Abstract
BACKGROUND
Hypoperfusion is associated with hyperfibrinolysis and early death from exsanguination, whereas tissue trauma is associated with hypofibrinolysis and delayed death from organ failure. We sought to elucidate the effects of injury patterns on fibrinolysis phenotypes using a nonhuman primate (NHP) model.
METHODS
NHPs were randomized to three injury groups (n = 8/group): 60 minutes severe pressure-targeted controlled hemorrhagic shock (HS); HS + soft tissue injury (HS+); or HS + soft tissue injury + femur fracture (HS++). Animals were resuscitated and monitored for 360 minutes. Blood samples were collected at baseline, end-of-shock, end-of-resuscitation (EOR), and T = 360 minutes for assessments of: severity of shock (lactate) and coagulation via prothrombin time, partial thromboplastin time, D-dimer, fibrinogen, antithrombin-III, von Willebrand factor, and viscoelastic testing (ROTEM). Results are reported as mean ± SEM; statistics: two-way analysis of variance and t-tests (significance: p < 0.05).
RESULTS
Blood loss, prothrombin time, partial thromboplastin time, antithrombin-III, fibrinogen, and von Willebrand factor were equivalent among groups and viscoelastic testing revealed few differences throughout the study. D-dimer increased approximately threefold, at EOR in the HS group, and at T = 360 minutes in the HS+ and HS++ groups (p < 0.05). At EOR, in the HS group compared with the HS+ and HS++ groups; the D-dimer-lactate ratio was twofold greater (2.2 ± 0.3 vs. 1.1 ± 0.3 and 1.1 ± 0.2, respectively; p < 0.05) and tissue factor-activated fibrin clot 30-minute lysis index was lower (98 ± 1% vs. 100 ± 0% and 100 ± 0%, respectively; p < 0.05).
CONCLUSION
NHPs in HS exhibit acute suppression of fibrinolysis in the presence of tissue injury. Additional assessments to more comprehensively evaluate the mechanisms linking tissue injury with the observed fibrinolysis shutdown response are warranted.
Keywords: Coagulation, clotting, hemostasis, injury, fibrin, shock, shutdown
Hemorrhage is the leading cause of potentially preventable death in military1 and civilian trauma.2 Coagulopathy is present in 20% to 40% of trauma patients after severe injury and associated with a fourfold increase in mortality.3 Trauma-induced coagulopathy (TIC) has been shown to increase with Injury Severity Score (ISS) from 10.8% (ISS < 14) to 33.1% (ISS > 15) and 61.7% (ISS > 45).4 Although progress has been made in understanding and treating dysregulation of the coagulation system in response to trauma, recent discoveries have generated a number of hypotheses addressing the mechanisms responsible for TIC.5 Although the majority of work related to TIC has focused on impairment of clot formation, abnormalities of clot degradation (i.e., fibrinolysis) have also been associated with increased mortality in trauma and sepsis.3,6–8 Hyperfibrinolysis (i.e., excessive clot degradation) is associated with early death from exsanguination,9,10 whereas impaired fibrinolysis (i.e., fibrinolysis shutdown) is associated with delayed death due to organ failure.6,11
Hypoperfusion is associated with hyperfibrinolysis through reduced plasminogen activator inhibitor-1 (PAI-1)3 via overwhelming tissue plasminogen activator (tPA) release.12 An initial report by Schöchl et al.13 suggested fibrinolysis was provoked by tissue injury, but subsequently, hyperfibrinolysis was found to be independent of tissue injury because it is common in patients with nontrauma-related cardiac arrest.14 On the other hand, a cohort of trauma patients exhibiting fibrinolysis shutdown had increased mortality associated with organ failure.6 Moreover, tissue injury has not been shown to promote fibrinolysis in rodents and swine and may, in fact, stimulate fibrinolysis shutdown.15,16 Recent advances in human cell/tissue-derived therapeutics (e.g., infusible human platelet-derived hemostatic agents [hPDHA]) have necessitated preclinical evaluations in a nonhuman primate (NHP) model due to xenogeneic compatibility,17 thus, a more thorough examination of coagulation system function in NHPs is prudent. Although high-fidelity studies can be conducted in lower-order species, such as rats and swine, there is great variability between species. For example, swine exhibit intrinsic hypercoagulation and lack of a hyperfibrinolytic response, thus justifying doing experiments with NHP, which more accurately reflect human response to injury. Furthermore, swine exhibit an hypercoagulable state, even in response to surgical catheter placement (i.e., sham animals). Additionally, xenogeneic incompatibility between swine models and PDHA has been demonstrated in one study that resulted in lethal pulmonary artery thrombi formation, thus hampering the evaluation of hPDHA in swine and necessitating xenogeneically compatible models.
NHPs (e.g., rhesus macaques) represent a viable species for evaluation of human-derived therapeutics for trauma, however, there is currently a paucity of information in the literature with regard to NHP coagulation system function and fibrinolysis phenotypes in response to trauma. The variability of the fibrinolytic response among different species becomes increasingly important not only in the understanding of the coagulation response but also when using the animal model for evaluations of human derived therapeutics. Additionally, a deeper understanding of the fibrinolytic mechanism in response to trauma in the NHP may provide insight to optimal use of anti-fibrinolytic therapy with tranexamic acid, as well as the development of novel therapeutic strategies to attenuate fibrinolysis inhibition. Therefore, we sought to elucidate the effects of injury pattern on clotting fibrinolysis in response to hemorrhage and multiple injuries in a NHP model and hypothesized that the addition of tissue injury to hemorrhagic shock (HS) would promote a state of impaired fibrinolysis.
MATERIALS AND METHODS
Ethical Approval and Accreditation
The study protocol was approved by the Institutional Animal Care and Use Committee at the 711th Human Performance Wing, Joint Base San Antonio-Fort Sam Houston, and conducted in accordance with the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council, National Academy Press, 2011. All procedures were performed in facilities accredited by the Association for Assessment and Accreditation for Laboratory Animal Care International.
Preoperative Preparation
Rhesus macaques (n = 24) weighing 7 to 14 kg were housed in compliance with the Secretary of the Navy Instruction (SECNAVINST) 3900.38C regulations. Feed was withheld 12 hours before surgery. Immediately before each procedure, animals were sedated and anesthetized as described below, transported to the operating room, surgical sites were sheared and cleaned with chlorohexidine, and animals were draped to create a sterile surgical field.
Anesthesia and Analgesia
Animals were sedated with Tiletamine-Zolazepam (Telazol; 3.0 mg/kg; Fort Dodge Animal Health, Overland Park, KS), premedicated with an analgesic, buprenorphine (Buprenex; 0.03 mg/kg; Reckitt Benkiser, Slough, Berkshire, UK), and weighed (Detecto Scale, Webb City, MO). Airway intubation was achieved with placement of a pediatric, size 4 to 5.5 mm, endotracheal tube (Rusch; Teleflex, Research Triangle Park, NC), and animals were placed on a Dräger Apollo Anesthesia Workstation (Draeger Medical Inc., Telford, PA) with volume-controlled respiration (10 mL/kg) at 12 to 15 breaths per minute, FIO2 of 21% to 25% and isoflurane (1.0–2.0%) inhalational anesthesia. Core body temperature was monitored continuously via 10 Fr rectal temperature probe connected to a Datex Ohmeda Cardiocap/5 (GE Healthcare Systems, Wauwatosa, WI) patient monitor and maintained between 36.0°C and 38.0°C.
Arterial and Venous Line Placement
The right femoral artery was cannulated with a SIL-C70 silicone catheter (Solomon Scientific, Skokie, IL) to facilitate blood sampling and invasive, continuous blood pressure monitoring using the Datex Ohmeda Cardiocap/5 patient monitor (General Electric Co., Fairfield, CT). The right femoral vein and left femoral artery were cannulated with a 14-gauge large bore catheter (Jorgensen Laboratories, Loveland, CO) for intravenous resuscitation fluid infusion and to facilitate hemorrhage, respectively. At no point in the study was heparin used to flush catheters nor were the catheters heparin-lined. To maintain patency, catheters were filled and periodically pulsed with small volumes (<1 mL) of 0.9% NaCl, normal saline (NS).
Soft Tissue and Musculoskeletal Injury
After baseline (BSLN) blood samples were drawn, a 10-minute “injury window” was used in all groups to standardize procedure times for soft tissue and musculoskeletal injuries. Within this time frame, the randomly assigned additional tissue injury, was produced (Fig. 1).
Figure 1.
Procedure summary. Figure 1 summarizes the overall sequence of events that occurred in each study as described in full detail in Materials and Methods.
Soft tissue injury was created by a 15-cm midline laparotomy incision spanning from the xiphoid process toward the umbilicus. The laparotomy incision remained open during the shock period and was closed in two layers (fascia and skin) with continuous sutures during the resuscitation phase.
The musculoskeletal injury was created on the left leg using a longitudinal 5 cm incision spanning the anterior mid-to distal-femur followed by blunt dissection to separate muscle and muscle insertions from a 4-cm span over the mid-femur. An oscillating power bone saw was used to transect the femur, gauze was placed in the wound for blood collection and the skin incision was closed with staples. During the resuscitation phase, the gauze was removed and weighed to quantify blood loss, the femur was approximated and stabilized with steel plates and screws, and the incision closed in two layers (fascia and skin) with sutures.
Pressure-Targeted Controlled HS
Hemorrhage was initiated by opening a stopcock in-line with the left arterial catheter allowing free-flow of blood until the mean arterial pressure (MAP) reached 20 mm Hg. This moment marked the beginning (T = 0 minute) of the shock period, additional blood was withdrawn as needed to maintain a MAP of 20 to 24 mm Hg until the end-of-shock (EOS) (T = 60 minutes). Blood was collected in a standard donor bag containing anticoagulant citrate dextrose solution-solution A (ACDA-A) at a 1:10 ratio for subsequent use in resuscitation.
Fluid Resuscitation
At T = 60 minutes blood samples were collected and animals were then resuscitated with NS, 2× shed blood volume [SBV]) infused over 30 minutes, followed by whole blood (50% SBV) infused over 30 minutes, and then 1× SBV of NS over 60 minutes. This resuscitation protocol, standardized to blood loss, was adapted from previous studies and used uniformly across all animals to minimize overall imbalance in terms of variability in volume resuscitation relative to blood loss and animal weight.18,19 An end-of-resuscitation (EOR) blood sample was collected at T = 180 minutes, and animals were monitored under anesthesia until T = 360 minutes.
Laboratory Analyses
Arterial blood samples were collected at scheduled intervals throughout the study: BSLN, T = 60 minutes (EOS), T = 180 minutes (EOR), and T = 360 minutes. Each blood sample was collected for whole blood analyses of viscoelastic clotting properties by rotational thromboelastrometry (ROTEM Delta system; TEM Systems Inc., Durham, NC), and plasma coagulation analyses (STAGO STA Compact; Diagnostica Stago Inc, Parsippany, NJ), complete blood cell counts (HemaTrue, HESKA, Loveland, CO), serum chemistries (DRI-CHEM 7000, HESKA), and arterial blood gas (ABG) (GEM Premier 4000, Instrumentation Laboratory, Bedford, MA). All samples were processed consistently throughout the study and in accordance with the manufacturer's recommendations per each device. All readouts were obtained from fresh whole blood, with the exception of STAGO analyses, which were conducted on frozen plasma samples stored at −80°C until analysis.
Plasmatic coagulation (STAGO) analyses included: prothrombin time (PT), partial thromboplastin time and concentrations of antithrombin-III, fibrinogen, D-Dimer, and von Willebrand factor. ROTEM analyses included evaluation of extrinsic coagulation pathway function (EXTEM) and fibrin clotting (FIBTEM). Clotting time, α angle, maximum clot firmness (MCF), and 30-minute, 45-minute, and 60-minute lysis index values (LI30, LI45, and LI60, respectively) were evaluated for EXTEM and FIBTEM.
In vitro studies were conducted using healthy rhesus macaque donor plasma to verify the sensitivity of the D-Dimer assay in NHP samples using tPA-induced fibrinolysis. Blood from eight healthy male rhesus macaques was collected and centrifuged twice at 3,000g for 10 minutes to collect plasma, then stored in 1 mL aliquots at −80°C until used. On the day of analysis, sample aliquots were rapidly thawed at 37°C in a water bath. Each aliquot was split for parallel analyses of fibrinolysis (D-dimer; STA-Liatest D-DI plus; Diagnostica Stago Inc.) analyzed on the STAGO STA-R Evolution coagulation analyzer (Diagnostica Stago, Inc.), and fibrin clot lysis (LI30, LI45, and LI60; ROTEM Delta system, TEM Systems Inc.). For assessment of D-dimer formation, each aliquot was induced to clot with the introduction of 15 mM CaCl2 and a mixture of tissue factor and phospholipid (Dade Innovin, Siemens Medical Solutions USA Inc., Malvern, PA; diluted 1:5,000) alongside a dose of human tPA (EMD Millipore, Billerica, MA) of either 0, 62.5, 125, or 250 pg/ml. After 5 minutes at 37°C, clotted samples were centrifuged at 1,0000g for 4 minutes, serum was collected, and D-dimer was quantified immediately. Clot lysis index via thromboelastometry (i.e., LI30, LI45, and LI60) was measured by mixing an aliquot of plasma with tPA (0, 62.5, 125, or 250 pg/mL) and analyzed with both EXTEM and APTEM tests.
Randomization and Blinding
Before each study, NHPs were randomized by drawing animal identification numbers and assigning them to one of three predetermined, injury pattern protocols (n = 8/group): 60 minutes of severe pressure-targeted controlled HS; HS + soft tissue injury (HS+); or HS + soft tissue injury + femur fracture (HS++). Hematologic assays were performed by research assistants blinded to the injury pattern utilizing instrumentation located in as separate laboratory space within the facility to which samples were transported.
Statistical Analysis
Statistical analyses were performed using Prism 6 (GraphPad Software, Inc., La Jolla, CA). Multiple time-point outcomes were analyzed by two-way analysis of variance with Bonferroni correction for multiple comparisons tests within and among groups. A p value less than 0.05 was considered to be statistically significant. All data are reported as mean ± SEM.
RESULTS
Survival, Blood Loss, Vital Signs, and Hematologic Parameters
All animals survived until T = 360 minutes. Blood loss was equivalent among injury groups and averaged 53 ± 2% of their estimated blood volume. Blood volume in milliliters was estimated by the equation: [animal weight (kg) × 0.054 (rhesus macaque blood volume is 54 ml/kg body weight). There were no BSLN differences among groups and all groups exhibited equivalent alterations from BSLN in: MAP, heart rate, core body temperature, hematocrit, platelet count, pH, lactate, bicarbonate, base excess, and arterial partial pressures of oxygen (PaO2) and carbon dioxide (PaCO2) (Table 1). At BSLN, MAP averaged 61 ± 4 mm Hg (n = 24) and following initiation of hemorrhage MAP was rapidly (134 ± 33 sec (n = 24)) reduced and maintained at 20–24 mm Hg, representing a ~60–65% decrease in MAP. After resuscitation and throughout the remainder of the study, MAP pressure values were restored to BSLN values and equivalent among all groups. Although differences from BSLN values were observed within groups, no differences among groups were observed in any of the measured parameters presented in Table 1.
TABLE 1.
Model Characteristics
| Parameter | Study Period Group | BSLN | EOS | EOR | T = 360 |
|---|---|---|---|---|---|
| MAP, mm Hg | HS | 66 ± 7 | 24 ± 2* | 76 ± 4 | 64 ± 4 |
| HS+ | 58 ± 9 | 24 ± 1* | 59 ± 5 | 55 ± 3 | |
| HS++ | 60 ± 3 | 23 ± 1* | 61 ± 2 | 56 ± 4 | |
| Heart rate, bpm | HS | 126 ± 4 | 150 ± 15* | 137 ± 8 | 166 ± 29 |
| HS+ | 123 ± 3 | 153 ± 4* | 134 ± 4 | 134 ± 5 | |
| HS++ | 115 ± 5 | 154 ± 7* | 139 ± 4 | 138 ± 6 | |
| Temperature, °C | HS | 35.6 ± 0.3 | 36.6 ± 0.2 | 36.5 ± 0.1 | 36.7 ± 0.1 |
| HS+ | 35.7 ± 0.1 | 36.6 ± 0.2 | 36.6 ± 0.1 | 37.1 ± 0.1 | |
| HS++ | 34.8 ± 0.4 | 35.8 ± 0.3 | 36.2 ± 0.2 | 36.9 ± 0.1 | |
| Hematocrit, % | HS | 32.9 ± 0.8 | 25.4 ± 1.2 | 24.0 ± 0.8 | 23. ± 2.0 |
| HS+ | 32.0 ± 0.9 | 24.7 ± 3.2 | 23.7 ± 1.1 | 25.4 ± 1.3 | |
| HS++ | 31.4 ± 0.9 | 26.4 ± 0.9 | 22.7 ± 0.7 | 24.0 ± 0.7 | |
| Platelet count, 103/µL | HS | 296 ± 19 | 224 ± 24* | 210 ± 14* | 215 ± 26* |
| HS+ | 283 ± 16 | 246 ± 22* | 199 ± 14* | 218 ± 12* | |
| HS++ | 264 ± 9 | 238 ± 13* | 192 ± 10* | 200 ± 9* | |
| pH | HS | 7.47 ± 0.01 | 7.48 ± 0.03 | 7.39 ± 0.01 | 7.40 ± 0.01 |
| HS+ | 7.47 ± 0.01 | 7.49 ± 0.03 | 7.39 ± 0.01 | 7.39 ± 0.01 | |
| HS++ | 7.47 ± 0.01 | 7.47 ± 0.01 | 7.40 ± 0.01 | 7.61 ± 0.20 | |
| Lactate, mmol/L | HS | 1.2 ± 0.1 | 2.8 ± 0.5* | 0.8 ± 0.1 | 0.9 ± 0.1 |
| HS+ | 1.4 ± 0.1 | 3.2 ± 0.6* | 1.0 ± 0.1 | 1.0 ± 0.1 | |
| HS++ | 1.4 ± 0.1 | 3.3 ± 0.8* | 1.2 ± 0.2 | 1.4 ± 0.2 | |
| Bicarbonate, mmol/L | HS | 30.6 ± 0.8 | 24.6 ± 1.1* | 23.1 ± 0.5* | 23.1 ± 0.5* |
| HS+ | 30.8 ± 0.3 | 25.8 ± 1.2* | 23.9 ± 0.4* | 24.2 ± 0.2* | |
| HS++ | 31.1 ± 0.6 | 25.1 ± 1.5* | 23.4 ± 0.4* | 23.9 ± 0.3* | |
| Base excess, mmol/L | HS | 5.9 ± 0.8 | 1.0 ± 1.2* | −1.9 ± 0.5* | −1.7 ± 0.6* |
| HS+ | 6.1 ± 0.4 | 2.0 ± 1.0* | −1.2 ± 0.4* | −0.7 ± 0.2* | |
| HS++ | 6.0 ± 0.5 | 1.2 ± 1.2* | −1.6 ± 0.4* | −0.7 ± 0.3* | |
| PaO2, mm Hg | HS | 115.8 ± 9.5 | 119.1 ± 9.6 | 118.3 ± 4.5 | 110.0 ± 9.2 |
| HS+ | 98.8 ± 10.4 | 92.0 ± 15.8 | 110.3 ± 9.1* | 104.0 ± 8.8 | |
| HS++ | 77.8 ± 12.4 | 94.6 ± 14.1 | 93.4 ± 10.3* | 98.3 ± 10.6 | |
| PaCO2, mm Hg | HS | 41.3 ± 1.1 | 33.4 ± 1.7* | 38.1 ± 1.1 | 37.3 ± 0.8* |
| HS+ | 41.9 ± 0.8 | 35.8 ± 3.3 | 39.6 ± 0.9* | 39.6 ± 1.0* | |
| HS++ | 42.0 ± 1.2 | 34.3 ± 2.2* | 37.9 ± 1.5* | 37.9 ± 1.6 |
Values for MAP, heart rate, core body temperature, hematocrit, platelet count, pH, blood lactate, bicarbonate, base excess, and arterial partial pressures of oxygen (PaO2) and carbon dioxide (PaCO2) for the HS, HS+, HS++ groups are shown. Data presented as mean ± SEM. No differences among groups were observed throughout the study.
Statistically significant differences (p < 0.05) from BSLN values within each group.
Plasma Coagulation
PT increased from BSLN in the HS group only at T = 360 minutes (15.3 ± 0.5 vs. 21.9 ± 5.3 sec) respectively), while partial thromboplastin time and von Willebrand factor did not change from BSLN values at any time point in any group (p > 0.05). Fibrinogen was reduced and equivalent in all groups at all time-points (p < 0.05), with the lowest average level at EOR of 123.5 ± 3.9 mg/dL (n = 24) compared to a BSLN value of 179.1 ± 4.9 mg/dL (n = 24). Compared to BSLN values, AT III was reduced at EOR in the HS group (87 ± 3 vs. 66 ± 3%) and the HS++ group (82 ± 4 vs. 61 ± 3% respectively); and this reduction was sustained at T = 360 minutes in the HS group (69 ± 3%; p < 0.05). D-dimer was elevated threefold in the HS group at EOR and in all groups by T = 360 minutes (Fig. 2). The calculated D-dimer to lactate ratio (p < 0.05; Fig. 3) was twofold higher in the HS group compared to the HS+ and HS++ groups at EOR.
Figure 2.
D-dimer. Values for the plasma concentration of D-dimer are shown for the HS group (solid line), HS+ group (coarse dashed line) and HS++ group (fine dashed line) Data presented as mean ± SEM. Statistically significant differences from BSLN values (p < 0.05) are indicated by degree symbol (HS), infinity symbol (HS+), and asterisks (*; HS++).
Figure 3.
D-dimer-lactate ratio. Values for the ratio of plasma concentration of D-dimer divided by the blood lactate concentration were calculated for the HS group (solid line), HS+ group (coarse dashed line) and HS++ group (fine dashed line). Data presented as mean ± SEM. Asterisks (*) indicate statistically significant differences (p < 0.05) between the HS group and the HS+ and HS++ groups.
Thromboelastometry
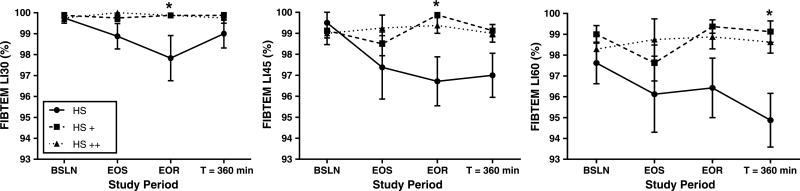
Clotting time, α angle, and MCF in the ROTEM EXTEM and FIBTEM tests did not change from BSLN values and were equivalent among groups throughout the study with few exceptions (see Table, Supplemental Digital Content 1, which shows thromboelastometry clot formation, http://links.lww.com/TA/A886). FIBTEM α angle was decreased at T = 360 minutes in the HS group compared to BSLN values, but unchanged in the HS+ and HS++ groups. FIBTEM MCF was greater in the HS group compared to the HS++ group at EOR (p < 0.05; SDC 1). As shown in Supplemental Digital Content 2 (see Table, Supplemental Digital Content 2, http://links.lww.com/TA/A887) thromboelastometry clot lysis, compared to BSLN values, EXTEMLI30 was higher in the HS group at T = 360 minutes (p < 0.05), but was not significantly different among groups throughout the study. Compared to the HS+ and HS++ groups in which no lysis was observed, the HS group exhibited significantly more lysis in FIBTEMLI30 and LI45 at EOR (p < 0.05), and FIBTEMLI60 at T = 360 minutes (p < 0.05; SDC2; Fig. 4).
Figure 4.
Fibrin clot lysis. Values for 30-minute, 45-minute, and 60-minute lysis index (LI30, LI45, and LI60, respectively) ROTEM FIBTEM test for the HS group (solid line), HS+ group (coarse dashed line) and HS++ group (fine dashed line). Data presented as mean ± SEM. Asterisks (*) indicate significant differences (p < 0.05) between the HS group and both the HS+ and HS++ groups.
In Vitro tPA-Induced Fibrinolysis
Healthy rhesus macaque donor plasma response to tPA was tempered; D-dimer was observed to increase monotonically with tPA dose, but significant differences between groups were only observed at the highest concentration (250 pg/mL; p < 0.01 versus other concentrations; Fig. 5A). Similarly, clot lysis was only observed at 250 pg/ml tPA in EXTEM assay measurements of LI30, LI60, and LI90 (p < 0.001; Fig. 5B), no lysis was observed in APTEM assays.
Figure 5.
In vitro tPA-mediated fibrinolysis. (A) D-dimer concentrations are shown for healthy donor rhesus macaque plasma (n = 8) exposed to tPA. Asterisk (*) indicates significant elevation in D-dimer due to the addition of tPA. (B) Lysis index values (LI30, LI45, and LI60) are shown for healthy donor rhesus macaque plasma (n = 8) exposed to tPA and ROTEM reagents (APTEM, light gray; EXTEM, dark gray). Asterisks (*) indicate significant differences (p < 0.05) between lysis index values between APTEM and EXTEM tests at a given lysis index time and tPA concentration.
DISCUSSION
The current study demonstrates that the addition of tissue injury to HS promotes a fibrinolysis shutdown phenotype in NHPs. The earlier elevation in D-dimer and increased lysis (versus no lysis) observed in the FIBTEM lysis tests in the HS group, compared to the HS+ and HS++ groups, indicate that soft tissue and musculoskeletal injuries inhibit the fibrinolytic process. The current findings are supported by a simplified assay described by Hartemink et al.7 in that NHPs with tissue injury in addition to HS have suppressed ratios of D-Dimer to lactate compared to animals with HS alone. Clinically, the diagnosis of fibrinolysis by ROTEM has been made based on the tissue factor-based EXTEM test.13 In the current study, differences in fibrinolysis were detected by FIBTEM, but not EXTEM, suggesting that platelets contribute to fibrinolysis resistance as has been previously reported.20 Additionally, impaired platelet function has been correlated to increased sensitivity to tPA.21 Furthermore, there is evidence that FIBTEM, and its thromboelastography (TEG) correlate, namely, functional fibrinogen, detect clot lysis sooner than the EXTEM test22 and increased sensitivity to hyperfibrinolysis has been shown in the FIBTEM versus EXTEM test in patients undergoing liver transplant.23 Therefore, in the current study, it is plausible that the resistance to fibrinolysis observed was in part mediated by platelet function, and that cytochalasin-D–mediated platelet inhibition in the FIBTEM assay revealed differences in fibrinolytic activity among injury groups.
Several investigations have elucidated the underlying mechanisms and treatment of hyperfibrinolysis; however, few studies have examined the mechanisms underlying the fibrinolysis shutdown phenotype in trauma.6,7,9,10,15,16,21 Previous work in rats has revealed an innate resistance to tPA-mediated fibrinolysis, which is decreased after HS but increased with tissue injury.16 This is further supported in a swine model of a severe blast injury which produces a hypercoagulable state rather than a bleeding coagulopathy.15 Furthermore, in a clinical study evaluating trauma patients within 12 hours of injury, 63% of trauma patients with an ISS greater than 15 exhibited fibrinolysis shutdown which was associated with an almost sixfold increase in mortality rate compared to patients with physiologic fibrinolysis.6 Interestingly, in that study, the difference in TEG LY30 values was only 3% between patients that exhibited hyperfibrinolysis and patients that exhibited fibrinolysis shutdown phenotypes. Yet this stratification, based on such seemingly subtle differences in lysis, was associated with a higher frequency of organ failure-related mortality in the fibrinolysis shutdown phenotype, and a high frequency of hemorrhage-related mortality in the hyperfibrinolysis phenotype.6 In a more recent multicenter analysis of 2,450 injured patients, using the same LY30 parameters to define fibrinolysis phenotypes, fibrinolysis shutdown was confirmed as the most common phenotype, and associated with 100 more deaths than patients with hyperfibrinolysis.8 That study also indicated that patients with blunt trauma were more likely to have a shutdown phenotype. In our animal study, the observed alterations in the magnitude of fibrinolysis were also subtle yet corroborate previous reports that increasing the extent of tissue injury promotes fibrinolysis shutdown. At the time of writing this report, we are not able to meaningfully stratify our animals into the three distinct fibrinolysis phenotypes previously reported clinically largely due to: limited number of animals/observations (i.e., n = 24 to date) and variability inherent to an outbred larger animal model. Further hampering efforts to stratify animals based on fibrinolysis phenotypes is that, despite the severity of HS and extent and range of tissue injuries used in the current study, it appears the NHP coagulation system exhibits a high degree of resilience to fibrinolysis, as reflected by the relatively uniform alterations in coagulation parameters observed among groups in the in vivo studies, as well as in the in vitro assessments of tPA-mediated fibrinolysis in plasma samples collected from healthy donor rhesus macaques.
Although the exact mechanisms by which fibrinolysis is regulated in response to various injury patterns are not well understood, a growing body of evidence implicates blood cells, namely, erythrocytes (red blood cells [RBC]) and thrombocytes (platelets). In trauma patients exhibiting hyperfibrinolysis, elevations in RBC degradation products were also identified.24 In an in vitro study in which blood from healthy donors was used, tPA increased fibrinolysis, tPA with RBC lysate augmented fibrinolysis threefold more than tPA alone, whereas platelet lysate was shown to inhibit tPA-mediated fibrinolysis.20 Additionally, platelet microparticles, which are abundant in plasma, express surface phosphatidyl serine which has been shown to elicit procoagulant effects in vitro. However, in vivo, phosphatidyl serine only exerts procoagulant effects in the presence of procoagulant enzymes, as would be the case in the circulating blood of patients after major trauma.25 Furthermore, human platelets produce and store large quantities of active PAI-1 in α-granules, and platelet derived PAI-1 has been shown to account for 50% of PAI-1 activity in circulation.26,27 It should also be noted that the endothelium is a source of PAI-1 and may play an important role in local regulation of fibrinolysis. In trauma patients exhibiting a hyperfibrinolytic phenotype, circulating free tPA concentrations are approximately 50 ng/mL, whereas in patients exhibiting a fibrinolysis shutdown phenotype free tPA levels were undetectable due to elevations in PAI-1 and formation of tPA-PAI-1 complex.21 Another mechanism by which platelets have been shown to inhibit fibrinolysis is through the secretion of inorganic phosphate polymers (polyP).28 Human platelet dense granules contain substantial amounts of polyP, which are secreted upon platelet activation and regulates blood clotting by activating the contact pathway and promoting the activation of factor V.29 Additionally, polyP has been shown to directly modify fibrin network architecture and downregulate fibrinolysis by attenuating binding of tPA and plasminogen to fibrin; however, this effect has been shown to be effective only when polyP is incorporated into the fibrin network at the time of formation.30,31 Given the central role of platelets in the cell-based model of coagulation,32 platelet contributions to the regulation of fibrinolysis warrant further investigation.
With the recent interest in antifibrinolytic therapeutics, such as tranexamic acid, especially in the context of trauma where diverging phenotypes have been documented and have been shown to be time-dependent, a deeper understanding of the innate mechanisms that regulate fibrinolysis is prudent to more effectively guide treatment.33 Understanding whether the mechanisms of action for various antifibrinolytic agents involves inhibition of fibrinolytic enzymes or modification of the fibrin architecture at the time of formation will further refine the development of therapeutics and clinical indications for their use.
Although these data are consistent with previous animal work, the type and degree of injury patterns used did not elicit alterations in coagulation as robust as those observed clinically in severe trauma patients.4 This may be due to a natural resilience of NHPs to severe hemorrhage and traumatic injury indicating some degree of inconsistency between species. Despite profound hemodynamic shock in these NHPs, lactate levels did not exceed 3.3 mmol/L and the greatest decrease in base excess from BSLN values observed in the study was at EOR (−7.5 ± 0.3 mmol/L; n = 24); this relatively mild metabolic effect may additionally contribute to the relatively mild overall effects in coagulation function observed. The MAP target for the current study was based on MAP values observed in a previous study by our group in which an average nadir in MAP of 21 mm Hg was observed within 5 minutes of initiating uncontrolled hemorrhage via liver transection.17 Additionally, some of the observed differences may be attributed to the need for anesthesia before injury and shock and indeed the surgical manipulations themselves. Surgical placement of vascular catheters alone provoke a hypercoagulable state in healthy male swine similar to that observed in animals exposed to HS and multiple injuries.34,35
Furthermore, surgery has been shown to elicit physiologic and coagulation responses similar to those observed in trauma clinically as postoperative fibrinolytic shutdown mediated by elevated PAI-1 has been reported.36,37 Though the present study corroborates fibrinolysis shutdown in response to tissue trauma in the setting of severe HS, in-depth quantification of several markers of interest (e.g., tPA, PAI-1, tPA-PAI-1 complex, Protein C, and polyP) could not be performed. Additional studies to include assay refinement for use in NHPs and animal model variations to expand capabilities and more closely replicate the clinical scenario to more comprehensively assess coagulation function and the role of platelets are needed.
In summary, the constellation of observations of tissue injury causing fibrinolysis shutdown is consistent with previous animal and clinical reports. Given the documented clinical relevance and promising implications of improved detection and management of fibrinolysis phenotypes in trauma patients, additional assessments to more comprehensively evaluate the mechanisms underlying the fibrinolytic shutdown phenotype in response to varying injury patterns are warranted.
Supplementary Material
Acknowledgments
We thank Darren Fryer, Kassandra Ozuna, Heather Grossman, Amanda Lanier, Peter Hemond and Ashley Turnmire at NAMRU-SA and Michael Scherer, Chriselda Fedyk, Maryanne Herzig, and Grantham Peltier at USAISR for their excellent technical support, as well as LCDR Jacob J. Glaser, Randy F. Crossland, and Leasha J. Schaub of NAMRU-SA for their expertise and valuable input in the preparation of this manuscript. Additionally, we are grateful for the steadfast support from the staff at the NAMRU-SA Veterinary Science Department under the guidance of Carrie Crane and MAJ Craig Koeller, DVM.
Funding: This work was supported by funding from Medical Research and Materiel Command: Joint Program Committee 6, work unit number G1403.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
AUTHORSHIP
A.R.M. was involved in conduction of study, data analysis and interpretation, and composition of the article. H.B.M. contributed to data interpretation, literature review, and critical revisions. A.P.C. contributed to data interpretation and critical revisions. M.A.M. contributed to data acquisition, interpretation, and critical revisions. E.E.M. contributed to data interpretation, critical revisions, and study design. F.R.S. was involved in conduction of study, interpretation of data, and critical revisions.
DISCLOSURE
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Army, Department of Defense, nor the U.S. Government. The experiments reported herein were conducted in compliance with the Animal Welfare Act and in accordance with the principles set forth in the “Guide for the Care and Use of Laboratory Animals,” Institute of Laboratory Animals Resources, National Research Council, National Academy Press, 1996. This work was supported by funding from the Medical Research and Materiel Command: Joint Program Committee 6, work unit number G1403. Dr. Antoni R. Macko and Dr. M. Adam Meledeo are civilian employees of the US Government, Dr. Hunter B. Moore and Dr. Ernest E. Moore are employees of Denver Health Medical Center and University of Colorado, Denver, and Dr. Andrew P. Cap and Dr. Forest R. Sheppard are military service members. This work was prepared as part of their official duties. Title 17 USC §105 provides that ‘copyright protection under this title is not available for any work of the US Government.’ Title 17 USC §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
References
- 1.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6):S431–S437. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 2.Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010;34(1):158–163. doi: 10.1007/s00268-009-0266-1. [DOI] [PubMed] [Google Scholar]
- 3.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13(6):680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 4.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 5.Dobson GP, Letson HL, Sharma R, Sheppard FR, Cap AP. Mechanisms of early trauma-induced coagulopathy: the clot thickens or not? J Trauma Acute Care Surg. 2015;79(2):301–309. doi: 10.1097/TA.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 6.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811–817. doi: 10.1097/TA.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartemink KJ, Hack CE, Groeneveld AB. Relation between coagulation/fibrinolysis and lactate in the course of human septic shock. J Clin Pathol. 2010;63(11):1021–1026. doi: 10.1136/jcp.2010.079707. [DOI] [PubMed] [Google Scholar]
- 8.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, Sauaia A, Cotton BA. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg. 2016;222(4):347–355. doi: 10.1016/j.jamcollsurg.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schöchl H, Wade CE, Holcomb JB, Matijevic N. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73(2):365–370. doi: 10.1097/TA.0b013e31825c1234. [DOI] [PubMed] [Google Scholar]
- 10.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013;75(6):961–967. doi: 10.1097/TA.0b013e3182aa9c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardaway RM. Disseminated intravascular coagulation with special reference to shock and its treatment. Mil Med. 1965;130:451–460. [PubMed] [Google Scholar]
- 12.Chapman MP, Moore EE, Moore HB, Gonzalez E, Gamboni F, Chandler JG, Mitra S, Ghasabyan A, Chin TL, Sauaia A, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg. 2016;80(1):16–23. doi: 10.1097/TA.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schöchl H, Frietsch T, Pavelka M, Jámbor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009;67(1):125–131. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 14.Schöchl H, Cadamuro J, Seidl S, Franz A, Solomon C, Schlimp CJ, Ziegler B. Hyperfibrinolysis is common in out-of-hospital cardiac arrest: results from a prospective observational thromboelastometry study. Resuscitation. 2013;84(4):454–459. doi: 10.1016/j.resuscitation.2012.08.318. [DOI] [PubMed] [Google Scholar]
- 15.Prat NJ, Montgomery R, Cap AP, Dubick MA, Sarron JC, Destombe C, May P, Magnan P. Comprehensive evaluation of coagulation in swine subjected to isolated primary blast injury. Shock. 2015;43(6):598–603. doi: 10.1097/SHK.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 16.Moore HB, Moore EE, Lawson PJ, Gonzalez E, Fragoso M, Morton AP, Gamboni F, Chapman MP, Sauaia A, Banerjee A, et al. Fibrinolysis shutdown phenotype masks changes in rodent coagulation in tissue injury versus hemorrhagic shock. Surgery. 2015;158(2):386–392. doi: 10.1016/j.surg.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macko AR, Crossland RF, Cap AP, Fryer DM, Mitchell TA, Pusateri AE, Sheppard FR. Control of severe intra-abdominal hemorrhage with an infusible platelet-derived hemostatic agent in a nonhuman primate (rhesus macaque) model. J Trauma Acute Care Surg. 2016;80(4):617–624. doi: 10.1097/TA.0000000000000964. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez RJ, Moore EE, Ciesla DJ, Biffl WL, Johnson JL, Silliman CC. Mesenteric lymph is responsible for post-hemorrhagic shock systemic neutrophil priming. J Trauma. 2001;51(6):1069–1072. doi: 10.1097/00005373-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Jordan JR, Moore EE, Sarin EL, Damle SS, Kashuk SB, Silliman CC, Banerjee A. Arachidonic acid in postshock mesenteric lymph induces pulmonary synthesis of leukotriene B4. J Appl Physiol (1985) 2008;104(4):1161–1166. doi: 10.1152/japplphysiol.00022.2007. [DOI] [PubMed] [Google Scholar]
- 20.Moore HB, Moore EE, Gonzalez E, Wiener G, Chapman MP, Dzieciatkowska M, Sauaia A, Banerjee A, Hansen KC, Silliman C. Plasma is the physiologic buffer of tissue plasminogen activator-mediated fibrinolysis: rationale for plasma-first resuscitation after life-threatening hemorrhage. J Am Coll Surg. 2015;220(5):872–879. doi: 10.1016/j.jamcollsurg.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore HB, Moore EE, Gonzalez E, Wiener G, Chapman MP, Dzieciatkowska M, Sauaia A, Banerjee A, Hansen KC, Silliman C. Plasma is the physiologic buffer of tissue plasminogen activator-mediated fibrinolysis: rationale for plasma-first resuscitation after life-threatening hemorrhage. J Am Coll Surg. 2015;220(5):872–879. doi: 10.1016/j.jamcollsurg.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harr JN, Moore EE, Chin TL, Chapman MP, Ghasabyan A, Stringham JR, Banerjee A, Silliman CC. Viscoelastic hemostatic fibrinogen assays detect fibrinolysis early. Eur J Trauma Emerg Surg. 2015;41(1):49–56. doi: 10.1007/s00068-014-0400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abuelkasem E, Lu S, Tanaka K, Planinsic R, Sakai T. Comparison between thrombelastography and thromboelastometry in hyperfibrinolysis detection during adult liver transplantation. Br J Anaesth. 2016;116(4):507–512. doi: 10.1093/bja/aew023. [DOI] [PubMed] [Google Scholar]
- 24.Peltz ED, D’Alessandro A, Moore EE, Chin T, Silliman CC, Sauaia A, Hansen KC, Banerjee A. Pathologic metabolism: An exploratory study of the plasma metabolome of critical injury. J Trauma Acute Care Surg. 2015;78(4):742–751. doi: 10.1097/TA.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park MS, Owen BA, Ballinger BA, Sarr MG, Schiller HJ, Zietlow SP, Jenkins DH, Ereth MH, Owen WG, Heit JA. Quantification of hypercoagulable state after blunt trauma: microparticle and thrombin generation are increased relative to injury severity, while standard markers are not. Surgery. 2012;151(6):831–836. doi: 10.1016/j.surg.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brogren H, Wallmark K, Deinum J, Karlsson L, Jern S. Platelets retain high levels of active plasminogen activator inhibitor 1. PLoS One. 2011;6(11):e26762. doi: 10.1371/journal.pone.0026762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booth NA, Croll A, Bennett B. The activity of plasminogen activator inhibitor-1 (PAI-1) of human platelet. Fibrinolysis. 1990;4(2):138–140. [Google Scholar]
- 28.Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139(6):1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006;103(4):903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutch NJ, Engel R, Uitte de Willige S, Philippou H, Ariëns RA. Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Thromb Haemost. 2009;115(19):3980–3988. doi: 10.1182/blood-2009-11-254029. [DOI] [PubMed] [Google Scholar]
- 31.Mutch NJ, Myles T, Leung LL, Morrissey JH. Polyphosphate binds with high affinity to exosite II of thrombin. J Thromb Haemost. 2010;8:548–555. doi: 10.1111/j.1538-7836.2009.03723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts HR, Hoffman M, Monroe DM. A cell-basedmodel of thrombin generation. Semin Thromb Hemost. 2006;32(Suppl 1):32–38. doi: 10.1055/s-2006-939552. [DOI] [PubMed] [Google Scholar]
- 33.Binz S, McCollester J, Thomas S, Miller J, Pohlman T, Waxman D, Shariff F, Tracy R, Walsh M. CRASH-2 study of tranexamic acid to treat bleeding in trauma patients: a controversy fueled by science and social media. J Blood Transfus. 2015;2015:874920. doi: 10.1155/2015/874920. Article ID 874920. 12 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan ML, Thorson CM, King DR, Van Haren RM, Manning RJ, Andrews DM, Livingstone AS, Proctor KG. Insertion of central venous catheters induces a hypercoagulable state. J Trauma Acute Care Surg. 2012;73(2):385–390. doi: 10.1097/TA.0b013e31825a0519. [DOI] [PubMed] [Google Scholar]
- 35.Mulier KE, Greenberg JG, Beilman GJ. Hypercoagulability in porcine hemorrhagic shock is present early after trauma and resuscitation. J Surg Res. 2012;174(1):e31–e35. doi: 10.1016/j.jss.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Dobson GP. Addressing the global burden of trauma in major surgery. Front Surg. 2015;2:43. doi: 10.3389/fsurg.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassis J, Hirsh J, Podor TJ. Evidence that postoperative fibrinolytic shutdown is mediated by plasma factors that stimulate endothelial cell type I plasminogen activator inhibitor biosynthesis. Blood. 1992;80(7):1758–1764. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.