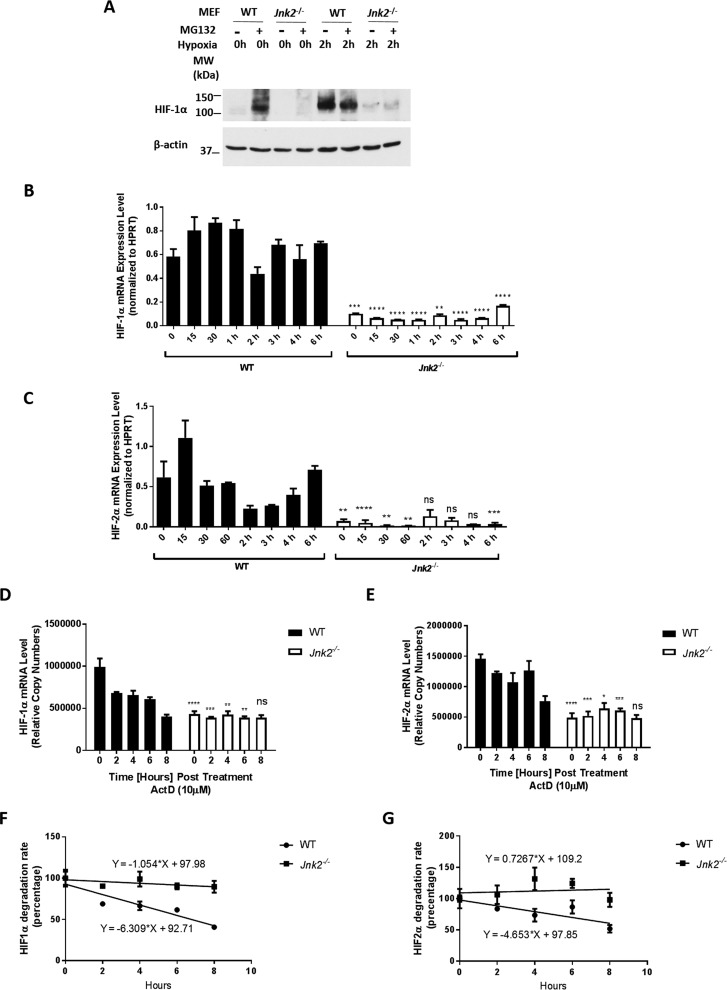
JNK2 up-regulates HIF by maintaining HIF mRNA levels
HIF-1α and HIF-2α proteins are constitutively degraded by the ubiquitin-proteasome system in oxygen-replete states, which is inhibited under hypoxic conditions, leading to their accumulation. We sought to determine whether our observation of decreased HIF-1α protein levels by loss of JNK2 under hypoxic conditions was due to increased HIF-1α proteasomal degradation. We treated wild-type and Jnk2−/− MEFs with the proteasome inhibitor MG132 under resting conditions and hypoxic conditions. In wild-type cells, MG132 treatment resulted in HIF-1α protein accumulation under resting conditions (Fig. 3A), consistent with the general notion that HIF-1α protein is subjected to constant proteasomal degradation in the steady state. Hypoxia treatment led to the accumulation of HIF-1α protein in wild-type cells, which was not further increased with MG132 (Fig. 3A). However, in Jnk2−/− cells, MG132 did not increase HIF-1α protein levels under either resting or hypoxic conditions (Fig. 3A), suggesting that the reduction in HIF-1α protein levels in Jnk2−/− cells is not due to accelerated proteasomal degradation. Interestingly, quantitative RT-PCR showed a drastic decrease in HIF-1α and HIF-2α mRNA expression levels in Jnk2−/− cells compared with wild-type cells under either resting or hypoxic conditions (Fig. 3, B and C). These data suggest that JNK2 is critical for maintaining HIF-1α and HIF-2α mRNA levels in vitro, which precedes hypoxia-induced accumulation of HIF proteins. To further investigate the role of JNK2 in maintaining HIF mRNA levels, we sought to determine whether JNK2 regulates the stability of HIF mRNAs. We treated wild-type or Jnk2−/− cells with actinomycin D, which blocks de novo mRNA synthesis (34). Using an equal amount of total RNA for cDNA preparation, we used the standard curve of dilutions to estimate mRNA copy number as described previously (35). As expected, HIF-1α and HIF-2α mRNA levels were lower in Jnk2−/− cells compared with wild-type cells at baseline (Fig. 3, D and E). After normalizing to the abundance of HIF-1α transcript at baseline, we observed a slower rate of degradation of HIF-1α transcript in Jnk2−/− cells compared with wild-type cells (Fig. 3F, slope of −1.054%/h in Jnk2−/− versus −6.309%/h in WT; p < 0.05). Similar results were obtained for HIF-2α transcript (Fig. 3G, −4.653%/h versus 0.7267%/h; not statistically significant). These results suggest that JNK2 up-regulation of HIF mRNAs is not mediated through stabilizing HIF transcripts.
Figure 3.
JNK2 up-regulates HIF by maintaining HIF mRNA levels. A, HIF-1α immunoblot from WT or Jnk2−/− MEFs treated with hypoxia in the absence or presence of MG132. B, quantitative PCR for relative HIF-1α mRNA expression after normalizing to HPRT with technical duplicates. C, quantitative PCR for relative HIF-2α mRNA expression after normalizing to HPRT with technical duplicates. D, quantitative PCR (relative copy number calculated from standard curve) of HIF-1α in WT or Jnk2−/− MEFs after treatment with actinomycin D (ActD; 10 μm) under normoxic conditions. E, quantitative PCR (relative copy number calculated from a standard curve) of HIF-2α in WT or Jnk2−/− MEFs after treatment with actinomycin D (10 μm) under normoxic conditions. F, linear regression with line of best fit of data in D after normalizing copy number of control (0 h) to 100%. G, linear regression with line of best fit of data in E after normalizing copy number of control (0 h) to 100%. All immunoblots and quantitative PCR data are representative of at least three independent experiments that yielded similar results. All data are presented as mean ± S.E. (error bars). Statistical significance was determined by one-way ANOVA with Tukey's post hoc test (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001; ns, non-significant for the comparisons indicated by brackets).
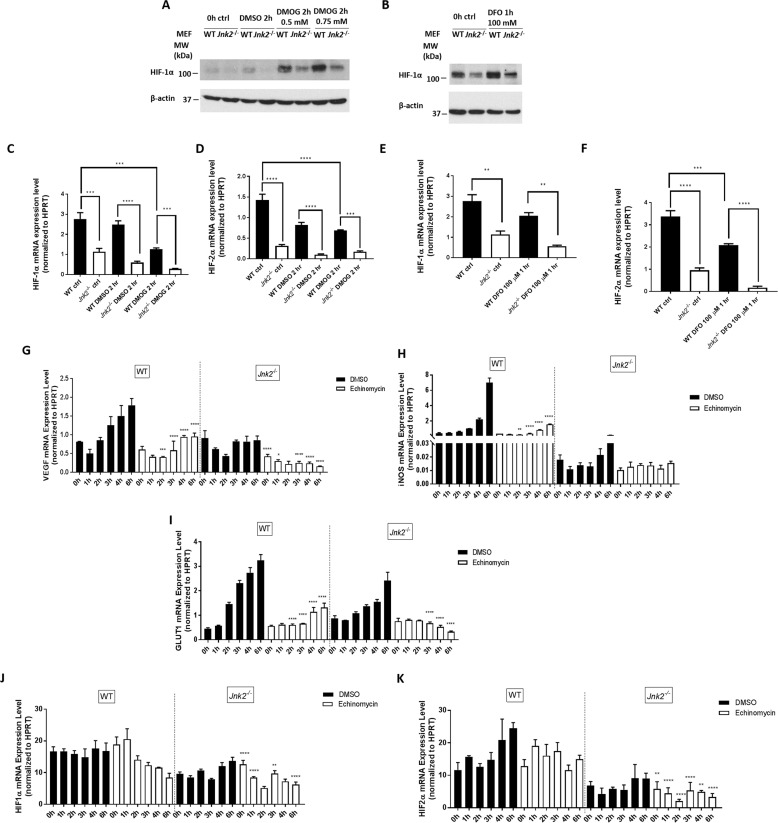
JNK2 up-regulates the expression of HIF under “pseudohypoxic” conditions
We then determined whether JNK2 also up-regulates HIF in vitro under pseudohypoxic conditions without the use of ambient low oxygen tension. We treated wild-type or Jnk2−/− cells with either the prolyl-4-hydroxylase inhibitor dimethyloxalylglycine (DMOG) or the iron chelator deferoxamine (DFO), both of which stabilize HIF protein, but by different mechanisms; DMOG inhibits the prolyl hydroxylases that suppress HIF expression through VHL-dependent ubiquitination and proteasomal degradation, whereas DFO is an iron chelator that displaces porphyrin iron and interferes with the heme oxygen sensor (36–40). Similar to the response to hypoxia, DMOG or DFO treatment increased HIF-1α protein levels in wild-type cells, which was again inhibited in Jnk2−/− cells (Fig. 4, A and B). Under these conditions, HIF-1α and HIF-2α mRNA levels were consistently lower in Jnk2−/− cells than in wild-type cells (DMOG (Fig. 4, C and D) and DFO (Fig. 4, E and F)). These data suggest that the regulation of HIF by JNK2 is not restricted to the stimulus of low oxygen tension, but is also observed in the context of alternative HIF-stabilizing mechanisms, including hydroxlase inhibition and iron chelation. To determine whether the regulation of HIF-1α and HIF-2α mRNA by JNK2 depends on the transcriptional activity of HIF per se, we treated wild-type and Jnk2−/− MEFs with the HIF-1α DNA-binding inhibitor, echinomycin, followed by hypoxia treatment. We first measured the mRNA levels of several well-established HIF target genes (Vegf, inducible nitric-oxide synthase (iNos), and glucose transporter 1 (Glut1)). Consistent with reduced HIF levels in Jnk2−/− cells, hypoxia-induced mRNA levels of Vegf, iNos, and Glut1 were decreased in Jnk2−/− cells compared with their wild-type counterparts (Fig. 4, G–I). As expected, echinomycin treatment dramatically inhibited the induction of Vegf, iNos, and Glut1 in hypoxic wild-type cells and further inhibited their induction in Jnk2−/− cells (Fig. 4, G–I). Under these conditions, the levels of HIF-1α or HIF-2α mRNA remained lower during hypoxia in Jnk2−/− cells compared with wild-type cells in the presence of echinomycin (Fig. 4, J and K). These data suggest that the JNK2 regulation of HIF mRNA occurs independently of HIF transcriptional activity.
Figure 4.
JNK2 up-regulates the expression of HIF-1α under pseudohypoxic conditions. A, HIF-1α immunoblot of wild-type or Jnk2−/− MEFs treated with DMOG (0.5 or 0.75 mm dissolved in DMSO) or vehicle control for 0 or 2 h. B, HIF-1α immunoblot of wild-type or Jnk2−/− MEFs treated with DFO (100 mm dissolved in water) for 0 h or 1 h. C, quantitative PCR for relative HIF-1α mRNA expression after normalizing to HPRT in wild-type or Jnk2−/− MEFs treated with DMOG (0.5 or 0.75 mm dissolved in DMSO) or vehicle control for 0 or 2 h. D, quantitative PCR for relative HIF-2α mRNA expression after normalizing to HPRT in wild-type or Jnk2−/− MEFs treated with DMOG (0.5 or 0.75 mm dissolved in DMSO) or vehicle control for 0 or 2 h. E, quantitative PCR for relative HIF-1α mRNA expression after normalizing to HPRT in wild-type or Jnk2−/− MEFs treated with DFO (100 mm dissolved in water) for 0 or 1 h. F, quantitative PCR for relative HIF-1α mRNA expression after normalizing to HPRT in wild-type or Jnk2−/− MEFs treated with DFO (100 mm dissolved in water) for 0 or 1 h. G, quantitative PCR for relative VEGF mRNA expression after normalizing to HPRT in wild-type or Jnk2−/− MEFs treated with hypoxia for 0–6 h following pretreatment with echinomycin or vehicle for 1 h. H, quantitative PCR for relative iNOS mRNA expression after normalizing to HPRT in wild-type or Jnk2−/− MEFs treated with hypoxia for 0–6 h following pretreatment with echinomycin or vehicle for 1 h. I, quantitative PCR for relative GLUT1 mRNA expression after normalizing to HPRT in wild-type or Jnk2−/− MEFs treated with hypoxia for 0–6 h following pretreatment with echinomycin or vehicle for 1 h. J, quantitative PCR for relative HIF-1α mRNA expression after normalizing to HPRT in wild-type or Jnk2−/− MEFs treated with hypoxia for 0–6 h following pretreatment with echinomycin or vehicle for 1 h. K, quantitative PCR for relative HIF-2α mRNA expression after normalizing to HPRT in wild-type or Jnk2−/− MEFs treated with hypoxia for 0–6 h following pretreatment with echinomycin or vehicle for 1 h. All immunoblots and quantitative PCR data are representative of at least three independent experiments that yielded similar results. All data are presented as mean ± S.E. (error bars). Statistical significance was determined by one-way ANOVA with Tukey's post hoc test (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001 for the comparisons indicated by brackets). For G–I, statistical analysis was performed by comparing WT pre-echinomycin with WT post-echinomycin or Jnk2−/− pre-echinomycin with Jnk2−/− post-echinomycin. For J and K, statistical analysis was performed by comparing WT post-echinomycin with Jnk2−/− post-echinomycin.
Jnk2−/− mice have decreased erythropoiesis under hypoxic conditions
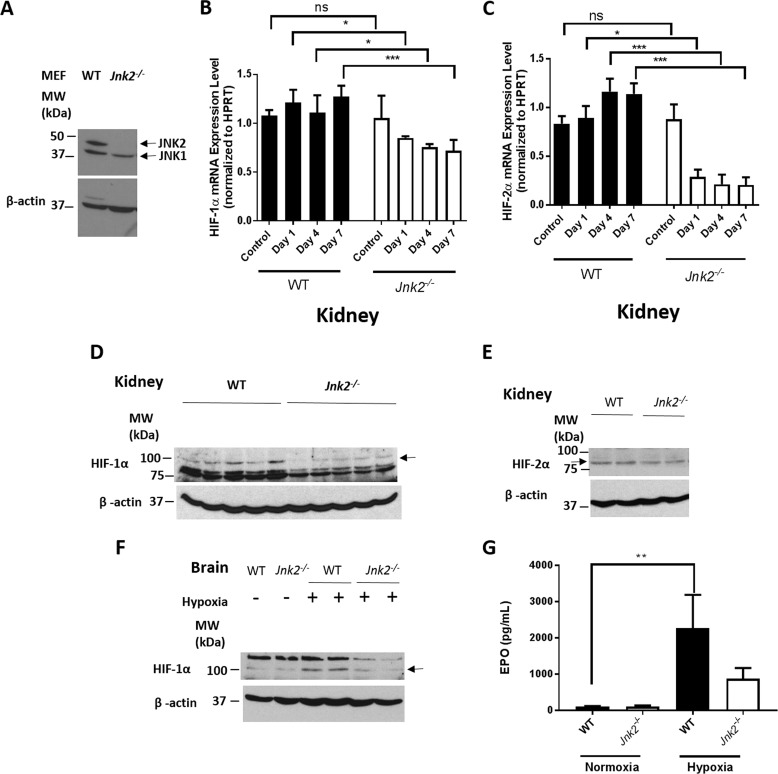
To determine whether the regulation of HIF by JNK2 occurs in vivo, we treated wild-type and Jnk2−/− mice (Fig. 5A) with hypoxia (7% oxygen) for 7 days. We analyzed the mRNA levels of HIFs in the kidney, where the HIF target gene Epo is produced (41). We observed decreased mRNA levels of HIF-1α and HIF-2α in Jnk2−/− mice as compared with wild-type mice under hypoxic conditions (Fig. 5, B and C), consistent with our in vitro results (Figs. 1–3). Accordingly, the protein levels of HIF-1α and HIF-2α in the kidney were reduced in hypoxic Jnk2−/− mice compared with wild-type mice under the same conditions (Fig. 5, D and E). The HIF protein levels in the brain were also decreased in hypoxic Jnk2−/− mice compared with hypoxic wild-type mice (Fig. 5F). We assessed the levels of EPO in the serum, and as expected, EPO was induced in wild-type mice under hypoxic conditions, which was suppressed in Jnk2−/− mice (Fig. 5G). These data suggest that JNK2 also maintains HIF expression in mice under hypoxic conditions.
Figure 5.
JNK2 up-regulates the expression of the HIF target gene Epo. A, JNK1 and JNK2 immunoblots from WT and Jnk2−/− mouse whole-lung homogenates. The blot shown is representative of the results of n = 4 in each genotype. B, quantitative PCR for relative HIF-1α mRNA expression after normalizing to HPRT in mouse kidney homogenates after 0, 1, 4, or 7 days of hypoxia (7% oxygen). n = 5 in control group, and n = 3 in each hypoxia group. C, quantitative PCR for relative HIF-2α mRNA expression after normalizing to HPRT in mouse kidney homogenates after 0, 1, 4, or 7 days of hypoxia (7% oxygen). n = 5 in control group, and n = 3 in each hypoxia group. D, HIF-1α immunoblot of whole-kidney homogenates from WT or Jnk2−/− mice kept in hypoxic conditions (7% oxygen) for 7 days. E, HIF-2α immunoblot of whole-kidney homogenates from WT or Jnk2−/− mice kept in hypoxic conditions (7% oxygen) for 7 days. F, HIF-1α immunoblot of whole-brain homogenates from WT or Jnk2−/− mice kept in normoxic or hypoxic conditions (7% oxygen) for 7 days. G, EPO levels in the serum of WT or Jnk2−/− mice kept in normoxic conditions or hypoxic conditions (7% oxygen) for 7 days. Data are pooled from two independent experiments. n = 9 in the WT control group, n = 6 in the Jnk2−/− control group, n = 8 in WT hypoxia group, and n = 9 in Jnk2−/− hypoxia group. Immunoblots shown are representative of three independent experiments conducted with at least three mice in each group. All data are presented as mean ± S.E. (error bars). Statistical significance was determined by one-way ANOVA with Tukey's post hoc test (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ns, non-significant for the comparisons indicated by brackets).
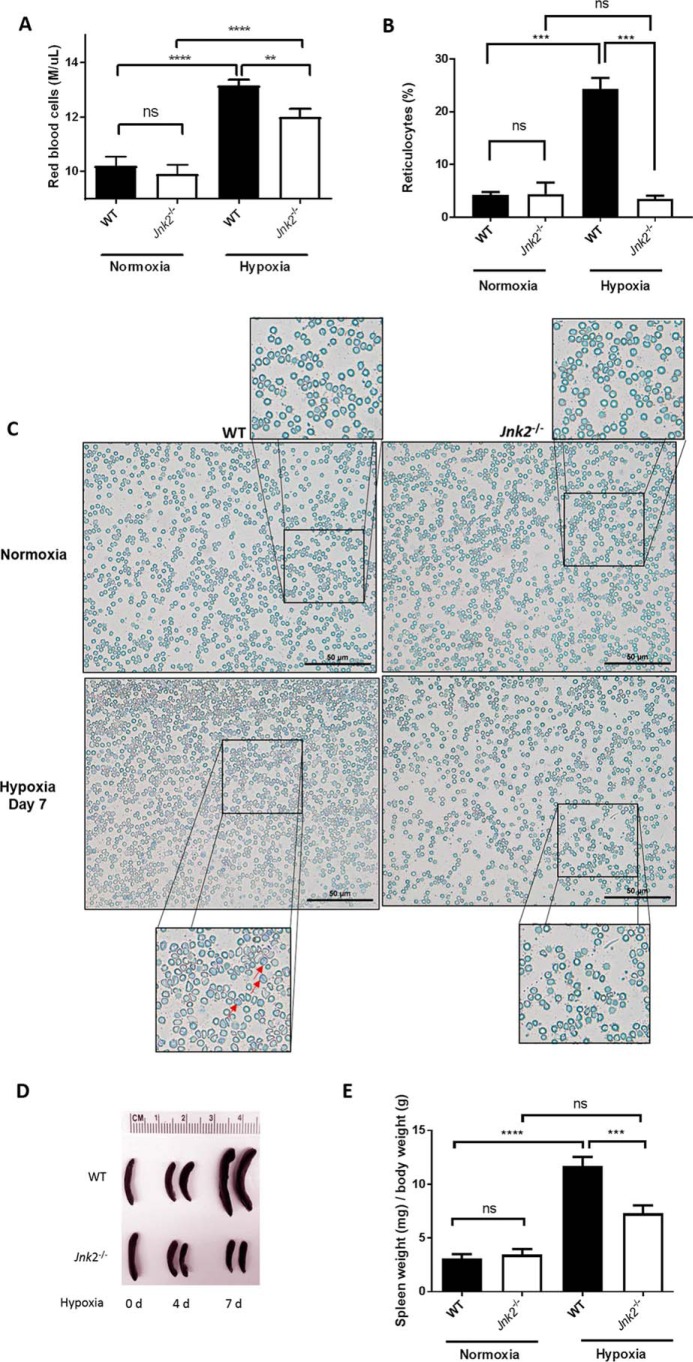
Under hypoxic conditions, up-regulation of HIF expression, predominantly HIF-2, induces the transcription of their target gene EPO, leading to increased erythropoiesis (19, 42–45). To determine the in vivo relevance of JNK2 regulation of HIFs, we treated wild-type and Jnk2−/− mice with hypoxia (7% oxygen) for 7 days and examined hypoxia-induced erythropoiesis. Hypoxia treatment resulted in a drastic increase in erythropoiesis in wild-type mice, as shown by increased red blood cell (RBC) counts (Fig. 6A) and reticulocytes (Fig. 6, B and C) in the peripheral blood. However, in hypoxic Jnk2−/− mice, the total numbers of RBC and reticulocytes in the peripheral blood were less than those from wild-type mice under the same conditions (Fig. 6, A–C). It has been reported that hypoxia treatment leads to splenomegaly in mice due to increased erythropoiesis in the spleen (46). Consistent with this, we observed that wild-type mice had increased spleen/body weight ratio after hypoxia treatment for 7 days (Fig. 6, D and E). As expected, splenomegaly was alleviated in Jnk2−/− mice under the same conditions (Fig. 6, D and E).
Figure 6.
Jnk2−/− mice have decreased RBC count, reticulocytosis, and splenomegaly under hypoxic conditions. A, red blood cell counts from WT or Jnk2−/− mice exposed to hypoxia (7% oxygen) for 7 days or kept in normoxic conditions. Data are pooled from five independent experiments. n = 19 in WT control group, n = 18 in Jnk2−/− control group, n = 50 in WT hypoxia group, and n = 37 in Jnk2−/− hypoxia group. B, reticulocyte percentage manually counted after staining with methylene blue dye. n = 2 in each control group, and n = 3 in each hypoxia (7% oxygen) group (a total of 500 cells were counted for each smear). C, representative reticulocyte stain with methylene blue dye of peripheral blood smears from WT or Jnk2−/− mice kept in normoxic conditions or treated with hypoxia (7% oxygen) for 7 days at ×40 magnification. Red arrows, reticulocytes. Images shown are representative of smears obtained from two independent experiments. D, representative photo of dissected spleens from WT or Jnk2−/− treated with hypoxia for 0, 4, or 7 days. E, spleen weight normalized to body weight of WT or Jnk2−/− mice treated with hypoxia (7% oxygen) for 7 days or kept in normoxic conditions. Data are pooled from five independent experiments. n = 16 in WT control group, n = 14 in Jnk2−/− control group, n = 52 in WT hypoxia group, and n = 39 in Jnk2−/− hypoxia group. All data are presented as mean ± S.E. (error bars). Statistical significance was determined by one-way ANOVA with Tukey's post hoc test (**, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001; ns, non-significant for the comparisons indicated by brackets).
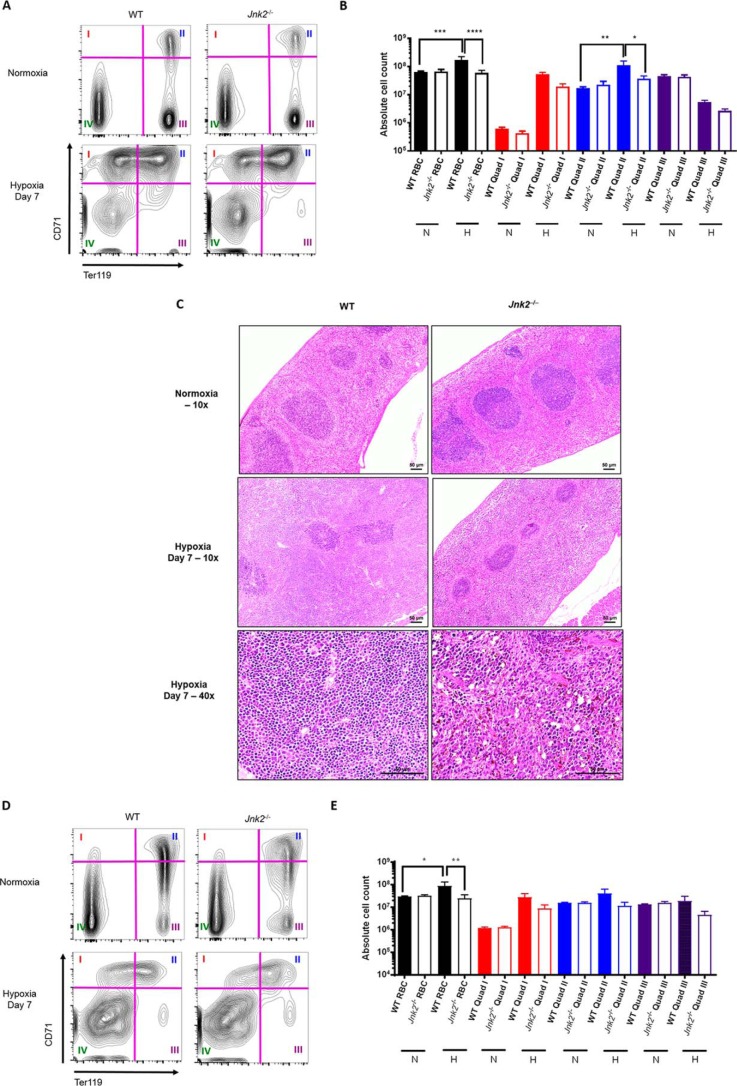
To further confirm that Jnk2−/− mice have decreased medullary and extramedullary erythropoiesis, we performed flow cytometry to trace erythroid precursors in the spleen and bone marrow using the markers of CD71 (transferrin receptor) and Ter119 (glycophorin A–associated protein). Ter119 is expressed at intermediate levels at the earliest, proerythroblast stage and is subsequently expressed at high levels during erythroid maturation (47). Meanwhile, CD71 expression declines with erythroid maturation (48). Using a gating strategy previously reported by others (19, 49, 50), we applied quadrants to define four subpopulations of erythroid cells in the spleen: Ter119lowCD71high (quadrant I, proerythroblasts), Ter119highCD71high (quadrant II, early erythroblasts), Ter119highCD71low (quadrant III, late erythroblasts), and Ter119lowCD71low (quadrant IV, non-erythroblasts) (Fig. 7A).
Figure 7.
Jnk2−/− mice have decreased splenic erythropoiesis under hypoxic conditions. A, representative flow cytometry gating strategy for mouse spleen erythroid precursors. Quadrants were used to divide the cell populations into four groups representing progressive erythroid precursor differentiation (see “Results”). B, absolute counts of erythroid precursors in the spleen measured by flow cytometry in WT or Jnk2−/− mice after treatment with 7 days of hypoxia (7% oxygen) or kept in normoxic conditions. Data are pooled from two independent experiments. n = 5 in WT control group, n = 5 in Jnk2−/− control group, n = 5 in WT hypoxia group, and n = 9 in Jnk2−/− hypoxia group. C, representative H&E-stained sections of WT or Jnk2−/− mice spleens treated with hypoxia (7% oxygen) for 7 days or kept in normoxic conditions (×10 or ×40 magnification). D, representative flow cytometry gating strategy for mouse bone marrow erythroid precursors. Quadrants were used to divide the cell populations into four groups representing progressive erythroid precursor differentiation (see “Results”). E, absolute counts of erythroid precursors in the bone marrow measured by flow cytometry in WT or Jnk2−/− mice after treatment with 7 days of hypoxia (7% oxygen) or kept in normoxic conditions. Data are pooled from two independent experiments. n = 5 in WT control group, n = 5 in Jnk2−/− control group, n = 5 in WT hypoxia group, n = 9 in Jnk2−/− hypoxia group. N, normoxia; H, hypoxia; RBC, red blood cell content calculated as the sum of quadrants I, II, and III. All data are presented as mean ± S.E. (error bars). Statistical significance was determined by one-way ANOVA with Tukey's post hoc test (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001; ns, non-significant for the comparisons indicated by brackets).
There were no differences in the numbers of total erythroid cells or different subpopulations of erythroid cells between wild-type and Jnk2−/− mice in the steady state (Fig. 7, A and B). Hypoxia treatment induced de novo erythropoiesis in wild-type mice, as shown by increased numbers in total erythroid cells as well as in the subpopulations of proerythroblasts and early erythroblasts (Fig. 7, A and B). In hypoxic Jnk2−/− mice, de novo erythropoiesis was impaired compared with wild-type mice, as shown by lesser numbers in total erythroid cells and the subpopulations of proerythroblasts and early and late erythroblasts (Fig. 7, A and B). Spleen histology showed red pulp expansion in hypoxic wild-type mice compared with normoxic controls, which is consistent with a state of increased extramedullary erythropoiesis (46, 51, 52). However, we did not observe red pulp expansion in the spleen of Jnk2−/− mice (Fig. 7C). Similar results were obtained in the bone marrow. There were no differences in marrow erythropoiesis between wild-type and Jnk2−/− mice in the steady state (Fig. 7, D and E). Hypoxia treatment induced de novo erythropoiesis in the bone marrow of wild-type mice, as shown by increased numbers in total erythroid cells as well as in the subpopulations of proerythroblasts and early erythroblasts (Fig. 7, D and E). Again, under hypoxic conditions, Jnk2−/− mice exhibited less de novo erythropoiesis compared with wild-type mice, as shown by reduced numbers of total erythroid cells and subpopulations of proerythroblasts and early and late erythroblasts (Fig. 7, D and E). Taken together, these data suggest that JNK2 contributes to de novo erythropoiesis in response to hypoxia, both medullary and extramedullary. Considering the role of HIFs in hypoxia-induced erythropoiesis (53, 54), our in vivo data are consistent with our in vitro observation that JNK2 up-regulates HIFs (Figs. 1–3).
Jnk2−/− mice are protected from hypoxia-induced pulmonary hypertension
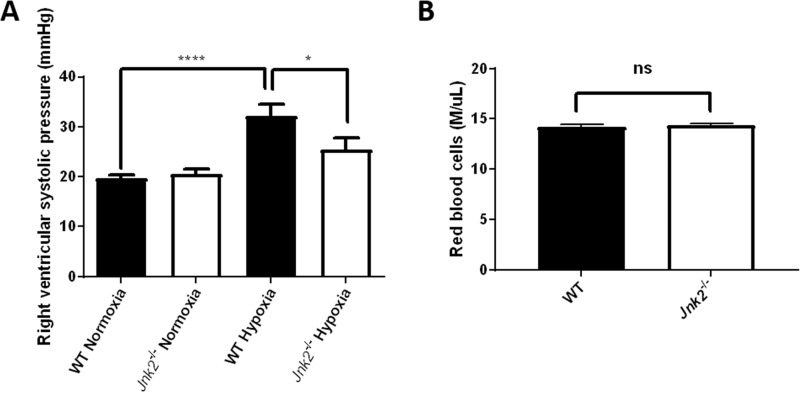
Our data suggest that JNK2 up-regulates HIF in vitro as well as in a mouse model of acute hypoxia-induced erythropoiesis. We sought to validate the regulation of HIF by JNK2 in vivo using a different mouse model of chronic hypoxia-induced PH. HIF-1α and HIF-2α are critical for the development of hypoxia-induced PH, as it has been reported that PH due to chronic hypoxia can be mitigated in either Hif-1α or Hif-2α heterozygous-null mice (15, 16). The positive regulation of HIFs by JNK2 prompted us to postulate that Jnk2−/− mice may be protected from chronic hypoxia-induced PH. Wild-type and Jnk2−/− mice were maintained at 10% oxygen for 21 days before hemodynamic measurements were obtained by directly measuring right ventricular systolic pressure using a micromanometer-tipped catheter. During these measurements, the mice were ventilated with 21% oxygen. As expected, Jnk2−/− mice were partially protected from hypoxia-induced PH, as shown by decreased right ventricular systolic pressures compared with wild-type mice (Fig. 8A). It is known that RBC content can influence viscosity, which in turn can lead to elevated pulmonary artery pressures. We measured the RBC count in the peripheral blood of wild-type and Jnk2−/− mice after chronic exposure to 10% oxygen for 21 days. Whereas RBC count in Jnk2−/− was decreased compared with wild-type mice under acute hypoxic conditions (7% oxygen for 7 days) (Fig. 6A), we observed no difference in the RBC count between these two groups of mice under chronic hypoxic conditions (10% oxygen for 21 days) (Fig. 8B). These data suggest that JNK2 is required to maintain de novo erythropoiesis under acute but not chronic hypoxic conditions, indicating that the hypoxia-induced erythropoietic response is only delayed and not diminished by the loss of JNK2. Furthermore, our data suggest that the reduction in the right ventricular systolic pressure observed in hypoxic Jnk2−/− mice compared with wild-type mice is not attributable to a lower vascular resistance caused by reduced erythropoiesis in hypoxia. It is interesting to note that Jnk2−/− mice recapitulate the phenotype of Hif-1α or Hif-2α heterozygous-null mice during hypoxic response, which also displayed a delayed erythropoietic response and reduced pulmonary hypertension (15, 16). Taken together, these data suggest that JNK2 deficiency protects mice from chronic hypoxia-induced PH, consistent with its role in HIF regulation.
Figure 8.
Jnk2−/− mice are protected from hypoxia-induced pulmonary hypertension. A, right ventricular systolic pressure measurements in WT or Jnk2−/− mice kept in normoxic conditions (21% oxygen) or chronic hypoxic conditions (10% oxygen) for 21 days. n = 8 in WT control group, n = 8 in Jnk2−/− control group, n = 7 in WT hypoxia group, and n = 7 in Jnk2−/− hypoxia group. B, red blood cell count in the peripheral blood of WT or Jnk2−/− mice kept at 10% oxygen for 21 days. n = 5 in each group. All data are presented as mean ± S.E. (error bars). Statistical significance was determined in A by one-way ANOVA with Tukey's post hoc test and in B by a t test (*, p ≤ 0.05; ****, p ≤ 0.0001; ns, non-significant for the comparisons indicated by brackets).
Discussion
Hypoxia from any cause, whether from low barometric pressure, anemia, or decreased alveolar–capillary diffusion, can have adverse effects on all organ systems and overall survival. As exemplified in chronic lung diseases, such as COPD, the presence of chronic hypoxia is associated with worse survival and quality of life and complications such as PH and polycythemia. In this context, the most commonly prescribed therapy is continuous oxygen supplementation (55), but disease progression and mortality are the norm. Here, we show that JNK2 mediates the up-regulation of HIF-1α and HIF-2α at the transcriptional level, which in turn contributes to the impact of hypoxia on erythropoiesis and PH. Thus, our data may provide a novel target for future therapies of hypoxia-associated diseases.
Our in vitro studies revealed that JNK2, but not JNK1, up-regulated HIF-1α and HIF-2α through increased mRNA expression rather than decreased post-translational degradation by the proteasome. In vivo, we observed decreased mRNA and protein levels of HIF-1α and/or HIF-2α in Jnk2−/− mice compared with wild-type mice under hypoxic conditions, which is consistent with our in vitro data. The in vivo relevance of JNK2 regulation of HIF was revealed by our observation that Jnk2−/− mice were alleviated from hypoxia-induced polycythemia and PH, two well-established phenotypes of overexpression of the HIF pathway. Thus, JNK2 may be an important mediator in the pathobiology of hypoxia-induced polycythemia and PH through the positive regulation of HIF-1α and HIF-2α expression.
A limited body of work has thus far implicated a role for JNK in the regulation of HIF and the hypoxic response in vitro (56–58). Comerford et al. (56) treated HeLa cells with hypoxia in the presence of a nonspecific JNK1/2/3 inhibitor and found decreased HIF-1α protein levels, decreased HIF-1 reporter activity, and decreased hypoxia response element binding. Subsequently, Zhang et al. (57) found that both JNK1 and JNK2 increased HIF-1α protein expression in nickel-treated MEFs but by different mechanisms; whereas JNK1 mediated HIF-1α protein stabilization through molecular chaperone Hsp70/Hsp90 activity, JNK2 stabilized the HIF-1α mRNA transcripts by up-regulating nucleolin expression (58). Paradoxically, we did not observe that JNK2 stabilized either HIF-1α or HIF-2α mRNA in vitro. This discrepancy may be due to differences in the assay used; whereas Zhang et al. (57) used relative densitometry of agarose gel–separated PCR products after staining with ethidium bromide, we used quantitative RT-PCR for our measurements. Combined with our findings that JNK2 did not regulate HIF-1α or HIF-2α through regulation of their proteosomal degradation, we conclude that JNK2 regulation of HIF mRNA may be mediated through de novo mRNA transcription. Here, with the use of cells and mice deficient in JNK2, as well as the animal models of hypoxia-induced erythropoiesis and PH, we demonstrate that JNK2 is an important physiological contributor to the hypoxic response by maintaining HIF expression.
Most in vitro studies indicate that HIF is predominantly regulated post-translationally through the inhibition of O2-dependent prolyl-4-hydroxylase domain proteins that drive HIF degradation under normoxia. Our results show that maintenance of HIF mRNA levels by JNK2, which precedes hypoxia-induced HIF protein accumulation, is of critical importance under pathophysiologically relevant conditions in vivo. Future studies are needed to investigate the molecular mechanisms by which JNK2 maintains HIF mRNA levels.
Several studies have shown that JNK is activated in response to hypoxia in a variety of biological systems to hypoxia, including the neurological (59–62), cardiovascular (63, 64), renal (65), hepatic (66), and oncological (67–69) systems. Specifically, Jin et al. (70) found that the activity of all MAPKs (JNK, ERK, and p38) was increased, with varying time courses, in the pulmonary arteries of hypoxia-treated rats. Our findings that mice deficient in JNK2 were relatively (but not completely) spared of hypoxia-induced polycythemia and PH are in line with these studies. The incomplete mitigation of the pulmonary hypertension by loss of JNK2 suggests contributions from additional factors.
In summary, we have demonstrated that the regulation of HIF-1α and HIF-2α by JNK2 is physiologically relevant in the hypoxic response. This regulation appears to occur by increasing HIF mRNA rather than through the inhibition of the canonical, post-translational ubiquitin–proteasomal degradation. Our data suggest that JNK2 may play a role in the development of hypoxia-induced polycythemia and PH, both common complications of chronic hypoxia in humans. Inhibition of JNK2 may be a novel therapeutic target for these conditions and possibly other HIF pathway-associated disease states.
Experimental procedures
Animal model of hypoxia
Wild-type or Jnk2−/− C57BL/6 mice (at least 8 weeks old) were treated with normobaric normoxia (21% oxygen) or in an enclosed normobaric hypoxia chamber (7% oxygen) for 1, 4, or 7 days (acute hypoxia model) or 10% oxygen for 21 days (chronic hypoxia model). Post-hypoxia, mice were weighed and then sacrificed with Euthasol euthanizing solution. Peripheral blood was collected directly into EDTA-lined tubes to proceed to automated cell counting using a Hemavet 950FS system (Drew Scientific, Inc.). For a separate subgroup of mice, spleens and femoral bone marrow were collected and made into single cell suspensions for flow cytometry (see below). For another subgroup of mice, spleens were fixed, embedded in paraffin, and analyzed by staining with hematoxylin and eosin. The animal care and experiments were performed in compliance with institutional and National Institutes of Health guidelines and were approved by the Northwestern University Animal Care and Use Committee.
Cell culture
Wild-type, Jnk1−/−, and Jnk2−/− MEFs have been described (25, 33). Cells were incubated at 37 °C in an atmosphere of 21% oxygen, 5% CO2 before experimental exposures. Cells were maintained according to ATCC (Manassas, VA) recommendations.
EPO analysis
The concentration of erythropoietin in the mouse serum was measured using multiplex immunoassay according to the manufacturer's instructions (eBioscience).
In vitro model of hypoxia
Cells were exposed to defined atmospheric conditions in environmental chambers (InvivO2 hypoxia workstation, Baker Co.). Normoxia was defined as 21% oxygen and 5% carbon dioxide, and hypoxia was defined as 1.5% oxygen and 5% carbon dioxide. Cell lysates were harvested immediately after the treatment course was completed. Starvation medium (2.5% FBS, 2.0% HEPES) was used for all hypoxia experiments and was pre-equilibrated overnight in the hypoxic atmosphere. For the MG132 experiments, cells were treated with 20 μm MG132 (dissolved in DMSO) 2 h before hypoxia exposure. For the echinomycin experiments, cells were treated with 10 nm echinomycin (dissolved in DMSO) (SML0477, Sigma-Aldrich) for 1 h before hypoxia exposure. For siRNA transfection experiments, the following siRNA oligonucleotides were from Thermo Scientific (Dharmacon products): control siRNA (D-001210-02), JNK1-specific siRNA (5′-UGAUUCAGAUGGAGUUAGATT-3′), and JNK2-specific siRNA (5′-CCGCAGAGUUCAUGAAGAATT-3′). HIF-1α-specific siRNA (NM_010431) was from Sigma-Aldrich.
Western blotting
Protein content was normalized with spectrophotometry and subsequently resolved on 10% polyacrylamide gels. Separated proteins were then transferred to PVDF membranes and incubated overnight with primary antibodies. The following primary antibodies were used: HIF-1α (10006421, 1:200; Cayman Chemical, Ann Arbor, MI), HIF-2α (ab199, 1:500; Abcam), JNK2 (4672, 1:500; Cell Signaling Technology, Beverly, MA), JNK1/JNK2 (G151-666, 1:500; BD Biosciences), or β-actin (A5441, 1:20,000; Sigma-Aldrich).
Flow cytometry
Spleens were enzymatically digested by instillation with a mixture of DNase I and collagenase D and then passed through a 40-μm filter to obtain single cell suspension. 2.5 × 106 cells were stained with Fixable viability dye, phycoerythrin-conjugated anti-Ter119 (12-5921-83, eBioscience), and allophycocyanin-conjugated anti-CD71 (17-0711-82, eBioscience). Femoral bone marrow was isolated as single cell suspension by passing through a 40-μm filter, and 2.5 × 106 cells were stained with the same fluorochromes. All data collection and sorting were performed using BD FACS Diva software (BD Biosciences), and data analyses were performed using FlowJo software (Tree Star, Ashland, OR). Compensation was performed using single-color controls prepared from OneComp Beads (eBioscience) for cell-surface staining or Arc Beads (Life Technologies) for live/dead discrimination. Compensation matrices were calculated and applied using FlowJo software (Tree Star).
Quantitative real-time PCR
Total RNA was isolated from MEFs or homogenized kidneys using TRIzol reagent (Invitrogen). RNA was converted into cDNA using M-MuLV reverse transcriptase, and mRNA abundance was quantified by quantitative PCR using SYBR Green (Bio-Rad) and normalized to HPRT using the following primers: HIF-1α (forward, 5′-ATGAGTTCTGAACGTCGAAAAGA-3′; reverse, 5′-GGGGAAGTGGCAACTGATGA-3′), HIF-2α (forward, 5′-CTGGACAAAGCCTCCATCAT-3′; reverse, 5′-TTGCTGATGTTTTCCGACAG-3′), JNK1 (forward,5′-GTTCCCCGATGTGCTTTTCC-3′; reverse, 5′-GGTGCTGGAGAGCTTCATCT-3′), JNK2 (forward, 5′-AGGTGGCGGACTCAACTTTC-3′; reverse, 5′-CGAGTTCACGGTAGGCTCTC-3′); iNOS (forward, 5′-AGGGCCACCTCTACATTTGC-3′; reverse, 5′-GTGCCAGAAGCTGGAACTCT-3′), VEGF (forward, 5′-CCACGTCAGAGAGCAACATCA-3′; reverse, 5′-TCATTCTCTCTATGTGCTGGCTTT-3′), GLUT1 (forward, 5′-CATCCTTATTGCCCAGGTGTTT-3′; reverse, 5′-GAAGACGACACTGAGCAGCAGA-3′), and HPRT (forward, 5′-AGGCCAGACTTTGTTGGATTTGAA-3′; reverse, CAACTTGCGCTCATCTTAGGCTTT-3′).
Right ventricular systolic pressure measurements
Mice were housed in normobaric normoxia (21% oxygen) or chronic hypoxia (10% oxygen) conditions for 21 days. Pulmonary artery pressure was quantified by right heart catheterization with a micromanometer-tipped catheter while being ventilated at 21% oxygen (Millar Instruments, Houston, TX) using a modification of a previous technique (17, 71) before euthanasia.
Statistical analysis
Data are summarized as mean ± S.E. for n independent experiments. Statistical significance was assessed using a t test or one-way ANOVA followed by the appropriate post test. Within the figures, levels of statistical significance are denoted as follows: *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001; ns, non-significant.
Author contributions
M. A. S. was responsible for study design, data collection and analysis, and manuscript writing; C. C., Q. Z., H. C. D.-U., and W. W. contributed to study design, data collection and analysis, and manuscript writing; A. V. M. contributed to study design and technical support for flow cytometry; G. B. W. and P. T. S. designed and conducted micromanometer hemodynamic measurements; D. F., G. R. S. B., S. L., N. S. C., and J. I. S. contributed to study design; J. L. contributed to study design, data analysis, and manuscript writing.
Supplementary Material
Acknowledgment
We thank M. Karin (University of California, San Diego) for kindly providing Jnk2−/− mice.
This work was supported by National Institutes of Health Grant R01 HL114763-01A1 (to J. L.) and American Lung Association Senior Research Training Grant RT-416649 (to M. S.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Fig. S1.
- PH
- pulmonary hypertension
- MEF
- mouse embryonic fibroblast
- COPD
- chronic obstructive pulmonary disease
- HIF
- hypoxia-inducible factor
- VHL
- von Hippel–Lindau
- EPO
- erythropoietin
- DMOG
- dimethyloxalylglycine
- DFO
- deferoxamine
- iNOS
- inducible nitric-oxide synthase
- RBC
- red blood cell(s)
- HPRT
- hypoxanthine-guanine phosphoribosyltransferase
- ANOVA
- analysis of variance.
References
- 1. Prabhakar N. R., and Semenza G. L. (2012) Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 92, 967–1003 10.1152/physrev.00030.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang G. L., Jiang B. H., Rue E. A., and Semenza G. L. (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U.S.A. 92, 5510–5514 10.1073/pnas.92.12.5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stenmark K. R., Fagan K. A., and Frid M. G. (2006) Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ. Res. 99, 675–691 10.1161/01.RES.0000243584.45145.3f [DOI] [PubMed] [Google Scholar]
- 4. Girgis R. E., and Mathai S. C. (2007) Pulmonary hypertension associated with chronic respiratory disease. Clinics in chest medicine 28, 219–232, x 10.1016/j.ccm.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 5. Cooper R., Ghali J., Simmons B. E., and Castaner A. (1991) Elevated pulmonary artery pressure: an independent predictor of mortality. Chest 99, 112–120 10.1378/chest.99.1.112 [DOI] [PubMed] [Google Scholar]
- 6. Weitzenblum E., Hirth C., Ducolone A., Mirhom R., Rasaholinjanahary J., and Ehrhart M. (1981) Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax 36, 752–758 10.1136/thx.36.10.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuttica M. J., Kalhan R., Shlobin O. A., Ahmad S., Gladwin M., Machado R. F., Barnett S. D., and Nathan S. D. (2010) Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir. Med. 104, 1877–1882 10.1016/j.rmed.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 8. Incalzi R. A., Fuso L., De Rosa M., Di Napoli A., Basso S., Pagliari G., and Pistelli R. (1999) Electrocardiographic signs of chronic cor pulmonale: a negative prognostic finding in chronic obstructive pulmonary disease. Circulation 99, 1600–1605 10.1161/01.CIR.99.12.1600 [DOI] [PubMed] [Google Scholar]
- 9. Barberà J. A., Peinado V. I., and Santos S. (2003) Pulmonary hypertension in chronic obstructive pulmonary disease. Eur. Respir. J. 21, 892–905 10.1183/09031936.03.00115402 [DOI] [PubMed] [Google Scholar]
- 10. Cote C., Zilberberg M. D., Mody S. H., Dordelly L. J., and Celli B. (2007) Haemoglobin level and its clinical impact in a cohort of patients with COPD. Eur. Respir. J. 29, 923–929 10.1183/09031936.00137106 [DOI] [PubMed] [Google Scholar]
- 11. Nakamura A., Kasamatsu N., Hashizume I., Shirai T., Hanzawa S., Momiki S., Sasaki K., Kinoshita M., Okada O., Tatsumi K., and Kuriyama T. (2000) Effects of hemoglobin on pulmonary arterial pressure and pulmonary vascular resistance in patients with chronic emphysema. Respiration 67, 502–506 10.1159/000067463 [DOI] [PubMed] [Google Scholar]
- 12. York E. L., Jones R. L., Menon D., and Sproule B. J. (1980) Effects of secondary polycythemia on cerebral blood flow in chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 121, 813–818 [DOI] [PubMed] [Google Scholar]
- 13. Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., and Ratcliffe P. J. (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 10.1038/20459 [DOI] [PubMed] [Google Scholar]
- 14. Masson N., Willam C., Maxwell P. H., Pugh C. W., and Ratcliffe P. J. (2001) Independent function of two destruction domains in hypoxia-inducible factor-α chains activated by prolyl hydroxylation. EMBO J. 20, 5197–5206 10.1093/emboj/20.18.5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu A. Y., Shimoda L. A., Iyer N. V., Huso D. L., Sun X., McWilliams R., Beaty T., Sham J. S., Wiener C. M., Sylvester J. T., and Semenza G. L. (1999) Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J. Clin. Invest. 103, 691–696 10.1172/JCI5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brusselmans K., Compernolle V., Tjwa M., Wiesener M. S., Maxwell P. H., Collen D., and Carmeliet P. (2003) Heterozygous deficiency of hypoxia-inducible factor-2α protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J. Clin. Invest. 111, 1519–1527 10.1172/JCI15496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ball M. K., Waypa G. B., Mungai P. T., Nielsen J. M., Czech L., Dudley V. J., Beussink L., Dettman R. W., Berkelhamer S. K., Steinhorn R. H., Shah S. J., and Schumacker P. T. (2014) Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am. J. Respir. Crit. Care Med. 189, 314–324 10.1164/rccm.201302-0302OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ang S. O., Chen H., Hirota K., Gordeuk V. R., Jelinek J., Guan Y., Liu E., Sergueeva A. I., Miasnikova G. Y., Mole D., Maxwell P. H., Stockton D. W., Semenza G. L., and Prchal J. T. (2002) Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat. Genet. 32, 614–621 10.1038/ng1019 [DOI] [PubMed] [Google Scholar]
- 19. Hickey M. M., Lam J. C., Bezman N. A., Rathmell W. K., and Simon M. C. (2007) von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2α signaling and splenic erythropoiesis. J. Clin. Invest. 117, 3879–3889 10.1172/JCI32614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davis R. J. (2000) Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252 10.1016/S0092-8674(00)00116-1 [DOI] [PubMed] [Google Scholar]
- 21. Hibi M., Lin A., Smeal T., Minden A., and Karin M. (1993) Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 7, 2135–2148 10.1101/gad.7.11.2135 [DOI] [PubMed] [Google Scholar]
- 22. Weston C. R., and Davis R. J. (2007) The JNK signal transduction pathway. Curr. Opin. Cell Biol. 19, 142–149 10.1016/j.ceb.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 23. Bogoyevitch M. A., and Kobe B. (2006) Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 70, 1061–1095 10.1128/MMBR.00025-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kallunki T., Su B., Tsigelny I., Sluss H. K., Dérijard B., Moore G., Davis R., and Karin M. (1994) JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 8, 2996–3007 10.1101/gad.8.24.2996 [DOI] [PubMed] [Google Scholar]
- 25. Liu J., Minemoto Y., and Lin A. (2004) c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for tumor necrosis factor α-induced c-Jun kinase activation and apoptosis. Mol. Cell Biol. 24, 10844–10856 10.1128/MCB.24.24.10844-10856.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sabapathy K., Hochedlinger K., Nam S. Y., Bauer A., Karin M., and Wagner E. F. (2004) Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol. Cell 15, 713–725 10.1016/j.molcel.2004.08.028 [DOI] [PubMed] [Google Scholar]
- 27. Fuchs S. Y., Adler V., Buschmann T., Yin Z., Wu X., Jones S. N., and Ronai Z. (1998) JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev. 12, 2658–2663 10.1101/gad.12.17.2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fuchs S. Y., Dolan L., Davis R. J., and Ronai Z. (1996) Phosphorylation-dependent targeting of c-Jun ubiquitination by Jun N-kinase. Oncogene 13, 1531–1535 [PubMed] [Google Scholar]
- 29. Fuchs S. Y., Xie B., Adler V., Fried V. A., Davis R. J., and Ronai Z. (1997) c-Jun NH2-terminal kinases target the ubiquitination of their associated transcription factors. J. Biol. Chem. 272, 32163–32168 10.1074/jbc.272.51.32163 [DOI] [PubMed] [Google Scholar]
- 30. Fuchs S. Y., Fried V. A., and Ronai Z. (1998) Stress-activated kinases regulate protein stability. Oncogene 17, 1483–1490 10.1038/sj.onc.1202184 [DOI] [PubMed] [Google Scholar]
- 31. Schofield C. J., and Ratcliffe P. J. (2004) Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 5, 343–354 10.1038/nrm1366 [DOI] [PubMed] [Google Scholar]
- 32. Bode A. M., and Dong Z. (2007) The functional contrariety of JNK. Mol. Carcinog. 46, 591–598 10.1002/mc.20348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Q., Kuang H., Chen C., Yan J., Do-Umehara H. C., Liu X. Y., Dada L., Ridge K. M., Chandel N. S., and Liu J. (2015) The kinase Jnk2 promotes stress-induced mitophagy by targeting the small mitochondrial form of the tumor suppressor ARF for degradation. Nat. Immunol. 16, 458–466 10.1038/ni.3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bensaude O. (2011) Inhibiting eukaryotic transcription: which compound to choose? How to evaluate its activity? Transcription 2, 103–108 10.4161/trns.2.3.16172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leclerc G. J., Leclerc G. M., and Barredo J. C. (2002) Real-time RT-PCR analysis of mRNA decay: half-life of β-actin mRNA in human leukemia CCRF-CEM and Nalm-6 cell lines. Cancer Cell Int. 2, 1 10.1186/1475-2867-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wanner R. M., Spielmann P., Stroka D. M., Camenisch G., Camenisch I., Scheid A., Houck D. R., Bauer C., Gassmann M., and Wenger R. H. (2000) Epolones induce erythropoietin expression via hypoxia-inducible factor-1α activation. Blood 96, 1558–1565 [PubMed] [Google Scholar]
- 37. Cummins E. P., Seeballuck F., Keely S. J., Mangan N. E., Callanan J. J., Fallon P. G., and Taylor C. T. (2008) The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134, 156–165 10.1053/j.gastro.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 38. Park J. Y., Jung J. Y., Kim H. J., Bae I. H., Kim D. Y., Lee T. R., and Shin D. W. (2016) Hypoxia leads to abnormal epidermal differentiation via HIF-independent pathways. Biochem. Biophys. Res. Commun. 469, 251–256 10.1016/j.bbrc.2015.11.111 [DOI] [PubMed] [Google Scholar]
- 39. Wang G. L., and Semenza G. L. (1993) Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood 82, 3610–3615 [PubMed] [Google Scholar]
- 40. Goldberg M. A., Dunning S. P., and Bunn H. F. (1988) Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science 242, 1412–1415 10.1126/science.2849206 [DOI] [PubMed] [Google Scholar]
- 41. Lacombe C., Da Silva J. L., Bruneval P., Fournier J. G., Wendling F., Casadevall N., Camilleri J. P., Bariety J., Varet B., and Tambourin P. (1988) Peritubular cells are the site of erythropoietin synthesis in the murine hypoxic kidney. J. Clin. Invest. 81, 620–623 10.1172/JCI113363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gruber M., Hu C. J., Johnson R. S., Brown E. J., Keith B., and Simon M. C. (2007) Acute postnatal ablation of Hif-2α results in anemia. Proc. Natl. Acad. Sci. U.S.A. 104, 2301–2306 10.1073/pnas.0608382104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Semenza G. L., and Wang G. L. (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 12, 5447–5454 10.1128/MCB.12.12.5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kampf D., Eckardt K. U., Fischer H. C., Schmalisch C., Ehmer B., and Schostak M. (1992) Pharmacokinetics of recombinant human erythropoietin in dialysis patients after single and multiple subcutaneous administrations. Nephron 61, 393–398 10.1159/000186955 [DOI] [PubMed] [Google Scholar]
- 45. Lee G., and Arcasoy M. O. (2015) The clinical and laboratory evaluation of the patient with erythrocytosis. Eur. J. Int. Med. 26, 297–302 10.1016/j.ejim.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 46. Beran M., and Tribukait B. (1971) The post hypoxic bone marrow and spleen composition. Scand. J. Haematol. 8, 5–15 [DOI] [PubMed] [Google Scholar]
- 47. Kina T., Ikuta K., Takayama E., Wada K., Majumdar A. S., Weissman I. L., and Katsura Y. (2000) The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br. J. Haematol. 109, 280–287 10.1046/j.1365-2141.2000.02037.x [DOI] [PubMed] [Google Scholar]
- 48. Lesley J., Hyman R., Schulte R., and Trotter J. (1984) Expression of transferrin receptor on murine hematopoietic progenitors. Cell Immunol. 83, 14–25 10.1016/0008-8749(84)90220-X [DOI] [PubMed] [Google Scholar]
- 49. Socolovsky M., Nam H., Fleming M. D., Haase V. H., Brugnara C., and Lodish H. F. (2001) Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood 98, 3261–3273 10.1182/blood.V98.12.3261 [DOI] [PubMed] [Google Scholar]
- 50. Dumitriu B., Bhattaram P., Dy P., Huang Y., Quayum N., Jensen J., and Lefebvre V. (2010) Sox6 is necessary for efficient erythropoiesis in adult mice under physiological and anemia-induced stress conditions. PLoS One 5, e12088 10.1371/journal.pone.0012088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ou L. C., Kim D., Layton W. M. Jr., and Smith R. P. (1980) Splenic erythropoiesis in polycythemic response of the rat to high-altitude exposure. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 48, 857–861 [DOI] [PubMed] [Google Scholar]
- 52. Tsuboi I., Yamashita T., Nagano M., Kimura K., To'a Salazar G., and Ohneda O. (2015) Impaired expression of HIF-2α induces compensatory expression of HIF-1α for the recovery from anemia. J. Cell Physiol. 230, 1534–1548 10.1002/jcp.24899 [DOI] [PubMed] [Google Scholar]
- 53. Wang G. L., and Semenza G. L. (1996) Molecular basis of hypoxia-induced erythropoietin expression. Curr. Opin. Hematol. 3, 156–162 10.1097/00062752-199603020-00009 [DOI] [PubMed] [Google Scholar]
- 54. Socolovsky M. (2007) Molecular insights into stress erythropoiesis. Curr. Opin. Hematol. 14, 215–224 10.1097/MOH.0b013e3280de2bf1 [DOI] [PubMed] [Google Scholar]
- 55. Anthonisen N. R. (1983) Long-term oxygen therapy. Ann. Intern. Med. 99, 519–527 10.7326/0003-4819-99-4-519 [DOI] [PubMed] [Google Scholar]
- 56. Comerford K. M., Cummins E. P., and Taylor C. T. (2004) c-Jun NH2-terminal kinase activation contributes to hypoxia-inducible factor 1α-dependent P-glycoprotein expression in hypoxia. Cancer Res. 64, 9057–9061 10.1158/0008-5472.CAN-04-1919 [DOI] [PubMed] [Google Scholar]
- 57. Zhang D., Li J., Costa M., Gao J., and Huang C. (2010) JNK1 mediates degradation HIF-1α by a VHL-independent mechanism that involves the chaperones Hsp90/Hsp70. Cancer Res. 70, 813–823 10.1158/0008-5472.CAN-09-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang D., Li J., Zhang M., Gao G., Zuo Z., Yu Y., Zhu L., Gao J., and Huang C. (2012) The requirement of c-Jun N-terminal kinase 2 in regulation of hypoxia-inducing factor-1α mRNA stability. J. Biol. Chem. 287, 34361–34371 10.1074/jbc.M112.365882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gozal E., Simakajornboon N., Dausman J. D., Xue Y. D., Corti M., El-Dahr S. S., and Gozal D. (1999) Hypoxia induces selective SAPK/JNK-2-AP-1 pathway activation in the nucleus tractus solitarii of the conscious rat. J. Neurochem. 73, 665–674 [DOI] [PubMed] [Google Scholar]
- 60. Guma M., Rius J., Duong-Polk K. X., Haddad G. G., Lindsey J. D., and Karin M. (2009) Genetic and pharmacological inhibition of JNK ameliorates hypoxia-induced retinopathy through interference with VEGF expression. Proc. Natl. Acad. Sci. U.S.A. 106, 8760–8765 10.1073/pnas.0902659106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mishra O. P., Zubrow A. B., and Ashraf Q. M. (2004) Nitric oxide-mediated activation of extracellular signal-regulated kinase (ERK) and c-jun N-terminal kinase (JNK) during hypoxia in cerebral cortical nuclei of newborn piglets. Neuroscience 123, 179–186 10.1016/j.neuroscience.2003.08.008 [DOI] [PubMed] [Google Scholar]
- 62. Nijboer C. H., van der Kooij M. A., van Bel F., Ohl F., Heijnen C. J., and Kavelaars A. (2010) Inhibition of the JNK/AP-1 pathway reduces neuronal death and improves behavioral outcome after neonatal hypoxic-ischemic brain injury. Brain Behav. Immun. 24, 812–821 10.1016/j.bbi.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 63. Shao Z., Bhattacharya K., Hsich E., Park L., Walters B., Germann U., Wang Y. M., Kyriakis J., Mohanlal R., Kuida K., Namchuk M., Salituro F., Yao Y. M., Hou W. M., Chen X., et al. (2006) c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ. Res. 98, 111–118 [DOI] [PubMed] [Google Scholar]
- 64. Shang L., Ananthakrishnan R., Li Q., Quadri N., Abdillahi M., Zhu Z., Qu W., Rosario R., Touré F., Yan S. F., Schmidt A. M., and Ramasamy R. (2010) RAGE modulates hypoxia/reoxygenation injury in adult murine cardiomyocytes via JNK and GSK-3β signaling pathways. PLoS One 5, e10092 10.1371/journal.pone.0010092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Luo F., Shi J., Shi Q., Xu X., Xia Y., and He X. (2016) Mitogen-activated protein kinases and hypoxic/ischemic nephropathy. Cell Physiol. Biochem. 39, 1051–1067 10.1159/000447812 [DOI] [PubMed] [Google Scholar]
- 66. McCloskey C. A., Kameneva M. V., Uryash A., Gallo D. J., and Billiar T. R. (2004) Tissue hypoxia activates JNK in the liver during hemorrhagic shock. Shock 22, 380–386 10.1097/01.shk.0000140660.78744.bf [DOI] [PubMed] [Google Scholar]
- 67. Kunz M., Ibrahim S., Koczan D., Thiesen H. J., Köhler H. J., Acker T., Plate K. H., Ludwig S., Rapp U. R., Bröcker E. B., van Muijen G. N., Flory E., and Gross G. (2001) Activation of c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) is critical for hypoxia-induced apoptosis of human malignant melanoma. Cell Growth Differ. 12, 137–145 [PubMed] [Google Scholar]
- 68. Le Y. J., and Corry P. M. (1999) Hypoxia-induced bFGF gene expression is mediated through the JNK signal transduction pathway. Mol. Cell. Biochem. 202, 1–8 10.1023/A:1007059806016 [DOI] [PubMed] [Google Scholar]
- 69. Tournier C. (2013) The 2 faces of JNK signaling in cancer. Genes Cancer 4, 397–400 10.1177/1947601913486349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jin N., Hatton N., Swartz D. R., Xia Xl., Harrington M. A., Larsen S. H., and Rhoades R. A. (2000) Hypoxia activates jun-N-terminal kinase, extracellular signal-regulated protein kinase, and p38 kinase in pulmonary arteries. Am. J. Respir. Cell Mol. Biol. 23, 593–601 10.1165/ajrcmb.23.5.3921 [DOI] [PubMed] [Google Scholar]
- 71. Pacher P., Nagayama T., Mukhopadhyay P., Bátkai S., and Kass D. A. (2008) Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 3, 1422–1434 10.1038/nprot.2008.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.