Abstract
Research on the biology of addiction has advanced significantly over the last 50 years expanding our understanding of the brain mechanisms underlying reward, reinforcement and craving. Novel experimental approaches and techniques have provided an ever increasing armory of tools to dissect behavioral processes, neural networks and molecular mechanisms. The ultimate goal is to reintegrate this knowledge into a coherent, mechanistic framework of addiction to help identify new treatment. This can be greatly facilitated by using tools that allow, with great spatial and temporal specificity, to link molecular changes with altered activation of neural circuits and behavior. Such specificity can now be achieved by using optogenetic tools. Our review describes the general principles of optogenetics and its use to understand the links between neural activity and behavior. We also provide an overview of recent studies using optogenetic tools in addiction and consider some outstanding questions of addiction research that are particularly amenable for optogenetic approaches.
Keywords: addiction, behavior, electrophysiology, optogenetics, photosensitive proteins
Introduction—How Technological Advancement Contributed to Addiction Research
Research on the biology of addiction has witnessed an enormous progress over the last 50 years. This progress has always been linked to the successful adaptation of new technologies and research tools to addiction research. Initial attempts to biologically understand addiction relied on approaches of classical pharmacology to measure subjective drug effects in humans and of behavioral pharmacology, using classical (Pavlovian) and operant (Skinnerian) conditioning in rodents, primates and in humans (Mello 1973; Brady & Griffiths 1976; Goldberg & Henningfield 1988). These studies revealed the basic behavioral mechanisms behind reward and reinforcement and opened up the field, by establishing objective and relatively quantitative behavioral methods, to specifically investigate the contribution of brain areas and neurotransmitter systems.
Targeted lesions of brain nuclei and administration of pharmacological agents, agonists and antagonists of endogenous neurotransmitters, into well-defined brain regions led to the discovery of the key role of the dopaminergic neurotransmission, the mesolimbic dopaminergic system in particular, in the mediation of acute non-conditioned and rewarding effects of different classes of drugs of abuse (Di Chiara et al. 2004). In-vivo microdialysis lent further credibility to the importance of dopamine release in the nucleus accumbens as a final common mechanism (Pontieri, Tanda & Di Chiara 1995). The pioneering application of human brain imaging to addiction research did not only confirm earlier discoveries in animal models, but it also provided an excellent translation research tool to better understand the relationship between activation of neurotransmitter systems in identified brain circuits with subjective effects of drugs and drug-associated cues in humans (Volkow, Fowler & Wang 2004; Heinz et al. 2009). Neuroendocrinological approaches revealed bidirectional links between the brain as the organ responsible for the initial processing of stressful stimuli from the environment and the hormone systems, the hypothalamic-pituitary-adrenal system in particular, that shape the behavioral responses to addictive drugs and craving through alterations in neurotransmitter signaling (Sarnyai, Shaham & Heinrichs 2001; Shalev, Erb & Shaham 2010).
By the late 1980s there was a good understanding of the basic neurochemistry and functional neuromorphology of addiction, but the knowledge of detailed molecular mechanisms beyond dopamine and opiate receptors was lacking. The main neurotransmitter receptors involved in drug addiction, such as dopamine and opiate receptors, are G-protein coupled receptors linked to cyclic adenosine monophosphate (cAMP) signaling. Based on earlier work by Collier (Collier & Francis 1975), the cAMP-PKA-CREB signaling was the first to be specifically studied from the point of view of addiction biology (Nestler 2004). Detailed analysis of drug- and withdrawal-induced alterations in this signaling system and downstream molecules such as DFosB, has firmly established its role in drug reward, tolerance and withdrawal (McClung et al. 2004). These initial discoveries have the other benefit of introducing new molecular biological techniques to identify the role of several other extra- and intracellular signaling systems, such as brain derived neurotrophic factor (BDNF) (Ghitza et al. 2010) and related mitogen-activated protein kinase-extracellular signal-regulated kinases (MARK-ERK) (Lu et al. 2006) signaling to further our understanding in the molecular basis of addiction as a disease of long-term neuroplasticity.
Drug addiction is primarily a behavioral disorder; therefore, the need to link genes and molecular changes with behavior in valid models has facilitated the introduction of transgenic and knockout mouse technologies into the field of addiction research. Some early knockout mouse studies, for example, dopamine transporter (DAT) knockout, confirmed unequivocally what had already been proposed on the basis of classing pharmacology studies that acute effects of cocaine are mediated by the DAT (Giros et al. 1996). However, this early study also showed that mice lacking DAT still self-administer cocaine, suggesting the previously unexpected contribution of factors other than DAT in the mediation of the rewarding effects of the drug (Rocha et al. 1998; Sora et al. 2001). More recent transgenic mouse technologies allow for a uniquely tight spatial and temporal control of the expression of the modified gene in a cell-type specific manner. For example, constraining the deletion the cAMP-responsive element binding protein 1 (CREB1) gene to cerebral cortical and hippocampal pyramidal neurons results normal locomotor responses to acute and chronic cocaine as well as normal development of cocaine place preference (McPherson et al. 2010). However, CREB1 mutants demonstrate a diminished drive to self-administer cocaine under operant conditions, which suggests that there is a specific role for CREB1 in telencephalic glutamatergic neurons regulating the motivational properties of cocaine (McPherson et al. 2010). Recent studies have achieved controlling the disruption of the gene of interest in a particular cell type. Using cell type-specific RNA interference, a novel mouse line with a selective knockdown of mGluR5 in dopamine D1 receptor-expressing neurons was generated (Novak et al. 2010). These mice self-administered cocaine, but the reinstatement of cocaine-seeking induced by a cocaine-paired stimulus was impaired. Deficits in specific incentive learning processes that enable a reward-paired stimulus to directly reinforce behavior and to become attractive, thus eliciting approach toward it, were identified in these mice, showing that glutamate signaling through mGluR5 located on dopamine D1 receptor-expressing neurons is necessary for incentive learning processes that contribute to cue-induced reinstatement of cocaine-seeking and which may underpin relapse in drug addiction (Novak et al. 2010).
These last two studies illustrate well that research in the field of addiction has achieved a very high level of sophistication to connect molecular changes with behavioral alterations. The ability to specifically activate or inhibit defined neuron populations with known connectivity to modify behaviors relevant to drug effects and addiction could provide a previously unprecedented power to understand the functional connectivity of the nervous system in addiction. Such approach in the past was only possible by the use of relatively ‘crude’ pharmacological tools, such as electrical stimulation or local microinjection of lidocaine into brain nuclei to activate or inhibit, respectively, a large, heterogeneous group of cells. During the last few years, a very powerful novel approach, optogenetics, has been introduced in neuroscience to specifically address such questions. This review introduces the basic concept of optical manipulation of neuronal activity and its specific application for the addiction research community. We will also describe some new data obtained by the use of optogenetics in neuroscience in general and in the area of addiction research in particular. Furthermore, we highlight some key outstanding questions in the biology of addiction, which looks particularly amenable to be studied by optogenetic tools.
Optogenetics—Basic Principles
Optogenetics refers to the use of optical methods to probe and control genetically targeted neurons within intact neural circuits (Miesenbock 2009). With optogenetics, researchers can now manipulate defined cell populations that have been rendered responsive to light and control their activity. The use of this technique to probe the basic workings of the brain as well as changes in brain function in disease states poses several advantages over current methodologies. First, optogenetics allows scientist to turn neurons on and off safely in intact invertebrate and vertebrate brains and in freely moving animals, avoiding any developmental compensation effects. In addition, it provides the ability to create defined events, either activation or inhibition, of neurons with high-cell type, location and/or projection specificity, as well as temporal resolution. Furthermore, it also allows the simultaneous manipulation of scattered yet functionally defined groups of neurons.
Optogenetics is based on the principle of expressing photosensitive proteins in a genetically defined cell group. Photosensitive proteins are ion channels that, when applied with light of a particular wavelength, open and allow the inflow of cations or anions for the excitation or inhibition of the cells in which it was expressed (Fig. 1a). The optogenetics toolbox includes the use of chemically modified ion channels and receptors (Banghart et al. 2004) as well as the use of natural photosensitive proteins (Oesterhelt & Stoeckenius 1971; Matsuno-Yagi & Mukohata 1977; Nagel et al. 2003). In this review, we will focus on the implementation of natural photosensitive proteins and its engineered variants on the study of the functional organization of the mammalian brain. A general overview of currently available photosensitive proteins and their delivery and expression methods is given. In addition, we will discuss how this technology has been implemented to answer questions in the field of neuroscience that traditional research tools are unable to tackle, and how these techniques can be used in addiction research. Lastly, we review studies in the field of addiction that have used this new technology to advance the general knowledge of the field, and discuss some of the limitations of optogenetics for addiction research.
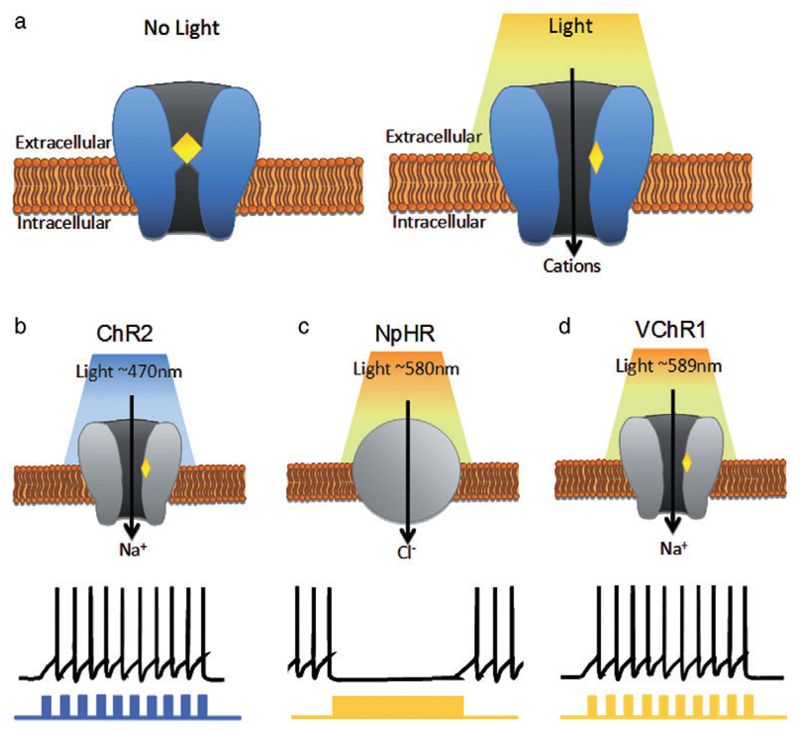
Figure 1. Principles of optogenetic tools.
(a) In general, natural photosensitive proteins are transmembrane ion channels with ATR bound at the core of the channel. In the presence of light of a specified wavelength, ATR is isomerized and creates a conformational change to open the channel pore and allow the influx or outflux of cations of anions. (b) ChR2 is an ion channel that is activated in the presence of blue light (~470 nm) and allows the influx of cations, with a particular preference for Na+. With a pulsed light stimulation protocol, ChR2 can elicit depolarization currents and action potentials. (c) NpHR is a chloride pump responsive to yellow light (~580 nm). Activation of NpHR allows the influx of Cl-, resulting in hyperpolarization of the cell. (d) VChR1, like ChR2, is an ion channel. VChR1, however, is activated by yellow light (~589 nm) to allow influx of Na+ and elicit action potentials or depolarization
Available optogenetic tools
Although the natural photosensitive proteins that would become the basis of optogenetics were described as early as the 1970s (Oesterhelt & Stoeckenius 1971), their use as a tool to manipulate neural tissue was not described until over three decades later. The first attempt at using natural photosensitive proteins to manipulate neural tissue involved the use of the ChARGe system developed by Zemelman et al. in 2002. ChARGe took advantage of the drosophila visual system cascade. However, this system was never widely adopted because of its low temporal resolution, as well as its complex three-component system (G-protein couple rhodopsin, arrestin, and a G protein), which made it too difficult to implement in mammalian brains in vivo.
Channelrhodopsin2 (ChR2) is a seven transmembrane non-selective cation channel from the unicellular green alga Chlamydomonas reinhardtii was first described by Nagel et al. in 2003. In the presence of the cofactor all-trans retinal (ATR), ChR2 is capable of light transduction. ATR is bound at the core of the channel, and illumination with blue light (~470 nm) isomerizes ATR to create a conformational change to open the channel pore, allowing the passage of several different cations, with a preference for Na+ (Fig. 1b). Since mammalian brains naturally express ATR, ChR2 could be used as a single gene, single component light activated regulator of transmembrane ion flow (Nagel et al. 2003).
In 2005, Karl Deisseroth’s group was first to report the use of ChR2 to manipulate the activity of mammalian neurons (Boyden et al. 2005). In this seminal paper, the group used a viral delivery method to express ChR2 in cultured rat CA3/CA1 hippocampal neurons and demonstrated that ChR2 enabled photoactivation of these neurons was able to depolarize these cells as well as induce action potentials. In addition, it was shown that the expression of ChR2 created very little basal activity in the absence of blue light, did not change cell properties such as resting potential or membrane resistance nor did light stimulation predispose neurons to cell death. The use of a pulsed light strategy allowed them to create precise patterns of spiking across a heterogeneous population of cells with the same precision and at a physiologically relevant range of firing frequencies (with high fidelity up to 40 Hz) as well as subthreshold depolarizations. A few years after the introduction of ChR2, its inhibitory counterpart, halorhodopsin (NpHR), was described (Zhang et al. 2007). NpHR is bacterial light driven chloride pump from the archeabacteria Natronomonas pharaonis (Kolbe et al. 2000). Like ChR2, NpHR requires ATR but is activated with yellow light (~580 nm) to produce hyperpolarizing currents in the cells expressing it (Fig. 1c). Since ChR2 and NpHR are activated by distinct wavelengths of light, the two can be expressed in the same cell populations to achieve multimodal bidirectional controls of neurons (Zhang et al. 2007). Cation-conducting channelrhodopsin from Volvox carteri (VChR1) is a second type of ChR2 from the green algae Volvox carteri (Zhang et al. 2008). Just like ChR2, VChR1 is a non-selective cation channel that can drive depolarization and spike activity in a cell when activated by light. However, VChR1 is activated by yellow light (~589 nm) instead of blue (Fig. 1d). Therefore, VChR1 can be used in combination with ChR2 for the simultaneous activation of distinct cell populations.
Since their original introduction as optogenetic tools, several engineering techniques have been used to create optimized versions of these original channels as well as new channels with different useful properties. Although ChR2 is very efficient on its own to induce activation of neurons, a few problems have been reported. High expression levels of ChR2 leads to the production of extra spikes in response to a single light pulse. In addition, spike fidelity declines with stimulations above 40 Hz, and plateau potentials were created after long optically driven spike trains (Boyden et al. 2005; Ishizuka et al. 2006). The new modified version of ChR2, ChETA, shows significant improvements in reduction of extra spikes, plateau potentials, as well as increased fidelity in spiking during optical stimulation of up to 200 Hz (Gunaydin et al. 2010). Two other versions of excitatory light-activated channels include channelrhodopsin green receiver (ChRGR) and channelrhodopsin chimera EF (ChIEF) with I170V mutation. ChRGR is a modified version of the proton pump ChR1 (Nagel et al. 2003), offering rapid ON-OFF kinetics and minimal desensitization, which can be useful for the implementation of opto-current clamp techniques, where current injection is predicted by the intensity of light stimulation (Wen et al. 2010). ChIEF, in turn, is a chimera of ChR1 and ChR2 that shows less inactivation with persistent light stimulation and higher closure rate, providing more consistent responses with higher frequency stimulations (Lin et al. 2009).
It has been previously reported that expression of NpHR resulted in intracellular accumulations and poor membrane tracking (Gradinaru, Thompson & Deisseroth 2008; Zhao et al. 2008). Inhibition by NpHR could also be overcome by strong excitation (Sohal et al. 2009). The second version of this channel, eNpHR (now termed NpHR 2.0) was able to reduce membrane trafficking issues. The latest version of this channel, NpHR 3.0, shows improvements in membrane trafficking, leading to a 20-fold increase in current conductance over the original (Gradinaru et al. 2010). Archaerhodopsin-3 (ArCh) is a light-driven outward proton pump from the archaebacteria Halorubrum sodomense that has been demonstrated to be capable of driving hyperpolarizing currents exceeding 900pA, sufficient to achieve near-total neuronal silencing in awake, behaving animals. In addition, unlike NpHR, ArCh is capable of spontaneously recovering from light-dependent inactivation, avoiding long-lasting inactive states in response to light (Chow et al. 2010). The improvement in the kinetics of the channels allows for more reliable patterns of spiking, as well as greater control over cell activation and lowering requirements in channel expression levels for cell activation, preventing potential cell toxicity issues.
Although action potential activation or inhibition is an important aspect of cell manipulation, biochemical modulations of cells can also contribute to behavior both in excitable and non-excitable cells. Therefore, methods to manipulate biochemical signaling in cells with light have been developed. The manipulation of G-protein coupled receptor signaling cascades can be accomplished using OptoXRs, which are opsin receptor chimeras. Airan et al. (2009) developed a Gs-OptoXR and a Gq-OptoXR by coupling a human alpha-1 adrenergic receptor or a beta-2 adrenergic receptor with a Gt-coupled bovine rhodopsin, respectively. They demonstrated that activation of these receptors resulted in light-induced regulation of appropriate signaling cascade, as evidenced by pCREB levels, as well as leading opposing effects on spike firing. New biochemical optogenetic control strategies are also being developed, which would allow for the control of GTPases. As this topic is beyond the scope of this review, we guide the reader to Toettcher et al. (2011).
Since its original introduction of ChR2 as a tool to manipulate neuronal activity, the optogenetic tool set has been significantly expanded to the point where it is now possible to cover the optical control of activity of practically every type of neuron present in the brain. Caution must be exercised when choosing which photoactivated proteins to use as there are advantages and disadvantages to each in their functionality. As new photoactivated proteins are found and new molecular engineering technologies are developed, the field of optogenetics should lead to the possibility of activating specific receptor types and biochemical molecules to explore their function.
Many techniques are available for the expression of these light sensitive channels and receptors. The most commonly used consists of delivering the genes for their expression to neurons through the use of viral vectors and stereotaxic surgery procedures. For detailed methodology, please refer to Zhang et al. (2010). Lentivirus delivery is advantageous in that the genes are incorporated into the DNA, leading to stable expression to occur with shorter expression time requirements (Boyden et al. 2005). Adeno-associated virus (AAV) vectors are also widely used as they allow greater spatial spread than lentiviruses (Zhang et al. 2007; Atasoy et al. 2008; Cardin et al. 2010; Kravitz et al. 2010). It is common practice to fuse a fluorescent reporter protein, such as mCherry or tdTomato (Atasoy et al. 2008; Aponte et al. 2010) with the channel in order to visually confirm the successful expression of the light sensitive channels in the neurons of interest. To render cell type specificity, genes can be expressed under the control of a particular promoter of interest as well as general neuronal promoters such as the Thy-1 promoter (Wang et al. 2007; Zhao et al. 2008; Gradinaru et al. 2009). The Cre-Lox recombination system (Fig. 2a) can also be exploited to achieve high levels of expression when the desired promoter is not particularly strong at driving expression (Atasoy et al. 2008; Cardin et al. 2010). In addition, by combining the Cre-LoxP system, retrogradely transported viral vectors such as pseudorabies virus (PRV) (Mazarakis et al. 2001), Herpex simplex virus 1 (HSV-1) (Lilley et al. 2001; Covington et al., 2010) or canine adenovirus (CAV) (Kremer et al. 2000) and spatial targeting, expression of photosensitive proteins can be achieved with an incredible level of specificity. For example, expression of ChR2 in dopaminergic cells that only project to the NAc shell can be achieved by injecting a retrograde Cre-dependent opsin virus into the NAc shell of TH-Cre animals. The virus will infect all cells that project to the NAc shell, but ChR2 will only be expressed in cell where Cre is present, in this case, dopaminergic cells. With the use of the wheat germ agglutinin (WGA)-Cre fusion system, it is possible to achieve expression specifically in neurons connected to particular neurons of interest (Gradinaru et al. 2010). For this, two injection sites are required, one with WGA-Cre fusion gene, and another with a Cre-dependent opsin virus. Cre will be delivered transynaptically from cells with the WGA-Cre fusion gene, but the opsin will only be expressed in connected cells containing the Cre-dependent opsin gene. In utero electroporation gene delivery can also be performed (Petreanu et al. 2007) to target developmentally defined cell types. This technique also allows for expression under a larger range of promoters as vectors are not required, eliminating DNA size restrictions (Zhang et al. 2010). In addition, although more time consuming, transgenic mouse lines expressing CHR2 in specific cell types can also be created. Existing ChR2 transgenic mouse lines indicate that expression of ChR2 have minimal effects on the animal’s development (Wang et al. 2007; Ayling et al. 2009).
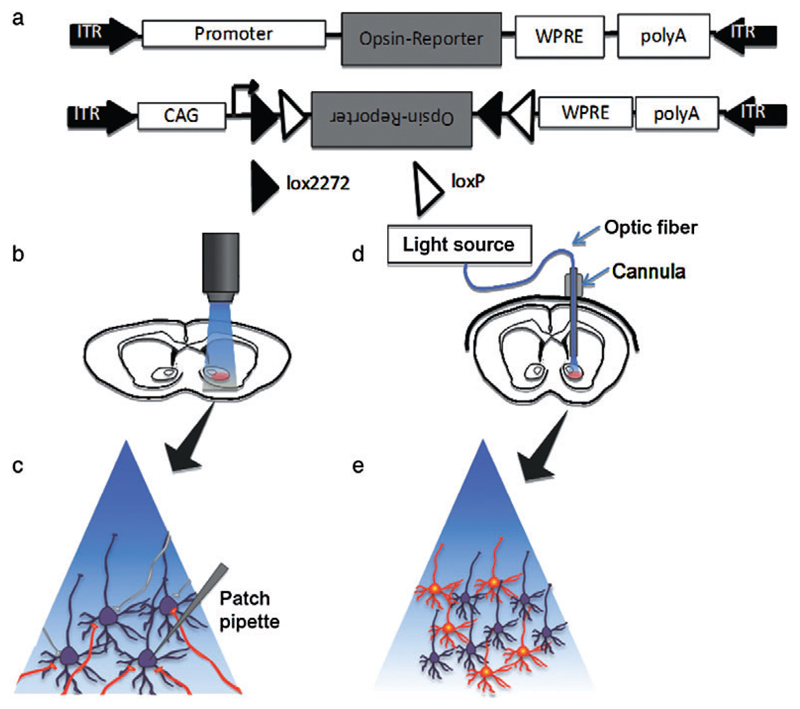
Figure 2. Opsin expression and stimulation strategies.
(a) AAV vector with a promoter, opsin and fluorescent marker gene to deliver opsins with cell-type specificity (top). Cre-dependent AAV vector carrying a doubly floxed inverted opsin and fluorescent marker gene (bottom). In the presence of Cre, the floxed gene is first inverted, and then one loxP and one lox2272 site are excised, leaving the opsin gene in the appropriate direction for expression, floxed by one loxP and one Lox2272. No further inversions of the opsin gene are possible. (b) For in vitro slice studies, light for photostimulation of ChR2 expressing cells can be provided by halogen/xenon lamps, LEDs, or two-photon excitation lasers and delivered through a microscope objective to the desired area. (c) In the presence of blue light, ChR2 expressing afferents (red) are activated while non-ChR2 expressing cells (purple) and axons (grey) are not affected. Activation of ChR2 expressing afferents, even severed from their cell bodies, are capable of eliciting action potentials and neurotransmitter release. Circuit mapping and neurotransmitter release studies can be conducted by stimulating ChR2 expressing afferents with patch clamping the post-synaptic cell. (d) For in vivo ChR2 stimulation in rodents, a cannula is fixed on the animal’s skull.A fiber optic cable attached to a light source is inserted through the cannula to deliver light to desired brain area. (e) In the presence of blue light, ChR2 expressing cells (red) are activated, allowing for the selective firing modulation of these cells, while non-ChR2 remain unaffected
Once expressed, halogen/xenon lamps, light emiting diodes (LED) (Campagnola, Wang & Zylka 2008) or two-photon excitation laser scanning methods (Mohanty et al. 2008; Rickgauer & Tank 2009) can be used for in vitro excitation (Fig. 2b). For in vivo studies in particular, those involving freely moving animals and the manipulation of behavior, the use of fiber optic technology is usually necessary. This consists of an optical fiber guide stereotaxically mounted on the skull with an optical fiber inserted through the guide to the desired structure in the brain. The optic fiber cable is coupled to high power lasers or LEDs for effective light delivery (Fig. 2d) (Aravanis et al. 2007; Gradinaru et al. 2010).
The use of optical activation of neuronal populations confers a high degree of flexibility as it can be used with a combination of read out options. In vitro, slice electrophysiology is one of the most common read out options. Its use as an effective tool in the circuit mapping and neurotransmitter release studies is described further in the next section. In addition, in vivo electrophysiological recordings are possible with the use of an optrode (Gradinaru et al. 2007), a hybrid of a fiber optic and electrodes, to study the correlation of cell firing with behavior. Optogenetic strategies can also be combined with imaging readout techniques, such as the use of voltage sensitive dyes (VSD), can allow the monitoring of electrical activity of large populations of neurons in both slice as well as in vivo with high temporal resolution (Peron & Svoboda 2011), although these types of studies in vivo are restricted to cortical areas near the surface of the brain. Access to brain tissue is achieved with the use of a chronic cranial window (Ayling et al. 2009) or a polished and reinforced thinned skull method (Drew et al. 2010). Global readout technologies such as electroencephalography (Adamantidis et al. 2007; Ayling et al. 2009), electromyograms (EMG) (Ayling et al. 2009), fMRI (Lee et al. 2010) can also be used to study changes in brain states with the activation of particular neuron populations in normal and diseased brains. In addition, photoactivation can also be correlated to biochemical activation readouts, such as the expression of early genes c-fos and arc (Sohal et al. 2009), as well as cyclic voltammetry for the measurement of neurotransmitter release (Tsai et al. 2009). Nonetheless, because optogenetic tools provide, arguably, the best way to manipulate cell activity in awake behaving animals, it is especially well suited to be used with behavioral paradigms. The use of optogenetics to probe the role of particular neuron populations in specific behaviors will be discussed in greater detail below.
Using Optogenetics
The application of optogenetic tools to the study of the brain has been varied. However, optognetics proves to be most useful in the study of a few particular topics: circuit mapping, neurotransmitter release and underlying mechanisms of behavior. Defining the connections of particular cell types is particularly hard to achieve. Current methods for determining synaptic connectivity include the reconstruction of axonal and dendritic arbors (Gilbert 1983; Binzegger, Douglas & Martin 2004) as well as the use of paired recordings (Thomson et al. 2002; Holmgren et al. 2003). However, reconstruction studies are incapable of determining cell type specificity nor the strength of functional connections, and paired recordings, although capable of estimating connection probabilities, is an incredibly slow and arduous process, making it highly inefficient. In addition, paired recording methods are not capable of determining long-range connections as cell bodies are severed from axons during the preparation of brain slices. Petreanu et al. (2007) demonstrated that the use of optogenetics would be a viable option and a more efficient way of conducting circuit-mapping studies. Since ChR2 is expressed in both cell bodies as well as projections, it is possible to excite severed axons of a defined cell type and record responses in postsynaptic cells in structures far apart from the original location of the cell bodies (Fig. 2c). By employing ChR2 assisted circuit mapping (CRACM), they were able to dissect the laminar specificity of long-range callosal projections from L2/3 of the somatosensory cortex (Petreanu et al. 2007). Atasoy et al. (2008), for example, used CRACM to determine the postsynaptic effects of activation of either AGRP or POMC neurons in the arcuate nucleus on postsynaptic cells in the PVN. Without ChR2, determining the contribution of postsynaptic effects from either AGRP or POMC neurons would have been difficult as these two cell populations and their axons are mixed. CRACM is also particularly useful in the study of synaptic transmission and neurotransmitter release. Like the study of synaptic connections, synaptic transmission studies also require the use of paired recordings which is highly inefficient. In addition, stimulation of presynaptic cells with electrodes can activate nearby cells as well as axon fibers, creating artifacts. Photoactivation of presynaptic axons can induce neurotransmitter release, creating activation of postsynaptic cells. With the use of specific channel blockers, it is possible to determine what neurotransmitter photoactivated cells are releasing that result in the postsynaptic effect (Bass et al. 2010; Higley & Sabatini 2010; Stuber et al. 2010; Tecuapetla et al. 2010; Schoenenberger, Scharer & Oertner 2011).
Optogenetics has been particularly useful in research aimed to determine the underpinning of cognition and behavior. Traditional methods of behavioral manipulation involved the use of pharmacology and mutant animal lines. As mentioned above, these two methods pose particular challenges. Pharmacological manipulations provide very low temporal resolution as well as cell type specificity, while global mutations of particular genes can create compensatory mechanisms during development, at times producing results that contradict pharmacological methods. The use of optogenetics to manipulate cells in vivo and observe effects on behavior are advantageous in that it provides high temporal resolution as well as cell type specificity (Fig. 2e). Many research groups have taken advantage of optogenetic tools to manipulate cell populations in vivo to study a wide array of behaviors. Of particular note are the studies carried out by Aponte et al. (2010) as well as Johansen et al. (2010) where light stimulated cellular activation produced dramatic effects on behavior. Aponte and colleagues sought to determine the contribution of AGRP and POMC neurons in the regulation of feeding behavior. Activation of AGRP neurons in the arcuate nucleus was sufficient to evoke voracious feeding in mice shortly after photostimulation demonstrating that AGRP neurons are directly engaging feeding circuits. Johansens et al., demonstrated that ChR2 assisted activation of lateral amygdala (LA) pyramidal neurons as an US (unconditioned stimulus), paired with an auditory CS (conditioned stimulus), was sufficient to induce fear learning in rats without the need of an aversive stimulus, leading to the conclusion that aversive stimuli induces activation of LA pyramidal cells during fear conditioning.
It is hard to tell whether optogenetics will one day become a viable method for disease treatment as numerous safety concerns over method of gene delivery and expression exist. However, the use of these tools have led to tremendous insight in the causes of a diverse range of neurological disorders, including depression (Gradinaru et al. 2009; Covington et al. 2010), narcolepsy (Adamantidis et al. 2007), epilepsy (Tonnesen et al. 2009) and blindness (Bi et al. 2006; Busskamp et al. 2010), as well as providing potential new targets for their treatment. For example, the use of ChR2 and NpHR has allowed researchers to determine that mPFC activation can create antidepressive effects (Covington et al. 2010), while frequency dependent excitation of orexin cells in the lateral hypothalamus can increase the probability of transition from one state of consciousness to another, with important implications for narcolepsy (Adamantidis et al. 2007). The most notable translational work using optogenetics is in the field of Parkinson’s disease (PD). Deep brain stimulation (DBS) has been employed as a form of therapy for the reduction of PD symptoms with great success. However, how DBS exerts its therapeutic effects was not understood until recently. The difficulty in deciphering its mode of action rested on the fact that DBS is able to activate a heterogeneous population of neurons as well as fibers of passage. Understanding its mode of action is, nonetheless, incredibly important as the creation of mixed responses in cells can affect the effectiveness of the therapy by creating side effects. By using ChR2 to activate potential cell populations that are potentially activated with DBS, Gradinaru et al. (2009) were able to determine that DBS exerted its therapeutic effects by activating M1 layer V projections in the STN, as well as demonstrating that high frequency stimulation of these cells is sufficient to ameliorate PD symptoms in hemiparkinsonian rats. In addition, Kravitz et al. (2010) demonstrated that activation of the dopamine D1 pathway in the striatum is sufficient to reduce motor symptoms in PD mouse models. These two studies have shed light on the circuitry of the PD brain as well as provided new, more specific targets to be exploited for the creation of more effective therapeutic strategies to ameliorate the symptoms of PD, with the potential of reduced side effects. As it is evidenced, the use of optogenetic tools in combination with other research approaches can be highly effective at answering questions about the functional organization of the mammalian brain that were not previously possible with traditional techniques.
Optogenetics and Addiction
Decades of study on the mechanisms of addiction have implicated the midbrain dopaminergic system as being the reward center of the brain. These studies have produced a vast amount of information on mechanistic aspects of dopamine (DA) signaling, in particular in the ventral tegmental area (VTA) that allows for the encoding and conditioning of reward signals, as well as how drugs of abuse might modulate the DAergic system in the development of addiction. Research to date has produced a number of theories as how addiction is developed, but they have not been properly tested because of the limitation of traditional methods. The use of optogenetics has provided a new avenue in which to validate these theories, and has the potential to propel the understanding of underlying mechanisms of addiction. For example, a prominent theory indicates that changes in DA neuron firing patterns between low-frequency (tonic) activity and high-frequency (phasic) bursts could provide the basis of reward prediction and incentive salience encoding (Schultz 2007). Nonetheless, it is still unclear whether tonic or phasic dopamine activity alone is capable of eliciting reward related behaviors. Recently, Tsai et al. (2009) demonstrated that activation of DA neurons in the VTA alone using ChR2 in freely behaving mice was capable of eliciting conditioned place preference (CPP). Of particular note was the result that only DA neuron stimulation at high frequencies, mimicking phasic DA firing, was capable of eliciting CPP, defining a causal role for phasic activity in DA neurons in conditioning behavior.
Furthermore, it has been theorized that synaptic adaptations triggered by the surge in DA levels during the consumption of drugs of abuse lead to circuit reorganization, including AMPA receptor (AMPAR) redistribution (Kauer & Malenka 2007), which may underlie addiction. As psychostimulants such as cocaine are capable of activating serotonergic systems and noradrenergic systems as well as dopaminergic systems, it was unclear whether the dopaminergic system in itself could induce AMPAR redistribution. In an elegant study, Christian Luscher’s group (Brown et al. 2010) was able to demonstrate that optical activation of DA neurons was sufficient to induce AMPAR redistribution through a D1 receptor mediated mechanisms.
Outside of the role of dopamine, the use of optogenetic tools has allowed researchers to tease out the contributions of other neurotransmitters in addiction-related behaviors and how they might modulate the reward system to exert their roles in addiction. Cholinergic transmission, for example, has been shown to play a role in reward learning (Hikida et al. 2001; Robbins, Ersche & Everitt 2008; Chen, Hopf & Bonci 2010). However, pharmacological studies and lesion studies have reported conflicting roles. By using optogenetics, Witten et al. (2010) demonstrated that stimulation and inhibition of choline acetyl transferase (ChAT) neurons in the nucleus accumbens (NAc) has opposing effects in medium spiny neurons (MSN) firing, and inhibition of ChAT neurons during a cocaine induced conditioned place preference (CPP) test could reduce preference for the cocaine paired chamber, consistent with the idea that pauses in NAc firing is required for reward-related conditioning. BDNF has also been demonstrated to modulate the reward system, as it can activate ERK signaling in D1 and D2 receptor expressing MSN in the NAc. In an elegant study carried by Lobo et al. (2010), optogenetic and genetic manipulations were used to determine the role of BDNF in cocaine-induced CPP. This study demonstrated that BDNF can activate both D1 and D2 MSNs but with opposing behavioral effects, indicating that BDNF is capable of regulating both D1 and D2 MSN excitability, resulting in either the enhancement or desensitization of rewarding effects of psychostimulants such as cocaine. Coincident DAergic and glutamatergic signaling has also been proposed to be crucial for synaptic plasticity and reward signaling (Moss & Bolam 2008). Although previous studies have suggested that some midbrain dopaminergic neurons express glutamate (Sulzer et al. 1998; Bourque & Trudeau 2000), the subject of whether DA neurons were capable of releasing glutamate remained controversial. Most recently, two groups unequivocally reported co-release of DA and glutamate by midbrain DAergic neurons. The studies reported that ChR2 assisted activation of DA neuron axons in the NAc and striatum resulted in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/N-methyl-D-aspartic acid (NMDA) mediated excitatory postsynaptic potentials (EPSPs) in MSN in the NAc only, and it is dependent on the presence of vesicular glutamate transporter 2 (VGLUT2) (Stuber et al. 2010; Tecuapetla et al., 2010). These studies shine light on glutamatergic transmission as novel mechanisms for rewards signaling, where the fast kinetics of glutamate signaling could potentially be important in providing information for reward prediction and incentive salience, as well as the regulation of plasticity.
A particular problem in treating addiction is an addict’s susceptibility to relapse. Studies on reinstatement mechanisms point to the contribution of other brain areas outside of the dopaminergic system, yet their roles are still not fully understood. Previous pharmacological studies have demonstrated the role of orexin-1 in the reinstatement of cocaine-seeking behavior yet a causal relationship has not been established. Tourino et al. (2010) are currently seeking to establish this connection. Their work to date demonstrates that activation of orexin neurons using ChR2 is capable of reinstating cocaine-seeking behavior in mice that have previously extinguished cocaine self-administration. Furthermore, the dorsomedial prefrontal cortex has been previously implicated in having a role in the initiation of cocaine seeking. Using optogenetic silencing of dmPFC neurons, Stefanik et al. (2010) found that inactivation of this area is capable of reducing cocaine prime-induced reinstatement but not cue-induced reinstatement of cocaine-seeking behavior. Current studies by the group seek to determine whether optically induced inhibition of the BLA is capable of reducing cue-induced reinstatement as previous studies have implicated the basolateral amygdala (BLA) in this particular type of reinstatement. These two studies highlight the use of optogenetic tools in deciphering the contributions of the orexin neurons and the dorsomedial prefrontal cortex in reinstatement, providing new therapeutic targets.
How distinct subsets of a particular type of neurons may differ in their role in addiction remains as an outstanding question in addiction research. VTA DAergic neurons as a whole have been implicated to play a crucial role in the development of addiction. Since VTA DA neurons project to more than one area in the brain, effects on these neurons by addictive drugs result in changes in many different downstream structures, which as a whole, lead to the development of addiction. However, it is not particularly clear how subsets of DAergic neurons, defined by their target projections, differ in their role in addiction development. What aspect of addiction do VTA DA neurons that project to the NAc play a role in as opposed to those that project to the amygdala or the mPFC? One of the greatest advantages optogenetics can confer in the field of addiction over traditional methods is the ability to selectively activate or inactivate genetically defined and projection-specific neuron types. As mentioned above, by combining the Cre-LoxP system with retrogradely transported viral vectors, or using the WGA-Cre fusion system and spatial targeting, expression of photosensitive proteins can be achieved with an incredible level of specificity (Gradinaru et al. 2010). These strategies allow for the probing of circuit specific contributions to the development of addictive behaviors. In addition, the high temporal resolution of photosensitive proteins allows for the activation or inactivation of particular cell populations during a particular event, such as a lever press or nose poke, allowing the study of the importance of temporal coincidence of neural activation and behavioral output in reward learning.
Despite the many advantages the use of optogenetics brings to the field of addiction research, it also has its limitations. An important aspect of the development of addiction is the learning of associations between environmental cues and rewarding substances. Hebbian theory postulates that these learned associations are encoded through neuronal ensembles. Therefore, understanding the role of neuronal ensembles in different areas of the brain could lead to the understanding of how addictive behaviors are developed. Because the expression of photosensitive proteins can only be targeted by genetic markers and/or location of neurons, it is not possible to selectively activate or inhibit neurons that have formed ensembles in response to a learned behavior to determine their role in addiction. Therefore, the Daun02 system (Kasof et al. 1995; Koya et al. 2009; Bossert et al. 2011) is more suitable for these types of studies than optogenetics. In the Daun02 system, beta-galactosidase (b-gal) and Fos are co-expressed in behaviorally activated neurons of c-fos-lacZ transgenic rats. These neurons can subsequently be silenced by injection of Daun02 into a brain area of interest. Daun02 is converted by bgal into daunorubicin, which disrupts neuronal function for several days. Moreover, due to light scattering properties as well as the need for fiber optic technology, activation in vivo of sparsely scattered neuron populations over a large area is impractical. The use of synthetic receptor-ligand pair systems, such as receptors activated solely by synthetic ligands (RASSLs), designer receptors exclusively activated by a designer drug (DREADDs), or chemical inducers of dimerization (CIDs) (Strader et al. 1991; Yang et al. 1998; Alexander et al. 2009), serve as better tools to tackle these issues. In these synthetic receptor-ligand pair systems, a receptor of interest is engineered to respond only to a synthetic ligand with no effects on endogenous receptors, and expressed in cells of interest. The synthetic ligand is then administered systemically or intracranially, depending on the system, to activate or inhibit the syntethic receptors. These systems, however, provide far lower temporal resolution than optogenetics. In the DREADD system, for example, cell activation is achieved after 10 minutes of ligand administration (Alexander et al. 2009). For a more thorough review on these systems, we guide you to Nichols & Roth 2009.
Since addiction is a disorder that is developed over time, long-term within subject studies are crucial in the study of the development of addiction. The use of optogenetics for these types of studies should be exercised with caution, however. It has been indicated that over expression of these channels can cause intracellular accumulation and membrane blebbing, which might lead to cell toxicity and loss of membrane integrity (Gradinaru et al. 2007). As of date, no systematic study has been conducted to test the stability of expression and cell toxicity using the variety of methods available for gene expression over a prolonged period of time. These issues could potentially be amealiorated by future version of these channels with enhanced membrane trafficking and current conductance in order to further reduce expression levels required for cell activation or inhibition.
Conclusions
The research on the biological basis of addiction has progressed a great deal over the past decades. The identification of the central role of dopaminergic neurotransmission in reward and reinforcement and further refinements of the dopaminergic theory of addiction have benefited considerably from the use of behavioral pharmacological, electrophysiological and functional neuroanatomical techniques. Molecular biology, the application of knockout and transgenic technologies in particular, has unraveled the role of neurotransmitter receptors, intracellular signaling events and transcription factors, as well as the importance of epigenetic modifications, in the short- and long-term neuroadaptation in addiction. Basic research using the techniques mentioned above, along with pre-clinical and clinical structural and functional neuro-imaging, have started to provide an initial understanding of the functional connections between brain regions and circuits as they relate to reward, reinforcement and craving/relapse. More recently, the combination of genetic and imaging approaches has been proven to be a fruitful new way of linking genes with brain circuits and behavior in a translationally relevant fashion (Schumann et al. 2010). We now have testable hypotheses regarding the role of neurotransmitter systems, e.g. dopamine and glutamate, and brain regions and circuits, e.g. mesolimbic dopamine system, prefrontal cortex, in different aspects of the addictive process. The use of optogenetic tools will certainly provide an unprecedented opportunity to test these hypotheses with a level of molecular, cell-type, spatial and temporal specificity that has previously been unimaginable. This is a very rapidly evolving field. What today are perceived as limitations of the optogenetic techniques could easily be overcome in the near future. Furthermore, new applications of these, and more improved tools may open up the use of optogenetics to conduct more directly translational research, first as research tools and potentially later as treatment modalities in addiction. With the increasingly widespread application of optogenetics, the field of addictions research will never be the same as it was before. Optogenetics will bring our understanding of the neurobiology of addiction to a different level, similar to what was achieved by the introduction of molecular biology to the field that had previously been dominated by classical behavioral pharmacology (Nestler 2004). These new tools will, on the other hand, not negate the use of established technologies. Rather, they will allow us to use our established technologies in a new context and to ask more refined questions, ultimately leading us to a better mechanistic understanding of addiction and to the development of novel treatment entities.
Acknowledgements
Research supported by an ERC FP7 grant for DB and by the Howard Hughes Medical Institute for ZFHC.
Footnotes
Disclosure/Conflict of Interest
None.
Authors Contribution
ZS was responsible for conceiving the idea of the paper. ZFHC and ZS wrote the manuscript. DB provided critical revision of the manuscript. All authors critically reviewed the content and approved the final version of the manuscript for publication.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2010;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–S156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayling OG, Harrison TC, Boyd JD, Goroshkov A, Murphy TH. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat Methods. 2009;6:219–224. doi: 10.1038/nmeth.1303. [DOI] [PubMed] [Google Scholar]
- Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass CE, Grinevich VP, Vance ZB, Sullivan RP, Bonin KD, Budygin EA. Optogenetic control of striatal dopamine release in rats. J Neurochem. 2010;114:1344–1352. doi: 10.1111/j.1471-4159.2010.06850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzegger T, Douglas RJ, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12:3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brady JV, Griffiths RR. Behavioral procedures for evaluating the relative abuse potential of CNS drugs in primates. Fed Proc. 1976;35:2245–2253. [PubMed] [Google Scholar]
- Brown MT, Bellone C, Mameli M, Labouebe G, Bocklisch C, Balland B, Dahan L, Lujan R, Deisseroth K, Luscher C. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLoS ONE. 2010;5:e15870. doi: 10.1371/journal.pone.0015870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busskamp V, Duebel J, Balya D, Fradot M, Viney TJ, Siegert S, Groner AC, Cabuy E, Forster V, Seeliger M, Biel M, et al. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science. 2010;329:413–417. doi: 10.1126/science.1190897. [DOI] [PubMed] [Google Scholar]
- Campagnola L, Wang H, Zylka MJ. Fiber-coupled light-emitting diode for localized photostimulation of neurons expressing channelrhodopsin-2. J Neurosci Methods. 2008;169:27–33. doi: 10.1016/j.jneumeth.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc. 2010;5:247–254. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Hopf FW, Bonci A. Synaptic plasticity in the mesolimbic system: therapeutic implications for substance abuse. Ann N Y Acad Sci. 2010;1187:129–139. doi: 10.1111/j.1749-6632.2009.05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier HO, Francis DL. Morphine abstinence is associated with increased brain cyclic AMP. Nature. 1975;255:159–162. doi: 10.1038/255159b0. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA, Neve RL, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Drew PJ, Shih AY, Driscoll JD, Knutsen PM, Blinder P, Davalos D, Akassoglou K, Tsai PS, Kleinfeld D. Chronic optical access through a polished and reinforced thinned skull. Nat Methods. 2010;7:981–984. doi: 10.1038/nmeth.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35:157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD. Microcircuitry of the visual cortex. Annu Rev Neurosci. 1983;6:217–247. doi: 10.1146/annurev.ne.06.030183.001245. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Henningfield JE. Reinforcing effects of nicotine in humans and experimental animals responding under intermittent schedules of i.v. drug injection. Pharmacol Biochem Behav. 1988;30:227–234. doi: 10.1016/0091-3057(88)90450-9. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of Parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008;36:129–139. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P. Ultrafast optogenetic control. Nat Neurosci. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grüsser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14:108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kaneko S, Isobe T, Kitabatake Y, Watanabe D, Pastan I, Nakanishi S. Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proc Natl Acad Sci USA. 2001;98:13351–13354. doi: 10.1073/pnas.231488998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren C, Harkany T, Svennenfors B, Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J Physiol. 2003;551:139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T, Kakuda M, Araki R, Yawo H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci Res. 2006;54:85–94. doi: 10.1016/j.neures.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci USA. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasof GM, Mandelzys A, Maika SD, Hammer RE, Curran T, Morgan JI. Kainic acid-induced neuronal death is associated with DNA damage and a unique immediate-early gene response in c-fos-lacZ transgenic rats. J Neurosci. 1995;15:4238–4249. doi: 10.1523/JNEUROSCI.15-06-04238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kolbe M, Besir H, Essen LO, Oesterhelt D. Structure of the light-driven chloride pump halorhodopsin at 1.8 A resolution. Science. 2000;288:1390–1396. doi: 10.1126/science.288.5470.1390. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of Parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer EJ, Boutin S, Chillon M, Danos O. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J Virol. 2000;74:505–512. doi: 10.1128/jvi.74.1.505-512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim DS, Fenno LE, Ramakrishnan C, Deisseroth K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley CE, Groutsi F, Han Z, Palmer JA, Anderson PN, Latchman DS, Coffin RS. Multiple immediate-early gene-deficient herpes simplex virus vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo. J Virol. 2001;75:4343–4356. doi: 10.1128/JVI.75.9.4343-4356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Matsuno-Yagi A, Mukohata Y. Two possible roles of bacteriorhodopsin; a comparative study of strains of Halobacterium halobium differing in pigmentation. Biochem Biophys Res Commun. 1977;78:237–243. doi: 10.1016/0006-291x(77)91245-1. [DOI] [PubMed] [Google Scholar]
- Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL, Carter EE, Barber RD, Baban DF, Kingsman SM, Kingsman AJ, et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum Mol Genet. 2001;10:2109–2121. doi: 10.1093/hmg/10.19.2109. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- McPherson CS, Mantamadiotis T, Tan SS, Lawrence AJ. Deletion of CREB1 from the dorsal telencephalon reduces motivational properties of cocaine. Cereb Cortex. 2010;20:941–952. doi: 10.1093/cercor/bhp159. [DOI] [PubMed] [Google Scholar]
- Mello NK. A review of methods to induce alcohol addiction in animals. Pharmacol Biochem Behav. 1973;1:89–101. doi: 10.1016/0091-3057(73)90061-0. [DOI] [PubMed] [Google Scholar]
- Miesenbock G. The optogenetic catechism. Science. 2009;326:395–399. doi: 10.1126/science.1174520. [DOI] [PubMed] [Google Scholar]
- Mohanty SK, Reinscheid RK, Liu X, Okamura N, Krasieva TB, Berns MW. In-depth activation of channelrhodopsin 2-sensitized excitable cells with high spatial resolution using two-photon excitation with a near-infrared laser microbeam. Biophys J. 2008;95:3916–3926. doi: 10.1529/biophysj.108.130187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J, Bolam JP. A dopaminergic axon lattice in the striatum and its relationship with cortical and thalamic terminals. J Neurosci. 2008;28:11221–11230. doi: 10.1523/JNEUROSCI.2780-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci. 2004;25:210–218. doi: 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Roth BL. Engineered G-protein coupled receptors are powerful tools to investigate biological processes and behaviors. Front Mol Neurosci. 2009;2:1–10. doi: 10.3389/neuro.02.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak M, Halbout B, O’Connor EC, Rodriguez Parkitna J, Su T, Chai M, Crombag HS, Bilbao A, Spanagel R, Stephens DN, et al. Incentive learning underlying cocaine-seeking requires mGluR5 receptors located on dopamine D1 receptor-expressing neurons. J Neurosci. 2010;30:11973–11982. doi: 10.1523/JNEUROSCI.2550-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D, Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971;233:149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Peron S, Svoboda K. From cudgel to scalpel: toward precise neural control with optogenetics. Nat Methods. 2011;8:30–34. doi: 10.1038/nmeth.f.325. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the ‘shell’ as compared with the ‘core’ of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–11238. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickgauer JP, Tank DW. Two-photon excitation of channelrhodopsin-2 at saturation. Proc Natl Acad Sci USA. 2009;106:15025–15030. doi: 10.1073/pnas.0907084106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Schoenenberger P, Scharer YP, Oertner TG. Channel-rhodopsin as a tool to investigate synaptic transmission and plasticity. Exp Physiol. 2011;96:34–39. doi: 10.1113/expphysiol.2009.051219. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, Conrod PJ, Dalley JW, Flor H, Gallinat J, Garavan H, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonintransporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Deisseroth K, Kalivas PW, Laluniere RT. Shinning the light on relapse neurocircuitry: modulating the reinstatement of drug-seeking behavior using an optogenetic approach. 2010 Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience; 2010. Program No. 888.1/XX6. Online. [Google Scholar]
- Strader CD, Gaffney T, Sugg EE, Candelore MR, Keys R, Patchett AA, Dixon RA. Allele-specific activation of genetically engineered receptors. J Biol Chem. 1991;266:5–8. [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, Rayport S. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, West DC, Wang Y, Bannister AP. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2-5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cereb Cortex. 2002;12:936–953. doi: 10.1093/cercor/12.9.936. [DOI] [PubMed] [Google Scholar]
- Toettcher JE, Voigt CA, Weiner OD, Lim WA. The promise of optogenetics in cell biology: interrogating molecular circuits in space and time. Nat Methods. 2011;8:35–38. doi: 10.1038/nmeth.f.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen J, Sorensen AT, Deisseroth K, Lundberg C, Kokaia M. Optogenetic control of epileptiform activity. Proc Natl Acad Sci USA. 2009;106:12162–12167. doi: 10.1073/pnas.0901915106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourino CM, Canella N, Adamantidis A, Boutrel B, De Lecea L. Optogenetic stimulation of hypocretin neurons reinstate cocaine-seeking behavior. 2010 Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience; 2010. Program No. 887.4/WW2. Online. [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Wang H, Peca J, Matsuzaki M, Matsuzaki K, Noguchi J, Qiu L, Wang D, Zhang F, Boyden E, Deisseroth K, Kasai H, et al. High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proc Natl Acad Sci USA. 2007;104:8143–8148. doi: 10.1073/pnas.0700384104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Wang H, Tanimoto S, Egawa R, Matsuzaka Y, Mushiake H, Ishizuka T, Yawo H. Opto-current-clamp actuation of cortical neurons using a strategically designed channel-rhodopsin. PLoS ONE. 2010;5:e12893. doi: 10.1371/journal.pone.0012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Symes K, Mercola M, Schreiber SL. Small-molecule control of insulin and PDGF receptor signaling and the role of membrane attachment. Curr Biol. 1998;8:11–18. doi: 10.1016/s0960-9822(98)70015-6. [DOI] [PubMed] [Google Scholar]
- Zemelman BV, Lee GA, Ng M, Miesenbock G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Prigge M, Beyriere F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11:631–633. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhao S, Cunha C, Zhang F, Liu Q, Gloss B, Deisseroth K, Augustine GJ, Feng G. Improved expression of halorhodopsin for light-induced silencing of neuronal activity. Brain Cell Biol. 2008;36:141–154. doi: 10.1007/s11068-008-9034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]