Abstract
Sensing of sugar by specialized ‘glucose-inhibited’ cells helps organisms to counteract swings in their internal energy levels. Evidence from several cell types in both vertebrates and invertebrates suggests that this process involves sugar-induced stimulation of plasma membrane K+ currents. However, the molecular composition and the mechanism of activation of the underlying channel(s) remain controversial. In mouse hypothalamic neurones and neurosecretory cells of the crab Cancer borealis, glucose stimulates K+ currents displaying leak-like properties. Yet knockout of some of the candidate ‘leak’ channel subunits encoded by the KCNK gene family so far failed to abolish glucose inhibition of hypothalamic cells. Moreover, in other tissues, such as the carotid body, glucose-stimulated K+ channels appear to be not leak-like but voltage-gated, suggesting that glucose-induced inhibition may engage multiple types of K+ channels. Other mechanisms of glucose-induced inhibition, such as hyperpolarization mediated by opening of Cl− channels and depolarization block caused by closure of KATP channels have also been proposed. Here we review known ionic and pharmacological features of glucose-induced inhibition in different cell types, which may help to identify its molecular correlates.
Keywords: appetite, glucose, hypocretin, hypothalamus, orexin, sleep
To sustain life, all organisms must compensate for their energy expenditure by energy intake. In higher animals, this requires sensing of internal energy levels by specialized organs that participate in orchestrating food intake, energy expenditure and hormone release. The concentration of glucose in the extracellular fluid is a good direct indicator of available energy, and specialized ‘glucose-sensor’ cells are part of a variety of organs such as the brain, pancreas and the specialized neurosecretory organs of invertebrates. These sensor cells are excitable cells that respond to increased glucose concentration with either an increase (glucose-excited cells) or a decrease (glucose-inhibited cells) in electrical activity (Oomura et al. 1969, Glowik et al. 1997, Burdakov et al. 2005). Glucose-excited cells, e.g. the β-cells of the mammalian pancreas, are much better understood than glucose-inhibited cells, and will not be discussed here (see instead Ashcroft & Rorsman 1989, Routh 2002, Burdakov et al. 2005). Here we will instead discuss the ionic mechanisms involved in glucose-induced inhibition, focusing predominantly on the role of glucose-stimulated K+ channels but also discussing some other proposed mechanisms.
Glucose-stimulated K+ currents in mammalian neurones
In the mammalian brain, glucose-inhibited neurones are found predominantly in the hypothalamus and the brainstem (Oomura et al. 1969, Adachi et al. 1995, Balfour et al. 2006). In the hypothalamus, some of these neurones are now behaviourally and neurochemically defined, and comprise cell populations such as wakefulness-promoting orexin/hypocretin neurones and appetite-promoting neuropeptide Y (NPY) neurones, which may potentially link glucose-induced inhibition to important behavioural adaptations (reviewed in Burdakov & González 2009). Other populations of hypothalamic glucose-inhibited neurones also exist, e.g. a neurochemically undefined set of cells in the ventromedial nucleus (Minami et al. 1986, Song et al. 2001), whose physiological importance is currently less understood. Here we focus on studies supporting the idea that glucose-stimulated K+ currents are involved in glucose-induced inhibition of lateral and mediobasal hypothalamic neurones.
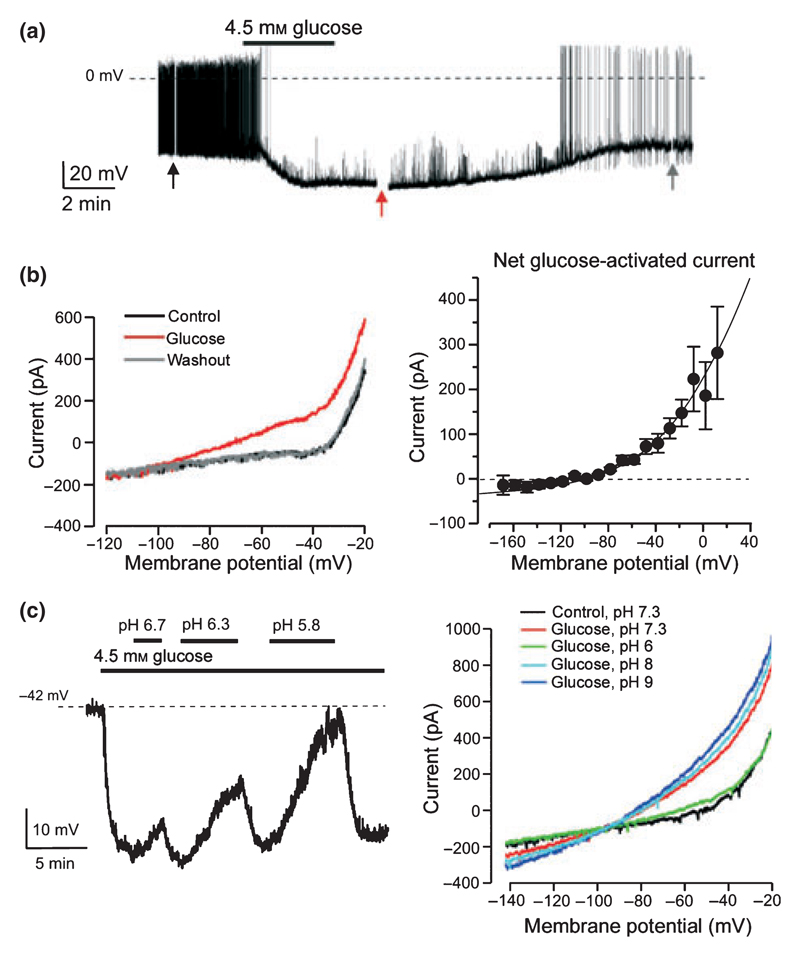
In cell bodies of lateral hypothalamic orexin neurones, glucose stimulates K+-selective whole-cell currents that are large enough to account for glucose-induced hyperpolarization and electrical silencing displayed by these cells (Fig. 1a,b; Burdakov et al. 2006). Glucose-induced hyperpolarization of orexin cells persists when they are dissociated from slice tissue (Yamanaka et al. 2003), indicating a direct action of glucose. Glucose-stimulated currents behaved like ‘leak’ currents lacking intrinsic voltage dependence (Fig. 1b), and were not blocked by 10 μm ruthenium red, an inhibitor of leak K+ channels made up of TASK3 subunits, or by reducing extracellular or intracellular Ca2+ concentration (Burdakov et al. 2006). However, they were inhibited by extracellular acidification (Fig. 1c), although currently it remains to be unequivocally established whether acidification acts on the channels themselves or some other part of the glucose-sensing pathway. The latter remains undefined, with indications from functional experiments that extracellular glucose but not intracellular glucose metabolism is required, unlike the traditional glucose-sensing pathways in glucose-excited neurones (discussed in González et al. 2009a). The agonist profile for activation of glucose-stimulated K+ currents in orexin neurones also appears different from most glucose-binding proteins classically thought to be involved in glucose-sensing: the currents are activated by d-glucose, 2-deoxyglucose, and mannose, but not by galactose, fructose, sucrose, l-glucose or alpha-methyl-d-glucoside (González et al. 2008).
Figure 1. Evidence for glucose-stimulated K+ currents in mouse orexin neurones.
(a) Membrane potential response to an increase in the concentration of glucose from 1 to 4.5 mm. (b) Left, membrane current–voltage relationships during different points of the experiment in (a). Right, net current activated by glucose. (c) Extracellular acidification reverses the effect of glucose on the membrane potential (left) and current (right). Data in (a)–(c) reproduced from Burdakov et al. (2006) with permission from Elsevier.
Recent experiments also suggest a role for glucose-stimulated K+ channels in a group of glucose-inhibited neurones of the mediobasal hypothalamus, whose neurochemical identity is not yet defined. In these cells, glucose stimulates leak-like whole-cell K+ currents and hyperpolarization that persists when other inhibitory currents (chloride channels) are biophysically disabled (Williams & Burdakov 2009). Like in orexin neurones, the activity of these K+ currents is suppressed by acidification, suggesting a similar kind of mechanism (Williams & Burdakov 2009). Attempts to characterize glucose-stimulated channels at the single-channel level have been made for both mediobasal and lateral hypothalamus. In mediobasal neurones, the single-channel conductance of putative glucose-stimulated K+ channels appeared to be around 130 pS (Rowe et al. 1996), whereas in lateral hypothalamic orexin neurones, the value of 40 pS was estimated (Burdakov et al. 2006). One interpretation of this is that different channels are involved; however, we acknowledge that a causal link between these single-channel measurements and membrane hyperpolarization has not yet been made, leaving open the possibility that other as yet uncharacterized K+ channels mediate glucose-induced hyperpolarization at the whole-cell level.
Glucose-stimulated K+ currents in invertebrate neurones
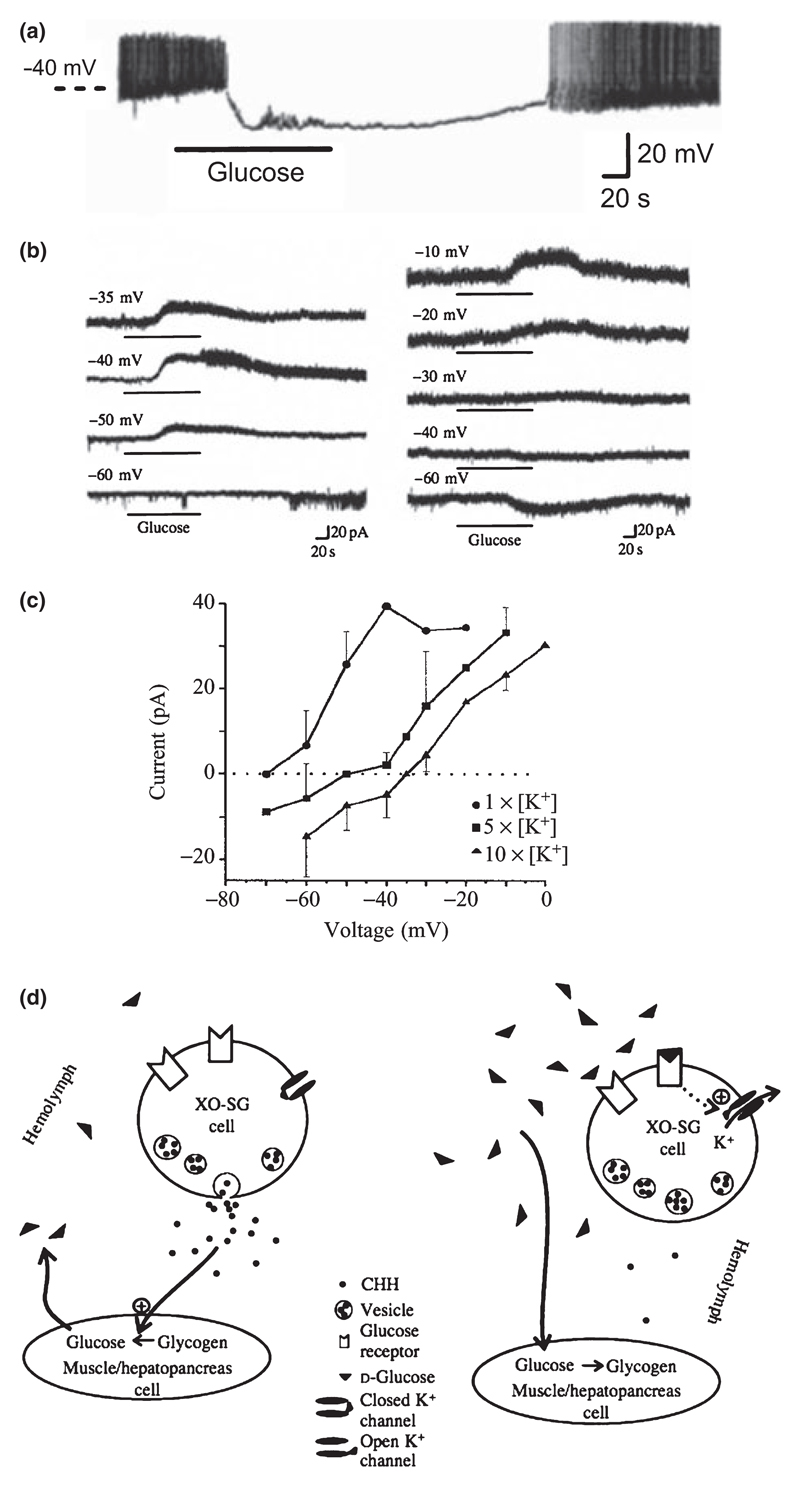
A discovery and characterization of glucose-induced inhibition in crab neurones, which went virtually unnoticed by mammalian neuroscientists and only recently came to the attention of the authors of this review, was reported by Glowik et al. (1997). These authors performed intracellular microelectrode recordings from isolated and cultured neurones from the X-organ of the crab Cancer borealis, which release the glucose-increasing hormone CHH into the crab haemolymph (Glowik et al. 1997). Similar to the orexin neurones described above, glucose induced large-amplitude membrane hyperpolarization which was due to activation of K+-selective currents with leak-like (voltage-independent) properties (Fig. 2a–c). Like in orexin neurones (González et al. 2008), no response was observed to l-glucose, sucrose, galactose and fructose; but in contrast to orexin neurones (González et al. 2008), the crustacean cells also did not respond to mannose (Glowik et al. 1997).
Figure 2. Evidence for glucose-stimulated K+ currents in crab neurosecretory neurones.
(a) Membrane potential response to an increase in the concentration of glucose from 0 to 5 mm. (b) Membrane current response to glucose at different holding potentials. (c) Current–voltage relationship of glucose-induced current (Note: the reversal potential was indistinguishable from that of K+ currents; see Glowik et al. 1997). (d) Hypothetical model of glucose-induced inhibition. (a)–(d) reproduced from Glowik et al. (1997) with permission from The Company of Biologists.
Glucose-induced K+ currents in crab neurones persisted when extracellular Na+ and Ca2+ were eliminated, and were blocked neither by the broad Ca2+ channel blocker Cd2+(200 μm) nor by 500 μm ouabain, the inhibitor of the Na+ pump historically proposed to mediate glucose-induced hyperpolarization (Oomura et al. 1974). The glucose-stimulated K+ currents were only partially (50%) blocked by 10 mm TEA+, which has different blocking effects on different types of K+ channels (Glowik et al. 1997). Interestingly, application of 3 mm of 4-AP, a blocker of A-type (voltage-gated) K+ channels, had variable effects: in three of eight cells glucose-stimulated K channels were totally blocked, three of eight cells displayed partial block, whereas two of eight cells showed no block (Glowik et al. 1997). The cause of this variability is unknown but it is consistent with a mixture of K+ channel types participating in the effect. No blocking effects were observed upon application of 1 mm tolbutamide, a blocker of ATP-sensitive K+ channels, or 5 mm Cs+ (Glowik et al. 1997). The authors concluded that glucose-stimulated K+ channels probably form a part of leak current in the crab cells, and speculated that they are activated by glucose binding to a plasma membrane site that shows different sensitivity and specificity for sugars than known passive glucose-transport systems (Fig. 2d).
Glucose-stimulated K+ currents in the mammalian carotid body
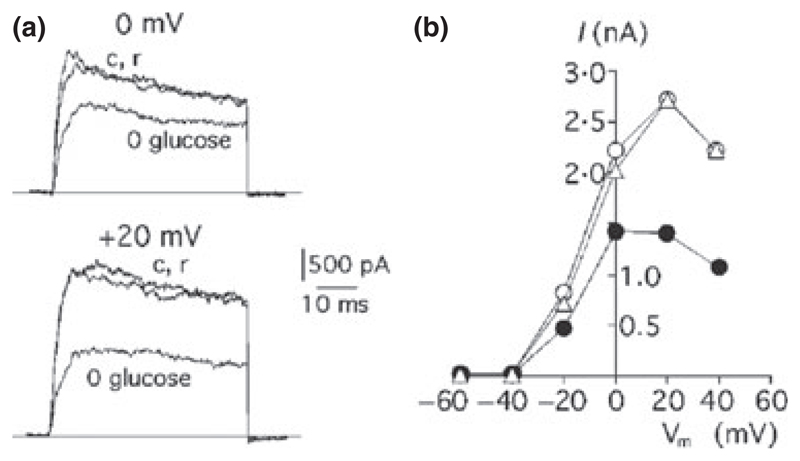
The mammalian carotid body is a collection of chemoreceptors and supporting cells located at the bifurcation of the carotid artery. Stimulation of glomus cells of the carotid body produces sympathetic activation. Although better known as sensors of O2 and CO2/H+ levels (Peers & Buckler 1995), some studies also indicated that carotid body glomus cells are stimulated by low glucose-levels (Pardal & Lopez-Barneo 2002, Garcia-Fernandez et al. 2007), and thus can be considered glucose-inhibited. While the physiological significance of this glucose-sensing remains to be resolved (Conde et al. 2007), glucose appears to stimulate K+ currents in the carotid body glomus cells. However, glucose-stimulated K+ channels in the glomus cells behave not like ‘leak’ but like voltage-gated channels, becoming activated at potentials above around −40 mV (Fig. 3). Their molecular identity remains to be determined, but they were blocked by 10 mm TEA+ (Garcia-Fernandez et al. 2007). In some (Garcia-Fernandez et al. 2007) but not other (Pardal & Lopez-Barneo 2002) experiments the glucose-stimulated currents were also sensitive to iberiotoxin, suggesting that Ca2+-activated maxi-K+ channels may make a variable contribution. Thus glucose-stimulated K+ channels in the carotid body appear to differ from those that make up glucose-stimulated K+ currents in vertebrate and invertebrate neurones, and may comprise a molecularly heterogeneous mixture.
Figure 3. Evidence for glucose-stimulated K+ currents in mammalian carotid body cells.
(a) Membrane current responses to a voltage step from −80 to 0 mV (top) or +20 mV (bottom) in 5 mm glucose (c, control solution), 0 mm glucose and return to 5 mm glucose (r). (b) Relationship between peak outward current and membrane potential in the same cell bathed in control solutions before (5 mm glucose, open circles) and after (5 mm glucose, triangles) exposure to low glucose (0 mm glucose, closed circles). Reproduced from Pardal & Lopez-Barneo (2002), with permission from Macmillan Publishers Ltd: Nature Neuroscience, © 2002.
What is the molecular identity of glucose-stimulated K+ channel(s)?
Is the pharmacology of glucose-stimulated currents in C. borealis neurones and in mouse orexin neurones helpful for the molecular identification of glucose-stimulated K+ channels? TEA, 4-AP, Ba2+and Cs+ are all non-selective blockers. Their application is known to affect a large number of cloned K+ channels to various extents. Variation in drug affinity for related channel isoforms between species and subunit heteromultimerization further complicates the relevance of predictions based on pharmacology. Nevertheless, glucose-stimulated K+ currents in orexin neurones of the mouse lateral hypothalamus were sufficiently well characterized to give rise to theories about their molecular basis. Lack of voltage dependence, inhibition by external acidification in the physiological pH range and stimulation by halothane suggested that some members of the KCNK family of leak K+ channels may contribute to these glucose-stimulated currents (Burdakov et al. 2006). In addition, channel insensitivity to ruthenium red and a 40 pS unitary conductance, as well as immunohistochemical data, suggested heterodimeric channels made up of TASK1 (KCNK3) and TASK3 (KCNK9) subunits as the most probable candidates (Burdakov et al. 2006). Much to our surprise, we found that the hyperpolarization induced by glucose in hypothalamic neurones was unaffected in TASK1, TASK3 and double TASK1/TASK3 KO mice (González et al. 2009b, Guyon et al. 2009). Voltage-clamp studies showed that in orexin hypothalamic neurones from TASK1/TASK3 KO mice, the glucose-sensitive currents were functionally similar to those in wild type mice (González et al. 2009b). We also tested the glucose response in mice lacking another class of KCNK channels activated by halothane. In these TREK1/TREK2/TRAAK triple KO mice, the glucose-induced hyperpolarization was also unaffected (Guyon et al. 2009). Finally, we found that Ba2+ was able to reverse the glucose-induced membrane potential hyperpolarization in wild type mice (Guyon et al. 2009). This occurred at concentrations lower than those expected to inhibit KCNK channels, and more like those expected to block the Kir subfamily (based on data on heterologously expressed cloned channels; Lesage 2003, Kubo et al. 2005, Goldstein et al. 2005). Taken together, these results seem to argue that KCNK channels are not essential for glucose-induced inhibition. However, the blocking effects of acidification and Ba2+ were rather slow compared with the effects usually observed with cloned K+ channels expressed in transfected cells (Guyon et al. 2009). Although it is difficult to infer from kinetics of response onset when using applications in brain slice tissue, this slow time course might indicate that pH and Ba2+ did not directly affect K+ channels but a remote glucosensor coupled to leak K+ channels. In this case, KCNK leak channels other that TASK and TREK channels remain good candidates.
Another possibility is that some other type of leak K+ channel(s) sensitive to pH and Ba2+ contribute to glucose-induced hyperpolarization. K+ channels form the largest family of ion channels with 77 genes encoding pore-forming subunits (Goldstein et al. 2005, Gutman et al. 2005, Kubo et al. 2005). Often, different subunits form heteromultimers with novel properties. Heteromultimers and homomultimers often interact with auxiliary and regulatory subunits that themselves modify the electrophysiological and/or pharmacological characteristics. KCNK channels are not the only subunits to produce leak channels. The association of KCNQ1 and KCNE2 subunits produce background currents (Tinel et al. 2000). There are five KCNQ subunits and five KCNE subunits, leaving room for more leak channels (Goldstein et al. 2005, Gutman et al. 2005). Another example of K+ channels producing voltage-independent currents, once activated, are the SK channels. They are activated by Ca2+ binding to calmodulins attached to pore-forming subunits, but other associated proteins regulate the Ca2+sensitivity (Allen et al. 2007). Finally, in physiological conditions, the I–V curve of weakly inwardly rectifying K+ channels such as ROMK1 fits the GHK prediction (Hebert 1995), in particular between −120 and +0 mV as measured in orexin neurones (Fig. 1b). The glucose-induced conductance may be produced by one or more of these channels, or by a yet uncharacterized combination of pore-forming subunits and regulatory proteins.
Do glucose-stimulated K+ currents explain all glucose-induced inhibition?
Although the data discussed above provide strong support for the idea that K+ currents mediate glucose-induced inhibition of certain neurones, there are several other glucose-inhibited cells where other mechanisms are likely to be involved. One of these is the α-cell of the pancreas, perhaps the best known glucose-inhibited cell, which is responsible for the secretion of the most important glucose-increasing hormone in mammals, glucagon. How glucose inhibits α-cell electrical activity, and hence glucagon secretion, is controversial and thought to be influenced by many factors such as secretion of inhibitory factors from the neighbouring glucose-excited β-cells and nerves (Rorsman et al. 2008). However, intrinsic glucose-sensing by the α-cell is also thought to play a role, with glucose acting directly on the cell to change its membrane potential. One current model postulates that this is due to glucose-induced depolarization block of action potential generation, wherein glucose causes closure of KATP channels, which depolarizes the membrane to such an extent that Na+ and Ca2+ channels become inactivated (see figure 6 in Gromada et al. 2004). Although controversial (see discussion in Rorsman et al. 2008), this model outlines a pathway of glucose-induced inhibition independent of glucose-stimulated K+ currents, as well as making an important general point of how, depending on the overall ion channel make-up of the cell, closure of KATP channels can lead to electrical inhibition rather than excitation. However, such ‘inhibition through depolarization block’ is unlikely to explain the glucose-induced inhibition of neurones discussed above, as the latter is associated with profound hyperpolarization and, at least in the crab, is insensitive to the KATP blocker tolbutamide (Glowik et al. 1997, Burdakov et al. 2006).
Another model to explain glucose-induced inhibition involves activation of Cl−) channels, and has been proposed to explain the effects of glucose on certain mediosal hypothalamic neurones (Song et al. 2001, Fioramonti et al. 2007). Although our recent experiments suggest that glucose-stimulated K+ channels also contribute to glucose-induced inhibition in the mediobasal hypothalamus (Williams & Burdakov 2009), the relative contribution of K+ vs. Cl−) channels in this brain region remains unresolved and would require further experimentation (see discussion in Burdakov & González 2009, Williams & Burdakov 2009).
Conclusions
Studies discussed above suggest that glucose-stimulated K+ channels are a conserved mechanism of energy sensing in both vertebrates and invertebrates, although other mechanisms also contribute to glucose-induced inhibition of some cells. However, glucose-stimulated K+ currents appear to have different functional properties in different tissues, suggesting that either different K+ channels are engaged in different cells or that more than one type of K+ channel is directly or indirectly modulated by glucose during glucose-induced inhibition. These possibilities remain to be investigated, for example by systematically screening the effects of a large array of specific K+ channel blockers and/or RNA interference methods on glucose-inhibited tissues. How glucose opens K+ channels also remains unresolved (for discussion, see González et al. 2008). It will be important to address these questions because glucose-stimulated K+ channels are likely to be directly involved in the control of behaviour and energy homeostasis in both vertebrates and invertebrates (Glowik et al. 1997, Burdakov & González 2009).
Footnotes
Conflict of interest
There is no conflict of interest.
References
- Adachi A, Kobashi M, Funahashi M. Glucose-responsive neurons in the brainstem. Obes Res. 1995;3(Suppl. 5):735S–740S. doi: 10.1002/j.1550-8528.1995.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Allen D, Fakler B, Maylie J, Adelman JP. Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J Neurosci. 2007;27:2369–2376. doi: 10.1523/JNEUROSCI.3565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol. 2006;570:469–484. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, González JA. Physiological functions of glucose-inhibited neurones. Acta Physiol (Oxf) 2009;195:71–78. doi: 10.1111/j.1748-1716.2008.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci. 2005;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Conde SV, Obeso A, González C. Low glucose effects on rat carotid body chemoreceptor cells’ secretory responses and action potential frequency in the carotid sinus nerve. J Physiol. 2007;585:721–730. doi: 10.1113/jphysiol.2007.144261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioramonti X, Contie S, Song Z, Routh VH, Lorsignol A, Penicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez M, Ortega-Saenz P, Castellano A, Lopez-Barneo J. Mechanisms of low-glucose sensitivity in carotid body glomus cells. Diabetes. 2007;56:2893–2900. doi: 10.2337/db07-0122. [DOI] [PubMed] [Google Scholar]
- Glowik RM, Golowasch J, Keller R, Marder E. d-glucose-sensitive neurosecretory cells of the crab Cancer borealis and negative feedback regulation of blood glucose level. J Exp Biol. 1997;200:1421–1431. doi: 10.1242/jeb.200.10.1421. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- González JA, Jensen LT, Fugger L, Burdakov D. Metabolism-independent sugar sensing in central orexin neurons. Diabetes. 2008;57:2569–2576. doi: 10.2337/db08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JA, Reimann F, Burdakov D. Dissociation between sensing and metabolism of glucose in sugar sensing neurones. J Physiol. 2009a;587:41–48. doi: 10.1113/jphysiol.2008.163410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JA, Jensen LT, Doyle SE, Miranda-Anaya M, Menaker M, Fugger L, Bayliss DA, Burdakov D. Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. Eur J Neurosci. 2009b doi: 10.1111/j.1460-9568.2009.06789.x. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Ma X, Hoy M, Bokvist K, Salehi A, Berggren PO, Rorsman P. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1–/– mouse alpha-cells. Diabetes. 2004;53(Suppl. 3):S181–S189. doi: 10.2337/diabetes.53.suppl_3.s181. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- Guyon A, Tardy MP, Rovere C, Nahon JL, Barhanin J, Lesage F. Glucose inhibition persists in hypothalamic neurons lacking tandem-pore K+ channels. J Neurosci. 2009;29:2528–2533. doi: 10.1523/JNEUROSCI.5764-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SC. An ATP-regulated, inwardly rectifying potassium channel from rat kidney (ROMK) Kidney Int. 1995;48:1010–1016. doi: 10.1038/ki.1995.383. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Adelman JP, Clapham DE, Jan LY, Karschin A, Kurachi Y, Lazdunski M, Nichols CG, Seino S, Vandenberg CA. International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol Rev. 2005;57:509–526. doi: 10.1124/pr.57.4.11. [DOI] [PubMed] [Google Scholar]
- Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Minami T, Oomura Y, Sugimori M. Electrophysiological properties and glucose responsiveness of guinea-pig ventromedial hypothalamic neurones in vitro. J Physiol. 1986;380:127–143. doi: 10.1113/jphysiol.1986.sp016276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature. 1974;247:284–286. doi: 10.1038/247284a0. [DOI] [PubMed] [Google Scholar]
- Pardal R, Lopez-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci. 2002;5:197–198. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- Peers C, Buckler KJ. Transduction of chemostimuli by the type I carotid body cell. J Membr Biol. 1995;144:1–9. doi: 10.1007/BF00238411. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Salehi SA, Abdulkader F, Braun M, MacDonald PE. K(ATP)-channels and glucose-regulated glucagon secretion. Trends Endocrinol Metab. 2008;19:277–284. doi: 10.1016/j.tem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Routh VH. Glucose-sensing neurons: are they physiologically relevant? Physiol Behav. 2002;76:403–413. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- Rowe IC, Treherne JM, Ashford ML. Activation by intracellular ATP of a potassium channel in neurones from rat basomedial hypothalamus. J Physiol. 1996;490(Pt 1):97–113. doi: 10.1113/jphysiol.1996.sp021129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre- and post-synaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2001;50:2673–2681. doi: 10.2337/diabetes.50.12.2673. [DOI] [PubMed] [Google Scholar]
- Tinel N, Diochot S, Borsotto M, Lazdunski M, Barhanin J. KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. EMBO J. 2000;19:6326–6330. doi: 10.1093/emboj/19.23.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RH, Burdakov D. Silencing of ventromedial hypothalamic neurons by glucose-stimulated K(+) currents. Pflugers Arch. 2009 doi: 10.1007/s00424-009-0650-6. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]