Abstract
Objective
Although the detrimental physical health effects of social isolation have been known for three decades, the answers to how and why social relationships generally improve health remain elusive. Social relationships are not always beneficial, and we examined a structural dimension that may bring about their salubrious effects: affiliative reciprocity during a stressor.
Methods
In a lifespan study, female rats lived with their sisters and were tested for temperament, affiliative reciprocity during an everyday stressor at puberty, corticosterone response to a stressor, mammary tumor development and diagnosis, and death.
Results
Rats that affiliated more reciprocally during a mild group stressor survived longer (p = .0005), having exhibited a lower corticosterone peak in response to an acute novel stressor in late adulthood (p = .0015), and longer time to the development of spontaneous mammary tumors (p = .02). These effects could not be explained solely by the number of affiliative interactions or individual temperament. Indeed, affiliative reciprocity and neophobia were independent and predicted mortality additively (p = .0002).
Conclusions
Affiliative reciprocity during a stressor, a structural quality of social interactions, protected females from early mammary tumor development (the primary pathology in Sprague-Dawley rats) and early all-cause mortality. Conversely, lack of reciprocity (whether disproportionately seeking or receiving attempted affiliation) was as potent a risk factor as neophobia. Thus a social role increased risk additively with individual temperament. Our data indicate that affiliative reciprocity functions as a buffer for everyday stressors and are likely mediated by attenuated reactivity of the hypothalamic-pituitary-adrenal axis.
Keywords: social support structure, affiliative reciprocity, corticosterone, individual differences, spontaneous mammary tumor, psychosocial risk factors
INTRODUCTION
Social isolation is deleterious for physical health (1–4) with an estimable risk of broad-based morbidity and mortality comparable to cigarette smoking (1). Yet answers to how and why this is so remain elusive (5,6). It is widely assumed that relationships facilitate good health primarily through providing social support (6). Two pathways have been proposed to explain how supportive relationships can promote better health: (1) socio-behaviorally, by promoting the adoption of a healthier lifestyle consisting of behaviors such as eating a balanced diet, exercising, and taking regular visits to the family physician; or (2) psychobiologically, via psychological mechanisms that coordinate appraisals, emotions, and a sense of control, and thereby influence biological processes (6–10). This distinction is often made by referring to the support as either instrumental or emotional, respectively (11). Supportive relationships are thought to augment physical health through psychological mechanisms by organizing the endogenous regulation of hormones, cytokines, and other chemical mediators. However, although there is strong evidence that health behaviors play a part (12), there is little direct evidence that psychological mechanisms affect mortality through these endogenous mediators (5). To address this paucity, we confine our focus to psychobiological pathways.
House et al.’s (1) seminal report on social relationships and health proposed that further theorizing would require distinguishing between: a) the existence or quantity of relationships, b) their formal structure, and c) their content. Although an abundance of research has explored the health impact of varying amounts of social support, few have examined the structural features. To our knowledge, in the nearly 30 years since Berkman and Syme (3) published the first prospective study linking social isolation with increased mortality, very little progress has been made toward understanding what features of the formal structure need to be present for social relationships to provide their health benefits.
One way to isolate the necessary structural features that make social relationships salubrious is to closely examine the quality of group interactions. There are a number of different dimensions along which social relationships can vary structurally, including, for example, reciprocity (1). If the variation in a structural dimension can predict the variation in mortality, all while the number of social partners and content of the interactions are held constant, then this is strong evidence that this feature of the formal structure is an integral part of what makes social relationships beneficial to health.
In the present report, we employ a rat model to study the structural features of social interactions. To study the social interactions most salient to support and health, we investigated social interactions in response to a mild common stressor experienced simultaneously by the whole group—relocation of the home cage. Such stressors occur at least daily over the lifespan for laboratory-housed rats (13), and thus, although relatively mild, creates a background of chronic stressors throughout the lifespan (14). Examining interactions during such a stressor also keeps the content of the interactions within a narrow range of behaviors that are expressed daily in response to common laboratory disruptions. Moreover, the number of social partners was held constant for each group to further ensure that we are isolating the effects of relationship structure. Rats are an excellent model for investigations of the connection between social relationships and health because rats exhibit complex socio-cognitive capacities, such as generalized reciprocity, previously thought only to exist in humans (15), and because they are relatively short-lived, allowing us to bring social and temperament characterizations made early in development to bear on morbidity and mortality.
We first set out to characterize the content of social behaviors enacted during the mild stressor of home cage relocation. We hypothesized that social contact under stressful conditions would not be equally initiated and received across group members, resulting in individual differences in reciprocity that may explain the variation in morbidity and mortality. The structural dimension of reciprocity has been linked to both physical and mental health outcomes. For example, in married couples in which one member develops a serious illness, targeted intervention to restore the equity of the relationship resulted in increased relationship quality and less psychological distress for the patient (16). Similarly, seriously ill patients receiving long-term care are less likely to report depression when they perceive the relationship with their primary caregiver as more reciprocal (17). In the context of the workplace, lack of reciprocity has been linked to declines in physical health. Imbalance in the amount of effort expended in relation to the rewards reaped is associated with increased risk of coronary heart disease (18). Given these findings, we expected that rats whose interactions after a stressor were reciprocal would experience better health outcomes.
Furthermore, since previous work in our laboratory has demonstrated that neophobia, a dimension of individual temperament, predicts mammary tumor incidence and mortality (19,20), we investigate whether social interactions and neo-phobia are independent risk factors for mammary tumors and death, or whether one operates solely through an effect on the other. For example, neophobic individuals might be more likely to seek the reassurance of affiliation more frequently than they are approached by their less neophobic cagemates.
Lastly, since we are primarily interested in psychobiological mechanisms, and have restricted any opportunity for individual differences in health behaviors, we examine whether the structure of social interactions early in life predicts stress-induced glucocorticoid reactivity or ovarian function late in life. Overexposure to glucocorticoids throughout the lifespan is widely thought to exacerbate declining health (21). Moreover, a history of prior exposure to repeated stressors (i.e., chronic stress) facilitates a heightened glucocorticoid response to a novel stressor (22,23). We hypothesized that reciprocal affiliative interactions with cagemates during the daily stressors they encounter in the lab over the course of the lifespan would buffer them from deleterious consequences. Thus, when presented with a novel stressor in adulthood, rats that consistently engaged in reciprocity would exhibit lower glucocorticoid levels than rats that lacked reciprocity in their interactions. Finally, because social interactions can affect ovarian function, and ovarian steroids modulate glucocorticoid levels, we assessed ovarian function in the weeks before corticosterone measurements as potentially confounding factor.
METHODS
Animals
Female Sprague-Dawley rats lived throughout their lives in solid bottom plastic cages (43.5 × 23.5 × 20.5 cm) on a 14L:10D lighting schedule (lights on at 20:00 hours) with food and water ad libitum. Rooms were maintained at 22 ± 1°C, with 13 to 15 room air changes an hour and humidity range 40% to 60%. The rats also contributed to a previous study focusing strictly on the relationships between temperament, corticosterone responses to stressors, and lifespan (20). For this reason, methodology concerning the protocol will be abbreviated with a focus on aspects relevant to the findings presented in this report. Fifty-four rats were originally in this longitudinal study, but five died before all data were collected (e.g., corticosterone measured at 15 months of age). Due to the longitudinal nature of the study, rats that died were not replaced.
Protocol Timeline
Females were bred in our laboratory and tested for neophobic or neophilic temperament at 20 days of age. After weaning at 22 days of age, they continued to live only with their sisters until 28 days of age, when we reorganized their home cages; a third remained with two of their sisters, a third were housed with two strange females, and a third were isolated. This reorganization did not affect the measures or outcome of this study and thus need not be discussed further. At 46 days of age, all resumed living with their sisters in trios, and remained together until death.
Having been reunited with their sisters for a week, each trio was exposed to home cage relocation at 53 days of age, a mild stressor that is part of standard husbandry procedures, and one of the common stressors that laboratory rats experience throughout their lives (13). Social interactions during this stressor were recorded. The home relocation stressor was repeated in early middle age (8.3 months). At 11 months of age, biweekly health checks and palpation for mammary tumors, along with daily vaginal smears. In late middle-age (15 months), during reproductive senescence, when ~89% of the rats were still disease free, the females were exposed to a 30-minute restraint stressor and repeated blood samples were collected to assess corticosterone response dynamics. Health checks continued until death (median = 22.3 months of age).
Temperament
At 20 days of age, we measured rats’ willingness to explore a complex novel environment by quantifying locomotion in a 940 cm2 square arena scattered with a few rat-sized objects. Each rat’s locomotor behavior was compared against her sisters to create three categories: the two most active sisters were identified as ‘neophilic,’ the two least active were identified as ‘neophobic,’ and those with values closest to the family mean were identified as ‘intermediate.’ In the current study, intermediates were not included in order to assess whether temperament and affiliative reciprocity are independent or overlapping constructs with respect to health measures. For details on the behavioral assay, see Ref. (20).
Each sister represented a different third of the temperament distribution. As in our previous report (20), which focused on the comparison between neophobia and neophilia to identify neophobia as a risk factor for early death, we excluded data from sisters with an intermediate temperament to maintain a “high risk versus low risk” comparative framework (yielding 54 subjects). Sisters with an intermediate temperament had lifespans that were roughly intermediate between their neophobic and neophilic sisters.
Social Interactions During a Common Stressor
Common Stressor
Home cage behavior was recorded during the first half of the dark period (11:00–15:00) after relocating the home cage to another room and setting it on a table next to a cage of unfamiliar rats, a manipulation designed to mildly stress each animal simultaneously, while not changing their normal social context. Affiliative reciprocity scores were established at 53 days of age, and their stability was quantified by retesting at 8.3 months of age.
Behavior was analyzed using the Noldus Observer (Noldus) video analysis system. An ethogram (see Table 1) was constructed to quantify the most common social behaviors after cage relocation. Coding for these social behaviors was corroborated by comparison to the existing literature on rat social behavior in both captive and naturalistic populations (24–26).
TABLE 1.
Ethogram
| Social Behavior | Definition |
|---|---|
| Touch | Places forepaws on conspecifics for less than one second |
| Holdfast | Places forepaws on conspecifics for greater than one second |
| Follow | Walks closely behind conspecific |
| Stand-by | Rears against cage wall within an arm’s length of a conspecific rearing against cage wall |
| Push-away | Uses forepaws or flank to move a conspecific from its position |
| Oral inspection | Sniffs at the side of conspecific’s mouth. Sniffing typically involves tilted head posture and short, repeated head nods |
| Anogenital inspection | Sniffs or licks anogenital region of conspecific. Sniffing typically involves tilted head posture and short, repeated head nods. Conspecific receiver may lift hindquarters |
Social behaviors consisted of four general categories, 1) affiliation received, 2) affiliation initiated, 3) tandem curiosity in what lies outside the home cage, and 4) mutual inspection. Since confirmatory factor analysis verified that initiation and reception of affiliation were factors different from each other as well as from the joint behaviors of tandem curiosity and inspection, we were able to quantify the degree to which affiliative interactions were reciprocated. Together, an oblique solution for these four conceptually related factors comprised 69.6% of the variance in overall social behavior (See Table 2).
TABLE 2.
Confirmatory Factor Analysis of Categories of Social Interactions During a Mild Stressor
| Social Behaviors | Initiate or Receive | Affiliation-Initiating | Affiliation-Receiving | Mutual Inspection | Tandem Curiosity |
|---|---|---|---|---|---|
| Touch | Initiate | 0.723 | 0.025 | −0.059 | 0.519 |
| Holdfast | Initiate | 0.866 | −0.041 | −0.018 | 0.231 |
| Follow | Initiate | 0.729 | 0.343 | 0.051 | −0.342 |
| Touch | Receive | 0.099 | 0.738 | 0.064 | 0.277 |
| Holdfast | Receive | −0.064 | 0.809 | −0.008 | 0.164 |
| Follow | Receive | 0.129 | 0.617 | 0.156 | −0.248 |
| Oral | Initiate | 0.089 | −0.118 | 0.756 | −0.032 |
| Oral | Receive | −0.253 | 0.271 | 0.678 | −0.150 |
| Anogenital | Initiate | 0.276 | −0.253 | 0.714 | 0.018 |
| Anogenital | Receive | 0.050 | 0.081 | 0.679 | 0.169 |
| Push-away | Initiate | 0.177 | 0.231 | −0.128 | 0.843 |
| Push-away | Receive | 0.104 | 0.451 | −0.146 | 0.747 |
| Stand-by | Initiate | 0.057 | −0.319 | 0.120 | 0.854 |
| Stand-by | Receive | 0.009 | 0.046 | 0.149 | 0.854 |
| Eigenvalue | 1.58 | 2.06 | 2.35 | 3.76 | |
| Percent of variance | 11.2 | 14.7 | 16.8 | 26.9 |
For affiliative interactions, initiating social contact was relatively independent of receiving it. Indeed, initiating and receiving affiliation comprised different factors (see Table 2), suggesting that asymmetry in affiliative interactions was feasible and occurred. In sharp contrast, Tandem Curiousity and Mutual Inspection are inherently joint social interactions in which initiating a behavior is more correlated with receiving that same behavior than with initiating or receiving other behaviors (see Table 2). Because affiliative interactions were the only asymmetrical social behavior manifest within the sister trios, these behaviors were used to construct a reciprocity score to quantify the primary source of social behavioral asymmetry within the group.
Affiliative Reciprocity
Affiliative reciprocity is defined here as the balance between initiated and received affiliation. For each member of the triad, the total percent of receptions was subtracted from the total percent of initiations to create an Affiliative Role Score, reflecting the overall receptiveness or initiativeness in all affiliative interactions. This score ranges theoretically from −1.0 to +1.0, with large negative and positive scores indicating a rat that is more receptive or more initiative, respectively. Scores close to zero indicate a rat that initiated and received affiliation in a relatively reciprocal manner. We quantified the reciprocity of affiliative social interactions with an Affiliative Reciprocity Index: 10 × (1 − Affiliative Role Score ). Ranging theoretically from 0 to 10, this Index operationalizes the affiliative reciprocity construct with a unitary, continuous variable. Values close to 10 reflect an even balance of social initiations and receptions, and values close to 0 reflect an uneven balance of social initiations and receptions. Individual reciprocity scores that were compared with corticosterone production, tumor onset and life span are from the 53 days measure.
Endocrine Measures
Corticosterone Response to Restraint Stressor in Middle Adulthood
We used the corticosterone response to restraint stress, a novel stressor, as a biomarker of the degree to which the daily husbandry events throughout adulthood had been experienced as stressful or potentially ameliorated by the buffering of affiliation interactions during these daily stressors. At 15 months of age, we determined whether females with high and low reciprocal affiliation, measured twice previously, had different corticosterone responses. Five of the original 54 rats in the study did not survive until this point and were excluded, yielding a total of 49 individuals that completed the study (Nneophobic & nonreciprocal = 11, Nneophobic & reciprocal = 12, Nneophilic & nonreciprocal = 13, Nneophilic & reciprocal = 13).
During the nadir of the corticosterone circadian rhythm at the end of the rats’ active period (0–3 hours after lights on), we collected repeated blood samples following a novel, 30-minute restraint stressor at 15 months of age. Samples were collected at 0, 30, 60, 90, and 150 minutes from the onset of the restraint period, according to methods described previously (20). Corticosterone was measured using a commercial radioimmunoassay kit (Rat and Mice Corticosterone kit, MP Biomedicals) as described previously (20).
Ovarian Function During Reproductive Senescence
Daily vaginal lavages and cytology analyses were initiated at 60 days of age and continued through reproductive senescence. To determine whether different rates of reproductive senescence could account for any observed differences between groups in corticosterone reactivity to a stressor, we measured ovarian function during the 3 weeks before administering the late middle-age restraint stressor according to methods described previously (20,27). The pattern of estrous cycles indicated the successive reproductive states of ovarian senescence: regular cycles, irregular cycles, constant estrus, persistent diestrus and anestrus (27). During regular cycles, prolonged irregular estrogenized cycles and constant estrus, females spend proportionally more time in an estrogenized state. In the later stages of reproductive senescence, when the ovaries become atrophic (irregular cycles and persistent diestrus/anestrus), females are significantly less estrogenized.
Tumor Growth and Mortality
The colony was allowed to undergo natural attrition as a variety of pathologies spontaneously developed. Mammary neoplasia, the most common pathology in this strain of rats, was assessed through regular health checks begun at 11 months of age. We defined onset of tumor detection as the age at which a growth was first reliably palpable and persisted for 3 weeks. Mammary tumor size averaged across all females was 77.02 g, approximately 19% of the mean body weight (i.e., 400 g).
At necropsy, tumors were excised, formalin-fixed, and paraffin-embedded. After deparaffinization in xylene, slides were rehydrated through consecutive graded alcohols to distilled water. After blocking endogenous peroxidase activity, sections were heated for antigen retrieval with citrate buffer. The sections were then incubated with the anti-Glucocorticoid Receptor primary antibody (3D5, 1:400, Abcam Inc., Cambridge, MA) for 1 hour at room temperature. The slides were then incubated with horse-radish peroxidase labeled Polymer, and the reactions were completed with the Envision detection system using 3 to 3′ diaminobenzidine as the chromogen (DakoCytomation, Carpinteria, CA).
Histological diagnosis was validated by concordance of two surgical pathologists specializing in breast cancer pathology in the Department of Pathology at the University of Chicago (28). Diagnoses included malignant cancer (e.g., in situ ductal carcinoma, invasive ductal carcinoma, and carcinosarcoma) as well as benign tumors (e.g., fibroadenoma, lactating adenoma, and papillary cystadenoma). Rats often developed a multiplicity of tumors, each with independent diagnoses; some tumors contained multiple segments, each with independent diagnoses.
Health of aging females was monitored biweekly by researchers and the institutional veterinarian, and weekly if not daily toward the end of life. Half of the rats died naturally. To preclude suffering, however, 29 of the 49 females were euthanized when they displayed symptoms indicating that they were within 1 week of death. Primary indicators were: shallowness of breath, behavioral lethargy, chromodachyrhea, drooping eyelids or partial closure of the eyes, wheezing or clicking in lungs, and urine-soaked fur (19). Secondary indicators were: nasal discharge, sticky or un-groomed fur, pale eye and skin color, and bloating (Cavigelli and McClintock, unpublished data). Decisions to euthanize based on these symptoms were made independently by a veterinarian making weekly health checks and by an observer making daily health checks. Both were blind to the animals’ temperament and style of social interaction. Approximately equal numbers of neophobic and neophilic females (N = 15, 14, respectively), and reciprocal and nonreciprocal females (N = 13, 16, respectively) were sacrificed.
Statistical Analyses
To assess the underlying structure to the social behaviors enacted in the 20 minutes during cage relocation, we analyzed each social behavior, initiated and received, using principal components factor analysis with orthotran/varimax rotation. The final factor solution was extracted by considering the eigenvalues (greater than 1.0), examining the scree plot (29), and verifying that the overall factor structure was consistent with a priori concepts (30).
To measure the effect of continuous predictors on temporal outcomes, we used Cox proportional hazards regression. We fulfilled the proportional hazards assumption—that the ratio of hazards is constant across time for levels of predictors—by comparing log-minus-log survival plots for all outcomes in which affiliative reciprocity was a significant predictor. Full versus partial models were tested with the likelihood ratio test. The nonparametric Kaplan-Meier survival analysis was used for group comparisons of temporal variables.
When appropriate, the affiliative reciprocity variable is dichotomized solely for descriptive purposes. Rats with scores above the median are referred to as “high reciprocity rats,” whereas those with scores below the median are referred to as “low reciprocity rats.” The dichotomized variable was never used for statistical purposes.
Reciprocity as a predictor of mammary tumor severity and type of reproductive senescence was assessed with ordered logistic regression. Mammary tumor severity was classified into three categories: none, benign, or malignant. The categories for reproductive senescence from most to least estrogenized were long regular cycles, constant estrus, irregular cycles, persistent diestrus and anestrus.
The amount of corticosterone produced in response to a stressor was summarized as the area under the curve with respect to ground (AUCG; cite Pruessner 2003) and the dynamics of its response with baseline (prestressor), peak (maximal response during 2 hours post-stress), and recovery (final minus baseline) levels. The effects of reciprocity on these variables were tested with simple linear regression.
All tests were two-tailed and statistical significance was achieved at p ≤ .05. Statistical trends were noted when p ≤ .10.
RESULTS
Affiliative Reciprocity
The average Affiliative Reciprocity Index score ranged from 1.67, indicating a large asymmetry between initiating and receiving affiliative contact, to 10.00, indicating a perfect balance (colony average = 6.94 ± 2.31). Each rat’s role in the balance of initiating or receiving affiliative contact with her sisters remained relatively consistent from puberty to late middle age (r[49] = 0.322, p = .02). However, we did not detect stability in the frequency of engaging in tandem curiosity and mutual inspection (tandem curiosity: r[49] = 0.155, p = .29; mutual inspection: r[49] = 0.006, p = .97), further indicating that these behaviors are not major indicators of stable social roles.
Affiliative reciprocity, a social role emergent from group interactions, might result from individual differences in neophobia, particularly during the mild stressor of home cage relocation However, females categorized as having high or low affiliative reciprocity (median split) were equally likely to have a neophobic temperament (48% versus 46% respectively; chi-square [1, N = 49] = 0.023, p = .88). Conversely, neophobic and neophilic rats did not differ in degree of affiliative reciprocity (t[47] = 0.13, p = .90). Thus, reciprocity of social contact, a style of social interaction, and infant temperament, an individual trait, are two independent psychosocial factors.
Females affiliating more reciprocally might be more socially active in general. However, the total number of social interactions in which a rat engaged was not significantly associated with her affiliative reciprocity (R2 = 0.063, F(1, 47) = 3.18; p = .08). Affiliative reciprocity might also reflect social dominance, although social dominance relationships among female rats are not typically stable (31). A correlate of social dominance, body weight, measured when social behavior was observed in puberty, was not associated with social reciprocity during a mild stressor as would be expected if engaging in reciprocal support were a product of dominance (R2 = 0.040, F(1, 47)=1.95 ; p = .17). Moreover, body weight was not associated with a rat’s role in initiating and receiving affiliation (R2 = 0.001, F(1, 47) = 0.68 ; p = .80). Finally, no fights were observed.
Mortality
Rats that did not have reciprocated affiliative contact during the brief group stressor died earlier than those whose affiliative interactions were reciprocated equally. Affiliative reciprocity predicted lifespan such that every 10% decrease in reciprocity resulted in an increased risk of death between 13% and 51% (HR = 1.31, 95% CI = 1.13–1.51; p = .0005). High reciprocity rats had a median lifespan of 25.9 months whereas that of low reciprocity rats was only 20.4 months, a difference of almost a half-year, one-third of the range in lifespan under laboratory conditions (1.5–3 years; Figure 1A). To verify that the effects of affiliative reciprocity were not mediated by the mere frequency of interactions, but rather its structure, univariate predictions were calculated for the frequency of initiating and receiving affiliative interactions, as well as total involvement in affiliative behaviors, and affiliative role. Only the frequency of receiving affiliation decreased the risk of death, albeit a weaker effect ranging from 0.002% to 5.8% for each additional affiliative behavior received (HR = 0.97, 95% CI = 0.94–0.99; p = .04). Moreover, frequency did not mediate the effect of reciprocity; when reception frequency is modeled along with affiliative reciprocity, it did not diminish reciprocity’s predictive power, as indicated by the lack of a decrease in z score going from the univariate model with reciprocity alone to a full model including both variables (Univariate: zreciprocity = 3.62; Full: zreciprocity = 3.75).
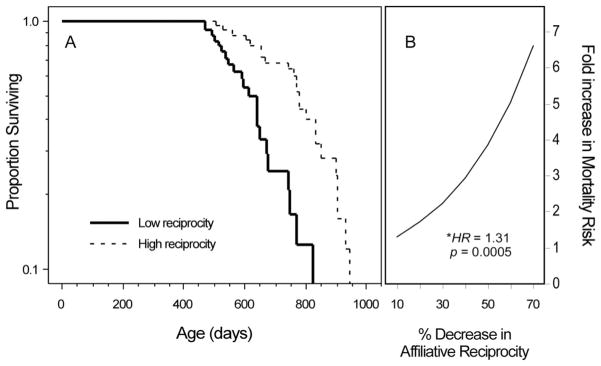
Figure 1.
Affiliative reciprocity predicts lifespan. A, Rats that affiliate more reciprocally live longer. Median survival for “High reciprocity” rats (dotted line) (upper 50th percentile of affiliative reciprocity scores) was 776 days; median survival for “Low reciprocity” rats (solid line) (lower 50th percentile) was 612 days. Affiliative reciprocity scores were dichotomized for graphical purposes only. B, Cox proportional hazards survival analysis was applied to estimate the effect of affiliative reciprocity on lifespan (HR = 1.31, 95% CI = 1.13–1.51; p = .0005).
Additive Effects of Affiliative Reciprocity and Temperament
Because affiliative reciprocity and temperament were independent traits, and neophobic temperament is a risk factor for early death (20), we sought to determine whether low affiliative reciprocity is also a risk factor for early death, acting independently of neophobic temperament. The variance in lifespan explained by affiliative reciprocity was not merely mediated by temperament as indicated by the lack of a decrease in z-score when temperament is added to the model; Reciprocity Univariate: zreciprocity = 3.62; Full: zreciprocity = 3.99).
Indeed, low affiliative reciprocity and neophobia each increased the risk of death and did so additively (multivariate model, Affiliative reciprocity: HR = 1.38, 95% CI = 1.18–1.61, p < .00003; Neophobia: HR = 2.00, 95% CI = 1.09–3.66, p = .025; Full model: Likelihood-ratio chi-square [2, N = 49] = 17.16, p = .0002). In fact, the full model including both neophobia and affiliative reciprocity explained more of the variance in lifespan than affiliative reciprocity alone (likelihood ratio test: chi-square [1, N = 49] = 4.95, p = .03). Rats exhibiting both risk factors died earliest (i.e., low reciprocity and neophobic temperament) and those with neither lived the longest (median lifespans were 19.0 and 26.8 months, respectively; Figure 2). Rats with only one risk factor had an intermediate life span (22.0 months), with no difference between those with a social or a temperament risk factor (Mantel-Cox logrank chi-square [1, n = 27] = 0.24, p = .62).
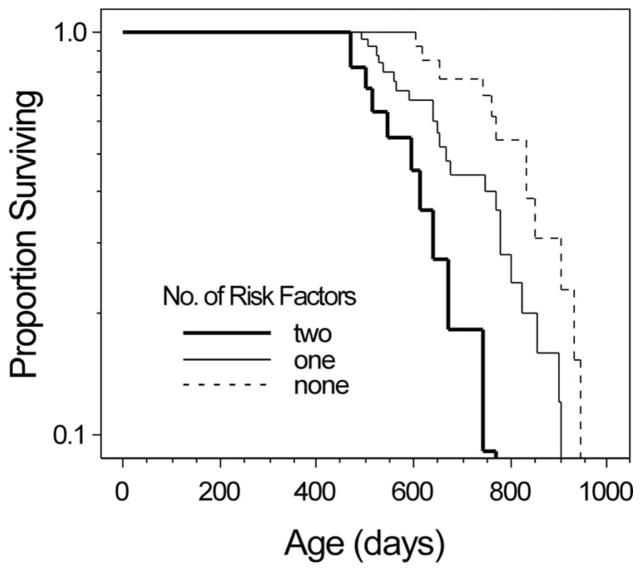
Figure 2.
Low affiliative reciprocity and neophobia additively predict shorter lifespan. Rats exhibiting the risk factors low affiliative reciprocity and neophobia die sooner. Median survival for rats exhibiting no risk factors (dotted line) was 805 days; median survival for rats exhibiting 1 risk factor (thin solid line) was 660 days; median survival for rats exhibiting two risk factors (wide solid line) was 571. Affiliative reciprocity scores were dichotomized for graphical purposes only. Cox proportional hazards survival analysis was applied to estimate the effect of affiliative reciprocity and neophobic temperament on lifespan (Affiliative reciprocity: HR = 1.38, 95% CI = 1.18–1.61, p = .00003; Neophobia: HR = 2.00, 95% CI = 1.09–3.66, p = .025; Full model: Likelihood-ratio (LR) chi-square = 17.16, df = 2, p = .0002).
Mammary Tumors
Mammary tumors are one of the major forms of pathology at death in the Sprague-Dawley strain. Of the 49 aging female rats included in the current study, 32 (65%) developed at least one palpable mammary tumor by the end of her natural life span. Rats that did not interact reciprocally during a brief group stressor developed palpable mammary tumors earlier than rats engaged in reciprocal affiliation with their cagemate. Affiliative reciprocity directly predicted the onset of palpable mammary tumors such that every 10% decrease in reciprocity resulted in an increased risk of tumor palpation between 3% and 43% (HR = 1.21, 95% CI = 1.03–1.43, p = .02). As with lifespan, it was social structure that was predictive—tumor onset was not predicted by the frequency of initiating or receiving affiliative interactions, total involvement in affiliative behaviors, or affiliative role (i.e., primarily initiating or primarily receiving) (Univariate models for: Initiation: HR = 1.02, 95% CI=0.99–1.06, p = .19; Reception: HR = 1.02, 95% CI = 0.98–1.05, p = .39; Involvement: HR = 1.01, 95% CI = 0.99–1.03, p = .20; Role: HR = 1.39, 95% CI = 0.42–4.63, p = .58).
The risk of developing at least one tumor by the end of the lifespan, however, was not predicted by affiliative reciprocity; 17 of the rats with at least one mammary tumor exhibited high reciprocity, and 15 exhibited low reciprocity (chi-square [1, n = 32] = 0.16, p = .69). Likewise, overall tumor burden at the end of life was not predicted by affiliative reciprocity as a continuous variable (R2 = 0.085, F(1, 47) = 2.78 ; p = .11). Although 73% of tumor-bearing low reciprocity rats developed malignancies compared with 47% of tumor-bearing high reciprocity rats that developed malignancies, this result was not statistically significant (Odds ratio = 3.18; 95% CI = 0.27–37.00; p = .35). Affiliative reciprocity did not predict survival once a mammary tumor had been palpated (HR = 0.89, 95% CI = 0.74–1.06, p = .18).
Corticosterone Response to Restraint Stressor at Late Middle Age
At 15 months of age, after an adulthood of exposure to daily mild stressors in the laboratory and yet before 93.9% of the rats had developed tumors and 7 months before the median age of death, rats that had been less reciprocally affiliative since adolescence had larger corticosterone responses to an acute novel restraint stressor (AUCG) than did rats that had been more reciprocally affiliative (R2 = 0.095; F(1, 47) = 4.92, p = .03). On closer examination of individual components of the corticosterone response, we found that it was the peak corticosterone concentration attained within 120 minutes of the stressor onset that was strongly associated with its degree of affiliative reciprocity (Figure 3; R2 = 0.194; F(1, 47) = 11.33, p = .0015). It was not associated with baseline or recovery concentrations (Figure 3; Baseline: R2 = 0.013, F(1, 47) = 0.63, p = .43; Recovery: R2 = 0.023, F(1, 47) = 1.09, p = .30), which explains the weaker association with the total amount of corticosterone produced (i.e., AUCG). There was no difference between high and low affiliative reciprocity groups in the latency to reach peak corticoste-rone levels (Mantel-Cox logrank chi-square [1, N = 49] = 0.334, p = .56).
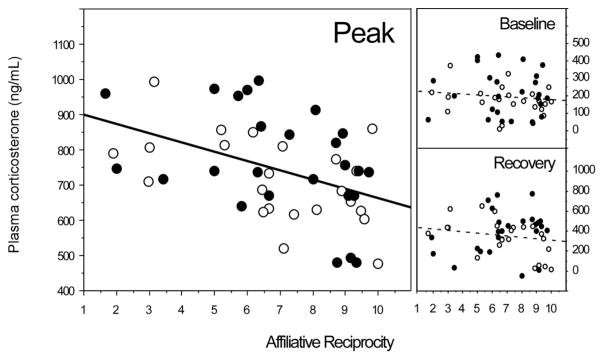
Figure 3.
Affiliative reciprocity predicts corticosterone response to restraint stress. Rats that affiliate more reciprocally in adolescence exhibit lower peak corticosterone concentrations in the 2 hours following a restraint stressor in adulthood (R2 = 0.194; F(1, 47)) = 11.33, p = .0015). Affiliative reciprocity, however, predicted neither baseline (R2 = 0.013, F(1, 47) = 0.63, p = .43) nor recovery (R2 = 0.023, F(1) = 1.09, p = .30) concentrations. Closed circles (●) represent sisters identified as “Neophilic”; open circles (○) represent sisters identified as “Neophobic”.
Ovarian Function During Reproductive Senescence
The larger rise in corticosterone concentration in females with low reciprocal affiliation could result from higher estrogen levels, as is observed during proestrus of a spontaneous ovarian cycle (20,32). On the day of corticosterone measurement, however, there was no difference in the level of estrogenization between dichotomized reciprocity groups (chi-square [1, N = 49] = 0.587, p = .44). Moreover, in contrast to temperament (20), affiliative reciprocity was not associated with rate of reproductive senescence (Odds ratio = 0.86; 95% CI = 0.63–1.19; p = .37), further suggesting that temperament and affiliative reciprocity are independent risk factors.
DISCUSSION
The results presented here are the first to demonstrate that affiliative reciprocity, an aspect of the formal structure of affiliative interactions, can predict the glucocorticoid response to stress in aging rats, onset of mammary tumors, and longevity. Affiliative reciprocity, a social structural trait measurable during puberty and maintained into adulthood, predicted morbidity and mortality prospectively. Based on the current evidence, our working hypothesis is that reciprocity of affiliative interactions, independent of their frequency, buffers the experience of everyday stressors, attenuates glucocorticoid reactivity over the lifespan, and hence, slows pathophysiological decline and the rate of aging.
To examine the meaning behind initiating and receiving affiliative interactions, we carefully considered their situational context. Reenacting a standard mildly-stressful laboratory procedure, each group’s home cage was taken from its rack and carried to a table where it was placed alongside another cage of three sisters. Once in the behavioral observation room, cage lids were removed and replaced with Plexiglas. This reenactment was chosen to present the group with novel and potentially threatening physical and social stimuli, and to this end, animals experienced unexpected disruptions to their housing conditions and were confronted with the sights, sounds, and smells of new conspecifics. In this potentially threatening context, affiliative contact could be supportive. Therefore, social contact during this novel and potentially threatening cage relocation may be conceptualized as social support insofar as the animals found the situation stressful and their female littermates a source of comfort (33–35).
The primary objective of this study was to characterize the pattern of home cage social interactions during this mild stressor of relocation to a relatively novel physical environment. Initiations of the affiliative behaviors “touch,” “holdfast,” and “follow,” were more highly intercorrelated than with reception of these same social behaviors. This pattern contrasted sharply with inspection behavior (oral and anogenital) and tandem curiosity (push away and stand by), where initiating and receiving of each these behaviors was highly correlated. Indeed, the initiation and reception of affiliation were not distributed equally among group members, and thus, there was considerable difference among the triad of sisters in the balance of giving and receiving social contact during the mild stressor. In other words, some sisters were highly reciprocal in initiating and receiving affiliation, whereas others gave more than they initiated or vice versa.
We construed social contact as affiliative, but also considered its association with social dominance. Body weight, however, a measure that is typically highly correlated with dominance status in rats, did not predict the reciprocity of females initiating or receiving affiliation. Moreover, the typical ingredients of antagonistic social relationships were absent. We never observed interactions that caused an animal to be physically wounded. This may be a result of the fact that there were no clear defendable resources. Females comprising the group were littermates whose access to resources (i.e., food and water) was unlimited. They were not allowed to mate, and in fact, were never exposed to colony rooms in which males were also housed. Dominance hierarchies have been known to manifest through more subtle behaviors in groups of female rats (31), but the semi-natural housing conditions necessary to elicit those behaviors were not available in this experiment. Moreover, behaviors that we labeled affiliative did not resemble these female-typic dominance behaviors.
Although further study of the temporal stability of affiliative reciprocity is necessary, we were able to show that affiliative reciprocity was stable across a long time interval (7 months), which spanned different developmental stages, from adolescence to adulthood. Recent progress has been made in the study of animal personality (36). For those studies on individual differences in rat behavior, few have assessed temporal stability in individual differences as was done in the current study (19,20,37). One such study reported that insect predation (a behavior involving interaction with another organism) in female rats exhibits a significant correlation (r = 0.447, p < .05) when testing 1 month apart (38). In the current study where we found behavioral stability across 7 months, the stability may have been due to the fact that cage composition was stable. Social groups were stable between affiliative reciprocity measurements and until natural death, thus providing little stimulus for behavioral fluctuation or change over time. This social stability is similar to the maintenance of dominance hierarchies among female baboons (Papio cynocephalus) as long as the group composition does not change (39,40). However, this is not to say that our methods were not strict enough to detect a lack of stability since two other types of behaviors measured in our study (tandem curiosity and mutual inspection) were not correlated across timepoints. Lastly, it is known that, in human personality research, there is fluctuation from one measure to the next, even when the interval between measures is day-to-day, or within-day (41).
If affiliative contact during group duress can be construed as social support, then our model provides an opportunity to prospectively contrast the health effects of the quantitative and qualitative aspects of social support. House et al.’s seminal reports (1,5) on social relationships and health indicated that further elucidation would require the clear distinction between 1) the existence or quantity of relationships, 2) their formal structure, and 3) their content. In our model, the size of each animal’s social network was held constant, and the frequency of engaging in affiliative behaviors did not predict morbidity and mortality. The content of social interactions was held relatively constant by observing social behaviors strictly while the group was experiencing a stressor, and analyzing only social behaviors that occurred in this context. Although holding these factors relatively constant, we found that affiliative reciprocity, an aspect of a relationship’s formal structure, predicted later health outcomes. To our knowledge, this is the first report directly comparing quantitative and qualitative aspects of social interactions that also demonstrates that it is the structural feature of social relationships that predicts morbidity and mortality.
Previously, we have shown that neophobic temperament is a risk factor for early death (20). Here, we show that low affiliative reciprocity during a mild group stressor is an equally potent risk factor. Moreover, low affiliative reciprocity can be regarded as a risk factor independent from neophobia since it is distributed evenly across temperaments. Furthermore, it can be considered an additive risk factor since models including both risk factors are significantly more predictive than either partial model. It is noteworthy that neophobia and nonreciprocal affiliation are independent, additive risk factors. This independence suggests that reciprocal social relationships could ameliorate early-appearing psychological predispositions toward mortality (e.g., neophobia).
In this rat model, we can think of no ways in which sibling cagemates could have provided sociobehavioral (i.e., instrumental) support. Rather, our data support psychobiological mediation whereby social support enhances physical health through psychological states affecting neuroendocrine and neuroimmune mechanisms, including the hypothalamic-pituitary-adrenal axis. Affiliative reciprocity during late puberty was associated with a low stress-induced rise in corticosterone in adulthood. This suggests that regular reciprocal relationships serve to alleviate everyday stressors, perhaps by altering perceptions of stressor severity. This finding is in line with the stress-buffering hypothesis that states that social support protects against the harmful physiological effects of repeated stressors. It is unlikely that the well-known effects of estrogen on glucocorticoid secretion (32,42,43) mediate the association between affiliative reciprocity and corticosterone since neither level of estrogenization on the day of restraint (indicated by vaginal cytology) or the state of reproductive senescence preceding restraint was associated with reciprocity.
It is beyond the scope of this study to describe the mechanism through which reciprocity influences corticosterone responses to stress, but future work could start by examining brain regions known to modulate the meaning of a stressor, such as the medial prefrontal cortex (44). It is worth noting that the results of this study do not diminish the contribution of sociobehavioral types of support; rather, by creating an animal model devoid of these influences, we show that psychobiological mechanisms may confer salubrious benefits on their own without the influence of sociobehavioral mechanisms.
Our data indicate that low affiliative reciprocity predicts not only early death but also early mammary tumor growth, the most common pathology at the time of death in this rat strain. There is accumulating evidence that stress and glucocorticoids could be involved in tumor growth processes. Conzen and coworkers have demonstrated in a xenograph mouse model of human breast cancer cells, that dexamethasone inhibits chemotherapy-induced apoptosis and also in vitro regulates genes mediating cell survival pathways (45,46). In a mouse model of spontaneous carcinomas, Tausk and coworkers demonstrated that repeated chronic stressors increased skin cancer risk (47). Kiecolt-Glaser and coworkers have shown that psychological stress suppresses apoptosis in cells that have sustained DNA damage in vitro (48,49). Finally, a lifespan study of isolated rats, which are at risk for spontaneous early development of rapidly growing mammary tumors (50), implicated a heightened corticosterone response to stressors (G.L. Hermes, MD, PhD, unpublished data, January 2008) and among group-housed rats the dynamics of the corticosterone stress response predicted not only tumor onset, but also malignancy and growth (JRY, unpublished data, 2008). Since females low in affiliative reciprocity in this study exhibited heightened corticosterone responses to a stressor, we examined whether affiliative reciprocity could predict mammary tumorigenesis, a process that is dependent on the suppression of apoptosis in damaged cells. This was indeed the case—females with a relative lack of reciprocal support developed tumors earlier. This finding adds to a growing literature implicating stress in the development and progression of neoplastic disease (51–53).
The study of reciprocity in social interactions has primarily fallen under the purview of human research given its complex nature. However, human studies are limited in a number of ways. Few are prospective in relation to predicting health outcomes. Surely, none can take a prospective lifespan approach where attributes early in life are used to predict health during senescence. In addition, few studies examine naturally-occurring reciprocity among individuals who already have a significant personal relationship (54), even though these relationships may be the most frequent and/or important social interactions an individual experiences. In patient contexts, separating emotional from instrumental support is difficult, if not unethical, to achieve. It is perhaps the inability to achieve conceptual precision that has made further mechanistic precision difficult. Objective measures of the structural qualities of social relationships are nearly impossible since self-report is preferred over time-intensive behavioral observations.
The current report aimed to address and improve each of these issues. By developing a rat model we were able to take a prospective lifespan approach, showing that social psychological characteristics present in adolescence could predict age of mammary tumor development and natural death. We examined the social interactions of sisters whose behavior was in response to a mild stressor not unlike those that commonly occur in the laboratory on a daily basis (e.g., cage change, transport) (13). Since there was no conceivable opportunity for sisters to exchange instrumental support, we were able to isolate the effect of what we have argued above to be akin to emotional support from other group members. Moreover, we were able to directly compare the quantity of affiliation to the quality of it, finding that affiliative reciprocity, a measure that represents a qualitative dimension of social relationships, predicts mammary tumor development and death while the raw number of affiliative interactions was unable to predict either outcome. The operational precision with which we defined reciprocity may have strengthened the mechanistic line of inquiry. We found that reciprocity is associated with the corticosterone response to an acute stressor during adulthood, and that this effect was not mediated by ovarian functioning. Future studies must determine if these associations are indeed causal.
It has been argued that rats are not capable of such complex sociality as to warrant the study of reciprocity. However, recent evidence indicates that rats are capable of generalized reciprocity, a social ability that has only been previously demonstrated in humans (15). Female rats were more likely provide food for another rat if they had received such instrumental support themselves in the past. Here we show behavior among sisters that is consistent with reciprocal emotional support. Until research demonstrates the contrary, we find no good reason not to assume that the behavior observed can be construed as emotional support and serve as a good animal model for human psychosocial amelioration of stress and its pathophysiological consequences. Future studies will hopefully characterize more fully the conditions that enable affiliative reciprocity, as well as achieve a higher level of mechanistic precision explicating the neuroendocrine systems and cellular pathways in this form of social modulation of disease and aging.
Acknowledgments
Supported by Grants from the National Institutes of Health CA130267 (to J.R.Y.), HD08693 (to S.A.C.), and ES012382 and AG018911 (to M.K.M.).
We thank Dr. L. Pyter for helpful comments on drafts of the manuscript, Dr. M. Kocherginsky for statistical assistance, and T. Brawn, J. Clarke, K. Eisenman, J. Hoffman, A. Lindner, E. Shaw-Taylor, M. Tsakalis, T. Whitney, H. You, and A. Wiley for their expert technical assistance.
Glossary
- AUC
area under the curve
- HR
hazard ratio
- CI
confidence interval
References
- 1.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–5. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 2.Cobb S. Presidential address—1976. Social support as a moderator of life stress. Psychosom Med. 1976;38:300–14. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 4.Cassel J. The contribution of the social environment to host resistance: the fourth Wade Hampton frost lecture. Am J Epidemiol. 1976;104:107–23. doi: 10.1093/oxfordjournals.aje.a112281. [DOI] [PubMed] [Google Scholar]
- 5.House JS. Social isolation kills, but how and why? Psychosom Med. 2001;63:273–4. doi: 10.1097/00006842-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29:377–87. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S. Psychosocial models of the role of social support in the etiology of physical disease. Health Psychol. 1988;7:269–97. doi: 10.1037//0278-6133.7.3.269. [DOI] [PubMed] [Google Scholar]
- 8.Lin N. Modeling the effects of social support. In: Lin N, Dean A, Ensel W, editors. Social support, life events, and depression. Orlando: Academic Press; 1986. pp. 173–209. [Google Scholar]
- 9.Gore S. Stress-buffering functions of social supports: an appraisal and clarification of research models. In: Dohrenwend B, Dohrenwend B, editors. Stressful life events and their context. New York: Prodist; 1981. pp. 202–22. [Google Scholar]
- 10.Silverstein M, Bengtson VL. Do close parent-child relations reduce the mortality risk of older parents? J Health Soc Behav. 1991;32:382–95. [PubMed] [Google Scholar]
- 11.Hernandez LM, Blazer DG. Genes, behavior, and the social environment: moving beyond the nature/nurture debate. Washington, DC: The National Academies Press; 2006. [PubMed] [Google Scholar]
- 12.Kaplan GA, Wilson TW, Cohen RD, Kauhanen J, Wu M, Salonen JT. Social functioning and overall mortality: prospective evidence from the Kuopio Ischemic Heart Disease Risk Factor Study. Epidemiology. 1994;5:495–500. [PubMed] [Google Scholar]
- 13.Sharp J, Zammit T, Azar T, Lawson D. Stress-like responses to common procedures in individually and group-housed female rats. Contemp Top Lab Anim Sci. 2003;42:9–18. [PubMed] [Google Scholar]
- 14.Steinberg H, Watson RH. Failure of growth in disturbed laboratory rats. Nature. 1960;185:615–6. doi: 10.1038/185615a0. [DOI] [PubMed] [Google Scholar]
- 15.Rutte C, Taborsky M. Generalized reciprocity in rats. PLoS Biol. 2007;5:e196. doi: 10.1371/journal.pbio.0050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuijer RG, Buunk BP, De Jong GM, Ybema JF, Sanderman R. Effects of a brief intervention program for patients with cancer and their partners on feelings of inequity, relationship quality and psychological distress. Psychooncology. 2004;13:321–34. doi: 10.1002/pon.749. [DOI] [PubMed] [Google Scholar]
- 17.Wolff JL, Agree EM. Depression among recipients of informal care: the effects of reciprocity, respect, and adequacy of support. J Gerontol B Psychol Sci Soc Sci. 2004;59B:S173–80. doi: 10.1093/geronb/59.3.s173. [DOI] [PubMed] [Google Scholar]
- 18.Kuper H, Singh-Manoux A, Siegrist J, Marmot M. When reciprocity fails: effort-reward imbalance in relation to coronary heart disease and health functioning within the Whitehall II study. Occup Environ Med. 2002;59:777–84. doi: 10.1136/oem.59.11.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc Natl Acad Sci USA. 2003;100:16131–6. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavigelli SA, Yee JR, McClintock MK. Infant temperament predicts life span in female rats that develop spontaneous tumors. Horm Behav. 2006;50:454–62. doi: 10.1016/j.yhbeh.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Brain Res Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 22.Young EA, Akana S, Dallman MF. Decreased sensitivity to glucocorticoid fast feedback in chronically stressed rats. Neuroendocrinology. 1990;51:536–42. doi: 10.1159/000125388. [DOI] [PubMed] [Google Scholar]
- 23.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–39. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 24.Timmermans PJA. Social Behavior in the Rat. Nijmegen: Katholieke Universiteit te Nijmegen; 1978. [Google Scholar]
- 25.Grant EC, Mackintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–81. [Google Scholar]
- 26.Barnett SA. Competition among wild rats. Nature. 1955;175:126–7. doi: 10.1038/175126b0. [DOI] [PubMed] [Google Scholar]
- 27.LeFevre J, McClintock MK. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol Reprod. 1988;38:780–9. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- 28.Russo J, Russo IH. Atlas and histologic classification of tumors of the rat mammary gland. J Mammary Gland Biol Neoplasia. 2000;5:187–200. doi: 10.1023/a:1026443305758. [DOI] [PubMed] [Google Scholar]
- 29.Cattell RB. The scree test for the number of factors. Multivariate Behav Res. 1966;1:245–76. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- 30.Hair JF, Anderson RE, Tatham RL, Black WC. Multivariate Data Analysis. Singapore: Pearson Education, Inc; 1998. [Google Scholar]
- 31.Ziporyn T, McClintock MK. Passing as an indicator of social dominance among female wild and domestic Norway rats. Behaviour. 1991;118:26–41. [Google Scholar]
- 32.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–11. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 33.Ditzen B, Neumann ID, Bodenmann G, von Dawans B, Turner RA, Ehlert U, Heinrichs M. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32:565–74. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Lonstein JS. Reduced anxiety in postpartum rats requires recent physical interactions with pups, but is independent of suckling and peripheral sources of hormones. Horm Behav. 2005;47:241–55. doi: 10.1016/j.yhbeh.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–29. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 36.Gosling S. From mice to men: what can we learn about personality from animal research? Psychol Bull. 2001;127:45–86. doi: 10.1037/0033-2909.127.1.45. [DOI] [PubMed] [Google Scholar]
- 37.Cavigelli SA, Stine MM, Kovacsics C, Jefferson A, Diep MN, Barrett CE. Behavioral inhibition and glucocorticoid dynamics in a rodent model. Physiol Behav. 2007;92:897–905. doi: 10.1016/j.physbeh.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Negrao N, Schmidek WR. Individual differences in the behavior of rats (Rattus norvegicus) J Comp Psychol. 1987;101:107–11. [Google Scholar]
- 39.Hausfater G, Altmann J, Altmann S. Long-term consistency of dominance relations among female baboons (Papio cynocephalus) Science. 1982;217:752–5. doi: 10.1126/science.217.4561.752. [DOI] [PubMed] [Google Scholar]
- 40.Samuels A, Silk JB, Altmann J. Continuity and change in dominance relations among female baboons. Anim Behav. 1987;35:785–93. [Google Scholar]
- 41.Brown KW, Moskowitz DS. Dynamic stability of behavior: the rhythms of our interpersonal lives. J Pers. 1998;66:105–34. doi: 10.1111/1467-6494.00005. [DOI] [PubMed] [Google Scholar]
- 42.Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adreno-corticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–9. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- 43.Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J Clin Invest. 1993;92:1896–902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 45.Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64:1757–64. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 46.Pang D, Kocherginsky M, Krausz T, Kim SY, Conzen SD. Dexamethasone decreases xenograft response to Paclitaxel through inhibition of tumor cell apoptosis. Cancer Biol Ther. 2006;5:933–40. doi: 10.4161/cbt.5.8.2875. [DOI] [PubMed] [Google Scholar]
- 47.Parker J, Klein SL, McClintock MK, Morison WL, Ye X, Conti CJ, Peterson N, Nousari CH, Tausk FA. Chronic stress accelerates ultraviolet-induced cutaneous carcinogenesis. J Am Acad Dermatol. 2004;51:919–22. doi: 10.1016/j.jaad.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 48.Tomei LD, Kiecolt-Glaser JK, Kennedy S, Glaser R. Psychological stress and phorbol ester inhibition of radiation-induced apoptosis in human peripheral blood leukocytes. Psychiatry Res. 1990;33:59–71. doi: 10.1016/0165-1781(90)90149-y. [DOI] [PubMed] [Google Scholar]
- 49.Kiecolt-Glaser JK, Glaser R. Psychoneuroimmunology and cancer: fact or fiction? Eur J Cancer. 1999;35:1603–7. doi: 10.1016/s0959-8049(99)00197-5. [DOI] [PubMed] [Google Scholar]
- 50.Hermes GL, McClintock MK. Isolation and the timing of mammary gland development, gonadarche, and ovarian senescence: implications for mammary tumor burden. Dev Psychobiol. 2008;50:353–60. doi: 10.1002/dev.20295. [DOI] [PubMed] [Google Scholar]
- 51.Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, Mc-Donald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–8. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. Am Psychol. 1994;49:389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heffner KL, Loving TJ, Robles TF, Kiecolt-Glaser JK. Examining psychosocial factors related to cancer incidence and progression: in search of the silver lining. Brain Behav Immun. 2003;17:S109–11. doi: 10.1016/s0889-1591(02)00076-4. [DOI] [PubMed] [Google Scholar]
- 54.Rook KS. Reciprocity of social-exchange and social satisfaction among older women. J Pers Soc Psychol. 1987;52:145–54. [Google Scholar]