Abstract
Echocardiography is increasingly recommended for the diagnosis and assessment of patients with severe cardiac disease, including acute heart failure. Although previously considered to be within the realm of cardiologists, the development of ultrasonography technology has led to the adoption of echocardiography by acute care clinicians across a range of specialties. Data from echocardiography and lung ultrasonography can be used to improve diagnostic accuracy, guide and monitor the response to interventions, and communicate important prognostic information in patients with acute heart failure. However, without the appropriate skills and a good understanding of ultrasonography, its wider application to the most acutely unwell patients can have substantial pitfalls. This Consensus Statement, prepared by the Acute Heart Failure Study Group of the ESC Acute Cardiovascular Care Association, reviews the existing and potential roles of echocardiography and lung ultrasonography in the assessment and management of patients with acute heart failure, highlighting the differences from established practice where relevant.
Heart failure is the primary cause of hospital admission in >1 million patients per year in the USA, with 25% of patients being readmitted within 1 month, and 10–20% mortality at 6 months after discharge1,2. Acute heart failure (AHF) — either a new diagnosis in patients with no history of cardiac disease, or as a result of acute decompensation in patients with known heart failure — is the leading cause of hospital admission in individuals aged >65 years in the UK3. According to data from Europe, approximately 50% of these patients will be readmitted within 12 months, and 30% will be deceased at the 1-year follow-up4. Despite numerous clinical trials to assess optimal treatment and management strategies for patients with AHF, little improvement has been made in AHF outcomes in the past 30 years1,4,5, with management decisions largely based on expert consensus rather than robust evidence. The burden of AHF is therefore substantial, both to individual patients and to society6,7. The successful management of patients with any acute condition involves early diagnosis, the identification of underlying reversible causes, and the implementation of effective therapies in a timely manner, all while avoiding harm; all these factors are associated with better in-hospital and short-term prognosis8. This Consensus Statement, prepared by the Acute Heart Failure Study Group of the ESC Acute Cardiovascular Care Association, reviews the existing and potential roles of echocardiography and lung ultrasonography (LUS) in the assessment and management of patients with AHF.
AHF: a diagnostic and management challenge
AHF is a syndrome rather than a diagnosis per se, caused by a wide array of pathologies that result in a spectrum of disease severity ranging from breathlessness to cardiogenic shock or cardiac arrest. AHF is a highly lethal condition, and studies have shown that minimizing the ‘time to appropriate therapy’ — the initiation of treatment as soon as possible, including in the prehospital setting — is potentially beneficial in improving outcomes9,10. AHF is variably defined as the rapid onset or acute worsening of symptoms and signs of heart failure that is associated with elevated plasma levels of natriuretic peptides4,11. However, substantial diagnostic uncertainty is inevitable when relying only on traditional clinical findings, and currently a lack of specificity exists in routine investigations for this condition. Indeed, although patients often present with a suggestive history, clinical features (such as shock, and pulmonary or peripheral congestion), and/or symptoms related to the underlying potential cause, these traditional clinical features are frequently absent; over-reliance on these factors might delay diagnosis and implementation of appropriate therapy, or contribute to a missed diagnosis in up to 20% of patients12,13. Furthermore, patients’ clinical features might vary according to the site of initial medical contact and the management strategies employed14,15.
The majority of patients with AHF present to emergency departments; however, many patient are also assessed and managed in other acute care settings such as in intensive care and inpatient cardiology units. Patients with AHF usually present with symptoms of congestion and breathlessness rather than cardiac arrest or shock16. Symptoms of breathlessness account for 3–5% of emergency department attendances in Europe and the USA, and the major causes of breathlessness and their prevalence include AHF (50%), pneumonia or bronchitis (20%), exacerbation of chronic obstructive pulmonary disease or asthma (20%), and pulmonary embolism (5–10%)16,17. Current guidelines recommend that clinical examination and investigations should be integrated to form the diagnosis, including the use of electrocardiogram (ECG), chest radiograph, and biomarkers such as natriuretic peptides, troponin, and D-dimer as indicated16,18,19. Unfortunately, these data can be challenging to interpret, in particular in the 10–15% of patients in whom two concomitant diagnoses exist1,4,20. Specifically, although included in the current definition of AHF, levels of natriuretic peptides can be elevated in respiratory disease and other acute conditions such as pulmonary embolism, sepsis, and anaemia21–24.
Any acute condition can be further complicated by the external factors present in emergency settings, such as high ambient noise and restrictive space, limiting a clinician’s ability to position the patient optimally for examination. Furthermore, the frequently atypical features of very severe pathology (in particular valvular disease), and the time pressures imposed by an acutely deteriorating patient can contribute to poor outcomes. These factors are further confounded by the presence of concomitant pathologies in the increasingly ageing patient population25.
Echocardiography and LUS are readily available and widely validated techniques that can be used to reveal anatomical and physiological abnormalities in patients with AHF, which when correctly applied in the acute setting, can improve patient assessment, management, and outcomes (FIGS 1,2)26. Unlike other biomarkers used in AHF, echocardiography and LUS can be used to identify not only inadequate cardiac output and/or the presence of congestion, but also the underlying cause, allowing the most appropriate, individualized interventions to be delivered immediately to the patient27. Furthermore, these imaging modalities can be used to monitor the effects of treatment (either beneficial or detrimental), as well as to guide patient disposition and interventions as indicated28. Pocket-sized echocardiography devices are practical for screening, and provide information to clinicians in addition to that gathered from auscultation by a stethoscope alone. When AHF is suspected, an integrative approach is recommended, including determination of cardiopulmonary instability and evaluation of congestion (pulmonary and peripheral) using a combination of techniques4. When image quality is inadequate, either transoesophageal echocardiography or the use of contrast should be considered.
Figure 1. Lung and pleural ultrasonography.
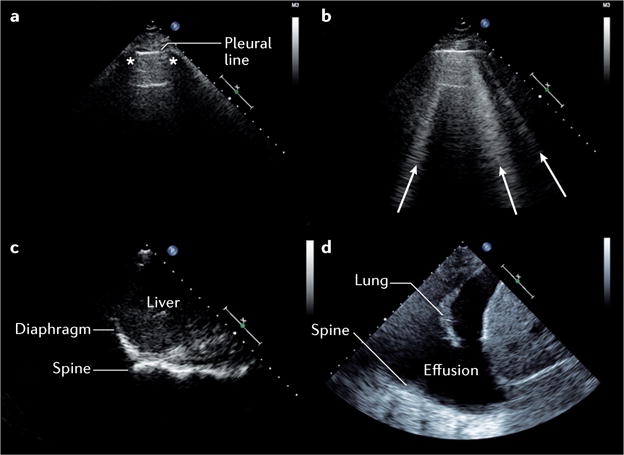
a | Normal lung with pleural line, and ribs (*) with shadowing. b | Pulmonary oedema with multiple vertical B-lines (arrows) arising from the pleural line. c | Diaphragmatic view with spine ending at the level of the diaphragm, with no pleural effusion. d | Pleural effusion seen as anechoic (echo-free) space above the diaphragm with atelectatic lung. Spine can be visualized beyond the diaphragm owing to the effusion.
Figure 2. Echocardiographic methods to estimate left atrial pressure.
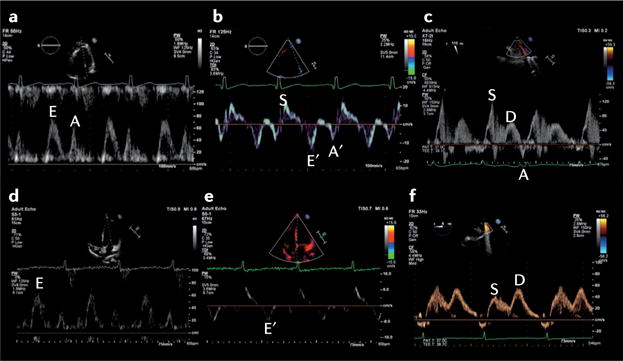
The upper panels show the echocardiographic scan of a patient aged 45 years admitted to hospital with dyspnoea owing to severe acute respiratory failure. a | Transthoracic echocardiogram (TTE) of the mitral inflow pattern showing a normal early (E) and late (A) transmitral flow pattern. b | Tissue Doppler imaging (TDI) of the lateral mitral valve annulus from the same patient; S is systolic annular velocity, E′ is early annular diastolic velocity, and A′ is late annular diastolic velocity (related to atrial contraction). c | Pulmonary venous Doppler (transesophageal echocardiography) demonstrating a dominant systolic wave (S) and smaller diastolic wave (D), with a normal deceleration time. The E/A ratio is >1 and the E/E′ is <8 cm/s with a dominant S wave on pulmonary vein, consistent with a normal left atrial pressure. The lower panels show the echocardiographic scan of a female patient aged 59 years admitted with dyspnoea owing to severe left ventricular dysfunction with pulmonary oedema. d | TTE of the mitral inflow pattern showing a dominant E wave with E/A ratio >2. e | TDI of the septal mitral valve annulus with a very low early diastolic velocity (E′), and f | pulmonary venous Doppler (transoesophageal echocardiography) showing a blunted systolic wave (S) and dominant diastolic wave (D). The E/E′ is 16.3 cm/s, and dominant D wave on pulmonary venous Doppler with D deceleration time <150 ms are consistent with an elevated left atrial pressure.
Lung ultrasonography
Based on the interpretation of a number of artefacts, specific ultrasonography appearances, and their distribution (FIG. 1), LUS allows for a rapid point-of-care evaluation of a number of conditions, including pulmonary oedema, lung consolidation, pleural effusion, and pneumothorax29. High intra-rater and inter-rater reproducibility, ease of learning, short exam duration (<5 min), and the noninvasive nature of this technique makes it an advantageous point-of-care tool30–32. LUS is increasingly used in the acute care setting, and has improved diagnostic accuracy compared with clinical assessment and chest radiography for the identification of a cardiac aetiology in patients presenting to the emergency department with undifferentiated dyspnoea33.
Interstitial fluid and pulmonary oedema
Quantification of B-lines (vertical artefacts that result from an increase in interstitial density; FIG. 1b) has been shown to be useful for the diagnosis, monitoring, and risk assessment of patients with known or suspected AHF34–36. Either curvilinear or phased array transducers can be used, typically at an imaging depth of 18 cm. Although the assessment of eight or more anterior and lateral thoracic zones (four on each hemithorax) has been recommended in a consensus statement29, a subsequent study demonstrated high diagnostic accuracy with examination of only six thoracic regions33. The visualization of three or more B-lines in two or more intercostal spaces bilaterally should be considered diagnostic for pulmonary oedema, with sensitivity of 94% (95% CI 81–98%) and specificity of 92% (95% CI 84–96%)33,37. By contrast, physical examination and chest radiography have a sensitivity of only 62% (95% CI 61–64%) and 57% (95% CI 55–59%), and a specificity of 68% (95% CI 67–69%) and 89% (95% CI 88–90%) for a diagnosis of pulmonary oedema, respectively38. The presence of multiple bilateral B-lines in AHF has been well-correlated with natriuretic peptide levels, and only variably correlated with pulmonary capillary wedge pressure and measures of extravascular lung water30,33,35,39–41. Given that studies to assess the incremental diagnostic value of LUS compared with natriuretic peptides for the identification of AHF in patients with dyspnoea reported variable results in different cohorts, this topic warrants further investigation31,33,42. The number of B-lines is thought to decrease with treatment for AHF and, therefore, this technique is potentially useful in the monitoring of pulmonary oedema in response to therapy35,36. For serial assessments, patient positioning (sitting versus supine) should be kept consistent43. Importantly, a higher number of B-lines on LUS at the time of discharge from hospital might help to identify patients with heart failure who have a worse prognosis36.
Pleural effusion
Similarly to B-lines, the presence of pleural effusions can be assessed using curvilinear or phased array transducers in the posterior–axillary line34 (FIG. 1d). Current data regarding the diagnostic utility of pleural effusions identified on ultrasonography in patients with AHF are less robust, but have been reported with sensitivities of 79–84% and specificities of 83–98% in small studies of patients with dyspnoea44,45.
Pneumothorax
LUS can be used to exclude pneumothorax in the area scanned with higher sensitivity than supine chest radiography by recognizing lung sliding, a slight horizontal movement of the pleural line with respiration; see Supplementary information S1 (video)46. In the setting of a pneumothorax, lung sliding is absent in the affected area of the chest. At the border of a pneumothorax, a transition point between normal lung surface (with lung sliding) and pneumothorax (without lung sliding) can sometimes be identified47. This so-called ‘lung point’ confirms the diagnosis. Lung sliding might be absent in several other pathological conditions (such as pleural adhesions or selective mainstem intubation) and, therefore, should not be used in isolation to make the diagnosis of pneumothorax, but rather in conjunction with the full range of sonographic features46.
Differential diagnosis and potential pitfalls
The major questions when using LUS for the assessment of patients with possible AHF include whether there is evidence of pulmonary oedema (such as multiple bilateral B-lines), whether there are other findings suggestive of AHF (such as pleural effusion), and finally, whether there are findings of alternate or concurrent conditions (such as pulmonary consolidation or pneumothorax). Despite its apparent simplicity, a number of caveats exist for the use of LUS. First, B-lines can resolve rapidly in response to treatment, and, therefore, LUS data must be interpreted in the context of previous interventions35. Second, B-lines can be seen in a number of pulmonary conditions, including pulmonary fibrosis or interstitial lung disease, acute respiratory distress syndrome, and pneumonitis29. The observation of B-lines together with other LUS abnormalities might indicate that two pathologies coexist, or that the B-lines are an expression of pathology other than AHF (for example, acute respiratory distress syndrome, or pulmonary oedema in patients receiving haemodialysis)48. Third, large pleural effusions might interfere with B-line quantification in the affected thoracic zones and induce lung consolidation (FIG. 1d). Together, these considerations outline why LUS should not be used in isolation, but rather integrated into clinical and laboratory assessment33,49,50.
Echocardiography in AHF
Driven by progressive advances in ultrasonography technology and an expanding evidence base, the use of echocardiography has extended beyond the traditional application in stable patients to become widespread in the acute and emergency settings51,52. Mirroring the concept of critical care, echocardiography is increasingly used as a tool to guide management of the most acutely unwell patients wherever they present along the management pathway. Pocket-sized devices have been recommended in the emergency department, intensive care unit, and coronary units for fast initial qualitative screening of ventricular and valvular function, pericardial and pleural effusion, or extravascular lung water. However, owing to the known limitations of this technique, they are not intended as a substitution for comprehensive echocardiography26,53. Remote expert review of images is now a possibility, and in the future, telemedicine will probably have an important role in guiding the assessment and management of these acutely unwell patients.
Echocardiography is used in AHF to help to confirm diagnosis, delineate potential underlying causes, identify associated pathophysiology, and monitor the response to therapy28,54. Echocardiography can also be used to guide specialist interventions in the catheter laboratory or operating room55–57. Furthermore, echocardiography can address several major questions, including whether a patient has a cardiac cause for their symptoms and signs, the severity of the cardiac impairment and its physiological effect, whether there is an underlying reversible cause, what the most appropriate initial treatment is, and how the patient responds to treatment.
Guidelines recommend immediate echocardiographic assessment for patients with suspected AHF with haemodynamic instability1,4; however, interpretation of echocardiographic data in these acutely unwell patients can be extremely complex (TABLE 1). First, the finding of a structurally or functionally abnormal heart does not necessarily mean the cause of dyspnoea is cardiac-related. Second, patients might be misdiagnosed as having primary respiratory disease, even in the presence of very severe cardiac pathology27,58. Third, substantial cardiac and respiratory disease might coexist, and determining the degree of cardiac contribution is frequently challenging in this setting59. These considerations are further compounded by the relative paucity of high-quality evidence to support the use of echocardiography techniques in the acute arena, as they have been predominantly validated in the outpatient clinic.
Table 1.
Challenges in using echocardiography to determine the underlying cause of AHF
| Underlying cause |
AHF-related clinical presentation |
Echo findings | Notes and potential pitfalls |
|---|---|---|---|
| ACS and ischaemic heart disease | Dyspnoea, as atypical presentation of ACS |
|
|
| Shock |
|
|
|
Severe MR:
|
|
||
|
|
||
Ventricular septal rupture:
|
|
||
|
|
||
| Myocarditis | Widely variable, might be within AHF spectrum |
|
|
| Takotsubo syndrome | Widely variable, might be within AHF spectrum |
|
Echocardiographically more heterogeneous than originally described
|
| Dissection | Shock |
|
|
| Cardiomyopathy | Full spectrum of AHF |
|
|
|
|
||
| Pulmonary embolism | Full spectrum of AHF |
|
|
| Pneumothorax | From dyspnoea to cardiac arrest |
|
|
| Valve disease | Mitral regurgitation; from dyspnoea to shock |
|
|
| Aortic regurgitation; from dyspnoea to shock |
|
|
|
| Mitral stenosis; might mimic ARDS |
|
|
|
| Aortic stenosis; from dyspnoea to shock to cardiac arrest |
|
|
|
| Valve prosthesis dysfunction; from dyspnoea to shock |
|
|
|
| Sepsis | Clinically septic, but inadequate CO |
|
|
| Tamponade | Dyspnoea to shock to cardiac arrest |
|
|
ACS, acute coronary syndrome; AHF, acute heart failure; AMI, acute myocardial infarction; AR, aortic regurgitation; ARDS, acute respiratory distress syndrome; AV, aortic valve; CO, cardiac output; Echo, echocardiography; ECMO, extracorporeal membrane oxygenation; EDV, end-diastolic velocity; EF, ejection fraction; GLS, global longitudinal strain; HCM, hypertrophic cardiomyopathy; LAP, left atrial pressure; LUS, lung ultrasonography; LV, left ventricular; LVOTO, left ventricular outflow tract obstruction; MCE, myocardial contrast echocardiography; MCS, mechanical circulatory support; MI, myocardial infarction; MR, mitral regurgitation; MV, mitral valve; PASP, pulmonary artery systolic pressure; PHT, pressure half-time; PPV, positive pressure ventilation; PR, pulmonary regurgitation; PVR, pulmonary vascular resistance; RV, right ventricular; RWMA, regional wall motion abnormality; STE, speckle-tracking echocardiography; TOE, transoesophageal echocardiography; TR, tricuspid regurgitation; TTE, transthoracic echocardiography.
Left-sided disease and elevated LAP
Dyspnoea resulting from left-sided cardiac disease is likely to be associated with elevated left atrial pressure (LAP) and pulmonary oedema. Historically, pulmonary capillary wedge pressure has been measured using a pulmonary artery catheter as a substitute for LAP measurement60–62. The use of the pulmonary artery catheter has greatly declined over the past decade, owing to a number of studies that showed potential harm or no improved outcomes in the perioperative and critical care settings63. Although absolute pressure values cannot be measured using echocardiography, a drive has occurred to find an echocardiography-derived parameter that can be used to estimate the LAP noninvasively. Indices that have been proposed include interrogation of the transmitral left ventricular (LV) filling pattern (E/A ratio, E wave deceleration time, and the isovolumic relaxation time), pulmonary venous Doppler diastolic deceleration time (FIG. 2), M-mode colour Doppler propagation velocities, the time interval between the onset of early diastolic mitral inflow (E) and annular early diastolic velocity (e′) by tissue Doppler imaging, and the E/e′ ratio64–69. None of these measures has been well-validated in the context of emergency medicine70,71; they all present technical challenges that must be carefully considered for accurate interpretation, and provide only estimates of a potential range of corresponding LAP values. Even when used in combination (as proposed in critical care), they can at best only indicate that the LAP is probably very high or normal.
LV ejection fraction has been the main parameter used for the diagnosis, treatment, and stratification of patients with heart failure. However, this parameter has several limitations that are particularly relevant in the acute setting, such as load-dependency and inotropydependency72,73. Even in the absence of high-quality 2D images, Doppler abnormalities in transmitral filling might provide an early indicator of important pathology72,74–76.
Unlike LUS, echocardiography might be challenging to perform well and interpret accurately, as a number of considerations add to the complexity of its application in the acute setting. First, in all parameters described for LAP estimation, the confounding factors imposed by critical illness (changes in heart rate, cardiac output, LV compliance, and volume and ventilatory status) have not been fully evaluated. Second, not only might patients with a relatively normal LAP have radiographic and sonographic evidence of pulmonary oedema, but conversely, patients with chronically elevated LAP might have no evidence of pulmonary oedema. Similarly to LUS, however, the echocardiographic findings should be integrated with those from clinical examination, laboratory investigations, and lung imaging data (radiographic and/or sonographic), and be assessed within the clinical context. The main value of echocardiography in this setting is to diagnose or exclude an underlying cardiac cause for dyspnoea and guide subsequent interventions.
Right-sided disease: pulmonary embolism
The diagnosis of pulmonary embolism can be challenging, because symptoms and signs are nonspecific. The transthoracic echocardiogram is normal in approximately 50% of unselected patients with acute pulmonary embolism, and has a sensitivity of 50–60% and specificity of 80–90%77. Therefore, other investigations are used to confirm the diagnosis, with echocardiography used as a complementary imaging technique19. The principal indirect echocardiographic findings are nonspecific, and include right heart dilatation, right ventricular (RV) hypokinesis (with or without apical sparing), abnormal septal motion, and inferior vena cava dilatation78 (FIG. 3a). Secondary tricuspid regurgitation might be present, allowing estimation of pulmonary arterial systolic pressure using the simplified Bernoulli equation79 (FIG. 3b). Given that the right ventricle can generate a pulmonary artery systolic pressure of only ≤60 mmHg acutely, a higher pressure suggests a more chronic process (either multiple repeated episodes or chronic pulmonary parenchymal disease, with or without pulmonary embolism)80. Although the peak tricuspid regurgitation gradient is the most commonly used parameter to assess pulmonary artery systolic pressure in clinical practice, difficulties in the detection of good tricuspid regurgitation envelope might occur. Pulsed Doppler recordings of pulmonary valve flow acceleration time, pre-ejection period, and ejection time at the RV outflow tract can also be used to estimate pulmonary artery pressure and resistance81,82.
Figure 3. Echocardiographic features in patients presenting with severe haemodynamic impairment.
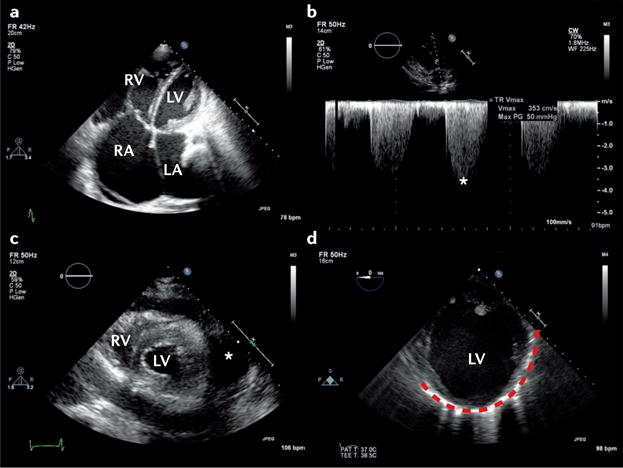
a | Transthoracic echocardiography in a patient with acute-on-chronic pulmonary embolism from an apical four-chamber view showing a severely dilated right ventricle (RV), and b | increased pulmonary systolic pressure estimated by applying the simplified Bernoulli equation using the measured tricuspid regurgitation peak velocity (50 mmHg; asterisk). c | Parasternal short axis view showing RV and left ventricle (LV) surrounded by a circumferential pericardial effusion (asterisk) that induced tamponade. d | Transoesophageal echocardiography (transgastric short-axis view) of a patient aged 42 years admitted with cardiogenic shock presenting with ST-segment elevation in the anterolateral electrocardiogram leads. Coronary angiography showed critical three-vessel coronary artery disease. The LV is severely dilated, and there is evidence of previous myocardial infarction, shown by the presence of thinned and akinetic myocardium (dotted red line). LA, left atrium; RA, right atrium.
Pericardial collection and tamponade
Echocardiography is pivotal for recognition of the haemodynamic consequences of a pericardial collection (FIG. 3c), allowing demonstration of features of tamponade including right atrial and/or RV diastolic collapse, in addition to guiding pericardiocentesis83. A number of potential pitfalls exist when interpreting the echocardiographic features of tamponade in the acute setting. These pitfalls include the effects of positive pressure ventilation (reversal of changes in transvalvular flows) and localized collections, in particular after cardiac surgery when substantial haemodynamic compromise might be present, even in the absence of echocardiographic features of tamponade84.
Monitoring of therapy
Echocardiography is not recommended for the monitoring of therapy in patients with AHF in the absence of cardiogenic shock4,9,11, given the complexity of LAP estimation using echocardiography, its lack of association with pulmonary congestion and symptoms, and superiority of natriuretic peptide levels in monitoring response to therapy. An emerging area in which echocardiography might be of use is in risk stratification before discharge from hospital. In patients with AHF with dyspnoea, persistent pulmonary congestion before discharge (demonstrated on LUS) has been shown to be an independent predictor of rehospitalization for AHF at 6 months after discharge36.
Cardiogenic shock
Cardiogenic shock is the most severe manifestation of AHF. Although relatively uncommon, the published prevalence (5% of patients with AHF) varies according to the point of initial contact and management (1–2% of patients with AHF in the prehospital or emergency setting versus 29% in intensive care)4,9,10,16. Precise definitions of cardiogenic shock can vary; however, the syndrome generally results from inadequate cardiac output for peripheral organ requirements85,86. Cardiogenic shock can manifest as hypotension despite adequate filling (with or without vasopressors), altered mentation, cool peripheries, oliguria, hyperlactataemia, metabolic acidaemia, and low mixed venous oxygen saturation86. In addition to standard evaluation of critically ill patients in parallel with resuscitation, echocardiography is mandated immediately in patients with cardiogenic shock, because without identification and treatment of the underlying cause, the outcome is usually fatal9,85 (FIG. 3d). Additional information that should be obtained from echocardiography includes estimation of stroke volume and cardiac output levels, because these data can provide guidance on how to maximize the cardiac output at the lowest filling pressures (see Supplementary information S2 (table)). These measurements should be taken during the echocardiogram, and should be performed repeatedly to monitor the response to therapeutic interventions and minimize potentially injurious treatment. Every study must be interpreted in the context of the level of inotropic and ventilatory support, as well as metabolic and arterial blood gas status, because these variables might have profound effects on echocardiographic findings.
Assessment of volume status
The physiological basis of providing ‘optimal’ filling in cardiogenic shock is that a critical decrease in intravascular-stressed volume reduces the difference between mean systemic venous and right atrial pressure, thereby limiting stroke volume. Although frequently used, invasive static pressure monitoring is not helpful for determining whether an individual patient is volume-responsive87,88. Static echocardiographic parameters are widely used to predict volume responsiveness in critically ill patients (FIG. 4); however, their use requires that a number of strict criteria (relating to the patient, their underlying pathology, and medical interventions) are met, otherwise the investigation becomes invalid (see Supplementary information S3 (table)). Similarly, although thought to be superior, dynamic echocardiographic parameters to predict volume responsiveness are valid only in fully mechanically ventilated patients in sinus rhythm and without chronic heart disease89. In the presence of cardiac disease (either left-sided and/or right-sided), these measurements can be misleading and should not be used. Conversely, tolerance to volume loading among different patients is variable. The conventional teaching to increase volume in RV failure has not been upheld by findings published in the past 3 years90,91. Physiological models suggest that in some patients, progressive fluid loading leads to a plateauing of cardiac output, with a progressive increase in pulmonary artery occlusion pressure. In addition, higher volume is associated with worse outcome in critically ill patients92–94.
Figure 4. Static 2D echocardiography parameters are used to evaluate potential volume responsiveness.
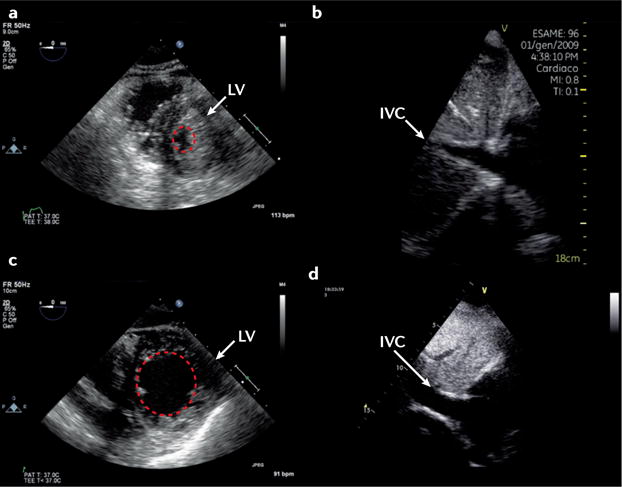
The upper panels show a patient who is severely hypovolaemic, and responded to volume loading with an increase in stroke volume. a | Short-axis view of the left ventricle (LV) is shown, where the left ventricular end-diastolic area (dotted red circle) is small. b | From a subcostal view, an obliterated inferior vena cava (IVC) at end-expiration (<1 cm) can be observed. The lower panels show a patient who, according to static 2D echocardiography parameters, would not be predicted to respond to volume loading by increasing stroke volume. c | Short-axis view of the LV with a normal left ventricular end-diastolic area (dotted red circle). d | Dilated IVC at end-expiration.
Inotropes and vasoactive agents
Although inotropes and vasopressors are commonly used to improve cardiac output and blood pressure in patients with cardiogenic shock, there is currently insufficient evidence to support the use of any particular agent in this context9,95,96. Dobutamine is generally the first-line inotrope of choice in the clinic9,95,96. The detrimental effects of positive inotropic agents have been extensively described in the literature97,98, and their use should, therefore, be restricted to the shortest possible duration and the lowest dose, both individualized to the patient. Although little guidance exists on how inotrope treatment should be individualized, echocardiography might be helpful in certain scenarios.
First, not all patients with cardiac disease respond to escalating doses of dobutamine by increasing their stroke volume; in some patients, dobutamine can result in an increase in the total isovolumic time (tIVT)99. Echocardiographic identification of an abnormally prolonged tIVT with dobutamine use, or an increase in tIVT in response to escalating inotropic support might indicate that inotropes are directly impairing myocardial performance, thereby prompting a reduction in dose or a change in treatment strategy99–101 (FIG. 5). Second, the combination of LV end-diastolic pressure (LVEDP) and low aortic root pressure might result in a mismatch of coronary perfusion and myocardial oxygen demand. If untreated, this mismatch can result in type 2 myocardial infarction102 (FIG. 3d). Echocardiographic demonstration of a dominant or isolated A wave on transmitral Doppler in combination with postejection shortening can also be diagnostic (FIG. 6a,b), and indicates that aortic root pressure should be increased and/or LVEDP reduced103,104. Third, physiological studies have demonstrated that the combination of RV ischaemia and increased RV afterload is particularly injurious to RV performance, resulting in a fall in systemic blood pressure and cardiac output levels105. Echocardiography can be used to estimate pulmonary artery systolic pressure and pulmonary vascular resistance, as well as measure RV dimensions and performance106. Echocardiographic identification of high pulmonary vascular resistance with or without pulmonary hypertension in combination with RV dysfunction in cardiogenic shock might necessitate the introduction of a pressor agent plus treatment to reduce RV after-load90,107 (FIG. 6c,d). Finally, in a patient with falling cardiac output levels despite escalating inotropic support, echocardiography can help to diagnose LV outflow tract obstruction (with or without associated mitral regurgitation)27,108. Treatment in this context involves reduction or cessation of positive inotropic agents, in combination with volume and pressor support.
Figure 5. Echocardiography-guided cardiac output optimization using pulsed-wave Doppler imaging.
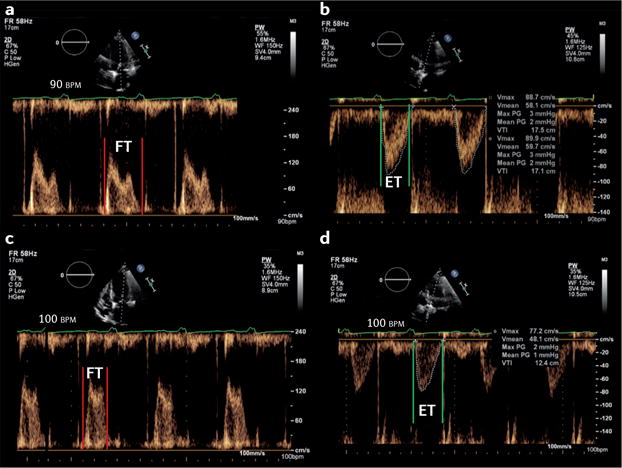
a,b | Transmitral and transaortic pulsed-wave Doppler imaging at 90 bpm. c,d | Transmitral and transaortic pulsed-wave Doppler imaging at 100 bpm. The filling time (FT) is measured from the start to the end of transmitral filling, and the ejection time (ET) from the start to the end of aortic ejection. The total ejection (t–ET) and filling (t–FT) periods are then derived as the product of the corresponding time interval and heart rate, and expressed in s/min. t–IVT (also in s/min) is calculated as 60–(t–FT + t–ET). A heart rate reduction of 10 bpm resulted in a reduction of t–IVT from 16.8 s/min to 10.0 s/min, and a corresponding increase in cardiac output from 3.6 l/min to 5.6 l/min.
Figure 6. The haemodynamic effects of thrombosis (coronary and pulmonary) as demonstrated by echocardiography.
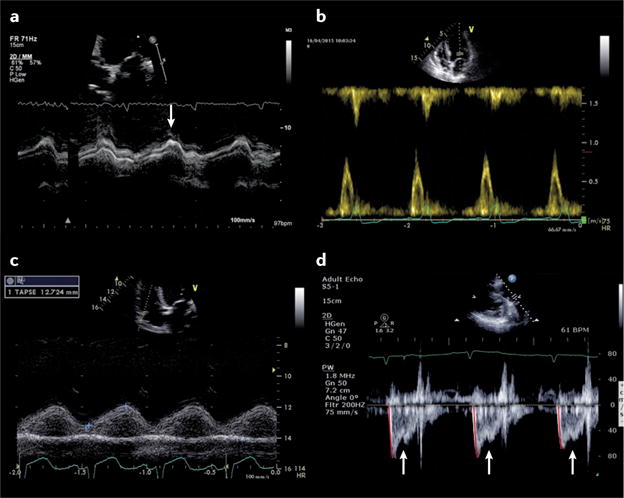
a | Early features of myocardial ischaemia can be demonstrated by the presence of prolonged long-axis shortening, measured by M-mode echocardiography across the base of the left ventricle (post-ejection shortening; arrow). b | Prolonged left ventricular wall tension suppresses early transmitral filling, resulting in an isolated late-diastolic transmitral A wave. c | Increased right ventricular afterload leads to a reduction in right ventricular systolic function, as demonstrated by tricuspid annular plane systolic excursion on M-mode echocardiography across the tricuspid annulus. d | A substantial increase in pulmonary vascular resistance might be associated with a midsystolic notch (arrows) on pulmonary valve pulsed-wave Doppler ejection wave and a short pulmonary valve acceleration time (78 ms; red lines).
Cardiac arrest
The most extreme presentation of cardiogenic shock is cardiac arrest. International evidence-based guidelines recommend the use of echocardiography to diagnose or exclude some of the causes of arrest109. However, echocardiography should not affect the delivery of high-quality cardiopulmonary resuscitation, and specific training in advanced cardiovascular life support is required, even for experienced practitioners. As images are obtained and recorded only during the pulse/rhythm check, studies performed during cardiac arrest are strictly time-limited, and therefore are dissimilar to comprehensive studies that use only focused 2D imaging aimed at diagnosis or exclusion of potentially reversible causes in a simple, binary manner. The pathology leading to arrest is likely to be extreme (tamponade, massive pulmonary embolism, severe LV and/or RV dysfunction, myocardial infarction/ischaemia, hypovolaemia, or tension pneumothorax) and fairly easy to diagnose without more sophisticated echocardiographic techniques. Whether the use of echocardiography in cardiac arrest (and as part of care after resuscitation) can improve outcomes is unknown, but its application in the prehospital setting has been found to change management strategies in up to 60% of patients110,111.
Acute mechanical circulatory support
The indications for mechanical circulatory support (MCS) in the acute setting are constantly changing112,113. Intra-aortic balloon pumps are no longer routinely recommended for cardiogenic shock114. A range of new percutaneous ventricular assist devices are available, in addition to extracorporeal membrane oxygenation (ECMO). These techniques can be used as a bridge to recovery or for longer-term support, and differ not only in terms of their technical aspects, but the degree and type of support provided (LV and/or RV support, with or without the addition of respiratory support)115–120. Echocardiography is critical for successful implementation of acute MCS121,122 (TABLE 2). MCS is not a treatment per se, but instead a supportive therapy for patients awaiting treatment or resolution of the underlying pathological process. As in all cases of AHF, the most important role of echocardiography is to diagnose the underlying cardiac cause. When the decision to institute MCS is made, echocardiography is then used to corroborate the decision regarding the type and level of support required. Although clear echocardiography parameters have been used to guide longer-term MCS for both the left and right heart123,124, these parameters are not yet available for devices designed for short-term use. Furthermore, clear contraindications to MCS exist that can be diagnosed only using echocardiography. Echocardiography is used in the initiation of MCS, including the use of vascular ultrasonography to guide safe vessel cannulation and steer device or cannula placement. Echocardiography is subsequently used to monitor MCS by ensuring the goals of support are met, and for detecting complications and assessing tolerance to assistance121. Unfortunately, peripheral ECMO can paradoxically worsen cardiac function by increasing LV afterload. Although a number of echocardiographic parameters exist that might indicate this complication (including lack of aortic valve opening, biphasic retrograde flow across the mitral valve in diastole, and retrograde systolic pulmonary venous flow; FIG. 7), the inherent limitations of echocardiography in estimating LAP and LVEDP, especially when the heart is partially bypassed, makes this strategy particularly challenging122. Echocardiography can be used, however, to guide interventions to ensure that the heart is adequately offloaded. Finally, a number of echocardiographic parameters are used in conjunction with clinical and haemodynamic assessment to predict which patients might be successfully weaned off MCS125,126.
Table 2.
Echocardiography for acute mechanical circulatory support
| Type of mechanical support | Indications | Contraindications | Role of echo |
|---|---|---|---|
| VA ECMO |
|
|
|
| Impella (Abiomed, USA) |
|
|
|
| Tandem Heart (Cardiac Assist, USA) |
|
|
|
| IABP |
|
|
|
BAV, balloon aortic valvuloplasty; Echo, echocardiography; IABP, intra-aortic balloon pump; LAP, left atrial pressure; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; MI, myocardial infarction; MR, mitral regurgitation; MV, mitral valve; PCI, percutaneous coronary intervention; RV, right ventricular; S′, peak systolic annular velocity; TDI, tissue Doppler imaging; TOE, transoesophageal echocardiography; VAD, ventricular assist device; VT, ventricular tachycardia; VTI, velocity time integral; VA ECMO, venoarterial extracorporeal membrane oxygenation.
Figure 7. Echocardiographic features in patients receiving extracorporeal support.
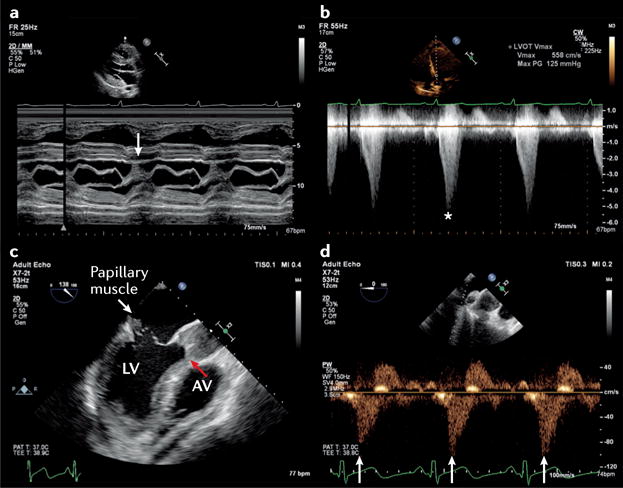
Transthoracic echocardiography in a patient with severe respiratory failure receiving venovenous extracorporeal membrane oxygenation (ECMO). a | Parasternal long axis M-mode echocardiography across the mitral valve showing systolic anterior motion of the mitral valve leaflets (arrow). b | This motion was associated with substantial left ventricular intracavity gradient of 125 mmHg (asterisk). c | A complication of ST-segment elevation myocardial infarction requiring peripheral ECMO is revealed on M-mode echocardiography; papillary muscle rupture had resulted in a flail anterior mitral valve leaflet (white arrow) with associated torrential mitral regurgitation. The increase in left ventricular afterload from ECMO has resulted in failure of the left ventricle (LV) to eject, with a persistently closed aortic valve (AV; red arrow) and stasis of blood in the aortic root. d | Reversal of systolic pulmonary venous flow (arrows) in a patient receiving peripheral venovenous ECMO, suggesting inadequate offloading of the LV.
Other indications
Transoesophageal echocardiograpy can also be used in the acute setting in patients with dynamic mitral regurgitation (see Supplementary information S4 (figure)). Furthermore, features of infective endocarditis caused by aortic prostheses or a device can be demonstrated using transoesophageal echocardiography (see Supplementary information S5 (figure)).
Quality assurance
A detailed overview of the necessary organizational structure and processes for use of ultrasonography and echocardiography in the acute setting is beyond the scope of this Review, and has been published previously26,127–130. However, when used in routine clinical care, training, education, protocols, and ongoing certification of practitioners are required, which should all be performed within existing governance structures.
Conclusions
Echocardiography and LUS can assist in the rapid assessment of patients with acute dyspnoea and hypotension, and have the potential to transform the way in which clinicians assess and manage critically ill patients with AHF and cardiogenic shock (TABLE 3). The current AHF guidelines are cautious in recommendations for the widespread use of advanced echocardiography techniques in the acute care setting because robust applicability data are lacking, interpretation of findings requires highly specialized, in-depth knowledge of cardiac pathophysiology, and there is potential for harm by injudicious application in this patient population. The opportunities to improve diagnostic accuracy, reduce delays in treatment, and improve outcomes through the use of advanced echocardiography need to be further explored.
Table 3.
Proposed initial focused cardiac and lung ultrasonography assessment for patients with suspected AHF in acute care setting
| Clinical question | Structural and functional assessment | Views (2D imaging) | Comments | Evidence |
|---|---|---|---|---|
| Focused echocardiography131,132 | ||||
| Alternative diagnoses for patient’s signs and symptoms? |
|
Subxiphoid, parasternal long-axis and short-axis views, apical four-chamber view | Absence of RV dilatation/dysfunction cannot exclude the presence of pulmonary emboli | |
| Evidence of impaired systolic function? | Global LV systolic function | Subxiphoid, parasternal long-axis and short-axis views, apical four-chamber view | Might be useful in new-onset HF for identification of reduced EF | Sensitivity and specificity for diagnosis of AHF depending on prevalence of HFrEF38,135 |
| Is there (additional) evidence of volume overload? | IVC assessment | IVC (subxiphoid) | IVC collapsibility <50% | Sensitivity 83%, specificity 81% for diagnosis of AHF in patients with dyspnoea in the ED135 |
| Gross structural abnormality as AHF aetiology? | Subxiphoid, parasternal long-axis and short-axis views, apical four-chamber view | AHF aetiology might be identified in rare cases | NA | |
| Lung and pleural ultrasonography37,38 | ||||
| Alternative diagnoses for patient’s signs and symptoms? | Pneumothorax assessment | Anterior, upper chest on each hemithorax | Presence of lung sliding along pleural line rules out pneumothorax in the scanned chest zones | Sensitivity 91%, specificity 98% for detection of pneumothorax136 |
| Evidence of pulmonary oedema? | Pulmonary oedema detection | Three or four anterior/lateral chest zones on each hemithorax | Three or more B-lines in two or more zones on each hemithorax considered diagnostic for AHF | Sensitivity 94%, specificity 92% for diagnosis of AHF in patients with dyspnoea in the ED33,38 |
| Evidence of pleural effusions? | Pleural effusion detection | Posterior axillary line on both hemithoraces | Echo-free space above the diaphragm | Sensitivity 79–84%, specificity 83–98% for diagnosis of AHF in patients with dyspnoea in the ED44,45 |
Valvular abnormalities recognizable with focused echocardiography (without the use of Doppler-based techniques) entail leaflet or cusp massive disruption or marked thickening, flail, or anatomical gaps.
Refers to large valve vegetations or visible intracardiac or IVC thrombi. AHF, acute heart failure; Echo, echocardiography; ED, emergency department; EF, ejection fraction; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; IVC, inferior vena cava; LV, left ventricular; NA, not available; PE, pulmonary embolism; RV, right ventricular.
Supplementary Material
Key points.
Over-reliance of traditional clinical findings and symptoms can potentially delay diagnosis of acute heart failure (AHF), prolonging the time to appropriate therapy
The use of echocardiography and lung ultrasonography can help to improve diagnostic accuracy and monitor responses to interventions in patients with AHF
Lung ultrasonography allows for rapid assessment of numerous conditions, including pulmonary oedema, pleural effusion, and pneumothorax
Use of echocardiography has extended beyond the traditional application in stable patients to become widespread in the acute and emergency settings
In the setting of AHF, echocardiography can be used to assess pericardial effusion, right ventricular dilatation, left ventricular systolic function, gross valvular abnormality, and potentially the presence of intracardiac masses
Echocardiography can also be used to monitor treatment in patients with cardiogenic shock
Footnotes
Author contributions
S.P., L.C., and G.T. researched data for the article. S.P., E.P., and G.T. wrote the manuscript. S.P., L.C., E.P., J.M., and W.F.P. substantially contributed to the discussion of content. All the authors reviewed and edited the manuscript before submission.
Competing interests statement
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION
S1 (video) | S2 (table) | S3 (table) | S4 (figure) | S5 (figure)
References
- 1.Yancy CW, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dworzynski K, Roberts E, Ludman A, Mant J, Guideline Development Group of the National Institute for Health and Care Excellence Diagnosing and managing acute heart failure in adults: summary of NICE guidance. BMJ. 2014;349:g5695. doi: 10.1136/bmj.g5695. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 5.Braunwald E. Heart failure. JACC Heart Fail. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosy AP, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 7.Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–376. doi: 10.1016/j.ijcard.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 8.Ray P, et al. Acute respiratory failure in the elderly: etiology, emergency diagnosis and prognosis. Crit Care. 2006;10:R82. doi: 10.1186/cc4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mebazaa A, et al. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med. 2016;42:147–163. doi: 10.1007/s00134-015-4041-5. [DOI] [PubMed] [Google Scholar]
- 10.Mebazaa A, et al. Recommendations on pre-hospital and early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail. 2015;17:544–558. doi: 10.1002/ejhf.289. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 12.Maisel AS, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol. 2008;52:534–540. doi: 10.1016/j.jacc.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Januzzi JL, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27:330–337. doi: 10.1093/eurheartj/ehi631. [DOI] [PubMed] [Google Scholar]
- 14.Miro O, et al. European Society of Cardiology — Acute Cardiovascular Care Association position paper on safe discharge of acute heart failure patients from the emergency department. Eur Heart J Acute Cardiovasc Care. 2016 doi: 10.1177/2048872616633853. http://dx.doi.org/10.1177/2048872616633853. [DOI] [PMC free article] [PubMed]
- 15.Mueller C, et al. European Society of Cardiology — Acute Cardiovascular Care Association position paper on acute heart failure: a call for interdisciplinary care. Eur Heart J Acute Cardiovasc Care. 2017;6:81–86. doi: 10.1177/2048872615593279. [DOI] [PubMed] [Google Scholar]
- 16.Mebazaa A, et al. Recommendations on pre-hospital and early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine — short version. Eur Heart J. 2015;36:1958–1966. doi: 10.1093/eurheartj/ehv066. [DOI] [PubMed] [Google Scholar]
- 17.Weintraub NL, et al. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122:1975–1996. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 18.Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944–1956. doi: 10.1001/jama.294.15.1944. [DOI] [PubMed] [Google Scholar]
- 19.Konstantinides SV, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 20.Mueller C, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350:647–654. doi: 10.1056/NEJMoa031681. [DOI] [PubMed] [Google Scholar]
- 21.Budweiser S, et al. NT-proBNP in chronic hypercapnic respiratory failure: a marker of disease severity, treatment effect and prognosis. Respir Med. 2007;101:2003–2010. doi: 10.1016/j.rmed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Medina AM, et al. Prognostic utility of NT-proBNP in acute exacerbations of chronic pulmonary diseases. Eur J Intern Med. 2011;22:167–171. doi: 10.1016/j.ejim.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Campo G, et al. Cardiovascular history and adverse events in patients with acute exacerbation of COPD. COPD. 2015;12:560–567. doi: 10.3109/15412555.2014.995293. [DOI] [PubMed] [Google Scholar]
- 24.Gaggin HK, Januzzi JL., Jr Biomarkers and diagnostics in heart failure. Biochim Biophys Acta. 2013;1832:2442–2450. doi: 10.1016/j.bbadis.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Peacock WF, Cannon CM, Singer AJ, Hiestand BC. Considerations for initial therapy in the treatment of acute heart failure. Crit Care. 2015;19:399. doi: 10.1186/s13054-015-1114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lancellotti P, et al. The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care. 2015;4:3–5. doi: 10.1177/2048872614568073. [DOI] [PubMed] [Google Scholar]
- 27.Price S, Nicol E, Gibson DG, Evans TW. Echocardiography in the critically ill: current and potential roles. Intensive Care Med. 2006;32:48–59. doi: 10.1007/s00134-005-2834-7. [DOI] [PubMed] [Google Scholar]
- 28.Porter TR, et al. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015;28:40–56. doi: 10.1016/j.echo.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Volpicelli G, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 30.Platz E, et al. Utility of lung ultrasound in predicting pulmonary and cardiac pressures. Eur J Heart Fail. 2012;14:1276–1284. doi: 10.1093/eurjhf/hfs144. [DOI] [PubMed] [Google Scholar]
- 31.Liteplo AS, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): sonographic B-lines and N-terminal pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med. 2009;16:201–210. doi: 10.1111/j.1553-2712.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 32.Anderson KL, et al. Inter-rater reliability of quantifying pleural B-lines using multiple counting methods. J Ultrasound Med. 2013;32:115–120. doi: 10.7863/jum.2013.32.1.115. [DOI] [PubMed] [Google Scholar]
- 33.Pivetta E, et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: a SIMEU multicenter study. Chest. 2015;148:202–210. doi: 10.1378/chest.14-2608. [DOI] [PubMed] [Google Scholar]
- 34.Platz E, Jhund PS, Campbell RT, McMurray JJ. Assessment and prevalence of pulmonary oedema in contemporary acute heart failure trials: a systematic review. Eur J Heart Fail. 2015;17:906–916. doi: 10.1002/ejhf.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volpicelli G, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585–591. doi: 10.1016/j.ajem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Gargani L, et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovasc Ultrasound. 2015;13:40. doi: 10.1186/s12947-015-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014;21:843–852. doi: 10.1111/acem.12435. [DOI] [PubMed] [Google Scholar]
- 38.Martindale JL, et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:223–242. doi: 10.1111/acem.12878. [DOI] [PubMed] [Google Scholar]
- 39.Agricola E, et al. “Ultrasound comet-tail images”: a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127:1690–1695. doi: 10.1378/chest.127.5.1690. [DOI] [PubMed] [Google Scholar]
- 40.Miglioranza MH, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. 2013;6:1141–1151. doi: 10.1016/j.jcmg.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Volpicelli G, et al. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology. 2014;121:320–327. doi: 10.1097/ALN.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 42.Gargani L, et al. Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: a comparison with natriuretic peptides. Eur J Heart Fail. 2008;10:70–77. doi: 10.1016/j.ejheart.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Frasure SE, et al. Impact of patient positioning on lung ultrasound findings in acute heart failure. Eur Heart J Acute Cardiovasc Care. 2014;4:326–332. doi: 10.1177/2048872614551505. [DOI] [PubMed] [Google Scholar]
- 44.Cibinel GA, et al. Diagnostic accuracy and reproducibility of pleural and lung ultrasound in discriminating cardiogenic causes of acute dyspnea in the emergency department. Intern Emerg Med. 2011;7:65–70. doi: 10.1007/s11739-011-0709-1. [DOI] [PubMed] [Google Scholar]
- 45.Russell FM, et al. Diagnosing acute heart failure in patients with undifferentiated dyspnea: a lung and cardiac ultrasound (LuCUS) protocol. Acad Emerg Med. 2015;22:182–191. doi: 10.1111/acem.12570. [DOI] [PubMed] [Google Scholar]
- 46.Volpicelli G. Sonographic diagnosis of pneumothorax. Intensive Care Med. 2011;37:224–232. doi: 10.1007/s00134-010-2079-y. [DOI] [PubMed] [Google Scholar]
- 47.Lichtenstein D, Meziere G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26:1434–1440. doi: 10.1007/s001340000627. [DOI] [PubMed] [Google Scholar]
- 48.Zoccali C, et al. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol. 2013;24:639–646. doi: 10.1681/ASN.2012100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gargani L. Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound. 2011;9:6. doi: 10.1186/1476-7120-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bataille B, et al. Integrated use of bedside lung ultrasound and echocardiography in acute respiratory failure: a prospective observational study in ICU. Chest. 2014;146:1586–1593. doi: 10.1378/chest.14-0681. [DOI] [PubMed] [Google Scholar]
- 51.Neskovic AN, et al. Emergency echocardiography: the European Association of Cardiovascular Imaging recommendations. Eur Heart J Cardiovasc Imaging. 2013;14:1–11. doi: 10.1093/ehjci/jes193. [DOI] [PubMed] [Google Scholar]
- 52.Cholley BP, Vieillard-Baron A, Mebazaa A. Echocardiography in the ICU: time for widespread use! Intensive Care Med. 2006;32:9–10. doi: 10.1007/s00134-005-2833-8. [DOI] [PubMed] [Google Scholar]
- 53.Sicari R, et al. The use of pocket-size imaging devices: a position statement of the European Association of Echocardiography. Eur J Echocardiogr. 2011;12:85–87. doi: 10.1093/ejechocard/jeq184. [DOI] [PubMed] [Google Scholar]
- 54.Flachskampf FA, et al. Recommendations for transoesophageal echocardiography: EACVI update 2014. Eur Heart J Cardiovasc Imaging. 2014;15:353–365. doi: 10.1093/ehjci/jeu015. [DOI] [PubMed] [Google Scholar]
- 55.Silvestry FE, et al. Echocardiography-guided interventions. J Am Soc Echocardiogr. 2009;22:213–231. doi: 10.1016/j.echo.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Zamorano JL, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur Heart J. 2011;32:2189–2214. doi: 10.1093/eurheartj/ehr259. [DOI] [PubMed] [Google Scholar]
- 57.Reeves ST, et al. Basic perioperative transesophageal echocardiography examination: a consensus statement of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26:443–456. doi: 10.1016/j.echo.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Hawkins NM, et al. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11:130–139. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferguson ND, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 60.Pinsky MR. Clinical significance of pulmonary artery occlusion pressure. Intensive Care Med. 2003;29:175–178. doi: 10.1007/s00134-002-1616-8. [DOI] [PubMed] [Google Scholar]
- 61.Luchsinger PC, Seipp HW, Jr, Patel DJ. Relationship of pulmonary artery-wedge pressure to left atrial pressure in man. Circ Res. 1962;11:315–318. doi: 10.1161/01.res.11.2.315. [DOI] [PubMed] [Google Scholar]
- 62.Nagy AI, et al. The pulmonary capillary wedge pressure accurately reflects both normal and elevated left atrial pressure. Am Heart J. 2014;167:876–883. doi: 10.1016/j.ahj.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 63.Chatterjee K. The Swan–Ganz catheters: past, present, and future. A viewpoint. Circulation. 2009;119:147–152. doi: 10.1161/CIRCULATIONAHA.108.811141. [DOI] [PubMed] [Google Scholar]
- 64.Oh JK, Park SJ, Nagueh SF. Established and novel clinical applications of diastolic function assessment by echocardiography. Circ Cardiovasc Imaging. 2011;4:444–455. doi: 10.1161/CIRCIMAGING.110.961623. [DOI] [PubMed] [Google Scholar]
- 65.Diwan A, McCulloch M, Lawrie GM, Reardon MJ, Nagueh SF. Doppler estimation of left ventricular filling pressures in patients with mitral valve disease. Circulation. 2005;111:3281–3289. doi: 10.1161/CIRCULATIONAHA.104.508812. [DOI] [PubMed] [Google Scholar]
- 66.Oh JK. Echocardiography as a noninvasive Swan–Ganz catheter. Circulation. 2005;111:3192–3194. doi: 10.1161/CIRCULATIONAHA.105.548644. [DOI] [PubMed] [Google Scholar]
- 67.Temporelli PL, Scapellato F, Eleuteri E, Imparato A, Giannuzzi P. Doppler echocardiography in advanced systolic heart failure: a noninvasive alternative to Swan–Ganz catheter. Circ Heart Fail. 2010;3:387–394. doi: 10.1161/CIRCHEARTFAILURE.108.809590. [DOI] [PubMed] [Google Scholar]
- 68.Ritzema JL, et al. Serial Doppler echocardiography and tissue Doppler imaging in the detection of elevated directly measured left atrial pressure in ambulant subjects with chronic heart failure. JACC Cardiovasc Imaging. 2011;4:927–934. doi: 10.1016/j.jcmg.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 69.Lester SJ, et al. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol. 2008;51:679–689. doi: 10.1016/j.jacc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 70.Vignon P, et al. Echocardiographic assessment of pulmonary artery occlusion pressure in ventilated patients: a transoesophageal study. Crit Care. 2008;12:R18. doi: 10.1186/cc6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vignon P. Hemodynamic assessment of critically ill patients using echocardiography Doppler. Curr Opin Crit Care. 2005;11:227–234. doi: 10.1097/01.ccx.0000159946.89658.51. [DOI] [PubMed] [Google Scholar]
- 72.Cikes M, Solomon SD. Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J. 2016;37:1642–1650. doi: 10.1093/eurheartj/ehv510. [DOI] [PubMed] [Google Scholar]
- 73.Blyakhman FA, et al. Validity of ejection fraction as a measure of myocardial functional state: impact of asynchrony. Eur J Echocardiogr. 2009;10:613–618. doi: 10.1093/ejechocard/jep010. [DOI] [PubMed] [Google Scholar]
- 74.Jones CJ, Raposo L, Gibson DG. Functional importance of the long axis dynamics of the human left ventricle. Br Heart J. 1990;63:215–220. doi: 10.1136/hrt.63.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henein MY, Gibson DG. Long axis function in disease. Heart. 1999;81:229–231. doi: 10.1136/hrt.81.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tavazzi G, Via G, Braschi A, Price S. An 82-year-old woman with ongoing dyspnea. Chest. 2016;150:e9–e11. doi: 10.1016/j.chest.2016.02.687. [DOI] [PubMed] [Google Scholar]
- 77.Perrier A, Tamm C, Unger PF, Lerch R, Sztajzel J. Diagnostic accuracy of Doppler-echocardiography in unselected patients with suspected pulmonary embolism. Int J Cardiol. 1998;65:101–109. doi: 10.1016/s0167-5273(98)00107-7. [DOI] [PubMed] [Google Scholar]
- 78.Casazza F, Bongarzoni A, Capozi A, Agostoni O. Regional right ventricular dysfunction in acute pulmonary embolism and right ventricular infarction. Eur J Echocardiogr. 2005;6:11–14. doi: 10.1016/j.euje.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Amsallem M, et al. Addressing the controversy of estimating pulmonary arterial pressure by echocardiography. J Am Soc Echocardiogr. 2016;29:93–102. doi: 10.1016/j.echo.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 80.Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120:992–1007. doi: 10.1161/CIRCULATIONAHA.106.674028. [DOI] [PubMed] [Google Scholar]
- 81.Lindqvist P, Calcutteea A, Henein M. Echocardiography in the assessment of right heart function. Eur J Echocardiogr. 2008;9:225–234. doi: 10.1016/j.euje.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 82.Bossone E, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr. 2013;26:1–14. doi: 10.1016/j.echo.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 83.Imazio M, Adler Y. Management of pericardial effusion. Eur Heart J. 2013;34:1186–1197. doi: 10.1093/eurheartj/ehs372. [DOI] [PubMed] [Google Scholar]
- 84.Adler Y, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC). Endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2015;36:2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015;36:1223–1230. doi: 10.1093/eurheartj/ehv051. [DOI] [PubMed] [Google Scholar]
- 86.Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117:686–697. doi: 10.1161/CIRCULATIONAHA.106.613596. [DOI] [PubMed] [Google Scholar]
- 87.Monnet X, Teboul JL. Assessment of volume responsiveness during mechanical ventilation: recent advances. Crit Care. 2013;17:217. doi: 10.1186/cc12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Somma S, et al. The emerging role of biomarkers and bio-impedance in evaluating hydration status in patients with acute heart failure. Clin Chem Lab Med. 2012;50:2093–2105. doi: 10.1515/cclm-2012-0289. [DOI] [PubMed] [Google Scholar]
- 89.Charron C, Caille V, Jardin F, Vieillard-Baron A. Echocardiographic measurement of fluid responsiveness. Curr Opin Crit Care. 2006;12:249–254. doi: 10.1097/01.ccx.0000224870.24324.cc. [DOI] [PubMed] [Google Scholar]
- 90.Mebazaa A, Karpati P, Renaud E, Algotsson L. Acute right ventricular failure — from pathophysiology to new treatments. Intensive Care Med. 2004;30:185–196. doi: 10.1007/s00134-003-2025-3. [DOI] [PubMed] [Google Scholar]
- 91.Inohara T, Kohsaka S, Fukuda K, Menon V. The challenges in the management of right ventricular infarction. Eur Heart J Acute Cardiovasc Care. 2013;2:226–234. doi: 10.1177/2048872613490122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bendjelid K, Romand JA. Fluid responsiveness in mechanically ventilated patients: a review of indices used in intensive care. Intensive Care Med. 2003;29:352–360. doi: 10.1007/s00134-002-1615-9. [DOI] [PubMed] [Google Scholar]
- 93.Pinsky MR. My paper 20 years later: effect of positive end-expiratory pressure on right ventricular function in humans. Intensive Care Med. 2014;40:935–941. doi: 10.1007/s00134-014-3294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reuse C, Vincent JL, Pinsky MR. Measurements of right ventricular volumes during fluid challenge. Chest. 1990;98:1450–1454. doi: 10.1378/chest.98.6.1450. [DOI] [PubMed] [Google Scholar]
- 95.Francis GS, Bartos JA, Adatya S. Inotropes. J Am Coll Cardiol. 2014;63:2069–2078. doi: 10.1016/j.jacc.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 96.Unverzagt S, et al. Inotropic agents and vasodilator strategies for acute myocardial infarction complicated by cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2014;1:CD009669. doi: 10.1002/14651858.CD009669.pub2. [DOI] [PubMed] [Google Scholar]
- 97.Bangash MN, Kong ML, Pearse RM. Use of inotropes and vasopressor agents in critically ill patients. Br J Pharmacol. 2012;165:2015–2033. doi: 10.1111/j.1476-5381.2011.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singer M. Catecholamine treatment for shock — equally good or bad? Lancet. 2007;370:636–637. doi: 10.1016/S0140-6736(07)61317-8. [DOI] [PubMed] [Google Scholar]
- 99.Duncan AM, Francis DP, Gibson DG, Henein MY. Limitation of exercise tolerance in chronic heart failure: distinct effects of left bundle-branch block and coronary artery disease. J Am Coll Cardiol. 2004;43:1524–1531. doi: 10.1016/j.jacc.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 100.Duncan AM, O’Sullivan CA, Gibson DG, Henein MY. Electromechanical interrelations during dobutamine stress in normal subjects and patients with coronary artery disease: comparison of changes in activation and inotropic state. Heart. 2001;85:411–416. doi: 10.1136/heart.85.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duncan AM, Francis DP, Henein MY, Gibson DG. Limitation of cardiac output by total isovolumic time during pharmacologic stress in patients with dilated cardiomyopathy: activation-mediated effects of left bundle branch block and coronary artery disease. J Am Coll Cardiol. 2003;41:121–128. doi: 10.1016/s0735-1097(02)02665-7. [DOI] [PubMed] [Google Scholar]
- 102.Thygesen K, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 103.Gibson DG, Francis DP. Clinical assessment of left ventricular diastolic function. Heart. 2003;89:231–238. doi: 10.1136/heart.89.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henein MY, Gibson DG. Suppression of left ventricular early diastolic filling by long axis asynchrony. Br Heart J. 1995;73:151–157. doi: 10.1136/hrt.73.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brooks H, Kirk ES, Vokonas PS, Urschel CW, Sonnenblick EH. Performance of the right ventricle under stress: relation to right coronary flow. J Clin Invest. 1971;50:2176–2183. doi: 10.1172/JCI106712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rudski LG, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 107.Hoeper MM, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med. 2011;184:1114–1124. doi: 10.1164/rccm.201104-0662CI. [DOI] [PubMed] [Google Scholar]
- 108.Chockalingam A, Tejwani L, Aggarwal K, Dellsperger KC. Dynamic left ventricular outflow tract obstruction in acute myocardial infarction with shock: cause, effect, and coincidence. Circulation. 2007;116:e110–e113. doi: 10.1161/CIRCULATIONAHA.107.711697. [DOI] [PubMed] [Google Scholar]
- 109.Soar J, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support. Resuscitation. 2015;95:100–147. doi: 10.1016/j.resuscitation.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 110.Monsieurs KG, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 1. Executive summary. Resuscitation. 2015;95:1–80. doi: 10.1016/j.resuscitation.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 111.Beaulieu Y. Bedside echocardiography in the assessment of the critically ill. Crit Care Med. 2007;35:S235–S249. doi: 10.1097/01.CCM.0000260673.66681.AF. [DOI] [PubMed] [Google Scholar]
- 112.Shah KB, et al. Mechanical circulatory support devices in the ICU. Chest. 2014;146:848–857. doi: 10.1378/chest.13-2645. [DOI] [PubMed] [Google Scholar]
- 113.Stewart GC, Givertz MM. Mechanical circulatory support for advanced heart failure: patients and technology in evolution. Circulation. 2012;125:1304–1315. doi: 10.1161/CIRCULATIONAHA.111.060830. [DOI] [PubMed] [Google Scholar]
- 114.Thiele H, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638–1645. doi: 10.1016/S0140-6736(13)61783-3. [DOI] [PubMed] [Google Scholar]
- 115.Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J. 2014;35:156–167. doi: 10.1093/eurheartj/eht248. [DOI] [PubMed] [Google Scholar]
- 116.Drakos SG, Uriel N. Spotlight on cardiogenic shock therapies in the era of mechanical circulatory support. Curr Opin Cardiol. 2014;29:241–243. doi: 10.1097/HCO.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 117.Peura JL, et al. Recommendations for the use of mechanical circulatory support: device strategies and patient selection: a scientific statement from the American Heart Association. Circulation. 2012;126:2648–2667. doi: 10.1161/CIR.0b013e3182769a54. [DOI] [PubMed] [Google Scholar]
- 118.Kapur NK, et al. Mechanical circulatory support for right ventricular failure. JACC Heart Fail. 2013;1:127–134. doi: 10.1016/j.jchf.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 119.Cheung AW, White CW, Davis MK, Freed DH. Short-term mechanical circulatory support for recovery from acute right ventricular failure: clinical outcomes. J Heart Lung Transplant. 2014;33:794–799. doi: 10.1016/j.healun.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 120.Kirklin JK, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 121.Platts DG, Sedgwick JF, Burstow DJ, Mullany DV, Fraser JF. The role of echocardiography in the management of patients supported by extracorporeal membrane oxygenation. J Am Soc Echocardiogr. 2012;25:131–141. doi: 10.1016/j.echo.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 122.Doufle G, Roscoe A, Billia F, Fan E. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit Care. 2015;19:326. doi: 10.1186/s13054-015-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ammar KA, et al. The ABCs of left ventricular assist device echocardiography: a systematic approach. Eur Heart J Cardiovasc Imaging. 2012;13:885–899. doi: 10.1093/ehjci/jes090. [DOI] [PubMed] [Google Scholar]
- 124.Stainback RF, et al. Echocardiography in the management of patients with left ventricular assist devices: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015;28:853–909. doi: 10.1016/j.echo.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 125.Aissaoui N, et al. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med. 2011;37:1738–1745. doi: 10.1007/s00134-011-2358-2. [DOI] [PubMed] [Google Scholar]
- 126.Aissaoui N, et al. Two-dimensional strain rate and Doppler tissue myocardial velocities: analysis by echocardiography of hemodynamic and functional changes of the failed left ventricle during different degrees of extracorporeal life support. J Am Soc Echocardiogr. 2012;25:632–640. doi: 10.1016/j.echo.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 127.Evangelista A, et al. European Association of Echocardiography recommendations for standardization of performance, digital storage and reporting of echocardiographic studies. Eur J Echocardiogr. 2008;9:438–448. doi: 10.1093/ejechocard/jen174. [DOI] [PubMed] [Google Scholar]
- 128.Price S, et al. Echocardiography practice, training and accreditation in the intensive care: document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS) Cardiovasc Ultrasound. 2008;6:49. doi: 10.1186/1476-7120-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spencer KT, et al. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567–581. doi: 10.1016/j.echo.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 130.Levitov A, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients — part II: cardiac ultrasonography. Crit Care Med. 2016;44:1206–1227. doi: 10.1097/CCM.0000000000001847. [DOI] [PubMed] [Google Scholar]
- 131.Labovitz AJ, et al. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23:1225–1230. doi: 10.1016/j.echo.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 132.Via G, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. 2014;27:e1–e33. doi: 10.1016/j.echo.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 133.Jones AE, Tayal VS, Kline JA. Focused training of emergency medicine residents in goal-directed echocardiography: a prospective study. Acad Emerg Med. 2003;10:1054–1058. doi: 10.1111/j.1553-2712.2003.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 134.Lucas BP, et al. Diagnostic accuracy of hospitalist-performed hand-carried ultrasound echocardiography after a brief training program. J Hosp Med. 2009;6:340–349. doi: 10.1002/jhm.438. [DOI] [PubMed] [Google Scholar]
- 135.Kajimoto K, et al. Rapid evaluation by lung-cardiac-inferior vena cava (LCI) integrated ultrasound for differentiating heart failure from pulmonary disease as the cause of acute dyspnea in the emergency setting. Cardiovasc Ultrasound. 2012;10:49. doi: 10.1186/1476-7120-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest. 2012;141:703–708. doi: 10.1378/chest.11-0131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


