Abstract
Physiological fluctuations in the levels of hormones, nutrients, and gasses are sensed in parallel by interacting control systems distributed throughout the brain and body. We discuss the logic of this arrangement and the definitions of “sensing”; and then focus on lateral hypothalamic (LH) control of energy balance and respiration. LH neurons control diverse behavioral and autonomic processes by projecting throughout the neuraxis. Three recently characterized types of LH cells are discussed here. LH orexin/hypocretin (ORX) neurons fire predominantly during wakefulness and are thought to promote reward-seeking, arousal, obesity resistance, and adaptive thermogenesis. Bidirectional control of ORX cells by extracellular macronutrients may add a new regulatory loop to these processes. ORX neurons also stimulate breathing and are activated by acid/CO2 in vivo and in vitro. LH melanin-concentrating hormone (MCH) neurons fire mostly during sleep, promote physical inactivity, weight gain, and may impair glucose tolerance. Reported stimulation of MCH neurons by glucose may thus modulate energy homeostasis. Leptin receptor (LepR) neurons of the LH are distinct from ORX and MCH neurons, and may suppress feeding and locomotion by signaling to the mesolimbic dopamine system and local ORX neurons. Integration within the ORX–MCH–LepR microcircuit is suggested by anatomical and behavioral data, but requires clarification with direct assays of functional connectivity. Further studies of how LH circuits counteract evolutionarily-relevant environmental fluctuations will provide key information about the logic and fragilities of brain controllers of healthy homeostasis.
Keywords: hypothalamus, sensing, control, CO2, glucose, leptin, orexin, MCH, Neurotensin
1. Introduction
The brain is in a constant dialog with the internal environment of the body, forming a control system that maintains homeostasis. This review discusses possible cellular correlates of this control, with a specific focus on the lateral hypothalamus (LH), the classical “hunger and wakefulness” brain center [1–3]. The modern understanding is that the LH is not cellularly homogeneous, but contains several functionally and molecularly distinct classes of widely-projecting neurons. These LH cells control a vast array of vital physiological processes, including food intake, locomotor activity, wakefulness, sleep, blood pressure, reward-seeking, and breathing [4–6]. In turn, the activity of LH neurons is regulated by an equally numerous array of neural, endocrine, and metabolic inputs [7–12].
Here, we focus on three chemical signals that directly regulate the activity of LH neurons: macronutrients, leptin, and acid/CO2. We will specifically discuss the effects of these signals on three distinct types of LH neurons, as recently defined by non-overlapping expression of orexin/hypocretin (ORX), melanin-concentrating hormone (MCH), and leptin receptor (LepR). Other types of LH neurons have also been described, e.g. GAD65 neurons and NPY neurons, which are both distinct from ORX and MCH cells [13,14]. However, it is currently unclear whether or not they overlap with LH LepR neurons, thus they will not be considered separately in the review. The important reciprocal interactions between LH and other brain circuits are also not discussed here in detail but are covered in other recent reviews (e.g. [15,16]).
Because a key focus of this review is on sensing of the internal state, we will briefly discuss general reasons for distributed (brain and peripheral) sensing of the internal environment, and revisit basic definitions of sensors. We will then discuss recent data on the ORX, MCH, and LepR neurons of the LH within a “sensor-regulator” framework.
2. Parallel and distributed sensing of vital variables inside and outside the brain
Experimental evidence for specialized brain sensors of basic physiological variables such as glucose existed for many decades [17]. Yet until recently, the action of nutrients and hormones on peripheral tissues was considered sufficient for stabilizing vital parameters such as blood glucose levels [15]. Indeed, sensors for many basic physiological variables are found in the periphery, e.g. the endocrine pancreas for glucose, and the carotid body for O2/CO2. Via neural and hormonal responses, such peripheral sensors form classical feedback loops safeguarding internal levels of gasses and glucose from perturbations. Why are brain sensors of the same variables required?
Recent experiments suggest that without inputs from the brain, peripheral control loops are insufficient to safeguard body homeostasis. Disrupting brain sensing of macronutrients such as glucose leads to metabolic disturbances, including defects in pancreatic glucose-stimulated insulin secretion (e.g. [18–20]). For hormones, the effects of deletion of receptors for insulin and leptin from specific populations of hypothalamic neurons demonstrate that hormone sensing by brain circuits is essential for peripheral glucose homeostasis and normal body weight [21–23]. For gasses, deleting hypothalamus-specific transmitters disrupts modulation of respiration by CO2 [24,25]. Thus a substantial body of evidence now indicates that brain sensors of homeostasis-related signals are required for normal health.
Placing direct sensors of internal variables within the brain has logical advantages for preparing for the future, for reducing reliance on individual links, and for monitoring the function of peripheral tissues. A key limitation of peripheral organs is that, beyond the anticipatory actions of intrinsic circadian clocks [26], they are largely reactive control systems that produce corrective actions after a change has occurred (e.g. release of insulin triggered by a rise in glucose). In contrast, a key function of the brain is to estimate the future from the present, and prepare the body for a change before it occurs (e.g. salivation at the sight of food). Such predictive actions need to occur in proportion to internal needs in order to be energy-efficient. It would thus be useful for the brain to adjust its predictive actions based on internal levels of vital variables.
Direct brain sensing of signals such as glucose also reduces ambiguity. For example, elevated blood insulin can signal either elevated glucose or an insulinoma. Measuring blood glucose would help the brain resolve this ambiguity, and also inform the brain about pancreatic input–output function. This logic may explain why the brain itself contains sensors for both “primary” (nutrients, gasses) and “secondary” (hormonal) signals. Finally, placing sensors in parallel (Fig. 1B) protects the system against collapse or “input blindness” that would be caused by failure in one sensor in a serial system (Fig. 1A). Indeed, there is now considerable experimental support for multiple distributed sensors for glucose [27], pH/CO2 [28], and hormones such as leptin and ghrelin [29,30]. In contrast, serial sensing systems such as those discussed in early models of brain leptin sensing [31], and depicted in Fig. 1A, are not supported by current experimental evidence.
Fig. 1.
Model system architectures for brain sensor-regulators. A) In sequential/serial sensing models, primary stimuli such as nutrients are sensed mostly outside the brain, and then this information is relayed via hormonal and neural signals to a sequence of brain centers. B) In parallel/distributed models, stimuli are directly sensed in parallel by several brain sensors distributed throughout diverse and mutually interconnected brain circuits.
3. Defining “sensing”: ubiquitous v specialized responses to vital variables
Extreme changes in life-supporting contents of the extracellular fluid can, by definition, have disruptive effects on all neuronal functions, and trigger general neuroprotective responses. We draw a distinction these non-specific effects from specialized “sensing” responses discussed here, even though the two types of responses can share molecular machinery. This section is intended as a clarification of this distinction.
Maintaining vital variables such as glucose and pH within a narrow physiological range (≈0.7–2.5 mM for brain glucose, ≈6.9–7.4 for brain pH, [32–35]) is crucial for normal neuronal activity. Outside these physiological ranges, neuronal function is reduced in ubiquitous and stereotyped ways. Such “life-preserving” effects differ from the more specialized sensing responses within physiological ranges. For the purposes of this review, we define “sensing” responses as steep changes in firing rate caused by normal physiological variations in the levels of the sensed parameter. For glucose, such specialized sensing responses probably occur above 0.2 mM (see below), and are most steep in the physiological range of glucose levels (≈4–7 mM for the plasma, ≈1–2.5 mM for the brain, [33]). Below 0.2 mM glucose, ubiquitous glucose-entry pathways stop being saturated and thus changes in [glucose] influence cellular function [36]. This is where general neuroprotective “shut down” responses are seen (Fig. 2).
Fig. 2.
Relationship between glucose and neuronal function. Most neurons reduce their activity (e.g. via K-ATP channel opening) when glucose levels drop below a critical value (≈0.2 mM, see text for detail). Above this life-threatening range, most cells are protected from glucose fluctuations due to saturation of glucose entry transporters. However, a smaller number of specialized “glucosensor” excitable cells continue to be steeply modulated by glucose in the physiological concentration range, allowing appropriate defenses to be initiated before [glucose] becomes life-threateningly low.
A famous example of a protein involved in both general and specific responses is the ATP-inhibited K channel (K-ATP). K-ATP channels have a dual role in responses of different excitable cells to glucose. The first role is general, and involves hyperpolarization caused by opening of K-ATP channels following a fall in cytosolic ATP/ADP ratio [36,37]. This ubiquitous neuroprotective response occurs in both glucosensing and non-glucosensing neurons, as well as in other cells, when extracellular glucose becomes unphysiologically low (reviewed in detail in [36]). This response is functionally distinct from the physiological glucosensing capability that may or may not be present in the same cell [36]. For example, K-ATP channels in ORX neurons open, and hyperpolarize/inhibit the cell, when extracellular glucose is reduced to 0.1 mM, a pathologically low concentration that would produce loss of consciousness [36]. This does not mean that ORX neurons will be “glucose-excited” in the physiological glucose range. Indeed, rises in [glucose] >0.2 mM have been reported to hyperpolarize and inhibit ORX cells in several independent studies [38–40]. This illustrates the presence of a general neuroprotective response, and of a specific glucosensing response, in the same cell. The two responses are clearly distinct in both direction (glucose-induced depolarization v hyperpolarization) and concentration-dependence.
The second function of K-ATP channel – the specialized glucosensing response – is similar to the first, since it also involves K-ATP channel closure by a rise in ATP/ADP ratio. However, it is also very different because it occurs within the physiological range of glucose levels and requires additional molecules. This specialized glucosensing response occurs only in a few “glucose-excited” cell types, such as the pancreatic β-cell. Here, the presence of additional molecules (e.g. glucokinase for the β-cell) extends K-ATP channel's dependence on extracellular glucose to physiological concentrations of glucose [41]. Without such additional molecules, K-ATP channels are maximally closed above ≈0.2 mM extracellular glucose (due to saturation of glucose entry, see [36]). This illustrates that the general and the specialized glucose responses can share molecular machinery (e.g. K-ATP channel), but glucosensing in the physiological glucose range requires additional components. The distinction between specialized and general glucose responses is summarized in Fig. 2.
Although less researched, the same may hold for other vital variables such as extracellular pH. For example, as reviewed by Chesler [32], extracellular acidosis has been widely observed to diminish neuronal activity. Presumably this serves a general feedback-neuroprotective function, since neuronal activity generally increases extracellular acidity [32]. In contrast, specialized chemosensory neurons steeply increase their firing rate upon extracellular acidification in the physiological range [42].
4. Overview of ORX, MCH, and LepR neurons of the LH
4.1. ORX and MCH neurons
Anatomical and physiological properties of ORX and MCH neurons of the LH have been the subject of several recent reviews [10,43,44]. Only some key points are briefly summarized here. In the brain, ORX and MCH neurons are found only in the LH, but their axons extend throughout the brain, and have largely overlapping projection fields. ORX and MCH neuropeptides are used as defining markers of ORX and MCH cells, but both cell types express numerous transmitters. ORX cells express glutamatergic markers, and can affect their projection targets on a millisecond time-scale via fast AMPA-receptor-mediated glutamate transmission [45]. Dynorphin and NARP are also expressed in ORX cells, and are lost concurrently with ORX in human narcoleptics, indicating that loss of ORX neurons (rather than only ORX peptides) is a cause of human narcolepsy [46]. In turn, MCH neurons contain transmitters such as GABA, nesfatin, and CART, which may have critical roles in glucose homeostasis [47].
ORX and MCH cells are physiologically antagonistic in at least some key respects. ORX cells promote wakefulness [48], while MCH cells may promote sleep [49]. Loss of ORX cells causes obesity [50], while loss of MCH cells causes leanness [51]. ORX has been linked to anxiety [52], while MCH is thought to be anxiolytic [53]. ORX peptides are predominantly neuroexcitatory [54], while MCH is considered to be an inhibitory peptide [55]. ORX cells fire action potentials mostly during wakefulness (especially active wakefulness), while MCH neurons fire predominantly during sleep (especially REM sleep) [56–58]. In terms of autonomic actions, ORX neurons may activate the sympathetic system to drive glucose release from the liver and glucose uptake by muscle, and may promote brown-adipose tissue thermogenesis and increased cardiovascular function [59]. In contrast, via the parasympathetic system, MCH neurons may promote bradycardia and increase fat deposition in the white adipose tissue [60,61]. The metabolic and respiratory functions of ORX and MCH are discussed more closely in Section 5.
Considering the diverse roles of ORX and MCH neurons, a key question is whether ORX and/or MCH neurons are subdivided into functionally specialized subpopulations. For ORX neurons, there is evidence of such subdivisions at the level of inputs [62,63] and electrophysiological properties [40,45]. Whether such different groups of ORX neurons have distinct projection targets is currently unclear. For example, in the mouse, ORX cells in different areas of the LH are similarly likely to project to the ventral tegmental area (VTA, a “reward” center) or the locus coeruleus (an “arousal center”) [64].
4.2. LH LepR neurons
Diverse types of leptin receptor-expressing (LepR) neurons are distributed throughout the brain [65], and their relative roles have been intensely researched during the past decade. Recent studies of the fluorescently-tagged LepR neurons in the LH indicate that LH LepR cells do not contain ORX or MCH, and thus represent a distinct group of LH neurons. Anterograde tracing between LHA and VTA suggests that LH LepR neurons project to VTA, but not to striatum or nucleus accumbens [11]. Interestingly, retrograde tracing with fluorogold beads injected into the VTA suggests that VTA receives inputs from LH LepR neurons, but not from the “classical” LepR neurons in the hypothalamic arcuate nucleus [11]. Infusion of leptin selectively into the LH reduces food intake and body weight, and increases VTA TH gene expression and mesolimbic DA content [11]. Thus, LH LepR cells are a distinct population of anorexigenic neurons that may signal adiposity levels to the VTA.
5. LH neurons and macronutrients
5.1. Glucose sensing by LH neurons
Historical in vivo electrophysiology experiments show that the LH contains both glucose-excited and glucose-inhibited neurons [17]. After ORX and MCH neurons of the LH were discovered, their electrophysiological glucose responses have been characterized in vitro [38,66], and, in the case of MCH neurons, the metabolic significance of these responses in the whole-animal has also been investigated [67]. The same physiological shifts in extracellular glucose concentration alter the firing of ORX and MCH cells in different directions: ORX cells are glucose-inhibited and MCH cells are glucose-excited [66]. These responses occur irrespective of whether or not the changes in [glucose] are osmotically compensated.
5.1.1. Glucose sensing by MCH neurons
Glucose-induced excitation of MCH cells has recently been shown to follow the classical (β-cell like) mechanism involving glucose-dependent closure of Kir6.2/SUR1-containing ATP-inhibited K+ (K-ATP) channels [67]. Expression of ATP-resistant (and thus overactive) K-ATP channels selectively in MCH neurons, chronically from birth, ablates MCH cell glucosensing and impairs systemic glucose tolerance [67]. This suggests that glucose excitation of MCH cells may improve glucose tolerance. However, the acute v chronic roles of MCH neurons in regulating glucose fluxes are not clear at present. While glucose tolerance worsens if MCH cells are made chronically glucose-insensitive from birth [67], it improves if MCH cells are destroyed in the adult [47]. In turn, MCH overexpression leads to obesity and insulin resistance [68], and increases insulin levels [69]. It is possible that, like ORX neurons (see below), MCH cells could have multiple and differentially-modulated effects on glucose release and uptake, a key subject for further investigation. Glucose actions of MCH in the context of other functions of MCH neurons (e.g. hypoactivity, anxiolytic effects) also remain to be explored.
5.1.2. Glucose sensing by ORX neurons
In contrast to MCH cells, ORX cells are activated following in vivo hypoglycemia [70,71], and are electrically inhibited by d-glucose, mannose, and 2-deoxyglucose (but not galactose, l-glucose, alpha-methyl-d-glucoside, or fructose) in vitro [72]. Glucose-induced hyperpolarization of ORX cells involves background K+ currents [73]. A similar biophysical mechanism for glucose-induced inhibition has been reported in glucose-inhibited invertebrate neurons [74]. Background channels could be encoded by members of “KCNK” gene family (also known as “K2P” or “tandem-pore” channels), which codes for at least 15 ion channel subunits in mammals, as well as by other genes (discussed in [75]). So far, when some (5 out of 15) of the KCNK have been knocked out from birth, ORX glucosensing persisted, suggesting that other candidate subunits, both within and outside KCNK family, are able to drive glucose-induced hyperpolarization of ORX cells [39,75,76].
In the mouse, ORX neurons are electrophysiologically dichotomous in both their electrical properties and glucose responses [40,45]. Some ORX cells adaptively reduce their glucose-stimulated K+ conductance when glucose levels are stable, thereby maintaining excitability and glucose-trend sensitivity at different glucose baselines [40]. Such stimulus adaptations are very common in the classical external sensory systems [77], and may serve a number of functions, such as tuning sensor sensitivity to stimulus baseline. Other ORX neurons respond to sustained change in [glucose] with sustained hyperpolarization, which means that their activity may track absolute levels of glucose rather than just changes in those levels [40]. Projections from sustained and adaptive ORX glucosensors converge on areas regulating arousal and reward [64].
Emerging evidence suggests that the sensitivity of ORX neurons to glucose varies based on the physiological composition of the extracellular space, allowing them to act as “conditional glucosensors”. ORX neurons clearly act as glucosensors at a physiological extracellular pH of ≈7.25 [76,73] (and as pH/CO2 sensors in physiological [glucose] [76], see Section 6.2). However, ORX glucosensing is dose-dependently prevented by lactate, pyruvate, ATP [78], reduced extracellular pH [73], and (through pH-independent effects) by nutritionally-relevant mixtures of dietary amino acids [79]. This suggests that ORX cells may reduce their glucose sensitivity in favor of integrating other signals, when energy levels are high and/or when CO2 levels are high. The dependence of ORX glucosensing on the levels of other energy substrates and on pH, as well the general responses of ORX cells to unphysiologically low glucose (Fig. 2) should be considered when interpreting the direction of ORX cell glucose responses [80,81].
The system-level implications of glucose-induced inhibition of ORX neurons remain to be clarified. The hypotheses that it could be involved in after-meal sleepiness [38] and/or in a feedback loop controlling peripheral glucose production and utilization [27], should be examined directly. Genetic tools for manipulating adult ORX neurons would help to explore these issues.
5.2. Amino acid sensing by LH neurons
5.2.1. Background: physiological levels of extracellular AAs and their brain actions
Ingested protein and AAs elevate AA concentrations in plasma [82,83] and the brain [84–87] on a time-scale of tens of minutes. Although to the best of our knowledge, similar direct measurements have not been made during starvation and dietary AA deficiencies, these conditions may also elevate brain AA levels. During starvation, tissue proteins are broken down for energy substrates, in particular to fuel hepatic gluconeogenesis [88]. This induces a rise in plasma AAs after around 3–4 days of starvation in rodents and humans [89,90]. Essential AA (eAA) deficiency leads to a rapid increase in protein degradation in order to replenish the missing eAA [91]. Several plasma eAAs (leucine, isoleucine, valine, tyrosine, phenylalanine, histidine, tryptophan and methionine) as well as the nonessential AAs (nAAs) glutamine, proline and alanine can suppress hepatic autophagy (large scale protein degradation in liver) to supply other tissues with AAs [91–93].
Increased dietary protein content increases satiety and decreases food intake in both the short and long-term [94–96]. However, diet deficient in an eAA suppresses food intake [97–100], presumably in order to locate diets more complete in eAAs. In terms of arousal, in humans, protein may be more effective than carbohydrate in increasing arousal and attention, although to the best of our knowledge the relative roles of eAAs and nAAs have not been examined. For example, protein-rich meals have been reported to be more effective at promoting cognitive arousal than isocaloric carbohydrate-rich meals [101,102].
Sensing of dietary protein is likely to occur directly in the brain, because vagotomy, which ablates communication from gut nutrient sensors, does not fully suppress satiety induced by high-protein diet [103]. Furthermore, i.c.v. injections of leucine or an AA mixture decrease food intake [104]. As reviewed thoroughly by Gietzen et al., considerable evidence points to direct sensing of dietary AAs in the anterior piriform cortex [105]. In addition, eAA-deficient diet increases c-fos expression in dorsomedial hypothalamus [106], and cutting fibers running anteriorly from dorsomedial hypothalamus increases intake of an eAA-deficient diet [107]. The essential AA leucine is sensed by neurons in the arcuate nucleus of the hypothalamus [108], and a further study showed c-fos activation in ARC POMC neurons, as well as in paraventricular nucleus oxytocin neurons and in the nucleus of the solitary tract, in response to leucine infusion near the ARC [87]. However, it is unclear how the leucine concentrations that reached putative sensor neurons in these studies compared to physiological baseline or postprandial values [84,87,109].
5.2.2. AA sensing by LH ORX cells
ORX cells in the LH are excited by physiological AA mixtures in vitro and in vivo, and AA gavage increases ORX receptor-dependent locomotion [79]. These effects are likely to be independent of pH effects described above, since physiological AA mixtures do not significantly change the pH of physiologically-buffered extracellular solutions used to investigate ORX cell activity (Mahesh Karnani, unpublished observations). Pharmacological and biophysical evidence suggests that stimulation of ORX neurons by dietary AAs involves a dual mechanism comprising activation of electrogenic (depolarizing) “system-A” transporters and closure of K-ATP channels [79]. Consistent with the substrate profile of system-A AA transporters [110,111], nAAs elicited larger responses than eAAs [79]. This may seem counterintuitive, since in terms of regulation of behavior, eAA levels may seem more critical to detect, because unlike nAAs, they cannot be made in the body and so have to be obtained through foraging. However, a rise in nAA levels in the brain may in fact indicate a fall in eAA levels in the blood, because essential and nAAs compete with each other for entry across the blood–brain barrier [112]. It is thus possible that increased excitation of ORX neurons by nAAs signals a fall in blood levels of eAAs, thereby (hypothetically) triggering a foraging or metabolic response required to counteract eAA deficiency.
Although AA sensing by ORX cells occurs in vitro and in vivo, its physiological role(s) on the whole-animal level remain to be determined. At the cellular level, it seems likely that ORX neurons are not “energy-meters” but may sense macronutrient balance, since some energy sources (glucose) are inhibitory and others (AAs, lactate) are excitatory. Interestingly, these opposing effects are reminiscent of human studies suggesting that protein-rich meals are more effective at promoting cognitive arousal than isocaloric carbohydrate-rich meals [101,102]. Furthermore, at the cellular level, the extracellular levels of AAs may be a permissive factor in determining whether ORX cells respond to glucose, since AAs suppress ORX glucose responses [79].
6. LH neurons, respiration, and CO2
6.1. Historical links between the LH and respiratory control
Classical lesion studies identified the LH not only as a key source of wakefulness and hunger signals, but also highlighted the importance of the LH in the regulation of breathing. In particular, the LH was historically proposed to be a source of respiration-facilitating impulses by Redgate and Gellhorn in the 1950s [6], who made electrolytic lesions in cat LH while monitoring the rate and depth of respiration. LH lesions produced rapid reductions in the rate and depth of respiration, while inhibition of the LH by barbiturates also reduced respiration [6]. This is supported by recent data revealing an anatomical ‘hotspot’ for breathing stimulation very close to the LH area where ORX neurons are found [113]. Importantly, it has been recently shown that focal microdialysis of CO2 into the LH increases breathing during wakefulness, suggesting that the LH is a central chemoreceptor site [114].
6.2. ORX neurons, breathing, and CO2
Analyses of in vivo phenotypes of ORX deficient mice, in vivo pharmacological evidence, and in vitro electrophysiological recordings from ORX neurons all suggest a role for ORX neurons in adjusting breathing to CO2 levels, especially during wakefulness [28]. Antagonism of ORX receptors inhibits CO2-induced ventilation [115], and ORX knockout can reduce CO2-induced ventilation by up to 50% [116]. At the cellular level, ORX neurons sense basic signals that are regulated by breathing, such as H+ and CO2, which in the body are always linked by the reaction [32]. In vivo, elevated CO2 stimulates the expression of the activation marker c-fos in ORX neurons [117]. In vitro, electrophysiological recordings of the membrane potential of ORX neurons in mouse brain slices show that the firing rate of some ORX cells is sensitive to the ambient levels of H+ and CO2 [12].
Brain interstitial pH is around 7.25 [34], but it can fluctuate between 6.9 and 7.4, for example during hypo or hyperventilation respectively [118]. Physiological acidosis (e.g. pH = 7) excites ORX neurons, while physiological alkalosis (e.g. pH = 7.4) causes electrical silencing (Fig. 3B). These responses resemble those of known chemosensory neurons, such as those in medullary raphe, in both direction (acidification is excitatory, alkalinization is inhibitory) and sensitivity (~100% change in firing rate per 0.1 unit change in pHe). These effects appear to be mediated, at least in part, by acid-induced inhibition of background K+ currents in the ORX cell membrane [12]. Although these currents functionally resemble TASK-like tandem-pore K+ channels proposed to mediate pH-sensing in certain classical chemosensory neurons such as retrotrapezoid nucleus, their molecular identity remains to be determined, since acid-sensing in ORX neurons – and in retrotrapezoid nucleus neurons – persists in TASK1/3 knockout mice [76,119]. Recent data suggest that other acid-sensing channels, such as ASICs, may contribute to regulation of breathing by ORX neurons [120], but it remains to be investigated whether they are responsible for electrophysiological effects of pH changes on ORX cell firing.
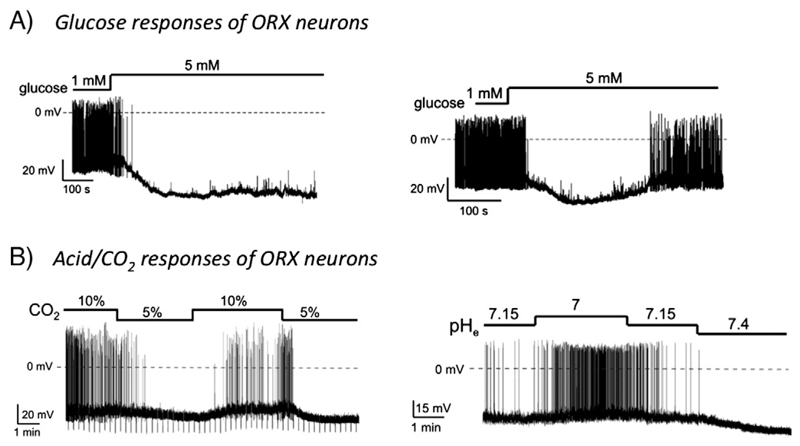
Fig. 3.
Glucose and CO2 responses of ORX neurons. A) ORX neurons can respond in a sustained elevation in extracellular glucose levels with either sustained (left) or transient (right) membrane hyperpolarization. Whole-cell patch-clamp recordings from acute brain slices from young mice. B) ORX cells are excited by extracellular acidification. The membrane potential and firing rate of mouse ORX neurons can reversibly modulated by changes in extracellular CO2 and pH levels.
Reproduced from [40] and [12] with permission from Proc Natl Acad Sci USA.
Overall, these data strongly suggest that, during wakefulness, ORX neurons regulate breathing in response to changes in CO2 levels. Whether the respiratory-related ORX neurons are a distinct population from “metabolic” and “arousal” ORX neurons remains to be clarified. The intriguing possibility that MCH and/or LepR neurons of the LH are also involved in respiratory control also deserves to be investigated.
7. LH neurons and leptin
7.1. Leptin directly regulates “non-ORX non-MCH” neurons in the LH
Early models of leptin action on the hypothalamus envisioned a serial arrangement where first-order leptin sensor neurons in the arcuate nucleus then synapsed onto second-order neurons in the LH and other areas [31]. It subsequently became clear, however, that LepR-expressing neurons are much more widely distributed throughout the brain [29]. The relative roles of LepR neurons in different brain areas are still being investigated. Neurochemically-speaking, the main antiobesity effects of leptin are thought to be mediated predominantly by GABAergic rather than glutamatergic neurons [22].
Recent data suggest that LH LepR neurons are important regulators of body energy status, and link anorexigenic leptin action to the mesolimbic DA system [121,122]. LH LepR neurons are neurochemically distinct from ORX and MCH cells, as indicated by lack of co-localization between ORX/MCH and GFP in LepR-GFP mice, and by lack of pSTAT3 (a marker of LepR activation) in ORX/MCH neurons [11]. Most LH LepR cells express GAD1, suggesting that they are inhibitory GABAergic neurons [11].
When applied by bath to LH brain slices, leptin rapidly depolarizes ≈35% but hyperpolarizes ≈20% of LH LepR neurons [11]. Both effects are thought to be postsynaptic, thus suggesting functionally distinct subpopulations among LH LepR cells [11]. Interestingly, leptin-induced c-fos immunoreactivity is not seen in VTA-projecting LH LepR neurons, suggesting that VTA-projecting LH LepR cells are not leptin-excited [11] (but see below). How the rapid (minutes) electrophysiological effects relate to the action of leptin on feeding is unclear, because the effects of intra-LH leptin infusion on feeding take several hours to develop [11]. This could suggest that the action of leptin of LH LepR cells is not a “stop-go” feeding signal but has a more permissive role, perhaps requiring a coincidence of some later signal(s) to elicit feeding. The role of the ≈50% of LH LepR cells that did not display rapid membrane potential responses to leptin also remains to be clarified.
7.2. Some LH LepR neurons express neurotensin
In addition to their electrophysiological and projection diversity, LH LepR cells are heterogenous neurochemically. Approximately 60% of them express the neuropeptide neurotensin (NTS) [122]. NTS-neurons are found throughout the brain, but LepRs are only present in LH NTS neurons [122]. Knocking out LepR in NTS neurons should thus affect only LH LepR-NTS cells [122]. The deletion of LepR from NTS cells in mice caused early-onset obesity, increased feeding, decreased locomotor activity, and reduced the weight-loss effects of exogenous leptin [122]. Electrophysiologically, leptin induces a rapid direct depolarization of LepR-expressing LH NTS cells [122]. Thus the action of leptin on LH NTS cells likely causes inhibition in their postsynaptic targets, such as ORX cells and the VTA [122], since all LH LepR cells are thought to be GABAergic [11].
The actions of leptin on leptin-excited LH LepR-NTS neurons [122], and perhaps on leptin-inhibited LH LepR-nonNTS neurons [11], thus play a key role in antiobesity effects of leptin. The underlying circuits may involve projections from LH LepR cells to the VTA and to local ORX neurons. However, many aspects remain to be clarified, in particular there are some discrepancies between electrophysiological and genetic data. For example, LH LepR-NTS neurons are directly activated by leptin in vitro and project to the VTA [122], yet in vivo leptin does not induce c-fos in VTA-projecting LH LepR neurons, suggesting that most VTA-projecting LH LepR cells are not leptin-activated [11]. It is also unclear how the antiobesity effects of leptin-excited LepR-NTS cells could be explained by their GABAergic (inhibitory) innervation of ORX cells [122], although – paradoxically – leptin increases ORX mRNA expression [122]. These ambiguities could reflect the unclear functional implications of some genetic measurements (see below), or could be related to projections of LH LepR neurons outside the LH and VTA [11].
8. Overview and concluding remarks
Several studies suggest that LH-specific sensing of glucose, leptin, and CO2 is vital for normal respiratory and metabolic function in vivo [67], but the broader physiological implications of these findings are not yet fully understood. For example, is there a significant cross-talk between the different signals, whereby a respiratory signal could influence metabolic function, e.g. as speculated in [123]? Some answers will undoubtedly arise from a better understanding of the LH as a functionally interconnected microcircuit. Gene-expression related methods, such as measurements of mRNA or c-fos may need to be complemented by more direct and “real-time” methods. This is because genetic changes may not always translate linearly into circuit function, especially at the level of release and action of transmitters from neurons. Moreover, gene expression is slow, while important interactions between LH neurons, the external world, and other brain circuits can occur at a very fast (seconds/milliseconds) timescale [58,124]. Direct measurements of functional connections between LH neurons and their targets with fast tools such as optogenetics and electrophysiology are well placed to fill in these gaps. Continuing investigations into these issues are important, because the emerging controllability of LH by factors linked to diet and respiration may suggest new strategies for improving whole-body energy balance.
Highlights.
LH regulates respiration via ORX cells.
LH regulates energy balance via ORX, MCH and LepR cells.
ORX, MHC, and LepR cells sense and integrate key feedback signals.
Acknowledgments
This article is based on a lecture during the 2012 Annual Meeting of the Society for the Study of Ingestive Behavior, Zurich, Switzerland, July 10–14, 2012, made possible in part by generous donations from Novo Nordisk A/S, Research Diets, Inc., Sanofi, Inc., and TSE, Inc.
References
- [1].Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77:323–4. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- [2].Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–31. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- [3].Gerashchenko D, Shiromani PJ. Different neuronal phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol Neurobiol. 2004;29 doi: 10.1385/MN:29:1:41. [DOI] [PubMed] [Google Scholar]
- [4].Kuwaki T. Orexin links emotional stress to autonomic functions. Auton Neurosci. 2011;161:20–7. doi: 10.1016/j.autneu.2010.08.004. [DOI] [PubMed] [Google Scholar]
- [5].Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: ingestive behavior. Neurosci Biobehav Rev. 1996;20:189–287. doi: 10.1016/0149-7634(95)00015-1. [DOI] [PubMed] [Google Scholar]
- [6].Redgate ES, Gellhorn E. Respiratory activity and the hypothalamus. Am J Physiol. 1958;193:189–94. doi: 10.1152/ajplegacy.1958.193.1.189. [DOI] [PubMed] [Google Scholar]
- [7].Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron—a potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–81. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- [8].van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–52. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- [9].Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [10].Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–81. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- [11].Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–90. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Marston OJ, Hurst P, Evans ML, Burdakov DI, Heisler LK. Neuropeptide Y cells represent a distinct glucose-sensing population in the lateral hypothalamus. Endocrinology. 2011;152:4046–52. doi: 10.1210/en.2011-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Karnani MM, Szabo G, Erdelyi F, Burdakov D. Lateral hypothalamic GAD65 neurons are spontaneously firing and distinct from orexin- and melanin-concentrating hormone neurons. J Physiol. 2013;591:933–53. doi: 10.1113/jphysiol.2012.243493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Myers MG, Jr, Olson DP. Central nervous system control of metabolism. Nature. 2012;491:357–63. doi: 10.1038/nature11705. [DOI] [PubMed] [Google Scholar]
- [16].Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15:1350–5. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–4. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- [18].Osundiji MA, Lam DD, Shaw J, Yueh CY, Markkula SP, Hurst P, et al. Brain glucose sensors play a significant role in the regulation of pancreatic glucose-stimulated insulin secretion. Diabetes. 2012;61:321–8. doi: 10.2337/db11-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Osundiji MA, Evans ML. Brain control of insulin and glucagon secretion. Endocrinol Metab Clin North Am. 2013;42:1–14. doi: 10.1016/j.ecl.2012.11.006. [DOI] [PubMed] [Google Scholar]
- [20].Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–32. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- [21].Vogt MC, Bruning JC. CNS insulin signaling in the control of energy homeostasis and glucose metabolism — from embryo to old age. Trends Endocrinol Metab. 2013;24:76–84. doi: 10.1016/j.tem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- [22].Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–54. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–91. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [24].Nattie E, Li A. Respiration and autonomic regulation and orexin. Prog Brain Res. 2012;198:25–46. doi: 10.1016/B978-0-444-59489-1.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kuwaki T, Li A, Nattie E. State-dependent central chemoreception: a role of orexin. Respir Physiol Neurobiol. 2010;173:223–9. doi: 10.1016/j.resp.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reddy AB, O'Neill JS. Healthy clocks, healthy body, healthy mind. Trends Cell Biol. 2010;20:36–44. doi: 10.1016/j.tcb.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Karnani M, Burdakov D. Multiple hypothalamic circuits sense and regulate glucose levels. Am J Physiol Regul Integr Comp Physiol. 2011;300:R47–55. doi: 10.1152/ajpregu.00527.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nattie E, Li A. Central chemoreception in wakefulness and sleep: evidence for a distributed network and a role for orexin. J Appl Physiol. 2010;108:1417–24. doi: 10.1152/japplphysiol.01261.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–32. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- [31].Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- [32].Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- [33].Routh VH. Glucose-sensing neurons: are they physiologically relevant? Physiol Behav. 2002;76:403–13. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- [34].Kintner DB, Anderson MK, Fitzpatrick JH, Jr, Sailor KA, Gilboe DD. 31P-MRS-based determination of brain intracellular and interstitial pH: its application to in vivo H+ compartmentation and cellular regulation during hypoxic/ischemic conditions. Neurochem Res. 2000;25:1385–96. doi: 10.1023/a:1007664700661. [DOI] [PubMed] [Google Scholar]
- [35].Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci. 2005;360:2227–35. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mobbs CV, Kow LM, Yang XJ. Brain glucose-sensing mechanisms: ubiquitous silencing by aglycemia vs. hypothalamic neuroendocrine responses. Am J Physiol Endocrinol Metab. 2001;281:E649–54. doi: 10.1152/ajpendo.2001.281.4.E649. [DOI] [PubMed] [Google Scholar]
- [37].Liss B, Roeper J. Molecular physiology of neuronal K-ATP channels (review) Mol Membr Biol. 2001;18:117–27. [PubMed] [Google Scholar]
- [38].Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–13. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- [39].Guyon A, Tardy MP, Rovere C, Nahon JL, Barhanin J, Lesage F. Glucose inhibition persists in hypothalamic neurons lacking tandem-pore K+ channels. J Neurosci. 2009;29:2528–33. doi: 10.1523/JNEUROSCI.5764-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci U S A. 2008;105:11975–80. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yang XJ, Kow LM, Funabashi T, Mobbs CV. Hypothalamic glucose sensor: similarities to and differences from pancreatic beta-cell mechanisms. Diabetes. 1999;48:1763–72. doi: 10.2337/diabetes.48.9.1763. [DOI] [PubMed] [Google Scholar]
- [42].Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO(2) and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- [43].Bittencourt JC. Anatomical organization of the melanin-concentrating hormone peptide family in the mammalian brain. Gen Comp Endocrinol. 2011;172:185–97. doi: 10.1016/j.ygcen.2011.03.028. [DOI] [PubMed] [Google Scholar]
- [44].de Lecea L, Carter ME, Adamantidis A. Shining light on wakefulness and arousal. Biol Psychiatry. 2012;71:1046–52. doi: 10.1016/j.biopsych.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schöne C, Venner A, Knowles D, Karnani MM, Burdakov D. Dichotomous cellular properties of mouse orexin/hypocretin neurons. J Physiol. 2011;589:2767–79. doi: 10.1113/jphysiol.2011.208637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Crocker A, Espana RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, et al. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–8. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Whiddon BB, Palmiter RD. Ablation of neurons expressing melanin-concentrating hormone (MCH) in adult mice improves glucose tolerance independent of MCH signaling. J Neurosci. 2013;33:2009–16. doi: 10.1523/JNEUROSCI.3921-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–4. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L, et al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- [51].Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–4. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- [52].Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–5. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, et al. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–30. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- [54].Burdakov D. Electrical signaling in central orexin/hypocretin circuits: tuning arousal and appetite to fit the environment. Neuroscientist. 2004;10:286–91. doi: 10.1177/1073858404263597. [DOI] [PubMed] [Google Scholar]
- [55].Wu M, Dumalska I, Morozova E, van den Pol A, Alreja M. Melanin-concentrating hormone directly inhibits GnRH neurons and blocks kisspeptin activation, linking energy balance to reproduction. Proc Natl Acad Sci U S A. 2009;106:17217–22. doi: 10.1073/pnas.0908200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep–wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–22. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14:478–90. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- [60].Brown SN, Chitravanshi VC, Kawabe K, Sapru HN. Microinjections of melanin concentrating hormone into the nucleus tractus solitarius of the rat elicit depressor and bradycardic responses. Neuroscience. 2007;150:796–806. doi: 10.1016/j.neuroscience.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Imbernon M, Beiroa D, Vazquez MJ, Morgan DA, Veyrat-Durebex C, Porteiro B, et al. Central melanin-concentrating hormone influences liver and adipose metabolism via specific hypothalamic nuclei and efferent autonomic/JNK1 pathways. Gastroenterology. 2013;144:636–49 (e6). doi: 10.1053/j.gastro.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–61. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci. 2002;22:6742–6. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Convergent inputs from electrically and topographically distinct orexin cells to locus coeruleus and ventral tegmental area. Eur J Neurosci. 2012;35:1426–32. doi: 10.1111/j.1460-9568.2012.08057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Leinninger GM, Myers MG., Jr LRb signals act within a distributed network of leptin-responsive neurones to mediate leptin action. Acta Physiol. 2008;192:49–59. doi: 10.1111/j.1748-1716.2007.01784.x. [DOI] [PubMed] [Google Scholar]
- [66].Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–33. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kong D, Vong L, Parton LE, Ye C, Tong Q, Hu X, et al. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 2010;12:545–52. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–86. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Pissios P, Ozcan U, Kokkotou E, Okada T, Liew CW, Liu S, et al. Melanin concentrating hormone is a novel regulator of islet function and growth. Diabetes. 2007;56:311–9. doi: 10.2337/db06-0708. [DOI] [PubMed] [Google Scholar]
- [70].Cai XJ, Evans ML, Lister CA, Leslie RA, Arch JR, Wilson S, et al. Hypoglycemia activates orexin neurons and selectively increases hypothalamic orexin-B levels: responses inhibited by feeding and possibly mediated by the nucleus of the solitary tract. Diabetes. 2001;50:105–12. doi: 10.2337/diabetes.50.1.105. [DOI] [PubMed] [Google Scholar]
- [71].Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- [72].Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Metabolism-independent sugar sensing in central orexin neurons. Diabetes. 2008;57:2569–76. doi: 10.2337/db08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O'Kelly I, et al. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–22. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- [74].Glowik RM, Golowasch J, Keller R, Marder E. d-glucose-sensitive neurosecretory cells of the crab Cancer borealis and negative feedback regulation of blood glucose level. J Exp Biol. 1997;200:1421–31. doi: 10.1242/jeb.200.10.1421. [DOI] [PubMed] [Google Scholar]
- [75].Burdakov D, Lesage F. Glucose-induced inhibition: how many ionic mechanisms? Acta Physiol. 2009;198:295–301. doi: 10.1111/j.1748-1716.2009.02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gonzalez JA, Jensen LT, Doyle SE, Miranda-Anaya M, Menaker M, Fugger L, et al. Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. Eur J Neurosci. 2009;30:57–64. doi: 10.1111/j.1460-9568.2009.06789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Carpenter R. Neurophysiology. 4th ed. London: Arnold; 2003. [Google Scholar]
- [78].Venner A, Karnani MM, Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Orexin neurons as conditional glucosensors: paradoxical regulation of sugar sensing by intracellular fuels. J Physiol. 2011;589:5701–8. doi: 10.1113/jphysiol.2011.217000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Karnani MM, Apergis-Schoute J, Adamantidis A, Jensen LT, de Lecea L, Fugger L, et al. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. 2011;72:616–29. doi: 10.1016/j.neuron.2011.08.027. [DOI] [PubMed] [Google Scholar]
- [80].Parsons MP, Hirasawa M. ATP-sensitive potassium channel-mediated lactate effect on orexin neurons: implications for brain energetics during arousal. J Neurosci. 2010;30:8061–70. doi: 10.1523/JNEUROSCI.5741-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Liu ZW, Gan G, Suyama S, Gao XB. Intracellular energy status regulates activity in hypocretin/orexin neurones: a link between energy and behavioural states. J Physiol. 2011;589:4157–66. doi: 10.1113/jphysiol.2011.212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Adibi SA, Mercer DW. Protein digestion in human intestine as reflected in luminal, mucosal, and plasma amino acid concentrations after meals. J Clin Invest. 1973;52:1586–94. doi: 10.1172/JCI107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gill GV, Baylis PH, Flear CT, Lawson JY. Changes in plasma solutes after food. J R Soc Med. 1985;78:1009–13. doi: 10.1177/014107688507801206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Choi YH, Chang N, Anderson GH. An intragastric amino acid mixture influences extracellular amino acid profiles in the lateral hypothalamic area of freely moving rats. Can J Physiol Pharmacol. 1999;77:827–34. [PubMed] [Google Scholar]
- [85].Choi YH, Chang N, Fletcher PJ, Anderson GH. Dietary protein content affects the profiles of extracellular amino acids in the medial preoptic area of freely moving rats. Life Sci. 2000;66:1105–18. doi: 10.1016/s0024-3205(00)00414-8. [DOI] [PubMed] [Google Scholar]
- [86].Choi YH, Fletcher PJ, Anderson GH. Extracellular amino acid profiles in the paraventricular nucleus of the rat hypothalamus are influenced by diet composition. Brain Res. 2001;892:320–8. doi: 10.1016/s0006-8993(00)03267-4. [DOI] [PubMed] [Google Scholar]
- [87].Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus–brainstem circuit. J Neurosci. 2009;29:8302–11. doi: 10.1523/JNEUROSCI.1668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wang T, Hung CC, Randall DJ. The comparative physiology of food deprivation: from feast to famine. Annu Rev Physiol. 2006;68:223–51. doi: 10.1146/annurev.physiol.68.040104.105739. [DOI] [PubMed] [Google Scholar]
- [89].Adibi SA. Influence of dietary deprivations on plasma concentration of free amino acids of man. J Appl Physiol. 1968;25:52–7. doi: 10.1152/jappl.1968.25.1.52. [DOI] [PubMed] [Google Scholar]
- [90].Felig P, Owen OE, Wahren J, Cahill GF., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969;48:584–94. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kadowaki M, Kanazawa T. Amino acids as regulators of proteolysis. J Nutr. 2003;133:2052S–6S. doi: 10.1093/jn/133.6.2052S. [DOI] [PubMed] [Google Scholar]
- [92].Buse MG, Reid SS. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975;56:1250–61. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Fulks RM, Li JB, Goldberg AL. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975;250:290–8. [PubMed] [Google Scholar]
- [94].Anderson GH. Control of protein and energy intake: role of plasma amino acids and brain neurotransmitters. Can J Physiol Pharmacol. 1979;57:1043–57. doi: 10.1139/y79-158. [DOI] [PubMed] [Google Scholar]
- [95].Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M. Protein, weight management, and satiety. Am J Clin Nutr. 2008;87:1558S–61S. doi: 10.1093/ajcn/87.5.1558S. [DOI] [PubMed] [Google Scholar]
- [96].Bertenshaw EJ, Lluch A, Yeomans MR. Dose-dependent effects of beverage protein content upon short-term intake. Appetite. 2009;52:580–7. doi: 10.1016/j.appet.2009.01.010. [DOI] [PubMed] [Google Scholar]
- [97].Leung PM, Rogers QR. Importance of prepyriform cortex in food-intake response of rats to amino acids. Am J Physiol. 1971;221:929–35. doi: 10.1152/ajplegacy.1971.221.3.929. [DOI] [PubMed] [Google Scholar]
- [98].Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–8. doi: 10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- [99].Koehnle TJ, Russell MC, Morin AS, Erecius LF, Gietzen DW. Diets deficient in indispensable amino acids rapidly decrease the concentration of the limiting amino acid in the anterior piriform cortex of rats. J Nutr. 2004;134:2365–71. doi: 10.1093/jn/134.9.2365. [DOI] [PubMed] [Google Scholar]
- [100].Koehnle TJ, Russell MC, Gietzen DW. Rats rapidly reject diets deficient in essential amino acids. J Nutr. 2003;133:2331–5. doi: 10.1093/jn/133.7.2331. [DOI] [PubMed] [Google Scholar]
- [101].Fischer K, Colombani PC, Langhans W, Wenk C. Carbohydrate to protein ratio in food and cognitive performance in the morning. Physiol Behav. 2002;75:411–23. doi: 10.1016/s0031-9384(01)00676-x. [DOI] [PubMed] [Google Scholar]
- [102].Spring B, Maller O, Wurtman J, Digman L, Cozolino L. Effects of protein and carbohydrate meals on mood and performance: interactions with sex and age. J Psychiatr Res. 1982;17:155–67. doi: 10.1016/0022-3956(82)90017-6. [DOI] [PubMed] [Google Scholar]
- [103].L'Heureux-Bouron D, Tome D, Rampin O, Even PC, Larue-Achagiotis C, Fromentin G. Total subdiaphragmatic vagotomy does not suppress high protein diet-induced food intake depression in rats. J Nutr. 2003;133:2639–42. doi: 10.1093/jn/133.8.2639. [DOI] [PubMed] [Google Scholar]
- [104].Morrison CD, Xi X, White CL, Ye J, Martin RJ. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab. 2007;293:E165–71. doi: 10.1152/ajpendo.00675.2006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Gietzen DW, Hao S, Anthony TG, Lajtha A, Johnson AD. Handbook of neurochemistry and molecular neurobiology sensory neurochemistry. US: Springer; 2007. Amino acid-sensing mechanisms: biochemistry and behavior; pp. 249–69. [Google Scholar]
- [106].Wang Y, Cummings SL, Gietzen DW. Temporal–spatial pattern of c-fos expression in the rat brain in response to indispensable amino acid deficiency. I. The initial recognition phase. Brain Res Mol Brain Res. 1996;40:27–34. doi: 10.1016/0169-328x(96)00032-0. [DOI] [PubMed] [Google Scholar]
- [107].Bellinger LL, Evans JF, Gietzen DW. Dorsomedial hypothalamic lesions alter intake of an imbalanced amino acid diet in rats. J Nutr. 1998;128:1213–7. doi: 10.1093/jn/128.7.1213. [DOI] [PubMed] [Google Scholar]
- [108].Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–30. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- [109].Nishimura F, Nishihara M, Mori M, Torii K, Takahashi M. Excitability of neurons in the ventromedial nucleus in rat hypothalamic slices: modulation by amino acids at cerebrospinal fluid levels. Brain Res. 1995;691:217–22. doi: 10.1016/0006-8993(95)00719-7. [DOI] [PubMed] [Google Scholar]
- [110].Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (system N/A) transporters of the SLC38 gene family. Pflugers Archiv. 2004;447:784–95. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- [111].Mackenzie B, Schafer MK, Erickson JD, Hediger MA, Weihe E, Varoqui H. Functional properties and cellular distribution of the system A glutamine transporter SNAT1 support specialized roles in central neurons. J Biol Chem. 2003;278:23720–30. doi: 10.1074/jbc.M212718200. [DOI] [PubMed] [Google Scholar]
- [112].Oldendorf WH, Szabo J. Amino acid assignment to one of three blood–brain barrier amino acid carriers. Am J Physiol. 1976;230:94–8. doi: 10.1152/ajplegacy.1976.230.1.94. [DOI] [PubMed] [Google Scholar]
- [113].Tanaka M, McAllen RM. Functional topography of the dorsomedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2008;294:R477–86. doi: 10.1152/ajpregu.00633.2007. [DOI] [PubMed] [Google Scholar]
- [114].Li N, Li A, Nattie E. Focal microdialysis of CO(2) in the perifornical–hypothalamic area increases ventilation during wakefulness but not NREM sleep. Respir Physiol Neurobiol. 2013 Jan 15;185(2):349–55. doi: 10.1016/j.resp.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Li A, Nattie E. Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. J Physiol. 2010;588:2935–44. doi: 10.1113/jphysiol.2010.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol. 2007;102:241–8. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- [117].Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol. 2009;166:184–6. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- [118].van Hulst RA, Hasan D, Lachmann B. Intracranial pressure, brain PCO2, PO2, and pH during hypo- and hyperventilation at constant mean airway pressure in pigs. Intensive Care Med. 2002;28:68–73. doi: 10.1007/s00134-001-1157-6. [DOI] [PubMed] [Google Scholar]
- [119].Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, et al. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–58. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Song N, Zhang G, Geng W, Liu Z, Jin W, Li L, et al. Acid sensing ion channel 1 in lateral hypothalamus contributes to breathing control. PLoS One. 2012;7:e39982. doi: 10.1371/journal.pone.0039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Louis GW, Leinninger GM, Rhodes CJ, Myers MG., Jr Direct innervation and modulation of orexin neurons by lateral hypothalamic LepRb neurons. J Neurosci. 2010;30:11278–87. doi: 10.1523/JNEUROSCI.1340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–23. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hersoug L-G, Sjödin A, Astrup A. A proposed potential role for increasing atmospheric CO2 as a promoter of weight gain and obesity. Nutr Diab. 2012;2:e31. doi: 10.1038/nutd.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Schone C, Cao ZF, Apergis-Schoute J, Adamantidis A, Sakurai T, Burdakov D. Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J Neurosci. 2012;32:12437–43. doi: 10.1523/JNEUROSCI.0706-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]