Abstract
Tissue remodeling is a crucial process in animal development and disease progression. Coordinately controlled by the two main insect hormones, juvenile hormone (JH) and 20-hydroxyecdysone (20E), tissues are remodeled context-specifically during insect metamorphosis. We previously discovered that two matrix metalloproteinases (Mmps) cooperatively induce fat body cell dissociation in Drosophila. However, the molecular events involved in this Mmp-mediated dissociation are unclear. Here we report that JH and 20E coordinately and precisely control the developmental timing of Mmp–induced fat body cell dissociation. We found that during the larval–prepupal transition, the anti-metamorphic factor Kr-h1 transduces JH signaling, which directly inhibited Mmp expression and activated expression of tissue inhibitor of metalloproteinases (timp) and thereby suppressed Mmp–induced fat body cell dissociation. We also noted that upon a decline in the JH titer, a prepupal peak of 20E suppresses Mmp–induced fat body cell dissociation through the 20E primary-response genes, E75 and Blimp-1, which inhibited expression of the nuclear receptor and competence factor βftz-F1. Moreover, upon a decline in the 20E titer, βftz-F1 expression was induced by the 20E early–late response gene DHR3, and then βftz-F1 directly activated Mmp expression and inhibited timp expression, causing Mmp–induced fat body cell dissociation during 6–12 h after puparium formation. In conclusion, coordinated signaling via JH and 20E finely tunes the developmental timing of Mmp–induced fat body cell dissociation. Our findings shed critical light on hormonal regulation of insect metamorphosis.
Keywords: development, juvenile hormone (JH), matrix metalloproteinase (Mmp), signal transduction, steroid hormone, 20-hydroxyecdysone, cell dissociation, fat body
Introduction
Tissue remodeling plays crucial roles during animal development and disease progression. The fat body in the fruit fly, Drosophila melanogaster, which is analogous to the vertebrate liver and adipose tissue, has emerged as an excellent model to study tissue remodeling (1, 2). The larval fat body is a single-cell layer and consists of only one cell type. During the prepupal–pupal transition, the larval fat body begins to lose its polygonal shape and becomes spherical, ∼6 h after puparium formation (APF)3 and completely dissociates into single and unattached cells during pupation (∼12 h APF) (1–3). Notably, both matrix metalloproteinases (Mmp1 and Mmp2) are required to induce fat body cell dissociation in Drosophila (2, 3). We previously showed that Mmp1 preferentially cleaves Drosphila epithelial-cadherin–mediated cell–cell junctions, whereas Mmp2 preferentially degrades basement membrane (BM) components and thus destroys cell–BM junctions, resulting in the complete dissociation of fat body tissues into individual cells (2).
MMPs are extracellular Zn2+-dependent endopeptidases that are responsible for degrading cell–cell junctions, cell–BM junctions, and BM components. In vivo, Mmp activities are finely tuned at different levels, from gene expression to zymogen activation and endogenous inhibition. In terms of endogenous inhibition, Mmps are typically regulated by TIMP (tissue inhibitor of metalloproteinases) (4). Although vertebrates contain more than 20 Mmps and 4 TIMPs, Drosophila possesses only two Mmps and a single timp (5, 6). In addition, although Mmps induce fat body cell dissociation, timp overexpression suppresses fat body cell dissociation in Drosophila (2).
Developmental transitions in Drosophila are regulated by two major insect hormones: the steroid 20-hydroxyecdysone (20E) and the sesquiterpenoid juvenile hormone (JH). Two pulses of 20E, in conjunction with the nuclear receptor complex consisting of the ecdysone receptor (EcR) and ultraspiracle, were shown to induce larval–prepupal–pupal metamorphosis (7). Specifically, during the larval–prepupal transition, 20E induces the expression of several 20E primary-response genes, including E74 (Ecdysone-induced protein 74), E75, E93, Br-C (Broad complex), and Blimp-1 (B lymphocyte-induced maturation protein 1) (8). The 20E early–late response gene DHR3 controls the termination of the 20E signal pulse during the larval–prepupal transition; DHR3 also induces the expression of βftz-F1, which acts as a competence factor for EcR-ultraspiracle to respond to the subsequent 20E signal pulse during the prepupal–pupal transition. Importantly, E75 prevents DHR3-mediated inhibition of 20E signaling and DHR3-induced βftz-F1 expression through physical interaction and competition for retinoic acid receptor–related response elements (9–13). Moreover, Blimp-1 acts as a transcriptional repressor to restrict βftz-F1 expression (14). Therefore, the 20E-induced transcriptional cascade, including E75, Blimp-1, DHR3, and βftz-F1, governs the two 20E signal pulses during Drosophila metamorphosis (8, 14). The two 20E pulses are likely involved in the regulation of fat body cell dissociation in Drosophila: blockade of the 20E receptor prevents fat body cell dissociation (15), whereas βftz-F1 overexpression induces Mmp2 expression and premature fat body cell dissociation (3). However, detailed studies are required to clarify the precise molecular mechanisms by which the two 20E pulses regulate fat body cell dissociation.
JH prevents 20E-induced metamorphosis via the JH receptor methoprene-tolerant (Met, a bHLH-PAS transcription factor) and the JH primary-response gene Kr-h1 (Krüppel homolog 1, encoding a zinc finger transcription factor) (16, 17). There are two JH receptors in Drosophila: Met and its paralog gce (germ-cell expressed) (18–21). In the presence of JH, Met/gce binds to a JH response region (JHRR) in the Kr-h1 promoter and directly induces Kr-h1 expression (22–24). Notably, Kr-h1 directly represses the expression of two crucial 20E primary-response genes: Br-C and E93 (22, 25, 26). Moreover, Kr-h1–binding sites (KBSs) were identified in the promoter regions of Br-C and E93 in the silkworm, Bombyx mori, with a consensus sequence TGACCTNNNNYAAC (27, 28). Kr-h1–mediated inhibition of the expression of 20E response genes at least partially accounts for the cross-talk between JH and 20E; thus, Kr-h1 is considered as an anti-metamorphic factor in insects (16, 17). Interestingly, we observed precocious fat body cell dissociation in both JH-deficient animals and Met gce double mutant animals (19, 29). However, nothing is known about whether and how Kr-h1 mediates JH signals to inhibit fat body cell dissociation in Drosophila.
During insect metamorphosis, a series of cellular events, including programmed cell death, cell proliferation, cell differentiation, and cell dissociation, occurs in a context-specific manner. Previous investigations indicate all of the events are coordinately controlled by JH and 20E (7, 16, 17, 27). However, our current understanding of how the same two hormones induce different cellular processes at distinct, yet precise, developmental timing is very limited. Here, we discovered that JH and 20E coordinately and precisely control the developmental timing of Mmp–induced fat body cell dissociation in Drosophila at both the mRNA and enzymatic levels. This study provides an example to better understand hormonal regulation of tissue remodeling during insect metamorphosis.
Results
Kr-h1 transduces JH signaling to repress Mmp expression
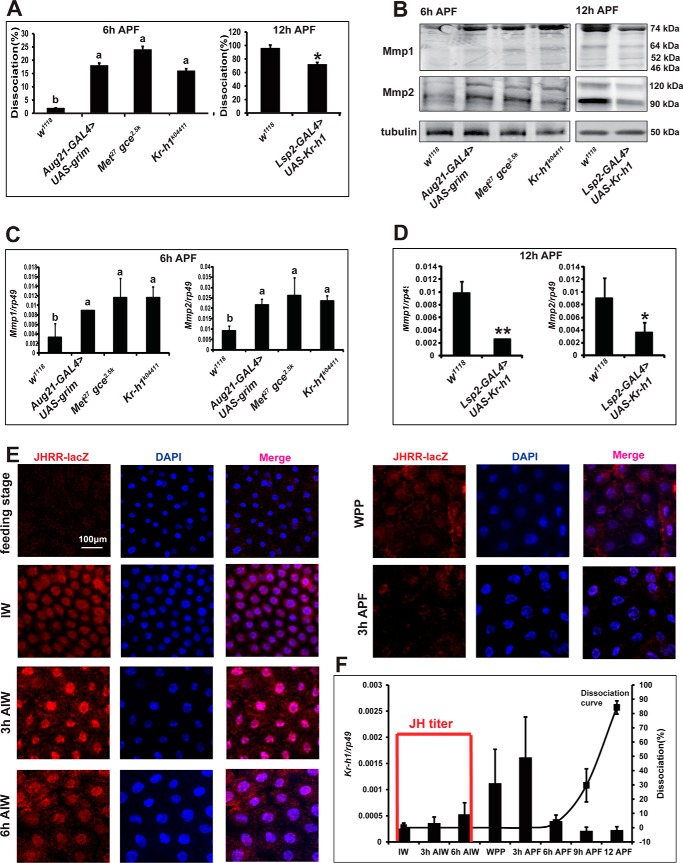
The developmental timing of fat body cell dissociation is strictly controlled in Drosophila and occurs only within a small window immediately before pupation, from 6 h APF to 12 h APF (1, 2). Consistent with our previous reports (19, 29), enhanced fat body cell dissociation was observed in both JH-deficient animals (Aug21-Gal4>UAS-grim) and Met gce double mutant (Met27gce2.5k) at 6 h APF compared with wild-type animals (w1118) (Fig. 1A and Fig. S1). Likewise, the Kr-h1 mutant (Kr-h1k04411) showed enhanced fat body cell dissociation at 6 h APF. By contrast, fat body cell dissociation was inhibited at 12 h APF when Kr-h1 was specifically overexpressed in the fat body (Lsp2-Gal4>UAS-Kr-h1) (Fig. 1A and Fig. S1).
Figure 1.
Kr-h1 transduces JH signaling to repress Mmp expression. A, compared with w1118 animals, fat body cell dissociation in JH signaling-deficient animals at 6 h APF (left) and Kr-h1–overexpressing animals at 12 h APF (right). B, compared with w1118 animals, Mmps protein levels in the fat body of JH signaling-deficient animals at 6 h APF (left) and in the fat body of Kr-h1–overexpressing animals at 12 h APF (right). C and D, compared with w1118 animals, Mmps mRNA levels in the fat body of JH signaling-deficient animals at 6 h APF (C) and in the fat body of Kr-h1–overexpressing animals at 12 h APF (D). E and F, developmental profiles of JHRR-LacZ (E), JH levels, Kr-h1 mRNA levels in fat body, and fat body cell dissociation (F) of w1118 animals at 3-h intervals. In F, the JH titer is depicted according to Dubrovsky 2005 (30); the columns show mRNA level of Kr-h1, and the curve shows the degree of fat body dissociation.
According to our previous study, Mmp1 and Mmp2 cooperatively induce Drosophila fat body cell dissociation, each assuming a distinct role (2). Therefore, we examined whether JH signaling prevents fat body cell dissociation by regulating Mmp expression. We performed a Western blot analysis (2), and detected increased protein levels for both Mmps at 6 h APF in the fat body of animals lacking JH signaling, including Aug21-Gal4 > UAS-grim, Met27gce2.5k, and Kr-h1k04411, whereas protein levels decreased at 12 h APF in the fat body of the Kr-h1–overexpressing animals, Lsp2-GAL4 > UAS-Kr-h1 (Fig. 1B). Using quantitative real-time PCR (qPCR), we also detected up-regulated mRNA levels for both Mmps at 6 h APF in the fat body of animals lacking JH signaling and down-regulated mRNA levels at 12 h APF in the fat body of the Kr-h1–overexpressing animals (Fig. 1, C and D).
After examining the role of Kr-h1 in regulating Mmp expression and fat body cell dissociation, we determined its developmental profile in the fat body. We previously reported that the JHRR-LacZ reporter recapitulates the responsiveness of Kr-h1 to JH and Met/gce (24). Immunohistochemistry indicated that JHRR-LacZ was non-detectable in the fat body of the feeding larvae, emerged upon the initiation of wandering (IW), peaked at 3 h after IW (AIW), gradually decreased from 6 h AIW to the white prepupal stage (WPP), and became barely detectable at 3 h APF (Fig. 1E). These results were consistent with previous reports describing developmental profiles for JH titers (21, 30). Interestingly, we found that the developmental profile of Kr-h1 expression was several hours delayed when compared with that of JHRR-LacZ. Kr-h1 mRNA levels in the fat body of w1118 animals gradually increased upon IW to 3 h APF but dramatically decreased thereafter (Fig. 1F). The developmental profile for Kr-h1 mRNA expression corroborates previous findings (22, 31), indicating that it should be activated by both JH and 20E with overlapping effects. The detailed molecular mechanisms by which JH and 20E coordinately induce Kr-h1 expression warrant further investigation. Nevertheless, the experimental data show that the anti-metamorphic factor Kr-h1 transduces JH signaling to repress Mmp expression and prevent fat body cell dissociation during the larval–prepupal transition in Drosophila.
Identification of KBS in Mmp1 promoter
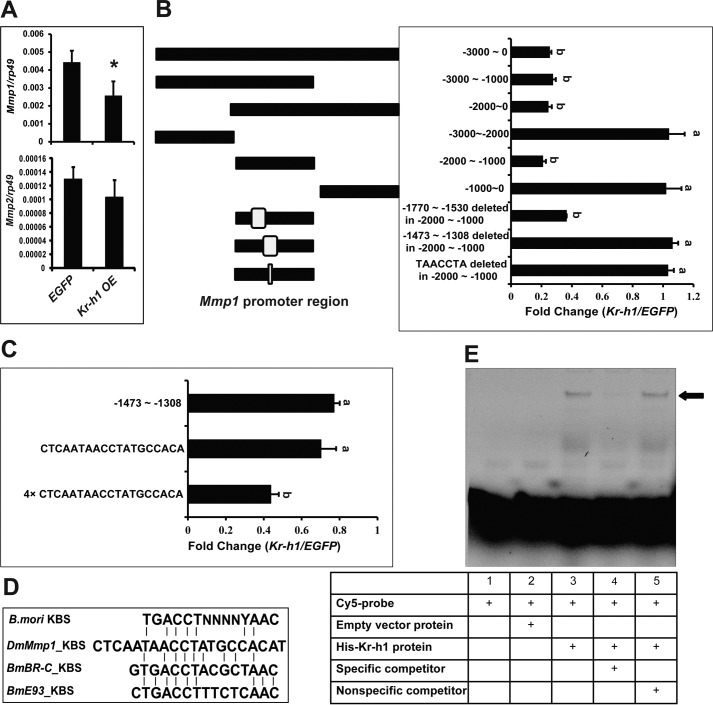
We next examined whether Kr-h1 overexpression in Drosophila Kc cells represses Mmp expression to the same extent as that in the fat body. Although Mmp2 expression was not significantly repressed, the down-regulation of Mmp1 expression was significant (Fig. 2A). Thus, we focused on investigating how Kr-h1 represses Mmp1 expression in Kc cells.
Figure 2.
Identification of a KBS in Mmp1 promoter. A, Mmps mRNA levels in EGFP-overexpressing (as a control) and Kr-h1–overexpressing Drosophila Kc cells. B and C, dual luciferase assay. Kc cells were co-transfected with a Kr-h1 expression construct (EGFP was used as a control) and pGL3-basic plasmids containing different lengths of the Mmp1 promoter region or the −2000 to −1000 fragment with specific deletions (B). From the localization in B, a sequence was identified in a fragment −1473 to −1308 and a specific sequence CTCAATAACCTATGCCACAT (KBS) in one or four copies (C). Dual luciferase assays were performed at 48 h post-transfection. The luciferase activity fold change was defined as the relative luciferase activity induced by Kr-h1 overexpression compared with EGFP overexpression. D, alignment of KBS in promoters of silkworm Br-C and E93 and Drosophila Mmp1. E, EMSA. His-Kr-h1 protein was purified from E. coli and incubated with a three times repeated KBS probe labeled with Cy5 for 2 h. Competition assays were performed using 100-fold molar excess of unlabeled specific or nonspecific probes.
To identify the Kr-h1 response region in the Mmp1 promoter, we employed a dual luciferase assay system and Kc cells. A 3-kb promoter region of Mmp1 (−3000 to 0 upstream of the transcriptional start site) was cloned into the pGL3-Basic vector. Upon Kr-h1 overexpression, this promoter region exhibited ∼25% of the luciferase activity achieved with EGFP overexpression (Fig. 2B). Five truncated regions were also cloned into the pGL3-Basic vector, and Kr-h1 overexpression inhibited three truncated regions (−3000 to −1000, −2000 to 0, and −2000 to −1000) the same degree as the 3-kb promoter region (Fig. 2B). Considering some preliminary ChIP and qPCR results, we deleted two small fragments (−1770 to −1530 and −1473 to −1308) from the −2000 to −1000 region in the pGL3-Basic vector. Although the former construct still exhibited similar inhibitory responses, the latter showed no response to Kr-h1 overexpression (Fig. 2B). The −1473 to −1308 fragment was then cloned into the pGL3-promoter vector, which responded to Kr-h1 inhibition (Fig. 2C).
KBSs were previously identified in the promoter regions of Br-C and E93 in the silkworm (27, 28), and we searched to determine whether the consensus sequence TGACCTNNNNYAAC was also conserved in the −1473 to −1308 region of the Drosophila Mmp1 promoter. We found a sequence, CTCAATAACCTATGCCACAT (−1407 to −1427), that was similar to the consensus sequence above (Fig. 2D). After the core consensus sequence TAACCTA was deleted from the −2000 to −1000 fragment-containing pGL3-Basic vector, the promoter region did not respond to Kr-h1 inhibition any longer (Fig. 2B). Then either one copy or four copies of CTCAATAACCTATGCCACAT were individually cloned into the pGL3-promoter vector. One copy of the sequence produced an inhibitory response similar to the −1473 to −1308 fragment, whereas four copies of the sequence resulted in luciferase activity that was reduced by half compared with that with EGFP overexpression (Fig. 2C), suggesting the sequence CTCAATAACCTATGCCACAT in the Mmp1 promoter is a KBS.
To validate this hypothesis, we performed EMSA with three tandem repeats of CTCAATAACCTATGCCACAT and His tag–purified Kr-h1 protein. Importantly, Kr-h1 and CTCAATAACCTATGCCACAT formed a protein–DNA complex. In competition assays, the specific band disappeared upon the addition of 100-fold molar excess of an unlabeled probe but not a nonspecific competitor (Fig. 2E). Thus, our experimental data identified a true KBS in the Mmp1 promoter. In conclusion, Kr-h1 transduces JH signaling to repress fat body cell dissociation through the direct inhibition of Mmp1 expression during the larval–prepupal transition.
EcR and βftz-F1 are required for Mmp expression
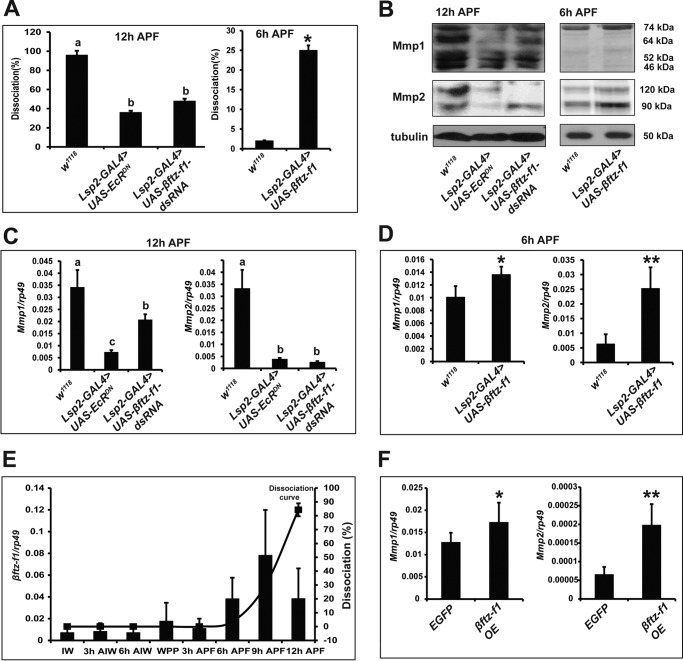
To better understand how the two 20E pulses regulate fat body cell dissociation during Drosophila metamorphosis, we first re-examined the roles of EcR and βftz-F1 in this process (3, 15). When a dominant-negative mutant of EcR (EcRDN) was overexpressed (Lsp2-Gal4>UAS-EcRDN) or βftz-F1 expression was reduced by RNAi (Lsp2-Gal4>UAS-βftz-F1-dsRNA), fat body cell dissociation was inhibited at 12 h APF. By contrast, when βftz-F1 was overexpressed (Lsp2-Gal4>UAS-βftz-F1), fat body cell dissociation increased 10-fold at 6 h APF (Fig. 3A and Fig. S2). According to the results of Western blotting analyses, protein levels of both Mmps decreased in EcRDN-overexpressing and βftz-F1-RNAi fat body and increased in βftz-F1-overexpressing fat body (Fig. 3B). The results of qPCR analysis revealed that mRNA levels of both Mmps were similarly regulated by EcR and βftz-F1 (Fig. 3, C and D). It is of note that the regulatory effect of EcR and βftz-F1 on Mmp2 is much stronger than Mmp1 (Fig. 3, B–D). Significantly, βftz-F1 mRNA levels in the fat body were comparatively low from IW to 3 h APF, sharply increased until 9h APF, and decreased at 12 h APF (Fig. 3E), indicating that the developmental profile of βftz-F1 mRNA is similar to but slightly ahead of that for fat body cell dissociation. Finally, we determined whether βftz-F1 overexpression in Kc cells increased Mmp expression to the same extent as that in the fat body. Again, expression of both Mmps was up-regulated, but the up-regulation of Mmp2 expression was much more significant (Fig. 3F). We next investigated whether E75, DHR3, and Blimp-1 convert signals of the first 20E pulse to βftz-F1 at the second 20E pulse to induce Mmp expression and thus Mmp–induced fat body cell dissociation before pupation.
Figure 3.
EcR and βftz-F1 are required for Mmp expression. A, fat body cell dissociation in EcRDN-overexpressing and βftz-F1 down-regulated animals at 12 h APF (left panel) and βftz-F1-overexpressing animals at 6 h APF (right panel). B, Mmps protein levels in the fat body of EcRDN-overexpressing and βftz-F1 RNAi animals at 12 h APF (left panel) and in the fat body of βftz-F1-overexpressing animals at 6 h APF (right panel). C and D, Mmps mRNA levels in the fat body of EcRDN-overexpressing and βftz-F1 down-regulated animals at 12 h APF (C) and in the fat body of βftz-F1-overexpressing animals at 6 h APF (D). E, the columns show developmental changes in the mRNA levels of βftz-F1 in fat body, and the curve shows the degree of fat body dissociation of w1118 animals at 3-h intervals. βftz-F1 mRNA levels peak at 9 h APF. F, Mmps mRNA levels in EGFP-overexpressing (as a control) and βftz-F1-overexpressing Kc cells.
E75, DHR3, and Blimp-1 elaborately regulate expression of βftz-F1 and Mmps
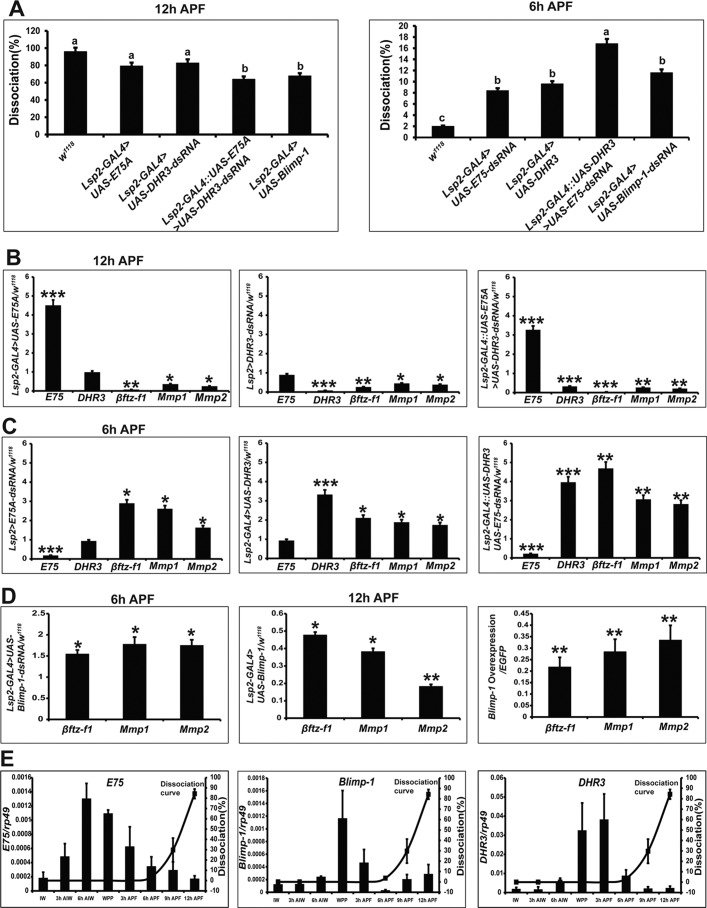
Previous reports indicate the 20E primary-response gene E75 prevents DHR3 transactivation of βftz-F1 expression (9–13). Therefore, we verified whether the 20E-triggered transcriptional cascade, involving E75, DHR3, and βftz-F1, was conserved in the regulation of fat body cell dissociation. Although fat body dissociation visually appeared to be reduced upon E75A overexpression or DHR3 depletion alone (Fig. S3), only the combination of both treatments inhibited the tissue dissociation significantly when quantified (Fig. 4A). By contrast, depletion of E75 or overexpression of DHR3 in the fat body led to increased tissue dissociation at 6 h APF, and combined manipulation of both genes had an additive effect (Fig. 4A and Fig. S3).
Figure 4.
E75, DHR3, and Blimp-1 regulate the expression of Mmps and βftz-F1. A, fat body cell dissociation in animals in which E75A was overexpressed, DHR3 expression was reduced by RNAi, combined E75A overexpression and DHR3 RNAi, and Blimp was overexpressed at 12 h APF (left panel). Fat body cell dissociation in animals in which DHR3 was overexpressed, E75 expression was reduced by RNAi, combined DHR3 overexpression and E75 RNAi, and Blimp expression was reduced by RNAi at 6 h APF (right panel). B, βftz-F1 and Mmp expression in the fat body of E75A overexpression (left panel), DHR3 RNAi (middle panel), and combined E75A overexpression and DHR3 RNAi (right panel) animals. C, βftz-F1 and Mmp expression in the fat body of E75 RNAi (left panel), DHR3 overexpression (middle panel), and combined E75-RNAi and DHR3 overexpression animals (right panel). D, βftz-F1 and Mmp expression levels in the Blimp-1 RNAi fat body (left panel); βftz-F1 and Mmp expression levels in the Blimp-1 overexpressing fat body (middle panel) and Kc cells (right panel). E, the columns show developmental changes in mRNA levels of E75 (left panel), Blimp-1 (middle panel), and DHR3 (right panel) in the fat body, and the curve shows the degree of fat body dissociation in w1118 animals at 3-h intervals.
We then investigated the role of E75 and DHR3 in regulating the expression of E75, DHR3, βftz-F1, and Mmps in the fat body. At 12 h APF, E75A overexpression had no effect on DHR3 expression but significantly repressed the expression of βftz-F1 and Mmps; DHR3 RNAi had no effect on E75 expression but significantly repressed the expression of βftz-F1 and Mmps; and simultaneous E75A overexpression and DHR3 RNAi exerted additive effects (Fig. 4B). By contrast, at 6 h APF, E75 RNAi had no effect on DHR3 expression but significantly induced the expression of βftz-F1 and Mmps, DHR3 overexpression had no effect on E75 expression but significantly induced the expression of βftz-F1 and Mmps, and simultaneous E75 RNAi and DHR3 overexpression exerted additive effects (Fig. 4C).
The 20E primary-response gene Blimp-1 acts as a transcriptional repressor for βftz-F1 (14). When Blimp-1 expression was inhibited by RNAi (Lsp2-Gal4>UAS-Blimp-1-dsRNA) or Blimp-1 was overexpressed (Lsp2-Gal4>UAS-Blimp-1), fat body cell dissociation was inhibited at 12 h APF and increased at 6 h APF, respectively (Fig. 4A and Fig. S4). Blimp-1 RNAi increased the expression of βftz-F1 and Mmps, whereas Blimp-1 overexpression had the opposite effect. In addition, Blimp-1 overexpression in Kc cells repressed the expression of βftz-F1 and Mmps (Fig. 4D).
To gain additional insights into the regulation of fat body cell dissociation by E75, DHR3, and Blimp-1, we determined developmental profiles of mRNA expression in the fat body at 3-h intervals. Expression of E75, Blimp-1, and DHR3 peaked at 6 h AIW, WPP, and 3 h APF, respectively (Fig. 4E). Thus, we assume that E75 represses DHR3 transactivation of βftz-F1 expression, and Blimp-1 directly represses βftz-F1 expression during the larval–prepupal transition; moreover, DHR3 directly induces βftz-F1 expression during the prepupal–pupal transition.
Identification of a F1-binding site (FBS) in Mmp2 promoter
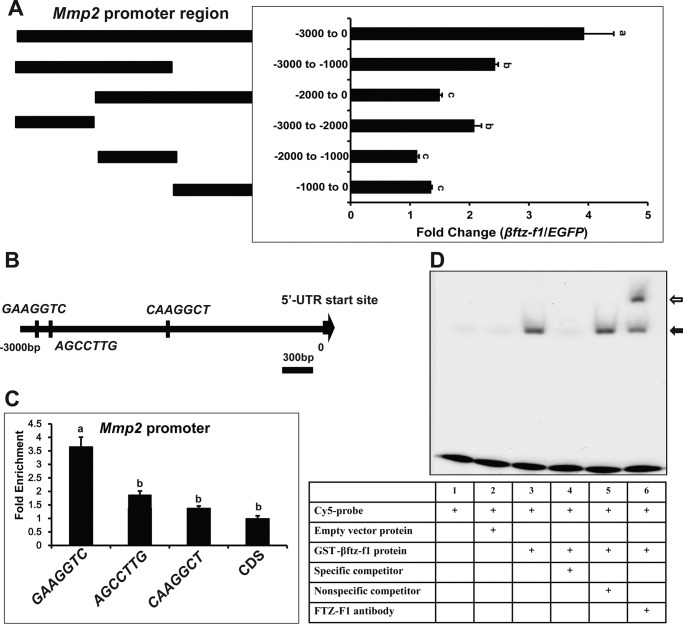
To this end, we examined whether βftz-F1 induces Mmp2 expression directly or indirectly using the dual luciferase assay system and Kc cells. A 3-kb Mmp2 promoter region was cloned into the pGL3-Basic vector. Upon βftz-F1 overexpression, this promoter region supported an ∼4-fold increase in luciferase activity (Fig. 5A). Five truncated regions (−3000 to −1000, −2000 to 0, −3000 to −2000, −2000 to −1000, and −1000 to 0) were cloned into the pGL3-Basic vector. Based on the results of dual luciferase assay, βftz-F1 response elements exist in a distal region of the Mmp2 promoter (−3000 to −2000) (Fig. 5A).
Figure 5.
Identification of a FBS in Mmp2 promoter. A, dual luciferase assay. Kc cells were co-transfected with a βftz-F1 expression construct (EGFP was used as a control), along with pGL3-basic plasmids containing Mmp2 promoter regions of different lengths. After 48 h of transfection, dual luciferase assays were performed. The luciferase activity fold change is defined as the relative luciferase activity induced by βftz-F1 overexpression compared with EGFP overexpression. B, locations of three βftz-F1 putative binding sites within the Mmp2 3-kb promoter. C, ChIP-qPCR. At 48 h after transfection, FLAG-βftz-F1-overexpressing Kc cells were fixed and subjected to ChIP using a FLAG mouse monoclonal antibody. Mock immunoprecipitations with preimmune serum were performed as negative controls. The precipitated DNA (different fragments in the 3-kb promoter region of Mmp2) and input were analyzed by qPCR to detect binding ability. D, EMSA. We used a three times repeated TGGGGGAAGGTCAAAT sequence (KBS), corresponding to a site located in the −2604 to −2595 region upstream of the Mmp2 transcription start site. A Cy5-labeled βftz-F1 binding site was added to a mixture with GST-βftz-F1 fusion proteins, in the presence (lane 4) or absence (lane 5) of an unmodified competitor. A supershift band was observed when a FTZ-F1 antibody was added into the reaction mixture.
Previous studies suggest the monomeric FBS consensus sequence is PyCAAGGPyCPu or PyGAAGGPyCPu (36). Three possible FBSs were predicted in the 3-kb promoter region of Mmp2: GGAAGGTCA (−2604 to −2595), AGGCCTTGA (−2326 to −2317), and TCAAGGCTG (−1254 to −1263) (Fig. 5B). After FLAG-βftz-F1 was overexpressed in Kc cells, ChIP-qPCR was performed to examine whether βftz-F1 directly binds to these potential FBSs to induce Mmp2 expression. As measured by qPCR, a FLAG antibody increased the precipitation of the first possible FBS but not the other two (Fig. 5C).
To verify that this sequence was a genuine FBS, we examined whether three tandem repeats of TGGGGGAAGGTCAAAT (−2607 to −2592) bound to GST-purified βftz-F1 protein in an EMSA experiment (Fig. 5D). βftz-F1 and TGGGGGAAGGTCAAAT formed a protein–DNA complex with a specific band shift that was supershifted by the addition of a βftz-F1 antibody. Competition assays showed that the specific band disappeared upon the addition of 100-fold molar excess of an unlabeled probe but not a nonspecific competitor. Thus, a genuine FBS was identified from the Mmp2 promoter.
Altogether, βftz-F1 expression is activated during the prepupal–pupal transition because of elaborate control exerted by the 20E-triggered transcriptional cascade, including E75, Blimp-1, and DHR3. When JH titer declines, the prepupal peak of 20E suppresses Mmp–induced fat body cell dissociation through the 20E primary-response genes, E75 and Blimp-1, which inhibit βftz-F1 expression indirectly or directly. Until 20E titer declines, βftz-F1 expression is induced by the 20E early–late response gene DHR3; then βftz-F1 directly activates Mmp expression and causes Mmp–induced fat body cell dissociation occurring from 6 h APF to 12 h APF.
Kr-h1 activates and βftz-F1 inhibits timp expression
As mentioned in our previous reports (2), overexpression of timp in the fat body (Lsp2-Gal4>UAS-timp) completely blocks its cell dissociation, resulting in pupal lethality. By contrast, precocious fat body cell dissociation was observed in the timp mutant (timp28) (Fig. S5B). The above results agree with the notion that timp inhibits the enzymatic activity of Mmps in the fat body (2). Thus, we investigated whether and how JH- and 20E-mediated regulation of timp expression and thus the enzymatic activity of Mmps.
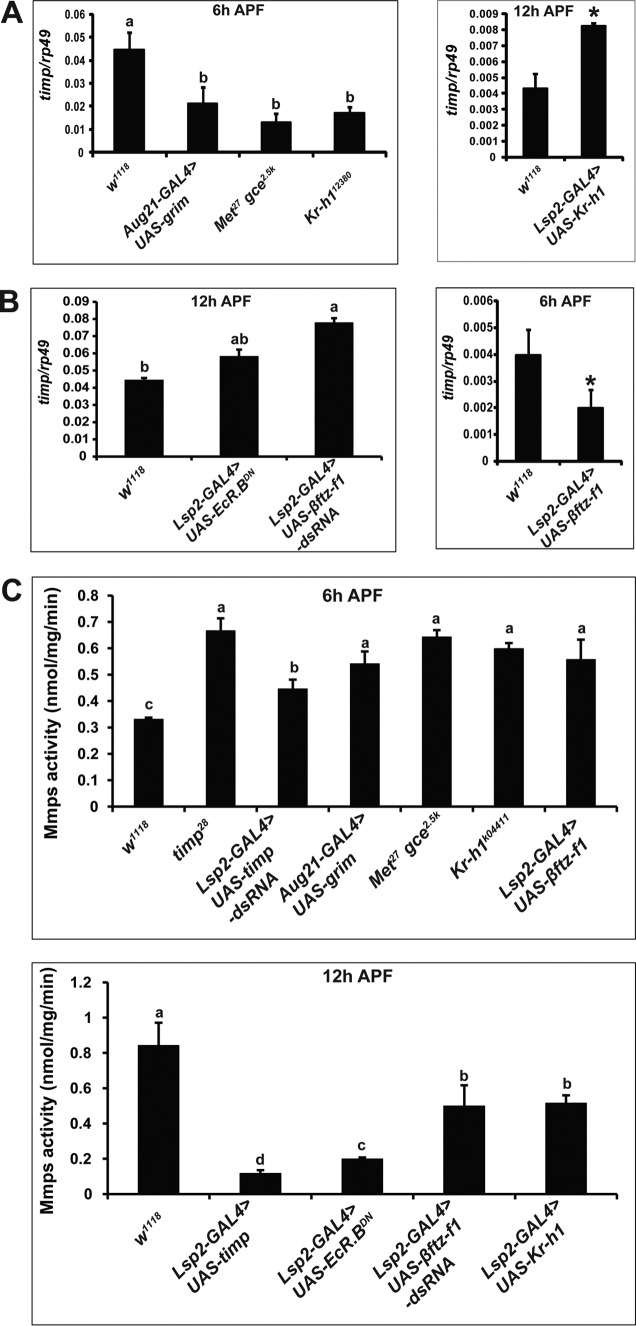
The mRNA levels of timp were down-regulated at 6 h APF in the fat body of animals lacking JH signaling and up-regulated at 12 h APF in the fat body of Kr-h1–overexpressing animals (Fig. 6A). By contrast, the mRNA levels of timp were up-regulated in EcRDN-overexpressing and ftz-F1-RNAi fat body at 12 h APF and down-regulated in βftz-F1-overexpressing fat body at 6 h APF (Fig. 6B). The experimental data indicate that Kr-h1 transduces JH signaling to activate and βftz-F1 mediates 20E signaling to inhibit timp expression, which could inhibit the enzymatic activity of Mmps and thus fat body cell dissociation.
Figure 6.
JH signaling promotes timp expression, and βftz-F1 inhibits timp expression. A, timp expression in the fat body of the JH signaling-deficient animals at 6 h APF (left panel) and in the fat body of Kr-h1–overexpressing animals at 12 h APF (right panel). B, timp expression in the fat body of EcRDN-overexpressing and βftz-F1 RNAi animals at 12 h APF (left panel) and in the fat body of βftz-F1-overexpressing animals at 6 h APF (right panel). C, Mmps enzymatic activity in the fat body of JH signaling-deficient animals and βftz-F1-overexpressing animals at 6 h APF (upper panel) as well as in the fat body of EcRDN-overexpressing, βftz-F1 RNAi, and Kr-h1–overexpressing animals (lower panel).
Finally, we examined the enzymatic activity of Mmps in the above genotypes. Mmps activity significantly increased at 6 h APF in the fat body of the timp mutant, animals lacking JH signaling, and βftz-F1-overexpressing animals and decreased at 12 h APF in the fat body of timp-overexpressing, EcRDN-overexpressing, βftz-F1-RNAi, and Kr-h1–overexpressing animals (Fig. 6C). Thus, the enzymatic activity of Mmps for each genotype results from changes in the expression of both Mmps and timp. In summary, JH and 20E coordinately and precisely control Mmps activity at both mRNA and enzymatic levels so that fat body cell dissociation occurs within 6 h before pupation (Fig. 7).
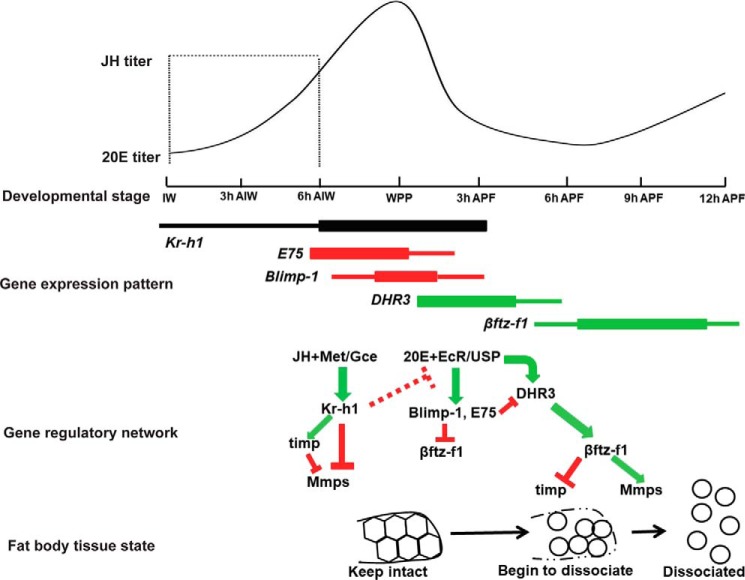
Figure 7.
Model showing developmental timing of Mmp–induced fat body cell dissociation is coordinately and precisely controlled by JH and 20E in Drosophila. During larval–prepupal transition, the anti-metamorphic factor Kr-h1 transduces JH signaling to directly inhibit Mmp expression and to activate timp expression and thus suppresses Mmp–induced fat body cell dissociation. When JH titer declines, the prepupal peak of 20E suppresses Mmp–induced fat body cell dissociation through the 20E primary-response genes, E75 and Blimp-1, which inhibit βftz-F1 expression indirectly or directly. Until 20E titer declines, βftz-F1 expression is induced by the 20E early–late response gene DHR3; then βftz-F1 directly activates Mmp expression and inhibits timp expression and causes Mmp–induced fat body cell dissociation occurring from 6 h APF to 12 h APF. The JH and 20E titers are depicted according to Dubrovsky 2005 (30).
Discussion
MMPs and TIMPs play crucial roles in regulating tissue remodeling in both vertebrates and Drosophila (4–6). Our previous work has demonstrated the collaborative functions of Mmp1 and Mmp2 in inducing fat body cell dissociation in Drosophila (2). timp mutant adults show autolyzed tissue in the abdominal cavity and inflated wings, a phenotype consistent with the role of timp in BM integrity and remodeling (33). The current study clarified the role of timp in inhibiting the enzymatic activity of Mmps and thus, Mmp–induced fat body cell dissociation (Fig. 6 and Fig. S4). In mammals, Mmps activity in vivo is controlled at different levels, including the regulation by gene expression, the zymogens activation, and the inhibition of active enzymes by TIMPs (33, 34). These studies unify the important inhibitory roles of timp/TIMP in regulating tissue remodeling in both Drosophila and mammals. In addition to regulating Mmp expression (Figs. 1–5), JH and 20E signals differentially regulate timp expression, with the stimulatory role of Kr-h1 and the inhibitory role of βftz-F1 (Fig. 6). Because timp inhibits the enzymatic activity of Mmps in the Drosophila fat body (Fig. 6), we conclude that JH and 20E coordinately control Mmps activity at both the mRNA and enzymatic levels (Fig. 7).
We previously reported the requirement of both JH and its receptors to inhibit fat body cell dissociation in Drosophila (19, 29). Here, we demonstrated the ability of Kr-h1 to transduce JH signaling to decrease Mmp expression and to induce timp expression during larval–prepupal transition (Figs. 1 and 6). Moreover, a KBS was identified in the Mmp1 promoter, indicating that Kr-h1 directly represses Mmp1 expression (Fig. 2). Interestingly, Kr-h1 expression gradually increases from IW to 3 h APF when induced by JH and 20E in an overlapping manner (Fig. 1F), thus inhibiting the enzymatic activity of Mmps and Mmp–induced fat body cell dissociation during the larval–prepupal transition. Moreover, Kr-h1 acts as an anti-metamorphic factor by inhibiting 20E signaling (16, 17). We propose, in addition to directly affecting the expression of Mmps and timp, that Kr-h1 might also indirectly regulate their expression by inhibiting 20E signaling (Fig. 7).
Two consecutive 20E pulses control timely metamorphosis in Drosophila (8). Together with previous findings (3, 15), our results show that the conserved 20E transcriptional cascade precisely controls the timing of Mmp–induced fat body cell dissociation (Fig. 7). In general, the first 20E signal pulse plays an inhibitory role during the larval–prepupal transition; however, it is a prerequisite for the expression of βftz-F1, which induces the second 20E signal pulse during the prepupal–pupal transition and the expression of Mmps. Because of the requirement for the first 20E signal pulse, blockade of the 20E receptor prevents fat body cell dissociation (Fig. 3). When JH titer declines, the prepupal peak of 20E activates expression of two 20E primary-response genes, E75 and Blimp-1, to inhibit fat body cell dissociation: E75 represses DHR3 transactivation of βftz-F1 expression, and Blimp-1 directly represses βftz-F1 expression. During the prepupal–pupal transition, DHR3 directly induces βftz-F1 expression from 6 h APF to 12 APF (Fig. 4). Before pupation, βftz-F1 induces Mmp expression and represses timp expression (Figs. 3 and 6). Moreover, an FBS was identified in the Mmp2 promoter, demonstrating that βftz-F1 directly induces Mmp2 expression (Fig. 5). Finally, within 6 h before pupation, Mmp1 and Mmp2 cooperatively induce fat body cell dissociation, with each assuming a distinct role (2).
Insect metamorphosis is coordinately controlled by JH and 20E, whereas the hormonal control of tissue remodeling is strictly context-specific. Different larval tissues and adult organs might have distinct, yet precise, developmental fates and timing (7, 8, 16, 17). Our knowledge regarding this question is poor. Based on previous preliminary information, we clarified the detailed molecular mechanisms by which JH and 20E precisely control the developmental timing of Mmp–induced fat body cell dissociation at both mRNA and enzymatic levels in Drosophila, and we provided a working model of hormonal control of tissue remodeling in animals (Fig. 7). In summary, at first, Kr-h1 transduces JH signaling to inhibit Mmp–induced fat body cell dissociation during larval–prepupal transition. Then when JH titer declines, the prepupal peak of 20E suppresses Mmp–induced fat body cell dissociation through E75 and Blimp-1, which inhibit βftz-F1 expression. Finally, until 20E titer declines, DHR3 induces βftz-F1 expression, and βftz-F1 covers the 20E-triggered transcriptional cascade to activate Mmp–induced fat body cell dissociation within 6 h before pupation. This study provides an excellent sample for better understanding the hormonal regulation of insect metamorphosis.
Experimental procedures
Flies and genetics
All fly strains were grown at 25 °C on standard cornmeal/molasses/agar medium. Synchronization was performed at IW or WPP as previously described (35). UAS-Kr-h1-V5 and UAS-Blimp-1 transgenic flies were produced via P-element–mediated germ-line transformation (29). w1118, Aug21-GAL4, Lsp2-GAL4, UAS-grim, UAS-EcRDN, Met27 gce2.5k, and Kr-h1k04411 were reported previously (2, 19, 21, 23, 24, 29, 35). Notably, Lsp2-Gal4 is specifically expressed in the fat body during larval–pupal metamorphosis (15). UAS-ftz-f1-RNAi (v2959), UAS-Blimp-1-dsRNA (v108374), and UAS-timp-dsRNA (v109427) flies were obtained from the Vienna Drosophila RNAi Center. The UAS-E75A, UAS-E75-dsRNA, UAS-DHR3, and UAS-DHR3-dsRNA flies were generously provided by Dr. Jiong Chen (36). The UAS-βftz-F1 fly was presented by Dr. Rosa Barrio (37). The timp mutant flies was presented by Dr. Buchner (38). All flies were crossed with the wild-type w1118 eight times to minimize the effects of the genetic background. Other flies used in this paper were generated by recombination.
Quantitative measurements of fat body cell dissociation
The degree of fat body cell dissociation was measured as previously described in detail (2). Fat body tissues at 6 h APF from different genotypes were used to evaluate whether premature fat body dissociation happened. Fat body tissues at 12 h APF from different genotypes were used to evaluate whether delayed fat body cell dissociation happened. In this assay, 10 animals were used for each independent genotype, and three independent replications were carried out.
Cell culture and transient transfection
Drosophila Kc cells were cultured in Schneider's Drosophila medium (Sigma–Aldrich) supplemented with 5% fetal bovine serum (HyClone). The pActin-GAL4 plasmid was constructed in our laboratory. Kr-h1 and Blimp-1 cDNA was cloned into the pUAST vector to obtain pUAST-Kr-h1-V5 and pUAST-Blimp-1, respectively. The pUAST-3×flag-βftz-F1 (37) construct was generously provided by Dr. Rosa Barrio. Transient transfection in Kc cells was performed as previously described in detail (2, 24).
qPCR and Western blotting
qPCR and Western blotting were performed as described previously (2). Rp49 was chosen as the reference gene for qPCR analysis. Our previous studies showed that Western blotting detected four major bands (46, 52, 64, and 74 kDa) and two major bands (90 and 120 kDa) for Mmp1 and Mmp2, respectively, in the Drosophila fat body (2).
Dual luciferase assay
To identify KBS, a 3-kb region of the Mmp1 promoter upstream of the transcription start site was cloned into the SmaI and BglII sites of the pGL3-Basic vector. Likewise, to identify βftz–FBS, a 3-kb region of the Mmp2 promoter upstream of the transcription start site was cloned into the SmaI and BglII sites of the pGL3-Basic vector. Deletions and mutations in Mmp1 promoter regions were also constructed in the pGL3-Basic vector. The −1473 to −1308 fragment, the KBS CTCAATAACCTATGCCACAT and 4×CTCAATAACCTATGCCACAT sequence were cloned into the SmaI and BglII sites of the pGL3-promoter vector. After transient transfection into Kc cells with the pGL3 reporter vector and the pRL reference vector, dual luciferase assays were performed as previously described (13, 24, 39).
Electrophoretic mobility shift assay
Kr-h1 cDNA was inserted into a pET28a vector with a 6×His tag at the N terminus. Then the plasmid was transformed into the Escherichia coli Rosetta strain. The transformed bacterial cells were grown in LB medium supplemented with kanamycin (0.1 mg/ml) at 37 °C and induced by 0.25 mm isopropyl β-d-thiogalactopyranoside for 12 h at 16 °C. The cells were harvested and resuspended in buffer A (20 m Tris-HCl (H8.0) and 100 m NaCl) supplemented with 1 mm PMSF. Cells were lysed using a high-pressure cell disruptor at 18,000 p.s.i., and the lysate was centrifuged at 16,000 × g for 45 min. The supernatant was loaded onto a Ni2+-nitrilotriacetic acid affinity column (Qiagen) and washed with buffer A plus 20 mm imidazole. Proteins were eluted with buffer A plus 250 mm imidazole and purified further by gel filtration using a Superdex 200 column (GE Healthcare) in buffer A. Peak fractions were collected and concentrated for subsequent studies (40). βftz-F1 cDNA was cloned into the pGEX-4T-1 vector, and GST-βftz-F1 proteins were expressed and purified in Rosetta cells using standard methods as previously described (32). EMSA was performed according to our previous publication (41).
Chromatin immunoprecipitation
At 48 h after transfection, 3× FLAG-βftz-F1-overexpressing Kc cells were fixed and subjected to ChIP using the PierceTM agarose ChIP kit (26156; Thermo) and the FLAG mouse monoclonal antibody (F3156; Sigma–Aldrich). Mock immunoprecipitations with preimmune serum were performed as negative controls. The precipitated DNA and input were analyzed by qPCR to detect binding ability (13, 39).
Statistics
Experimental data were analyzed with analysis of variance and Student's t test. Analysis of variance is shown as the bars labeled with different lowercase letters as significantly different (p < 0.05; t test: *, p < 0.05; **, p < 0.01; ***, p < 0.001). Throughout the paper, values are represented as the means ± standard deviation of 3–10 independent experiments.
Author contributions
S. Li, J. W., and Q. J. conceived the study and wrote the paper. Q. J., S. Liu, D. W., Y. C., W. G. B., and S. Li performed and analyzed the experiments. All authors reviewed the results and approved the final version of the manuscript
Supplementary Material
This work was supported by National Science Foundation of China Grants 31620103917, 31560609, 31330072, and 31572325 and National Key Research and Development Program of China Grant 2016YFD0101900 (to S. Li and D. W.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S4.
- APF
- after puparium formation
- 20E
- 20-hydroxyecdysone
- JH
- juvenile hormone
- Mmp
- matrix metalloproteinase
- TIMP
- tissue inhibitor of matrix metalloproteinase
- BM
- basement membrane
- EcR
- ecdysone receptor
- Met
- methoprene-tolerant
- JHRR
- JH response region
- KBS
- Kr-h1–binding sites
- qPCR
- quantitative real-time PCR
- IW
- initiation of wandering
- AIW
- after IW
- WPP
- white prepupal stage
- FBS
- βftz-F1-binding site.
References
- 1. Nelliot A., Bond N., and Hoshizaki D. K. (2006) Fat-body remodeling in Drosophila melanogaster. Genesis 44, 396–400 [DOI] [PubMed] [Google Scholar]
- 2. Jia Q., Liu Y., Liu H., and Li S. (2014) Mmp1 and Mmp2 cooperatively induce Drosophila fat body cell dissociation with distinct roles. Sci. Rep. 4, 7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bond N. D., Nelliot A., Bernardo M. K., Ayerh M. A., Gorski K. A., Hoshizaki D. K., and Woodard C. T. (2011) βftz-F1 and matrix metalloproteinase 2 are required for fat-body remodeling in Drosophila. Dev. Biol. 360, 286–296 [DOI] [PubMed] [Google Scholar]
- 4. Gaffney J., Solomonov I., Zehorai E., and Sagi I. (2015) Multilevel regulation of matrix metalloproteinases in tissue homeostasis indicates their molecular specificity in vivo. Matrix Biol. 44, 191–199 [DOI] [PubMed] [Google Scholar]
- 5. Page-McCaw A., Serano J., Santé J. M., and Rubin G. M. (2003) Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev. Cell 4, 95–106 [DOI] [PubMed] [Google Scholar]
- 6. Page-McCaw A., Ewald A. J., and Werb Z. (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 8, 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riddiford L. M., Cherbas P., and Truman J. W. (2000) Ecdysone receptors and their biological actions. Vitam. Horm. 60, 1–73 [DOI] [PubMed] [Google Scholar]
- 8. Yamanaka N., Rewitz K. F., and O'Connor M. B. (2013) Ecdysone control of developmental transitions: lessons from Drosophila research. Annu. Rev. Entomol. 58, 497–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lam G. T., Jiang C., and Thummel C. S. (1997) Coordination of larval and prepupal gene expression by the DHR3 orphan receptor during Drosophila metamorphosis. Development 124, 1757–1769 [DOI] [PubMed] [Google Scholar]
- 10. Lam G., Hall B. L., Bender M., and Thummel C. S. (1999) DHR3 is required for the prepupal–pupal transition and differentiation of adult structures during Drosophila metamorphosis. Dev. Biol. 212, 204–216 [DOI] [PubMed] [Google Scholar]
- 11. White K. P., Hurban P., Watanabe T., and Hogness D. S. (1997) Coordination of Drosophila metamorphosis by two ecdysone-induced nuclear receptors. Science 276, 114–117 [DOI] [PubMed] [Google Scholar]
- 12. Parvy J. P., Wang P., Garrido D., Maria A., Blais C., Poidevin M., and Montagne J. (2014) Forward and feedback regulation of cyclic steroid production in Drosophila melanogaster. Development 141, 3955–3965 [DOI] [PubMed] [Google Scholar]
- 13. Li K., Tian L., Guo Z., Guo S., Zhang J., Gu S.-H., Palli S. R., Cao Y., and Li S. (2016) 20-hydroxyecdysone (20E) primary response gene E75 isoforms mediate steroidogenesis autoregulation and regulate developmental timing in Bombyx. J. Biol. Chem. 291, 18163–18175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akagi K., Sarhan M., Sultan A. R., Nishida H., Koie A., Nakayama T., and Ueda H. (2016) A biological timer in the fat body comprising Blimp-1, βftz-F1 and Shade regulates pupation timing in Drosophila melanogaster. Development 143, 2410–2416 [DOI] [PubMed] [Google Scholar]
- 15. Cherbas L., Hu X., Zhimulev I., Belyaeva E., and Cherbas P. (2003) EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockage and rescue. Development 130, 271–284 [DOI] [PubMed] [Google Scholar]
- 16. Jindra M., Palli S. R., and Riddiford L. M. (2013) The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 58, 181–204 [DOI] [PubMed] [Google Scholar]
- 17. Jindra M., Bellés X., and Shinoda T. (2015) Molecular basis of juvenile hormone signaling. Curr. Opin. Insect Sci. 11, 39–46 [DOI] [PubMed] [Google Scholar]
- 18. Godlewski J., Wang S., and Wilson T. G. (2006) Interaction of bHLH-PAS proteins involved in juvenile hormone reception in Drosophila. Biochem. Biophys. Res. Commun. 342, 1305–1311 [DOI] [PubMed] [Google Scholar]
- 19. Abdou M. A., He Q., Wen D., Zyaan O., Wang J., Xu J., Baumann A. A., Joseph J., Wilson T. G., Li S., and Wang J. (2011) Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 41, 938–945 [DOI] [PubMed] [Google Scholar]
- 20. Jindra M., Uhlirova M., Charles J. P., Smykal V., and Hill R. J. (2015) Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet. 11, e1005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wen D., Rivera-Perez C., Abdou M., Jia Q., He Q., Liu X., Zyaan O., Xu J., Bendena W. G., Tobe S. S., Noriega F. G., Palli S. R., Wang J., and Li S. (2015) Methyl farnesoate plays a dual role in regulating Drosophila metamorphosis. PLoS Genet. 11, e1005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Minakuchi C., Zhou X., and Riddiford L. M. (2008) Kruppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech. Dev. 125, 91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang J., Tian L., Peng C., Abdou M., Wen D., Wang Y., Li S., and Wang J. (2011) DPP-mediated TGF-β signaling regulates juvenile hormone biosynthesis by upregulating expression of JH acid methyltransferase. Development 138, 2283–2291 [DOI] [PubMed] [Google Scholar]
- 24. He Q., Wen D., Jia Q., Cui C., Wang J., Palli S. R., and Li S. (2014) Heat shock protein 83 (Hsp83) facilitates methoprene-tolerant (Met) nuclear import to modulate juvenile hormone signaling. J. Biol. Chem. 289, 27874–27885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belles X., and Santos C. G. (2014) The MEKRE93 (methoprene tolerant-Kruppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect Biochem. Mol. Biol. 52, 60–68 [DOI] [PubMed] [Google Scholar]
- 26. Ureña E., Chafino S., Manjón C., Franch-Marro X., and Martín D. (2016) The occurrence of the holometabolous pupal stage requires the interaction between E93, Krüppel-homolog 1 and Broad-complex. PLoS Genet. 12, e1006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kayukawa T., Nagamine K., Ito Y., Nishita Y., Ishikawa Y., and Shinoda T. (2016) Krüppel homolog 1 inhibits insect metamorphosis via direct transcriptional repression of Broad-complex, a pupal specifier gene. J. Biol. Chem. 291, 1751–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kayukawa T., Jouraku A., Ito Y., and Shinoda T. (2017) Molecular mechanism underlying juvenile hormone-mediated repression of precocious larval-adult metamorphosis. Proc. Natl. Acad. Sci. U.S.A. 114, 1057–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Y., Sheng Z., Liu H., Wen D., He Q., Wang S., Shao W., Jiang R.-J., An S., Sun Y., Bendena W. G., Wang J., Gilbert L. I., Wilson T. G., Song Q., et al. (2009) Juvenile hormone counteracts the bHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. Development 136, 2015–2025 [DOI] [PubMed] [Google Scholar]
- 30. Dubrovsky E. B. (2005) Hormonal cross talk in insect development. Trends Endocrinol. Metab. 16, 6–11 [DOI] [PubMed] [Google Scholar]
- 31. Pecasse F., Beck Y., Ruiz C., and Richards G. (2000) Kruppel-homolog, a stage-specific modulator of the prepupal ecdysone response, is essential for Drosophila metamorphosis. Dev. Biol. 221, 53–67 [DOI] [PubMed] [Google Scholar]
- 32. Bowler T., Kosman D., Licht J. D., and Pick L. (2006) Computational identification of Ftz/Ftz-f1 downstream target genes. Dev. Biol. 299, 78–90 [DOI] [PubMed] [Google Scholar]
- 33. Pearson J. R., Zurita F., Tomás-Gallardo L., Díaz-Torres A., Díaz de la Loza M., Franze K., Martín-Bermudo M., and González-Reyes A. (2016) ECM-regulator timp is required for stem cell niche organization and cyst production in the Drosophila ovary. PLoS Genet. 12, e1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clark I. M., Swingler T. E., Sampieri C. L., and Edwards D. R. (2008) The regulation of matrix metalloproteinases and their inhibitors. Int. J. Biochem. Cell Biol. 40, 1362–1378 [DOI] [PubMed] [Google Scholar]
- 35. Liu H., Jia Q., Tettamanti G., and Li S. (2013) Balancing crosstalk between 20-hydroxyecdysone-induced autophagy and caspase activity in the fat body during Drosophila larval–prepupal transition. Insect Biochem. Mol. Biol. 43, 1068–1078 [DOI] [PubMed] [Google Scholar]
- 36. Luo J., Zuo J., Wu J., Wan P., Kang D., Xiang C., Zhu H., and Chen J. (2015) In vivo RNAi screen identifies candidate signaling genes required for collective cell migration in Drosophila ovary. Sci. China Life Sci. 58, 379–389 [DOI] [PubMed] [Google Scholar]
- 37. Talamillo A., Herboso L., Pirone L., Pérez C., González M., Sánchez J., Mayor U., Lopitz-Otsoa F., Rodriguez M. S., Sutherland J. D., and Barrio R. (2013) Scavenger receptors mediate the role of SUMO and Ftz-f1 in Drosophila steroidogenesis. PLoS Genet. 9, e1003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Godenschwege T. A., Pohar N., Buchner S., and Buchner E. (2000) Inflated wings, tissue autolysis and early death in tissue inhibitor of metalloproteinases mutants of Drosophila. Eur. J. Cell Biol. 79, 495–501 [DOI] [PubMed] [Google Scholar]
- 39. Liu X., Dai F., Guo E., Li K., Ma L., Tian L., Cao Y., Zhang G., Palli S. R., and Li S. (2015) 20-hydroxyecdysone (20E) primary-response gene E93 modulates 20E signaling to promote Bombyx larval–pupal metamorphosis. J. Biol. Chem. 290, 27370–27383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qi X., Lin W., Ma M., Wang C., He Y., He N., Gao J., Zhou H., Xiao Y., Wang Y., and Zhang P. (2016) Structural basis of rifampin inactivation by rifampin phosphotransferase. Proc. Natl. Acad. Sci. U.S.A. 113, 3803–3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tian L., Ma L., Guo E., Deng X., Ma S., Xia Q., Cao Y., and Li S. (2013) 20-hydroxyecdysone upregulates Atg genes to induce autophagy in the Bombyx fat body. Autophagy 9, 1172–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.